Scienceddd13.com
ddd13.com 时间:2021-04-08 阅读:()
FullPaperAconcisesynthesisof-carboxy--lactonesJ.
Gopalakrishna1,SubashC.
Jonnalagadda2*,VenkatramR.
Mereddy1*1DepartmentofChemistryandBiochemistry,UniversityofMinnesotaDuluth,1039UniversityDrive,Duluth,MN55812,(U.
S.
A.
)2DepartmentofChemistryandBiochemistry,RowanUniversity,201MullicaHillRoad,Glassboro,NJ08028,(U.
S.
A.
)E-mail:vmereddy@d.
umn.
eduReceived:25thNovember,2008;Accepted:30thNovember,2008OCAIJ,4(12),2008[513-517]INTRODUCTION-Carboxy--lactonemoietyisanextremelyim-portantmotiffoundinseveralnaturalproducts[1].
Asapartofourongoingprojectinvolvingthesynthesisofbiologicallyactivesmallmolecules,weundertookthesynthesisof-carboxy--lactonesviatheallylborationofá-ketoesters[1].
Asymmetricallylborationofcarbo-nylcompoundswithseveralchiralauxiliarieshasbeenextensivelystudiedforstereoselectiveformationofhomoallylicalcoholsandofallthesereagents,B-allyldiisopinocampheylborane(Ipc2BAllyl,1)hasproventobeoneofthepracticalandwidelyusedreagentsintermsofthecostandlevelofstereoselectivityob-served[1].
EXPERIMENTALPreparationofethyl2-oxo-2-phenylacetate,(2a)Thionylchloride(8mL,8mmol,1Msolutioninether)wasaddedtoasolutionofbenzoylformicacid(1.
0g,6.
66mmol)andpyridine(0.
5g)inanhydrousether(10mL)atroomtemperatureandstirredfor1.
5h.
Etherealsolutionwasdecantedandconcentrated.
Hexane(8mL)wasaddedtoresultingsolutionandconcentratedagain.
Theremainingthionylchloridewasremovedunderreducedpressuretoyieldphenylglyoxylylchloride(1.
06g).
Toasolutionofthecrudephenylglyoxylylchloride(1g,5.
95mmol)in12mLoftoluene,wasaddedpyridine(0.
94g,8mmol)andethanol(0.
36g,7.
1mmol)andstirredovernight.
Theresultingsolutionwasdecanted,concentratedunderreducedpressureandpurifiedbycolumnchromatography(ethylacetate:hexane1:19ratio)toyieldethyl2-oxo-2-phenylacetate(2a)(0.
9g,79%yield).
1HNMR(CDCl3,500MHz):7.
98-8.
00(m,2H),7.
47-7.
64(m,3H),4.
43(q,J=7.
2Hz,2H),1.
40(t,J=7.
0Hz,3H);13CNMR(CDCl3,125MHz)186.
7,164.
1,135.
1,132.
8,130.
0,129.
1,62.
4,14.
2.
Preparationofisopropyl2-oxo-2-phenylacetate,(2b)Proceduresimilartothatof(2a).
(76%yield)1HNMR(CDCl3,500MHz)7.
88(d,J=7.
5Hz,2H),7.
53(t,J=7.
5Hz,1H),7.
40(m,2H),5.
25(m,1H),1.
30(d,J=6.
0Hz,6H);13CNMR(CDCl3,125MHz):187.
0,KEYWORDS-Hydroxy-4-pentenoate;,-Dihydroxyesters;-Carboxy--lactone;Hydroboration;Oxidation.
ABSTRACTAshortprotocolforthesynthesisof-carboxy--lactoneshasbeendevel-opedviahydroborationfollowedbyoxidationof-hydroxy-4-pentenoates.
2008TradeScienceInc.
-INDIAAnIndianJournalTradeScienceInc.
Volume4Issue12December2008OrganicCHEMISTRYOrganicCHEMISTRY514FullPaperAconcisesynthesisof-carboxy--lactonesOCAIJ,4(12)December2008SubashC.
Jonnalagaddaetal.
515FullPaperOCAIJ,4(12)December2008OrganicCHEMISTRYOrganicCHEMISTRYAnIndianJournal5.
60(m,1H),5.
00-5.
08(m,2H),3.
74(s,3H),3.
61(dd,J=8.
0,15.
5Hz,1H),3.
30(dd,J=6.
5,13.
5Hz,1H);13CNMR(CDCl3,125MHz):172.
8,171.
1,165.
9,137.
5,132.
4,132.
1,131.
7,131.
6,131.
4,130.
0,129.
7,128.
8,128.
5,125.
4,119.
8,84.
9,53.
1,40.
3.
Preparationof2-((1-methoxy-1-oxo-2-methyl-pent-4-ene-2-yloxy)carbonylbenzoicacid,(5d)Proceduresimilartothatof(5a).
(30%yield)1HNMR(CDCl3,500MHz):7.
71-7.
78(m,1H),7.
61-7.
68(m,1H),7.
44-7.
54(m,2H),5.
75-5.
83(m,1H),5.
08-5.
12(m,2H),3.
72(s,3H),2.
75(dd,J=6.
5,13.
5Hz,1H),2.
59(dd,J=7.
5,14.
0Hz,1H),1.
66(s,3H).
PreparationofMethyl-2,5-dihydroxy-2-phenyl-pentanoate,7:BH3.
Me2S(2.
0mmol,0.
2mL,10Msolution)wasaddedtocooledsolutionoftheolefin3a(0.
2g,0.
97mmol)inTHF(2mL)at00Candstirredfor15min.
Excessboranewasquenchedslowlybyaddingmethanol(1mL)atthesametemperature.
Thereactionmixturewasoxidizedwith1mLof3MNaOHand1mLof30%hydrogenperoxideandstirredfor6hatroomtemperature.
TheproductwasextractedwithEt2OanddriedoverMgSO4.
Thesolventwasremovedunderaspiratorvacuum.
Thecrudeproductwaspurifiedbycolumnchromatography(silicagel,hexane:ethylacetate(4:1))toyield73%(0.
16g)ofdiol7.
1HNMR(CDCl3,500MHz):7.
57-7.
59(m,2H),7.
27-7.
36(m,3H),4.
41(brs,1H),3.
76(s,3H),3.
61(t,J=6.
2Hz,2H),2.
82(brs,1H),2.
30(ddd,J=5.
5,9.
0,14.
0Hz,1H),2.
15(ddd,J=5.
5,9.
5,14.
5Hz,1H),1.
52-1.
69(m,2H);13CNMR(CDCl3,125MHz):175.
8,141.
8,128.
5,128.
0,125.
7,78.
6,62.
7,53.
5,36.
7,27.
2.
Preparationoftetrahydro-5-oxo-2-phenyl-2-furancarboxylicacidmethylester,(9)Tetrapropylammoniumperruthenate(TPAP)(10mg,0.
03mmol)wasaddedtoastirredsolutionofthediol7(100mg,0.
45mmol),andNMO(192mg,1.
64mmol)indicholoromethane(2mL)atrtfor24h.
Aftercompletionofthereaction(TLC),CH2Cl2wasevaporatedandtheblackresiduewaspurifiedbycolumnchromatography(silicagel,hexane:ethylacetate(4:1))toafford(88mg)89%ofthelactone9.
1HNMR(CDCl3,500MHz):7.
38-7.
40(m,2H),7.
22-7.
29(m,3H),3.
61(s,3H),2.
93-2.
98(m,1H),2.
40-2.
59(m,3H);13CNMR(CDCl3,125MHz):175.
3,171.
0,138.
3,129.
0,128.
9,125.
3,87.
0,53.
5,33.
6,28.
3.
RESULTSANDDISCUSSIONOwingtotheimportanceofallylborationreactioninsyntheticorganicandmedicinalchemistry,wewantedtofurtherexpandthescopeof1.
-Ketoestersarere-activeprochiralketonesthatuponasymmetricnucleo-philicadditionsprovidequaternarychiralcentersandarehighlyusefulforthesynthesisofvarietyofstructur-allyintriguingnaturalproducts.
Allylationof-ketoestersprovidestertiary-carboxyhomoallylicalcohols,how-ever,stereoselectiveallylmetallationof-ketoestershasnotbeenawellstudiedprotocol[1].
AsymmetricallylborationofIpc2Ballyl1withaldehydesprovidesthehomoallylicalcoholsinveryhighee[3].
However,asymmetricallylborationofketoneswith1givesverylowtomoderateenantiomericexcess[3].
-Ketoestersarestereo-electronicallydifferentfromnormalketonesduetothepresenceofanelectronwithdrawingcarboxyestermoiety.
Itisknownintheliteraturethatá-ketoestersaremorereactivethannormalketoneswithareactivitypatternalmostsimilartothatofaldehydes.
ReactionssuchasBaylisHillmanreaction[1],Barbierallylation[1],etc.
thatarefacilewithaldehydesandsluggishwithke-tones,takeplaceveryreadilywith-ketoesters/á-iminoesters.
Basedonthisgeneralobservation,wehy-pothesizethat-ketoesterscouldundergofacileallylborationwithIpc2BAllyl1withthereactivityverysimilartothatofaldehydes.
Wehypothesizethattheproducthomoallylicalcoholsuponhydroborationfollowedbyoxidationoftheresultingprimaryalcoholsshouldprovide-carboxy--lactonemoiety.
Withtheabovehypothesisinmind,weinitiatedtheprojectwithstereoselectiveasymmet-ricallylborationofmethylbenzoylformate(2)withIpc2BAllyl1.
Thereagent1waspreparedbytreatingcommerciallyavailableB-methoxydiisopinocampheylboranewithallylmagnesiumbromideat00Cfollowedbyfiltrationoftheresultingsolidmethoxymagnesiumbromide(Mg(OMe)Br).
Thefiltratethusobtainedwasconcentratedunderinertatmosphereandstoredat40Casa1Mstocksolutioninpentane.
Thereagentwasessentiallypureasanalyzedby11BNMRspectroscopy(withabroadsinglepeakat78ppm).
Theinitialre-516FullPaperAconcisesynthesisof-carboxy--lactonesOCAIJ,4(12)December2008SubashC.
Jonnalagaddaetal.
517FullPaperOCAIJ,4(12)December2008OrganicCHEMISTRYOrganicCHEMISTRYAnIndianJournalperruthenate(TPAP)andN-methylmorpholine-N-ox-ide(NMO)ledtotheformationofthe-lactone9viatheintermediate-lactol8(SCHEME3).
CONCLUSIONSInconclusion,wehavedevelopedasimplechemi-calmethodologyforthesynthesisof-carboxysubsti-tuted-lactoneviathehydroborationandlactonizationofhomoallylicalcoholsobtainedfromallylborationof-ketoesters.
Furtherstudiesareunderwayfortheex-tensionofthisprotocolforthesynthesisofthe-lac-tonebasednaturalproducts.
ACKNOWLEDGMENTSWethanktheDepartmentsofChemistryandBio-chemistry,RowanUniversityandUniversityofMinne-sotaDuluthforthefunding.
REFERENCES[1]M.
Seitz,O.
Reiser;CurrentOpinioninChemicalBiology,9,285(2005).
[2](a)D.
S.
Gunasekera,D.
Gerold,N.
C.
Aalderks,C.
A.
Maanu,S.
C.
Jonnalagadda,V.
V.
Zhdankin,P.
Kiprof,V.
R.
Mereddy;Tetrahedron,63,9401(2007).
(b)V.
J.
Reddy,S.
C.
Jonnalagadda,V.
R.
Mereddy;Org.
Biomol.
Chem.
,5,889(2007).
(c)V.
J.
Reddy,M.
F.
Roforth,C.
Tan,V.
R.
Mereddy;Inorg.
Chem.
,47,381(2007).
[3](a)R.
W.
Hoffmann;PureAppl.
Chem.
,60,123(1988).
(b)Y.
Yamamoto,N.
AsaoChem.
Rev.
,93,2207(1993).
(c)W.
R.
Roush;InMethodsofOrganicChemistry(Houben-Weyl),GeorgThieme:Stuttgart,E21,1410(1995).
(d)H.
C.
Brown,P.
V.
RamachandranJ.
Organomet.
Chem.
,500,1(1995).
(e)H.
C.
Brown,P.
V.
Ramachandran,A.
Hassaner;'AdvancesinAsymmetricSynthesis',JAI:Green-wich,CT,1,Chapter5(1995).
[4](a)K.
Zheng,B.
Qin,X.
Liu,X.
Feng;J.
Org.
Chem.
,72,8478-8483(2007).
(b)S.
Hanessian,R.
Y.
Yang;TetrahedronLett.
,37,8997-9000(1996).
(c)N.
A.
Kulkarni,K.
Chen;TetrahedronLett.
,47,611-613(2006).
[5]D.
Basavaiah,A.
J.
Rao,T.
Satyanarayana;Chem.
Rev.
,103,811(2003).
[6]A.
Jogi,U.
Maeorg;Molecules,6,964-968(2001).
[7]T.
Ooi,K.
Fukumoto,K.
Maruoka;Angew.
Chem.
,Int.
Ed.
,45,3839-3842(2006).
Gopalakrishna1,SubashC.
Jonnalagadda2*,VenkatramR.
Mereddy1*1DepartmentofChemistryandBiochemistry,UniversityofMinnesotaDuluth,1039UniversityDrive,Duluth,MN55812,(U.
S.
A.
)2DepartmentofChemistryandBiochemistry,RowanUniversity,201MullicaHillRoad,Glassboro,NJ08028,(U.
S.
A.
)E-mail:vmereddy@d.
umn.
eduReceived:25thNovember,2008;Accepted:30thNovember,2008OCAIJ,4(12),2008[513-517]INTRODUCTION-Carboxy--lactonemoietyisanextremelyim-portantmotiffoundinseveralnaturalproducts[1].
Asapartofourongoingprojectinvolvingthesynthesisofbiologicallyactivesmallmolecules,weundertookthesynthesisof-carboxy--lactonesviatheallylborationofá-ketoesters[1].
Asymmetricallylborationofcarbo-nylcompoundswithseveralchiralauxiliarieshasbeenextensivelystudiedforstereoselectiveformationofhomoallylicalcoholsandofallthesereagents,B-allyldiisopinocampheylborane(Ipc2BAllyl,1)hasproventobeoneofthepracticalandwidelyusedreagentsintermsofthecostandlevelofstereoselectivityob-served[1].
EXPERIMENTALPreparationofethyl2-oxo-2-phenylacetate,(2a)Thionylchloride(8mL,8mmol,1Msolutioninether)wasaddedtoasolutionofbenzoylformicacid(1.
0g,6.
66mmol)andpyridine(0.
5g)inanhydrousether(10mL)atroomtemperatureandstirredfor1.
5h.
Etherealsolutionwasdecantedandconcentrated.
Hexane(8mL)wasaddedtoresultingsolutionandconcentratedagain.
Theremainingthionylchloridewasremovedunderreducedpressuretoyieldphenylglyoxylylchloride(1.
06g).
Toasolutionofthecrudephenylglyoxylylchloride(1g,5.
95mmol)in12mLoftoluene,wasaddedpyridine(0.
94g,8mmol)andethanol(0.
36g,7.
1mmol)andstirredovernight.
Theresultingsolutionwasdecanted,concentratedunderreducedpressureandpurifiedbycolumnchromatography(ethylacetate:hexane1:19ratio)toyieldethyl2-oxo-2-phenylacetate(2a)(0.
9g,79%yield).
1HNMR(CDCl3,500MHz):7.
98-8.
00(m,2H),7.
47-7.
64(m,3H),4.
43(q,J=7.
2Hz,2H),1.
40(t,J=7.
0Hz,3H);13CNMR(CDCl3,125MHz)186.
7,164.
1,135.
1,132.
8,130.
0,129.
1,62.
4,14.
2.
Preparationofisopropyl2-oxo-2-phenylacetate,(2b)Proceduresimilartothatof(2a).
(76%yield)1HNMR(CDCl3,500MHz)7.
88(d,J=7.
5Hz,2H),7.
53(t,J=7.
5Hz,1H),7.
40(m,2H),5.
25(m,1H),1.
30(d,J=6.
0Hz,6H);13CNMR(CDCl3,125MHz):187.
0,KEYWORDS-Hydroxy-4-pentenoate;,-Dihydroxyesters;-Carboxy--lactone;Hydroboration;Oxidation.
ABSTRACTAshortprotocolforthesynthesisof-carboxy--lactoneshasbeendevel-opedviahydroborationfollowedbyoxidationof-hydroxy-4-pentenoates.
2008TradeScienceInc.
-INDIAAnIndianJournalTradeScienceInc.
Volume4Issue12December2008OrganicCHEMISTRYOrganicCHEMISTRY514FullPaperAconcisesynthesisof-carboxy--lactonesOCAIJ,4(12)December2008SubashC.
Jonnalagaddaetal.
515FullPaperOCAIJ,4(12)December2008OrganicCHEMISTRYOrganicCHEMISTRYAnIndianJournal5.
60(m,1H),5.
00-5.
08(m,2H),3.
74(s,3H),3.
61(dd,J=8.
0,15.
5Hz,1H),3.
30(dd,J=6.
5,13.
5Hz,1H);13CNMR(CDCl3,125MHz):172.
8,171.
1,165.
9,137.
5,132.
4,132.
1,131.
7,131.
6,131.
4,130.
0,129.
7,128.
8,128.
5,125.
4,119.
8,84.
9,53.
1,40.
3.
Preparationof2-((1-methoxy-1-oxo-2-methyl-pent-4-ene-2-yloxy)carbonylbenzoicacid,(5d)Proceduresimilartothatof(5a).
(30%yield)1HNMR(CDCl3,500MHz):7.
71-7.
78(m,1H),7.
61-7.
68(m,1H),7.
44-7.
54(m,2H),5.
75-5.
83(m,1H),5.
08-5.
12(m,2H),3.
72(s,3H),2.
75(dd,J=6.
5,13.
5Hz,1H),2.
59(dd,J=7.
5,14.
0Hz,1H),1.
66(s,3H).
PreparationofMethyl-2,5-dihydroxy-2-phenyl-pentanoate,7:BH3.
Me2S(2.
0mmol,0.
2mL,10Msolution)wasaddedtocooledsolutionoftheolefin3a(0.
2g,0.
97mmol)inTHF(2mL)at00Candstirredfor15min.
Excessboranewasquenchedslowlybyaddingmethanol(1mL)atthesametemperature.
Thereactionmixturewasoxidizedwith1mLof3MNaOHand1mLof30%hydrogenperoxideandstirredfor6hatroomtemperature.
TheproductwasextractedwithEt2OanddriedoverMgSO4.
Thesolventwasremovedunderaspiratorvacuum.
Thecrudeproductwaspurifiedbycolumnchromatography(silicagel,hexane:ethylacetate(4:1))toyield73%(0.
16g)ofdiol7.
1HNMR(CDCl3,500MHz):7.
57-7.
59(m,2H),7.
27-7.
36(m,3H),4.
41(brs,1H),3.
76(s,3H),3.
61(t,J=6.
2Hz,2H),2.
82(brs,1H),2.
30(ddd,J=5.
5,9.
0,14.
0Hz,1H),2.
15(ddd,J=5.
5,9.
5,14.
5Hz,1H),1.
52-1.
69(m,2H);13CNMR(CDCl3,125MHz):175.
8,141.
8,128.
5,128.
0,125.
7,78.
6,62.
7,53.
5,36.
7,27.
2.
Preparationoftetrahydro-5-oxo-2-phenyl-2-furancarboxylicacidmethylester,(9)Tetrapropylammoniumperruthenate(TPAP)(10mg,0.
03mmol)wasaddedtoastirredsolutionofthediol7(100mg,0.
45mmol),andNMO(192mg,1.
64mmol)indicholoromethane(2mL)atrtfor24h.
Aftercompletionofthereaction(TLC),CH2Cl2wasevaporatedandtheblackresiduewaspurifiedbycolumnchromatography(silicagel,hexane:ethylacetate(4:1))toafford(88mg)89%ofthelactone9.
1HNMR(CDCl3,500MHz):7.
38-7.
40(m,2H),7.
22-7.
29(m,3H),3.
61(s,3H),2.
93-2.
98(m,1H),2.
40-2.
59(m,3H);13CNMR(CDCl3,125MHz):175.
3,171.
0,138.
3,129.
0,128.
9,125.
3,87.
0,53.
5,33.
6,28.
3.
RESULTSANDDISCUSSIONOwingtotheimportanceofallylborationreactioninsyntheticorganicandmedicinalchemistry,wewantedtofurtherexpandthescopeof1.
-Ketoestersarere-activeprochiralketonesthatuponasymmetricnucleo-philicadditionsprovidequaternarychiralcentersandarehighlyusefulforthesynthesisofvarietyofstructur-allyintriguingnaturalproducts.
Allylationof-ketoestersprovidestertiary-carboxyhomoallylicalcohols,how-ever,stereoselectiveallylmetallationof-ketoestershasnotbeenawellstudiedprotocol[1].
AsymmetricallylborationofIpc2Ballyl1withaldehydesprovidesthehomoallylicalcoholsinveryhighee[3].
However,asymmetricallylborationofketoneswith1givesverylowtomoderateenantiomericexcess[3].
-Ketoestersarestereo-electronicallydifferentfromnormalketonesduetothepresenceofanelectronwithdrawingcarboxyestermoiety.
Itisknownintheliteraturethatá-ketoestersaremorereactivethannormalketoneswithareactivitypatternalmostsimilartothatofaldehydes.
ReactionssuchasBaylisHillmanreaction[1],Barbierallylation[1],etc.
thatarefacilewithaldehydesandsluggishwithke-tones,takeplaceveryreadilywith-ketoesters/á-iminoesters.
Basedonthisgeneralobservation,wehy-pothesizethat-ketoesterscouldundergofacileallylborationwithIpc2BAllyl1withthereactivityverysimilartothatofaldehydes.
Wehypothesizethattheproducthomoallylicalcoholsuponhydroborationfollowedbyoxidationoftheresultingprimaryalcoholsshouldprovide-carboxy--lactonemoiety.
Withtheabovehypothesisinmind,weinitiatedtheprojectwithstereoselectiveasymmet-ricallylborationofmethylbenzoylformate(2)withIpc2BAllyl1.
Thereagent1waspreparedbytreatingcommerciallyavailableB-methoxydiisopinocampheylboranewithallylmagnesiumbromideat00Cfollowedbyfiltrationoftheresultingsolidmethoxymagnesiumbromide(Mg(OMe)Br).
Thefiltratethusobtainedwasconcentratedunderinertatmosphereandstoredat40Casa1Mstocksolutioninpentane.
Thereagentwasessentiallypureasanalyzedby11BNMRspectroscopy(withabroadsinglepeakat78ppm).
Theinitialre-516FullPaperAconcisesynthesisof-carboxy--lactonesOCAIJ,4(12)December2008SubashC.
Jonnalagaddaetal.
517FullPaperOCAIJ,4(12)December2008OrganicCHEMISTRYOrganicCHEMISTRYAnIndianJournalperruthenate(TPAP)andN-methylmorpholine-N-ox-ide(NMO)ledtotheformationofthe-lactone9viatheintermediate-lactol8(SCHEME3).
CONCLUSIONSInconclusion,wehavedevelopedasimplechemi-calmethodologyforthesynthesisof-carboxysubsti-tuted-lactoneviathehydroborationandlactonizationofhomoallylicalcoholsobtainedfromallylborationof-ketoesters.
Furtherstudiesareunderwayfortheex-tensionofthisprotocolforthesynthesisofthe-lac-tonebasednaturalproducts.
ACKNOWLEDGMENTSWethanktheDepartmentsofChemistryandBio-chemistry,RowanUniversityandUniversityofMinne-sotaDuluthforthefunding.
REFERENCES[1]M.
Seitz,O.
Reiser;CurrentOpinioninChemicalBiology,9,285(2005).
[2](a)D.
S.
Gunasekera,D.
Gerold,N.
C.
Aalderks,C.
A.
Maanu,S.
C.
Jonnalagadda,V.
V.
Zhdankin,P.
Kiprof,V.
R.
Mereddy;Tetrahedron,63,9401(2007).
(b)V.
J.
Reddy,S.
C.
Jonnalagadda,V.
R.
Mereddy;Org.
Biomol.
Chem.
,5,889(2007).
(c)V.
J.
Reddy,M.
F.
Roforth,C.
Tan,V.
R.
Mereddy;Inorg.
Chem.
,47,381(2007).
[3](a)R.
W.
Hoffmann;PureAppl.
Chem.
,60,123(1988).
(b)Y.
Yamamoto,N.
AsaoChem.
Rev.
,93,2207(1993).
(c)W.
R.
Roush;InMethodsofOrganicChemistry(Houben-Weyl),GeorgThieme:Stuttgart,E21,1410(1995).
(d)H.
C.
Brown,P.
V.
RamachandranJ.
Organomet.
Chem.
,500,1(1995).
(e)H.
C.
Brown,P.
V.
Ramachandran,A.
Hassaner;'AdvancesinAsymmetricSynthesis',JAI:Green-wich,CT,1,Chapter5(1995).
[4](a)K.
Zheng,B.
Qin,X.
Liu,X.
Feng;J.
Org.
Chem.
,72,8478-8483(2007).
(b)S.
Hanessian,R.
Y.
Yang;TetrahedronLett.
,37,8997-9000(1996).
(c)N.
A.
Kulkarni,K.
Chen;TetrahedronLett.
,47,611-613(2006).
[5]D.
Basavaiah,A.
J.
Rao,T.
Satyanarayana;Chem.
Rev.
,103,811(2003).
[6]A.
Jogi,U.
Maeorg;Molecules,6,964-968(2001).
[7]T.
Ooi,K.
Fukumoto,K.
Maruoka;Angew.
Chem.
,Int.
Ed.
,45,3839-3842(2006).
- Scienceddd13.com相关文档
- blockingddd13.com
- protectsddd13.com
- 1.0ddd13.com
- 61.8ddd13.com
- readdd13.com
- Ethylbenzeneddd13.com
MOACK:韩国服务器/双E5-2450L/8GB内存/1T硬盘/10M不限流量,$59.00/月
Moack怎么样?Moack(蘑菇主机)是一家成立于2016年的商家,据说是国人和韩国合资开办的主机商家,目前主要销售独立服务器,机房位于韩国MOACK机房,网络接入了kt/lg/kinx三条线路,目前到中国大陆的速度非常好,国内Ping值平均在45MS左右,而且商家的套餐比较便宜,针对国人有很多活动。不过目前如果购买机器如需现场处理,由于COVID-19越来越严重,MOACK办公楼里的人也被感染...
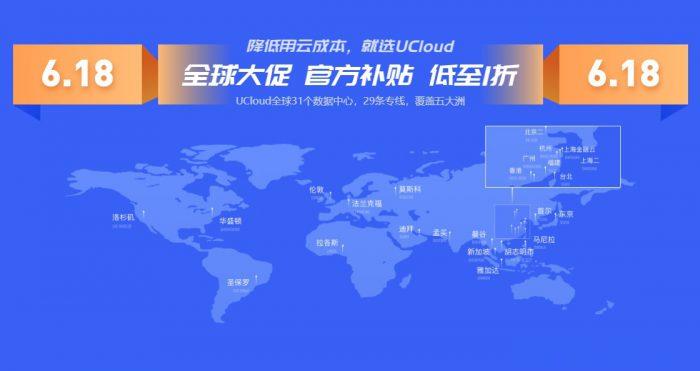
UCloud 618活动:香港云服务器月付13元起;最高可购3年,AMD/Intel系列
ucloud6.18推出全球大促活动,针对新老用户(个人/企业)提供云服务器促销产品,其中最低配快杰云服务器月付5元起,中国香港快杰型云服务器月付13元起,最高可购3年,有AMD/Intel系列。当然这都是针对新用户的优惠。注意,UCloud全球有31个数据中心,29条专线,覆盖五大洲,基本上你想要的都能找到。注意:以上ucloud 618优惠都是新用户专享,老用户就随便看看!点击进入:uclou...
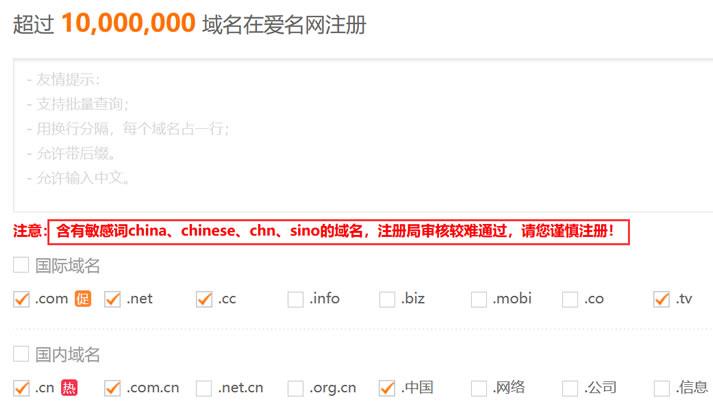
域名注册需要哪些条件(新手注册域名考虑的问题)
今天下午遇到一个网友聊到他昨天新注册的一个域名,今天在去使用的时候发现域名居然不见。开始怀疑他昨天是否付款扣费,以及是否有实名认证过,毕竟我们在国内域名注册平台注册域名是需要实名认证的,大概3-5天内如果不验证那是不可以使用的。但是如果注册完毕的域名找不到那也是奇怪。同时我也有怀疑他是不是忘记记错账户。毕竟我们有很多朋友在某个商家注册很多账户,有时候自己都忘记是用哪个账户的。但是我们去找账户也不办...
ddd13.com为你推荐
-
云爆发养兵千日用兵千日这个说法对不对lunwenjiance知网论文检测查重系统杰景新特杰普特长笛JFL-511SCE是不是有纯银的唇口片??价格怎样??psbc.com邮政储蓄卡如何激活www.5any.comwww.qbo5.com 这个网站要安装播放器lcoc.toptop weenie 是什么?www.henhenlu.com有一个两位数,十位数字是个位数字的二分之一,将十位数字与个位数字对调,新的两位数比原来大36,这个两位数彪言彪语()言() 语盗车飞侠侠盗飞车罪恶都市警车任务怎么做ename.com趫 是什么意思?