Coronaryco
co 时间:2021-03-03 阅读:()
CO-1Victoza(liraglutide)InjectionEvaluationofCardiovascularOutcomeResultsfromLEADEREndocrinologicandMetabolicDrugsAdvisoryCommitteeJune20,2017NovoNordiskCO-2LiraglutideEffectandActioninDiabetes:EvaluationofCardiovascularOutcomeResultsfromLEADERRobertClarkVicePresident,RegulatoryAffairsNovoNordiskCO-3Victoza:establishedpositivebenefit-riskforT2DMApprovedin>100countriesEUin2009USin2010OriginalNDAevaluated6885patientswithT2DM>6millionpatient-yearsexperienceSaxenda:higherdose(3mg)approvedforweightmanagementVictoza(liraglutide)isMostWidelyPrescribedGLP-1ReceptorAgonistGloballyCO-4ActivateshighlyspecificGLP-1cellsurfacereceptorwithdefinedcellandorgandistributionImprovementofglycemiccontrolandbodyweightbasedonGLP-1physiologyScientificliteraturehypothesizesindependenteffectonatherosclerosisLiraglutide:MechanismofActionLiraglutideCO-5PreapprovalevidenceofCVsafetyfornewT2DMdrugsPost-hocmeta-analysisofPhase3adata1didnotsuggestCVharmHR(95%CI)=0.
73(0.
38,1.
41)NovoNordiskconductedalarge,randomizedCVOTwith3-componentMACEasprimaryendpoint:LEADERLiraglutideNDASubmittedPriorto2008FDAGuidanceRegardingCVSafetyCV=cardiovascular;T2DM=type2diabetesmellitus;HR=hazardratio;CVOT=cardiovascularoutcomestrial;MACE=majoradversecardiovascularevents1.
Marsoetal.
,DiabVascDisRes,2011.
CO-6EstablishCVsafety(event-driven)throughnon-inferiority≥611firstMACEinpatientswithT2DMandhighCVriskTestforCVbenefitthroughsuperiorityEstablishlong-termsafetybygatheringdataonmedicaleventsofspecialinterest(MESI)Treatmentperiodof≥42monthsLEADERFollowedPre-SpecifiedAnalysisPlanwith3GoalsCO-7DemonstratessuperiorityvsplaceboforreductioninMACEAchievedstatisticallysignificant13%reductioninMACEHR(95%CI)=0.
87(0.
78,0.
97)22%reductioninCVdeathReaffirmsoverallsafetyprofileReinforcesfavorablebenefit-riskLEADERSupportsCVBenefitforPatientswithT2DMandHighCVRiskCO-8Inpatientswithlong-standingsuboptimallycontrolledtype2diabetesandestablishedatheroscleroticcardiovasculardisease,empagliozinorliraglutideshouldbeconsideredastheyhavebeenshowntoreducecardiovascularandall-causemortalitywhenaddedtostandardofcare.
2017ADAStandardsofMedicalCareinDiabetesIncludesLiraglutideADA,DiabetesCare,2017.
CO-9CurrentapprovedindicationAsanadjuncttodietandexercisetoimproveglycemiccontrolinadultswithtype2diabetesmellitus.
ProposedindicationAsanadjuncttodietandexercisetoimproveglycemiccontrolinadultswithtype2diabetesmellitus.
Asanadjuncttostandardtreatmentofcardiovascularriskfactorstoreducetheriskofmajoradversecardiovascularevents(cardiovasculardeath,non-fatalmyocardialinfarction,ornon-fatalstroke)inadultswithtype2diabetesmellitusandhighcardiovascularrisk.
LEADERResultsSupportProposedAdditionalIndicationCO-10AgendaLEADERClinicalDesignSteveMarso,M.
D.
MedicalDirector,CardiovascularServicesHCAMidwestHealthHeartandVascularInstituteLEADERResultsAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskGeneralSafetyToddHobbs,M.
D.
USChiefMedicalOfficerNovoNordiskClinicalImplicationsJohnB.
Buse,M.
D.
,Ph.
D.
VerneS.
CavinessDistinguishedProfessorChief,DivisionofEndocrinologyUniversityofNorthCarolinaSchoolofMedicineBenefit-RiskConclusionAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-11AdditionalExpertsAnilK.
Rustgi,M.
D.
Chief,DivisionofGastroenterologyT.
GrierMillerProfessorofMedicineUniversityofPennsylvaniaJanetWittes,Ph.
D.
PresidentStatisticsCollaborative,IncCO-12LEADERClinicalDesignSteveMarso,M.
D.
MedicalDirector,CardiovascularServicesHCAMidwestHealthHeartandVascularInstituteCo-chair,LEADERSteeringCommitteeCO-13LEADER:Randomized,Double-Blind,Placebo-ControlledTrialLiraglutide1.
8mg/day+StandardofCare(N=4,668)Placebo+StandardofCare(N=4,672)Duration3.
5-5yearsPlaceboInjectionRun-in(2-3weeks)DetermineeffectofliraglutideonCVeventsinadultswithT2DMathighCVriskInitialdoseof0.
6mg/dayliraglutideorplaceboescalatedweeklytoreach1.
8mg/daytargetdoseperlabelTime(minimumdurationof42months)andevent(atleast611firstMACE)driventrialEffortsundertakentolimitmissingdataSafetyFollow-up(30days)CO-14LEADERIncludedRecommendedStandardofCareforPatientswithT2DMMultifactorialapproachPlateletinhibitionBloodpressurecontrolRydénLetal.
,EurHeartJ,2013;FoxCSetal.
,DiabetesCare,2015;PiepoliMFetal.
,EurHeartJ,2016.
LifestylemodificationGlycemiccontrolManagementofdyslipidemiaTarget:IncludeweightTarget:Aspirinandotheranti-thromboticswithpriorCVeventTarget:HbA1cCO-15T2DMwithHbA1c≥7.
0%HighCVriskpopulationLEADER:KeyInclusionCriteriatoEnrichPopulationforCVRiskAtleastone:PriorMI,StrokeorTIA,orarterialrevascularization>50%stenosisonangiographyHistoryofsymptomaticcoronaryheartdiseaseDocumentedasymptomaticcardiacischemiaChronicheartfailureNYHAclassII-IIIChronickidneydiseaseAndage≥50yearsAtleastone:MicroalbuminuriaormacroalbuminuriaHypertensionandleftventricularhypertrophyLeftventricularsystolicordiastolicdysfunctionAnkle/brachialindexCO-16Timetofirst3-componentMACECVdeathNon-fatalmyocardialinfarction(MI)Non-fatalstrokeFullanalysisset(FAS):Intention-to-treatAllrandomizedpatientsPrimaryEndpoint:MACECO-17ExpandedMACE3-componentMACEHospitalizationforheartfailureHospitalizationforunstableanginaCoronaryrevascularizationIndividualcomponentsofExpandedMACEAll-causemortalityOtherkeyendpointsMicrovascularevents,HbA1cKeySecondaryEndpointsCO-18PrimaryEndpointandTimetoEventAnalysisRandomizationDateNon-fatalMICVDeathTimeto1stMACE(primaryendpoint)TimetoCV-deathNon-fatalStrokeTimeto1stNon-fatalstrokeTimeto1stNon-fatalMICO-19CommitteeFunctionsSteeringCommittee(StC)DesignedprotocolProvidedscientificleadershipDataMonitoringCommittee(DMC)MonitoredpatientsafetyEvaluatedefficacyandsafetydataEventAdjudicationCommittee(EAC)PerformedongoingadjudicationofdeathsandpredefinedMESILEADER:StructureofTrialOversightCO-20ComprehensiveApproachtoIdentifyEventsforAdjudicationInvestigatorDirectreportingofdeathand/orMESIsLabMeasuresScreeningoflaboratorymeasurementsEAC/CROAdditionaleventsidentifiedinsourcedocumentsMedDRAScreeningofAEsnotreporteddirectlyforadjudicationbyinvestigatorIndependentContractResearchOrganization(CRO)ECGCentralreviewofECGsLabMeasuresScreeningoflaboratorymeasurementsEAC/CROAdditionaleventsidentifiedinsourcedocumentsMedDRAScreeningofAEsnotreporteddirectlyforadjudicationbyinvestigatorECGCentralreviewofECGsInvestigatorDirectreportingofdeathand/orMESIsCO-21ExternalEACCardiovascularsubcommitteeMicrovascularsubcommitteePancreatitissubcommitteeNeoplasmsubcommitteeDeath,ACS,CVA,CoronaryRevasc.
,HeartFailureNephropathyRetinopathyPancreatitisNeoplasmincludingThyroidExternal,Independent,BlindedEventAdjudicationCommittee(EAC)EventsACS=AcutecoronarysyndromeCVA=CerebrovascularaccidentCO-221.
Testofnon-inferiorityConfirmedifupperboundof95%CIConfirmedifupperboundof95%CICO-23PerProtocolPatientswhotook≥1dose;accumulateddaysofftreatmentCO-24PatientswhohadeitherPrimaryendpoint(MACE)Diedduetonon-CVcausesDirectcontactatlastplannedfollow-upCompleterDefinitionCO-25PatientDisposition:HighCompletionRateinLEADERFAS=fullanalysissetCompleter:apatientwhohad1.
aprimaryevent(MACE),or2.
diedduetonon-cardiovascularcauses,or3.
withwhomdirectcontactwasestablishedatoraftertheplannedfollow-upvisitRandomised(FAS)N=9340Placebo+StandardofCareN=4672Liraglutide+StandardofCareN=4668CompletedStudyN=4513(96.
6%)CompletedStudyN=4529(97.
0%)DidnotcompleteN=139(3.
0%)AliveN=127(2.
7%)VitalstatusunknownN=12(0.
3%)WithdrawnconsentN=4(0.
1%)Losttofollow-upN=8(0.
2%)DidnotcompleteN=159(3.
4%)AliveN=142(3.
0%)VitalstatusunknownN=17(0.
4%)WithdrawnconsentN=8(0.
2%)Losttofollow-upN=9(0.
2%)CO-26RelevanthighriskdiabetespopulationLongdurationoftrialStructuredexternaloversightExperiencedsteeringcommitteeIndependentdatamonitoringcommitteeSmallamountofmissingdataLEADER:RobustDesignandExecutionCO-27LEADERCVOTResultsAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-28EstablishedcardiovascularsafetyStatisticallysignificantreductioninMACEReductionsinexpandedMACEandall-causemortalityConsistentbenefitacrossallCVcomponentsBenefitsseenacrosssubgroupsSubstantialevidenceofliraglutidesuperiorityversusstandardofcareLEADERDemonstratedCVBenefitsinT2DMCO-29LiraglutideN=4668PlaceboN=4672Age64years64yearsSex,male65%64%RaceWhite78%78%BlackorAfricanAmerican8%9%Other15%14%BMI,kg/m232.
532.
5LEADER:BalancedBaselineDemographicsandCharacteristicsRegionNorthAmerica(UnitedStates+Canada)30%31%Europe35%36%Asia8%8%RestofWorld27%26%HbA1c(%)8.
78.
7Diabetesduration12.
8years12.
9yearsEstablishedCVD/CKD(inclusioncriterion3a)82%81%CO-30LEADER:BalancedBaselineCardiovascularProfile*ChronickidneydiseasedefinedaseGFRcoronary,carotidorperipheralarterialrevascularization39%39%>50%stenosisofcoronary,carotidorlowerextremityarteries25%26%Historyofsymptomaticcoronaryheartdisease9%9%Documentedasymptomaticcardiacischemia27%26%ChronicheartfailureNYHAIIorIII14%14%Chronickidneydisease*25%24%Microalbuminuriaormacroalbuminuria25%27%Hypertensionandleftventricularhypertrophy25%24%Leftventricularsystolicordiastolicdysfunction24%24%Ankle/brachialindexCO-31LEADER:BalancedDistributionofPatientsbyBaselineRenalFunction~2000patientswithmoderaterenalimpairment224patientswithsevererenalimpairmentLiraglutideN=4668PlaceboN=4672Normalrenalfunction(eGFR≥90)35%35%Mildimpairment(eGFR60-89)41%42%Moderateimpairment(eGFR30-59)21%20%Severeimpairment(eGFRCO-32ObservationTimeandTrialDrugExposureTimeFullanalysissetExposuretimetotrialdrugisdefinedasdurationoftreatmentwithinvestigationalproductintrialexcludingperiodsoff-treatment*Including30dayssafetyfollow-upafterendofrandomizedtreatmentperiod;**DuringtheobservationperiodLiraglutideN=4668PlaceboN=4672Observationtime(median)*3.
8years3.
8yearsExposuretimetotrialdrug(median)3.
5years3.
5yearsExposuretimetotrialdrug**%ofPatientsCO-33LiraglutideN=4668PlaceboN=4672n%n%MACE60813.
0%69414.
9%CVdeath1813.
9%2274.
9%Non-fatalMI2755.
9%3046.
5%Non-fatalstroke1523.
3%1633.
5%ReductioninFirstMACEWhenLiraglutideAddedtoStandardofCareFullanalysisset;EAC-confirmedevents;onlyfirsteventsbetweenrandomizationandfollow-upareincluded1302firstMACEeventsCO-34StatisticallySignificantReductioninTimetoFirstMACEFullanalysisset;Coxproportionalhazardsmodelwithtreatmentasfactor;1-sidedp-values0%5%10%15%20%061218243036424854PlaceboLiraglutideHR(95%CI)0.
87(0.
78,0.
97)p(non-inferiority)CO-35LiraglutidePlaceboN=4668N=4672608694219278281317159177TimetoFirstEventforIndividualComponentsofPrimaryEndpointHazardRatio(95%CI)TimetofirstMACE(Primaryanalysis)0.
87(0.
78,0.
97)TimetoCVdeath0.
78(0.
66,0.
93)Timetofirstnon-fatalMI0.
88(0.
75,1.
03)Timetofirstnon-fatalstroke0.
89(0.
72,1.
11)0.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboLiraglutidePlaceboN=4668N=4672608694219278281317159177FullanalysissetLiraglutidePlaceboN=4668N=4672608694219278281317159177CO-36Pre-specifiedSensitivityAnalysesSupportMACEPrimaryEndpointSensitivityAnalysisHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672MACE–primaryanalysis(FAS)0.
87(0.
78,0.
97)608694Perprotocol0.
86(0.
76,0.
97)493564Ontreatment0.
83(0.
73,0.
95)414482Ontreatment+30days0.
83(0.
73,0.
94)469549Excludingeventsafterendoftreatment0.
86(0.
77,0.
95)598690Adjustedforadditionalcovariates0.
87(0.
78,0.
97)605692Adjustedforrandomcountryeffect0.
86(0.
77,0.
96)605692Stratifiedforsevererenalimpairment0.
87(0.
78,0.
97)6086940.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFAS=fullanalysisset;Numberofpatientsinperprotocolanalysisis4657forliraglutideand4664forplaceboCO-37Losttofollow-upanalysis12missingliraglutidepatientsassumedtohaveCVdeath17missingplacebopatientsassumedaliveHR(95%CI)=0.
89(0.
79,0.
99)Non-completeranalysisTolosesuperioritywouldneedtoassume21/139non-completerliraglutidepatientsand0/159inplacebogrouphadnon-fatalMIorstrokeUnlikelyscenariosbasedonreportingofeventsTippingPointAnalysesSupportiveofPrimaryResultsCO-38SecondaryOutcomesCO-39BenefitinExpandedMACE(6Components)0%5%10%15%20%25%30%061218243036424854PlaceboLiraglutideTimefromrandomization(months)Fullanalysisset;totalof2010firstevents;1-sidedp-valueAtRisk(N)Liraglutide466845154356422140633914379336821452395Placebo467245064336415740023857369735811410366HR(95%CI)0.
88(0.
81,0.
96)p-value0.
005Patientswithanevent(%)CO-40219278281317159177122124405441218248CVdeath0.
78(0.
66,0.
93)Non-fatalMI0.
88(0.
75,1.
03)Non-fatalstroke0.
89(0.
72,1.
11)Unstableanginapectorisleadingtohospitalization0.
98(0.
76,1.
26)Coronaryrevascularization0.
91(0.
80,1.
04)Hospitalizationforheartfailure0.
87(0.
73,1.
05)TimetoFirstEventforIndividualComponentsofExpandedMACESupportsCompositeResultsLiraglutidePlaceboN=4668N=4672MACE–primaryanalysis0.
87(0.
78,0.
97)608694ExpandedMACE0.
88(0.
81,0.
96)94810620.
61.
3FavorsLiraglutideFavorsPlacebo1221244054412182481.
0Fullanalysisset219278281317159177HazardRatio(95%CI)122124405441218248219278281317159177CO-41FactorNHazardRatio(95%CI)MACE–primaryanalysis934013.
90.
87(0.
78,0.
97)SexFemale333711.
70.
88(0.
72,1.
08)Male600315.
20.
86(0.
75,0.
98)AgeConsistentEffectonTimetoFirstMACEAcrossSubgroups0.
00.
51.
01.
5FavorsLiraglutideFavorsPlacebo%withMACEFullanalysissetCO-42BaselinedemographicsDiseasecharacteristicsConcomitantCVordiabetesmedicationsHbA1cBodyweightSystolicbloodpressureCovariatesAssessedDidNotExplainDifferencesBetweenNorthAmericaandPrimaryAnalysisCO-43CVEffectinUSPatientsinLinewithOverallPopulationWhenonRandomizedTreatmentLiraglutidePlaceboN%N%MACE–primaryanalysis(FAS)0.
87(0.
78,0.
97)60813.
069414.
9USPopulation(FAS)12471001267100MACE1.
03(0.
84,1.
25)19615.
719315.
2On-treatment0.
89(0.
69,1.
14)1169.
31259.
9On-treatment+30days0.
89(0.
70,1.
12)13110.
514211.
20.
60.
81.
01.
21.
4FAS=FullanalysissetFavorsLiraglutideFavorsPlaceboHazardRatio(95%CI)CO-44FactorNHazardRatio(95%CI)MACE–primaryanalysis934013.
90.
87(0.
78,0.
97)BMI≤30kg/m2357414.
00.
96(0.
81,1.
15)>30kg/m2575713.
90.
82(0.
71,0.
94)HbA1c≤8.
3%476813.
00.
89(0.
76,1.
05)>8.
3%457214.
90.
84(0.
72,0.
98)Diabetesduration≤11years442913.
10.
82(0.
70,0.
97)>11years489214.
60.
90(0.
78,1.
04)RenalfunctioneGFRConsistentEffectonTimetoFirstMACEAcrossSubgroupsFavorsLiraglutideFavorsPlacebo%withMACEFullanalysisset;eGFR(mL/min/1.
73m2)aspermodificationofdietinrenaldiseaseformulaCO-45FavorableTrendinMACEinPatientsWithorWithoutPriorMIorStroke0510152025300102030405060Timefromrandomization(months)HR(95%CI)0.
89(0.
76,1.
05)0510152025300102030405060Timefromrandomization(months)HR(95%CI)0.
84(0.
72,0.
97)N=3692Patientswithanevent(%)PriorMI,StrokeN=5648NoPriorMI,StrokePlaceboLiraglutidePlaceboLiraglutideCO-46TreatmentEffectConsistentforAll-CauseDeathandCVDeathHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672All-causedeath0.
85(0.
74,0.
97)381447Cardiovasculardeath0.
78(0.
66,0.
93)219278Non-cardiovasculardeath0.
95(0.
77,1.
18)1621690.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFullanalysissetCO-47ReductioninAll-CauseDeath0%2%4%6%8%10%12%14%061218243036424854PlaceboLiraglutideTimefromrandomization(months)FullanalysissetHR(95%CI)0.
85(0.
74,0.
97)p-value0.
017Patientswithanevent(%)AtRisk(N)Liraglutide466846414599455845054445438243221723484Placebo467246484601454644794407433842681709465CO-480%1%2%3%4%5%6%061218243036424854PlaceboLiraglutideTimetoNon-CVDeathTimefromrandomization(months)FullanalysissetHR(95%CI)0.
95(0.
77,1.
18)p-value0.
66Patientswithanevent(%)AtRisk(N)Liraglutide466846414599455845054445438243221719484Placebo467246484601454644794407433842671708465CO-49OverviewofMicrovascularEvents:NephropathyandRetinopathyHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672Microvascularcomposite0.
84(0.
73,0.
97)355416Nephropathycomposite0.
78(0.
67,0.
92)265337Newonsetofpersistentmacroalbuminuria0.
74(0.
60,0.
91)161215Persistentdoublingofcreatinine*0.
89(0.
67,1.
19)8797Continuousrenalreplacementtherapy0.
87(0.
61,1.
24)5664Deathduetorenaldisease1.
59(0.
52,4.
87)85Retinopathycomposite1.
15(0.
87,1.
52)10692Photocoagulation/intravitrealagent1.
16(0.
87,1.
55)10086Vitreoushemorrhage1.
45(0.
84,2.
50)32220.
52.
05.
01.
0FavorsLiraglutideFavorsPlacebo*andeGFR≤45mL/min/1.
73m2perMDRDFullanalysisset;OneeventofdiabetesrelatedblindnessreportedinplaceboCO-50RapidandSustainedHbA1cReductionforLiraglutidevsPlaceboWhenAddedtoStandardofCare5.
05.
56.
06.
57.
07.
58.
08.
59.
00612182430364248546066Timefromrandomization(months)EachVisit(N)Liraglutide466843554295413540343877381023498091013705Placebo46724355423540303905374236402303756873561Fullanalysisset;*Endoftreatment(EOT)maybeanytimefrommonth42onwardsEOT*EstimatedMeanHbA1c(%)Baseline8.
7%CO-51LiraglutidePatientsLessLikelytoInitiateNewDiabetesMedications0%10%20%30%40%50%01020304050PlaceboLiraglutideProportionofpatients(%)Timefromrandomization(months)HR(95%CI)0.
64(0.
60,0.
69)54AtRisk(N)Liraglutide46683991367833193037677294Placebo46723453295426302379497227Fullanalysisset;Timetofirstinitiationofinsulinoranyneworalanti-diabeticCO-52SuperiorityconfirmedfortimetofirstMACE(p=0.
005)SignificantlyreducedriskofMACEby13%ReducedriskofexpandedMACEby12%ConsistentreductionsinMACEacrosssensitivityanalysesConsistentreductionsacrossMACEcomponents,withpointestimatecomesforLiraglutideCO-53GeneralSafetyToddHobbs,M.
D.
USChiefMedicalOfficerNovoNordiskCO-54CardiovascularsafetyLong-termsafetyGatheradditionaldataonmedicaleventsofspecialinterest(MESI)LEADERDesignedwithTwoMajorSafetyAreasCO-55DatafromT2DMpatientswithhighcardiovascularriskreaffirmsexistingsafetyprofileClinicaldevelopmentprograms7yearspost-marketingexperienceLabeladequatelyaddressespotentialrisksLEADER5-YearDataReinforcesExistingLiraglutideSafetyProfileCO-56MedicalEventsofSpecialInterest(MESI)AdjudicationMedDRAsearchElectronicdatacaptureformsLaboratorymeasurementsAcutecoronarysyndromeCoronaryrevascularizationCerebrovasculareventsHospitalizationforheartfailureDeathRetinopathyNephropathyNeoplasms/ThyroidneoplasmsPancreatitisAcutegallbladderdiseaseThyroiddiseaseDiabeticfootulcersImmunogenicityeventsMedicationerrorsSeverehypoglycemiceventsAEsleadingtopermanenttreatmentdiscontinuationCalcitonin≥20ng/LAnti-liraglutideantibodiesAcutecoronarysyndromeCoronaryrevascularizationCerebrovasculareventsHospitalizationforheartfailureDeathRetinopathyNephropathyNeoplasms/ThyroidneoplasmsPancreatitisAcutegallbladderdiseaseThyroiddiseaseDiabeticfootulcersImmunogenicityeventsMedicationerrorsSeverehypoglycemiceventsAEsleadingtopermanenttreatmentdiscontinuationCO-57LEADER:AdverseEventsReportedin>2%PatientsLiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs62.
3%60.
8%Nausea3.
7%0.
9%Anginaunstable3.
4%3.
5%Acutemyocardialinfarction3.
3%4.
1%Cardiacfailure3.
0%3.
4%Cardiacfailurecongestive2.
9%3.
2%Pneumonia2.
9%3.
0%Diabeticfoot2.
8%3.
3%Coronaryrevascularization2.
7%3.
1%Coronaryarterialstentinsertion2.
5%2.
7%Acutekidneyinjury2.
4%2.
1%Hypoglycemia2.
3%2.
8%Anginapectoris2.
2%2.
2%CO-58LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs9.
6%7.
3%Serious4.
2%5.
3%Severe3.
6%4.
0%LEADER:AdverseEventsLeadingtoPermanentTreatmentDiscontinuationCO-59LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs9.
6%7.
3%Nausea1.
6%0.
4%Vomiting0.
7%comfort0.
2%0Abdominalpainupper0.
2%congestive0.
2%0.
1%Chronickidneydisease0.
2%0.
2%LEADER:MostCommonAdverseEventsLeadingtoPermanentDiscontinuation≥0.
2%PatientsCO-60AcutegallbladderdiseasePancreatitisNeoplasmsHypoglycemiaAdditionalSafetyAreasofInterestCO-61LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs3.
1%1.
9%Cholelithiasis1.
5%1.
1%Cholecystitisacute0.
8%0.
4%Cholecystitis0.
3%0.
3%Cholecystitischronic0.
2%0.
1%Biliarycolic0.
2%CO-62LEADER:NoIncreasedOccurrenceofPancreatitiswithLiraglutide*SeveritybymodifiedAtlantaCriteriaLiraglutideN=4668PlaceboN=4672Acutepancreatitis0.
4%0.
5%Mild*0.
3%0.
4%Moderately-severe*00.
1%Severe*CO-63LiraglutidePlaceboHazardRatio(95%CI)N%N%Totalneoplasms47010.
14199.
01.
12(0.
99,1.
28)Benign1683.
61453.
11.
16(0.
93,1.
44)Malignant2966.
32796.
01.
06(0.
90,1.
25)MalignantneoplasmsbytissuetypeSkin(non-melanoma)781.
7621.
31.
25(0.
90,1.
75)Prostate260.
9471.
60.
54(0.
34,0.
88)Breast211.
3201.
21.
06(0.
57,1.
96)Lung/bronchus280.
6330.
70.
85(0.
51,1.
40)Colorectal280.
6280.
60.
99(0.
59,1.
68)Other130.
3140.
30.
92(0.
43,1.
97)Urinarybladder150.
3120.
31.
24(0.
58,2.
66)Kidney/renalpelvis170.
490.
21.
88(0.
84,4.
22)Hepatic/biliary130.
380.
21.
62(0.
67,3.
90)Leukemias50.
1140.
30.
36(0.
13,0.
99)Pancreatic130.
350.
12.
59(0.
92,7.
27)Melanomaoftheskin130.
350.
12.
59(0.
92,7.
27)Cervical/vaginal60.
420.
13.
03(0.
61,15.
0)Lymphoma80.
260.
11.
33(0.
46,3.
82)Oralcavity/pharynx70.
160.
11.
16(0.
39,3.
46)Uterine20.
130.
20.
68(0.
11,4.
05)Oesophageal40.
160.
10.
66(0.
19,2.
34)Gastric40.
150.
10.
80(0.
21,2.
97)Thyroid50.
130.
11.
66(0.
40,6.
95)Bone/softtissue20.
050.
10.
40(0.
08,2.
05)Ovarian10.
120.
10.
51(0.
05,5.
58)LEADER:IncidencesofNeoplasmsSimilarBetweenLiraglutideandPlacebo0.
00.
21.
05.
025.
0FavorsLiraglutideFavorsPlacebo0.
04CO-64LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LEADER:EACConfirmedMalignantPancreaticNeoplasmsLiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311CO-65AdditionalDataSourcesShowNoIncreaseinRiskofPancreaticNeoplasmswithLiraglutideClinicaldevelopmentprogramsType2diabetesWeightmanagementPost-marketingdata>6millionpatient-yearsofexposureClaimsdatabasestudy(OPTUM)Non-clinicalstudiesshownoincreasedriskConsistentwith2014FDAandEMAjointstatementEMA=EuropeanMedicinesAgencyCO-66LEADER:NoEvidenceofMalignantThyroidNeoplasmRiskwithLiraglutideNocasesofMedullaryThyroidCarcinoma(MTC)inliraglutideexposedpopulationinLEADER1MTCinplacebogroupMonitoringforMTCthroughongoingUSregistryIncludesallapprovedlong-actingGLP-1receptoragonistsLiraglutideN=4668PlaceboN=4672n%n%Malignantthyroidneoplasms50.
1%30.
1%CO-67LEADER:LessHypoglycemiaWhenLiraglutideAddedtoStandardofCareConfirmedhypoglycemiaisdefinedasseverehypoglycemiaaccordingtotheADAdefinitionand/orplasmaglucoseConfirmedhypoglycemia43.
7%45.
6%Severehypoglycemia2.
4%3.
3%CO-680102030405060700102030405060PlaceboLiraglutideLEADER:LowerRiskofSevereHypoglycemiainPatientsTreatedWithLiraglutideMeannumberofepisodesper1000patientsTimefromrandomization(months)Fullanalysisset;RR=rateratioRR(95%CI)0.
69(0.
51,0.
93)CO-69SafetyprofileofliraglutideconfirmedGallbladderdisorderscurrentlyaddressedinliraglutidelabelSimilarfrequencyofacutepancreatitiswithliraglutideandplaceboSimilarriskforneoplasmswithliraglutideandplaceboLEADER:LiraglutideSafetyAlignswithLabelandPost-MarketingExperienceCO-70ClinicalImplicationsJohnB.
Buse,M.
D.
,Ph.
D.
VerneS.
CavinessDistinguishedProfessorChief,DivisionofEndocrinologyUniversityofNorthCarolinaSchoolofMedicineCO-71T2DMprevalencedoubledoverlast20years129millionaffectedinUS215%adults,26%≥65yrs,1~30%lifetimerisk3Hypoglycemiaisamajorissueindiabetescare4Glycemiccontrolreducesmicrovascularcomplications5CVeventrates~2foldhigherinpatientswithdiabetesthaninthosewithout2Type2DiabetesMellitus:MajorMedicalandSocietalChallenge1.
NCDRiskFactorCollaboration(NCD-RisC)JAMA,2016.
2.
AmericanDiabetesAssociation,http://www.
diabetes.
org/diabetes-basics/statistics.
3.
MenkeAetal.
,JAMA,2015;Narayanetal.
,JAMA,2003.
4.
WangJ,etal.
,PLoSOne,2015.
5.
DiabetesControlandComplicationsTrialResearchGroup,NEnglJMed,1993.
CO-721.
01.
52.
02.
53.
0CVdeathAll-causemortalityCVDiseaseLeadstoIncreasedMortalityinPeoplewithDiabetesMortalityriskassociatedwithdiabetes(n=820,900)11.
Seshasai,NEnglJMed,2011.
Non-diabeticpatientsHazardratio(95%CI)CO-73ImprovesglucosecontrolLowriskofhypoglycemiaNoweightgainProvidespositiveimpactonCVrisk,particularlyinolder,highriskindividualsNeedDiabetesTherapiestoAddressSpecificParametersandCVRiskCO-74LiraglutideReducedCVRiskonTopofStandardofCareRydénLetal.
,EurHeartJ,2013;FoxCSetal.
,DiabetesCare,2015;PiepoliMFetal.
,EurHeartJ,2016.
MultifactorialapproachPlateletinhibitionBloodpressurecontrolLifestylemodificationGlycemiccontrolManagementofdyslipidemiaTarget:IncludeweightTarget:Aspirinandotheranti-thromboticswithpriorCVeventTarget:HbA1cCO-75Statisticallysignificantresultonprimaryendpointof3-componentMACECVdeath,non-fatalMI,non-fatalstrokeEnhancingrobustnessofCVbenefitEachMACEcomponentHRCO-76HazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672TimetofirstMACE(Primaryanalysis)0.
87(0.
78,0.
97)608694TimetoCVdeath0.
78(0.
66,0.
93)219278Timetonon-fatalMI0.
88(0.
75,1.
03)281317Timetonon-fatalstroke0.
89(0.
72,1.
11)159177LEADER:ImprovingPatient-CenteredEndpointsOverMeaningfulTimeFrames0.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFullanalysissetCO-77ChangingParadigminDiabetesCareMedicationNNTPreventedAll-CauseDeathStatins(for5years)83Anti-hypertensives(for5years)125Aspirin(for2years)333NNT=numberneededtotreattopreventoneeventoveranintervaloftimeTheNNTGroup,2010-2017,http://www.
thennt.
comLiraglutide(for3years)98CO-78LonghistoryofuseExtensiveexposureLEADERresultsconsistentwithpreviousobservationsBlindedassessmentsObjectiveadjudicationofendpointsExpandedindicationtoincludeCVriskreductionwillincreasepatients'opportunitiesLEADERImplicationsforPatientCareCO-79Benefit-RiskConclusionAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-80LEADERCVOTResultsStrengthenFavorableBenefit-RiskofLiraglutideBenefitsReductioninMACEincludingCVdeathDecreaseinall-causemortalityDecreaseinHbA1cLoweruseofinsulinLowerrateofhypoglycemiaWeightlossSafetyinelderlyandchronickidneydiseaseandheartfailureRisksGItolerabilityGallbladdereventsResidualuncertaintiesMedullarythyroidcarcinomaPancreatitisPancreaticneoplasmsCO-81Victoza(liraglutide)InjectionEvaluationofCardiovascularOutcomeResultsfromLEADEREndocrinologicandMetabolicDrugsAdvisoryCommitteeJune20,2017NovoNordiskCO-82Back-upSlidesCO-83CO-84CO-85CO-86CO-87CO-88CO-89CO-90CO-91CO-92CO-93CO-94CO-95CO-96CO-97CO-98CO-99CO-100CO-101CO-102CO-103CO-104CO-105CO-106CO-107CO-108CO-109CO-110CO-111CO-112
73(0.
38,1.
41)NovoNordiskconductedalarge,randomizedCVOTwith3-componentMACEasprimaryendpoint:LEADERLiraglutideNDASubmittedPriorto2008FDAGuidanceRegardingCVSafetyCV=cardiovascular;T2DM=type2diabetesmellitus;HR=hazardratio;CVOT=cardiovascularoutcomestrial;MACE=majoradversecardiovascularevents1.
Marsoetal.
,DiabVascDisRes,2011.
CO-6EstablishCVsafety(event-driven)throughnon-inferiority≥611firstMACEinpatientswithT2DMandhighCVriskTestforCVbenefitthroughsuperiorityEstablishlong-termsafetybygatheringdataonmedicaleventsofspecialinterest(MESI)Treatmentperiodof≥42monthsLEADERFollowedPre-SpecifiedAnalysisPlanwith3GoalsCO-7DemonstratessuperiorityvsplaceboforreductioninMACEAchievedstatisticallysignificant13%reductioninMACEHR(95%CI)=0.
87(0.
78,0.
97)22%reductioninCVdeathReaffirmsoverallsafetyprofileReinforcesfavorablebenefit-riskLEADERSupportsCVBenefitforPatientswithT2DMandHighCVRiskCO-8Inpatientswithlong-standingsuboptimallycontrolledtype2diabetesandestablishedatheroscleroticcardiovasculardisease,empagliozinorliraglutideshouldbeconsideredastheyhavebeenshowntoreducecardiovascularandall-causemortalitywhenaddedtostandardofcare.
2017ADAStandardsofMedicalCareinDiabetesIncludesLiraglutideADA,DiabetesCare,2017.
CO-9CurrentapprovedindicationAsanadjuncttodietandexercisetoimproveglycemiccontrolinadultswithtype2diabetesmellitus.
ProposedindicationAsanadjuncttodietandexercisetoimproveglycemiccontrolinadultswithtype2diabetesmellitus.
Asanadjuncttostandardtreatmentofcardiovascularriskfactorstoreducetheriskofmajoradversecardiovascularevents(cardiovasculardeath,non-fatalmyocardialinfarction,ornon-fatalstroke)inadultswithtype2diabetesmellitusandhighcardiovascularrisk.
LEADERResultsSupportProposedAdditionalIndicationCO-10AgendaLEADERClinicalDesignSteveMarso,M.
D.
MedicalDirector,CardiovascularServicesHCAMidwestHealthHeartandVascularInstituteLEADERResultsAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskGeneralSafetyToddHobbs,M.
D.
USChiefMedicalOfficerNovoNordiskClinicalImplicationsJohnB.
Buse,M.
D.
,Ph.
D.
VerneS.
CavinessDistinguishedProfessorChief,DivisionofEndocrinologyUniversityofNorthCarolinaSchoolofMedicineBenefit-RiskConclusionAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-11AdditionalExpertsAnilK.
Rustgi,M.
D.
Chief,DivisionofGastroenterologyT.
GrierMillerProfessorofMedicineUniversityofPennsylvaniaJanetWittes,Ph.
D.
PresidentStatisticsCollaborative,IncCO-12LEADERClinicalDesignSteveMarso,M.
D.
MedicalDirector,CardiovascularServicesHCAMidwestHealthHeartandVascularInstituteCo-chair,LEADERSteeringCommitteeCO-13LEADER:Randomized,Double-Blind,Placebo-ControlledTrialLiraglutide1.
8mg/day+StandardofCare(N=4,668)Placebo+StandardofCare(N=4,672)Duration3.
5-5yearsPlaceboInjectionRun-in(2-3weeks)DetermineeffectofliraglutideonCVeventsinadultswithT2DMathighCVriskInitialdoseof0.
6mg/dayliraglutideorplaceboescalatedweeklytoreach1.
8mg/daytargetdoseperlabelTime(minimumdurationof42months)andevent(atleast611firstMACE)driventrialEffortsundertakentolimitmissingdataSafetyFollow-up(30days)CO-14LEADERIncludedRecommendedStandardofCareforPatientswithT2DMMultifactorialapproachPlateletinhibitionBloodpressurecontrolRydénLetal.
,EurHeartJ,2013;FoxCSetal.
,DiabetesCare,2015;PiepoliMFetal.
,EurHeartJ,2016.
LifestylemodificationGlycemiccontrolManagementofdyslipidemiaTarget:IncludeweightTarget:Aspirinandotheranti-thromboticswithpriorCVeventTarget:HbA1cCO-15T2DMwithHbA1c≥7.
0%HighCVriskpopulationLEADER:KeyInclusionCriteriatoEnrichPopulationforCVRiskAtleastone:PriorMI,StrokeorTIA,orarterialrevascularization>50%stenosisonangiographyHistoryofsymptomaticcoronaryheartdiseaseDocumentedasymptomaticcardiacischemiaChronicheartfailureNYHAclassII-IIIChronickidneydiseaseAndage≥50yearsAtleastone:MicroalbuminuriaormacroalbuminuriaHypertensionandleftventricularhypertrophyLeftventricularsystolicordiastolicdysfunctionAnkle/brachialindexCO-16Timetofirst3-componentMACECVdeathNon-fatalmyocardialinfarction(MI)Non-fatalstrokeFullanalysisset(FAS):Intention-to-treatAllrandomizedpatientsPrimaryEndpoint:MACECO-17ExpandedMACE3-componentMACEHospitalizationforheartfailureHospitalizationforunstableanginaCoronaryrevascularizationIndividualcomponentsofExpandedMACEAll-causemortalityOtherkeyendpointsMicrovascularevents,HbA1cKeySecondaryEndpointsCO-18PrimaryEndpointandTimetoEventAnalysisRandomizationDateNon-fatalMICVDeathTimeto1stMACE(primaryendpoint)TimetoCV-deathNon-fatalStrokeTimeto1stNon-fatalstrokeTimeto1stNon-fatalMICO-19CommitteeFunctionsSteeringCommittee(StC)DesignedprotocolProvidedscientificleadershipDataMonitoringCommittee(DMC)MonitoredpatientsafetyEvaluatedefficacyandsafetydataEventAdjudicationCommittee(EAC)PerformedongoingadjudicationofdeathsandpredefinedMESILEADER:StructureofTrialOversightCO-20ComprehensiveApproachtoIdentifyEventsforAdjudicationInvestigatorDirectreportingofdeathand/orMESIsLabMeasuresScreeningoflaboratorymeasurementsEAC/CROAdditionaleventsidentifiedinsourcedocumentsMedDRAScreeningofAEsnotreporteddirectlyforadjudicationbyinvestigatorIndependentContractResearchOrganization(CRO)ECGCentralreviewofECGsLabMeasuresScreeningoflaboratorymeasurementsEAC/CROAdditionaleventsidentifiedinsourcedocumentsMedDRAScreeningofAEsnotreporteddirectlyforadjudicationbyinvestigatorECGCentralreviewofECGsInvestigatorDirectreportingofdeathand/orMESIsCO-21ExternalEACCardiovascularsubcommitteeMicrovascularsubcommitteePancreatitissubcommitteeNeoplasmsubcommitteeDeath,ACS,CVA,CoronaryRevasc.
,HeartFailureNephropathyRetinopathyPancreatitisNeoplasmincludingThyroidExternal,Independent,BlindedEventAdjudicationCommittee(EAC)EventsACS=AcutecoronarysyndromeCVA=CerebrovascularaccidentCO-221.
Testofnon-inferiorityConfirmedifupperboundof95%CIConfirmedifupperboundof95%CICO-23PerProtocolPatientswhotook≥1dose;accumulateddaysofftreatmentCO-24PatientswhohadeitherPrimaryendpoint(MACE)Diedduetonon-CVcausesDirectcontactatlastplannedfollow-upCompleterDefinitionCO-25PatientDisposition:HighCompletionRateinLEADERFAS=fullanalysissetCompleter:apatientwhohad1.
aprimaryevent(MACE),or2.
diedduetonon-cardiovascularcauses,or3.
withwhomdirectcontactwasestablishedatoraftertheplannedfollow-upvisitRandomised(FAS)N=9340Placebo+StandardofCareN=4672Liraglutide+StandardofCareN=4668CompletedStudyN=4513(96.
6%)CompletedStudyN=4529(97.
0%)DidnotcompleteN=139(3.
0%)AliveN=127(2.
7%)VitalstatusunknownN=12(0.
3%)WithdrawnconsentN=4(0.
1%)Losttofollow-upN=8(0.
2%)DidnotcompleteN=159(3.
4%)AliveN=142(3.
0%)VitalstatusunknownN=17(0.
4%)WithdrawnconsentN=8(0.
2%)Losttofollow-upN=9(0.
2%)CO-26RelevanthighriskdiabetespopulationLongdurationoftrialStructuredexternaloversightExperiencedsteeringcommitteeIndependentdatamonitoringcommitteeSmallamountofmissingdataLEADER:RobustDesignandExecutionCO-27LEADERCVOTResultsAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-28EstablishedcardiovascularsafetyStatisticallysignificantreductioninMACEReductionsinexpandedMACEandall-causemortalityConsistentbenefitacrossallCVcomponentsBenefitsseenacrosssubgroupsSubstantialevidenceofliraglutidesuperiorityversusstandardofcareLEADERDemonstratedCVBenefitsinT2DMCO-29LiraglutideN=4668PlaceboN=4672Age64years64yearsSex,male65%64%RaceWhite78%78%BlackorAfricanAmerican8%9%Other15%14%BMI,kg/m232.
532.
5LEADER:BalancedBaselineDemographicsandCharacteristicsRegionNorthAmerica(UnitedStates+Canada)30%31%Europe35%36%Asia8%8%RestofWorld27%26%HbA1c(%)8.
78.
7Diabetesduration12.
8years12.
9yearsEstablishedCVD/CKD(inclusioncriterion3a)82%81%CO-30LEADER:BalancedBaselineCardiovascularProfile*ChronickidneydiseasedefinedaseGFRcoronary,carotidorperipheralarterialrevascularization39%39%>50%stenosisofcoronary,carotidorlowerextremityarteries25%26%Historyofsymptomaticcoronaryheartdisease9%9%Documentedasymptomaticcardiacischemia27%26%ChronicheartfailureNYHAIIorIII14%14%Chronickidneydisease*25%24%Microalbuminuriaormacroalbuminuria25%27%Hypertensionandleftventricularhypertrophy25%24%Leftventricularsystolicordiastolicdysfunction24%24%Ankle/brachialindexCO-31LEADER:BalancedDistributionofPatientsbyBaselineRenalFunction~2000patientswithmoderaterenalimpairment224patientswithsevererenalimpairmentLiraglutideN=4668PlaceboN=4672Normalrenalfunction(eGFR≥90)35%35%Mildimpairment(eGFR60-89)41%42%Moderateimpairment(eGFR30-59)21%20%Severeimpairment(eGFRCO-32ObservationTimeandTrialDrugExposureTimeFullanalysissetExposuretimetotrialdrugisdefinedasdurationoftreatmentwithinvestigationalproductintrialexcludingperiodsoff-treatment*Including30dayssafetyfollow-upafterendofrandomizedtreatmentperiod;**DuringtheobservationperiodLiraglutideN=4668PlaceboN=4672Observationtime(median)*3.
8years3.
8yearsExposuretimetotrialdrug(median)3.
5years3.
5yearsExposuretimetotrialdrug**%ofPatientsCO-33LiraglutideN=4668PlaceboN=4672n%n%MACE60813.
0%69414.
9%CVdeath1813.
9%2274.
9%Non-fatalMI2755.
9%3046.
5%Non-fatalstroke1523.
3%1633.
5%ReductioninFirstMACEWhenLiraglutideAddedtoStandardofCareFullanalysisset;EAC-confirmedevents;onlyfirsteventsbetweenrandomizationandfollow-upareincluded1302firstMACEeventsCO-34StatisticallySignificantReductioninTimetoFirstMACEFullanalysisset;Coxproportionalhazardsmodelwithtreatmentasfactor;1-sidedp-values0%5%10%15%20%061218243036424854PlaceboLiraglutideHR(95%CI)0.
87(0.
78,0.
97)p(non-inferiority)CO-35LiraglutidePlaceboN=4668N=4672608694219278281317159177TimetoFirstEventforIndividualComponentsofPrimaryEndpointHazardRatio(95%CI)TimetofirstMACE(Primaryanalysis)0.
87(0.
78,0.
97)TimetoCVdeath0.
78(0.
66,0.
93)Timetofirstnon-fatalMI0.
88(0.
75,1.
03)Timetofirstnon-fatalstroke0.
89(0.
72,1.
11)0.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboLiraglutidePlaceboN=4668N=4672608694219278281317159177FullanalysissetLiraglutidePlaceboN=4668N=4672608694219278281317159177CO-36Pre-specifiedSensitivityAnalysesSupportMACEPrimaryEndpointSensitivityAnalysisHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672MACE–primaryanalysis(FAS)0.
87(0.
78,0.
97)608694Perprotocol0.
86(0.
76,0.
97)493564Ontreatment0.
83(0.
73,0.
95)414482Ontreatment+30days0.
83(0.
73,0.
94)469549Excludingeventsafterendoftreatment0.
86(0.
77,0.
95)598690Adjustedforadditionalcovariates0.
87(0.
78,0.
97)605692Adjustedforrandomcountryeffect0.
86(0.
77,0.
96)605692Stratifiedforsevererenalimpairment0.
87(0.
78,0.
97)6086940.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFAS=fullanalysisset;Numberofpatientsinperprotocolanalysisis4657forliraglutideand4664forplaceboCO-37Losttofollow-upanalysis12missingliraglutidepatientsassumedtohaveCVdeath17missingplacebopatientsassumedaliveHR(95%CI)=0.
89(0.
79,0.
99)Non-completeranalysisTolosesuperioritywouldneedtoassume21/139non-completerliraglutidepatientsand0/159inplacebogrouphadnon-fatalMIorstrokeUnlikelyscenariosbasedonreportingofeventsTippingPointAnalysesSupportiveofPrimaryResultsCO-38SecondaryOutcomesCO-39BenefitinExpandedMACE(6Components)0%5%10%15%20%25%30%061218243036424854PlaceboLiraglutideTimefromrandomization(months)Fullanalysisset;totalof2010firstevents;1-sidedp-valueAtRisk(N)Liraglutide466845154356422140633914379336821452395Placebo467245064336415740023857369735811410366HR(95%CI)0.
88(0.
81,0.
96)p-value0.
005Patientswithanevent(%)CO-40219278281317159177122124405441218248CVdeath0.
78(0.
66,0.
93)Non-fatalMI0.
88(0.
75,1.
03)Non-fatalstroke0.
89(0.
72,1.
11)Unstableanginapectorisleadingtohospitalization0.
98(0.
76,1.
26)Coronaryrevascularization0.
91(0.
80,1.
04)Hospitalizationforheartfailure0.
87(0.
73,1.
05)TimetoFirstEventforIndividualComponentsofExpandedMACESupportsCompositeResultsLiraglutidePlaceboN=4668N=4672MACE–primaryanalysis0.
87(0.
78,0.
97)608694ExpandedMACE0.
88(0.
81,0.
96)94810620.
61.
3FavorsLiraglutideFavorsPlacebo1221244054412182481.
0Fullanalysisset219278281317159177HazardRatio(95%CI)122124405441218248219278281317159177CO-41FactorNHazardRatio(95%CI)MACE–primaryanalysis934013.
90.
87(0.
78,0.
97)SexFemale333711.
70.
88(0.
72,1.
08)Male600315.
20.
86(0.
75,0.
98)AgeConsistentEffectonTimetoFirstMACEAcrossSubgroups0.
00.
51.
01.
5FavorsLiraglutideFavorsPlacebo%withMACEFullanalysissetCO-42BaselinedemographicsDiseasecharacteristicsConcomitantCVordiabetesmedicationsHbA1cBodyweightSystolicbloodpressureCovariatesAssessedDidNotExplainDifferencesBetweenNorthAmericaandPrimaryAnalysisCO-43CVEffectinUSPatientsinLinewithOverallPopulationWhenonRandomizedTreatmentLiraglutidePlaceboN%N%MACE–primaryanalysis(FAS)0.
87(0.
78,0.
97)60813.
069414.
9USPopulation(FAS)12471001267100MACE1.
03(0.
84,1.
25)19615.
719315.
2On-treatment0.
89(0.
69,1.
14)1169.
31259.
9On-treatment+30days0.
89(0.
70,1.
12)13110.
514211.
20.
60.
81.
01.
21.
4FAS=FullanalysissetFavorsLiraglutideFavorsPlaceboHazardRatio(95%CI)CO-44FactorNHazardRatio(95%CI)MACE–primaryanalysis934013.
90.
87(0.
78,0.
97)BMI≤30kg/m2357414.
00.
96(0.
81,1.
15)>30kg/m2575713.
90.
82(0.
71,0.
94)HbA1c≤8.
3%476813.
00.
89(0.
76,1.
05)>8.
3%457214.
90.
84(0.
72,0.
98)Diabetesduration≤11years442913.
10.
82(0.
70,0.
97)>11years489214.
60.
90(0.
78,1.
04)RenalfunctioneGFRConsistentEffectonTimetoFirstMACEAcrossSubgroupsFavorsLiraglutideFavorsPlacebo%withMACEFullanalysisset;eGFR(mL/min/1.
73m2)aspermodificationofdietinrenaldiseaseformulaCO-45FavorableTrendinMACEinPatientsWithorWithoutPriorMIorStroke0510152025300102030405060Timefromrandomization(months)HR(95%CI)0.
89(0.
76,1.
05)0510152025300102030405060Timefromrandomization(months)HR(95%CI)0.
84(0.
72,0.
97)N=3692Patientswithanevent(%)PriorMI,StrokeN=5648NoPriorMI,StrokePlaceboLiraglutidePlaceboLiraglutideCO-46TreatmentEffectConsistentforAll-CauseDeathandCVDeathHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672All-causedeath0.
85(0.
74,0.
97)381447Cardiovasculardeath0.
78(0.
66,0.
93)219278Non-cardiovasculardeath0.
95(0.
77,1.
18)1621690.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFullanalysissetCO-47ReductioninAll-CauseDeath0%2%4%6%8%10%12%14%061218243036424854PlaceboLiraglutideTimefromrandomization(months)FullanalysissetHR(95%CI)0.
85(0.
74,0.
97)p-value0.
017Patientswithanevent(%)AtRisk(N)Liraglutide466846414599455845054445438243221723484Placebo467246484601454644794407433842681709465CO-480%1%2%3%4%5%6%061218243036424854PlaceboLiraglutideTimetoNon-CVDeathTimefromrandomization(months)FullanalysissetHR(95%CI)0.
95(0.
77,1.
18)p-value0.
66Patientswithanevent(%)AtRisk(N)Liraglutide466846414599455845054445438243221719484Placebo467246484601454644794407433842671708465CO-49OverviewofMicrovascularEvents:NephropathyandRetinopathyHazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672Microvascularcomposite0.
84(0.
73,0.
97)355416Nephropathycomposite0.
78(0.
67,0.
92)265337Newonsetofpersistentmacroalbuminuria0.
74(0.
60,0.
91)161215Persistentdoublingofcreatinine*0.
89(0.
67,1.
19)8797Continuousrenalreplacementtherapy0.
87(0.
61,1.
24)5664Deathduetorenaldisease1.
59(0.
52,4.
87)85Retinopathycomposite1.
15(0.
87,1.
52)10692Photocoagulation/intravitrealagent1.
16(0.
87,1.
55)10086Vitreoushemorrhage1.
45(0.
84,2.
50)32220.
52.
05.
01.
0FavorsLiraglutideFavorsPlacebo*andeGFR≤45mL/min/1.
73m2perMDRDFullanalysisset;OneeventofdiabetesrelatedblindnessreportedinplaceboCO-50RapidandSustainedHbA1cReductionforLiraglutidevsPlaceboWhenAddedtoStandardofCare5.
05.
56.
06.
57.
07.
58.
08.
59.
00612182430364248546066Timefromrandomization(months)EachVisit(N)Liraglutide466843554295413540343877381023498091013705Placebo46724355423540303905374236402303756873561Fullanalysisset;*Endoftreatment(EOT)maybeanytimefrommonth42onwardsEOT*EstimatedMeanHbA1c(%)Baseline8.
7%CO-51LiraglutidePatientsLessLikelytoInitiateNewDiabetesMedications0%10%20%30%40%50%01020304050PlaceboLiraglutideProportionofpatients(%)Timefromrandomization(months)HR(95%CI)0.
64(0.
60,0.
69)54AtRisk(N)Liraglutide46683991367833193037677294Placebo46723453295426302379497227Fullanalysisset;Timetofirstinitiationofinsulinoranyneworalanti-diabeticCO-52SuperiorityconfirmedfortimetofirstMACE(p=0.
005)SignificantlyreducedriskofMACEby13%ReducedriskofexpandedMACEby12%ConsistentreductionsinMACEacrosssensitivityanalysesConsistentreductionsacrossMACEcomponents,withpointestimatecomesforLiraglutideCO-53GeneralSafetyToddHobbs,M.
D.
USChiefMedicalOfficerNovoNordiskCO-54CardiovascularsafetyLong-termsafetyGatheradditionaldataonmedicaleventsofspecialinterest(MESI)LEADERDesignedwithTwoMajorSafetyAreasCO-55DatafromT2DMpatientswithhighcardiovascularriskreaffirmsexistingsafetyprofileClinicaldevelopmentprograms7yearspost-marketingexperienceLabeladequatelyaddressespotentialrisksLEADER5-YearDataReinforcesExistingLiraglutideSafetyProfileCO-56MedicalEventsofSpecialInterest(MESI)AdjudicationMedDRAsearchElectronicdatacaptureformsLaboratorymeasurementsAcutecoronarysyndromeCoronaryrevascularizationCerebrovasculareventsHospitalizationforheartfailureDeathRetinopathyNephropathyNeoplasms/ThyroidneoplasmsPancreatitisAcutegallbladderdiseaseThyroiddiseaseDiabeticfootulcersImmunogenicityeventsMedicationerrorsSeverehypoglycemiceventsAEsleadingtopermanenttreatmentdiscontinuationCalcitonin≥20ng/LAnti-liraglutideantibodiesAcutecoronarysyndromeCoronaryrevascularizationCerebrovasculareventsHospitalizationforheartfailureDeathRetinopathyNephropathyNeoplasms/ThyroidneoplasmsPancreatitisAcutegallbladderdiseaseThyroiddiseaseDiabeticfootulcersImmunogenicityeventsMedicationerrorsSeverehypoglycemiceventsAEsleadingtopermanenttreatmentdiscontinuationCO-57LEADER:AdverseEventsReportedin>2%PatientsLiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs62.
3%60.
8%Nausea3.
7%0.
9%Anginaunstable3.
4%3.
5%Acutemyocardialinfarction3.
3%4.
1%Cardiacfailure3.
0%3.
4%Cardiacfailurecongestive2.
9%3.
2%Pneumonia2.
9%3.
0%Diabeticfoot2.
8%3.
3%Coronaryrevascularization2.
7%3.
1%Coronaryarterialstentinsertion2.
5%2.
7%Acutekidneyinjury2.
4%2.
1%Hypoglycemia2.
3%2.
8%Anginapectoris2.
2%2.
2%CO-58LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs9.
6%7.
3%Serious4.
2%5.
3%Severe3.
6%4.
0%LEADER:AdverseEventsLeadingtoPermanentTreatmentDiscontinuationCO-59LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs9.
6%7.
3%Nausea1.
6%0.
4%Vomiting0.
7%comfort0.
2%0Abdominalpainupper0.
2%congestive0.
2%0.
1%Chronickidneydisease0.
2%0.
2%LEADER:MostCommonAdverseEventsLeadingtoPermanentDiscontinuation≥0.
2%PatientsCO-60AcutegallbladderdiseasePancreatitisNeoplasmsHypoglycemiaAdditionalSafetyAreasofInterestCO-61LiraglutideN=4668PlaceboN=4672SAEsornon-seriousMESIs3.
1%1.
9%Cholelithiasis1.
5%1.
1%Cholecystitisacute0.
8%0.
4%Cholecystitis0.
3%0.
3%Cholecystitischronic0.
2%0.
1%Biliarycolic0.
2%CO-62LEADER:NoIncreasedOccurrenceofPancreatitiswithLiraglutide*SeveritybymodifiedAtlantaCriteriaLiraglutideN=4668PlaceboN=4672Acutepancreatitis0.
4%0.
5%Mild*0.
3%0.
4%Moderately-severe*00.
1%Severe*CO-63LiraglutidePlaceboHazardRatio(95%CI)N%N%Totalneoplasms47010.
14199.
01.
12(0.
99,1.
28)Benign1683.
61453.
11.
16(0.
93,1.
44)Malignant2966.
32796.
01.
06(0.
90,1.
25)MalignantneoplasmsbytissuetypeSkin(non-melanoma)781.
7621.
31.
25(0.
90,1.
75)Prostate260.
9471.
60.
54(0.
34,0.
88)Breast211.
3201.
21.
06(0.
57,1.
96)Lung/bronchus280.
6330.
70.
85(0.
51,1.
40)Colorectal280.
6280.
60.
99(0.
59,1.
68)Other130.
3140.
30.
92(0.
43,1.
97)Urinarybladder150.
3120.
31.
24(0.
58,2.
66)Kidney/renalpelvis170.
490.
21.
88(0.
84,4.
22)Hepatic/biliary130.
380.
21.
62(0.
67,3.
90)Leukemias50.
1140.
30.
36(0.
13,0.
99)Pancreatic130.
350.
12.
59(0.
92,7.
27)Melanomaoftheskin130.
350.
12.
59(0.
92,7.
27)Cervical/vaginal60.
420.
13.
03(0.
61,15.
0)Lymphoma80.
260.
11.
33(0.
46,3.
82)Oralcavity/pharynx70.
160.
11.
16(0.
39,3.
46)Uterine20.
130.
20.
68(0.
11,4.
05)Oesophageal40.
160.
10.
66(0.
19,2.
34)Gastric40.
150.
10.
80(0.
21,2.
97)Thyroid50.
130.
11.
66(0.
40,6.
95)Bone/softtissue20.
050.
10.
40(0.
08,2.
05)Ovarian10.
120.
10.
51(0.
05,5.
58)LEADER:IncidencesofNeoplasmsSimilarBetweenLiraglutideandPlacebo0.
00.
21.
05.
025.
0FavorsLiraglutideFavorsPlacebo0.
04CO-64LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LEADER:EACConfirmedMalignantPancreaticNeoplasmsLiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311LiraglutideN=4668PlaceboN=4672AllStageIVAllStageIVN=13N=7N=5N=2Timetoonset0to18months844118to60months5311CO-65AdditionalDataSourcesShowNoIncreaseinRiskofPancreaticNeoplasmswithLiraglutideClinicaldevelopmentprogramsType2diabetesWeightmanagementPost-marketingdata>6millionpatient-yearsofexposureClaimsdatabasestudy(OPTUM)Non-clinicalstudiesshownoincreasedriskConsistentwith2014FDAandEMAjointstatementEMA=EuropeanMedicinesAgencyCO-66LEADER:NoEvidenceofMalignantThyroidNeoplasmRiskwithLiraglutideNocasesofMedullaryThyroidCarcinoma(MTC)inliraglutideexposedpopulationinLEADER1MTCinplacebogroupMonitoringforMTCthroughongoingUSregistryIncludesallapprovedlong-actingGLP-1receptoragonistsLiraglutideN=4668PlaceboN=4672n%n%Malignantthyroidneoplasms50.
1%30.
1%CO-67LEADER:LessHypoglycemiaWhenLiraglutideAddedtoStandardofCareConfirmedhypoglycemiaisdefinedasseverehypoglycemiaaccordingtotheADAdefinitionand/orplasmaglucoseConfirmedhypoglycemia43.
7%45.
6%Severehypoglycemia2.
4%3.
3%CO-680102030405060700102030405060PlaceboLiraglutideLEADER:LowerRiskofSevereHypoglycemiainPatientsTreatedWithLiraglutideMeannumberofepisodesper1000patientsTimefromrandomization(months)Fullanalysisset;RR=rateratioRR(95%CI)0.
69(0.
51,0.
93)CO-69SafetyprofileofliraglutideconfirmedGallbladderdisorderscurrentlyaddressedinliraglutidelabelSimilarfrequencyofacutepancreatitiswithliraglutideandplaceboSimilarriskforneoplasmswithliraglutideandplaceboLEADER:LiraglutideSafetyAlignswithLabelandPost-MarketingExperienceCO-70ClinicalImplicationsJohnB.
Buse,M.
D.
,Ph.
D.
VerneS.
CavinessDistinguishedProfessorChief,DivisionofEndocrinologyUniversityofNorthCarolinaSchoolofMedicineCO-71T2DMprevalencedoubledoverlast20years129millionaffectedinUS215%adults,26%≥65yrs,1~30%lifetimerisk3Hypoglycemiaisamajorissueindiabetescare4Glycemiccontrolreducesmicrovascularcomplications5CVeventrates~2foldhigherinpatientswithdiabetesthaninthosewithout2Type2DiabetesMellitus:MajorMedicalandSocietalChallenge1.
NCDRiskFactorCollaboration(NCD-RisC)JAMA,2016.
2.
AmericanDiabetesAssociation,http://www.
diabetes.
org/diabetes-basics/statistics.
3.
MenkeAetal.
,JAMA,2015;Narayanetal.
,JAMA,2003.
4.
WangJ,etal.
,PLoSOne,2015.
5.
DiabetesControlandComplicationsTrialResearchGroup,NEnglJMed,1993.
CO-721.
01.
52.
02.
53.
0CVdeathAll-causemortalityCVDiseaseLeadstoIncreasedMortalityinPeoplewithDiabetesMortalityriskassociatedwithdiabetes(n=820,900)11.
Seshasai,NEnglJMed,2011.
Non-diabeticpatientsHazardratio(95%CI)CO-73ImprovesglucosecontrolLowriskofhypoglycemiaNoweightgainProvidespositiveimpactonCVrisk,particularlyinolder,highriskindividualsNeedDiabetesTherapiestoAddressSpecificParametersandCVRiskCO-74LiraglutideReducedCVRiskonTopofStandardofCareRydénLetal.
,EurHeartJ,2013;FoxCSetal.
,DiabetesCare,2015;PiepoliMFetal.
,EurHeartJ,2016.
MultifactorialapproachPlateletinhibitionBloodpressurecontrolLifestylemodificationGlycemiccontrolManagementofdyslipidemiaTarget:IncludeweightTarget:Aspirinandotheranti-thromboticswithpriorCVeventTarget:HbA1cCO-75Statisticallysignificantresultonprimaryendpointof3-componentMACECVdeath,non-fatalMI,non-fatalstrokeEnhancingrobustnessofCVbenefitEachMACEcomponentHRCO-76HazardRatio(95%CI)LiraglutidePlaceboN=4668N=4672TimetofirstMACE(Primaryanalysis)0.
87(0.
78,0.
97)608694TimetoCVdeath0.
78(0.
66,0.
93)219278Timetonon-fatalMI0.
88(0.
75,1.
03)281317Timetonon-fatalstroke0.
89(0.
72,1.
11)159177LEADER:ImprovingPatient-CenteredEndpointsOverMeaningfulTimeFrames0.
60.
81.
01.
2FavorsLiraglutideFavorsPlaceboFullanalysissetCO-77ChangingParadigminDiabetesCareMedicationNNTPreventedAll-CauseDeathStatins(for5years)83Anti-hypertensives(for5years)125Aspirin(for2years)333NNT=numberneededtotreattopreventoneeventoveranintervaloftimeTheNNTGroup,2010-2017,http://www.
thennt.
comLiraglutide(for3years)98CO-78LonghistoryofuseExtensiveexposureLEADERresultsconsistentwithpreviousobservationsBlindedassessmentsObjectiveadjudicationofendpointsExpandedindicationtoincludeCVriskreductionwillincreasepatients'opportunitiesLEADERImplicationsforPatientCareCO-79Benefit-RiskConclusionAlanMoses,M.
D.
GlobalChiefMedicalOfficerNovoNordiskCO-80LEADERCVOTResultsStrengthenFavorableBenefit-RiskofLiraglutideBenefitsReductioninMACEincludingCVdeathDecreaseinall-causemortalityDecreaseinHbA1cLoweruseofinsulinLowerrateofhypoglycemiaWeightlossSafetyinelderlyandchronickidneydiseaseandheartfailureRisksGItolerabilityGallbladdereventsResidualuncertaintiesMedullarythyroidcarcinomaPancreatitisPancreaticneoplasmsCO-81Victoza(liraglutide)InjectionEvaluationofCardiovascularOutcomeResultsfromLEADEREndocrinologicandMetabolicDrugsAdvisoryCommitteeJune20,2017NovoNordiskCO-82Back-upSlidesCO-83CO-84CO-85CO-86CO-87CO-88CO-89CO-90CO-91CO-92CO-93CO-94CO-95CO-96CO-97CO-98CO-99CO-100CO-101CO-102CO-103CO-104CO-105CO-106CO-107CO-108CO-109CO-110CO-111CO-112
buyvm美国大硬盘VPS,1Gbps带宽不限流量
buyvm正式对外开卖第四个数据中心“迈阿密”的块存储服务,和前面拉斯维加斯、纽约、卢森堡一样,依旧是每256G硬盘仅需1.25美元/月,最大支持10T硬盘。配合buyvm自己的VPS,1Gbps带宽、不限流量,在vps上挂载块存储之后就可以用来做数据备份、文件下载、刷BT等一系列工作。官方网站:https://buyvm.net支持信用卡、PayPal、支付宝付款,支付宝付款用的是加元汇率,貌似...
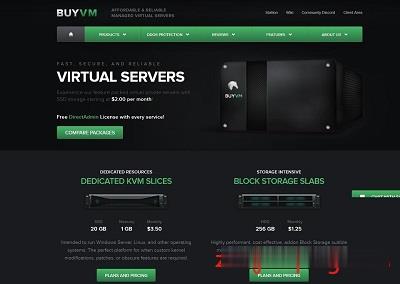
OneTechCloud(31元),美国CN2 GIA高防VPS月
OneTechCloud发布了本月促销信息,全场VPS主机月付9折,季付8折,优惠后香港VPS月付25.2元起,美国CN2 GIA线路高防VPS月付31.5元起。这是一家2019年成立的国人主机商,提供VPS主机和独立服务器租用,产品数据中心包括美国洛杉矶和中国香港,Cera的机器,VPS基于KVM架构,采用SSD硬盘,其中美国洛杉矶回程CN2 GIA,可选高防。下面列出部分套餐配置信息。美国CN...

SugarHosts新增Windows云服务器sugarhosts六折无限流量云服务器六折优惠
SugarHosts糖果主机商我们较早的站长们肯定是熟悉的,早年是提供虚拟主机起家的,如今一直还在提供虚拟主机,后来也有增加云服务器、独立服务器等。数据中心涵盖美国、德国、香港等。我们要知道大部分的海外主机商都只提供Linux系统云服务器。今天,糖果主机有新增SugarHosts夏季六折的优惠,以及新品Windows云服务器/云VPS上线。SugarHosts Windows系统云服务器有区分限制...
co为你推荐
-
中国电信互联星空中国电信宽带于互联星空的区别安卓应用平台哪个手机应用平台的软件比较正版,安全?彩信中心短信中心的号码是多少开机滚动条开机滚动条要很长时间怎么解决?神雕侠侣礼包大全神雕侠侣手游每天送的元宝买什么合适保护气球气球保护液可以用什么来代替?srv记录SRV记录的定义blogcn哪种博客更好...sina.baidu.blogcn还是.............?网站推广外链网站推广,免费的超级外链有用吗?seo还应该做什么厦门铁通厦门铁通固定电话的收费标准?