membrane7788kk.com
7788kk.com 时间:2021-03-21 阅读:()
ReviewWWdomaininteractionsregulatetheHippotumorsuppressorpathwayZSalah1andRIAqeilan*,1TheHippokinasepathwayisemergingasaconservedsignalingpathwaythatisessentialfororgangrowthandtumorigenesisinDrosophilaandmammalians.
Althoughthesignalingofthecorekinasesisrelativelywellunderstood,lessisknownabouttheupstreaminputs,downstreamoutputsandregulationofthewholecascade.
EnrichmentoftheHippopathwaycomponentswithWWdomainsandtheircognateproline-richinteractingmotifsprovidesaversatileplatformforfurtherunderstandingthemechanismsthatregulateorgangrowthandtumorigenesis.
Here,wereviewrecentlydiscoveredmechanismsofWWdomain-mediatedinteractionsthatcontributetotheregulationoftheHipposignalingpathwayintumorigenesis.
Wefurtherdiscussnewinsightsandfuturedirectionsontheemergingroleofsuchregulation.
CellDeathandDisease(2011)2,e172;doi:10.
1038/cddis.
2011.
53;publishedonline16June2011SubjectCategory:CancerThemechanismscontrollingmammalianorgansizehavebeentheinterestofscientistsforalongtime.
Duringthelastfewyears,immenseprogresshasbeenmadeindecipheringthesemechanismsandtheirimplicationsindiseasedevelopment,includingcancer.
Theregulationoforgangrowthiscontrolledbythenumberofcelldivisionsandtherateofcelldeath.
Theseprocessesregulatetissuehomeostasisandmaintaintheproperfunctionoforgans.
TherecentdiscoveryoftheHippopathwayasakeyregulatoroforgangrowthinfruitieshasgenerateddeeperinsightsintothemechanismoforgansize.
1,2Moreover,deregulationoftheHippopathwaycomponentsinmanydifferenttypesofcancersfurthersitscriticalroleintumorigenesis(reviewedinZhaoetal.
3).
AlthoughsignicantprogresshasbeenmadeinunderstandingthecoresignalingcascadeoftheHippopathway,muchlesshasbeenachievedinexploringtheregulationofthepathway.
Recently,muchattentionwasgiventotheunusualabundanceofWWmodulesandtheirinteractingcognateswithinsignalingmoleculesoftheHippopathway.
4,5ThisprevalenceofWWdomain-mediatedcomplexesintheHippopathwayperhapsfacilitatesitsmolecularanalysis,aidsinpredictionofnewpathwaycomponentsanduncoversnewmechanismsofregulation.
WWDomainsManyofthesignalingproteinscontainmodulardomainsthatfacilitateprotein-proteininteractions,oftenthroughtherecognitionofspecicandshortpeptidemotifsintheirbindingpartners.
Theseinteractionsaremostlyregulatedbypost-translationalmodications,forexample,phosphorylation.
Specicprotein-proteininteractionscantherebycontrolthesubcellularlocalization,enzymaticactivityandtheassemblyofmulti-proteincomplexes,thusallowingtheowofinforma-tionthroughsignalingpathways.
OnesuchexampleistheWWdomainmodules'interactions.
WWdomain,thesmallestmodulethatnaturallyoccurs,consistsofB35–40aminoacidresidues,includingtwohighlyconservedtryptophan(W)residuesseparatedby20–23aminoacidsinthepolypeptidechain.
6–8ThesetwoWaminoacidsgivethedomainitsname,WWdomain.
Originally,WWdomainswereidentiedthroughdetailedcharacterizationoftheYes-associatedprotein(YAP)basedoncomputer-aidedanalysisofimperfectlyrepeatedsequencesinthemouseisoformofYAP,andinyeastfactorRSP5.
7,8FunctionalscreenofacDNAexpressionlibraryidentiedthersttwoputativeWWdomainligands,WBP1and2.
9,10Todate,WWdomainsconstituteveclassesdependingonthecontentoftheircognateproline-richbindingmotifs(PRM).
11–14ThemostabundanttypeofWWdomainsareclass-IWWdomains,whichbindtoPPxYmotifs,wherePisproline,xisanyaminoacidandYistyrosine.
AlthoughWWdomainswithindifferentproteinsmighthaveaverysimilarstructure,theyhavedifferentialbindingtovariousligands.
Moreover,differentWWdomainsfallinginatandemrepeatmannerhavedifferentReceived28.
3.
11;revised28.
4.
11;accepted29.
4.
11;EditedbyGMelino1TheLautenbergCenterforGeneralandTumorImmunology,DepartmentofImmunologyandCancerResearch-IMRIC,TheHebrewUniversity–HadassahMedicalSchool,Jerusalem,Israel*Correspondingauthor:RIAqeilan,TheLautenbergCenterforGeneralandTumorImmunology,DepartmentofImmunologyandCancerResearch-IMRIC,TheHebrewUniversity–HadassahMedicalSchool,POBox12272,Jerusalem91120,Israel.
Tel:97226758609;Fax:97226424653;E-mail:aqeilan@cc.
huji.
ac.
ilKeywords:WWdomain;Hippopathway;protein-proteininteraction;ITCH;LATS1Abbreviations:WBP1and2,WWdomainbindingprotein1/2;YAP,Yes-associatedprotein;PQBP1,polyglutaminetract-bindingprotein1;MST1/2,mammalianSTE20-likekinase1/2;LATS1/2,largetumorsuppressor,homolog1/2;PRM,proline-richbindingmotifs;Yki,Yorki;Sav,Salvador;Dchs,Dachsous;Ex,Expanded;Mer,merlin;TAZ,transcriptionalcoactivatorwithPDZbindingmotif;EMT,epithelial-to-mesenchymaltransition;CTGF,connectivetissuegrowthfactor;AMOTL1/2,angiomotin-likeproteins1and2;AMOT,Angiomotin;ASPP1/2,apoptosis-stimulatingproteinofp531and2;Dvl-2,dishevelled2;RUNX2,runt-relatedtranscriptionfactor2;ERBB4,erythroblasticleukemiaviraloncogenehomolog4Citation:CellDeathandDisease(2011)2,e172;doi:10.
1038/cddis.
2011.
53&2011MacmillanPublishersLimitedAllrightsreserved2041-4889/11www.
nature.
com/cddisbindingpropertiestodifferentproteins,suggestingthatWWdomainsbindtoavastrepertoireofdifferentproteinsandthattheymightbepartofcomplexesbridgingblocks.
15–17WWdomain-containingproteinsappeartobeveryimportantinhomeostasisastheyoccurinproteinsinvolvedinawidearrayofbiologicalprocessesincludingtranscription,apoptosis,differentiation,splicingandubiquitination.
Infact,thesedomainsgainedtheiressentialroleafterbeingshowntobeinvolvedinhumandiseasesincluding,Liddle'ssyndromeofhypertension,wheretheWWdomainligand(PPxYdomain)isdeletedormutated,18,19musculardystrophy,20,21Alzheimer's,22–24Huntington'sdiseases,25,26Golabi-Ito-Hallsyndromeofmentalretardation,inwhichthebindingofY65C-mutatedWWdomainofpolyglutaminetract-bindingprotein1(PQBP1)toitscognateproline-richligandsisabrogated,27andmorerecentlycancer.
3,28–30Moreover,WWdomain-containingproteinshavegainedfurtherinterestafterbeingidentiedintheHippotumorsuppressorpathway.
HippoTumorSuppressorPathwayThefactthatseparateWWdomainsfromthesameprotein,orcloselyrelatedproteins,canhavedifferentspecicitiesforproteinligands,andthatasinglepolypeptidecanbindmultipleclassesofWWdomainsthroughseparatePRMsuggestedthatWWdomainsprovideaversatileplatformtolinkindividualproteinsintophysiologicallyimportantnetworks.
16,17OnesuchimportantnetworkthathasreceivedmuchattentioninthelastfewyearsistheHippotumorsuppressorpathway.
TheHippopathwayisahighlyconservedpathwaythatregulatestissuegrowthandorgansizebyregulatingcellgrowth,proliferation,differentiationandapoptosis.
3,29Inactivationormutationsofsomecomponentsofthepathwaywereidentiedindifferenttypesofcancer.
3,29,31TheHippopathwayiscomposedofakinasecascadecorethatincludesMST1/2serine/threoninekinase(orthologofHpo),WW45scaffoldprotein(Sav),MOB(Mts)andLATS1/2kinases(Wts)(Figure1).
Thiskinasecascadeisactivatedbyamechanismthatisnotyetfullyestablished,althoughsomeproteinswereidentiedtofeedintothecoreHippokinasecassette-likeFat,Dachsous(Dchs),Kibra,Expanded(Ex),Merlin(Mer)andothers(reviewedinGruscheetal.
32).
ActivationofthecorecascadeleadstophosphorylationofYAP33–35andTAZ36(Ykiinies)leadingtotheirsequestrationinthecytoplasm,preventingtheirtranslocationtothenucleusandbindingtoTEADtranscriptionfactor,therebyinhibitingtranscriptionofdownstreamtargetgenesimplicatedinproliferation,anti-apoptosisandepithelial-to-mesenchymaltransition(EMT).
37AuniquefeatureoftheHippopathwayisthehighprevalenceofWWdomain-mediatedcomplexes,denedrecentlyasWWmodularityoftheHippopathway.
4TheWWdomaincontainingproteinsoccuratdifferentlevelsoftheHippopathway.
InthecorecomponentsoftheHippopathwayinbothDrosophilaandmammals,theinteractionsaremediatedviaPPxYmotifsandWWdomains.
InDrosophila,HpoandWtseachcontainPPxYmotifs,andSavcontainstwoWWdomains.
Inmammals,thecorecassettealsocontainseitherPPxY/Fmotifs(Table1),asinthecaseofLATS1/2andMST1/2,orWWdomains,asincaseofWW45.
4Inaddition,thenucleareffectorsofthepathway,YkiiniesandYAPorTAZinmammals,functionthroughWW–PPxYinteraction.
Indeed,ithasalsobeenshownthattheWWdomainsofYAParecrucialforYAPtranscriptionalco-activationfunction+ProliferationgenesAnti-apoptoticgenesEMT-relatedgenesUBMST1/2LATS1ITCHPTEADYAPYAPProteasomaldegradation14-3-3PYAP-TRCPPYAPUBFigure1ITCHregulatestheHippopathwaybydegradingLATS1.
TheE3ubiquitinligaseITCHinteractswithLATS1byWWdomain–PPxYmotif-dependentmannerleadingtoubiquitinationandprotaesomaldegradationofLATS1.
ThisresultsinreducedYAPS127phosphorylation,thuslesscytoplasmicsequestrationbybindingto14-3-3protein,reducedYAPprotaesomaldegradationmediatedbyb-TRCPE3ligaseandconsequentlyenhancedYAPtranslocationtothenucleustomediateYAPdependentco-activationofTEAD-responsivegenes,includingthoseimplicatedinproliferation,anti-apoptosisandEMT.
Uponactivationofthepathway,ITCH–LATS1interactionisenhancedleadingtomoreefcientdegradationofLATS1attenuatingitsphosphorylationactivityofYAP.
Thisfunctionalassociationmighthavearoleinne-tuningtheoutcomeoftheHippopathwayandcouldbederegulatedinspecicsettingsuchasintumorigenesis.
Table1ExamplesofWWdomainandPPxY-containingproteinsintheHippopathwayWWDomainproteinsYAP1/21-WWand2-WW3,33–35,37,46TAZ1-WW29,36,59KIBRA2-WW38–40WW45(SAV1)2-WW60,61ITCH4-WW42,43PPXY/F-containingproteinsDCHS1/24-PPxFand2-PPxFa4,32,62FT1/2PPxYandPPxFa4,63–65CRB1/21-PPxYand2-PPxFa4,66,67MST1/21-PPxFand1-PPxFa4,60,68,69LATS1/22-PPxYand1-PPxY34,35WBP23-PPxY47,48AMOT2-PPxY49,51AMOTL1/22-PPxY50ASPP1/21-PPxYand1-PPxFa4,52,53,70P731-PPxY28,30,44,45ERBB43-PPxY28,30,71–73SMAD11-PPxY74RUNX21-PPxY28,30,59,75DVL21-PPxY76aPPxFmotifwassuggestedbySudolandHarvey4asapotentialWWdomainligandbasedoninvitroresults.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan2CellDeathandDiseasedownstreamoftheHippopathway.
37NotonlydothecorecomponentsorthedownstreameffectorscontainWWdomainsbutalsoseveralupstreamregulatorsoftheHippopathway,inbothDrosophilaandmammals,containeitherWWorPPxYmotifs.
Forexample,theWWdomainproteinKibraisaHipposignalingcomponentupstreamofHpo/MSTandMerlin.
38,39ThismodularityintheHippopathwaymightintendthatthispathwayisregulatedbyWWdomain-containingproteinsatdifferentlevelsinthepathway,fromthemediatorsdowntothecorecomponentsandeffectors.
WWDomainProteinsRegulateMembersoftheHippoPathwayWWdomainsofkibraregulateHippopathwayproteins.
Recently,differentreportshavedescribedgrowingevidenceofanumberofproteinsthatregulatethecorecomponentsoftheHippopathway.
SomeoftheseproteinscanbebroadlytermedupstreamHippopathwayregulatorsandincludeproteinsthatsignalviatheatypicalcadherin,Fat,whichfunctionsasatransmembranereceptorfortheHippopathway.
32Additionally,theKibra–Expanded–Merlincomplexlinkstheapicalmembranetothecoreofthepathwayproteinsandtheapicobasalpolarityproteins.
32Theseupstreamregulatorsmakedifferentphysicalinteractionswiththepathwaytomanipulateitsfunctions.
OneexampleoftheseinteractionsistheWWdomain–PPxYmotifinteractioninducedbyKibra.
Recently,ithasbeenshownthatdifferentnullmutantsoftheKibrageneareassociatedwithincreasedcellnumberleadingtotissueovergrowth.
Ontheotherhand,Kibraoverexpressingclonescontainfewercellsthancontrolclonesassociatedwithinducedapoptosis.
40KibrafunctionsprimarilyupstreamofMerandcontributestoMer-independentregulationofYkiactivity.
ThiseffectonMerseemedtobemediatedbyphysicalinteractionofthetwoproteins.
ThisinteractionwasfoundtobeindependentoftheWWdomainsofKibra.
40OntheotherhandLingXiaoetal.
41showedthattheKibraWWdomainsareessentialforKibra–LATSinteractionandregulationofLATS1/2functionsinthecontextofthemammalianHippopathway.
Uponitsexpression,KibraactivatesLATS1/2asrevealedbyitsincreasedphos-phorylation,leadingtoincreasedphosphorylationoftheultimateeffectorofthepathway,YAP.
41NotonlywasKibrashowntoenhanceLATSfunctionbutitwasalsoshowntoberesponsibleforincreasedLATS2proteinlevels.
Kibra-LATS2associationincreasesLATS2half-life,atleastinpart,byinhibitingLATS2ubiquitinationanditsproteasomaldegradation.
41Implicationofthisfunctionalinteractionontumorigenesisinvivoisstilltobedetermined.
WWdomainsofITCHregulatesLATS1stability.
Re-cently,tworeportsidentiedtheE3ligaseresponsiblefortheproteasomaldegradationofLATS1.
Therst,comingfromourlab,identiedITCHasaWWdomain-containingproteinthatregulatesthestabilityofLATS1usingWWdomainarrays.
42ThesendingswereconrmedlaterbyanothergroupthatutilizedSILAC(StableIsotopeLabelingwithAminoAcidsincellculture).
43Botharticlescametothesameconclusion,identifyingLATS1asatargetoftheE3ligaseITCH(Figure1).
Inourwork,wedemonstratedthatITCH,mostlyviaitsrstWWdomain,interactswiththePPxYmotifsofLATS1andenhancesitsubiqitinationandproteasomaldegradation.
42Ofnote,ITCHinteractionwithLATS1wasincreaseduponactivationoftheHippopathwayeitherbyMST2overexpressionorbyhigh-celldensityculture.
ThisinteractionwasassociatedwithenhanceddegradationofLATS1andsuggestthatITCHmightspecicallytargettheactivatedformofLATS1.
42Expressionofakinase-deadmutantofMST2(MSTD-KD),whichisincapableofphos-phorylatingandactivatingLATS1,indeedrescued,atleastinpart,ITCH-mediatedLATS1degradation(Unpublisheddata,SalahandAqeilan).
WhetherITCHexpressionand/orfunctionisaffectedbyLATSkinasesisstillanopenquestion.
Collectively,thismaysuggestthatITCHmightfunctionasane-tuningregulatoroftheHippopathwayunderphysiologicalconditions.
ITCH-mediatedLATS1degradationisalsoaccompaniedbyreducedYAPphosphorylationonSer127,mildYAPaccumu-lationinthenucleusandincreasedco-activationfunctionofTEAD-responsivegenes.
42AsYAPphosphorylationhasbeenshowntotriggeritsdegradationbySCF-(bTRCP)E3ubiquitinligase,ourresultsmaysuggestthatITCHexpressionmightsignalforYAPstabilizationandTEADco-activation.
42ThendingsbySalahetal.
42furtherdemonstratedthatLATS1degradationbyITCHenhancesEMTinHeLaandMCF10Acells,phenocopyingoverexpressionofYAP.
1,3IncreasedlevelsofYAP-relatedEMTgenes,includingCTGFandbronectin,andincreasedcellularmigrationandinvasionarehallmarksofITCHoverexpression.
NotonlydidthecellsshowmoreEMTphenotypesbutalsoITCH-manipulatedcellsaremoretumorigenicbothinvitroandinvivo.
ThendingsofHoetal.
43alsoconrmedthatITCHnegativelyregulatesLATS1levelandfunctionasrelatedtocellproliferationandapoptosisinthesamewayasdemonstratedearlier.
42BecauseITCH,asanE3ligase,targetsmanysubstrates,44,45itispossibletospeculatethatthephenotypesobservedafterITCHoverexpressionarerelatedtotheregulationofthedifferenttargetsinagivencontext.
Nevertheless,thesephenotypeswererescued,atleastinpart,inoursettingswhenmanipulatingLATS1expression,suggestingthatLATS1isacriticaltargetofITCH-mediatedtumorgrowthandprogressionbyregulatingtheHippopathway.
AsdifferentWWdomainproteinsmaysharecommontargets,itislikelytoassumethatchangingthelevel,stabilityorsubcellularlocalizationofoneWWproteinwouldalterthefunctionandoutcomeofWWdomaintargets,dependingonthecellularcontextortheexpressionofthedifferentproteins.
17,30Forexample,p73isacommonligandforITCHandYAP.
Ononehand,ITCHdegradesp73,44whileontheotherhanditleadstoenhancedYAPtranslocationtothenucleustopromoteTEAD-dependenttranscription.
3Inaddi-tion,YAPisanimportantco-factorforp73-dependenttranscriptionalactivityandexertsatumorsuppressorroleinthiscontext.
45Therefore,ITCHoverexpressionmightserveasamolecularswitchbetweenopposingYAPfunctions.
WhetherYAPrelocatesbetweenp73/YAPtargetsandTEAD/YAPtargetsinresponsetoITCHistobedeterminedinfutureWWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan3CellDeathandDiseasestudies.
ItwouldalsobenecessarytodeterminewhethertargetedmanipulationofWWdomainproteinsortheirinteractingpartnersintheHippopathwaywouldtilttheoutcomeoforgansizeand/ortumorigenicity.
AsITCHbehavesasaproto-oncogene,itmightalsocontributetotheobserveddownregulationofLATS1levelsincancer,andpossiblyothercomponentsoftheHippotumorsuppressorpathway.
Insummary,thesendingssuggestthatnovelWWdomainscouldregulatethecorecomponentsoftheHippopathwaytherebyaffectingtumorigenesisand,perhaps,organgrowth.
PPxY-containingproteinsregulateeffectorsoftheHippopathway.
AnotherlevelwhereWWdomainsappeartoregulatetheHippopathwayisontheleveloftheeffectors,YAPandTAZ.
Indeed,LATSproteins,viatheirPPxYmotifs,havebeenshowntobindtoWWdomainsofYAPleadingtoYAPphosphorylation,sequestrationinthecytoplasmandinactivation.
33,34,46ThisleadstoreduceYAP-inducedEMTphenotypesandisassociatedwithreducedtumor-igenicity.
1,34Infact,itwasshownthattheWWdomainofYAPhasacriticalroleininducingasubsetofYAPtargetgenesindependentof,orincooperationwith,TEAD.
37Inaddition,mutagenesisoftheWWdomainsdiminishestheabilityofYAPtostimulatecellproliferationandoncogenictransformation.
37Insupportofthisnotion,tworecentpapersshowedthatWWdomain-mediatedinteractionwithWBP2isimportantforthephenotypesinducedbybothYki47andTAZ.
48Intherstwork,Zhangetal.
47reportedthatYki,viaitsWWdomain,bindstothePPxYmotifsofWbp2.
ImportantlythisinteractionleadstoincreasedYkitranscriptionalco-activationfunctionandisassociatedwithYki-driventissueovergrowth.
KnockdownofWbp2expressionbyRNAiinawts-decientbackgroundreversedthelethalovergrowthphenotypesinwtsnullorganisms,suggestingthatYkifunctionismediatedbyWbp2.
47Inmammaliancells,TAZ'sWWdomains'interactionwithPPxYmotifsofWBP2suggestedanindispensableroleofWBP2inTAZtransformingability.
48AlthoughknockdownofWBP2suppressedTAZ-driventransformation,itsoverexpressionenhancedthistransformation.
48Recently,thePPxY-containingAngiomotin(AMOT)-likeproteins1and2(AMOTL1/AMOTL2)wereidentiedasregulatorsofthedownstreameffectorsoftheHippopathway,YAPandTAZ.
49–51ThreearticleshighlightthesignicanceofthisinteractionandshedlightontheroleofAMOTcelljunctionproteinsinregulatingYAPandTAZfunction.
49–51TheseproteinswerefoundtospecicallyinteractwithYAPinaWWdomain-PPxYmotif-dependentmanner.
ThisinteractionwasfoundtobesufcienttosequesterYAPandTAZinthecytoplasm,independentoftheirphosphorylationstatus.
Specically,AMOTexpressionleadstoYAPlocalizationatthetightjunctionandcellmembrane,preventingYAPnucleartranslocation.
51Moreover,itwasshownthatknockdownofAMOTL2phenocopiesYAP-inducedEMTinMCF10Acells.
51Consideringthisscenario,lossoftightjunction-localizedYAPandTAZincreasedtheirnuclearlocalizationandwasaccompaniedbyinductionofYAP/TAZtargetgeneexpres-sion,andmostimportantly,transformationandlossofcellcontactinhibition.
Furthermore,AMOTL2knockdown-depen-dentphenotypeswereblockedbysimultaneousknockdownofYAPandTAZ,demonstratingthattheAMOTfamilyproteinsarenewcomponentsoftheHippopathwaywithtumor-suppressingpotential,indicatinganewmodeofYAPandTAZregulation.
51Inadifferentmanner,WWdomain-PPxYmotifinteractionwasinvolvedintheregulationofthedownstreameffectorsoftheHippopathwaybyinvolvingmorethantwoproteins.
Forexample,ASPP2wasshowntostimulateTAZdepho-sphorylation,partlybypromotingtheinteractionbetweenTAZandPP1;thisfunctionofASPP2requirestheTAZWWdomain.
ASPP2–TAZinteractionpromotesTAZnuclearlocalizationandTAZtargetgeneexpression.
52Inanotherexample,itwasshownthatASPP1wasabletoinhibitYAP/TAZinteractionwithLATS1,leadingtoenhancednuclearaccumulationofYAP/TAZandYAP/TAZ-dependenttranscriptionalregulation.
ThisresultsinYAP/TAZactivationandthusinhibitsapoptosis,inpart,throughthedownregula-tionofBimexpression,leadingtoresistancetoanoikisandenhancedcellmigration.
53ConcludingRemarksandFutureDirectionsTheuniquefeatureoftheHippopathwayoverothersignalingpathwaysisitshighmodularityrepresentedbythegreatprevalenceofWW–PPxYinteractions,whichmightstronglysuggestthatotherWWdomainandPPxYmotif-containingproteinsregulate,orarepartof,theHippopathway.
ThestudyofWWdomainsandHippopathwayinrecentyearsfurtherhighlightedimportantaspectsofWWdomainproteinsignalingincludingdimerizationcapability,regulationofWWdomain-PRMinteractionandnetworking(reviewedinSudol5).
WWdomainsarepresentinawidevarietyofcellularproteinsincludingE3ligases,co-activators,co-repressorsandadapterproteinsthatcouldpotentiallyregulatemembersoftheHippopathway.
Takingintoconsiderationtheimportantroleofthispathwayintissuegrowthandhomeostasis,furthereffortsshouldbeinvestedinidentifyingnewregulatorsandcompo-nentsofthispathway.
TheuseofGFP-expressingtumorcellsinfreshtissueorliveanimalsshallfacilitatebetterchara-cterizationoftheHippopathwayproteinsandtheirrole,bothinvitroandinvivo,intumorinitiationandprogression.
54–57ExpansionofthisinformationmayaidindevelopingnewtherapeuticstrategiesbasedontheWWdomaininteractionsinthispathway.
Infact,thedesignofinhibitorsoractivatorsofWWdomainsignalingcomplexesintheHippopathwaycouldbefacilitatedbytheconsiderabledataavailableontheWWdomainstructure,themechanismofinteractionwithitsrigidligands,andthecomplexesitforms.
58OwingtothefactthattheWWdomainanditsligands'coremotifsarerelativelyshort,itmightbepossibletousesmallmoleculesthatfunctionasactivatorsorinhibitorsfortheHippopathwaysignalingproteins;thatis,smallchemicals/peptidesthatinhibitYAPandTAZoncogenicfunction.
However,beforethinkingabouttherapeuticstrategiesbasedonWWdomaininteractions,furtheranalysisoftheWWdomain-mediatedcomplexesintheHippopathwaymustbeelucidatedtobetterdesignnoveltherapeuticstrategiesformalfunctionsthatinvolvetheWWdomain.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan4CellDeathandDiseaseConictofInterestTheauthorsdeclarenoconictofinterest.
Acknowledgements.
WearegratefultoMsSherriCohenandMrsAlizaFormanforcriticalreadingofthemanuscript.
Weapologizetothosecolleagueswhoseworkwecouldnotcitebecauseofspacelimitation.
ThisworkwassupportedinpartbytheIsraeliScienceFoundationgrant(ISF#1331-08)andMarieCurie-EuropeanRe-integrationGrant(Project#224848)toAqeilanRIandIsraeliCancerResearchFund(ICRF)toSalahZ.
1.
DongJ,FeldmannG,HuangJ,WuS,ZhangN,ComerfordSAetal.
Elucidationofauniversalsize-controlmechanisminDrosophilaandmammals.
Cell2007;130:1120–1133.
2.
McNeillH,WoodgettJR.
Whenpathwayscollide:collaborationandconnivanceamongsignallingproteinsindevelopment.
NatRevMolCellBiol2010;11:404–413.
3.
ZhaoB,LiL,LeiQ,GuanKL.
TheHippo-YAPpathwayinorgansizecontrolandtumorigenesis:anupdatedversion.
GenesDev2010;24:862–874.
4.
SudolM,HarveyKF.
ModularityintheHipposignalingpathway.
TrendsBiochemSci2010;35:627–633.
5.
SudolM.
NewcomerstotheWWdomain–mediatednetworkoftheHippotumorsuppressorpathway.
GenesCancer2011;1:1115–1118.
6.
BorkP,SudolM.
TheWWdomain:asignallingsiteindystrophinTrendsBiochemSci1994;19:531–533.
7.
SudolM,BorkP,EinbondA,KasturyK,DruckT,NegriniMetal.
CharacterizationofthemammalianYAP(Yes-associatedprotein)geneanditsroleindeninganovelproteinmodule,theWWdomain.
JBiolChem1995;270:14733–14741.
8.
SudolM.
WWdomainin''modularproteindomains''In:CesareniG,GimonaM,SudolM,andYaffeM(eds).
ModularProteinDomains.
Wiley-VCHVerlagGmBH&Co,KGaA:Weinheim,FRG,2004,pp59–72.
9.
ChenHI,SudolM.
TheWWdomainofYes-associatedproteinbindsaproline-richligandthatdiffersfromtheconsensusestablishedforSrchomology3-bindingmodules.
ProcNatlAcadSciUSA1995;92:7819–7823.
10.
SudolM.
StructureandfunctionoftheWWdomain.
ProgBiophysMolBiol1996;65:113–132.
11.
ChenHI,EinbondA,KwakSJ,LinnH,KoepfE,PetersonSetal.
CharacterizationoftheWWdomainofhumanyes-associatedproteinanditspolyproline-containingligands.
JBiolChem1997;272:17070–17077.
12.
MaciasMJ,WiesnerS,SudolM.
WWandSH3domains,twodifferentscaffoldstorecognizeproline-richligands.
FEBSLett2002;513:30–37.
13.
OtteL,WiedemannU,SchlegelB,PiresJR,BeyermannM,SchmiederPetal.
WWdomainsequenceactivityrelationshipsidentiedusingligandrecognitionpropensitiesof42WWdomains.
ProteinSci2003;12:491–500.
14.
SudolM,HunterT.
NeWwrinklesforanolddomain.
Cell2000;103:1001–1004.
15.
FotiaAB,DinudomA,ShearwinKE,KochJP,KorbmacherC,CookDIetal.
TheroleofindividualNedd4-2(KIAA0439)WWdomainsinbindingandregulatingepithelialsodiumchannels.
FASEBJ2003;17:70–72.
16.
InghamRJ,ColwillK,HowardC,DettwilerS,LimCS,YuJetal.
WWdomainsprovideaplatformfortheassemblyofmultiproteinnetworks.
MolCellBiol2005;25:7092–7106.
17.
SalahZ,AlianA,AqeilanR.
WWdomain-containingproteins:retrospectivesandthefuture.
FrontBiosci2011.
18.
HanssonJH,SchildL,LuY,WilsonTA,GautschiI,ShimketsRetal.
AdenovomissensemutationofthebetasubunitoftheepithelialsodiumchannelcauseshypertensionandLiddlesyndrome,identifyingaproline-richsegmentcriticalforregulationofchannelactivity.
ProcNatlAcadSciUSA1995;92:11495–11499.
19.
InoueJ,IwaokaT,TokunagaH,TakamuneK,NaomiS,ArakiMetal.
AfamilywithLiddle'ssyndromecausedbyanewmissensemutationinthebetasubunitoftheepithelialsodiumchannel.
JClinEndocrinolMetab1998;83:2210–2213.
20.
ChungW,CampanelliJT.
WWandEFhanddomainsofdystrophin-familyproteinsmediatedystroglycanbinding.
MolCellBiolResCommun1999;2:162–171.
21.
HuangX,PoyF,ZhangR,JoachimiakA,SudolM,EckMJ.
StructureofaWWdomaincontainingfragmentofdystrophinincomplexwithbeta-dystroglycan.
NatStructBiol2000;7:634–638.
22.
LiuF,LiB,TungEJ,Grundke-IqbalI,IqbalK,GongCX.
Site-speciceffectsoftauphosphorylationonitsmicrotubuleassemblyactivityandself-aggregation.
EurJNeurosci2007;26:3429–3436.
23.
MandelkowEM,MandelkowE.
TauinAlzheimer'sdisease.
TrendsCellBiol1998;8:425–427.
24.
Morishima-KawashimaM,HasegawaM,TakioK,SuzukiM,YoshidaH,WatanabeAetal.
HyperphosphorylationoftauinPHF.
NeurobiolAging1995;16:365–371;discussion371-380.
25.
FaberPW,BarnesGT,SrinidhiJ,ChenJ,GusellaJF,MacDonaldME.
HuntingtininteractswithafamilyofWWdomainproteins.
HumMolGenet1998;7:1463–1474.
26.
PassaniLA,BedfordMT,FaberPW,McGinnisKM,SharpAH,GusellaJFetal.
Huntingtin'sWWdomainpartnersinHuntington'sdiseasepost-mortembrainfulllgeneticcriteriafordirectinvolvementinHuntington'sdiseasepathogenesis.
HumMolGenet2000;9:2175–2182.
27.
TapiaVE,NicolaescuE,McDonaldCB,MusiV,OkaT,InayoshiYetal.
Y65CmissensemutationintheWWdomainoftheGolabi-Ito-HallsyndromeproteinPQBP1affectsitsbindingactivityandderegulatespre-mRNAsplicing.
JBiolChem2010;285:19391–19401.
28.
DelMareS,SalahZ,AqeilanRI.
WWOX:itsgenomics,partners,andfunctions.
JCellBiochem2009;108:737–745.
29.
PanD.
Thehipposignalingpathwayindevelopmentandcancer.
DevCell2010;19:491–505.
30.
SalahZ,AqeilanR,HuebnerK.
WWOXgeneandgeneproduct:tumorsuppressionthroughspecicproteininteractions.
FutureOncol2010;6:249–259.
31.
HarveyK,TaponN.
TheSalvador-Warts-Hippopathway-anemergingtumour-suppressornetwork.
NatRevCancer2007;7:182–191.
32.
GruscheFA,RichardsonHE,HarveyKF.
Upstreamregulationofthehipposizecontrolpathway.
CurrBiol2010;20:R574–R582.
33.
HaoY,ChunA,CheungK,RashidiB,YangX.
TumorsuppressorLATS1isanegativeregulatorofoncogeneYAP.
JBiolChem2008;283:5496–5509.
34.
ZhangJ,SmolenGA,HaberDA.
NegativeregulationofYAPbyLATS1underscoresevolutionaryconservationoftheDrosophilaHippopathway.
CancerRes2008;68:2789–2794.
35.
ZhaoB,WeiX,LiW,UdanRS,YangQ,KimJetal.
InactivationofYAPoncoproteinbytheHippopathwayisinvolvedincellcontactinhibitionandtissuegrowthcontrol.
GenesDev2007;21:2747–2761.
36.
LeiQY,ZhangH,ZhaoB,ZhaZY,BaiF,PeiXHetal.
TAZpromotescellproliferationandepithelial-mesenchymaltransitionandisinhibitedbythehippopathway.
MolCellBiol2008;28:2426–2436.
37.
ZhaoB,KimJ,YeX,LaiZC,GuanKL.
BothTEAD-bindingandWWdomainsarerequiredforthegrowthstimulationandoncogenictransformationactivityofyes-associatedprotein.
CancerRes2009;69:1089–1098.
38.
GenevetA,WehrMC,BrainR,ThompsonBJ,TaponN.
KibraisaregulatoroftheSalvador/Warts/Hipposignalingnetwork.
DevCell2010;18:300–308.
39.
YuJ,ZhengY,DongJ,KluszaS,DengWM,PanD.
KibrafunctionsasatumorsuppressorproteinthatregulatesHipposignalinginconjunctionwithMerlinandExpanded.
DevCell2010;18:288–299.
40.
BaumgartnerR,PoernbacherI,BuserN,HafenE,StockerH.
TheWWdomainproteinKibraactsupstreamofHippoinDrosophila.
DevCell2010;18:309–316.
41.
XiaoL,ChenY,JiM,DongJ.
KIBRAregulatesHipposignalingactivityviainteractionswithlargetumorsuppressorkinases.
JBiolChem2011;286:7788–7796.
42.
SalahZ,MelinoG,AqeilanRI.
NegativeregulationoftheHippopathwaybyE3ubiquitinligaseItchissufcienttopromotetumorigenicity.
CancerRes2011;71:2010–2020.
43.
HoKC,ZhouZ,SheYM,ChunA,CyrTD,YangX.
ItchE3ubiquitinligaseregulateslargetumorsuppressor1tumor-suppressorstability.
ProcNatlAcadSciUSA2011;108:4870–4875.
44.
RossiM,DeLaurenziV,MunarrizE,GreenDR,LiuYC,VousdenKHetal.
Theubiquitin-proteinligaseItchregulatesp73stability.
EMBOJ2005;24:836–848.
45.
StranoS,MunarrizE,RossiM,CastagnoliL,ShaulY,SacchiAetal.
PhysicalinteractionwithYes-associatedproteinenhancesp73transcriptionalactivity.
JBiolChem2001;276:15164–15173.
46.
OkaT,MazackV,SudolM.
Mst2andLatskinasesregulateapoptoticfunctionofYeskinase-associatedprotein(YAP).
JBiolChem2008;283:27534–27546.
47.
ZhangX,MiltonCC,PoonCL,HongW,HarveyKF.
Wbp2cooperateswithYorkietodrivetissuegrowthdownstreamoftheSalvador-Warts-Hippopathway.
CellDeathDiffer2011.
48.
ChanSW,LimCJ,HuangC,ChongYF,GunaratneHJ,HogueKAetal.
WWdomain-mediatedinteractionwithWbp2isimportantfortheoncogenicpropertyofTAZ.
Oncogene2011;30:600–610.
49.
ChanSW,LimCJ,ChongYF,PobbatiAV,HuangC,HongW.
Hippopathway-independentrestrictionofTAZandYAPbyangiomotin.
JBiolChem2011;286:7018–7026.
50.
WangW,HuangJ,ChenJ.
Angiomotin-likeproteinsassociatewithandnegativelyregulateYAP1.
JBiolChem2011;286:4364–4370.
51.
ZhaoB,LiL,LuQ,WangLH,LiuCY,LeiQetal.
AngiomotinisanovelHippopathwaycomponentthatinhibitsYAPoncoprotein.
GenesDev2011;25:51–63.
52.
LiuCY,LvX,LiT,XuY,ZhouX,ZhaoSetal.
PP1cooperateswithASPP2todephosphorylateandactivateTAZ.
JBiolChem2011;286:5558–5566.
53.
VigneronAM,LudwigRL,VousdenKH.
CytoplasmicASPP1inhibitsapoptosisthroughthecontrolofYAP.
GenesDev2010;24:2430–2439.
54.
HoffmanRM.
Themultipleusesofuorescentproteinstovisualizecancerinvivo.
NatRevCancer2005;5:796–806.
55.
HoffmanRM,YangM.
Subcellularimaginginthelivemouse.
NatProtoc2006;1:775–782.
56.
HoffmanRM,YangM.
Color-codeduorescenceimagingoftumor-hostinteractions.
NatProtoc2006;1:928–935.
57.
HoffmanRM,YangM.
Whole-bodyimagingwithuorescentproteins.
NatProtoc2006;1:1429–1438.
58.
MaciasMJ,HyvonenM,BaraldiE,SchultzJ,SudolM,SarasteMetal.
StructureoftheWWdomainofakinase-associatedproteincomplexedwithaproline-richpeptide.
Nature1996;382:646–649.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan5CellDeathandDisease59.
HongJH,HwangES,McManusMT,AmsterdamA,TianY,KalmukovaRetal.
TAZ,atranscriptionalmodulatorofmesenchymalstemcelldifferentiation.
Science2005;309:1074–1078.
60.
HarveyKF,PegerCM,HariharanIK.
TheDrosophilaMstortholog,Hippo,restrictsgrowthandcellproliferationandpromotesapoptosis.
Cell2003;114:457–467.
61.
TaponN,HarveyKF,BellDW,WahrerDC,SchiripoTA,HaberDAetal.
SalvadorpromotesbothcellcycleexitandapoptosisinDrosophilaandismutatedinhumancancercelllines.
Cell2002;110:467–478.
62.
BrittleAL,RepisoA,CasalJ,LawrencePA,StruttD.
Four-jointedmodulatesgrowthandplanarpolaritybyreducingtheafnityofdachsousforfat.
CurrBiol2010;20:803–810.
63.
ChoE,FengY,RauskolbC,MaitraS,FehonR,IrvineKD.
Delineationofafattumorsuppressorpathway.
NatGenet2006;38:1142–1150.
64.
FengY,IrvineKD.
ProcessingandphosphorylationoftheFatreceptor.
ProcNatlAcadSciUSA2009;106:11989–11994.
65.
SopkoR,SilvaE,ClaytonL,GardanoL,Barrios-RodilesM,WranaJetal.
PhosphorylationofthetumorsuppressorfatisregulatedbyitsligandDachsousandthekinasediscsovergrown.
CurrBiol2009;19:1112–1117.
66.
ChenCL,GajewskiKM,HamaratogluF,BossuytW,Sansores-GarciaL,TaoCetal.
Theapical-basalcellpolaritydeterminantCrumbsregulatesHipposignalinginDrosophila.
ProcNatlAcadSciUSA2010;107:15810–15815.
67.
LingC,ZhengY,YinF,YuJ,HuangJ,HongYetal.
TheapicaltransmembraneproteinCrumbsfunctionsasatumorsuppressorthatregulatesHipposignalingbybindingtoExpanded.
ProcNatlAcadSciUSA2010;107:10532–10537.
68.
SongH,MakKK,TopolL,YunK,HuJ,GarrettLetal.
MammalianMst1andMst2kinasesplayessentialrolesinorgansizecontrolandtumorsuppression.
ProcNatlAcadSciUSA2010;107:1431–1436.
69.
ZhouD,ConradC,XiaF,ParkJS,PayerB,YinYetal.
Mst1andMst2maintainhepatocytequiescenceandsuppresshepatocellularcarcinomadevelopmentthroughinactivationoftheYap1oncogene.
CancerCell2009;16:425–438.
70.
PatelS,GeorgeR,AutoreF,FraternaliF,LadburyJE,NikolovaPV.
MolecularinteractionsofASPP1andASPP2withthep53proteinfamilyandtheapoptoticpromotersPUMAandBax.
NucleicAcidsRes2008;36:5139–5151.
71.
KomuroA,NagaiM,NavinNE,SudolM.
WWdomain-containingproteinYAPassociateswithErbB-4andactsasaco-transcriptionalactivatorforthecarboxyl-terminalfragmentofErbB-4thattranslocatestothenucleus.
JBiolChem2003;278:33334–33341.
72.
WebbC,UpadhyayA,GiuntiniF,EgglestonI,Furutani-SeikiM,IshimaRetal.
StructuralfeaturesandligandbindingpropertiesoftandemWWdomainsfromYAPandTAZ,nucleareffectorsoftheHippopathway.
Biochemistry2011;50:3300–3309.
73.
AqeilanRI,DonatiV,PalamarchukA,TrapassoF,KaouM,PekarskyYetal.
WWdomain-containingproteins,WWOXandYAP,competeforinteractionwithErbB-4andmodulateitstranscriptionalfunction.
CancerRes2005;65:6764–6772.
74.
AlarconC,ZaromytidouAI,XiQ,GaoS,YuJ,FujisawaSetal.
NuclearCDKsdriveSmadtranscriptionalactivationandturnoverinBMPandTGF-betapathways.
Cell2009;139:757–769.
75.
ZaidiSK,SullivanAJ,MedinaR,ItoY,vanWijnenAJ,SteinJLetal.
TyrosinephosphorylationcontrolsRunx2-mediatedsubnucleartargetingofYAPtorepresstranscription.
EMBOJ2004;23:790–799.
76.
VarelasX,MillerBW,SopkoR,SongS,GregorieffA,FellouseFAetal.
TheHippopathwayregulatesWnt/beta-cateninsignaling.
DevCell2010;18:579–591.
CellDeathandDiseaseisanopen-accessjournalpublishedbyNaturePublishingGroup.
ThisworkislicensedundertheCreativeCommonsAttribution-Noncommercial-NoDerivativeWorks3.
0UnportedLicense.
Toviewacopyofthislicense,visithttp://creativecommons.
org/licenses/by-nc-nd/3.
0/WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan6CellDeathandDisease
Althoughthesignalingofthecorekinasesisrelativelywellunderstood,lessisknownabouttheupstreaminputs,downstreamoutputsandregulationofthewholecascade.
EnrichmentoftheHippopathwaycomponentswithWWdomainsandtheircognateproline-richinteractingmotifsprovidesaversatileplatformforfurtherunderstandingthemechanismsthatregulateorgangrowthandtumorigenesis.
Here,wereviewrecentlydiscoveredmechanismsofWWdomain-mediatedinteractionsthatcontributetotheregulationoftheHipposignalingpathwayintumorigenesis.
Wefurtherdiscussnewinsightsandfuturedirectionsontheemergingroleofsuchregulation.
CellDeathandDisease(2011)2,e172;doi:10.
1038/cddis.
2011.
53;publishedonline16June2011SubjectCategory:CancerThemechanismscontrollingmammalianorgansizehavebeentheinterestofscientistsforalongtime.
Duringthelastfewyears,immenseprogresshasbeenmadeindecipheringthesemechanismsandtheirimplicationsindiseasedevelopment,includingcancer.
Theregulationoforgangrowthiscontrolledbythenumberofcelldivisionsandtherateofcelldeath.
Theseprocessesregulatetissuehomeostasisandmaintaintheproperfunctionoforgans.
TherecentdiscoveryoftheHippopathwayasakeyregulatoroforgangrowthinfruitieshasgenerateddeeperinsightsintothemechanismoforgansize.
1,2Moreover,deregulationoftheHippopathwaycomponentsinmanydifferenttypesofcancersfurthersitscriticalroleintumorigenesis(reviewedinZhaoetal.
3).
AlthoughsignicantprogresshasbeenmadeinunderstandingthecoresignalingcascadeoftheHippopathway,muchlesshasbeenachievedinexploringtheregulationofthepathway.
Recently,muchattentionwasgiventotheunusualabundanceofWWmodulesandtheirinteractingcognateswithinsignalingmoleculesoftheHippopathway.
4,5ThisprevalenceofWWdomain-mediatedcomplexesintheHippopathwayperhapsfacilitatesitsmolecularanalysis,aidsinpredictionofnewpathwaycomponentsanduncoversnewmechanismsofregulation.
WWDomainsManyofthesignalingproteinscontainmodulardomainsthatfacilitateprotein-proteininteractions,oftenthroughtherecognitionofspecicandshortpeptidemotifsintheirbindingpartners.
Theseinteractionsaremostlyregulatedbypost-translationalmodications,forexample,phosphorylation.
Specicprotein-proteininteractionscantherebycontrolthesubcellularlocalization,enzymaticactivityandtheassemblyofmulti-proteincomplexes,thusallowingtheowofinforma-tionthroughsignalingpathways.
OnesuchexampleistheWWdomainmodules'interactions.
WWdomain,thesmallestmodulethatnaturallyoccurs,consistsofB35–40aminoacidresidues,includingtwohighlyconservedtryptophan(W)residuesseparatedby20–23aminoacidsinthepolypeptidechain.
6–8ThesetwoWaminoacidsgivethedomainitsname,WWdomain.
Originally,WWdomainswereidentiedthroughdetailedcharacterizationoftheYes-associatedprotein(YAP)basedoncomputer-aidedanalysisofimperfectlyrepeatedsequencesinthemouseisoformofYAP,andinyeastfactorRSP5.
7,8FunctionalscreenofacDNAexpressionlibraryidentiedthersttwoputativeWWdomainligands,WBP1and2.
9,10Todate,WWdomainsconstituteveclassesdependingonthecontentoftheircognateproline-richbindingmotifs(PRM).
11–14ThemostabundanttypeofWWdomainsareclass-IWWdomains,whichbindtoPPxYmotifs,wherePisproline,xisanyaminoacidandYistyrosine.
AlthoughWWdomainswithindifferentproteinsmighthaveaverysimilarstructure,theyhavedifferentialbindingtovariousligands.
Moreover,differentWWdomainsfallinginatandemrepeatmannerhavedifferentReceived28.
3.
11;revised28.
4.
11;accepted29.
4.
11;EditedbyGMelino1TheLautenbergCenterforGeneralandTumorImmunology,DepartmentofImmunologyandCancerResearch-IMRIC,TheHebrewUniversity–HadassahMedicalSchool,Jerusalem,Israel*Correspondingauthor:RIAqeilan,TheLautenbergCenterforGeneralandTumorImmunology,DepartmentofImmunologyandCancerResearch-IMRIC,TheHebrewUniversity–HadassahMedicalSchool,POBox12272,Jerusalem91120,Israel.
Tel:97226758609;Fax:97226424653;E-mail:aqeilan@cc.
huji.
ac.
ilKeywords:WWdomain;Hippopathway;protein-proteininteraction;ITCH;LATS1Abbreviations:WBP1and2,WWdomainbindingprotein1/2;YAP,Yes-associatedprotein;PQBP1,polyglutaminetract-bindingprotein1;MST1/2,mammalianSTE20-likekinase1/2;LATS1/2,largetumorsuppressor,homolog1/2;PRM,proline-richbindingmotifs;Yki,Yorki;Sav,Salvador;Dchs,Dachsous;Ex,Expanded;Mer,merlin;TAZ,transcriptionalcoactivatorwithPDZbindingmotif;EMT,epithelial-to-mesenchymaltransition;CTGF,connectivetissuegrowthfactor;AMOTL1/2,angiomotin-likeproteins1and2;AMOT,Angiomotin;ASPP1/2,apoptosis-stimulatingproteinofp531and2;Dvl-2,dishevelled2;RUNX2,runt-relatedtranscriptionfactor2;ERBB4,erythroblasticleukemiaviraloncogenehomolog4Citation:CellDeathandDisease(2011)2,e172;doi:10.
1038/cddis.
2011.
53&2011MacmillanPublishersLimitedAllrightsreserved2041-4889/11www.
nature.
com/cddisbindingpropertiestodifferentproteins,suggestingthatWWdomainsbindtoavastrepertoireofdifferentproteinsandthattheymightbepartofcomplexesbridgingblocks.
15–17WWdomain-containingproteinsappeartobeveryimportantinhomeostasisastheyoccurinproteinsinvolvedinawidearrayofbiologicalprocessesincludingtranscription,apoptosis,differentiation,splicingandubiquitination.
Infact,thesedomainsgainedtheiressentialroleafterbeingshowntobeinvolvedinhumandiseasesincluding,Liddle'ssyndromeofhypertension,wheretheWWdomainligand(PPxYdomain)isdeletedormutated,18,19musculardystrophy,20,21Alzheimer's,22–24Huntington'sdiseases,25,26Golabi-Ito-Hallsyndromeofmentalretardation,inwhichthebindingofY65C-mutatedWWdomainofpolyglutaminetract-bindingprotein1(PQBP1)toitscognateproline-richligandsisabrogated,27andmorerecentlycancer.
3,28–30Moreover,WWdomain-containingproteinshavegainedfurtherinterestafterbeingidentiedintheHippotumorsuppressorpathway.
HippoTumorSuppressorPathwayThefactthatseparateWWdomainsfromthesameprotein,orcloselyrelatedproteins,canhavedifferentspecicitiesforproteinligands,andthatasinglepolypeptidecanbindmultipleclassesofWWdomainsthroughseparatePRMsuggestedthatWWdomainsprovideaversatileplatformtolinkindividualproteinsintophysiologicallyimportantnetworks.
16,17OnesuchimportantnetworkthathasreceivedmuchattentioninthelastfewyearsistheHippotumorsuppressorpathway.
TheHippopathwayisahighlyconservedpathwaythatregulatestissuegrowthandorgansizebyregulatingcellgrowth,proliferation,differentiationandapoptosis.
3,29Inactivationormutationsofsomecomponentsofthepathwaywereidentiedindifferenttypesofcancer.
3,29,31TheHippopathwayiscomposedofakinasecascadecorethatincludesMST1/2serine/threoninekinase(orthologofHpo),WW45scaffoldprotein(Sav),MOB(Mts)andLATS1/2kinases(Wts)(Figure1).
Thiskinasecascadeisactivatedbyamechanismthatisnotyetfullyestablished,althoughsomeproteinswereidentiedtofeedintothecoreHippokinasecassette-likeFat,Dachsous(Dchs),Kibra,Expanded(Ex),Merlin(Mer)andothers(reviewedinGruscheetal.
32).
ActivationofthecorecascadeleadstophosphorylationofYAP33–35andTAZ36(Ykiinies)leadingtotheirsequestrationinthecytoplasm,preventingtheirtranslocationtothenucleusandbindingtoTEADtranscriptionfactor,therebyinhibitingtranscriptionofdownstreamtargetgenesimplicatedinproliferation,anti-apoptosisandepithelial-to-mesenchymaltransition(EMT).
37AuniquefeatureoftheHippopathwayisthehighprevalenceofWWdomain-mediatedcomplexes,denedrecentlyasWWmodularityoftheHippopathway.
4TheWWdomaincontainingproteinsoccuratdifferentlevelsoftheHippopathway.
InthecorecomponentsoftheHippopathwayinbothDrosophilaandmammals,theinteractionsaremediatedviaPPxYmotifsandWWdomains.
InDrosophila,HpoandWtseachcontainPPxYmotifs,andSavcontainstwoWWdomains.
Inmammals,thecorecassettealsocontainseitherPPxY/Fmotifs(Table1),asinthecaseofLATS1/2andMST1/2,orWWdomains,asincaseofWW45.
4Inaddition,thenucleareffectorsofthepathway,YkiiniesandYAPorTAZinmammals,functionthroughWW–PPxYinteraction.
Indeed,ithasalsobeenshownthattheWWdomainsofYAParecrucialforYAPtranscriptionalco-activationfunction+ProliferationgenesAnti-apoptoticgenesEMT-relatedgenesUBMST1/2LATS1ITCHPTEADYAPYAPProteasomaldegradation14-3-3PYAP-TRCPPYAPUBFigure1ITCHregulatestheHippopathwaybydegradingLATS1.
TheE3ubiquitinligaseITCHinteractswithLATS1byWWdomain–PPxYmotif-dependentmannerleadingtoubiquitinationandprotaesomaldegradationofLATS1.
ThisresultsinreducedYAPS127phosphorylation,thuslesscytoplasmicsequestrationbybindingto14-3-3protein,reducedYAPprotaesomaldegradationmediatedbyb-TRCPE3ligaseandconsequentlyenhancedYAPtranslocationtothenucleustomediateYAPdependentco-activationofTEAD-responsivegenes,includingthoseimplicatedinproliferation,anti-apoptosisandEMT.
Uponactivationofthepathway,ITCH–LATS1interactionisenhancedleadingtomoreefcientdegradationofLATS1attenuatingitsphosphorylationactivityofYAP.
Thisfunctionalassociationmighthavearoleinne-tuningtheoutcomeoftheHippopathwayandcouldbederegulatedinspecicsettingsuchasintumorigenesis.
Table1ExamplesofWWdomainandPPxY-containingproteinsintheHippopathwayWWDomainproteinsYAP1/21-WWand2-WW3,33–35,37,46TAZ1-WW29,36,59KIBRA2-WW38–40WW45(SAV1)2-WW60,61ITCH4-WW42,43PPXY/F-containingproteinsDCHS1/24-PPxFand2-PPxFa4,32,62FT1/2PPxYandPPxFa4,63–65CRB1/21-PPxYand2-PPxFa4,66,67MST1/21-PPxFand1-PPxFa4,60,68,69LATS1/22-PPxYand1-PPxY34,35WBP23-PPxY47,48AMOT2-PPxY49,51AMOTL1/22-PPxY50ASPP1/21-PPxYand1-PPxFa4,52,53,70P731-PPxY28,30,44,45ERBB43-PPxY28,30,71–73SMAD11-PPxY74RUNX21-PPxY28,30,59,75DVL21-PPxY76aPPxFmotifwassuggestedbySudolandHarvey4asapotentialWWdomainligandbasedoninvitroresults.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan2CellDeathandDiseasedownstreamoftheHippopathway.
37NotonlydothecorecomponentsorthedownstreameffectorscontainWWdomainsbutalsoseveralupstreamregulatorsoftheHippopathway,inbothDrosophilaandmammals,containeitherWWorPPxYmotifs.
Forexample,theWWdomainproteinKibraisaHipposignalingcomponentupstreamofHpo/MSTandMerlin.
38,39ThismodularityintheHippopathwaymightintendthatthispathwayisregulatedbyWWdomain-containingproteinsatdifferentlevelsinthepathway,fromthemediatorsdowntothecorecomponentsandeffectors.
WWDomainProteinsRegulateMembersoftheHippoPathwayWWdomainsofkibraregulateHippopathwayproteins.
Recently,differentreportshavedescribedgrowingevidenceofanumberofproteinsthatregulatethecorecomponentsoftheHippopathway.
SomeoftheseproteinscanbebroadlytermedupstreamHippopathwayregulatorsandincludeproteinsthatsignalviatheatypicalcadherin,Fat,whichfunctionsasatransmembranereceptorfortheHippopathway.
32Additionally,theKibra–Expanded–Merlincomplexlinkstheapicalmembranetothecoreofthepathwayproteinsandtheapicobasalpolarityproteins.
32Theseupstreamregulatorsmakedifferentphysicalinteractionswiththepathwaytomanipulateitsfunctions.
OneexampleoftheseinteractionsistheWWdomain–PPxYmotifinteractioninducedbyKibra.
Recently,ithasbeenshownthatdifferentnullmutantsoftheKibrageneareassociatedwithincreasedcellnumberleadingtotissueovergrowth.
Ontheotherhand,Kibraoverexpressingclonescontainfewercellsthancontrolclonesassociatedwithinducedapoptosis.
40KibrafunctionsprimarilyupstreamofMerandcontributestoMer-independentregulationofYkiactivity.
ThiseffectonMerseemedtobemediatedbyphysicalinteractionofthetwoproteins.
ThisinteractionwasfoundtobeindependentoftheWWdomainsofKibra.
40OntheotherhandLingXiaoetal.
41showedthattheKibraWWdomainsareessentialforKibra–LATSinteractionandregulationofLATS1/2functionsinthecontextofthemammalianHippopathway.
Uponitsexpression,KibraactivatesLATS1/2asrevealedbyitsincreasedphos-phorylation,leadingtoincreasedphosphorylationoftheultimateeffectorofthepathway,YAP.
41NotonlywasKibrashowntoenhanceLATSfunctionbutitwasalsoshowntoberesponsibleforincreasedLATS2proteinlevels.
Kibra-LATS2associationincreasesLATS2half-life,atleastinpart,byinhibitingLATS2ubiquitinationanditsproteasomaldegradation.
41Implicationofthisfunctionalinteractionontumorigenesisinvivoisstilltobedetermined.
WWdomainsofITCHregulatesLATS1stability.
Re-cently,tworeportsidentiedtheE3ligaseresponsiblefortheproteasomaldegradationofLATS1.
Therst,comingfromourlab,identiedITCHasaWWdomain-containingproteinthatregulatesthestabilityofLATS1usingWWdomainarrays.
42ThesendingswereconrmedlaterbyanothergroupthatutilizedSILAC(StableIsotopeLabelingwithAminoAcidsincellculture).
43Botharticlescametothesameconclusion,identifyingLATS1asatargetoftheE3ligaseITCH(Figure1).
Inourwork,wedemonstratedthatITCH,mostlyviaitsrstWWdomain,interactswiththePPxYmotifsofLATS1andenhancesitsubiqitinationandproteasomaldegradation.
42Ofnote,ITCHinteractionwithLATS1wasincreaseduponactivationoftheHippopathwayeitherbyMST2overexpressionorbyhigh-celldensityculture.
ThisinteractionwasassociatedwithenhanceddegradationofLATS1andsuggestthatITCHmightspecicallytargettheactivatedformofLATS1.
42Expressionofakinase-deadmutantofMST2(MSTD-KD),whichisincapableofphos-phorylatingandactivatingLATS1,indeedrescued,atleastinpart,ITCH-mediatedLATS1degradation(Unpublisheddata,SalahandAqeilan).
WhetherITCHexpressionand/orfunctionisaffectedbyLATSkinasesisstillanopenquestion.
Collectively,thismaysuggestthatITCHmightfunctionasane-tuningregulatoroftheHippopathwayunderphysiologicalconditions.
ITCH-mediatedLATS1degradationisalsoaccompaniedbyreducedYAPphosphorylationonSer127,mildYAPaccumu-lationinthenucleusandincreasedco-activationfunctionofTEAD-responsivegenes.
42AsYAPphosphorylationhasbeenshowntotriggeritsdegradationbySCF-(bTRCP)E3ubiquitinligase,ourresultsmaysuggestthatITCHexpressionmightsignalforYAPstabilizationandTEADco-activation.
42ThendingsbySalahetal.
42furtherdemonstratedthatLATS1degradationbyITCHenhancesEMTinHeLaandMCF10Acells,phenocopyingoverexpressionofYAP.
1,3IncreasedlevelsofYAP-relatedEMTgenes,includingCTGFandbronectin,andincreasedcellularmigrationandinvasionarehallmarksofITCHoverexpression.
NotonlydidthecellsshowmoreEMTphenotypesbutalsoITCH-manipulatedcellsaremoretumorigenicbothinvitroandinvivo.
ThendingsofHoetal.
43alsoconrmedthatITCHnegativelyregulatesLATS1levelandfunctionasrelatedtocellproliferationandapoptosisinthesamewayasdemonstratedearlier.
42BecauseITCH,asanE3ligase,targetsmanysubstrates,44,45itispossibletospeculatethatthephenotypesobservedafterITCHoverexpressionarerelatedtotheregulationofthedifferenttargetsinagivencontext.
Nevertheless,thesephenotypeswererescued,atleastinpart,inoursettingswhenmanipulatingLATS1expression,suggestingthatLATS1isacriticaltargetofITCH-mediatedtumorgrowthandprogressionbyregulatingtheHippopathway.
AsdifferentWWdomainproteinsmaysharecommontargets,itislikelytoassumethatchangingthelevel,stabilityorsubcellularlocalizationofoneWWproteinwouldalterthefunctionandoutcomeofWWdomaintargets,dependingonthecellularcontextortheexpressionofthedifferentproteins.
17,30Forexample,p73isacommonligandforITCHandYAP.
Ononehand,ITCHdegradesp73,44whileontheotherhanditleadstoenhancedYAPtranslocationtothenucleustopromoteTEAD-dependenttranscription.
3Inaddi-tion,YAPisanimportantco-factorforp73-dependenttranscriptionalactivityandexertsatumorsuppressorroleinthiscontext.
45Therefore,ITCHoverexpressionmightserveasamolecularswitchbetweenopposingYAPfunctions.
WhetherYAPrelocatesbetweenp73/YAPtargetsandTEAD/YAPtargetsinresponsetoITCHistobedeterminedinfutureWWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan3CellDeathandDiseasestudies.
ItwouldalsobenecessarytodeterminewhethertargetedmanipulationofWWdomainproteinsortheirinteractingpartnersintheHippopathwaywouldtilttheoutcomeoforgansizeand/ortumorigenicity.
AsITCHbehavesasaproto-oncogene,itmightalsocontributetotheobserveddownregulationofLATS1levelsincancer,andpossiblyothercomponentsoftheHippotumorsuppressorpathway.
Insummary,thesendingssuggestthatnovelWWdomainscouldregulatethecorecomponentsoftheHippopathwaytherebyaffectingtumorigenesisand,perhaps,organgrowth.
PPxY-containingproteinsregulateeffectorsoftheHippopathway.
AnotherlevelwhereWWdomainsappeartoregulatetheHippopathwayisontheleveloftheeffectors,YAPandTAZ.
Indeed,LATSproteins,viatheirPPxYmotifs,havebeenshowntobindtoWWdomainsofYAPleadingtoYAPphosphorylation,sequestrationinthecytoplasmandinactivation.
33,34,46ThisleadstoreduceYAP-inducedEMTphenotypesandisassociatedwithreducedtumor-igenicity.
1,34Infact,itwasshownthattheWWdomainofYAPhasacriticalroleininducingasubsetofYAPtargetgenesindependentof,orincooperationwith,TEAD.
37Inaddition,mutagenesisoftheWWdomainsdiminishestheabilityofYAPtostimulatecellproliferationandoncogenictransformation.
37Insupportofthisnotion,tworecentpapersshowedthatWWdomain-mediatedinteractionwithWBP2isimportantforthephenotypesinducedbybothYki47andTAZ.
48Intherstwork,Zhangetal.
47reportedthatYki,viaitsWWdomain,bindstothePPxYmotifsofWbp2.
ImportantlythisinteractionleadstoincreasedYkitranscriptionalco-activationfunctionandisassociatedwithYki-driventissueovergrowth.
KnockdownofWbp2expressionbyRNAiinawts-decientbackgroundreversedthelethalovergrowthphenotypesinwtsnullorganisms,suggestingthatYkifunctionismediatedbyWbp2.
47Inmammaliancells,TAZ'sWWdomains'interactionwithPPxYmotifsofWBP2suggestedanindispensableroleofWBP2inTAZtransformingability.
48AlthoughknockdownofWBP2suppressedTAZ-driventransformation,itsoverexpressionenhancedthistransformation.
48Recently,thePPxY-containingAngiomotin(AMOT)-likeproteins1and2(AMOTL1/AMOTL2)wereidentiedasregulatorsofthedownstreameffectorsoftheHippopathway,YAPandTAZ.
49–51ThreearticleshighlightthesignicanceofthisinteractionandshedlightontheroleofAMOTcelljunctionproteinsinregulatingYAPandTAZfunction.
49–51TheseproteinswerefoundtospecicallyinteractwithYAPinaWWdomain-PPxYmotif-dependentmanner.
ThisinteractionwasfoundtobesufcienttosequesterYAPandTAZinthecytoplasm,independentoftheirphosphorylationstatus.
Specically,AMOTexpressionleadstoYAPlocalizationatthetightjunctionandcellmembrane,preventingYAPnucleartranslocation.
51Moreover,itwasshownthatknockdownofAMOTL2phenocopiesYAP-inducedEMTinMCF10Acells.
51Consideringthisscenario,lossoftightjunction-localizedYAPandTAZincreasedtheirnuclearlocalizationandwasaccompaniedbyinductionofYAP/TAZtargetgeneexpres-sion,andmostimportantly,transformationandlossofcellcontactinhibition.
Furthermore,AMOTL2knockdown-depen-dentphenotypeswereblockedbysimultaneousknockdownofYAPandTAZ,demonstratingthattheAMOTfamilyproteinsarenewcomponentsoftheHippopathwaywithtumor-suppressingpotential,indicatinganewmodeofYAPandTAZregulation.
51Inadifferentmanner,WWdomain-PPxYmotifinteractionwasinvolvedintheregulationofthedownstreameffectorsoftheHippopathwaybyinvolvingmorethantwoproteins.
Forexample,ASPP2wasshowntostimulateTAZdepho-sphorylation,partlybypromotingtheinteractionbetweenTAZandPP1;thisfunctionofASPP2requirestheTAZWWdomain.
ASPP2–TAZinteractionpromotesTAZnuclearlocalizationandTAZtargetgeneexpression.
52Inanotherexample,itwasshownthatASPP1wasabletoinhibitYAP/TAZinteractionwithLATS1,leadingtoenhancednuclearaccumulationofYAP/TAZandYAP/TAZ-dependenttranscriptionalregulation.
ThisresultsinYAP/TAZactivationandthusinhibitsapoptosis,inpart,throughthedownregula-tionofBimexpression,leadingtoresistancetoanoikisandenhancedcellmigration.
53ConcludingRemarksandFutureDirectionsTheuniquefeatureoftheHippopathwayoverothersignalingpathwaysisitshighmodularityrepresentedbythegreatprevalenceofWW–PPxYinteractions,whichmightstronglysuggestthatotherWWdomainandPPxYmotif-containingproteinsregulate,orarepartof,theHippopathway.
ThestudyofWWdomainsandHippopathwayinrecentyearsfurtherhighlightedimportantaspectsofWWdomainproteinsignalingincludingdimerizationcapability,regulationofWWdomain-PRMinteractionandnetworking(reviewedinSudol5).
WWdomainsarepresentinawidevarietyofcellularproteinsincludingE3ligases,co-activators,co-repressorsandadapterproteinsthatcouldpotentiallyregulatemembersoftheHippopathway.
Takingintoconsiderationtheimportantroleofthispathwayintissuegrowthandhomeostasis,furthereffortsshouldbeinvestedinidentifyingnewregulatorsandcompo-nentsofthispathway.
TheuseofGFP-expressingtumorcellsinfreshtissueorliveanimalsshallfacilitatebetterchara-cterizationoftheHippopathwayproteinsandtheirrole,bothinvitroandinvivo,intumorinitiationandprogression.
54–57ExpansionofthisinformationmayaidindevelopingnewtherapeuticstrategiesbasedontheWWdomaininteractionsinthispathway.
Infact,thedesignofinhibitorsoractivatorsofWWdomainsignalingcomplexesintheHippopathwaycouldbefacilitatedbytheconsiderabledataavailableontheWWdomainstructure,themechanismofinteractionwithitsrigidligands,andthecomplexesitforms.
58OwingtothefactthattheWWdomainanditsligands'coremotifsarerelativelyshort,itmightbepossibletousesmallmoleculesthatfunctionasactivatorsorinhibitorsfortheHippopathwaysignalingproteins;thatis,smallchemicals/peptidesthatinhibitYAPandTAZoncogenicfunction.
However,beforethinkingabouttherapeuticstrategiesbasedonWWdomaininteractions,furtheranalysisoftheWWdomain-mediatedcomplexesintheHippopathwaymustbeelucidatedtobetterdesignnoveltherapeuticstrategiesformalfunctionsthatinvolvetheWWdomain.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan4CellDeathandDiseaseConictofInterestTheauthorsdeclarenoconictofinterest.
Acknowledgements.
WearegratefultoMsSherriCohenandMrsAlizaFormanforcriticalreadingofthemanuscript.
Weapologizetothosecolleagueswhoseworkwecouldnotcitebecauseofspacelimitation.
ThisworkwassupportedinpartbytheIsraeliScienceFoundationgrant(ISF#1331-08)andMarieCurie-EuropeanRe-integrationGrant(Project#224848)toAqeilanRIandIsraeliCancerResearchFund(ICRF)toSalahZ.
1.
DongJ,FeldmannG,HuangJ,WuS,ZhangN,ComerfordSAetal.
Elucidationofauniversalsize-controlmechanisminDrosophilaandmammals.
Cell2007;130:1120–1133.
2.
McNeillH,WoodgettJR.
Whenpathwayscollide:collaborationandconnivanceamongsignallingproteinsindevelopment.
NatRevMolCellBiol2010;11:404–413.
3.
ZhaoB,LiL,LeiQ,GuanKL.
TheHippo-YAPpathwayinorgansizecontrolandtumorigenesis:anupdatedversion.
GenesDev2010;24:862–874.
4.
SudolM,HarveyKF.
ModularityintheHipposignalingpathway.
TrendsBiochemSci2010;35:627–633.
5.
SudolM.
NewcomerstotheWWdomain–mediatednetworkoftheHippotumorsuppressorpathway.
GenesCancer2011;1:1115–1118.
6.
BorkP,SudolM.
TheWWdomain:asignallingsiteindystrophinTrendsBiochemSci1994;19:531–533.
7.
SudolM,BorkP,EinbondA,KasturyK,DruckT,NegriniMetal.
CharacterizationofthemammalianYAP(Yes-associatedprotein)geneanditsroleindeninganovelproteinmodule,theWWdomain.
JBiolChem1995;270:14733–14741.
8.
SudolM.
WWdomainin''modularproteindomains''In:CesareniG,GimonaM,SudolM,andYaffeM(eds).
ModularProteinDomains.
Wiley-VCHVerlagGmBH&Co,KGaA:Weinheim,FRG,2004,pp59–72.
9.
ChenHI,SudolM.
TheWWdomainofYes-associatedproteinbindsaproline-richligandthatdiffersfromtheconsensusestablishedforSrchomology3-bindingmodules.
ProcNatlAcadSciUSA1995;92:7819–7823.
10.
SudolM.
StructureandfunctionoftheWWdomain.
ProgBiophysMolBiol1996;65:113–132.
11.
ChenHI,EinbondA,KwakSJ,LinnH,KoepfE,PetersonSetal.
CharacterizationoftheWWdomainofhumanyes-associatedproteinanditspolyproline-containingligands.
JBiolChem1997;272:17070–17077.
12.
MaciasMJ,WiesnerS,SudolM.
WWandSH3domains,twodifferentscaffoldstorecognizeproline-richligands.
FEBSLett2002;513:30–37.
13.
OtteL,WiedemannU,SchlegelB,PiresJR,BeyermannM,SchmiederPetal.
WWdomainsequenceactivityrelationshipsidentiedusingligandrecognitionpropensitiesof42WWdomains.
ProteinSci2003;12:491–500.
14.
SudolM,HunterT.
NeWwrinklesforanolddomain.
Cell2000;103:1001–1004.
15.
FotiaAB,DinudomA,ShearwinKE,KochJP,KorbmacherC,CookDIetal.
TheroleofindividualNedd4-2(KIAA0439)WWdomainsinbindingandregulatingepithelialsodiumchannels.
FASEBJ2003;17:70–72.
16.
InghamRJ,ColwillK,HowardC,DettwilerS,LimCS,YuJetal.
WWdomainsprovideaplatformfortheassemblyofmultiproteinnetworks.
MolCellBiol2005;25:7092–7106.
17.
SalahZ,AlianA,AqeilanR.
WWdomain-containingproteins:retrospectivesandthefuture.
FrontBiosci2011.
18.
HanssonJH,SchildL,LuY,WilsonTA,GautschiI,ShimketsRetal.
AdenovomissensemutationofthebetasubunitoftheepithelialsodiumchannelcauseshypertensionandLiddlesyndrome,identifyingaproline-richsegmentcriticalforregulationofchannelactivity.
ProcNatlAcadSciUSA1995;92:11495–11499.
19.
InoueJ,IwaokaT,TokunagaH,TakamuneK,NaomiS,ArakiMetal.
AfamilywithLiddle'ssyndromecausedbyanewmissensemutationinthebetasubunitoftheepithelialsodiumchannel.
JClinEndocrinolMetab1998;83:2210–2213.
20.
ChungW,CampanelliJT.
WWandEFhanddomainsofdystrophin-familyproteinsmediatedystroglycanbinding.
MolCellBiolResCommun1999;2:162–171.
21.
HuangX,PoyF,ZhangR,JoachimiakA,SudolM,EckMJ.
StructureofaWWdomaincontainingfragmentofdystrophinincomplexwithbeta-dystroglycan.
NatStructBiol2000;7:634–638.
22.
LiuF,LiB,TungEJ,Grundke-IqbalI,IqbalK,GongCX.
Site-speciceffectsoftauphosphorylationonitsmicrotubuleassemblyactivityandself-aggregation.
EurJNeurosci2007;26:3429–3436.
23.
MandelkowEM,MandelkowE.
TauinAlzheimer'sdisease.
TrendsCellBiol1998;8:425–427.
24.
Morishima-KawashimaM,HasegawaM,TakioK,SuzukiM,YoshidaH,WatanabeAetal.
HyperphosphorylationoftauinPHF.
NeurobiolAging1995;16:365–371;discussion371-380.
25.
FaberPW,BarnesGT,SrinidhiJ,ChenJ,GusellaJF,MacDonaldME.
HuntingtininteractswithafamilyofWWdomainproteins.
HumMolGenet1998;7:1463–1474.
26.
PassaniLA,BedfordMT,FaberPW,McGinnisKM,SharpAH,GusellaJFetal.
Huntingtin'sWWdomainpartnersinHuntington'sdiseasepost-mortembrainfulllgeneticcriteriafordirectinvolvementinHuntington'sdiseasepathogenesis.
HumMolGenet2000;9:2175–2182.
27.
TapiaVE,NicolaescuE,McDonaldCB,MusiV,OkaT,InayoshiYetal.
Y65CmissensemutationintheWWdomainoftheGolabi-Ito-HallsyndromeproteinPQBP1affectsitsbindingactivityandderegulatespre-mRNAsplicing.
JBiolChem2010;285:19391–19401.
28.
DelMareS,SalahZ,AqeilanRI.
WWOX:itsgenomics,partners,andfunctions.
JCellBiochem2009;108:737–745.
29.
PanD.
Thehipposignalingpathwayindevelopmentandcancer.
DevCell2010;19:491–505.
30.
SalahZ,AqeilanR,HuebnerK.
WWOXgeneandgeneproduct:tumorsuppressionthroughspecicproteininteractions.
FutureOncol2010;6:249–259.
31.
HarveyK,TaponN.
TheSalvador-Warts-Hippopathway-anemergingtumour-suppressornetwork.
NatRevCancer2007;7:182–191.
32.
GruscheFA,RichardsonHE,HarveyKF.
Upstreamregulationofthehipposizecontrolpathway.
CurrBiol2010;20:R574–R582.
33.
HaoY,ChunA,CheungK,RashidiB,YangX.
TumorsuppressorLATS1isanegativeregulatorofoncogeneYAP.
JBiolChem2008;283:5496–5509.
34.
ZhangJ,SmolenGA,HaberDA.
NegativeregulationofYAPbyLATS1underscoresevolutionaryconservationoftheDrosophilaHippopathway.
CancerRes2008;68:2789–2794.
35.
ZhaoB,WeiX,LiW,UdanRS,YangQ,KimJetal.
InactivationofYAPoncoproteinbytheHippopathwayisinvolvedincellcontactinhibitionandtissuegrowthcontrol.
GenesDev2007;21:2747–2761.
36.
LeiQY,ZhangH,ZhaoB,ZhaZY,BaiF,PeiXHetal.
TAZpromotescellproliferationandepithelial-mesenchymaltransitionandisinhibitedbythehippopathway.
MolCellBiol2008;28:2426–2436.
37.
ZhaoB,KimJ,YeX,LaiZC,GuanKL.
BothTEAD-bindingandWWdomainsarerequiredforthegrowthstimulationandoncogenictransformationactivityofyes-associatedprotein.
CancerRes2009;69:1089–1098.
38.
GenevetA,WehrMC,BrainR,ThompsonBJ,TaponN.
KibraisaregulatoroftheSalvador/Warts/Hipposignalingnetwork.
DevCell2010;18:300–308.
39.
YuJ,ZhengY,DongJ,KluszaS,DengWM,PanD.
KibrafunctionsasatumorsuppressorproteinthatregulatesHipposignalinginconjunctionwithMerlinandExpanded.
DevCell2010;18:288–299.
40.
BaumgartnerR,PoernbacherI,BuserN,HafenE,StockerH.
TheWWdomainproteinKibraactsupstreamofHippoinDrosophila.
DevCell2010;18:309–316.
41.
XiaoL,ChenY,JiM,DongJ.
KIBRAregulatesHipposignalingactivityviainteractionswithlargetumorsuppressorkinases.
JBiolChem2011;286:7788–7796.
42.
SalahZ,MelinoG,AqeilanRI.
NegativeregulationoftheHippopathwaybyE3ubiquitinligaseItchissufcienttopromotetumorigenicity.
CancerRes2011;71:2010–2020.
43.
HoKC,ZhouZ,SheYM,ChunA,CyrTD,YangX.
ItchE3ubiquitinligaseregulateslargetumorsuppressor1tumor-suppressorstability.
ProcNatlAcadSciUSA2011;108:4870–4875.
44.
RossiM,DeLaurenziV,MunarrizE,GreenDR,LiuYC,VousdenKHetal.
Theubiquitin-proteinligaseItchregulatesp73stability.
EMBOJ2005;24:836–848.
45.
StranoS,MunarrizE,RossiM,CastagnoliL,ShaulY,SacchiAetal.
PhysicalinteractionwithYes-associatedproteinenhancesp73transcriptionalactivity.
JBiolChem2001;276:15164–15173.
46.
OkaT,MazackV,SudolM.
Mst2andLatskinasesregulateapoptoticfunctionofYeskinase-associatedprotein(YAP).
JBiolChem2008;283:27534–27546.
47.
ZhangX,MiltonCC,PoonCL,HongW,HarveyKF.
Wbp2cooperateswithYorkietodrivetissuegrowthdownstreamoftheSalvador-Warts-Hippopathway.
CellDeathDiffer2011.
48.
ChanSW,LimCJ,HuangC,ChongYF,GunaratneHJ,HogueKAetal.
WWdomain-mediatedinteractionwithWbp2isimportantfortheoncogenicpropertyofTAZ.
Oncogene2011;30:600–610.
49.
ChanSW,LimCJ,ChongYF,PobbatiAV,HuangC,HongW.
Hippopathway-independentrestrictionofTAZandYAPbyangiomotin.
JBiolChem2011;286:7018–7026.
50.
WangW,HuangJ,ChenJ.
Angiomotin-likeproteinsassociatewithandnegativelyregulateYAP1.
JBiolChem2011;286:4364–4370.
51.
ZhaoB,LiL,LuQ,WangLH,LiuCY,LeiQetal.
AngiomotinisanovelHippopathwaycomponentthatinhibitsYAPoncoprotein.
GenesDev2011;25:51–63.
52.
LiuCY,LvX,LiT,XuY,ZhouX,ZhaoSetal.
PP1cooperateswithASPP2todephosphorylateandactivateTAZ.
JBiolChem2011;286:5558–5566.
53.
VigneronAM,LudwigRL,VousdenKH.
CytoplasmicASPP1inhibitsapoptosisthroughthecontrolofYAP.
GenesDev2010;24:2430–2439.
54.
HoffmanRM.
Themultipleusesofuorescentproteinstovisualizecancerinvivo.
NatRevCancer2005;5:796–806.
55.
HoffmanRM,YangM.
Subcellularimaginginthelivemouse.
NatProtoc2006;1:775–782.
56.
HoffmanRM,YangM.
Color-codeduorescenceimagingoftumor-hostinteractions.
NatProtoc2006;1:928–935.
57.
HoffmanRM,YangM.
Whole-bodyimagingwithuorescentproteins.
NatProtoc2006;1:1429–1438.
58.
MaciasMJ,HyvonenM,BaraldiE,SchultzJ,SudolM,SarasteMetal.
StructureoftheWWdomainofakinase-associatedproteincomplexedwithaproline-richpeptide.
Nature1996;382:646–649.
WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan5CellDeathandDisease59.
HongJH,HwangES,McManusMT,AmsterdamA,TianY,KalmukovaRetal.
TAZ,atranscriptionalmodulatorofmesenchymalstemcelldifferentiation.
Science2005;309:1074–1078.
60.
HarveyKF,PegerCM,HariharanIK.
TheDrosophilaMstortholog,Hippo,restrictsgrowthandcellproliferationandpromotesapoptosis.
Cell2003;114:457–467.
61.
TaponN,HarveyKF,BellDW,WahrerDC,SchiripoTA,HaberDAetal.
SalvadorpromotesbothcellcycleexitandapoptosisinDrosophilaandismutatedinhumancancercelllines.
Cell2002;110:467–478.
62.
BrittleAL,RepisoA,CasalJ,LawrencePA,StruttD.
Four-jointedmodulatesgrowthandplanarpolaritybyreducingtheafnityofdachsousforfat.
CurrBiol2010;20:803–810.
63.
ChoE,FengY,RauskolbC,MaitraS,FehonR,IrvineKD.
Delineationofafattumorsuppressorpathway.
NatGenet2006;38:1142–1150.
64.
FengY,IrvineKD.
ProcessingandphosphorylationoftheFatreceptor.
ProcNatlAcadSciUSA2009;106:11989–11994.
65.
SopkoR,SilvaE,ClaytonL,GardanoL,Barrios-RodilesM,WranaJetal.
PhosphorylationofthetumorsuppressorfatisregulatedbyitsligandDachsousandthekinasediscsovergrown.
CurrBiol2009;19:1112–1117.
66.
ChenCL,GajewskiKM,HamaratogluF,BossuytW,Sansores-GarciaL,TaoCetal.
Theapical-basalcellpolaritydeterminantCrumbsregulatesHipposignalinginDrosophila.
ProcNatlAcadSciUSA2010;107:15810–15815.
67.
LingC,ZhengY,YinF,YuJ,HuangJ,HongYetal.
TheapicaltransmembraneproteinCrumbsfunctionsasatumorsuppressorthatregulatesHipposignalingbybindingtoExpanded.
ProcNatlAcadSciUSA2010;107:10532–10537.
68.
SongH,MakKK,TopolL,YunK,HuJ,GarrettLetal.
MammalianMst1andMst2kinasesplayessentialrolesinorgansizecontrolandtumorsuppression.
ProcNatlAcadSciUSA2010;107:1431–1436.
69.
ZhouD,ConradC,XiaF,ParkJS,PayerB,YinYetal.
Mst1andMst2maintainhepatocytequiescenceandsuppresshepatocellularcarcinomadevelopmentthroughinactivationoftheYap1oncogene.
CancerCell2009;16:425–438.
70.
PatelS,GeorgeR,AutoreF,FraternaliF,LadburyJE,NikolovaPV.
MolecularinteractionsofASPP1andASPP2withthep53proteinfamilyandtheapoptoticpromotersPUMAandBax.
NucleicAcidsRes2008;36:5139–5151.
71.
KomuroA,NagaiM,NavinNE,SudolM.
WWdomain-containingproteinYAPassociateswithErbB-4andactsasaco-transcriptionalactivatorforthecarboxyl-terminalfragmentofErbB-4thattranslocatestothenucleus.
JBiolChem2003;278:33334–33341.
72.
WebbC,UpadhyayA,GiuntiniF,EgglestonI,Furutani-SeikiM,IshimaRetal.
StructuralfeaturesandligandbindingpropertiesoftandemWWdomainsfromYAPandTAZ,nucleareffectorsoftheHippopathway.
Biochemistry2011;50:3300–3309.
73.
AqeilanRI,DonatiV,PalamarchukA,TrapassoF,KaouM,PekarskyYetal.
WWdomain-containingproteins,WWOXandYAP,competeforinteractionwithErbB-4andmodulateitstranscriptionalfunction.
CancerRes2005;65:6764–6772.
74.
AlarconC,ZaromytidouAI,XiQ,GaoS,YuJ,FujisawaSetal.
NuclearCDKsdriveSmadtranscriptionalactivationandturnoverinBMPandTGF-betapathways.
Cell2009;139:757–769.
75.
ZaidiSK,SullivanAJ,MedinaR,ItoY,vanWijnenAJ,SteinJLetal.
TyrosinephosphorylationcontrolsRunx2-mediatedsubnucleartargetingofYAPtorepresstranscription.
EMBOJ2004;23:790–799.
76.
VarelasX,MillerBW,SopkoR,SongS,GregorieffA,FellouseFAetal.
TheHippopathwayregulatesWnt/beta-cateninsignaling.
DevCell2010;18:579–591.
CellDeathandDiseaseisanopen-accessjournalpublishedbyNaturePublishingGroup.
ThisworkislicensedundertheCreativeCommonsAttribution-Noncommercial-NoDerivativeWorks3.
0UnportedLicense.
Toviewacopyofthislicense,visithttp://creativecommons.
org/licenses/by-nc-nd/3.
0/WWdomainproteinsregulatetheHippopathwayZSalahandRIAqeilan6CellDeathandDisease
- membrane7788kk.com相关文档
- respect7788kk.com
- chromatid7788kk.com
- unable7788kk.com
- unbound7788kk.com
- retrofit7788kk.com
- myeloproliferation7788kk.com
NameCheap 2021年新年首次活动 域名 域名邮局 SSL证书等
NameCheap商家如今发布促销活动也是有不小套路的,比如会在提前一周+的时间告诉你他们未来的活,比如这次2021年的首次活动就有在一周之前看到,但是这不等到他们中午一点左右的时候才有正式开始,而且我确实是有需要注册域名,等着看看是否有真的折扣,但是实际上.COM域名力度也就一般需要51元左右,其他地方也就55元左右。当然,这次新年的首次活动不管如何肯定是比平时便宜一点点的。有新注册域名、企业域...

7月RAKsmart独立服务器和站群服务器多款促销 G口不限量更低
如果我们熟悉RAKsmart商家促销活动的应该是清楚的,每个月的活动看似基本上一致。但是有一些新品或者每个月还是有一些各自的特点的。比如七月份爆款I3-2120仅30美金、V4新品上市,活动期间5折、洛杉矶+硅谷+香港+日本站群恢复销售、G口不限流量服务器比六月份折扣力度更低。RAKsmart 商家这个月依旧还是以独立服务器和站群服务器为主。当然也包括有部分的低至1.99美元的VPS主机。第一、I...
酷番云78元台湾精品CN2 2核 1G 60G SSD硬盘
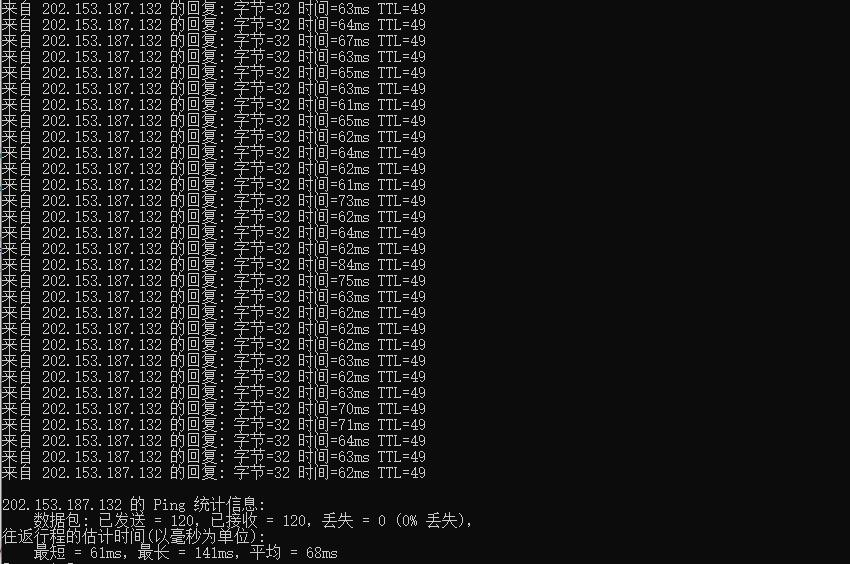
酷番云怎么样?酷番云就不讲太多了,介绍过很多次,老牌商家完事,最近有不少小伙伴,一直问我台湾VPS,比较难找好的商家,台湾VPS本来就比较少,也介绍了不少商家,线路都不是很好,有些需求支持Windows是比较少的,这里我们就给大家测评下 酷番云的台湾VPS,支持多个版本Linux和Windows操作系统,提供了CN2线路,并且还是原生IP,更惊喜的是提供的是无限流量。有需求的可以试试。可以看到回程...
7788kk.com为你推荐
-
Baby被问婚变绯闻小s在黄晓明婚礼上问了什么问题原代码什么叫源代码,源代码有什么作用百度关键词分析如何正确分析关键词?777k7.com怎么在这几个网站上下载图片啊www.777mu.com www.gangguan23.com同一服务器网站服务器建设:一个服务器有多个网站该如何设置?www.44ri.comwww.yydcsjw.comjavmoo.com找下载JAV软件格式的网站www.123qqxx.com我的首页http://www.hao123.com被改成了http://www.669dh.cn/?yhcwww4399com4399是什么网站啊???www.mfav.orgwww.osta.org.cn国家职业资格证书全国联网查询,为什么随便输入什么都可以查,都要验证码