proportionalTo do List
Gas Laws Practice Test
Solutio n Set
1. A Which ofthe following conditions would cause a gas to deviate most from idealbehavior?
A) -200oC and 50 atm; B)STP; C)200 K and 500 psi; D)2000oC and 500 mmHg.
(low T&high P)
2. F Which ofthe following gases would have the greatest average kinetic energy,assuming that allgases are at the same average temperature?
A)helium; B)xenon; C)carbon dioxide; D)ammonia; E)carbon monoxide;
F)all gases have the same average kinetic energy at a given temperature.
3. E Assume that you have 2.0 moles of gas at a certain temperature,pressure,and volume. If both thepressure and absolute temperature are tripled,what will be the final volume?
A)nine times as great as the initial volume; B)nine times less than the initial voume;
C) three times as great as the initial volume; D) three times less than the initial voume;
E) there will be no change in volume; F)final volume will be greater,but not nine times greater.
4. C One way to increase the average speed of the molecules ofan ideal gas is to:
A)decrease the temperature; B)expand the gas against a piston; C) increase the pressureby increasing the number of molecules in a confined volume(a container that has a fixed volume);
D)expanding the gas into a vacuum.
5. A Density is a characteristic property for any gas,but the temperature and pressure must also bespecified because:
A)density increases with increasing pressure at a given temperature;
B)density decrease s with increasing pre ssure at a given temp erature;
C)density increases with increasing temperature at a given pre ssure;
D)density increases with decreasing pres sure and increased temperature.
6. E Which ofthe following would cause an increase inthe pressure ofa gaseous system?
A)container is made larger,with temperature and moles constant;
B)additional amount of the gas is added,with volume and temperature kept constant;
C) temperature is decreased,with moles and volume constant;
D)another gas is added to the system,with volume and temperature kept constant;
E)both B and D would increase pressure.
7. C What is the relationship ofthe mass ofa gaseous molecule compared to its velocity,assuming thattemperature,pressure,and volume are kept constant?
A) inversely proportional; B)directly proportional; C) inversely proportional to the squareroot; D)directly proportional to the square of the velocity.
8. E At extemely highpressures,which of the following molecules would be expected to show themost deviation from ideal behavior?
A)neo n; B)hydro ge n; C)argo n; D)o xyge n; E)d initro ge n pe nto xide;
F)all would deviate equally.
-2-
9. B What is the molecular weight ofa gas if 0.3350 grams in a 30.0 ml container exerts a pressure of
20.3 psi at a temperature of-20.0?
A)88.6; B) 168; C) 127; D)32.7; E)355; F)22.9
MW=gRT/PV=[(0.3350 g)(0.0821)(253 K)] / [(20.3 psi/14.7 psi)(0.0300 liters)]
MW=167.96g/mol
10. B How many seconds will it take for 750 ml of xenon gas to effuse from an effusion chamber if ittakes 23.3 seconds for 750 ml of nitrogen gas to effuse?
A)71.4; B)50.5; C) 109; D) 10.8; E)4.69; F)23.3
=> Time Xen on/23.3 = 131.3/28.0
TimeXenon=50.5 sec.
11. E A sample of gas is put into a container that has a movable piston. Initially the volume is 4.00 literswhenthe pressure is 60.0 cm Hg and the temperature is 50.0the pressure is tripled(up to 180.0 cm Hg)and the temperature is also tripled(up to 150.0)?
A)0.444; B)36.0; C)4.00; D)8.85; E) 1.75; F)2.85
V1 P1 /T1 =V2 P2 /T2
V2 = [(4.00 liters)(60.0 cm)/ (323 K) ] / [(423 K)/(180.0 cm)] = 1.75 liters
12. A A three-gas system composed of neon,helium,and oxygen gas exerts a total pressure of 990.0 torr.Ifthe combined number of moles ofboth helium and neon is 10.0 moles,and the mass ofoxygenis 160.0 grams,what is the partialpressure exerted bythe oxygen gas?
A)330; B) 155; C) 165; D)660; E)495; F)990noxygen=160.0 g/32.0 g/mol=5.00 moles
Poxygen= 990.0 torr(5.00 mol/15.0 mol)=330.0 torr
-3-
13. C A student uses a manometer to determine the pressure of a confined gas in the lab. The barometershows that the atmosphe ric pressure is 28.80 inches Hg. The height ofthe mercury at the closedend of the manometer is 8.85 inches lower than the height of mercury at the open end.What isthe pressure of the confined gas (in atmospheres)?
A)597; B)225; C) 1.26; D)0.667; E) 1.33; F)0.963
Pgas=Patm+Ph=28.80 in.Hg+8.85 in.Hg =37.65 in.Hg = 1.26 atm.
14. D What is the density of bromine vapor(in grams/liter)at 775?
A)70.1; B)0.0556; C)3.38; D) 1.38; E)2.99; F) 1.72.
D= g/V=MWx P/RT
D= [(159.8 g/mol)(75.5 kPa/101.3 kPa)] / [(0.0821)(1048 K)]
D= 1.38 g/lite r
15. D 775 ml ofa gas is collected over water at a temperature of 23.0pressure of 12.20 psi.What would be the volume(in ml)of the dry gas at STP?
(Equilibrium vapor pressure ofwater vapor at 23.0.)
A)682; B)415; C)521; D)563; E)329; F)619.
V1 P1 /T1 =V2 P2 /T2
V2 = [(775 ml)(12.20 psi-0.62 psi)/(296 K) ] / [(273 K)/ (14.7 psi)] = 563 ml
16. B What is the density(in grams per liter)of sulfur dichloride vapor at STP conditions?
A) 1.24; B)4.60; C)22.4; D) 103.1; E)3.84; F)0.896
103.1 g/mol x 1 mol /22.4 liters = 4.60 g/liter
17. 82.8% A student reacts 1.50 moles of solid aluminum metal with an excess of 6.0 M sulfuric acid at
55.
What is the percent yield of this reaction? The aqueous vapor pressure at 55atmospheres. The other product is aluminum sulfate,but you knew that!
2 Al(s) +3 H2SO4(aq) --> 3 H2(g) + Al2(SO4)3(s)
1.50 mol Al x[3 mol H2 /2 mol Al ]=2.25 moles H2
V=nRT/P = [(2.25 mol)(0.0821)(328 K)] / [(0.445-0.045 atm)]V= 151 liters (theoretically) %yield=125/151 x 100=82.8%
- proportionalTo do List相关文档
- reflowTO DO List - VILLA MARTELLI
- saltTo do List
- blendedTo do list - Wikispaces
- formationHow do you go to the black head to shrink the pores and make a list of the products
- tickHAPPY TO DO LIST - Southern Area Hospice
- 的人【海归找工作】零经验小白想要进顶尖咨询公司?完整版to do list在这里
Vultr新用户省钱福利,最新可用优惠码/优惠券更新
如今我们无论线上还是线下选择商品的时候是不是习惯问问是不是有优惠活动,如果有的话会加速购买欲望。同样的,如果我们有准备选择Vultr商家云服务器的时候,也会问问是不是有Vultr优惠码或者优惠券这类。确实,目前Vultr商家有一些时候会有针对新注册用户赠送一定的优惠券活动。那就定期抽点时间在这篇文章中专门整理最新可用Vultr优惠码和商家促销活动。不过需要令我们老用户失望的,至少近五年我们看到Vu...

raksmart:全新cloud云服务器系列测评,告诉你raksmart新产品效果好不好
2021年6月底,raksmart开发出来的新产品“cloud-云服务器”正式上线对外售卖,当前只有美国硅谷机房(或许以后会有其他数据中心加入)可供选择。或许你会问raksmart云服务器怎么样啊、raksm云服务器好不好、网络速度快不好之类的废话(不实测的话),本着主机测评趟雷、大家受益的原则,先开一个给大家测评一下!官方网站:https://www.raksmart.com云服务器的说明:底层...
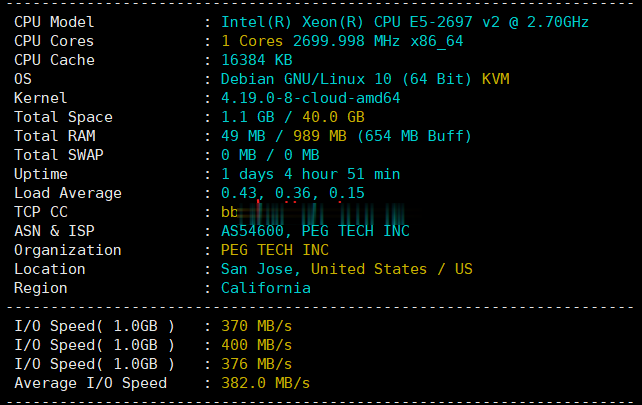
inux国外美老牌PhotonVPS月$2.5 ,Linux系统首月半价
PhotonVPS 服务商我们是不是已经很久没有见过?曾经也是相当的火爆的,我们中文习惯称作为饭桶VPS主机商。翻看之前的文章,在2015年之前也有较多商家的活动分享的,这几年由于服务商太多,乃至于有一些老牌的服务商都逐渐淡忘。这不有看到PhotonVPS商家发布促销活动。PhotonVPS 商家七月份推出首月半价Linux系统VPS主机,首月低至2.5美元,有洛杉矶、达拉斯、阿什本机房,除提供普...

-
可爱桌面背景图片卡通壁纸谁有聚酯纤维和棉哪个好聚酯纤维和纯棉的相比,哪个好?杀毒软件哪个好杀毒软件什么最好免费阅读小说app哪个好有什么免费读小说的软件?录音软件哪个好有什么录音软件好用??宝来和朗逸哪个好大众朗逸好还是宝来好ps软件哪个好怎么ps啊,哪个软件好oppo和vivo哪个好买oppo手机好还是vivo的好?红茶和绿茶哪个好红茶和绿茶哪个比较好?辽宁联通网上营业厅中国移动辽宁营业厅