Timco
co 时间:2021-03-03 阅读:()
CO-1EversenseContinuousGlucoseMonitoring(CGM)SystemMarch29,2018Senseonics,Inc.
ClinicalChemistryandClinicalToxicologyDevicePanelCO-2IntroductionMukulJain,PhDChiefOperatingOfficerSenseonics,Inc.
CO-3EversenseContinuousGlucoseMonitoring(CGM)System90-dayImplantableSensorsubcutaneousRemovableTransmitterwornoverskinMobileApplicationhandhelddeviceCO-4Forcontinuallymeasuringglucoselevelsinadults(age≥18)withdiabetesforoperatinglifeofsensorSystemprovides:Real-timeglucosereadingsGlucosetrendinformationAlertsfordetectionandpredictionofepisodesoflowbloodglucose(hypoglycemia)andhighbloodglucose(hyperglycemia)Adjunctivedevicetocomplement,notreplace,informationobtainedfromstandardhomebloodglucosemonitoringdevicesProposedIndicationforUseCO-5InsertedintoupperarmLastsupto90daysMeasuresglucoseevery5minSiliconecollarcontaining1.
75mgdexamethasoneacetate(DXA)ReduceinflammationaroundsensorSystemComponents:SensorCO-6SensorTechnologyBasedonFluorescenceCO-7SystemComponents:TransmitterCalculatesglucosevaluesandtrendsWornexternallyoverinsertedsensorSecuredwithadhesivepatchVibratesforalertsandnotificationsRechargeableCO-8SystemComponents:MobileMedicalApplicationDisplaysglucoseinformationfromtransmitterValuesandtrendsAlertsandnotificationsRunsonsmartphoneRemindsusertocalibrate(2x/day)OptiontouploaddatatoSenseonics'DataManagementSystemCO-9ThresholdIdentifyglucoselevelsbeloworabovepre-setvaluesPredictiveSignalwhenalertlevelisexpectedtobecrossedinimmediatefuture(e.
g.
10minutesprior)RateofchangeIdentifyrisingorfallingglucoseexceedingpre-setrateofchangeMultipleAlertTypestoEnsureSafetyCO-10Vibratory,Visual,andAudioAlertsTransmittervibrateswhethermobileappisactiveorinvicinityUniquevibrationpatternsAudiblealertANDvisualmessageonhandhelddeviceCO-11SensorInsertedinUpperArmDuringSimple,Office-BasedProcedureSensorinserted/removedbyHCPBrief,office-basedprocedureCustominsertiontoolsProcedure:SkinanesthetizedanddisinfectedSmallincisioninupperarmBluntdissectorcreatessubcutaneouspocketSensortransferredtopocketSimilarremovalprocedureCO-12CEMarkreceivedMay2016Availablein14countries1686patientscommercially2386insertionsUpto7sequentialsensorsPMAsubmittedtoFDAinOctober2016EversenseSystemRegulatoryStatusAsofFebruary2,2018CO-13ClinicalProgram:2224PatientsFeasibilityStudiesN=332PRECISE(EU)Nov2015N=81PRECISEII(US)Jul2016N=90EuropeanPatientRegistryOngoingN=1686PRECISION(US)Feb2018N=35=OngoingstudyCO-14DesignchangessincePRECISEIITransmitterGlucosealgorithmSensorendcapBluntdissectortoolStudyresultsestablishEversenseissafeandeffectiveChangesareincrementalinnatureContinuousimprovementindesignFDADiscussionTopics:DesignChangesCO-15DesignChanges:TransmitterMoreergonomicdesignThinnerLighterLessobtrusiveWater-resistantPassedverificationandvalidationtestingExtensiveEUcommercialexperienceGeneration1PRECISEII+PRECISIONGeneration2PRECISIONCO-16GlucosealgorithmupdatedtoimproveperformanceinEarlysensorwearHypoglycemicrangeRawsensordataindependentofalgorithmintransmitterAlgorithmdevelopedwithdatafromEUpivotalstudy(PRECISE)PosthocprocessingofUSdatacollectedwithSW602EversenseperformanceaccurateandreliablewithStudySWandSW602DesignChanges:GlucoseAlgorithmSMBGCalibrationsValuesFluorescenceMeasurementsGlucoseDeterminationAlgorithmCGMValuesCO-17Amountofdatarelativetosensorlife(90days)AccuracyinearlywearperiodFDADiscussionTopics:SensorAccuracyCO-18Demonstratedaccuracy8.
5%meanabsoluterelativedifference(MARD)87%ofreadingswithin15mg/dLor15%ofreferenceExcursionsconsistentlydetected96%ofhypoglycemicexcursions*98%ofhyperglycemicexcursions*Durationofuse91%ofsensorsfunctionedfor90daysPRECISEII:EversenseSystemisHighlyAccurate*Includes70mg/dLand180mg/dLthresholdsand10-minutepredictivealertsCO-19Nodevice-relatedSAEs1procedure-relatedSAEthrough90dayspost-insertionNounanticipatedAEsLowrateofinfectionsandadhesivepatchskinreactionsAEsconsistentwithotherCGMsandsubcutaneousimplantsEversenseSystemisSafeCO-20RiskanalysisRisksareconsistent,predictable,canbemitigatedSingleinsertioncharacterizesimpact,90-dayuse,removal,andhealingClinicalstudyresultsDeviceandinsertion/removalprocedurearesafeNominal/completehealingfollowingsensorremovalPost-marketingstudiesofrepeatuseEURegistry(1686patients,upto7sequentialsensors)RepeatsensornotassociatedwithincreasedAEsRepeatSensorUseisSafeCO-21AgendaUnmetNeedJeremyH.
Pettus,MDUniversityofCaliforniaatSanDiegoStudyDesignTimGoodnow,PhDSenseonics,Inc.
EffectivenessSafetyLynneKelley,MD,FACSSenseonics,Inc.
Post-approval/TrainingClinicalPerspectiveStevenJ.
Russell,MD,PhDHarvardMedicalSchoolCO-22AdditionalExpertsClinicalPharmacologyNicholasFleischer,RPhPhDVicePresidentClinicalPharmacologyandBiopharmaceuticsTheWeinbergGroupStatisticsRichardHolcomb,PhDConsultantStudyConductKatherineTweden,PhDSenseonics,Inc.
DermatologyHowardI.
Maibach,MDDermatologistProfessorofDermatologyUniversityofCalifornia,SanFranciscoPathologyRenuVirmani,MDFACCPresidentCVPathInstituteCO-23UnmetNeedJeremyH.
Pettus,MDAssistantProfessorofMedicineEndocrinology,DiabetesandMetabolismUniversityofCalifornia,SanDiego(UCSD)CO-24CGMbenefitsImprovedoverallglucosecontrollowerHbA1clevelsIncreasedtimespentwithinnormalglucoserangeImprovedqualityoflifeCGMusesupportedbysocietyguidelines*GreatlyunderutilizedCGMOverviewPolonskyetal.
(2017)*AmericanDiabetesAssociation,EndocrineSociety,andAmericanAssociationofClinicalEndocrinologistsCO-25TARGETRANGE010020030040004812162024IntermittentMonitoringwithHomeBloodGlucoseMeterLeadstoUnnoticedHighsandLowsGlucose(mg/dL)TimeFingersticksCGM4:00AM8:00AM12:00PM4:00PM8:00PM12:00AMCO-26-0.
6-0.
5-0.
4-0.
3-0.
2-0.
100.
10.
2CO-270%20%40%60%80%100%NoCGMCGMCGMProtectsAgainstSevereHypoglycemiaPatientswithSevereHypo-glycemiaperYearA1c:7.
21A1c:7.
121.
DCCTResearchGroup(1993);2.
JDRFCGMStudyGroup(2008)1event/19months1event/60monthsCO-28CGMSystemsAreUnderutilized:76%ofPatientsDoNotUseCGMType1Diabetes(T1D)Exchangeregistry7%24%0%20%40%60%80%100%2010-20122015-2017PatientsUsingCGM(%)YearsCO-2927%ofPatientsDiscontinueCGMUseWithin1YearType1Diabetes(T1D)ExchangeRegistry(2016)ReasonN=262CGMnotworkingproperly/accurateenough71%Problemswithadhesive/insertion61%Tooexpensive/notcoveredbyinsurance58%Uncomfortabletowear41%Usingpump/don'twanttwositesonbody33%CGMtoobig28%CO-30LongersensorlifeLessfrequentsensorinsertionsCurrentsystemsrequire25–50replacements/yearEasytowearandeasilyremovedForphysicalactivitiesordiscretionAdvancementsNeededinCGMSystemsCO-31ProvenclinicalbenefitManypatientshavenotadoptedCGMtechnologyorquicklyabandonitPatientsmissingopportunitytoimprovediabetesstatusandqualityoflifeNeedmoreCGMoptionstoincreasepatientaccessNaturalEvolutionofSensorTechnology:Longer-Lasting,LessIntrusiveCO-32StudyDesignandEffectivenessTimGoodnow,PhDChiefExecutiveOfficerSenseonics,Inc.
CO-33EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-34Non-randomized,single-arm,multi-centerstudyN=90patientsn=75onesensorinsertedn=15twosensorsinserted(oneineacharm)Sensorscalibrated2x/dayusinghomeglucosemeterGlucosereadingsandhigh/lowalertswereblindedduringstudyPRECISEII:PivotalStudyDesignCO-35PRECISEII:PivotalStudyScheduleClinicVisit1234567Day-3001306090100Screening/Follow-upAccuracy(in-clinic)Challenges**Meal,exercise,compressionchallengesInsertionAt-homewearfor90daysRemovalCO-36Meanabsoluterelativedifference(MARD)ComparessensorreadingwithreferenceglucoseSmallerMARD=higheraccuracyPercentofsensorvalueswithin15mg/dLor15%ofreferencePRECISEII:PrimaryEndpointBasedonMARDCO-37Sensoraccuracyacross90daysofuseAgreementofsensorreadingswithinaccuracylimitsHighandlowglucosealertperformanceImpactofcompressionPairedprecisionKaplan-MeieranalysisofsensorlifeMethodcomparison,biasanalysis,Clarke&ConsensusErrorAnalysisPRECISEII:AdditionalEffectivenessCharacterizationCO-38Adultsdiagnosedwithdiabetesmellitusforatleast1yearNoseverehypoglycemiawithinlast6monthsNodiabeticketoacidosisrequiringhospitalizationwithin6monthsPRECISEII:KeyEnrollmentCriteriaCO-39PRECISEIIDemographics:RepresentativeStudySampleParameterN=90SexMale60%AgeMean45yearsRaceCaucasian86%BlackorAfricanAmerican8%Asian3%Other3%BodyMassIndexMean29kg/m2GlycosylatedHemoglobin(HbA1c)Mean7.
6%TimesincediabetesdiagnosisMean20yearsDiabetestypeType168%Type232%TypeofinsulintherapyContinuousinsulininfusionpump48%Multipledailyinjections27%None(Type2,notoninsulin)22%Other(long-actinginsulinonly)3%CO-40PRECISEII:DispositionConsentedN=114CompletedSensorInsertionN=90CompletedDay1VisitN=90CompletedDay30VisitN=89CompletedDay60VisitN=86CompletedDay90VisitN=82Screenfailures(n=17)Withdrawnpriortoinsertion(n=7)Losttofollowup(n=1)Withdrawnconsent(n=2)Sensorreplacementalert(n=4)Sensorreplacementalert(n=1)CO-41PRECISEII:PrimaryEffectivenessEndpointMetUsingStudySoftwareSoftwareVersionUniquePatientsNPairedValuesNMeanAbsoluteRelativeDifference(95%CI)p-valueStudySW9015,7048.
8%(8.
3%,9.
4%)cosereadingbetweenprimarysensorandreferencevaluesCO-42PRECISEII:PrimaryEffectivenessEndpointMetUsingSW602BasedonallevaluabledatafromallpatientswithatleastonepairedglucosereadingbetweenprimarysensorandreferencevaluesSoftwareVersionUniquePatientsNPairedValuesNMeanAbsoluteRelativeDifference(95%CI)p-valueSW6029015,7538.
5%(8.
0%,9.
1%)CO-43PRECISEII:SensorAccuratethrough90DaysofUseStudyTimePointUniquePatientsNPairedValuesN%ofCGMReadingswithin15/15%ofReference(95%CI)Overall9015,75387%(84.
7%,88.
6%)Day190170877%(72.
8%,80.
4%)Day3088508191%(88.
3%,92.
6%)Day6085472587%(83.
4%,90.
4%)Day9077423985%(81.
8%,88.
4%)020406080100SW602CO-44EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-45PRECISIONStudyDesignClinicVisit123456789Day-3001714306090100Screening/Follow-upAccuracy(in-clinic)Challenges**Mealchallenges;OvernightchallengeswereperformedonDays7and14InsertionAt-homewearfor90daysRemovalCO-463USsites35patientswithsensorsinserted27patientshad2sensorsinsertedUnblindedsensorglucosevaluesandactivehigh/lowalertsPRECISIONDifferencesfromPRECISEIICO-47PRECISION:SensorAccurateover90DaysofUseStudyTimePointUniquePatientsNPairedValuesN%ofCGMReadingswithin15/15%ofReference(95%CI)Overall3515,17085%(82.
9%,87.
5%)Day135266579%(74.
5%,83.
1%)Day735292686%(81.
5%,89.
7%)Day1435299788%(84.
7%,90.
7%)Day3035228488%(81.
3%,92.
6%)Day6035213387%(79.
7%,91.
8%)Day9035216584%(78.
4%,88.
2%)020406080100SW602CO-48AccuracyComparisonwithApprovedCGMsthroughSensorLifeDataSourcePercentofSystemReadingsWithin15/15%ofReferenceDeviceDay1Day3-4Day7Day10Day14Day30Day60Day90Eversense(SW602)PRECISEII77%91%87%85%PRECISION79%--86%--88%88%87%84%DexcomG5*--77%89%90%MedtronicGuardian(3)*--68%87%82%FreeStyleLibre*--76%82%85%85%*SummaryofSafetyandEffectivenessData(SSED)-MedicalDeviceDatabases-http://www.
fda.
govResultsbasedoncalibrationevery12hoursCO-49AlertSettingPRECISEIIDetectionRateFalseAlertRateLowGlucoseAlertat70mg/dL*96%16%HighGlucoseAlertat180mg/dL*98%7%AccurateDetectionofGlucoseExcursionsPRECISIONDetectionRateFalseAlertRate95%8%99%7%SW602*Includesthresholdand10-minutepredictivealertsCO-50PRECISEII:KMsurvivalprobabilityof91%atDay90PRECISION:Allsensorsfunctioned90daysPRECISEIIandPRECISION:EversenseSensorLongevity0.
00.
20.
40.
60.
81.
00306090SensorSurvivalProbabilityTimesinceImplant(days)0.
00.
20.
40.
60.
81.
00306090PRECISIONPRECISEIICO-51PRECISEIIandPRECISION:SystemAdherencePRECISEIIPRECISIONMedianweartime23.
4hours23.
4hours%transmittersworn>20hours/day87%91%CO-52PRECISEII:87%ofsensorreadingswith15/15%ofreferencePRECISION:85%ofsensorreadingswith15/15%ofreferenceAccurateateachmeasuredtimepointNodegradationofsensorperformance91%ofsensorsfunctionthrough90days"SensorReplacement"alertappropriatelyproducedOver95%detectionratesforglycemicexcursionsHigh(180mg/dL)andlow(70mg/dL)glucoseEffectivenessSummary:EversenseisAccuratefor90DaysCO-53ClinicalSafetyLynneKelley,MD,FACSChiefMedicalOfficerSenseonics,Inc.
CO-54EversensesystemhasacceptablesafetyprofileSimilartoothermarketedCGMsystemsProceduralrisksofimplantablesensormitigatedDevicedesign,training,andcontinuedimprovementsbasedonpost-marketsurveillanceEversensereducessomeknownrisksassociatedwithotherCGMsystemsOverviewofSafetyProfileCO-55EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-56PRECISEIIandPRECISION:DeviceExposurePRECISEIIPRECISIONSensorsinsertedandremoved106sensors(90patients)62sensors(35patients)Proceduresperformed212procedures124proceduresSensoruse(meanduration)92.
2days91daysSensorexposure9,773days6,148daysCO-57Incidenceofdevice-relatedorinsertion/removalprocedure-relatedseriousadverseevents(SAEs)atanypointduringsensorusePRECISEIIandPRECISION:PrimarySafetyEndpointCO-58Non-seriousrelatedadverseeventsAEsofspecialintereste.
g.
infection,adhesivereactionsDexamethasoneexposureovertimePRECISEIIandPRECISION:AdditionalSafetyAnalysesCO-59PRECISEIINodevice-relatedSAEsOneprocedure-relatedSAEreportedSensorremovalsensorunsuccessful(withandwithoutultrasound)SensorsuccessfullyremovedbysurgeonundergeneralanesthesiaPRECISIONNodevice-orprocedure-relatedSAEs3unrelatedSAEsGastroenteritis,hypoglycemicepisode,cellulitisofleftfootPRECISEIIandPRECISION:SeriousAdverseEvents(SAEs)CO-60AdverseEventPRECISEIIPRECISIONEventsPatientsN=90EventsPatientsN=35AllEvents14785Pain/discomfort4222Bruising22----Erythema22----Devicefragmentnotrecovered22----Syncope11----Tingling11----Delayedreportofpain11----Secondaryproceduretoremovesensor1121Dermatitisatpatchlocation----21Skinhyperpigmentation----21PRECISEIIandPRECISION:Device-orInsertion/Removal-RelatedAEsCO-61Allremovedsensorsreturnedtosponsorforinspection2devicesdidnothavecapuponreturnCorrectiveandpreventativeactionplanimplementedImplementingenhancedqualityprocedures(capadhesion)Capmaterial:PMMAhighlybiocompatible,permanentimplantOrthopedic,dental,andophthalmologicCapsize:3.
2mmx0.
8mmPRECISEII:TwoEventsRelatedtoDeviceFragmentCO-62NoinfectionsAllrelatedAEsconsideredexpectedandcommonforsubcutaneousimplantAllrelatedAEsresolvedfullyPRECISEIIandPRECISION:AdditionalSafetyOutcomesCO-63Contains1.
75mgdexamethasoneacetate(DXA)Water-insolublecorticosteroidReduceslocalinflammatoryresponseExtendssensorlifeControlledandslowDXAreleaseconeCollarCO-64BloodassayedforDXAto50pg/mLlevelNodetectableplasmalevelsofDXAobservedNosystemiceffectsDXAcollarsexaminedafterremovalMinimalDXAexposureconfirmed3μg/day2eventsoftransienthyperpigmentationResolveduponsensorremovalImpactofDexamethasoneExposureCO-65EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-66Threemulti-centerstudiesPRECISEII,PRECISION,andPRECISE206subjects335sensors670insertion/removalprocedures22,529patient-daysofsensorwearIntegratedDeviceExposureCO-67IntegratedSummaryofRelatedAdverseEventsAdverseEventEventsPatientsN=206AllEvents4126(13%)Pain/discomfort108(4%)Redness/erythema66(3%)Secondaryproceduretoremovesensor43(1%)Infection33(1%)Bruising/hematoma33(1%)Devicefragmentnotrecovered22(1%)Dermatitisatpatchlocation32(1%)Skinhyperpigmentation21(cope,hypertension,andnauseaCO-68Aggregateinfectionrate1%ImprovedincisioncareinstructionsPRECISE:leavebandagefor24hoursPRECISEII:leavebandagefor48hoursInfectionrateobservedisbelowliteraturereportsforsimilarimplantsandminorprocedures:2–4%*LowRateofInfectionsObservedinStudies*Buprenorphine,BraeburnPharmaceuticals,Inc.
*http://www.
worldwidewounds.
com/2005/september/Gottrup/Surgical-Site-Infections-Overview.
htmlCO-69EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-70AllpatientsinsertedcommerciallyenrolledinregistryEnrollmentcompletedwhen100patientsreach4insertionsAllpatientsenrolledtobefollowedthrough8insertionsandremovalsEuropeanPatientRegistryCO-71LowRateofAEswithRepeatInsertionsEvents,n(%)PostInsertion#1N=16862N=4433N=1434N=585N=396N=147N=3SAEs0000000Device-,procedure-relatedAEsInfection(atsensorsite)8(0.
5%)4(1%)2(1%Secondaryproceduretoremovesensor72Adhesivepatchsiteirritation5-2----Prolongedwoundhealing3Redness/reactiontodressing3Sensorbrokeduringremoval3Skinatrophyoversensorw/skindiscoloration21Skinatrophyoversensor1Skindiscoloration12Sensorsitepain/discomfort1Bruising11-1---Patientfaintedduringprocedure1Hematoma---1---CO-72ProposedPost-ApprovalStudyCO-73Serialsensorinsertionsandremovalsfor2years175patientsinupto20clinicalsitesPrimarysafetyendpointRateofdevice-relatedandinsertion/removalprocedure-relatedSAEsthrough12months≤7%PrimaryeffectivenessendpointTimeinrange(between70mg/dLand180mg/dL),12monthsvs.
firstmonthProposedU.
S.
PostApprovalStudyDesignCO-74AllrelatedAEsthrough2yearsPlasmadexamethasonelevelsevery6monthsEffectivenessoftrainingprogramSuccessrateofinsertions/removalsDiabetesdistressscaleandCGMsatisfactionscaleBaselineandannuallyProposedU.
S.
PostApprovalStudy:OtherOutcomeMeasuresCO-75DesignChangesSensorendcapBluntdissectortoolCO-76SensorEndCapImprovementOriginalDesignNewDesignEndcapredesignedtobeflushwithendofsensorDesignverificationCompressiveforcesTorqueMaintainsfunctionalcompatibilitywithinsertiontoolCO-77BluntDissectorDesignImprovementSamefunctionConsistentplacementfacilitatesremovalProperentryanglePocketdepth/lengthParalleltoskinValidatedwithHumanFactorstestingNewBluntDissectorOriginalBluntDissectorNewguidesCO-78TrainingforCliniciansCO-79MandatorycomprehensivetrainingCertificationprocessledbySenseonicsapprovedtrainersDidacticsessionPracticeswithsimulatedskinInitialinsertionsandremovalsareobservedTrainingProgramOverviewTrainingresources:CGMSensorInsertionandRemovalInstructionsInsertionvideosRemovalvideosSimulationstationProcedureposterTake-homeinstructionsCO-80TrainingChecklistforCertificationPre-WorkbeforeSimulationTrainingInsertionSimulationTrainingPre-WorkbeforeRemovalReviewRemovalSimulationTrainingLearningcurve:~2to3procedures3patientsscheduledinsamedaytofamiliarizewithprocedureCO-81Hands-OnPracticeSessionwithSimulatedSkin,SterileField,andRequiredSuppliesCO-82ExamplesofTrainingMaterialsCO-83461clinicianstrainedoninsertions94%certifiedtodoinsertionsindependently258clinicianstrainedonremovals86%certifiedtodoremovalsindependentlyTrainedProvidersOutsideU.
S.
CO-84ProcedureeasilylearnedbyphysicianswithnopriorEversenseexperience99%ofremovalssuccessfulonfirstattemptLowinfectionrate(0.
5%)Europe:SuccessfulInsertionandRemovalTrainingCO-85100%ofinsertionsand99%ofremovalssuccessfulonfirstattempt91%ofinsertionsand80%ofremovalscompletedinCO-86NounanticipatedadverseeventsLimitedAEsrelatedtodeviceorprocedureAllAEsreportedresolvedfullyNoInfectionsinUSclinicaltrialsNodetectablebloodlevelsofdexamethasoneOneprocedure-relatedSAE,resolvedNodevice-relatedSAEsEversenseissafeforintendeduseEversenseSystemHasAcceptableSafetyProfileCO-87ClinicalPerspective/Benefit-RiskStevenJRussell,MD,PhDAssociateProfessorofMedicineHarvardMedicalSchoolCO-88ExperiencewithallcurrentlyapprovedCGMs(since2004)PublishedaccuracycomparisonstudiesofCGMsDevelopingbionicpancreasDependsonCGMaccuracyandreliabilityMotivatesinterestinnewCGMtechnologiesClinicalinvestigatorinartificialpancreastrialsUsedEversensesystemInsertedandremovedsensorsTrainedquicklyRelevantCGMExperienceCO-8970%notatA1ctargets*HypoglycemiastillverycommonCGMsystemsareproventohelpImproveglucosecontrolLowerriskofhypoglycemiaImprovepatients'livesCurrentSituation:MajorityofPatientsDoNotMeetGlycemicGoals*Type1DiabetesExchangeCO-90TheCurrentSituation:Only3Outof10PatientswithT1DUseCGMPerceivedburdenofrepeatinsertionFearofpainCO-91TheCurrentSituation:1outof3CGMUsersDiscontinuewithin1YearProblemswithadhesive/insertionUncomfortableCO-92Longersensorlife(90days)LessfrequentsensorinsertionsEversense:4timesperyearCurrentsystems:25–50timesperyearEasytowearandeasilyremovedForphysicalactivitiesordiscretionOn-bodyvibrationfromtransmitterprovidesextrasafetymeasureEversenseAddressesManyBarrierstoCGMUseCO-93TheGoal:IncreaseUseofCGMImproveglucosecontrolLowerriskofhypoglycemiaCO-94EversenseContinuousGlucoseMonitoring(CGM)SystemMarch29,2018Senseonics,Inc.
ClinicalChemistryandClinicalToxicologyDevicePanel
ClinicalChemistryandClinicalToxicologyDevicePanelCO-2IntroductionMukulJain,PhDChiefOperatingOfficerSenseonics,Inc.
CO-3EversenseContinuousGlucoseMonitoring(CGM)System90-dayImplantableSensorsubcutaneousRemovableTransmitterwornoverskinMobileApplicationhandhelddeviceCO-4Forcontinuallymeasuringglucoselevelsinadults(age≥18)withdiabetesforoperatinglifeofsensorSystemprovides:Real-timeglucosereadingsGlucosetrendinformationAlertsfordetectionandpredictionofepisodesoflowbloodglucose(hypoglycemia)andhighbloodglucose(hyperglycemia)Adjunctivedevicetocomplement,notreplace,informationobtainedfromstandardhomebloodglucosemonitoringdevicesProposedIndicationforUseCO-5InsertedintoupperarmLastsupto90daysMeasuresglucoseevery5minSiliconecollarcontaining1.
75mgdexamethasoneacetate(DXA)ReduceinflammationaroundsensorSystemComponents:SensorCO-6SensorTechnologyBasedonFluorescenceCO-7SystemComponents:TransmitterCalculatesglucosevaluesandtrendsWornexternallyoverinsertedsensorSecuredwithadhesivepatchVibratesforalertsandnotificationsRechargeableCO-8SystemComponents:MobileMedicalApplicationDisplaysglucoseinformationfromtransmitterValuesandtrendsAlertsandnotificationsRunsonsmartphoneRemindsusertocalibrate(2x/day)OptiontouploaddatatoSenseonics'DataManagementSystemCO-9ThresholdIdentifyglucoselevelsbeloworabovepre-setvaluesPredictiveSignalwhenalertlevelisexpectedtobecrossedinimmediatefuture(e.
g.
10minutesprior)RateofchangeIdentifyrisingorfallingglucoseexceedingpre-setrateofchangeMultipleAlertTypestoEnsureSafetyCO-10Vibratory,Visual,andAudioAlertsTransmittervibrateswhethermobileappisactiveorinvicinityUniquevibrationpatternsAudiblealertANDvisualmessageonhandhelddeviceCO-11SensorInsertedinUpperArmDuringSimple,Office-BasedProcedureSensorinserted/removedbyHCPBrief,office-basedprocedureCustominsertiontoolsProcedure:SkinanesthetizedanddisinfectedSmallincisioninupperarmBluntdissectorcreatessubcutaneouspocketSensortransferredtopocketSimilarremovalprocedureCO-12CEMarkreceivedMay2016Availablein14countries1686patientscommercially2386insertionsUpto7sequentialsensorsPMAsubmittedtoFDAinOctober2016EversenseSystemRegulatoryStatusAsofFebruary2,2018CO-13ClinicalProgram:2224PatientsFeasibilityStudiesN=332PRECISE(EU)Nov2015N=81PRECISEII(US)Jul2016N=90EuropeanPatientRegistryOngoingN=1686PRECISION(US)Feb2018N=35=OngoingstudyCO-14DesignchangessincePRECISEIITransmitterGlucosealgorithmSensorendcapBluntdissectortoolStudyresultsestablishEversenseissafeandeffectiveChangesareincrementalinnatureContinuousimprovementindesignFDADiscussionTopics:DesignChangesCO-15DesignChanges:TransmitterMoreergonomicdesignThinnerLighterLessobtrusiveWater-resistantPassedverificationandvalidationtestingExtensiveEUcommercialexperienceGeneration1PRECISEII+PRECISIONGeneration2PRECISIONCO-16GlucosealgorithmupdatedtoimproveperformanceinEarlysensorwearHypoglycemicrangeRawsensordataindependentofalgorithmintransmitterAlgorithmdevelopedwithdatafromEUpivotalstudy(PRECISE)PosthocprocessingofUSdatacollectedwithSW602EversenseperformanceaccurateandreliablewithStudySWandSW602DesignChanges:GlucoseAlgorithmSMBGCalibrationsValuesFluorescenceMeasurementsGlucoseDeterminationAlgorithmCGMValuesCO-17Amountofdatarelativetosensorlife(90days)AccuracyinearlywearperiodFDADiscussionTopics:SensorAccuracyCO-18Demonstratedaccuracy8.
5%meanabsoluterelativedifference(MARD)87%ofreadingswithin15mg/dLor15%ofreferenceExcursionsconsistentlydetected96%ofhypoglycemicexcursions*98%ofhyperglycemicexcursions*Durationofuse91%ofsensorsfunctionedfor90daysPRECISEII:EversenseSystemisHighlyAccurate*Includes70mg/dLand180mg/dLthresholdsand10-minutepredictivealertsCO-19Nodevice-relatedSAEs1procedure-relatedSAEthrough90dayspost-insertionNounanticipatedAEsLowrateofinfectionsandadhesivepatchskinreactionsAEsconsistentwithotherCGMsandsubcutaneousimplantsEversenseSystemisSafeCO-20RiskanalysisRisksareconsistent,predictable,canbemitigatedSingleinsertioncharacterizesimpact,90-dayuse,removal,andhealingClinicalstudyresultsDeviceandinsertion/removalprocedurearesafeNominal/completehealingfollowingsensorremovalPost-marketingstudiesofrepeatuseEURegistry(1686patients,upto7sequentialsensors)RepeatsensornotassociatedwithincreasedAEsRepeatSensorUseisSafeCO-21AgendaUnmetNeedJeremyH.
Pettus,MDUniversityofCaliforniaatSanDiegoStudyDesignTimGoodnow,PhDSenseonics,Inc.
EffectivenessSafetyLynneKelley,MD,FACSSenseonics,Inc.
Post-approval/TrainingClinicalPerspectiveStevenJ.
Russell,MD,PhDHarvardMedicalSchoolCO-22AdditionalExpertsClinicalPharmacologyNicholasFleischer,RPhPhDVicePresidentClinicalPharmacologyandBiopharmaceuticsTheWeinbergGroupStatisticsRichardHolcomb,PhDConsultantStudyConductKatherineTweden,PhDSenseonics,Inc.
DermatologyHowardI.
Maibach,MDDermatologistProfessorofDermatologyUniversityofCalifornia,SanFranciscoPathologyRenuVirmani,MDFACCPresidentCVPathInstituteCO-23UnmetNeedJeremyH.
Pettus,MDAssistantProfessorofMedicineEndocrinology,DiabetesandMetabolismUniversityofCalifornia,SanDiego(UCSD)CO-24CGMbenefitsImprovedoverallglucosecontrollowerHbA1clevelsIncreasedtimespentwithinnormalglucoserangeImprovedqualityoflifeCGMusesupportedbysocietyguidelines*GreatlyunderutilizedCGMOverviewPolonskyetal.
(2017)*AmericanDiabetesAssociation,EndocrineSociety,andAmericanAssociationofClinicalEndocrinologistsCO-25TARGETRANGE010020030040004812162024IntermittentMonitoringwithHomeBloodGlucoseMeterLeadstoUnnoticedHighsandLowsGlucose(mg/dL)TimeFingersticksCGM4:00AM8:00AM12:00PM4:00PM8:00PM12:00AMCO-26-0.
6-0.
5-0.
4-0.
3-0.
2-0.
100.
10.
2CO-270%20%40%60%80%100%NoCGMCGMCGMProtectsAgainstSevereHypoglycemiaPatientswithSevereHypo-glycemiaperYearA1c:7.
21A1c:7.
121.
DCCTResearchGroup(1993);2.
JDRFCGMStudyGroup(2008)1event/19months1event/60monthsCO-28CGMSystemsAreUnderutilized:76%ofPatientsDoNotUseCGMType1Diabetes(T1D)Exchangeregistry7%24%0%20%40%60%80%100%2010-20122015-2017PatientsUsingCGM(%)YearsCO-2927%ofPatientsDiscontinueCGMUseWithin1YearType1Diabetes(T1D)ExchangeRegistry(2016)ReasonN=262CGMnotworkingproperly/accurateenough71%Problemswithadhesive/insertion61%Tooexpensive/notcoveredbyinsurance58%Uncomfortabletowear41%Usingpump/don'twanttwositesonbody33%CGMtoobig28%CO-30LongersensorlifeLessfrequentsensorinsertionsCurrentsystemsrequire25–50replacements/yearEasytowearandeasilyremovedForphysicalactivitiesordiscretionAdvancementsNeededinCGMSystemsCO-31ProvenclinicalbenefitManypatientshavenotadoptedCGMtechnologyorquicklyabandonitPatientsmissingopportunitytoimprovediabetesstatusandqualityoflifeNeedmoreCGMoptionstoincreasepatientaccessNaturalEvolutionofSensorTechnology:Longer-Lasting,LessIntrusiveCO-32StudyDesignandEffectivenessTimGoodnow,PhDChiefExecutiveOfficerSenseonics,Inc.
CO-33EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-34Non-randomized,single-arm,multi-centerstudyN=90patientsn=75onesensorinsertedn=15twosensorsinserted(oneineacharm)Sensorscalibrated2x/dayusinghomeglucosemeterGlucosereadingsandhigh/lowalertswereblindedduringstudyPRECISEII:PivotalStudyDesignCO-35PRECISEII:PivotalStudyScheduleClinicVisit1234567Day-3001306090100Screening/Follow-upAccuracy(in-clinic)Challenges**Meal,exercise,compressionchallengesInsertionAt-homewearfor90daysRemovalCO-36Meanabsoluterelativedifference(MARD)ComparessensorreadingwithreferenceglucoseSmallerMARD=higheraccuracyPercentofsensorvalueswithin15mg/dLor15%ofreferencePRECISEII:PrimaryEndpointBasedonMARDCO-37Sensoraccuracyacross90daysofuseAgreementofsensorreadingswithinaccuracylimitsHighandlowglucosealertperformanceImpactofcompressionPairedprecisionKaplan-MeieranalysisofsensorlifeMethodcomparison,biasanalysis,Clarke&ConsensusErrorAnalysisPRECISEII:AdditionalEffectivenessCharacterizationCO-38Adultsdiagnosedwithdiabetesmellitusforatleast1yearNoseverehypoglycemiawithinlast6monthsNodiabeticketoacidosisrequiringhospitalizationwithin6monthsPRECISEII:KeyEnrollmentCriteriaCO-39PRECISEIIDemographics:RepresentativeStudySampleParameterN=90SexMale60%AgeMean45yearsRaceCaucasian86%BlackorAfricanAmerican8%Asian3%Other3%BodyMassIndexMean29kg/m2GlycosylatedHemoglobin(HbA1c)Mean7.
6%TimesincediabetesdiagnosisMean20yearsDiabetestypeType168%Type232%TypeofinsulintherapyContinuousinsulininfusionpump48%Multipledailyinjections27%None(Type2,notoninsulin)22%Other(long-actinginsulinonly)3%CO-40PRECISEII:DispositionConsentedN=114CompletedSensorInsertionN=90CompletedDay1VisitN=90CompletedDay30VisitN=89CompletedDay60VisitN=86CompletedDay90VisitN=82Screenfailures(n=17)Withdrawnpriortoinsertion(n=7)Losttofollowup(n=1)Withdrawnconsent(n=2)Sensorreplacementalert(n=4)Sensorreplacementalert(n=1)CO-41PRECISEII:PrimaryEffectivenessEndpointMetUsingStudySoftwareSoftwareVersionUniquePatientsNPairedValuesNMeanAbsoluteRelativeDifference(95%CI)p-valueStudySW9015,7048.
8%(8.
3%,9.
4%)cosereadingbetweenprimarysensorandreferencevaluesCO-42PRECISEII:PrimaryEffectivenessEndpointMetUsingSW602BasedonallevaluabledatafromallpatientswithatleastonepairedglucosereadingbetweenprimarysensorandreferencevaluesSoftwareVersionUniquePatientsNPairedValuesNMeanAbsoluteRelativeDifference(95%CI)p-valueSW6029015,7538.
5%(8.
0%,9.
1%)CO-43PRECISEII:SensorAccuratethrough90DaysofUseStudyTimePointUniquePatientsNPairedValuesN%ofCGMReadingswithin15/15%ofReference(95%CI)Overall9015,75387%(84.
7%,88.
6%)Day190170877%(72.
8%,80.
4%)Day3088508191%(88.
3%,92.
6%)Day6085472587%(83.
4%,90.
4%)Day9077423985%(81.
8%,88.
4%)020406080100SW602CO-44EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-45PRECISIONStudyDesignClinicVisit123456789Day-3001714306090100Screening/Follow-upAccuracy(in-clinic)Challenges**Mealchallenges;OvernightchallengeswereperformedonDays7and14InsertionAt-homewearfor90daysRemovalCO-463USsites35patientswithsensorsinserted27patientshad2sensorsinsertedUnblindedsensorglucosevaluesandactivehigh/lowalertsPRECISIONDifferencesfromPRECISEIICO-47PRECISION:SensorAccurateover90DaysofUseStudyTimePointUniquePatientsNPairedValuesN%ofCGMReadingswithin15/15%ofReference(95%CI)Overall3515,17085%(82.
9%,87.
5%)Day135266579%(74.
5%,83.
1%)Day735292686%(81.
5%,89.
7%)Day1435299788%(84.
7%,90.
7%)Day3035228488%(81.
3%,92.
6%)Day6035213387%(79.
7%,91.
8%)Day9035216584%(78.
4%,88.
2%)020406080100SW602CO-48AccuracyComparisonwithApprovedCGMsthroughSensorLifeDataSourcePercentofSystemReadingsWithin15/15%ofReferenceDeviceDay1Day3-4Day7Day10Day14Day30Day60Day90Eversense(SW602)PRECISEII77%91%87%85%PRECISION79%--86%--88%88%87%84%DexcomG5*--77%89%90%MedtronicGuardian(3)*--68%87%82%FreeStyleLibre*--76%82%85%85%*SummaryofSafetyandEffectivenessData(SSED)-MedicalDeviceDatabases-http://www.
fda.
govResultsbasedoncalibrationevery12hoursCO-49AlertSettingPRECISEIIDetectionRateFalseAlertRateLowGlucoseAlertat70mg/dL*96%16%HighGlucoseAlertat180mg/dL*98%7%AccurateDetectionofGlucoseExcursionsPRECISIONDetectionRateFalseAlertRate95%8%99%7%SW602*Includesthresholdand10-minutepredictivealertsCO-50PRECISEII:KMsurvivalprobabilityof91%atDay90PRECISION:Allsensorsfunctioned90daysPRECISEIIandPRECISION:EversenseSensorLongevity0.
00.
20.
40.
60.
81.
00306090SensorSurvivalProbabilityTimesinceImplant(days)0.
00.
20.
40.
60.
81.
00306090PRECISIONPRECISEIICO-51PRECISEIIandPRECISION:SystemAdherencePRECISEIIPRECISIONMedianweartime23.
4hours23.
4hours%transmittersworn>20hours/day87%91%CO-52PRECISEII:87%ofsensorreadingswith15/15%ofreferencePRECISION:85%ofsensorreadingswith15/15%ofreferenceAccurateateachmeasuredtimepointNodegradationofsensorperformance91%ofsensorsfunctionthrough90days"SensorReplacement"alertappropriatelyproducedOver95%detectionratesforglycemicexcursionsHigh(180mg/dL)andlow(70mg/dL)glucoseEffectivenessSummary:EversenseisAccuratefor90DaysCO-53ClinicalSafetyLynneKelley,MD,FACSChiefMedicalOfficerSenseonics,Inc.
CO-54EversensesystemhasacceptablesafetyprofileSimilartoothermarketedCGMsystemsProceduralrisksofimplantablesensormitigatedDevicedesign,training,andcontinuedimprovementsbasedonpost-marketsurveillanceEversensereducessomeknownrisksassociatedwithotherCGMsystemsOverviewofSafetyProfileCO-55EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-56PRECISEIIandPRECISION:DeviceExposurePRECISEIIPRECISIONSensorsinsertedandremoved106sensors(90patients)62sensors(35patients)Proceduresperformed212procedures124proceduresSensoruse(meanduration)92.
2days91daysSensorexposure9,773days6,148daysCO-57Incidenceofdevice-relatedorinsertion/removalprocedure-relatedseriousadverseevents(SAEs)atanypointduringsensorusePRECISEIIandPRECISION:PrimarySafetyEndpointCO-58Non-seriousrelatedadverseeventsAEsofspecialintereste.
g.
infection,adhesivereactionsDexamethasoneexposureovertimePRECISEIIandPRECISION:AdditionalSafetyAnalysesCO-59PRECISEIINodevice-relatedSAEsOneprocedure-relatedSAEreportedSensorremovalsensorunsuccessful(withandwithoutultrasound)SensorsuccessfullyremovedbysurgeonundergeneralanesthesiaPRECISIONNodevice-orprocedure-relatedSAEs3unrelatedSAEsGastroenteritis,hypoglycemicepisode,cellulitisofleftfootPRECISEIIandPRECISION:SeriousAdverseEvents(SAEs)CO-60AdverseEventPRECISEIIPRECISIONEventsPatientsN=90EventsPatientsN=35AllEvents14785Pain/discomfort4222Bruising22----Erythema22----Devicefragmentnotrecovered22----Syncope11----Tingling11----Delayedreportofpain11----Secondaryproceduretoremovesensor1121Dermatitisatpatchlocation----21Skinhyperpigmentation----21PRECISEIIandPRECISION:Device-orInsertion/Removal-RelatedAEsCO-61Allremovedsensorsreturnedtosponsorforinspection2devicesdidnothavecapuponreturnCorrectiveandpreventativeactionplanimplementedImplementingenhancedqualityprocedures(capadhesion)Capmaterial:PMMAhighlybiocompatible,permanentimplantOrthopedic,dental,andophthalmologicCapsize:3.
2mmx0.
8mmPRECISEII:TwoEventsRelatedtoDeviceFragmentCO-62NoinfectionsAllrelatedAEsconsideredexpectedandcommonforsubcutaneousimplantAllrelatedAEsresolvedfullyPRECISEIIandPRECISION:AdditionalSafetyOutcomesCO-63Contains1.
75mgdexamethasoneacetate(DXA)Water-insolublecorticosteroidReduceslocalinflammatoryresponseExtendssensorlifeControlledandslowDXAreleaseconeCollarCO-64BloodassayedforDXAto50pg/mLlevelNodetectableplasmalevelsofDXAobservedNosystemiceffectsDXAcollarsexaminedafterremovalMinimalDXAexposureconfirmed3μg/day2eventsoftransienthyperpigmentationResolveduponsensorremovalImpactofDexamethasoneExposureCO-65EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-66Threemulti-centerstudiesPRECISEII,PRECISION,andPRECISE206subjects335sensors670insertion/removalprocedures22,529patient-daysofsensorwearIntegratedDeviceExposureCO-67IntegratedSummaryofRelatedAdverseEventsAdverseEventEventsPatientsN=206AllEvents4126(13%)Pain/discomfort108(4%)Redness/erythema66(3%)Secondaryproceduretoremovesensor43(1%)Infection33(1%)Bruising/hematoma33(1%)Devicefragmentnotrecovered22(1%)Dermatitisatpatchlocation32(1%)Skinhyperpigmentation21(cope,hypertension,andnauseaCO-68Aggregateinfectionrate1%ImprovedincisioncareinstructionsPRECISE:leavebandagefor24hoursPRECISEII:leavebandagefor48hoursInfectionrateobservedisbelowliteraturereportsforsimilarimplantsandminorprocedures:2–4%*LowRateofInfectionsObservedinStudies*Buprenorphine,BraeburnPharmaceuticals,Inc.
*http://www.
worldwidewounds.
com/2005/september/Gottrup/Surgical-Site-Infections-Overview.
htmlCO-69EversenseClinicalProgramStudyDurationPatientsSitesRolePRECISEII90days908USPivotalPRECISION90days353USSupportivePRECISE180days817EUSupportiveEuropeanPatientRegistry(ongoing)2years1686350EUPost-marketFeasibilityStudiesVaried33210PilotTotal2224CO-70AllpatientsinsertedcommerciallyenrolledinregistryEnrollmentcompletedwhen100patientsreach4insertionsAllpatientsenrolledtobefollowedthrough8insertionsandremovalsEuropeanPatientRegistryCO-71LowRateofAEswithRepeatInsertionsEvents,n(%)PostInsertion#1N=16862N=4433N=1434N=585N=396N=147N=3SAEs0000000Device-,procedure-relatedAEsInfection(atsensorsite)8(0.
5%)4(1%)2(1%Secondaryproceduretoremovesensor72Adhesivepatchsiteirritation5-2----Prolongedwoundhealing3Redness/reactiontodressing3Sensorbrokeduringremoval3Skinatrophyoversensorw/skindiscoloration21Skinatrophyoversensor1Skindiscoloration12Sensorsitepain/discomfort1Bruising11-1---Patientfaintedduringprocedure1Hematoma---1---CO-72ProposedPost-ApprovalStudyCO-73Serialsensorinsertionsandremovalsfor2years175patientsinupto20clinicalsitesPrimarysafetyendpointRateofdevice-relatedandinsertion/removalprocedure-relatedSAEsthrough12months≤7%PrimaryeffectivenessendpointTimeinrange(between70mg/dLand180mg/dL),12monthsvs.
firstmonthProposedU.
S.
PostApprovalStudyDesignCO-74AllrelatedAEsthrough2yearsPlasmadexamethasonelevelsevery6monthsEffectivenessoftrainingprogramSuccessrateofinsertions/removalsDiabetesdistressscaleandCGMsatisfactionscaleBaselineandannuallyProposedU.
S.
PostApprovalStudy:OtherOutcomeMeasuresCO-75DesignChangesSensorendcapBluntdissectortoolCO-76SensorEndCapImprovementOriginalDesignNewDesignEndcapredesignedtobeflushwithendofsensorDesignverificationCompressiveforcesTorqueMaintainsfunctionalcompatibilitywithinsertiontoolCO-77BluntDissectorDesignImprovementSamefunctionConsistentplacementfacilitatesremovalProperentryanglePocketdepth/lengthParalleltoskinValidatedwithHumanFactorstestingNewBluntDissectorOriginalBluntDissectorNewguidesCO-78TrainingforCliniciansCO-79MandatorycomprehensivetrainingCertificationprocessledbySenseonicsapprovedtrainersDidacticsessionPracticeswithsimulatedskinInitialinsertionsandremovalsareobservedTrainingProgramOverviewTrainingresources:CGMSensorInsertionandRemovalInstructionsInsertionvideosRemovalvideosSimulationstationProcedureposterTake-homeinstructionsCO-80TrainingChecklistforCertificationPre-WorkbeforeSimulationTrainingInsertionSimulationTrainingPre-WorkbeforeRemovalReviewRemovalSimulationTrainingLearningcurve:~2to3procedures3patientsscheduledinsamedaytofamiliarizewithprocedureCO-81Hands-OnPracticeSessionwithSimulatedSkin,SterileField,andRequiredSuppliesCO-82ExamplesofTrainingMaterialsCO-83461clinicianstrainedoninsertions94%certifiedtodoinsertionsindependently258clinicianstrainedonremovals86%certifiedtodoremovalsindependentlyTrainedProvidersOutsideU.
S.
CO-84ProcedureeasilylearnedbyphysicianswithnopriorEversenseexperience99%ofremovalssuccessfulonfirstattemptLowinfectionrate(0.
5%)Europe:SuccessfulInsertionandRemovalTrainingCO-85100%ofinsertionsand99%ofremovalssuccessfulonfirstattempt91%ofinsertionsand80%ofremovalscompletedinCO-86NounanticipatedadverseeventsLimitedAEsrelatedtodeviceorprocedureAllAEsreportedresolvedfullyNoInfectionsinUSclinicaltrialsNodetectablebloodlevelsofdexamethasoneOneprocedure-relatedSAE,resolvedNodevice-relatedSAEsEversenseissafeforintendeduseEversenseSystemHasAcceptableSafetyProfileCO-87ClinicalPerspective/Benefit-RiskStevenJRussell,MD,PhDAssociateProfessorofMedicineHarvardMedicalSchoolCO-88ExperiencewithallcurrentlyapprovedCGMs(since2004)PublishedaccuracycomparisonstudiesofCGMsDevelopingbionicpancreasDependsonCGMaccuracyandreliabilityMotivatesinterestinnewCGMtechnologiesClinicalinvestigatorinartificialpancreastrialsUsedEversensesystemInsertedandremovedsensorsTrainedquicklyRelevantCGMExperienceCO-8970%notatA1ctargets*HypoglycemiastillverycommonCGMsystemsareproventohelpImproveglucosecontrolLowerriskofhypoglycemiaImprovepatients'livesCurrentSituation:MajorityofPatientsDoNotMeetGlycemicGoals*Type1DiabetesExchangeCO-90TheCurrentSituation:Only3Outof10PatientswithT1DUseCGMPerceivedburdenofrepeatinsertionFearofpainCO-91TheCurrentSituation:1outof3CGMUsersDiscontinuewithin1YearProblemswithadhesive/insertionUncomfortableCO-92Longersensorlife(90days)LessfrequentsensorinsertionsEversense:4timesperyearCurrentsystems:25–50timesperyearEasytowearandeasilyremovedForphysicalactivitiesordiscretionOn-bodyvibrationfromtransmitterprovidesextrasafetymeasureEversenseAddressesManyBarrierstoCGMUseCO-93TheGoal:IncreaseUseofCGMImproveglucosecontrolLowerriskofhypoglycemiaCO-94EversenseContinuousGlucoseMonitoring(CGM)SystemMarch29,2018Senseonics,Inc.
ClinicalChemistryandClinicalToxicologyDevicePanel
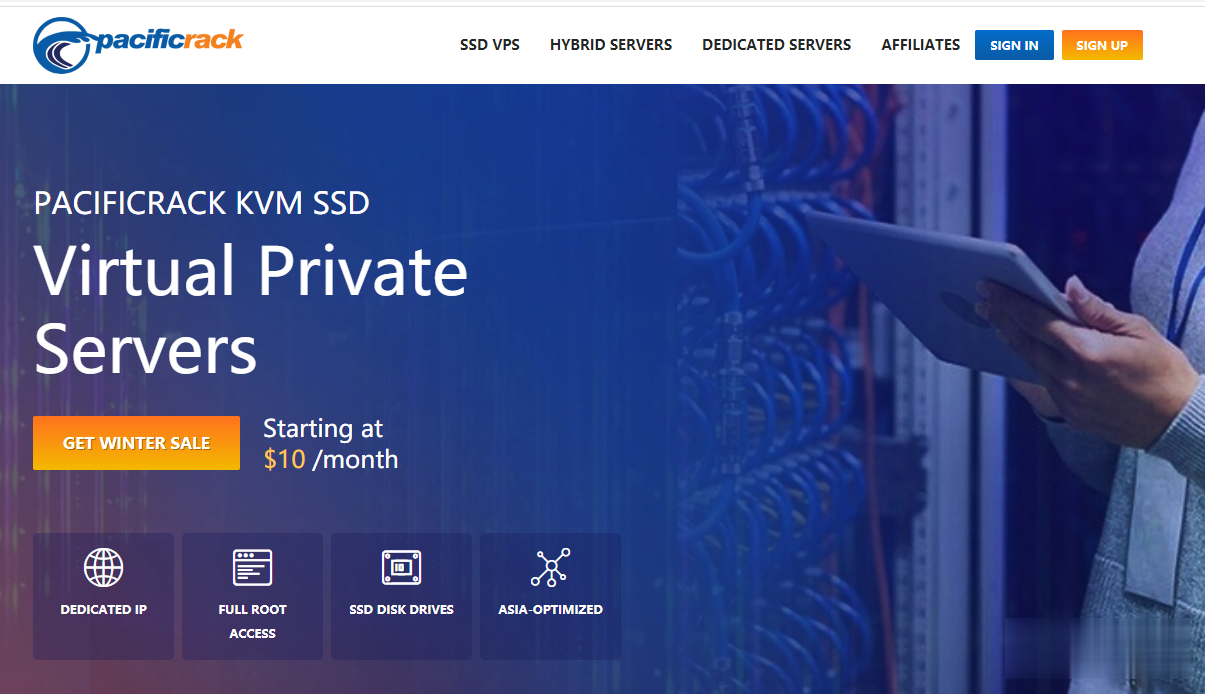
Pacificrack:新增三款超级秒杀套餐/洛杉矶QN机房/1Gbps月流量1TB/年付仅7美刀
PacificRack最近促销上瘾了,活动频繁,接二连三的追加便宜VPS秒杀,PacificRack在 7月中下旬已经推出了五款秒杀VPS套餐,现在商家又新增了三款更便宜的特价套餐,年付低至7.2美元,这已经是本月第三波促销,带宽都是1Gbps。PacificRack 7月秒杀VPS整个系列都是PR-M,也就是魔方的后台管理。2G内存起步的支持Windows 7、10、Server 2003\20...
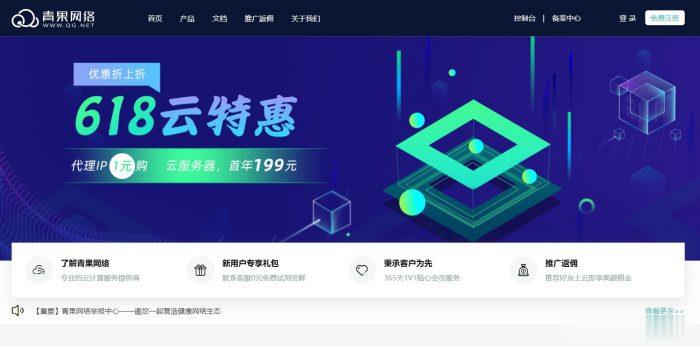
青果网络618:洛杉矶CN2 GIA/东京CN2套餐年付199元起,国内高防独服套餐66折
青果网络怎么样?青果网络隶属于泉州市青果网络科技有限公司,青果网络商家成立于2015年4月1日,拥有工信部颁发的全网IDC/ISP/IP-VPN资质,是国内为数不多具有IDC/ISP双资质的综合型云计算服务商。青果网络是APNIC和CNNIC地址分配联盟成员,泉州市互联网协会会员单位,信誉非常有保障。目前,青果网络商家正式开启了618云特惠活动,针对国内外机房都有相应的优惠。点击进入:青果网络官方...
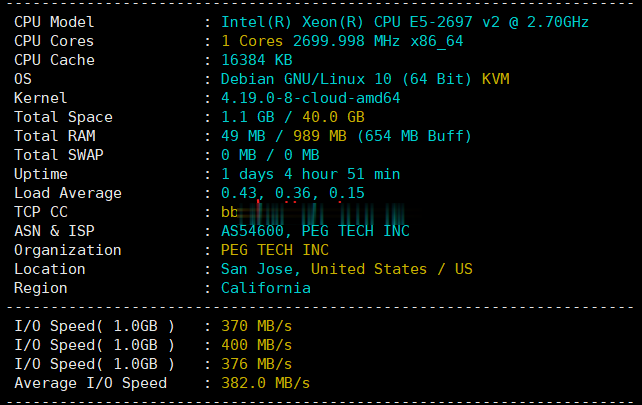
raksmart:全新cloud云服务器系列测评,告诉你raksmart新产品效果好不好
2021年6月底,raksmart开发出来的新产品“cloud-云服务器”正式上线对外售卖,当前只有美国硅谷机房(或许以后会有其他数据中心加入)可供选择。或许你会问raksmart云服务器怎么样啊、raksm云服务器好不好、网络速度快不好之类的废话(不实测的话),本着主机测评趟雷、大家受益的原则,先开一个给大家测评一下!官方网站:https://www.raksmart.com云服务器的说明:底层...
co为你推荐
-
万维读者网用QQ邮箱向《读者》投稿具体格式手游运营手册游戏策划新手应该看那些书籍?flash导航条如何添加FLASH导航条bluestacksbluestacks怎么用iphone越狱后怎么恢复苹果越狱后如何恢复bluestack安卓模拟器bluestacks怎么用?三星s8什么时候上市三星盖乐世S8上市时间公布 三星盖乐世s8多少钱网络虚拟机如何设置vmware虚拟机网络声母是什么什么是声母,什么是音母?尚易企业邮局尚易企业邮箱的服务怎么样