incorporatesphp的cms
php的cms 时间:2021-01-17 阅读:()
ORIGINALRESEARCHARTICLEDrugSafetyMonitoringinChildren:PerformanceofSignalDetectionAlgorithmsandImpactofAgeStraticationOsemekeU.
Osokogu1CaitlinDodd1AlexandraPacurariu1,3FlorentiaKaguelidou1,2DanielWeibel1MiriamC.
J.
M.
Sturkenboom1Publishedonline:2June2016TheAuthor(s)2016.
ThisarticleispublishedwithopenaccessatSpringerlink.
comAbstractIntroductionSpontaneousreportsofsuspectedadversedrugreactions(ADRs)canbeanalyzedtoyieldadditionaldrugsafetyevidenceforthepediatricpopulation.
Signaldetectionalgorithms(SDAs)arerequiredfortheseanaly-ses;however,theperformanceofSDAsinthepediatricpopulationspecicallyisunknown.
Wetestedtheperfor-manceoftwoSDAsonpediatricdatafromtheUSFDAAdverseEventReportingSystem(FAERS)andinvesti-gatedtheimpactofagestraticationandageadjustmentontheperformanceofSDAs.
MethodsWetestedtheperformanceoftwoestablishedSDAs:theproportionalreportingratio(PRR)andtheempiricalBayesgeometricmean(EBGM)onapediatricdatasetfromFAERS(2004–2012).
Wecomparedtheper-formanceoftheSDAswithapublishedpediatric-specicreferencesetbycalculatingdiagnostictest-relatedstatis-tics,includingtheareaunderthecurve(AUC)ofreceiveroperatingcharacteristics.
Impactofagestraticationandage-adjustmentontheperformanceoftheSDAswasassessed.
Ageadjustmentwasperformedbypooling(Mantel-Hanszel)stratum-specicestimates.
ResultsAtotalof115,674pediatricreports(patientsaged0–18years)comprising893,587drug–eventcombinations(DECs)wereanalysed.
CrudevaluesoftheAUCweresimilarforbothSDAs:0.
731(PRR)and0.
745(EBGM).
StraticationunmaskedfourDECs,e.
g.
,'ibuprofenandthrombocytopenia'.
Ageadjustmentdidnotimproveperformance.
ConclusionTheperformanceofthetwotestedSDAswassimilarinthepediatricpopulation.
Ageadjustmentdoesnotimproveperformanceandisthereforenotrecommendedtobeperformedroutinely.
Straticationcanrevealnewasso-ciations,andthereforeisrecommendedwheneitherdruguseisage-specicorwhenanage-specicriskissuspected.
KeyPointsDetectionofdrugsafetysignalsinchildren,whorepresentaheterogeneouspopulation,whereagemaybeaconfounderoreffectmodier,isanareainwhichonlylimitedresearchhasbeencarriedout.
Thesignaldetectionalgorithms(SDAs)showedgoodperformanceonpediatricdataandcanbeutilizedforpediatricsignaldetection.
AgeadjustmentdidnotimprovetheperformanceoftheSDAs.
Agestraticationshowedthatsomesignalsmaybedetectedonlyinspecicpediatricagegroups.
Forroutinesurveillance,checkingforeffectmodicationacrossagestratamaygenerateusefulinformation.
OsemekeU.
OsokoguandCaitlinDoddcontributedequallytothisarticle.
ElectronicsupplementarymaterialTheonlineversionofthisarticle(doi:10.
1007/s40264-016-0433-x)containssupplementarymaterial,whichisavailabletoauthorizedusers.
&CaitlinDoddc.
dodd@erasmusmc.
nl1DepartmentofMedicalInformatics,ErasmusUniversityMedicalCenter,Rotterdam3015CN,TheNetherlands2DepartmentofPediatricPharmacologyandPharmacogenetics,HopitalRobertDebre,APHP,UnivParis7-Diderot,SorbonneParisCite,EA08,INSERMCIC1426,Paris75019,France3DutchMedicinesEvaluationBoard,Utrecht,TheNetherlandsDrugSaf(2016)39:873–881DOI10.
1007/s40264-016-0433-x1IntroductionSpontaneousreportsofsuspectedadversedrugreactions(ADRs)canyieldimportantinformationregardingthesafetyofdrugs[1].
Usually,suchreportsarescreenedforemergingsafetyissuesbyapplyingstatisticalmethodscalledsignaldetectionalgorithms(SDAs).
CurrentSDAscomparethereportingrateofadrug–eventcombination(DEC)ofinterestwiththeexpectedcountcalculatedfromtheoverallreportingrateofthatreactionintheentiredatabase[1,2].
AlthoughSDAsareroutinelyappliedtoreportspertainingtothegeneralpopulation,theperfor-manceofSDAsinthepediatricpopulationspecicallyhasnotbeeninvestigatedtodate.
Comparedwithadults,thepatternofdruguseandoccurrenceofADRsinpediatricpatientsmaydiffer[3–5]sincethelatterpopulationcom-prisesaheterogeneousgroupofsubjectsatvariousstagesofdevelopmentwithage-dependentorganmaturationandhormonalchanges[6].
SeveralstudiesinvestigatingADRreportinginchildrenhaveidentieddifferentreportingpatternsinthispopulationthaninadults[3,5,7,8].
SinceADRsmaybeagespecic,adjustmentforageseemstobealogicalstepwheninvestigatingpediatricADRsandhasbeenadvocatedbysomeresearchers[4].
Themajoraimofstraticationisvericationofconfoundingandeffectmodicationwhichotherwisemaymasktruesignals[9].
Confoundingbyagecanbedealtwithbystratifyingforagecategoriesandpoolingstratum-specicestimates.
How-ever,ifage-specicestimatesdiffer(incaseofeffectmodication)pooling/adjustmentshouldnotbedone;instead,avericationofeachindividualstratumshouldbeperformed.
Whilestraticationhasbeeninvestigatedbysomeresearchers[10],adjustmentisroutinelyimple-mentedinsomeBayesianbutnotinfrequentistSDAs[11–13].
Fewstudieshavesystematicallyaddressedtheimpactofagestraticationoradjustmentandtheresultsarecontradictory[9,14,15].
WithinthecontextoftheGlobalResearchinPediatrics(GRiP)NetworkofExcellence[16],weaimedtoevaluatetheperformanceoftwowell-establishedSDAsinthepediatricpopulationanddetermineifagestraticationoradjustmentimpactssignaldetectioninthispopulation.
2Methods2.
1DataSourceDatawereretrievedfromthepubliclyavailableversionoftheUSFDAAdverseEventReportingSystem(FAERS),whichcomprisesspontaneousreportsofsuspectedADRssubmittedbymanufacturers,healthcareprofessionals,andpatients.
FAERSisoneofthelargestrepositoriesofspontaneousreportsintheworld[17,18].
Inthisstudy,weanalyzedreportsreceivedfromtherstquarterof2004throughtothethirdquarterof2012.
Forperformanceanalysis,onlyreportsofADRsoccur-ringinchildrenandadolescents(\18yearsofage)wereretained.
TheADRsinFAERSarecodedaccordingtotheMedicalDictionaryforRegulatoryActivities(MedDRA)[19].
Toimprovethequalityofthedataset,weexcludedreportswithmissingage,themainvariableinourstudy.
Also,reportswithreportedageequaltozeroandwithaMedDRApreferredtermindicatingprenatalexposurewereremoved,astheseimplyinuterodrugexposureandwerethereforenotrelevantforourstudy.
Weminimizedthenumberofduplicates(i.
e.
,thesamereportsubmittedbydifferentreporters)byapplyinganalgorithmbasedoncaseidentier,reportidentier,anddrugandeventnames.
Formultiplereports(i.
e.
,thesamereportisreportedatalatertime,withadditionalandupdatedinformation)[20],themostrecent(andmostupdated)reportwasretainedforanalysis.
AsdrugnamesincludedinFAERSarenotstandardized,aharmonizationprocedurewasimplemented.
Briey,thisconsistedofremovingsuperuouscharactersandapplyingageneralizededitdistancematchingalgorithm[21]tomapfree-textdrugnamestosynonymsandnallytothecor-respondingactivesubstanceandWorldHealthOrganiza-tion–AnatomicTherapeuticChemical(WHO-ATC)code.
Inthisstudy,onlythosedrugsreportedastheprimaryorsecondarysuspectintheFAERSdatabasewereretainedforanalysis.
AnalysiswasperformedatDEClevel,meaningthatwithineachreport,everysuspectdrugwascombinedwithallreportedADRs.
Thus,onereportmaycomprisemorethanoneDEC.
2.
2SignalDetectionAlgorithms(SDAs)Wetestedtwowell-establishedSDAsthatareroutinelyusedbyvariousnationalandinternationalregulatoryand/orresearchinstitutionsforsignaldetection:thepropor-tionalreportingratio(PRR)[2]andtheempiricalBayesgeometricmean(EBGM)[13](seeTable1).
Wealsotestedcountofreportsasapositivecontrol.
Inordertodeneasignalofdisproportionatereporting[22,23],weselectedthresholdsthatarecurrentlyappliedinroutinepractice.
WeappliedtheSDAsattheendofthestudyperiod,whenthemaximumnumberofreportshadaccrued.
2.
3PerformanceAssessmentMeasuresTheperformanceoftheSDAswasassessedbycalculatingdiagnostictest-relatedstatistics,namelyspecicityand874O.
U.
Osokoguetal.
sensitivity,positivepredictivevalue(PPV),andnegativepredictivevalue(NPV)[24,25].
Sensitivityistheabilityofthemethodtoidentifytruesignalscorrectly,whilespeci-cityistheabilitytoexcludefalsesignalscorrectly.
PPVandNPVareposteriorprobabilities,describinghowmanyofthesignalsclassiedaspositiveornegativeareindeedcorrectlyclassied[24,25].
Sincediagnostictest-relatedstatisticsaredependentonthethresholdchoice,theirindividualcomparisonhasonlyalimited,albeitpractical,value.
Therefore,wealsoesti-matedtheareaunderthecurve(AUC)ofreceiveroperatingcharacteristics(ROC)inordertocomparetheperformanceoftheSDAs[26];theAUCincorporatesbothsensitivityandspecicityacrossallthepossiblevaluesforacertainSDA.
CalculationofAUCswasconductedbyvaryingonlythepointestimateofeachSDAanddidnottakeintoaccounttheothercomponentsoftheSDA.
Forthepurposeofperformanceevaluation,apreviouslyconstructedpediatric-specicGRiPreferencesetofposi-tiveandnegativeDECswasused.
Itconsistsof37positiveand90negativeDECsandincludesdrugsthatareadmin-isteredtochildrenandeventsthatareregardedasimportantforthispopulation.
ThepositiveDECsarethosethatwereconrmedtooccurbasedonevidencefromSummaryofProductCharacteristics(SmPC)andthepublishedlitera-ture,whilethenegativeDECsarethosethatcouldnotbeconrmedatthetimeofliteraturereviewbyeithertheSmPCorthepublishedliterature.
Forafulldescriptionofthereferenceset,seeOsokoguetal.
[27].
2.
4StraticationandAdjustmentforAgeTheimpactofagestraticationandadjustmentontheperformanceoftheSDAswasinvestigated.
First,wecheckedforpossibleeffectmodicationacrossagestrata,bystratifyingthedataaccordingtoagecategoriesdenedbytheInternationalConferenceonHarmonization(ICH)[28]andcalculatingstratum-specicmeasuresforeachSDA.
Secondly,wecalculatedage-adjustedestimatesforPRRandEBGMbycombiningthestratum-specicesti-matesinanoverallmeasure[29].
TheperformanceofeachSDAwasreassessedafteradjustment.
2.
5StatisticalAnalysisDifferencesintheperformance(AUC)ofeachSDA,crudeversusage-adjustedandcrudeversuscountofreports(positivecontrol)weretestedusingpairedchi-squaredtests.
Stratum-speciccontingencytablesweretestedforhomogeneityusingtheBreslowDayTaronetest[30].
TheMantel-Haenszelapproachwasusedforpoolingandcalculatingage-adjustedestimates[29].
ThelowerboundoftheEBGM95%condenceinterval(EBGM05)wascalculatedusingthelowerboundofthe95%condenceinterval(EB05)foreachstratumandthencomputingaMantel-HaenszelaveragebasedonZeinounetal.
[31].
Statisticalsignicancewasdenedbyp\0.
05.
AnalysiswasperformedusingSASsoftwareversion9.
2(SASInstitute,Cary,NC,USA).
GraphsweremadeinSASsoftwareversion9.
2andRversion3.
1.
3.
3Results3.
1DescriptiveAnalysisForthestudyperiod(rstquarterof2004throughtothethirdquarterof2012),atotalof4,285,088reportswereretrievedfromFAERS.
Aftereliminatingduplicates(n=43,125)andremovalofadultreports(n=2,686,530)andreportswithmissingage(n=1,419,524)orageequaltozerowithaMedDRApreferredtermindicatingpre-natalexposure(n=20,235),115,674reportscorrespond-ingto893,587individualDECswereretainedforanalysisofpediatricspontaneousreports(seeTable2).
ThetotalnumberofpediatricreportsthatincludedtheinvestigateddrugsandADRsfromthereferencesetcanbeobservedinFig.
1,whichalsoshowsdataregardingadults(forcomparisonpurposes).
ThenumberofchildrenTable1SignaldetectionalgorithmsandcorrespondingthresholdsappliedSignaldetectionalgorithmAppliedthresholdaInstitutionwherethemethodandtherespectivethresholdiscurrentlyusedNumberofreportsC5NAPRRPRRlowerbound95%CIC1andnC5reportsEuropeanMedicinesAgency(EMA)EBGMEB05CIC1.
8,nC3reports,andEBGMC2.
5MedicinesandHealthcareproductsRegulatoryAgency(MHRA)CIcondenceinterval,EB05lowerboundofthe95%condenceinterval,EBGMempiricalBayesgeometricmean,NAnotavailable,PRRproportionalreportingratioaThresholdswereobtainedfromCandoreetal.
[23]PediatricSignalDetection875exposedtothedrugsofinterest,forwhomanyoftheinvestigatedADRswasreported,variedfrom26patients(forpraziquantel)to7535patients(foribuprofen),withamedianof781patientsexposedacrossalldrugs.
ThenumberofeventsofinterestinFAERSrangedfrom164reports(ventriculararrhythmia)to14,777(anaphylaxis),withamedianof1004reportsacrossallevents.
ForamoredetaileddescriptionofreportscountspleaserefertoElec-tronicSupplementarymaterialTable1.
3.
2OverallPerformanceofSDAsBothSDAsshowedhighspecicityandlowsensitivity.
Theybothhadsimilarspecicityvalues(PRR:83.
8%andEBGM:91.
9%),whilesensitivitywaslowerforEBGMthanforPRR(17.
2vs.
37.
9%).
TheNPVandPPVweresimilarforbothSDAs.
Whenweappliedthethreshold-independent(AUC-based)approach,thetestedSDAsshowedsimilarperformanceinthepediatricpopulation,althoughtheAUCvalueforEBGM(0.
745)wasslightlyhigherthanforPRR(0.
731).
NoneoftheSDAsperformedbetterthanthesimplereportcount(AUC=0.
634;p=0.
27forPRRandp=0.
14forEBGM)3.
3StraticationandAdjustmentforAgeanditsImpactonPerformanceUponcalculatingSDAvaluesperagestratumandtestingforheterogeneityacrossstrata,weobservedeffectmodi-cationforsomeassociations.
Somefalsenegatives(pos-itiveDECsthatfailedtobehighlightedassignalswhenanalyzingdatapertainingtotheentirepediatricpopulation)wereunmaskedinsomestrata.
FourDECswereunmaskedintotal:ibuprofen–thrombocytopeniaandisoniazid–sei-zure(byPRR)andclarithromycin–erythemamultiformeandibuprofen–erythemamultiforme(byEBGM).
Con-versely,'ibuprofen–acuteliverinjury',alsoapositiveDEC,washighlightedwhenweanalyzeddatapertainingtotheentirepediatricpopulation,butitbecameclearafterstratifyingthatthisDECwashighlightedonlyinolderchildren(adolescents)andnotinyoungerchildren(seeFig.
2).
ForanoverviewofSDAvaluesacrossagestrataandresultsofheterogeneitytestspleaserefertoElectronicSupplementaryMaterialFigures1Aand1B.
Weevaluatedtheperformanceofthemethodswithinindividualagestrata(seeTable3).
Onaverage,perfor-manceoftheSDAswaslowerwithinagestratathanintheentirepediatricpopulationandperformanceimprovedwithincreasingstratumsize.
Forinfantsandneonates,theper-formancewasverylow,notbetterthanchance(p[0.
5forbothSDAs).
Theadolescentgroupexhibitedthebestper-formance,whichwassimilartotheoverallperformance.
Afteradjustingforagebypoolingthestratum-specicestimates,theperformanceoftheSDAsdecreased,althoughnotsignicantly(seeFig.
3;crudevs.
adjustedAUCforPRR:0.
731vs.
0.
688,p=0.
267;crudevs.
adjustedAUCforEBGM:0.
745vs.
0.
683,p=0.
216).
4DiscussionInthisstudy,wehavedemonstratedthatagestraticationfordetectionofdrugsafetysignalsinchildrenmayunmasksomesignalsthatdonotappearineithercrudeoradjustedanalysis.
Adjustmentforagedoesnotimproveperfor-manceofthePRRandEBGM.
Fortheinvestigatedevents,similarreportingpatternswereobservedforchildrenandadults,whiletheinvesti-gateddrugsappearedtohavedifferentreportingpatterns(seeFig.
1).
Differentdrug-relatedreportingpatternsinchildrenversusadultshavebeenreportedpreviously[5].
Consequently,reportedDECsforchildrenmaydifferfromadults[3,5],underliningtheneedforpediatric-specicapproachestosignaldetection,especiallywhenwecon-siderthatreporteddrugsmayvarybyagegroupevenwithinthepediatricpopulation[3,32].
Overall,thePRRandEBGMshowedgoodperfor-mance,althoughresultswereslightlylowerthanresultsreportedonother(notpediatric-specic)referencesets[32,33].
ThesimilarityinperformancebetweenPRRandEBGMisinaccordancewithrecentresultsfromthePROTECT(PharmacoepidemiologicalResearchonOutcomesofTherapeuticsbyaEuropeanConsortium)project[23].
Thefactthattheperformance(basedonAUC)ofPRRandEBGMwasnotstatisticallysigni-cantlybetterthansimplereportcountmaybeduetothelackofpower.
Withinagestrata,performanceseemedtocorrelatewithstratumsize:thepoorestresultswereobservedforinfantsandneonates(thesmallergroups),slightlyimprovingforchildren,whilethebestperfor-mancewasobservedforadolescents,theagestratumwiththehighestnumberoftestedDECs.
DecreaseinpowerduetofewerreportsandthereforeDECsmayaccountforthisobservation.
Thefactthatweusedlowerboundsofcondenceintervalsforsignalinginsteadofpointestimatesmighthaveexacerbatedtheinuenceofsamplesizeontheresults,sincesmallerstratawillhaveTable2DescriptionofpediatricreportsbyagecategoriesAgegroupNumberofreports[n(%)]Neonates:0–27days5091(4.
40)Infants:28days–23months12,566(10.
86)Children:2–11years49,982(43.
21)Adolescents:12–17years48,035(41.
53)Total115,674(100)876O.
U.
Osokoguetal.
highervariability.
Inneonatesandinfantsforwhomexpectedcountsweredifculttocalculatebecauseoffewreports,weobservedthatsimplereportcountsper-formedsimilarorevenbetterthantheSDAsandmightbeanalternativetocommonlyusedSDAs.
ThefactthatsimplereportcountperformedbetterthanSDAsmayhavebeenbecausethereferencesetcomprisedknownDECs(whichinturnmayhaveinuencedreporting)ratherthanemergingsafetyissues,ahypothesispro-posedbyNorenetal.
[34].
InspectionofSDAvaluesacrosschild-specicstrata(agestratication)revealedsomeheterogeneityinesti-mates,pointingtosomeeffectmodication.
Forexample,'ibuprofen–thrombocytopenia'wasfoundasasignalintheFig.
1Countofreportsinthepediatricandadultpopulationfortheinvestigatedadversedrugreactions(a)anddrugs(b),cumulativelyfortheperiodquarter12004toquarter32012.
Thenumberofreportsinchildrenisrepresentedbybarsandplottedontheleftaxis,whilethenumberofreportsinadultsisrepresentedbytheredlineandplottedontherightaxis;reportswithmissingageorage=0wereexcluded.
OnlyreportsmentioninganyofthedrugsoreventsinthereferencesetwereconsideredPediatricSignalDetection877adolescents'groupbutnotdetectedintheentirepediatricpopulationortheyoungeragecategories.
Thissuggeststhatage-specicSDAcalculationsaresometimesneeded,ratherthanage-adjustedSDAestimates.
Theage-adjustedestimatesdidnotimproveperformance;infact,evenPPVunexpectedlydecreased.
Simulationstudieshaveshownthatwhenadjustedforstrata,BayesianmethodssuchasEBGMtendtobeunderestimatedwhentherearesparsestrata[15];thiswasalsothecaseinourstudy.
Previousstudiesinadultsshowcontradictoryresults,withsomeshowingabenecialeffect[9]whileothersdidnot[15].
Thereasonforourndingisnotentirelyclear;apossibleexplanationisthatageisnotastrongconfounderfortheinvestigatedDECs.
Also,themethodofweighting(Mantel-Haenszelapproach)mayhaveplayedarolesincemoreweightwasassignedtoagegroupswithmorereports(adolescentsandchildren).
Thismayhavemaskedsignalsoccurringinagegroupswithfewerreports.
ThelimitationsofdatamininginFAERSincludethoseinherenttospontaneousreportingdatabases:under-report-ing,lackofdenominatordataandcontrolgroup,biasesinreporting,aswellasmissingandpoor-qualitydata[35].
Missinginformationregardingagesubstantiallyreducedthestudysamplesizesincewecouldnotdeterminewhe-therthesereportsdescribedpatientsagedlessthan18yearsold.
Whilethesebiasesarewellacknowledgedandhaveadeniteimpact,theycannotbecompletelyavoided.
Comparedwithadults,therearefewerreportsanddifferentreportingpatternsforchildren[3,36,37],whichmaycomplicatesignaldetectioninthepediatricpopulation.
EvaluatingperformanceofSDAsisaconstantchallengeduetolackofstandardmethodologies,imperfectreferencestandards,anduncertaintyregardingthebestthresholds(seetheElectronicSupplementaryMaterialformeasuresofperformanceusingalternativethresholds).
SomeofthedrugsandeventsinthereferencesetarespecictooneagegroupwithinpediatricsandthisisobviousinFig.
1,eventhoughthereferencesetwasdesignedtoberelevantfortheentirepediatricpopulation.
Weacknowledgethattheref-erencesetused,althoughspecicallyconstructedforthisp-valueswerecalculatedwithBreslowDayTaronetestforhomogeneityp<0.
0001p=0.
001p=0.
339Fig.
2VariationofproportionalreportingratioandempiricalBayesgeometricmeanestimatesacrosspediatricspecicstrata—selectedexamples.
EBGMempiricalBayesgeometricmean,PRRproportionalreportingratio,SDAsignaldetectionalgorithmTable3PerformanceofsignaldetectionalgorithmsacrossagestrataAgegroupsandsignaldetectionalgorithmsSizeoftheagestratum(numberofreports)AUCNeonates5091Numberofreports0.
625EBGM0.
600PRR0.
65Infants12,566Numberofreports0.
667EBGM0.
548PRR0.
554Children49,982Numberofreports0.
654EBGM0.
698PRR0.
649Adolescents48,035Numberofreports0.
698EBGM0.
771PRR0.
718EntirepediatricpopulationNumberofreports115,6740.
634EBGM0.
746PRR0.
733AUCareaunderthecurve,EBGMempiricalBayesgeometricmean,PRRproportionalreportingratio878O.
U.
Osokoguetal.
purpose,doesnotincludealltheADRsthatarehighlyspecicforpediatrics.
Thishighlightstheneedforpedi-atric-specicapproachestosignaldetection,accountingfornotjusttheentirepediatricpopulationbutalsothedifferentagestratawithinpediatrics.
Still,thereferencesetcapturesvariousdruguseandADRspatterns[38]andiscurrentlytheonlyavailablepediatric-specicreferenceset.
Thethresholdsappliedtodeneasignalwereobtainedfrompreviouspublicationsandothercut-offpointsmaygeneratebetterresults;furtherresearchonpediatric-specicthresholdsshouldbeencouraged.
5ConclusionOurstudyrevealedthatageadjustmentdidnotimprovetheperformanceoftheSDAs.
However,straticationrevealedsomevariationinthevaluesofSDAsacrossstrata(effectmodication)andinspectionofstratum-specicestimatesmightsometimesyieldusefulinformationduringroutinesurveillance.
CompliancewithEthicalStandardsFundingTheGlobalResearchinPediatricsNetworkofExcellenceisfundedundertheEuropeanUnion'sSeventhFrameworkProgram(FP7/2007–2013)forresearch,technologicaldevelopment,anddemonstrationundergrantagreementnumber261060.
Fundingforthisstudywasalsoreceivedfromthe''PriorityMedicinesKinderenprojectZONMW:EVIPED:novelmethodstoassessandcomparedrugeffectsinpediatrics''(grantagreementnumber113201007).
Thefundershadnorolewhatsoeverindesigningandconductingthestudy,thecollectionandmanagementofdata,andpreparation,review,orapprovalofthemanuscript.
ConictofinterestMiriamSturkenboomleadsaresearchunitthatoccasionallyconductsresearchforpharmaceuticalcompanies,includingNovartis,Boehringer,Lilly,andPzer.
Noneofthisworkisrelatedtotheseactivities.
AlexandraPacurariuisanemployeeoftheDutchMedicinesEvaluationBoard.
Theviewsexpressedinthisarticlearethepersonalviewsoftheauthor(s)andmaynotbeunderstoodorquotedasbeingmadeonbehalfoforreectingthepositionoftheDutchMedicinesAgency.
OsemekeU.
Osokogu,CaitlinDodd,FlorentiaKaguelidou,andDanielWeibelhavenoconictsofinterestthataredirectlyrelatedtothecontentofthisstudy.
SDASensitivitySpecificityPPVNPVAUCp-valuebNumberofreports58.
6267.
5758.
6267.
570.
634referencePRR37.
9383.
7864.
7163.
270.
7310.
266EBGM17.
2491.
8962.
5058.
620.
7450.
144Afterageadjustmenta(reference-crudePRR/EBGM)PRR34.
4886.
4966.
6762.
750.
6880.
267EBGM10.
3497.
3075.
0058.
060.
6830.
216SDA-signaldetectionalgorithm;PRR=Proportionalreportingratio;EBGM=EmpiricalBayesGeometricMean;AUC=areaunderthecurve;PPV=positivepredictivevalue;NPV-negativepredictivevalueaadjustedPRR/RORvaluescalculatedbycombiningtheindividualestimatesfromeachagestratumintoonemeasureaccordingtotheMantel-Haenszelapproach.
bpairedchi-squaretestFig.
3PerformanceofsignaldetectionalgorithmswithintheentirepediatricpopulationPediatricSignalDetection879OpenAccessThisarticleisdistributedunderthetermsoftheCreativeCommonsAttribution-NonCommercial4.
0InternationalLicense(http://creativecommons.
org/licenses/by-nc/4.
0/),whichper-mitsanynoncommercialuse,distribution,andreproductioninanymedium,providedyougiveappropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreativeCommonslicense,andindicateifchangesweremade.
References1.
BateA,EvansSJW.
Quantitativesignaldetectionusingsponta-neousADRreporting.
PharmacoepidemiolDrugSaf.
2009;18:427–36.
2.
EvansSJW,WallerPC,DavisS.
Useofproportionalreportingratios(PRRs)forsignalgenerationfromspontaneousadversedrugreactionreports.
PharmacoepidemiolDrugSaf.
2001;10:483–6.
3.
BlakeKV,ZaccariaC,DomergueF,LaMacheE,Saint-Ray-mondA,Hidalgo-SimonA.
ComparisonbetweenpaediatricandadultsuspectedadversedrugreactionsreportedtotheEuropeanMedicinesAgency:implicationsforpharmacovigilance.
PaediatrDrugs.
2014;16:309–19.
4.
StarK,EdwardsIR.
Pharmacovigilanceforchildren'ssake.
DrugSaf.
2014;37:91–8.
5.
StarK,NorenGN,NordinK,EdwardsIR.
Suspectedadversedrugreactionsreportedforchildrenworldwide:anexploratorystudyusingVigiBase.
DrugSaf.
2011;34:415–28.
6.
KearnsGL,Abdel-RahmanSM,AlanderSW,BloweyDL,LeederJS,KauffmanRE.
Developmentalpharmacology—drugdisposition,action,andtherapyininfantsandchildren.
NEnglJMed.
2003;349:1157–67.
7.
AagaardL,WeberCB,HansenEH.
AdversedrugreactionsinthepaediatricpopulationinDenmark:aretrospectiveanalysisofreportsmadetotheDanishMedicinesAgencyfrom1998to2007.
DrugSaf.
2010;33:327–39.
8.
KimlandE,RaneA,UferM,PanagiotidisG.
PaediatricadversedrugreactionsreportedinSwedenfrom1987to2001.
Pharma-coepidemiolDrugSaf.
2005;14:493–9.
9.
WooEJ,BallR,BurwenDR,BraunMM.
EffectsofstraticationondataminingintheUSVaccineAdverseEventReportingSystem(VAERS).
DrugSaf.
2008;31:667–74.
10.
OrreR,LansnerA,BateA,LindquistM.
Bayesianneuralnet-workswithcondenceestimationsappliedtodatamining.
ComputStatDataAnal.
2000;34(4):473–93.
11.
AlmenoffJS,LaCroixKK,YuenNA,FramD,DuMouchelW.
Comparativeperformanceoftwoquantitativesafetysignallingmethods.
DrugSaf.
2006;29(10):875–87.
12.
NorenGN,BateA,OrreR,EdwardsIR.
ExtendingthemethodsusedtoscreentheWHOdrugsafetydatabasetowardsanalysisofcomplexassociationsandimprovedaccuracyforrareevents.
StatMed.
2006;25:3740–57.
13.
DuMouchelW.
Bayesiandatamininginlargefrequencytables,withanapplicationtotheFDAspontaneousreportingsystem.
AmStat.
1999;53(3):177–90.
14.
AlmenoffJS,PattishallEN,GibbsTG,DuMouchelW,EvansSJW,YuenN.
Novelstatisticaltoolsformonitoringthesafetyofmarketeddrugs.
ClinPharmacolTher.
2007;82(2):157–66.
15.
HopstadiusJ,NorenGN,BateA,EdwardsIR.
Impactofstrati-cationonadversedrugreactionsurveillance.
DrugSaf.
2008;31(11):1035–48.
16.
GlobalResearchinPaediatrics.
NetworkofExcellence.
http://www.
grip-network.
org/index.
php/cms/en/home.
Accessed28Feb2016.
17.
MooreTJ,FurbergCD.
Thesafetyrisksofinnovation:theFDA'sExpeditedDrugDevelopmentPathway.
JAMA.
2012;308(9):869–70.
18.
Weiss-SmithS,DeshpandeG,ChungS,GogolakV.
TheFDAdrugsafetysurveillanceprogram:adverseeventreportingtrends.
ArchInternMed.
2011;171:591–3.
19.
MedDRAMSSO.
Introductoryguide:MedDRAversion15.
1.
2012.
http://www.
meddra.
org/sites/default/les/guidance/le/intguide_15_1_English_0.
pdf.
Accessed28Feb2016.
20.
PoluzziE,PiccinniC,RaschiE,DePontiF.
Dataminingtech-niquesinpharmacovigilance:analysisofthepubliclyaccessibleFDAAdverseEventReportingSystem(AERS).
Rijeka:INTECHOpenAccessPublisher;2012.
21.
SAS.
COMPGEDFunction.
Returnsthegeneralizededitdistancebetweentwostrings.
ComputingtheGeneralizedEditDistance.
In:SAS9.
2languagereference:dictionary,fourthedition.
http://support.
sas.
com/documentation/cdl/en/lrdict/64316/HTML/default/viewer.
htm#a002206133.
htm.
Accessed3Mar2015.
22.
HaubenM,AronsonJK.
Dening'signal'anditssubtypesinpharmacovigilancebasedonasystematicreviewofpreviousdenitions.
DrugSaf.
2009;32(2):99–110.
23.
CandoreG,JuhlinK,ManlikK,ThakrarB,QuarcooN,SeabrokeS,etal.
Comparisonofstatisticalsignaldetectionmethodswithinandacrossspontaneousreportingdatabases.
DrugSaf.
2015;38(6):577–87.
24.
ZhouX-H,McClishDK,ObuchowskiNA.
Statisticalmethodsindiagnosticmedicine.
Hoboken:Wiley;2009.
25.
StromBL,KimmelSE,HennessyS.
Pharmacoepidemiology.
5thed.
Chichester:Wiley-Blackwell;2012.
26.
vanPuijenbroekEP,BateA,LeufkensHGM,LindquistM,OrreR,EgbertsACG.
Acomparisonofmeasuresofdispro-portionalityforsignaldetectioninspontaneousreportingsystemsforadversedrugreactions.
PharmacoepidemiolDrugSaf.
2002;11:3–10.
27.
OsokoguOU,FregoneseF,FerrajoloC,VerhammeK,deBieS,CatapanoM,etal.
Pediatricdrugsafetysignaldetection:anewdrug–eventreferencesetforperformancetestingofdata-miningmethodsandsystems.
DrugSaf.
2015;38(2):207–17.
28.
InternationalConferenceonHarmonisationofTechnicalRequirementsforRegistrationofPharmaceuticalsforHumanUse.
ICHHarmonisedTripartiteGuideline.
ClinicalInvestigationofMedicinalProductsinthePediatricPopulationE11.
CurrentStep4version.
20Jul2000.
http://www.
ich.
org/leadmin/Public_Web_Site/ICH_Products/Guidelines/Efcacy/E11/Step4/E11_Guideline.
pdf.
Accessed28Feb2016.
29.
MantelN,HaenszelW.
Statisticalaspectsoftheanalysisofdatafromretrospectivestudiesofdisease.
JNatlCancerInst.
1959;22:719–48.
30.
BreslowNE,DayNE,editors.
Statisticalmethodsincancerresearch.
VolumeI—theanalysisofcase-controlstudies.
IARCScienticPublicationsNo.
32.
Lyon:InternationalAgencyforResearchonCancer;1980.
31.
ZeinounZ,SeifertH,VerstraetenT.
Quantitativesignaldetectionforvaccines:effectsofstratication,backgroundandmaskingonGlaxoSmithKline'sspontaneousreportsdatabase.
HumVaccin.
2009;5(9):599–607.
32.
deBieS,FerrajoloC,StrausSMJM,VerhammeKMC,Bonho-efferJ,WongICK,etal.
PediatricdrugsafetysurveillanceinFDA-AERS:adescriptionofadverseeventsfromGRiPProject.
PloSOne.
2015;10(6):e0130399.
33.
HarpazR,DuMouchelW,LePenduP,Bauer-MehrenA,RyanP,ShahNH.
Performanceofpharmacovigilancesignal-detectionalgorithmsfortheFDAAdverseEventReportingSystem.
ClinPharmacolTher.
2013;93:539–46.
34.
NorenGN,CasterO,JuhlinK,LindquistM.
ZooorsavannahChoiceoftraininggroundforevidence-basedpharmacovigilance.
DrugSaf.
2014;37:655–9.
880O.
U.
Osokoguetal.
35.
AlmenoffJ,TonningJM,GouldAL,SzarfmanA,HaubenM,Ouellet-HellstromR,etal.
Perspectivesontheuseofdatamininginpharmacovigilance.
DrugSaf.
2005;28(11):981–1007.
36.
AagaardL,StrandellJ,MelskensL,PetersenPSG.
HolmeHansenE.
Globalpatternsofadversedrugreactionsoveradecade:analysesofspontaneousreportstoVigiBaseTM.
DrugSaf.
2012;35:1171–82.
37.
Morales-OlivasFJ,Martnez-MirI,FerrerJM,RubioE,PalopV.
AdversedrugreactionsinchildrenreportedbymeansoftheyellowcardinSpain.
JClinEpidemiol.
2000;53:1076–80.
38.
ImpicciatoreP,ChoonaraI,ClarksonA,ProvasiD,PandolniC,BonatiM.
Incidenceofadversedrugreactionsinpaediatricin/out-patients:asystematicreviewandmeta-analysisofprospec-tivestudies.
BrJClinPharmacol.
2001;52:77–83.
PediatricSignalDetection881
Osokogu1CaitlinDodd1AlexandraPacurariu1,3FlorentiaKaguelidou1,2DanielWeibel1MiriamC.
J.
M.
Sturkenboom1Publishedonline:2June2016TheAuthor(s)2016.
ThisarticleispublishedwithopenaccessatSpringerlink.
comAbstractIntroductionSpontaneousreportsofsuspectedadversedrugreactions(ADRs)canbeanalyzedtoyieldadditionaldrugsafetyevidenceforthepediatricpopulation.
Signaldetectionalgorithms(SDAs)arerequiredfortheseanaly-ses;however,theperformanceofSDAsinthepediatricpopulationspecicallyisunknown.
Wetestedtheperfor-manceoftwoSDAsonpediatricdatafromtheUSFDAAdverseEventReportingSystem(FAERS)andinvesti-gatedtheimpactofagestraticationandageadjustmentontheperformanceofSDAs.
MethodsWetestedtheperformanceoftwoestablishedSDAs:theproportionalreportingratio(PRR)andtheempiricalBayesgeometricmean(EBGM)onapediatricdatasetfromFAERS(2004–2012).
Wecomparedtheper-formanceoftheSDAswithapublishedpediatric-specicreferencesetbycalculatingdiagnostictest-relatedstatis-tics,includingtheareaunderthecurve(AUC)ofreceiveroperatingcharacteristics.
Impactofagestraticationandage-adjustmentontheperformanceoftheSDAswasassessed.
Ageadjustmentwasperformedbypooling(Mantel-Hanszel)stratum-specicestimates.
ResultsAtotalof115,674pediatricreports(patientsaged0–18years)comprising893,587drug–eventcombinations(DECs)wereanalysed.
CrudevaluesoftheAUCweresimilarforbothSDAs:0.
731(PRR)and0.
745(EBGM).
StraticationunmaskedfourDECs,e.
g.
,'ibuprofenandthrombocytopenia'.
Ageadjustmentdidnotimproveperformance.
ConclusionTheperformanceofthetwotestedSDAswassimilarinthepediatricpopulation.
Ageadjustmentdoesnotimproveperformanceandisthereforenotrecommendedtobeperformedroutinely.
Straticationcanrevealnewasso-ciations,andthereforeisrecommendedwheneitherdruguseisage-specicorwhenanage-specicriskissuspected.
KeyPointsDetectionofdrugsafetysignalsinchildren,whorepresentaheterogeneouspopulation,whereagemaybeaconfounderoreffectmodier,isanareainwhichonlylimitedresearchhasbeencarriedout.
Thesignaldetectionalgorithms(SDAs)showedgoodperformanceonpediatricdataandcanbeutilizedforpediatricsignaldetection.
AgeadjustmentdidnotimprovetheperformanceoftheSDAs.
Agestraticationshowedthatsomesignalsmaybedetectedonlyinspecicpediatricagegroups.
Forroutinesurveillance,checkingforeffectmodicationacrossagestratamaygenerateusefulinformation.
OsemekeU.
OsokoguandCaitlinDoddcontributedequallytothisarticle.
ElectronicsupplementarymaterialTheonlineversionofthisarticle(doi:10.
1007/s40264-016-0433-x)containssupplementarymaterial,whichisavailabletoauthorizedusers.
&CaitlinDoddc.
dodd@erasmusmc.
nl1DepartmentofMedicalInformatics,ErasmusUniversityMedicalCenter,Rotterdam3015CN,TheNetherlands2DepartmentofPediatricPharmacologyandPharmacogenetics,HopitalRobertDebre,APHP,UnivParis7-Diderot,SorbonneParisCite,EA08,INSERMCIC1426,Paris75019,France3DutchMedicinesEvaluationBoard,Utrecht,TheNetherlandsDrugSaf(2016)39:873–881DOI10.
1007/s40264-016-0433-x1IntroductionSpontaneousreportsofsuspectedadversedrugreactions(ADRs)canyieldimportantinformationregardingthesafetyofdrugs[1].
Usually,suchreportsarescreenedforemergingsafetyissuesbyapplyingstatisticalmethodscalledsignaldetectionalgorithms(SDAs).
CurrentSDAscomparethereportingrateofadrug–eventcombination(DEC)ofinterestwiththeexpectedcountcalculatedfromtheoverallreportingrateofthatreactionintheentiredatabase[1,2].
AlthoughSDAsareroutinelyappliedtoreportspertainingtothegeneralpopulation,theperfor-manceofSDAsinthepediatricpopulationspecicallyhasnotbeeninvestigatedtodate.
Comparedwithadults,thepatternofdruguseandoccurrenceofADRsinpediatricpatientsmaydiffer[3–5]sincethelatterpopulationcom-prisesaheterogeneousgroupofsubjectsatvariousstagesofdevelopmentwithage-dependentorganmaturationandhormonalchanges[6].
SeveralstudiesinvestigatingADRreportinginchildrenhaveidentieddifferentreportingpatternsinthispopulationthaninadults[3,5,7,8].
SinceADRsmaybeagespecic,adjustmentforageseemstobealogicalstepwheninvestigatingpediatricADRsandhasbeenadvocatedbysomeresearchers[4].
Themajoraimofstraticationisvericationofconfoundingandeffectmodicationwhichotherwisemaymasktruesignals[9].
Confoundingbyagecanbedealtwithbystratifyingforagecategoriesandpoolingstratum-specicestimates.
How-ever,ifage-specicestimatesdiffer(incaseofeffectmodication)pooling/adjustmentshouldnotbedone;instead,avericationofeachindividualstratumshouldbeperformed.
Whilestraticationhasbeeninvestigatedbysomeresearchers[10],adjustmentisroutinelyimple-mentedinsomeBayesianbutnotinfrequentistSDAs[11–13].
Fewstudieshavesystematicallyaddressedtheimpactofagestraticationoradjustmentandtheresultsarecontradictory[9,14,15].
WithinthecontextoftheGlobalResearchinPediatrics(GRiP)NetworkofExcellence[16],weaimedtoevaluatetheperformanceoftwowell-establishedSDAsinthepediatricpopulationanddetermineifagestraticationoradjustmentimpactssignaldetectioninthispopulation.
2Methods2.
1DataSourceDatawereretrievedfromthepubliclyavailableversionoftheUSFDAAdverseEventReportingSystem(FAERS),whichcomprisesspontaneousreportsofsuspectedADRssubmittedbymanufacturers,healthcareprofessionals,andpatients.
FAERSisoneofthelargestrepositoriesofspontaneousreportsintheworld[17,18].
Inthisstudy,weanalyzedreportsreceivedfromtherstquarterof2004throughtothethirdquarterof2012.
Forperformanceanalysis,onlyreportsofADRsoccur-ringinchildrenandadolescents(\18yearsofage)wereretained.
TheADRsinFAERSarecodedaccordingtotheMedicalDictionaryforRegulatoryActivities(MedDRA)[19].
Toimprovethequalityofthedataset,weexcludedreportswithmissingage,themainvariableinourstudy.
Also,reportswithreportedageequaltozeroandwithaMedDRApreferredtermindicatingprenatalexposurewereremoved,astheseimplyinuterodrugexposureandwerethereforenotrelevantforourstudy.
Weminimizedthenumberofduplicates(i.
e.
,thesamereportsubmittedbydifferentreporters)byapplyinganalgorithmbasedoncaseidentier,reportidentier,anddrugandeventnames.
Formultiplereports(i.
e.
,thesamereportisreportedatalatertime,withadditionalandupdatedinformation)[20],themostrecent(andmostupdated)reportwasretainedforanalysis.
AsdrugnamesincludedinFAERSarenotstandardized,aharmonizationprocedurewasimplemented.
Briey,thisconsistedofremovingsuperuouscharactersandapplyingageneralizededitdistancematchingalgorithm[21]tomapfree-textdrugnamestosynonymsandnallytothecor-respondingactivesubstanceandWorldHealthOrganiza-tion–AnatomicTherapeuticChemical(WHO-ATC)code.
Inthisstudy,onlythosedrugsreportedastheprimaryorsecondarysuspectintheFAERSdatabasewereretainedforanalysis.
AnalysiswasperformedatDEClevel,meaningthatwithineachreport,everysuspectdrugwascombinedwithallreportedADRs.
Thus,onereportmaycomprisemorethanoneDEC.
2.
2SignalDetectionAlgorithms(SDAs)Wetestedtwowell-establishedSDAsthatareroutinelyusedbyvariousnationalandinternationalregulatoryand/orresearchinstitutionsforsignaldetection:thepropor-tionalreportingratio(PRR)[2]andtheempiricalBayesgeometricmean(EBGM)[13](seeTable1).
Wealsotestedcountofreportsasapositivecontrol.
Inordertodeneasignalofdisproportionatereporting[22,23],weselectedthresholdsthatarecurrentlyappliedinroutinepractice.
WeappliedtheSDAsattheendofthestudyperiod,whenthemaximumnumberofreportshadaccrued.
2.
3PerformanceAssessmentMeasuresTheperformanceoftheSDAswasassessedbycalculatingdiagnostictest-relatedstatistics,namelyspecicityand874O.
U.
Osokoguetal.
sensitivity,positivepredictivevalue(PPV),andnegativepredictivevalue(NPV)[24,25].
Sensitivityistheabilityofthemethodtoidentifytruesignalscorrectly,whilespeci-cityistheabilitytoexcludefalsesignalscorrectly.
PPVandNPVareposteriorprobabilities,describinghowmanyofthesignalsclassiedaspositiveornegativeareindeedcorrectlyclassied[24,25].
Sincediagnostictest-relatedstatisticsaredependentonthethresholdchoice,theirindividualcomparisonhasonlyalimited,albeitpractical,value.
Therefore,wealsoesti-matedtheareaunderthecurve(AUC)ofreceiveroperatingcharacteristics(ROC)inordertocomparetheperformanceoftheSDAs[26];theAUCincorporatesbothsensitivityandspecicityacrossallthepossiblevaluesforacertainSDA.
CalculationofAUCswasconductedbyvaryingonlythepointestimateofeachSDAanddidnottakeintoaccounttheothercomponentsoftheSDA.
Forthepurposeofperformanceevaluation,apreviouslyconstructedpediatric-specicGRiPreferencesetofposi-tiveandnegativeDECswasused.
Itconsistsof37positiveand90negativeDECsandincludesdrugsthatareadmin-isteredtochildrenandeventsthatareregardedasimportantforthispopulation.
ThepositiveDECsarethosethatwereconrmedtooccurbasedonevidencefromSummaryofProductCharacteristics(SmPC)andthepublishedlitera-ture,whilethenegativeDECsarethosethatcouldnotbeconrmedatthetimeofliteraturereviewbyeithertheSmPCorthepublishedliterature.
Forafulldescriptionofthereferenceset,seeOsokoguetal.
[27].
2.
4StraticationandAdjustmentforAgeTheimpactofagestraticationandadjustmentontheperformanceoftheSDAswasinvestigated.
First,wecheckedforpossibleeffectmodicationacrossagestrata,bystratifyingthedataaccordingtoagecategoriesdenedbytheInternationalConferenceonHarmonization(ICH)[28]andcalculatingstratum-specicmeasuresforeachSDA.
Secondly,wecalculatedage-adjustedestimatesforPRRandEBGMbycombiningthestratum-specicesti-matesinanoverallmeasure[29].
TheperformanceofeachSDAwasreassessedafteradjustment.
2.
5StatisticalAnalysisDifferencesintheperformance(AUC)ofeachSDA,crudeversusage-adjustedandcrudeversuscountofreports(positivecontrol)weretestedusingpairedchi-squaredtests.
Stratum-speciccontingencytablesweretestedforhomogeneityusingtheBreslowDayTaronetest[30].
TheMantel-Haenszelapproachwasusedforpoolingandcalculatingage-adjustedestimates[29].
ThelowerboundoftheEBGM95%condenceinterval(EBGM05)wascalculatedusingthelowerboundofthe95%condenceinterval(EB05)foreachstratumandthencomputingaMantel-HaenszelaveragebasedonZeinounetal.
[31].
Statisticalsignicancewasdenedbyp\0.
05.
AnalysiswasperformedusingSASsoftwareversion9.
2(SASInstitute,Cary,NC,USA).
GraphsweremadeinSASsoftwareversion9.
2andRversion3.
1.
3.
3Results3.
1DescriptiveAnalysisForthestudyperiod(rstquarterof2004throughtothethirdquarterof2012),atotalof4,285,088reportswereretrievedfromFAERS.
Aftereliminatingduplicates(n=43,125)andremovalofadultreports(n=2,686,530)andreportswithmissingage(n=1,419,524)orageequaltozerowithaMedDRApreferredtermindicatingpre-natalexposure(n=20,235),115,674reportscorrespond-ingto893,587individualDECswereretainedforanalysisofpediatricspontaneousreports(seeTable2).
ThetotalnumberofpediatricreportsthatincludedtheinvestigateddrugsandADRsfromthereferencesetcanbeobservedinFig.
1,whichalsoshowsdataregardingadults(forcomparisonpurposes).
ThenumberofchildrenTable1SignaldetectionalgorithmsandcorrespondingthresholdsappliedSignaldetectionalgorithmAppliedthresholdaInstitutionwherethemethodandtherespectivethresholdiscurrentlyusedNumberofreportsC5NAPRRPRRlowerbound95%CIC1andnC5reportsEuropeanMedicinesAgency(EMA)EBGMEB05CIC1.
8,nC3reports,andEBGMC2.
5MedicinesandHealthcareproductsRegulatoryAgency(MHRA)CIcondenceinterval,EB05lowerboundofthe95%condenceinterval,EBGMempiricalBayesgeometricmean,NAnotavailable,PRRproportionalreportingratioaThresholdswereobtainedfromCandoreetal.
[23]PediatricSignalDetection875exposedtothedrugsofinterest,forwhomanyoftheinvestigatedADRswasreported,variedfrom26patients(forpraziquantel)to7535patients(foribuprofen),withamedianof781patientsexposedacrossalldrugs.
ThenumberofeventsofinterestinFAERSrangedfrom164reports(ventriculararrhythmia)to14,777(anaphylaxis),withamedianof1004reportsacrossallevents.
ForamoredetaileddescriptionofreportscountspleaserefertoElec-tronicSupplementarymaterialTable1.
3.
2OverallPerformanceofSDAsBothSDAsshowedhighspecicityandlowsensitivity.
Theybothhadsimilarspecicityvalues(PRR:83.
8%andEBGM:91.
9%),whilesensitivitywaslowerforEBGMthanforPRR(17.
2vs.
37.
9%).
TheNPVandPPVweresimilarforbothSDAs.
Whenweappliedthethreshold-independent(AUC-based)approach,thetestedSDAsshowedsimilarperformanceinthepediatricpopulation,althoughtheAUCvalueforEBGM(0.
745)wasslightlyhigherthanforPRR(0.
731).
NoneoftheSDAsperformedbetterthanthesimplereportcount(AUC=0.
634;p=0.
27forPRRandp=0.
14forEBGM)3.
3StraticationandAdjustmentforAgeanditsImpactonPerformanceUponcalculatingSDAvaluesperagestratumandtestingforheterogeneityacrossstrata,weobservedeffectmodi-cationforsomeassociations.
Somefalsenegatives(pos-itiveDECsthatfailedtobehighlightedassignalswhenanalyzingdatapertainingtotheentirepediatricpopulation)wereunmaskedinsomestrata.
FourDECswereunmaskedintotal:ibuprofen–thrombocytopeniaandisoniazid–sei-zure(byPRR)andclarithromycin–erythemamultiformeandibuprofen–erythemamultiforme(byEBGM).
Con-versely,'ibuprofen–acuteliverinjury',alsoapositiveDEC,washighlightedwhenweanalyzeddatapertainingtotheentirepediatricpopulation,butitbecameclearafterstratifyingthatthisDECwashighlightedonlyinolderchildren(adolescents)andnotinyoungerchildren(seeFig.
2).
ForanoverviewofSDAvaluesacrossagestrataandresultsofheterogeneitytestspleaserefertoElectronicSupplementaryMaterialFigures1Aand1B.
Weevaluatedtheperformanceofthemethodswithinindividualagestrata(seeTable3).
Onaverage,perfor-manceoftheSDAswaslowerwithinagestratathanintheentirepediatricpopulationandperformanceimprovedwithincreasingstratumsize.
Forinfantsandneonates,theper-formancewasverylow,notbetterthanchance(p[0.
5forbothSDAs).
Theadolescentgroupexhibitedthebestper-formance,whichwassimilartotheoverallperformance.
Afteradjustingforagebypoolingthestratum-specicestimates,theperformanceoftheSDAsdecreased,althoughnotsignicantly(seeFig.
3;crudevs.
adjustedAUCforPRR:0.
731vs.
0.
688,p=0.
267;crudevs.
adjustedAUCforEBGM:0.
745vs.
0.
683,p=0.
216).
4DiscussionInthisstudy,wehavedemonstratedthatagestraticationfordetectionofdrugsafetysignalsinchildrenmayunmasksomesignalsthatdonotappearineithercrudeoradjustedanalysis.
Adjustmentforagedoesnotimproveperfor-manceofthePRRandEBGM.
Fortheinvestigatedevents,similarreportingpatternswereobservedforchildrenandadults,whiletheinvesti-gateddrugsappearedtohavedifferentreportingpatterns(seeFig.
1).
Differentdrug-relatedreportingpatternsinchildrenversusadultshavebeenreportedpreviously[5].
Consequently,reportedDECsforchildrenmaydifferfromadults[3,5],underliningtheneedforpediatric-specicapproachestosignaldetection,especiallywhenwecon-siderthatreporteddrugsmayvarybyagegroupevenwithinthepediatricpopulation[3,32].
Overall,thePRRandEBGMshowedgoodperfor-mance,althoughresultswereslightlylowerthanresultsreportedonother(notpediatric-specic)referencesets[32,33].
ThesimilarityinperformancebetweenPRRandEBGMisinaccordancewithrecentresultsfromthePROTECT(PharmacoepidemiologicalResearchonOutcomesofTherapeuticsbyaEuropeanConsortium)project[23].
Thefactthattheperformance(basedonAUC)ofPRRandEBGMwasnotstatisticallysigni-cantlybetterthansimplereportcountmaybeduetothelackofpower.
Withinagestrata,performanceseemedtocorrelatewithstratumsize:thepoorestresultswereobservedforinfantsandneonates(thesmallergroups),slightlyimprovingforchildren,whilethebestperfor-mancewasobservedforadolescents,theagestratumwiththehighestnumberoftestedDECs.
DecreaseinpowerduetofewerreportsandthereforeDECsmayaccountforthisobservation.
Thefactthatweusedlowerboundsofcondenceintervalsforsignalinginsteadofpointestimatesmighthaveexacerbatedtheinuenceofsamplesizeontheresults,sincesmallerstratawillhaveTable2DescriptionofpediatricreportsbyagecategoriesAgegroupNumberofreports[n(%)]Neonates:0–27days5091(4.
40)Infants:28days–23months12,566(10.
86)Children:2–11years49,982(43.
21)Adolescents:12–17years48,035(41.
53)Total115,674(100)876O.
U.
Osokoguetal.
highervariability.
Inneonatesandinfantsforwhomexpectedcountsweredifculttocalculatebecauseoffewreports,weobservedthatsimplereportcountsper-formedsimilarorevenbetterthantheSDAsandmightbeanalternativetocommonlyusedSDAs.
ThefactthatsimplereportcountperformedbetterthanSDAsmayhavebeenbecausethereferencesetcomprisedknownDECs(whichinturnmayhaveinuencedreporting)ratherthanemergingsafetyissues,ahypothesispro-posedbyNorenetal.
[34].
InspectionofSDAvaluesacrosschild-specicstrata(agestratication)revealedsomeheterogeneityinesti-mates,pointingtosomeeffectmodication.
Forexample,'ibuprofen–thrombocytopenia'wasfoundasasignalintheFig.
1Countofreportsinthepediatricandadultpopulationfortheinvestigatedadversedrugreactions(a)anddrugs(b),cumulativelyfortheperiodquarter12004toquarter32012.
Thenumberofreportsinchildrenisrepresentedbybarsandplottedontheleftaxis,whilethenumberofreportsinadultsisrepresentedbytheredlineandplottedontherightaxis;reportswithmissingageorage=0wereexcluded.
OnlyreportsmentioninganyofthedrugsoreventsinthereferencesetwereconsideredPediatricSignalDetection877adolescents'groupbutnotdetectedintheentirepediatricpopulationortheyoungeragecategories.
Thissuggeststhatage-specicSDAcalculationsaresometimesneeded,ratherthanage-adjustedSDAestimates.
Theage-adjustedestimatesdidnotimproveperformance;infact,evenPPVunexpectedlydecreased.
Simulationstudieshaveshownthatwhenadjustedforstrata,BayesianmethodssuchasEBGMtendtobeunderestimatedwhentherearesparsestrata[15];thiswasalsothecaseinourstudy.
Previousstudiesinadultsshowcontradictoryresults,withsomeshowingabenecialeffect[9]whileothersdidnot[15].
Thereasonforourndingisnotentirelyclear;apossibleexplanationisthatageisnotastrongconfounderfortheinvestigatedDECs.
Also,themethodofweighting(Mantel-Haenszelapproach)mayhaveplayedarolesincemoreweightwasassignedtoagegroupswithmorereports(adolescentsandchildren).
Thismayhavemaskedsignalsoccurringinagegroupswithfewerreports.
ThelimitationsofdatamininginFAERSincludethoseinherenttospontaneousreportingdatabases:under-report-ing,lackofdenominatordataandcontrolgroup,biasesinreporting,aswellasmissingandpoor-qualitydata[35].
Missinginformationregardingagesubstantiallyreducedthestudysamplesizesincewecouldnotdeterminewhe-therthesereportsdescribedpatientsagedlessthan18yearsold.
Whilethesebiasesarewellacknowledgedandhaveadeniteimpact,theycannotbecompletelyavoided.
Comparedwithadults,therearefewerreportsanddifferentreportingpatternsforchildren[3,36,37],whichmaycomplicatesignaldetectioninthepediatricpopulation.
EvaluatingperformanceofSDAsisaconstantchallengeduetolackofstandardmethodologies,imperfectreferencestandards,anduncertaintyregardingthebestthresholds(seetheElectronicSupplementaryMaterialformeasuresofperformanceusingalternativethresholds).
SomeofthedrugsandeventsinthereferencesetarespecictooneagegroupwithinpediatricsandthisisobviousinFig.
1,eventhoughthereferencesetwasdesignedtoberelevantfortheentirepediatricpopulation.
Weacknowledgethattheref-erencesetused,althoughspecicallyconstructedforthisp-valueswerecalculatedwithBreslowDayTaronetestforhomogeneityp<0.
0001p=0.
001p=0.
339Fig.
2VariationofproportionalreportingratioandempiricalBayesgeometricmeanestimatesacrosspediatricspecicstrata—selectedexamples.
EBGMempiricalBayesgeometricmean,PRRproportionalreportingratio,SDAsignaldetectionalgorithmTable3PerformanceofsignaldetectionalgorithmsacrossagestrataAgegroupsandsignaldetectionalgorithmsSizeoftheagestratum(numberofreports)AUCNeonates5091Numberofreports0.
625EBGM0.
600PRR0.
65Infants12,566Numberofreports0.
667EBGM0.
548PRR0.
554Children49,982Numberofreports0.
654EBGM0.
698PRR0.
649Adolescents48,035Numberofreports0.
698EBGM0.
771PRR0.
718EntirepediatricpopulationNumberofreports115,6740.
634EBGM0.
746PRR0.
733AUCareaunderthecurve,EBGMempiricalBayesgeometricmean,PRRproportionalreportingratio878O.
U.
Osokoguetal.
purpose,doesnotincludealltheADRsthatarehighlyspecicforpediatrics.
Thishighlightstheneedforpedi-atric-specicapproachestosignaldetection,accountingfornotjusttheentirepediatricpopulationbutalsothedifferentagestratawithinpediatrics.
Still,thereferencesetcapturesvariousdruguseandADRspatterns[38]andiscurrentlytheonlyavailablepediatric-specicreferenceset.
Thethresholdsappliedtodeneasignalwereobtainedfrompreviouspublicationsandothercut-offpointsmaygeneratebetterresults;furtherresearchonpediatric-specicthresholdsshouldbeencouraged.
5ConclusionOurstudyrevealedthatageadjustmentdidnotimprovetheperformanceoftheSDAs.
However,straticationrevealedsomevariationinthevaluesofSDAsacrossstrata(effectmodication)andinspectionofstratum-specicestimatesmightsometimesyieldusefulinformationduringroutinesurveillance.
CompliancewithEthicalStandardsFundingTheGlobalResearchinPediatricsNetworkofExcellenceisfundedundertheEuropeanUnion'sSeventhFrameworkProgram(FP7/2007–2013)forresearch,technologicaldevelopment,anddemonstrationundergrantagreementnumber261060.
Fundingforthisstudywasalsoreceivedfromthe''PriorityMedicinesKinderenprojectZONMW:EVIPED:novelmethodstoassessandcomparedrugeffectsinpediatrics''(grantagreementnumber113201007).
Thefundershadnorolewhatsoeverindesigningandconductingthestudy,thecollectionandmanagementofdata,andpreparation,review,orapprovalofthemanuscript.
ConictofinterestMiriamSturkenboomleadsaresearchunitthatoccasionallyconductsresearchforpharmaceuticalcompanies,includingNovartis,Boehringer,Lilly,andPzer.
Noneofthisworkisrelatedtotheseactivities.
AlexandraPacurariuisanemployeeoftheDutchMedicinesEvaluationBoard.
Theviewsexpressedinthisarticlearethepersonalviewsoftheauthor(s)andmaynotbeunderstoodorquotedasbeingmadeonbehalfoforreectingthepositionoftheDutchMedicinesAgency.
OsemekeU.
Osokogu,CaitlinDodd,FlorentiaKaguelidou,andDanielWeibelhavenoconictsofinterestthataredirectlyrelatedtothecontentofthisstudy.
SDASensitivitySpecificityPPVNPVAUCp-valuebNumberofreports58.
6267.
5758.
6267.
570.
634referencePRR37.
9383.
7864.
7163.
270.
7310.
266EBGM17.
2491.
8962.
5058.
620.
7450.
144Afterageadjustmenta(reference-crudePRR/EBGM)PRR34.
4886.
4966.
6762.
750.
6880.
267EBGM10.
3497.
3075.
0058.
060.
6830.
216SDA-signaldetectionalgorithm;PRR=Proportionalreportingratio;EBGM=EmpiricalBayesGeometricMean;AUC=areaunderthecurve;PPV=positivepredictivevalue;NPV-negativepredictivevalueaadjustedPRR/RORvaluescalculatedbycombiningtheindividualestimatesfromeachagestratumintoonemeasureaccordingtotheMantel-Haenszelapproach.
bpairedchi-squaretestFig.
3PerformanceofsignaldetectionalgorithmswithintheentirepediatricpopulationPediatricSignalDetection879OpenAccessThisarticleisdistributedunderthetermsoftheCreativeCommonsAttribution-NonCommercial4.
0InternationalLicense(http://creativecommons.
org/licenses/by-nc/4.
0/),whichper-mitsanynoncommercialuse,distribution,andreproductioninanymedium,providedyougiveappropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreativeCommonslicense,andindicateifchangesweremade.
References1.
BateA,EvansSJW.
Quantitativesignaldetectionusingsponta-neousADRreporting.
PharmacoepidemiolDrugSaf.
2009;18:427–36.
2.
EvansSJW,WallerPC,DavisS.
Useofproportionalreportingratios(PRRs)forsignalgenerationfromspontaneousadversedrugreactionreports.
PharmacoepidemiolDrugSaf.
2001;10:483–6.
3.
BlakeKV,ZaccariaC,DomergueF,LaMacheE,Saint-Ray-mondA,Hidalgo-SimonA.
ComparisonbetweenpaediatricandadultsuspectedadversedrugreactionsreportedtotheEuropeanMedicinesAgency:implicationsforpharmacovigilance.
PaediatrDrugs.
2014;16:309–19.
4.
StarK,EdwardsIR.
Pharmacovigilanceforchildren'ssake.
DrugSaf.
2014;37:91–8.
5.
StarK,NorenGN,NordinK,EdwardsIR.
Suspectedadversedrugreactionsreportedforchildrenworldwide:anexploratorystudyusingVigiBase.
DrugSaf.
2011;34:415–28.
6.
KearnsGL,Abdel-RahmanSM,AlanderSW,BloweyDL,LeederJS,KauffmanRE.
Developmentalpharmacology—drugdisposition,action,andtherapyininfantsandchildren.
NEnglJMed.
2003;349:1157–67.
7.
AagaardL,WeberCB,HansenEH.
AdversedrugreactionsinthepaediatricpopulationinDenmark:aretrospectiveanalysisofreportsmadetotheDanishMedicinesAgencyfrom1998to2007.
DrugSaf.
2010;33:327–39.
8.
KimlandE,RaneA,UferM,PanagiotidisG.
PaediatricadversedrugreactionsreportedinSwedenfrom1987to2001.
Pharma-coepidemiolDrugSaf.
2005;14:493–9.
9.
WooEJ,BallR,BurwenDR,BraunMM.
EffectsofstraticationondataminingintheUSVaccineAdverseEventReportingSystem(VAERS).
DrugSaf.
2008;31:667–74.
10.
OrreR,LansnerA,BateA,LindquistM.
Bayesianneuralnet-workswithcondenceestimationsappliedtodatamining.
ComputStatDataAnal.
2000;34(4):473–93.
11.
AlmenoffJS,LaCroixKK,YuenNA,FramD,DuMouchelW.
Comparativeperformanceoftwoquantitativesafetysignallingmethods.
DrugSaf.
2006;29(10):875–87.
12.
NorenGN,BateA,OrreR,EdwardsIR.
ExtendingthemethodsusedtoscreentheWHOdrugsafetydatabasetowardsanalysisofcomplexassociationsandimprovedaccuracyforrareevents.
StatMed.
2006;25:3740–57.
13.
DuMouchelW.
Bayesiandatamininginlargefrequencytables,withanapplicationtotheFDAspontaneousreportingsystem.
AmStat.
1999;53(3):177–90.
14.
AlmenoffJS,PattishallEN,GibbsTG,DuMouchelW,EvansSJW,YuenN.
Novelstatisticaltoolsformonitoringthesafetyofmarketeddrugs.
ClinPharmacolTher.
2007;82(2):157–66.
15.
HopstadiusJ,NorenGN,BateA,EdwardsIR.
Impactofstrati-cationonadversedrugreactionsurveillance.
DrugSaf.
2008;31(11):1035–48.
16.
GlobalResearchinPaediatrics.
NetworkofExcellence.
http://www.
grip-network.
org/index.
php/cms/en/home.
Accessed28Feb2016.
17.
MooreTJ,FurbergCD.
Thesafetyrisksofinnovation:theFDA'sExpeditedDrugDevelopmentPathway.
JAMA.
2012;308(9):869–70.
18.
Weiss-SmithS,DeshpandeG,ChungS,GogolakV.
TheFDAdrugsafetysurveillanceprogram:adverseeventreportingtrends.
ArchInternMed.
2011;171:591–3.
19.
MedDRAMSSO.
Introductoryguide:MedDRAversion15.
1.
2012.
http://www.
meddra.
org/sites/default/les/guidance/le/intguide_15_1_English_0.
pdf.
Accessed28Feb2016.
20.
PoluzziE,PiccinniC,RaschiE,DePontiF.
Dataminingtech-niquesinpharmacovigilance:analysisofthepubliclyaccessibleFDAAdverseEventReportingSystem(AERS).
Rijeka:INTECHOpenAccessPublisher;2012.
21.
SAS.
COMPGEDFunction.
Returnsthegeneralizededitdistancebetweentwostrings.
ComputingtheGeneralizedEditDistance.
In:SAS9.
2languagereference:dictionary,fourthedition.
http://support.
sas.
com/documentation/cdl/en/lrdict/64316/HTML/default/viewer.
htm#a002206133.
htm.
Accessed3Mar2015.
22.
HaubenM,AronsonJK.
Dening'signal'anditssubtypesinpharmacovigilancebasedonasystematicreviewofpreviousdenitions.
DrugSaf.
2009;32(2):99–110.
23.
CandoreG,JuhlinK,ManlikK,ThakrarB,QuarcooN,SeabrokeS,etal.
Comparisonofstatisticalsignaldetectionmethodswithinandacrossspontaneousreportingdatabases.
DrugSaf.
2015;38(6):577–87.
24.
ZhouX-H,McClishDK,ObuchowskiNA.
Statisticalmethodsindiagnosticmedicine.
Hoboken:Wiley;2009.
25.
StromBL,KimmelSE,HennessyS.
Pharmacoepidemiology.
5thed.
Chichester:Wiley-Blackwell;2012.
26.
vanPuijenbroekEP,BateA,LeufkensHGM,LindquistM,OrreR,EgbertsACG.
Acomparisonofmeasuresofdispro-portionalityforsignaldetectioninspontaneousreportingsystemsforadversedrugreactions.
PharmacoepidemiolDrugSaf.
2002;11:3–10.
27.
OsokoguOU,FregoneseF,FerrajoloC,VerhammeK,deBieS,CatapanoM,etal.
Pediatricdrugsafetysignaldetection:anewdrug–eventreferencesetforperformancetestingofdata-miningmethodsandsystems.
DrugSaf.
2015;38(2):207–17.
28.
InternationalConferenceonHarmonisationofTechnicalRequirementsforRegistrationofPharmaceuticalsforHumanUse.
ICHHarmonisedTripartiteGuideline.
ClinicalInvestigationofMedicinalProductsinthePediatricPopulationE11.
CurrentStep4version.
20Jul2000.
http://www.
ich.
org/leadmin/Public_Web_Site/ICH_Products/Guidelines/Efcacy/E11/Step4/E11_Guideline.
pdf.
Accessed28Feb2016.
29.
MantelN,HaenszelW.
Statisticalaspectsoftheanalysisofdatafromretrospectivestudiesofdisease.
JNatlCancerInst.
1959;22:719–48.
30.
BreslowNE,DayNE,editors.
Statisticalmethodsincancerresearch.
VolumeI—theanalysisofcase-controlstudies.
IARCScienticPublicationsNo.
32.
Lyon:InternationalAgencyforResearchonCancer;1980.
31.
ZeinounZ,SeifertH,VerstraetenT.
Quantitativesignaldetectionforvaccines:effectsofstratication,backgroundandmaskingonGlaxoSmithKline'sspontaneousreportsdatabase.
HumVaccin.
2009;5(9):599–607.
32.
deBieS,FerrajoloC,StrausSMJM,VerhammeKMC,Bonho-efferJ,WongICK,etal.
PediatricdrugsafetysurveillanceinFDA-AERS:adescriptionofadverseeventsfromGRiPProject.
PloSOne.
2015;10(6):e0130399.
33.
HarpazR,DuMouchelW,LePenduP,Bauer-MehrenA,RyanP,ShahNH.
Performanceofpharmacovigilancesignal-detectionalgorithmsfortheFDAAdverseEventReportingSystem.
ClinPharmacolTher.
2013;93:539–46.
34.
NorenGN,CasterO,JuhlinK,LindquistM.
ZooorsavannahChoiceoftraininggroundforevidence-basedpharmacovigilance.
DrugSaf.
2014;37:655–9.
880O.
U.
Osokoguetal.
35.
AlmenoffJ,TonningJM,GouldAL,SzarfmanA,HaubenM,Ouellet-HellstromR,etal.
Perspectivesontheuseofdatamininginpharmacovigilance.
DrugSaf.
2005;28(11):981–1007.
36.
AagaardL,StrandellJ,MelskensL,PetersenPSG.
HolmeHansenE.
Globalpatternsofadversedrugreactionsoveradecade:analysesofspontaneousreportstoVigiBaseTM.
DrugSaf.
2012;35:1171–82.
37.
Morales-OlivasFJ,Martnez-MirI,FerrerJM,RubioE,PalopV.
AdversedrugreactionsinchildrenreportedbymeansoftheyellowcardinSpain.
JClinEpidemiol.
2000;53:1076–80.
38.
ImpicciatoreP,ChoonaraI,ClarksonA,ProvasiD,PandolniC,BonatiM.
Incidenceofadversedrugreactionsinpaediatricin/out-patients:asystematicreviewandmeta-analysisofprospec-tivestudies.
BrJClinPharmacol.
2001;52:77–83.
PediatricSignalDetection881
- incorporatesphp的cms相关文档
- tissuephp的cms
- 郑州商业技师学院-教材采购项目
- actorsphp的cms
- entitiesphp的cms
- 项目php的cms
- 2020年在晋招生院校咨询电话、网址登记表
火数云 55元/月BGP限时三折,独立服务器及站群限时8折,新乡、安徽、香港、美国
火数云怎么样?火数云主要提供数据中心基础服务、互联网业务解决方案,及专属服务器租用、云服务器、专属服务器托管、带宽租用等产品和服务。火数云提供洛阳、新乡、安徽、香港、美国等地骨干级机房优质资源,包括BGP国际多线网络,CN2点对点直连带宽以及国际顶尖品牌硬件。专注为个人开发者用户,中小型,大型企业用户提供一站式核心网络云端服务部署,促使用户云端部署化简为零,轻松快捷运用云计算!多年云计算领域服务经...

Virmach款低价VPS可选可以选择多个机房,新增多款低价便宜VPS主机7.2美元起
Virmach商家我们是不是比较熟悉?速度一般,但是人家价格低,而且机房是比较多的。早年的时候有帮助一个有做外贸也许需要多个机房且便宜服务商的时候接触到这个商家,有曾经帮助够买过上百台这样的低价机器。这里需要提醒的,便宜但是速度一般,尤其是中文业务速度确实不快,如果是外贸业务,那肯定是没有问题。这几天,我们有看到Virmach推出了夏季优惠促销,VPS首年8折,最低年付仅7.2美元,多机房可选,如...

极光KVM(限时16元),洛杉矶三网CN2,cera机房,香港cn2
极光KVM创立于2018年,主要经营美国洛杉矶CN2机房、CeRaNetworks机房、中国香港CeraNetworks机房、香港CMI机房等产品。其中,洛杉矶提供CN2 GIA、CN2 GT以及常规BGP直连线路接入。从名字也可以看到,VPS产品全部是基于KVM架构的。极光KVM也有明确的更换IP政策,下单时选择“IP保险计划”多支付10块钱,可以在服务周期内免费更换一次IP,当然也可以不选择,...
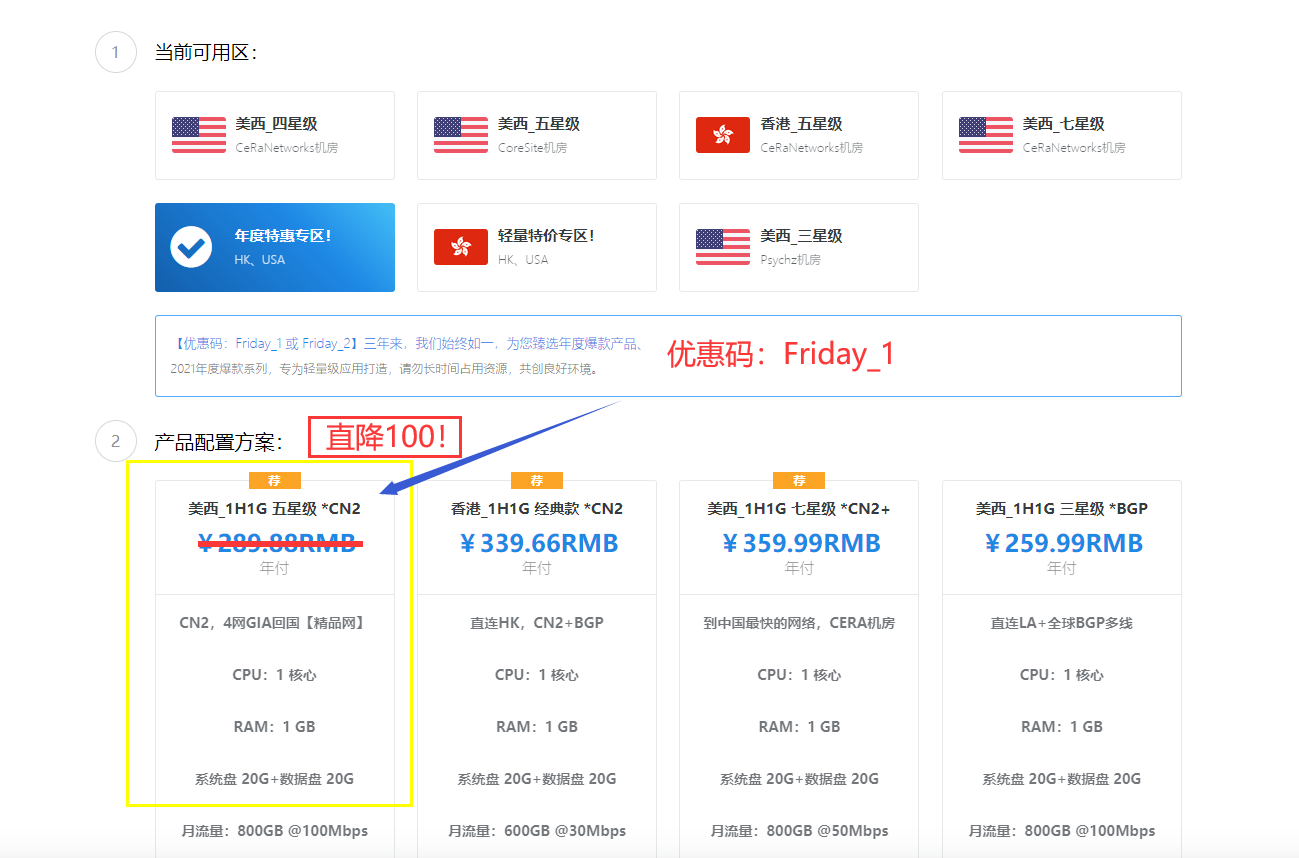
php的cms为你推荐
-
主机空间什么是网站虚拟主机空间?免费云主机永久免费的云主机哎或者空间或者vps国外虚拟空间哪里买的100m海外虚拟空间便宜稳定?代理主机如何将我工作的电脑设置为代理主机 让我回家以后可以用家里的电脑连接店里的主机访问网络虚拟空间哪个好虚拟内存设在哪个盘最好100m虚拟主机一般100-200M虚拟主机一天最多支持多少人访问啊?深圳虚拟主机深圳市虚拟主机深圳双线虚拟主机深圳主机合租深圳合租主机空推荐有哪?美国虚拟主机购买美国虚拟主机在国内那家卖的便宜,稳定,功能全??虚拟主机提供商虚拟主机必须与域名提供商在一家买吗?花生壳域名花生壳添加新域名