spindlewww.299pp.com
www.299pp.com 时间:2021-03-19 阅读:()
Availableonlineatwww.
ijpcr.
comInternationalJournalofPharmaceuticalandClinicalResearch2015;7(1):102-104ISSN-09751556ResearchArticle*AuthorforCorrespondenceIn-VivoAcuteDermalToxicityStudyofFormulationsonRabbits*ShetkarM.
A.
,MoreR.
R.
,KumbharS.
P.
,AuteA.
A.
,PoulB.
NDepartmentofPharmaceutics,MaharashtraCollegeofPharmacy,Nilanga,Dist.
Latur(MS)413521,IndiaAvailableOnline:1stJanuary,2015ABSTRACTPurpose:TodevelopanAceclofenactransdermalgelwithacapabilityfortopicaldrugdeliveryandThepurposeofthisstudyistoobtainscientificinformationregardinghealthhazardsthatmayresultfromaacutedermalexposuretotheagriculturalchemicalforprovidingthebasisoflayingdownthemeasureofsafetyprotectioninapplication.
Methods:Aceclofenacgelformulations,incorporatingvariouspermeationenhancers,werepreparedusingcarbomerasagellingagent.
Theformulationswereexaminedfortheirinvitrocharacteristicsincludingviscosity,pHanddrugreleaseaswellasin-vivopharmacologicalactivities.
Results:Theformulationscontaining5%ofeitherPropyleneglycolaspermeationenhancersgavedrugreleasepatternscomparabletothatofthereferenceproduct.
Propanolincreasedtheapparentviscosityofthetestgelstothesameextentasthatofthereference.
DrugreleasefromtheformulationsfittedbesttotheHiguchimodel.
Asignificantinvivoanalgesiceffectwasproducedbythetestformulationsandtheeffectwassuperiortothatobtainedwiththereferenceproduct.
Conclusion:Aceclofenacgelpreparationscontainingpropyleneglycolrespectively,exhibitedpronouncedanalgesicactivityandcouldbefurtherdevelopedfortopicaldeliveryofAceclofenac.
Keywords:Transdermalgel,toxicitystudy,PrimarydermalirritationstudyAceclofenac,PenetrationenhancerINTRODUCTIONThetestsubstanceisappliedtotheskinofthetestanimalsindifferentdosesandtheanimalsareobservedforeffectsandmortality.
Animalsshowingseveresignsoftoxicity/distressarehumanelykilled.
Attheendofthetestallsurvivinganimalsaretobesacrificed.
Necropsyisperformedonalldead,killedandsacrificedanimals.
TotesttheirritancypotentialofexperimentalAceclofenacgels,rabbitswereused,theselectedgelswereappliedtotheshavedskinontherabbitsback.
Theselectionofrabbitsforthisstudyisdescribedasfollows.
MATERIALSANDMETHODSPreparationofAceclofenacgelsRequiredquantitiesofCarbomer940,971,974weresoakedinsomeamountofdistilledwaterfor2to3hrs.
Thenitwasneutralizedbyaddingsufficientquantityoftriethanolaminewhilestirringwithhelpofoverheadstirrer(PhaseI).
Aceclofenacwasdissolvedinthemixtureofethanol(99.
9%),eitherofthePEG200,400,600andpropyleneglycol(PhaseII),Transcutol&DMSOwereaddedinsomeformulationsaspenetrationenhancers.
(PhaseII)wasaddedslowlytothe(phaseI)whilestirringwithhelpofoverheadstirrer,theremainingquantityofdistilledwaterwasthenaddedtomakeupthefinal100gmweight.
pHofallformulationswasmaintainedintherangeof6.
8to7.
4.
DeterminationofgelviscosityTheviscosityoftheformulationsandreferencewasperformedusingaBrookfielddigitalviscometer(ModelDV-II,USA)equippedwithspindleS27.
Theapparentviscositywasmeasuredat17seconds1shearrate(50rpm)androomtemperature,aftera3-minresttime.
EvaluationofinvitroibuprofenreleaseAsynthetichydrophilicmembranewasmountedonaFranzdiffusioncell(PermeGear,Riegelsville,PA,USA).
Thereceptorcompartmentcontained6.
5mLofphosphatebuffer(pH7.
4).
Onegramofthetestformulationorreferencewasappliedtothemembraneoveranareaof1.
131cm2areaacrossthedonorcompartment.
Thedonorcellwasexposedtoambienttemperatureandcoveredwithparafilmtopreventevaporation.
Thetemperatureofthereceptorcompartmentwasmaintainedat32CwhilethebuffersolutionwasstirredcontinuouslywithaTeflon-coatedmagneticbar.
Samples(0.
5ml)werewithdrawnfromthereleasemediumat0,0.
5,1,1.
5,2,3,4,6,and8handreplacedwithanequalvolumeoffreshbuffersolutiontomaintainsinkconditions.
Thesampleswereanalyzedbyhighperformanceliquidchromatography(HPLC)usingtheoperatingparametersdescribedbelow(see'HPLCanalysis').
Cumulativeamountofthedrugpermeatedversustime(Zeroorder)andalsoversussquarerootoftime(Higuchimodel)Housingandfeedingconditions:Temperature-22±3Cforrodentsand20°(±3°)forrabbitsRelativeHumidity–50-60%(However,humidityshouldnotbebelow30%orexceed70%atanygivenpointoftime).
Lighting–12hourslightanddarkcycleDietandwater–Standardlaboratorydietspecifictothespeciesandfilteredwaterfreefromcontamination.
Selectionandrandomization:Animalswererandomlyplacedincagesuponreceiptandthenrandomizedaccordingtothebodyweights,AnimalsShetkaret.
al.
/InVitroAcuteDermal…IJPCR,January2015–February2015,Volume7,Issue1,102-104Page103consideredunsuitablebecauseofoutlayingbodyweightswereexcludedfromthestudy.
Theanimalbackswerecarefullyshavedusingsterilizedshavingbladetoremovethefur.
Theselectedanimalsweregroupedinto4groups.
Andweremarkedwithidentificationcodesusingdilutedpicricacidsolution(1.
2%w/v).
Circularareasof2.
54cm(1inchdiameter)weremarkedonthebackofeachanimalusingmarkerinkonespotonrightsideandonespotonleftsideofvertebralcolumn.
Thespotonrightsidewasabradedusingshavingbladeandthespotonleftsidewaskeptintact1gmquantityoftheselectedgelswasappliedonmarkedspotsusingabsorbentcottonwool.
Thetoxicmanifestationsifanyontheskinwerethenassessedbyobservingtheskinatpre-selectedtimeintervalsaftertreatmente.
g.
1hrs,4hrs,12hrs,24hrs,48hrsand72hours,Theobservationswererecordedasnumericalscoresasforeachanimalasfollows0=novisiblereaction1=milderythema2=intenseerythema3=intenseerythemawithedema4=intenseerythemawithedemaandvesicularerosionThescorefortreatmentgroupandcontrolgroupwerethencomparedTable1:CompositionoftheAceclofenacgelformulationsNameofingredientWeight(gm)F1F2F3F4F5F6F7F8F9Aceclofenac111111111Ethanol(99.
9%)23.
9423.
9423.
9423.
9423.
9423.
9423.
9423.
9423.
94PEG40022.
4022.
4022.
4022.
4022.
4022.
4022.
4022.
4022.
40Carbopol9740.
512Carbopol971---0.
512---Carbopol9400.
512Propyleneglycol15.
5215.
5215.
5215.
5215.
5215.
5215.
5215.
5215.
52Triethanolamineq.
sq.
sq.
sq.
sq.
sq.
sq.
sq.
sq.
sDistilledwater(upto100gm\)100100100100100100100100100Table2:TheselectionofrabbitsforthisstudywithDosefortopicalApplication.
TestanimalHealthyRabbitsStrainNewZealandwhiterabbitsAge22weeksSexMaleWeight2-2.
5kgNoofanimalspergroups3animalsDoses1gmpersquareinchtopicallyNo.
ofGroupsGroupI=F1GelbasetreatedcontrolGroupII=1gmF3Formulation(withoutpenetrationenhancer)GroupIII=1gmF6Formulation(withpenetrationenhancer)DMSO1%w/w.
GroupIV=1gmF9Formulation(withpenetrationenhancer)Transcutol1%w/w,G-Gelbase,PEG200,400&Transcutol-penetrationenhancer,Carbopol974,940&971-asGellingAgentTable3:NumericalscoreforirritancypotentialforvehicletreatedcontrolGroup–I(F1Formulation)Sr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1A0000000000002B0000000000003C000000000000Shetkaret.
al.
/InVitroAcuteDermal…IJPCR,January2015–February2015,Volume7,Issue1,102-104Page104Table4:NumericalscoreforirritancypotentialforGroup-IItreatedwith[F3Formulation]whichcontainingnopenetrationenhancerSr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1D0000000000002E0000000000003F000000000000Table5:NumericalscoreforirritancypotentialforGroup-IIItreatedwith[F6Formulation]whichcontainingDMSOaspenetrationenhancerSr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1G0000000000002H0000000000003I000000000000Table6:NumericalscoreforirritancypotentialforGroup-IVtreatedwith[F9Formulation]whichcontainingTranscutolaspenetrationenhancerSr.
NoAnimalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1J0000000000002K0000000000003L000000000000RESULTSTableno1,2,3,4&5givesthesescoresforGroupI,GroupII,GroupIIIandGroupIVrespectivelyObservationsandExamination:Thefollowingfor72hrs.
Forvehiclecontrolgelbaseanimalsthosetreatedwithselectedformulationsdidn'tindicateanymanifestationofskinirritationsuchasrednessofskinorinflammationatsiteofapplication.
Boththeabradedareas&intactareaswhenappliedwithvehicleorselectedformulationwerefoundtobefreeofanysignofirritation.
Thusitcanbeconcludedthatalloftheselectedformulaearesafefortopicalapplication.
HoweverthisstudywasperformedusingasingleapplicationofgelproductsHence,itisneededtoconfirmsafetyafterrepeatedapplicationsalso.
Finding:Onlyaverysilenterythemareactionwasnotedonanimalsatexposureobservationtime.
Nodermalirritationwasnotedatthe24,48or72hourexamination.
REFERENCES1.
SethS.
D.
andMaulikM.
G.
,In;Analgesicsanti-pyreticsandanti-inflammatorydrugs,Eds;TextbookofPharmacology,1998;165.
2.
SatoskarandBhandarkar,In;Analgesic-AntipyreticsandNon-steroidalanti-inflammatorydrugs(NSAIDS)Eds;PharmacologyandPharmacoeherapeutics",1991;1(12),139-158.
3.
PalT,GanesanM.
BioavailabilityandBioequivalenceinPharmaceuticalTechnology.
India:CBSPublishers;2004.
4.
JagerW,BuchbauerG,JirovetzL,FritzerM.
PercutaneousAbsorptionofLavenderOilfromMassageOil.
JournaloftheSocietyofCosmeticChemists1992;43:49-54.
5.
PednekarPP,VakilBV,SaneRT,DatarAG.
AntimicrobialActivityofEssentialOilofBlumeaerianthaDCagainstSkinPathogens.
IntJPharmPharmSci2012;4:296-299.
6.
OrganizationforEconomicCo-operationandDevelopmentGuidelineforTestingofChemicals:RepeatedDoseDermalToxicity:21/28-DayStudy.
OECD.
1981;p.
410.
7.
SaiduYLS,BilbisML,IsezuoSA,HassanSW,AbbasAY.
AcuteAndSub-ChronicToxicityStudiesofCrudeAqueousExtractofAlbizziachevalieriharms(Leguminosae).
AsianJournalofBiochemistry2007;2:224–236.
8.
FallerC.
,BracherM.
,DamiN.
,RoguetR.
.
Predictiveabilityofreconstructedhumanepidermisequivalentsfortheassessmentofskinirritationofcosmetics.
ToxicologyinVitro2002;16,557-572.
9.
Fentem,J.
H.
,Briggs,D.
,Chesné,C.
,Elliott,G.
R.
,Harbell,J.
W.
,Heylings,J.
R.
,Portes,P.
,Roguet,R.
,vandeSandt,J.
J.
M.
&Botham,P.
A.
Aprevalidationstudyoninvitrotestsforacuteskinirritation:resultsandevaluationbytheManagementTeam.
ToxicologyinVitro2001;15,57-93.
10.
PortesP.
,GrandidierM.
-H.
,CohenC.
,RoguetR.
RefinementoftheEPISKINprotocolfortheassessmentofacuteskinirritationofchemicals:follow-uptotheECVAMprevalidationstudy.
ToxicologyinVitro2002;16,765-770
ijpcr.
comInternationalJournalofPharmaceuticalandClinicalResearch2015;7(1):102-104ISSN-09751556ResearchArticle*AuthorforCorrespondenceIn-VivoAcuteDermalToxicityStudyofFormulationsonRabbits*ShetkarM.
A.
,MoreR.
R.
,KumbharS.
P.
,AuteA.
A.
,PoulB.
NDepartmentofPharmaceutics,MaharashtraCollegeofPharmacy,Nilanga,Dist.
Latur(MS)413521,IndiaAvailableOnline:1stJanuary,2015ABSTRACTPurpose:TodevelopanAceclofenactransdermalgelwithacapabilityfortopicaldrugdeliveryandThepurposeofthisstudyistoobtainscientificinformationregardinghealthhazardsthatmayresultfromaacutedermalexposuretotheagriculturalchemicalforprovidingthebasisoflayingdownthemeasureofsafetyprotectioninapplication.
Methods:Aceclofenacgelformulations,incorporatingvariouspermeationenhancers,werepreparedusingcarbomerasagellingagent.
Theformulationswereexaminedfortheirinvitrocharacteristicsincludingviscosity,pHanddrugreleaseaswellasin-vivopharmacologicalactivities.
Results:Theformulationscontaining5%ofeitherPropyleneglycolaspermeationenhancersgavedrugreleasepatternscomparabletothatofthereferenceproduct.
Propanolincreasedtheapparentviscosityofthetestgelstothesameextentasthatofthereference.
DrugreleasefromtheformulationsfittedbesttotheHiguchimodel.
Asignificantinvivoanalgesiceffectwasproducedbythetestformulationsandtheeffectwassuperiortothatobtainedwiththereferenceproduct.
Conclusion:Aceclofenacgelpreparationscontainingpropyleneglycolrespectively,exhibitedpronouncedanalgesicactivityandcouldbefurtherdevelopedfortopicaldeliveryofAceclofenac.
Keywords:Transdermalgel,toxicitystudy,PrimarydermalirritationstudyAceclofenac,PenetrationenhancerINTRODUCTIONThetestsubstanceisappliedtotheskinofthetestanimalsindifferentdosesandtheanimalsareobservedforeffectsandmortality.
Animalsshowingseveresignsoftoxicity/distressarehumanelykilled.
Attheendofthetestallsurvivinganimalsaretobesacrificed.
Necropsyisperformedonalldead,killedandsacrificedanimals.
TotesttheirritancypotentialofexperimentalAceclofenacgels,rabbitswereused,theselectedgelswereappliedtotheshavedskinontherabbitsback.
Theselectionofrabbitsforthisstudyisdescribedasfollows.
MATERIALSANDMETHODSPreparationofAceclofenacgelsRequiredquantitiesofCarbomer940,971,974weresoakedinsomeamountofdistilledwaterfor2to3hrs.
Thenitwasneutralizedbyaddingsufficientquantityoftriethanolaminewhilestirringwithhelpofoverheadstirrer(PhaseI).
Aceclofenacwasdissolvedinthemixtureofethanol(99.
9%),eitherofthePEG200,400,600andpropyleneglycol(PhaseII),Transcutol&DMSOwereaddedinsomeformulationsaspenetrationenhancers.
(PhaseII)wasaddedslowlytothe(phaseI)whilestirringwithhelpofoverheadstirrer,theremainingquantityofdistilledwaterwasthenaddedtomakeupthefinal100gmweight.
pHofallformulationswasmaintainedintherangeof6.
8to7.
4.
DeterminationofgelviscosityTheviscosityoftheformulationsandreferencewasperformedusingaBrookfielddigitalviscometer(ModelDV-II,USA)equippedwithspindleS27.
Theapparentviscositywasmeasuredat17seconds1shearrate(50rpm)androomtemperature,aftera3-minresttime.
EvaluationofinvitroibuprofenreleaseAsynthetichydrophilicmembranewasmountedonaFranzdiffusioncell(PermeGear,Riegelsville,PA,USA).
Thereceptorcompartmentcontained6.
5mLofphosphatebuffer(pH7.
4).
Onegramofthetestformulationorreferencewasappliedtothemembraneoveranareaof1.
131cm2areaacrossthedonorcompartment.
Thedonorcellwasexposedtoambienttemperatureandcoveredwithparafilmtopreventevaporation.
Thetemperatureofthereceptorcompartmentwasmaintainedat32CwhilethebuffersolutionwasstirredcontinuouslywithaTeflon-coatedmagneticbar.
Samples(0.
5ml)werewithdrawnfromthereleasemediumat0,0.
5,1,1.
5,2,3,4,6,and8handreplacedwithanequalvolumeoffreshbuffersolutiontomaintainsinkconditions.
Thesampleswereanalyzedbyhighperformanceliquidchromatography(HPLC)usingtheoperatingparametersdescribedbelow(see'HPLCanalysis').
Cumulativeamountofthedrugpermeatedversustime(Zeroorder)andalsoversussquarerootoftime(Higuchimodel)Housingandfeedingconditions:Temperature-22±3Cforrodentsand20°(±3°)forrabbitsRelativeHumidity–50-60%(However,humidityshouldnotbebelow30%orexceed70%atanygivenpointoftime).
Lighting–12hourslightanddarkcycleDietandwater–Standardlaboratorydietspecifictothespeciesandfilteredwaterfreefromcontamination.
Selectionandrandomization:Animalswererandomlyplacedincagesuponreceiptandthenrandomizedaccordingtothebodyweights,AnimalsShetkaret.
al.
/InVitroAcuteDermal…IJPCR,January2015–February2015,Volume7,Issue1,102-104Page103consideredunsuitablebecauseofoutlayingbodyweightswereexcludedfromthestudy.
Theanimalbackswerecarefullyshavedusingsterilizedshavingbladetoremovethefur.
Theselectedanimalsweregroupedinto4groups.
Andweremarkedwithidentificationcodesusingdilutedpicricacidsolution(1.
2%w/v).
Circularareasof2.
54cm(1inchdiameter)weremarkedonthebackofeachanimalusingmarkerinkonespotonrightsideandonespotonleftsideofvertebralcolumn.
Thespotonrightsidewasabradedusingshavingbladeandthespotonleftsidewaskeptintact1gmquantityoftheselectedgelswasappliedonmarkedspotsusingabsorbentcottonwool.
Thetoxicmanifestationsifanyontheskinwerethenassessedbyobservingtheskinatpre-selectedtimeintervalsaftertreatmente.
g.
1hrs,4hrs,12hrs,24hrs,48hrsand72hours,Theobservationswererecordedasnumericalscoresasforeachanimalasfollows0=novisiblereaction1=milderythema2=intenseerythema3=intenseerythemawithedema4=intenseerythemawithedemaandvesicularerosionThescorefortreatmentgroupandcontrolgroupwerethencomparedTable1:CompositionoftheAceclofenacgelformulationsNameofingredientWeight(gm)F1F2F3F4F5F6F7F8F9Aceclofenac111111111Ethanol(99.
9%)23.
9423.
9423.
9423.
9423.
9423.
9423.
9423.
9423.
94PEG40022.
4022.
4022.
4022.
4022.
4022.
4022.
4022.
4022.
40Carbopol9740.
512Carbopol971---0.
512---Carbopol9400.
512Propyleneglycol15.
5215.
5215.
5215.
5215.
5215.
5215.
5215.
5215.
52Triethanolamineq.
sq.
sq.
sq.
sq.
sq.
sq.
sq.
sq.
sDistilledwater(upto100gm\)100100100100100100100100100Table2:TheselectionofrabbitsforthisstudywithDosefortopicalApplication.
TestanimalHealthyRabbitsStrainNewZealandwhiterabbitsAge22weeksSexMaleWeight2-2.
5kgNoofanimalspergroups3animalsDoses1gmpersquareinchtopicallyNo.
ofGroupsGroupI=F1GelbasetreatedcontrolGroupII=1gmF3Formulation(withoutpenetrationenhancer)GroupIII=1gmF6Formulation(withpenetrationenhancer)DMSO1%w/w.
GroupIV=1gmF9Formulation(withpenetrationenhancer)Transcutol1%w/w,G-Gelbase,PEG200,400&Transcutol-penetrationenhancer,Carbopol974,940&971-asGellingAgentTable3:NumericalscoreforirritancypotentialforvehicletreatedcontrolGroup–I(F1Formulation)Sr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1A0000000000002B0000000000003C000000000000Shetkaret.
al.
/InVitroAcuteDermal…IJPCR,January2015–February2015,Volume7,Issue1,102-104Page104Table4:NumericalscoreforirritancypotentialforGroup-IItreatedwith[F3Formulation]whichcontainingnopenetrationenhancerSr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1D0000000000002E0000000000003F000000000000Table5:NumericalscoreforirritancypotentialforGroup-IIItreatedwith[F6Formulation]whichcontainingDMSOaspenetrationenhancerSr.
No.
Animalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1G0000000000002H0000000000003I000000000000Table6:NumericalscoreforirritancypotentialforGroup-IVtreatedwith[F9Formulation]whichcontainingTranscutolaspenetrationenhancerSr.
NoAnimalcode1hrs4hrs12hrs24hrs48hrs72hrsIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbradedIntactAbraded1J0000000000002K0000000000003L000000000000RESULTSTableno1,2,3,4&5givesthesescoresforGroupI,GroupII,GroupIIIandGroupIVrespectivelyObservationsandExamination:Thefollowingfor72hrs.
Forvehiclecontrolgelbaseanimalsthosetreatedwithselectedformulationsdidn'tindicateanymanifestationofskinirritationsuchasrednessofskinorinflammationatsiteofapplication.
Boththeabradedareas&intactareaswhenappliedwithvehicleorselectedformulationwerefoundtobefreeofanysignofirritation.
Thusitcanbeconcludedthatalloftheselectedformulaearesafefortopicalapplication.
HoweverthisstudywasperformedusingasingleapplicationofgelproductsHence,itisneededtoconfirmsafetyafterrepeatedapplicationsalso.
Finding:Onlyaverysilenterythemareactionwasnotedonanimalsatexposureobservationtime.
Nodermalirritationwasnotedatthe24,48or72hourexamination.
REFERENCES1.
SethS.
D.
andMaulikM.
G.
,In;Analgesicsanti-pyreticsandanti-inflammatorydrugs,Eds;TextbookofPharmacology,1998;165.
2.
SatoskarandBhandarkar,In;Analgesic-AntipyreticsandNon-steroidalanti-inflammatorydrugs(NSAIDS)Eds;PharmacologyandPharmacoeherapeutics",1991;1(12),139-158.
3.
PalT,GanesanM.
BioavailabilityandBioequivalenceinPharmaceuticalTechnology.
India:CBSPublishers;2004.
4.
JagerW,BuchbauerG,JirovetzL,FritzerM.
PercutaneousAbsorptionofLavenderOilfromMassageOil.
JournaloftheSocietyofCosmeticChemists1992;43:49-54.
5.
PednekarPP,VakilBV,SaneRT,DatarAG.
AntimicrobialActivityofEssentialOilofBlumeaerianthaDCagainstSkinPathogens.
IntJPharmPharmSci2012;4:296-299.
6.
OrganizationforEconomicCo-operationandDevelopmentGuidelineforTestingofChemicals:RepeatedDoseDermalToxicity:21/28-DayStudy.
OECD.
1981;p.
410.
7.
SaiduYLS,BilbisML,IsezuoSA,HassanSW,AbbasAY.
AcuteAndSub-ChronicToxicityStudiesofCrudeAqueousExtractofAlbizziachevalieriharms(Leguminosae).
AsianJournalofBiochemistry2007;2:224–236.
8.
FallerC.
,BracherM.
,DamiN.
,RoguetR.
.
Predictiveabilityofreconstructedhumanepidermisequivalentsfortheassessmentofskinirritationofcosmetics.
ToxicologyinVitro2002;16,557-572.
9.
Fentem,J.
H.
,Briggs,D.
,Chesné,C.
,Elliott,G.
R.
,Harbell,J.
W.
,Heylings,J.
R.
,Portes,P.
,Roguet,R.
,vandeSandt,J.
J.
M.
&Botham,P.
A.
Aprevalidationstudyoninvitrotestsforacuteskinirritation:resultsandevaluationbytheManagementTeam.
ToxicologyinVitro2001;15,57-93.
10.
PortesP.
,GrandidierM.
-H.
,CohenC.
,RoguetR.
RefinementoftheEPISKINprotocolfortheassessmentofacuteskinirritationofchemicals:follow-uptotheECVAMprevalidationstudy.
ToxicologyinVitro2002;16,765-770
- spindlewww.299pp.com相关文档
- 8.www.299pp.com
- Ilwww.299pp.com
- MAXwww.299pp.com
- vFQwww.299pp.com
- putwww.299pp.com
- Int.J.Curr.Microbiol.App.Sci
优林云(53元)哈尔滨电信2核2G
优林怎么样?优林好不好?优林 是一家国人VPS主机商,成立于2016年,主营国内外服务器产品。云服务器基于hyper-v和kvm虚拟架构,国内速度还不错。今天优林给我们带来促销的是国内东北地区哈尔滨云服务器!全部是独享带宽!首月5折 续费5折续费!地区CPU内存硬盘带宽价格购买哈尔滨电信2核2G50G1M53元直达链接哈尔滨电信4核4G50G1M83元直达链接哈尔滨电信8核8G50G1M131元直...
CloudCone月付$48,MC机房可小时付费
CloudCone商家在前面的文章中也有多次介绍,他们家的VPS主机还是蛮有特点的,和我们熟悉的DO、Linode、VuLTR商家很相似可以采用小时时间计费,如果我们不满意且不需要可以删除机器,这样就不扣费,如果希望用的时候再开通。唯独比较吐槽的就是他们家的产品太过于单一,一来是只有云服务器,而且是机房就唯一的MC机房。CloudCone 这次四周年促销活动期间,商家有新增独立服务器业务。同样的C...

Raksmart VPS主机如何设置取消自动续费
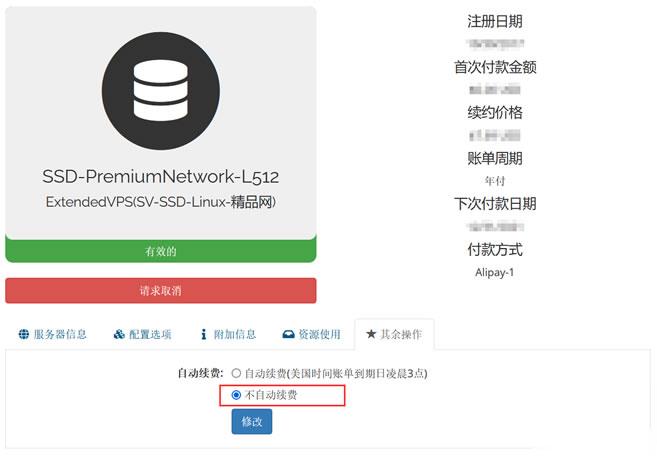
今天有看到Raksmart账户中有一台VPS主机即将到期,这台机器之前是用来测试评测使用的。这里有不打算续费,这不面对万一导致被自动续费忘记,所以我还是取消自动续费设置。如果我们也有类似的问题,这里就演示截图设置Raksmart取消自动续费。这里我们可以看到上图,在对应VPS主机的【其余操作】中可以看到默认已经是不自动续费,所以我们也不要担心被自动续费的。当然,如果有被自动续费,我们确实不想续费的...
www.299pp.com为你推荐
-
安徽汽车网在安徽那个市的二手车最好?丑福晋历史上真正的八福晋是什么样子的?seo优化工具SEO优化神器有什么比较好的?网站检测如何进行网站全面诊断www.119mm.comwww.kb119.com 这个网站你们能打开不?5xoy.com求个如月群真汉化版下载地址www.585ccc.com手机ccc认证查询,求网址99nets.com制作网络虚拟证件的网站 那里有呀?99nets.com99nets网游模拟娱乐社区怎么打不开了?????????谁能告诉我 ???、kb123.net连网方式:wap和net到底有什么不一样的