1.5nnt
nnt 时间:2021-01-11 阅读:()
ORIGINALRESEARCHReassessingtheSafetyProleofLesinuradinCombinationwithXanthineOxidaseInhibitorTherapyFernandoPerez-Ruiz.
TimL.
Jansen.
Anne-KathrinTausche.
PascalRichette.
FredericLiote.
AlexanderK.
So.
AustinStackReceived:December15,2018/Publishedonline:February14,2019TheAuthor(s)2019ABSTRACTIntroduction:Therateofadverserenaleventshasbeenshowntobehigherinpatientstreatedwithlesinuradplusaxanthine-oxidaseinhi-bitor(XOI)thaninpatientstreatedonlywithaXOI.
Wereassessedtherisksforvariousadverserenaleventsfromadifferentperspectiveanddevisedahypothesistoexplaintheresults.
Methods:Weuseddatafromphase3trialsthatwerepubliclyavailablefromthefullprescribinginformationdocumentandestimatedtherela-tiveriskandthenumberneededtotreatforincreasedserumcreatinine(sCri),renalfailure,andrenallithiasis.
Weexaminedtheserisksforeachtreatmentgroupandtherisksstratiedbyestimatedglomerularltrationrate(eGFR).
Results:Overall,therelativeriskforsCriwas[1.
0withthe400mg/daydoseoflesinuradandhigherwiththe200mg/daydose,butitwas\1.
0forbothlithiasisandrenalfailurewiththe200mg/daydose.
TherelativeriskwasonlystatisticallysignicantforsCriwiththehighestdoseoflesinurad.
WhenresultsstratiedbyeGFRwereconsidered,theratesofadverseeventsincreasedwithdecliningrenalfunction,buttherelativerisksdecreasedinparallel,astherateofadverseeventsincreasedmuchmoreintheplaceboarmthanintheactivearm(200mg/daydose).
Indeed,therelativeriskwasonlysignicantforthehighestdoseoflesinuradinpatientswithnormaleGFR.
Conclusion:TherateofsCrieventswashigherinpatientstreatedwithbothlesinuradandaXOIEnhancedDigitalFeaturesToviewenhanceddigitalfeaturesforthisarticlegotohttps://doi.
org/10.
6084/m9.
gshare.
7637195.
F.
Perez-Ruiz(&)RheumatologyDivision,CrucesUniversityHospital,BiocrucesBizkaiaHealthResearchInstituteandMedicineDepartment,MedicineandNurserySchool,UniversityoftheBasqueCountry,Bilbao,Vizcaya,Spaine-mail:fernando.
perezruiz@osakidetza.
eusT.
L.
JansenDepartmentofRheumatology,VieCuriMC,Teglseweg210,9012BLVenlo,TheNetherlandsA.
-K.
TauscheDepartmentofRheumatology,UniversityClinic''CarlGustavCarus''attheTechnicalUniversity,Dressden,GermanyP.
RichetteF.
LioteRheumatologyDepartmentandInsermURM1132,CentreViggoPetersen,HopitalLariboisie`re(AP-HP)andUniversiteParisDiderot,USPC,Paris,FranceA.
K.
SoUniversityofLausanne,Lausanne,SwitzerlandA.
StackNephrologyDivision,UniversityHospitalLimerick,GraduateEntryMedicalSchool,HealthResearchInstitute,UniversityofLimerick,Limerick,IrelandRheumatolTher(2019)6:101–108https://doi.
org/10.
1007/s40744-019-0143-9ratherthanaXOIalone.
ThisratewasfoundtoincreasewithdecreasingeGFR,butasitdoesinforbothactiveandplaceboarmstherelativeriskisnotdifferentfromthatobservedintheplaceboarmsinthelabeled200mg/daydose.
Thismaybeexplainedbypathophysiologicalchangesthatdevelopinchronickidneydisease.
Keywords:Creatinineincrease;Gout;Lesinurad;Safety;Relativerisk;Renalfailure;RenallithiasisINTRODUCTIONLesinuradisaselectiveuricacidreabsorptioninhibitor(SURI)medicationthatwasrecentlyapprovedbytheEuropeanCommissionfol-lowingapositiveevaluationfromtheEuropeanMedicinesAgency(EMA)oftheEuropeanUnionandtheUnitedStatesFoodandDrugAdministration(FDA)[1].
Lesinuradexertsitsurate-loweringactionbyselectivelyinhibitinguratetransporters(principallyURAT-1andOAT-4)intheproximalrenaltubuleandblockinguricacidreabsorption,therebyincreasingneturicacidrenalexcretion[2].
Thesafetyprolepanelestablishedduringtheclinicaldevelopmentoflesinuradincludedrenalevents,aswouldbeexpectedforanyuri-cosuricmedication.
Renaleventswereclassiedasincreasesinserumcreatinine(sCr)concen-trationsabovebaseline,renalfailure(whichencompassedabroadrangeofevents,includingacuteorchronicrenalfailureandacutepre-re-nalfailure),ornephrolithiasis.
Renalevents,andspecicallyincreasesinsCrconcentration,weremorefrequentinpatientsonlesinuradthaninthoseonaplacebo(PBO),withadose-dependentrelationship[3],andtheseeventsweremorecommoninpatientswhodidnotreceiveaxanthineoxidaseinhibitor(XOI)whileonlesinuradtherapy[4]andinthosewithmoderatekidneyimpairment[5].
Thesend-ingsledtheEMAandFDAtoapprovelesinuradonlywhenusedincombinationwithaXOIandatadoseof200mg/day,andtorecommendedthatitshould/mustnotbeinitiatedinpatientswithanestimatedglomerularltrationrate(eGFR)below45mL/min/1.
73m2(summaryofproductcharacteristicsorSmPC,FDA)[6]or30mL/min/1.
73m2(SmPC,EU)[7]perminute.
Intriguinglyandimportantly,eventhoughelevationsinsCRweremorecommoninlesinu-rad-treatedpatientsthaninthosereceivingaplacebo,theincidenceratesofrenalfailureandrenalnephrolithiasisdidnotdifferbetweenthesegroups,althoughpatientswithaprevioushistoryofrenallithiasiswerenotexcluded[8].
WhileseveralhypotheseshavebeenproposedforthedevelopmentofelevatedsCRinlesinurad-treatedpatients,thereisnowidelyacceptedexplanationforthisoccurrence.
Consequently,recommen-dationsarelackinginriskassessmentsofpatientswhomaybeatriskofadverserenaleventswhentreatedwithlesinuradcombinationtherapy.
Ourobjectivewastoreassesstherelativerisksofvariousadverserenaleventsinlesinu-rad-treatedpatientsfromthreepivotalplacebo-controlledcombinationclinicaltrialsstratiedaccordingtorenalfunction;toreview,critique,andattempttoexplaintheresults;andtopro-posemonitoringstrategies.
METHODSWeanalyzeddatafromthreemajorphase3clinicaltrialsthattestedtheefcacyandsafetyoflesinuradinclinicalpractice.
IncidenceratesofadverserenaleventswerederivedfromTables2and3inthefullprescribinginforma-tionforZurampic[6].
Table2oftheprescribinginformationshowsdataontheincidenceratesofadverserenaleventsinallthreemajorplacebo-controlledclinicalstudies(CLEAR1,CLEAR2,andCRYSTAL)inwhichlesinuradincombinationwithaXOI(ei-therallopurinolorfebuxostat)wascomparedwithXOIalone[9–11].
Adverserenaleventsweredenedasfollows:(1)increasedbloodcreatinine,denedasagreaterthan1.
5-foldincreaseinsCrascomparedtothebaselinemeasurementattrialentry;(2)renalfailure(acompositetermthatdescribedanyoccurrenceofacuteorchronicrenalfailureandacuteprerenalfailure);and(3)nephrolithiasis.
Thethreestudiestogetherpro-vided516,511,and510patientsintheXOIPBO,XOIlesinurad200mg/day,andXOIlesinurad400mg/daygroups,respectively.
102RheumatolTher(2019)6:101–108Table3oftheSmPCforZurampicincludesdataontheoccurrenceofadverseeventsinallthreeclinicaltrialsaccordingtostudyarm(XOIPBO,n=510;XOIlesinurad200mg/day,n=509;andXOIlesinurad400mg/day,n=508)andstratiedbybaselinerenalfunctioncategory(C90,C60to\90,C30to\60mL/min/1.
73m2).
StatisticalAnalysisWedeterminedthepatient-yearexposureforeachstudyarmfromarecentlypublishedreportonthesafetyoflesinurad[3]as408,396,and390patient-years,respectively.
ThesevalueswerethenusedtocomputetheincidencerateofeachadverserenaleventstratiedbyGFRcategory(C90mL/min,C60to\90mL/min,C30to\60mL/min/1.
73m2)ineachtreatmentarm.
Riskratios(RRs)andnumbersneededtotreat(NNTs)werecalculatedtodeterminetheimpactofeachtreatmentontheratesofeachspeciedadverserenalevent.
HarmwasdenedasRR[1andbenetasRR\1.
Calculationswereper-formedusingtheMedCalcsoftware(MedcalcSoftwarebvba,Ostend,Belgium,https://www.
medcalc.
org/calc/relative_risk.
php).
TheseanalyseswererepeatedtodeterminetheRRsofadverserenaleventsstratiedbykidneyfunc-tion.
WhentheRRforastudycannotbecal-culatedbecausetherearenoeventsinthecontrolgroupofastudyandthestandarderrorscannotbecomputed,itiscustomarytoadd0.
5toeachcellofthe292contingencytable[12].
CompliancewithEthicsGuidelinesDatawereretrievedfromapublicresource:thefullprescribinginformationforZurampic.
Thisarticledoesnotcontainanystudieswithhumanparticipantsoranimalsperformedbyanyoftheauthors.
RESULTSTheincidenceofincreasedsCrwasnumericallyhigheramongpatientsallocatedtoLES200mgdailythanamongpatientsallocatedtoaplacebo(4.
3%vs.
2.
3%respectively).
Thecor-respondingRR(1.
85,0.
93–3.
70)wasnotstatis-ticallysignicant(Table1).
Incontrast,patientsallocatedtotheLES400mgdoseshowedasig-nicantlyhigherincidenceofincreasedsCRthanpatientsonaplacebo,alongwithastatis-ticallysignicantRRof3.
37(1.
79–6.
35)(Table1).
Ontheotherhand,theincidenceratesforrenalfailureandnephrolithiasiswerenumeri-callylowerforthe200mgdailydosevs.
PBO,butthiswasnotastatisticallysignicantdif-ference.
Similarly,patientsallocatedtotheLES400mgdoseexperiencedratesofrenalfailureandnephrolithiasisthatwerenotsignicantlydifferentfromthoseintheplaceboarm(Table1).
TheanalysisofadverserenaleventsaccordingtoeGFRcategoryispresentedinTable2.
ForeachGFRcategory,therateofincreasedsCRwasnumericallyhigherfortheLES200mggroupthanfortheplaceboarm(3.
0,3.
8,and6.
9vs.
0.
6,1.
7,and5.
9forpatientsinthecategoriesCrClC90,CrClC60toB90,andCrClC30toB60mL/min,respectively)buttherewerenostatisticallysignicantdif-ferences.
Intriguingly,theRRofincreasedsCRdecreasedwithworseningGFR,from5.
34forpatientswithCrClC90mL/minto1.
17forpatientswithCrClC30toB60mL/min;thenumberneededtotreatwasover100forthelattergroup.
Furthermore,analysisoftheRRofrenalfailurerevealedasimilarpattern,withtheRRdecreasingfrom6.
30inpatientswithCrClC90mL/minto0.
40forpatientswithCrClC30toB60mL/min.
TheRRofrenalfailurefortheLES200mggroupversustheplacebogroupwasnotstatisticallysignicant.
Comparedtotheplacebogroup,patientstreatedwithXOIplusLES400mgdailypre-sentedsignicantlyhigherincidenceratesofincreasedsCR(5.
9,9.
9,and10.
9vs.
0.
6,1.
7,and5.
9forpatientsinthecategoriesCrClC90mL,CrClC60toB90,andCrClC30toB60mL/min,respectively).
Differencesbetweengroupsdidnotreachstatisticalsignif-icanceinthelowerestimatedglomerularltra-tionratestrata,asthemagnitudeoftheriskratiodecreasedwithworseningGFR,from10.
64(1.
40–81.
03)to5.
64(1.
97–16.
18)to1.
83(0.
69–4.
84).
AsimilarpatternwasobservedforRheumatolTher(2019)6:101–108103renalfailure.
Figure1providesavisualrepre-sentationoftheincidenceofincreasedsCRandthecorrespondingriskratioforeachtreatmentallocationstratiedbyGFRcategory.
DISCUSSIONUsingdatareleasedpubliclyforusebyhealth-careproviders[6],thisanalysisfoundthattherateofadverserenaleventswasnotsignicantlydifferentfortheLES200mggroupandtheplacebogroup.
WhiletheincidenceofincreasedsCRshowedamodestincreasefortheLES200mggroupascomparedtotheplacebogroup,theincidenceratesofrenaloutcomesotherthanincreasedserumcreatinine(sCri:renalfailureandnephrolithiasis)werenumeri-callylowerfortheLES200mgarmthanfortheplaceboarm,withnoneofthesedifferencesbetweengroupsbeingstatisticallysignicant.
Incontrast,treatmentwiththehigherLES400mgdoseyieldedsignicantlyhigherratesofincreasedsCRcomparedtotheplacebogroup,but—surprisingly—nosignicantlyincreasedriskofrenalfailureofnephrolithiasis.
Ingen-eral,adverserenaleventrateswerelowestamongpatientswithnormalrenalfunctionratherthanamongthosewithdecreasedGFR.
However,weobservedthattherateofadverserenaleventswithincreasedsCRroseatafasterrateintheplaceboarmthanintheLES200mgand400mgarmswithworseningGFR,leadingtoareductionintherelativerisk.
Itshouldbenotedthatwhiletheelevationofserumcreatininefromthebaselinewasmea-suredaspartofasafetyanalysisperformedbythesponsor,clinicaldiagnosesweremadebytheinvestigatorsandcompiledusingpredenedpreferredtermstocompilerenalsafetyreportsandincludedassupplementalmaterialin[3].
Anotherlimitationinherenttothisanalysisisthatitisnotameta-analysis.
Whilesuchameta-analysisincludingveclinicaltrialswasrecentlyreported[13],itdidnotprovideanyfreshper-spectiveontheoptimalclinicalapproachtothesafetyoflesinuradorinsightintopathophysio-logicalmechanisms.
Anotherlimitationofourstudyisthefactthatwecouldonlyusepubliclyavailabledatacompiledfromphase3trials,andthatthenumberofeventswassmall.
Therefore,toevaluatetheriskofadverserenaleventsbetweentheplaceboandactivearms,weemployedtherelativeriskorriskratio,Table1OverallincidenceratesofadverserenaleventsinphaseIIIclinicaltrialscomparinglesinuradplusXOItoplaceboplusXOIXOI1PBON=516XOI1LES200N=511RR(95%CI)NNTpEvents(%)Events(%)IncreasedsCr12(2.
3)22(4.
3)1.
85(0.
93–3.
70)-510.
081Renalfailure11(2.
1)6(1.
2)0.
55(0.
20–1.
48)1040.
236Nephrolithiasis9(1.
7)3(0.
6)0.
34(0.
09–1.
24)860.
101XOI1PBON=516XOI1LES400N=510RR(95%CI)NNTpEvents(%)Events(%)IncreasedsCr12(2.
3)40(7.
8)3.
37(1.
79–6.
35)-18\0.
000Renalfailure11(2.
1)18(3.
5)1.
65(0.
79–3.
47)-720.
182Nephrolithiasis9(1.
7)13(2.
5)1.
46(0.
63–3.
39)-1240.
377IncreasedsCrserumcreatinine[1.
59baselinevalue,RRrelativeriskorriskratio(absoluteriskofaneventintheactivegroup/absoluteriskforeventinthecontrolgroup),NNTnumberneededtotreat(-forharm,forbenet),XOIxanthineoxidaseinhibitor,PBOplacebo,LES200lesinurad200mgonceaday,LES400lesinurad400mgonceaday104RheumatolTher(2019)6:101–108Table2AnalysisofadverserenaleventsinpatientsstratiedbyestimatedglomerularltrationrateCrCl90mL/minXOI1PBON=510XOI1LES200N=509RR(95%CI)NNTpEvents(%)N=180Events(%)N=200IncreasedsCr1(0.
6)6(3.
0)5.
34(0.
65–43.
98)-410.
199Renalfailure03(1.
5)6.
30(0.
32–121.
21)-290.
222CrCl60toNNTpEvents(%)N=229Events(%)N=208IncreasedsCr4(1.
7)8(3.
8)2.
20(0.
67–7.
21)-480.
192Renalfailure4(1.
7)1(0.
5)0.
28(0.
03–2.
44)790.
247CrCl30toNNTpEvents(%)N=101Events(%)N=101IncreasedsCr6(5.
9)7(6.
9)1.
17(0.
40–3.
35)-1010.
775Renalfailure5(5.
0)2(2.
0)0.
40(0.
08–2.
01)490.
267CrCl90mL/minXOI1PBON=510XOI1LES400N=508RR(95%CI)NNTpEvents(%)N=180Events(%)N=203IncreasedsCr1(0.
6)12(5.
9)10.
64(1.
40–81.
03)-190.
022Renalfailure07(3.
4)13.
31(0.
76–231.
40)-290.
0756CrCl60toNNTpEvents(%)N=229Events(%)N=213IncreasedsCr4(1.
7)21(9.
9)5.
64(1.
97–16.
18)-120.
001Renalfailure4(1.
7)7(3.
3)1.
88(0.
59–6.
34)-650.
308CrCl30toNNTpEvents(%)N=101Events(%)N=92IncreasedsCr6(5.
9)10(10.
9)1.
83(0.
69–4.
84)-200.
223Renalfailure5(5.
0)7(4.
2)0.
87(0.
24–3.
17)-1660.
843CrClcreatinineclearance,IncreasedsCrserumcreatininemorethan1.
59baselinevalue,RRrelativeriskorriskratio(absoluteriskfortheeventintheactivegroup/absoluteriskfortheeventinthecontrolgroup),NNTnumberneededtotreat(-forharm,forbenet),XOIxanthineoxidaseinhibitor,PBOplacebo,LES200lesinurad200mgonceaday,LES400lesinurad400mgonceadayRheumatolTher(2019)6:101–108105asrecommendedbytheCochranecollaborationtoevaluateinterventions[12].
Patient-yearexposurewassimilarforallgroups,aswasthenumberofpatientsallocatedtoeachgroup,thusfacilitatingunbiasedestimates.
Ineachanalysis,wefoundthattheRRsforLES200mgvs.
placeboandLES400mgvspla-cebodecreasedsubstantiallywithworseningkidneyfunction,suggestingthattheriskofadverserenaleventsdiminishedorwaselimi-natedinpatientswithmoderatekidneyimpairment.
Iflesinuradexhibiteddirectnephrotoxicity[5],anincreasedriskofadverseeventsinpatientswiththeworstkidneyfunc-tionwouldbeexpected,ashappenswithNSAIDs[14].
Incontrast,weobservedapatternofdecreasingrelativeriskofadverserenaleventswithdecreasingeGFR,suggestingthatchronickidneydisease(CKD)isassociatedwithpathophysiologicalchangesthatmayprotectagainstanypotentialnephrotoxicityassociatedwithlesinurad/XOIcombinations.
Bycontrast,theUS[6]andEU[7]recommendeithernotprescribinginsomepatientswithmoderaterenalimpairment(GFR\45ml/min)ortakingspecialprecautionsifitisprescribed,respectively.
Riskfactorsforadverserenaleventsassoci-atedwiththeuseofuricosuricmedicationsincludethelteredloadofuricacid(theproductoftheserumurateconcentra-tion9GFR),urinarypH(relatedtotheacidi-cationcapacity),andurinaryacidconcentration(relatedtotheconcentrationcapacity).
Indeed,higherclearanceofuricacid(associatedwithbetterrenalfunction)andundissociatedurinaryurate(relatedtobothacidicurinarypHandtheurinaryuricacidconcentration)havebothbeenassociatedwithanincreasedriskofnephrolithiasisduringuricosurictreatmentinmonotherapy[15].
Moreover,theappearanceofalteredurinesediment,namelythepresenceofredcells,whilestartinguricosuricmedicationswasalsoassociatedwithincreasedurinedensityandurinarycreatinineanduricacidconcen-trations[16].
PatientswithsignicantCKDhavereducedeGFR,areducedcapacitytoconcen-trateurine,andadecreasedcapacitytoacidifyurine[17,sotheyshouldbenetfromacollec-tivereductioninmajorriskfactorsforurate-inducedadverseevents.
Moreover,thecon-comitantuseofaXOIwithlesinuradreducestheserumurateconcentrationandthereforethelteredloadofuricacidintheglomerulus[18].
Althoughnotderivedfromthisanalysis,ourempiricalapproachasprecticingclinicianstotherenalsafetyoflesinuradtreatment—untilfurtherandspecicallyderivedevidenceisavailable—istoencourageadherencetotheXOIFig.
1PlotshowingthattheincidencerateofincreasedserumcreatinineincreasedwithdecreasingeGFR,butthattheRRdecreasedwithdecreasingeGFR(becausetheincidencerateofincreasedserumcreatinineincreasesmorerapidlywithdecreasingeGFRinthePBOarmthanintheLESarms).
RRrelativerisk,PBOplacebo,LESlesinurad106RheumatolTher(2019)6:101–108treatmentwhenusedincombinationwithlesinurad,toensurethatadequateuidintakeismaintained,initialevaluationfollowedbyreg-ularsurveillanceorrenalfunctionisuntertakenatleastusingspoturinesamplesforanalysisincludingabnormals,uricacid,creatinine,density,andpHforallpatients,asweusedtodowithotheruricosurics.
CONCLUSIONSTherateofadverserenaleventswassimilarintheplaceboandlesinurad200mggroups,whiletheratesofrenalfailureandnephrolithiasiswerenumericallylowerinthelesinurad200mggroupthanintheplacebogroup.
TheriskratioofsCriwithlesinuradversusplacebodecreasedwithworseningrenalfunction.
ACKNOWLEDGEMENTSFunding.
Thisworkwaspartiallysupportedbyagrant(FPR)fromtheAsociaciondeReu-matologosdelHospitaldeCruces;noothersourcesoffundingwereused.
Nofundingorsponsorshipwasreceivedforthepublicationofthisarticle.
Authorship.
AllnamedauthorsmeettheInternationalCommitteeofMedicalJournalEditors(ICMJE)criteriaforauthorshipforthisarticle,takeresponsibilityfortheintegrityoftheworkasawhole,andhavegiventheirapprovalforthisversiontobepublished.
MedicalWriting,Editorial,andOtherAssistance.
Allauthorssharedthepreliminaryidea.
FernandoPerez-Ruizperformedthesta-tisticalanalysisandwroteadraftpaperwith-outanyexternalsupport.
Allauthorsactivelydiscussedtheresults,signicantlycontributedtothemanuscript,andapprovedthenalversion.
Disclosures.
FernandoPerezRuiz:AdvisororspeakerfeesfromAstellas,Gru¨nenthal,Hori-zon,JapanTobacco,Logarithm,Menarini.
TimL.
Jansen:AdvisororspeakerfeesfromAbbVie,Celgene,Gru¨nenthal,Novartis.
Anne-KathrinTausche:SpeakerfeesfromBer-linChemieMenarini,Gru¨nenthal,Novartis.
ResearchsupportfromArdeaBiosciences/As-traZeneca,BerlinChemieMenarini.
AlexanderK.
So:AdvisoryboardforSOBI,Gru¨nenthal.
SpeakersboardforMenarini,Gru¨nenthalSwitzerland.
PascalRichette:AdvisororspeakerfeesfromGru¨nenthal,IpsenPharma,MenariniFrance,AstraZeneca.
FredericLiote:AdvisororspeakerfeesfromGru¨nenthalFrance,Gru¨nenthalGlobal,IpsenPharma,MenariniFrance,NovartisFrance;unrestrictededucationalgrantsforsupportingannualworkshopsoftheEuropeanCrystalNetwork,Paris:Gru¨nenthalGlobal,IpsenPhar-ma,MenariniFrance,Novartis,SOBI.
AustinStack:AdvisororspeakerfeesfromAstellas,Gru¨nenthal,AstraZeneca,andMenar-ini.
UnrestrictededucationalgrantfromMenar-ini.
CompliancewithEthicsGuidelines.
Datawereretrievedfrompublicresources(fullpre-scribinginformationforZurampic).
Thisarticledoesnotcontainanystudieswithhumanpar-ticipantsoranimalsperformedbyanyoftheauthors.
OpenAccess.
ThisarticleisdistributedunderthetermsoftheCreativeCommonsAttribution-NonCommercial4.
0InternationalLicense(http://creativecommons.
org/licenses/by-nc/4.
0/),whichpermitsanynon-commercialuse,distribution,andreproductioninanymedium,providedyougiveappropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreativeCommonslicense,andindicateifchangesweremade.
REFERENCES1.
JansenTL,Perez-RuizF,TauscheAK,RichetteP.
Internationalpositionpaperontheappropriateuseofuricosuricswiththeintroductionoflesinurad.
ClinRheumatol.
2018;37(12):3159–65.
https://doi.
org/10.
1007/s10067-018-4306-9.
RheumatolTher(2019)6:101–1081072.
MinerJ,TanPK,HyndmanD,LiuS,IversonC,NanavatiP,etal.
Lesinurad,anovel,oralcom-poundforgout,actstodecreaseserumuricacidthroughinhibitionofuratetransportersinthekidney.
ArthritisResTher.
2016;18(1):214.
3.
TerkeltaubR,SaagKG,GoldfarbDS,BaumgartnerS,SchechterBM,ValiyilR,etal.
Integratedsafetystudiesoftheuratereabsorptioninhibitorlesinuradintreatmentofgout.
Rheumatology(Oxford).
2018;58(1):61–9.
https://doi.
org/10.
1093/rheumatology/key245.
4.
TauscheAK,AltenR,DalbethN,KopickoJ,FungM,AdlerS,etal.
Lesinuradmonotherapyingoutpatientsintoleranttoaxanthineoxidaseinhibitor:a6monthphase3clinicaltrialandextensionstudy.
Rheumatology(Oxford).
2017;56(12):2170–8.
5.
Sanchez-NinoMD,Zheng-LinB,Valino-RivasL,SanzAB,RamosAM,LunoJ,etal.
Lesinurad:whatthenephrologistshouldknow.
ClinKidneyJ.
2017;10(5):679–87.
6.
FDA.
Zurampic.
Fullprescribinginformation.
2018.
https://www.
accessdata.
fda.
gov/drugsatfda_docs/label/2015/207988lbl.
pdf.
Accessed14Dec20187.
EMA.
Zurampic:summaryofproductcharacteris-tics.
2018.
https://www.
ema.
europa.
eu/documents/product-information/zurampic-epar-product-information_en.
pdf.
Accessed14Dec20188.
TerkeltaubR,SaagKG,GoldfarbDS,BaumgartnerS,SchechterBM,ValiyilR,etal.
Integratedsafetystudiesoftheuratereabsorptioninhibitorlesinuradintreatmentofgout.
Rheumatology(Oxford).
2019;58:61–9.
9.
BardinT,KeenanRT,KhannaPP,KopickoJ,FungM,BhaktaN,etal.
Lesinuradincombinationwithallopurinol:arandomised,double-blind,placebo-controlledstudyinpatientswithgoutwithinade-quateresponsetostandardofcare(themultina-tionalCLEAR2study).
AnnRheumDis.
2017;76(5):811–20.
10.
DalbethN,JonesG,TerkeltaubR,KhannaD,KopickoJ,BhaktaN,etal.
Lesinurad,aselectiveuricacidreabsorptioninhibitor,incombinationwithfebuxostatinpatientswithtophaceousgout:aphaseIIIclinicaltrial.
ArthritisRheumatol.
2017;69(9):1903–13.
11.
SaagKG,Fitz-PatrickD,KopickoJ,FungM,BhaktaN,AdlerS,etal.
Lesinuradcombinedwithallop-urinol:arandomized,double-blind,placebo-con-trolledstudyingoutpatientswithaninadequateresponsetostandard-of-careallopurinol(aUS-basedstudy).
ArthritisRheumatol.
2017;69(1):203–12.
12.
Cochrane.
Cochranehandbookforsystematicreviewsofinterventions,version5.
1.
0[updatedMarch2011](9.
2.
2.
2).
2011.
https://handbook-5-1.
cochrane.
org/index.
htm#chapter_9/9_8_chapter_information.
htm.
Accessed14Dec201813.
WuJY,ChangYT,LinYC,LeeCH,LohEW,WuMY,etal.
Efcacyandsafetyoflesinuradinpatientswithhyperuricemiaassociatedwithgout:asys-tematicreviewandmeta-analysisofrandomizedcontrolledtrials.
Pharmacotherapy.
2018;38:1106–19.
14.
MoonKW,KimJ,KimJH,SongR,LeeEY,SongYW,etal.
Riskfactorsforacutekidneyinjurybynon-steroidalanti-inammatorydrugsinpatientswithhyperuricaemia.
Rheumatology(Oxford).
2011;50(12):2278–82.
15.
Perez-RuizF,Hernandez-BaldizonS,Herrero-BeitesAM,Gonzalez-GayMA.
Riskfactorsassociatedwithrenallithiasisduringuricosurictreatmentofhype-ruricemiainpatientswithgout.
ArthritisCareRes(Hoboken).
2010;62(9):1299–305.
16.
Perez-RuizF,CalabozoRaluyM,HerreroBeitesA,DurueloJ,RuibalA,GarciaErauskinG.
Utilityofurinespotsamplesforthefollow-upofuricosurictherapy.
RevEspRheumatol.
2001;28:57–61.
17.
HarrisRC,NeilsonEG.
Adaptationofthekidneytorenalinjury.
In:LongoDL,KasperDL,JamesonJL,FauciAS,HauserSL,LoscalzoJ,editors.
Harrison'sprinciplesofinternalmedicine.
18thed.
NewYork:McGraw-Hill;2012.
p.
2289–93.
18.
Perez-RuizF,CalabozoM,Garcia-ErauskinG,RuibalA,Herrero-BeitesAM.
Renalunderexcretionofuricacidispresentinpatientswithapparenthighuri-naryuricacidoutput.
ArthritisRheumatol.
2002;47(6):610–3.
108RheumatolTher(2019)6:101–108
TimL.
Jansen.
Anne-KathrinTausche.
PascalRichette.
FredericLiote.
AlexanderK.
So.
AustinStackReceived:December15,2018/Publishedonline:February14,2019TheAuthor(s)2019ABSTRACTIntroduction:Therateofadverserenaleventshasbeenshowntobehigherinpatientstreatedwithlesinuradplusaxanthine-oxidaseinhi-bitor(XOI)thaninpatientstreatedonlywithaXOI.
Wereassessedtherisksforvariousadverserenaleventsfromadifferentperspectiveanddevisedahypothesistoexplaintheresults.
Methods:Weuseddatafromphase3trialsthatwerepubliclyavailablefromthefullprescribinginformationdocumentandestimatedtherela-tiveriskandthenumberneededtotreatforincreasedserumcreatinine(sCri),renalfailure,andrenallithiasis.
Weexaminedtheserisksforeachtreatmentgroupandtherisksstratiedbyestimatedglomerularltrationrate(eGFR).
Results:Overall,therelativeriskforsCriwas[1.
0withthe400mg/daydoseoflesinuradandhigherwiththe200mg/daydose,butitwas\1.
0forbothlithiasisandrenalfailurewiththe200mg/daydose.
TherelativeriskwasonlystatisticallysignicantforsCriwiththehighestdoseoflesinurad.
WhenresultsstratiedbyeGFRwereconsidered,theratesofadverseeventsincreasedwithdecliningrenalfunction,buttherelativerisksdecreasedinparallel,astherateofadverseeventsincreasedmuchmoreintheplaceboarmthanintheactivearm(200mg/daydose).
Indeed,therelativeriskwasonlysignicantforthehighestdoseoflesinuradinpatientswithnormaleGFR.
Conclusion:TherateofsCrieventswashigherinpatientstreatedwithbothlesinuradandaXOIEnhancedDigitalFeaturesToviewenhanceddigitalfeaturesforthisarticlegotohttps://doi.
org/10.
6084/m9.
gshare.
7637195.
F.
Perez-Ruiz(&)RheumatologyDivision,CrucesUniversityHospital,BiocrucesBizkaiaHealthResearchInstituteandMedicineDepartment,MedicineandNurserySchool,UniversityoftheBasqueCountry,Bilbao,Vizcaya,Spaine-mail:fernando.
perezruiz@osakidetza.
eusT.
L.
JansenDepartmentofRheumatology,VieCuriMC,Teglseweg210,9012BLVenlo,TheNetherlandsA.
-K.
TauscheDepartmentofRheumatology,UniversityClinic''CarlGustavCarus''attheTechnicalUniversity,Dressden,GermanyP.
RichetteF.
LioteRheumatologyDepartmentandInsermURM1132,CentreViggoPetersen,HopitalLariboisie`re(AP-HP)andUniversiteParisDiderot,USPC,Paris,FranceA.
K.
SoUniversityofLausanne,Lausanne,SwitzerlandA.
StackNephrologyDivision,UniversityHospitalLimerick,GraduateEntryMedicalSchool,HealthResearchInstitute,UniversityofLimerick,Limerick,IrelandRheumatolTher(2019)6:101–108https://doi.
org/10.
1007/s40744-019-0143-9ratherthanaXOIalone.
ThisratewasfoundtoincreasewithdecreasingeGFR,butasitdoesinforbothactiveandplaceboarmstherelativeriskisnotdifferentfromthatobservedintheplaceboarmsinthelabeled200mg/daydose.
Thismaybeexplainedbypathophysiologicalchangesthatdevelopinchronickidneydisease.
Keywords:Creatinineincrease;Gout;Lesinurad;Safety;Relativerisk;Renalfailure;RenallithiasisINTRODUCTIONLesinuradisaselectiveuricacidreabsorptioninhibitor(SURI)medicationthatwasrecentlyapprovedbytheEuropeanCommissionfol-lowingapositiveevaluationfromtheEuropeanMedicinesAgency(EMA)oftheEuropeanUnionandtheUnitedStatesFoodandDrugAdministration(FDA)[1].
Lesinuradexertsitsurate-loweringactionbyselectivelyinhibitinguratetransporters(principallyURAT-1andOAT-4)intheproximalrenaltubuleandblockinguricacidreabsorption,therebyincreasingneturicacidrenalexcretion[2].
Thesafetyprolepanelestablishedduringtheclinicaldevelopmentoflesinuradincludedrenalevents,aswouldbeexpectedforanyuri-cosuricmedication.
Renaleventswereclassiedasincreasesinserumcreatinine(sCr)concen-trationsabovebaseline,renalfailure(whichencompassedabroadrangeofevents,includingacuteorchronicrenalfailureandacutepre-re-nalfailure),ornephrolithiasis.
Renalevents,andspecicallyincreasesinsCrconcentration,weremorefrequentinpatientsonlesinuradthaninthoseonaplacebo(PBO),withadose-dependentrelationship[3],andtheseeventsweremorecommoninpatientswhodidnotreceiveaxanthineoxidaseinhibitor(XOI)whileonlesinuradtherapy[4]andinthosewithmoderatekidneyimpairment[5].
Thesend-ingsledtheEMAandFDAtoapprovelesinuradonlywhenusedincombinationwithaXOIandatadoseof200mg/day,andtorecommendedthatitshould/mustnotbeinitiatedinpatientswithanestimatedglomerularltrationrate(eGFR)below45mL/min/1.
73m2(summaryofproductcharacteristicsorSmPC,FDA)[6]or30mL/min/1.
73m2(SmPC,EU)[7]perminute.
Intriguinglyandimportantly,eventhoughelevationsinsCRweremorecommoninlesinu-rad-treatedpatientsthaninthosereceivingaplacebo,theincidenceratesofrenalfailureandrenalnephrolithiasisdidnotdifferbetweenthesegroups,althoughpatientswithaprevioushistoryofrenallithiasiswerenotexcluded[8].
WhileseveralhypotheseshavebeenproposedforthedevelopmentofelevatedsCRinlesinurad-treatedpatients,thereisnowidelyacceptedexplanationforthisoccurrence.
Consequently,recommen-dationsarelackinginriskassessmentsofpatientswhomaybeatriskofadverserenaleventswhentreatedwithlesinuradcombinationtherapy.
Ourobjectivewastoreassesstherelativerisksofvariousadverserenaleventsinlesinu-rad-treatedpatientsfromthreepivotalplacebo-controlledcombinationclinicaltrialsstratiedaccordingtorenalfunction;toreview,critique,andattempttoexplaintheresults;andtopro-posemonitoringstrategies.
METHODSWeanalyzeddatafromthreemajorphase3clinicaltrialsthattestedtheefcacyandsafetyoflesinuradinclinicalpractice.
IncidenceratesofadverserenaleventswerederivedfromTables2and3inthefullprescribinginforma-tionforZurampic[6].
Table2oftheprescribinginformationshowsdataontheincidenceratesofadverserenaleventsinallthreemajorplacebo-controlledclinicalstudies(CLEAR1,CLEAR2,andCRYSTAL)inwhichlesinuradincombinationwithaXOI(ei-therallopurinolorfebuxostat)wascomparedwithXOIalone[9–11].
Adverserenaleventsweredenedasfollows:(1)increasedbloodcreatinine,denedasagreaterthan1.
5-foldincreaseinsCrascomparedtothebaselinemeasurementattrialentry;(2)renalfailure(acompositetermthatdescribedanyoccurrenceofacuteorchronicrenalfailureandacuteprerenalfailure);and(3)nephrolithiasis.
Thethreestudiestogetherpro-vided516,511,and510patientsintheXOIPBO,XOIlesinurad200mg/day,andXOIlesinurad400mg/daygroups,respectively.
102RheumatolTher(2019)6:101–108Table3oftheSmPCforZurampicincludesdataontheoccurrenceofadverseeventsinallthreeclinicaltrialsaccordingtostudyarm(XOIPBO,n=510;XOIlesinurad200mg/day,n=509;andXOIlesinurad400mg/day,n=508)andstratiedbybaselinerenalfunctioncategory(C90,C60to\90,C30to\60mL/min/1.
73m2).
StatisticalAnalysisWedeterminedthepatient-yearexposureforeachstudyarmfromarecentlypublishedreportonthesafetyoflesinurad[3]as408,396,and390patient-years,respectively.
ThesevalueswerethenusedtocomputetheincidencerateofeachadverserenaleventstratiedbyGFRcategory(C90mL/min,C60to\90mL/min,C30to\60mL/min/1.
73m2)ineachtreatmentarm.
Riskratios(RRs)andnumbersneededtotreat(NNTs)werecalculatedtodeterminetheimpactofeachtreatmentontheratesofeachspeciedadverserenalevent.
HarmwasdenedasRR[1andbenetasRR\1.
Calculationswereper-formedusingtheMedCalcsoftware(MedcalcSoftwarebvba,Ostend,Belgium,https://www.
medcalc.
org/calc/relative_risk.
php).
TheseanalyseswererepeatedtodeterminetheRRsofadverserenaleventsstratiedbykidneyfunc-tion.
WhentheRRforastudycannotbecal-culatedbecausetherearenoeventsinthecontrolgroupofastudyandthestandarderrorscannotbecomputed,itiscustomarytoadd0.
5toeachcellofthe292contingencytable[12].
CompliancewithEthicsGuidelinesDatawereretrievedfromapublicresource:thefullprescribinginformationforZurampic.
Thisarticledoesnotcontainanystudieswithhumanparticipantsoranimalsperformedbyanyoftheauthors.
RESULTSTheincidenceofincreasedsCrwasnumericallyhigheramongpatientsallocatedtoLES200mgdailythanamongpatientsallocatedtoaplacebo(4.
3%vs.
2.
3%respectively).
Thecor-respondingRR(1.
85,0.
93–3.
70)wasnotstatis-ticallysignicant(Table1).
Incontrast,patientsallocatedtotheLES400mgdoseshowedasig-nicantlyhigherincidenceofincreasedsCRthanpatientsonaplacebo,alongwithastatis-ticallysignicantRRof3.
37(1.
79–6.
35)(Table1).
Ontheotherhand,theincidenceratesforrenalfailureandnephrolithiasiswerenumeri-callylowerforthe200mgdailydosevs.
PBO,butthiswasnotastatisticallysignicantdif-ference.
Similarly,patientsallocatedtotheLES400mgdoseexperiencedratesofrenalfailureandnephrolithiasisthatwerenotsignicantlydifferentfromthoseintheplaceboarm(Table1).
TheanalysisofadverserenaleventsaccordingtoeGFRcategoryispresentedinTable2.
ForeachGFRcategory,therateofincreasedsCRwasnumericallyhigherfortheLES200mggroupthanfortheplaceboarm(3.
0,3.
8,and6.
9vs.
0.
6,1.
7,and5.
9forpatientsinthecategoriesCrClC90,CrClC60toB90,andCrClC30toB60mL/min,respectively)buttherewerenostatisticallysignicantdif-ferences.
Intriguingly,theRRofincreasedsCRdecreasedwithworseningGFR,from5.
34forpatientswithCrClC90mL/minto1.
17forpatientswithCrClC30toB60mL/min;thenumberneededtotreatwasover100forthelattergroup.
Furthermore,analysisoftheRRofrenalfailurerevealedasimilarpattern,withtheRRdecreasingfrom6.
30inpatientswithCrClC90mL/minto0.
40forpatientswithCrClC30toB60mL/min.
TheRRofrenalfailurefortheLES200mggroupversustheplacebogroupwasnotstatisticallysignicant.
Comparedtotheplacebogroup,patientstreatedwithXOIplusLES400mgdailypre-sentedsignicantlyhigherincidenceratesofincreasedsCR(5.
9,9.
9,and10.
9vs.
0.
6,1.
7,and5.
9forpatientsinthecategoriesCrClC90mL,CrClC60toB90,andCrClC30toB60mL/min,respectively).
Differencesbetweengroupsdidnotreachstatisticalsignif-icanceinthelowerestimatedglomerularltra-tionratestrata,asthemagnitudeoftheriskratiodecreasedwithworseningGFR,from10.
64(1.
40–81.
03)to5.
64(1.
97–16.
18)to1.
83(0.
69–4.
84).
AsimilarpatternwasobservedforRheumatolTher(2019)6:101–108103renalfailure.
Figure1providesavisualrepre-sentationoftheincidenceofincreasedsCRandthecorrespondingriskratioforeachtreatmentallocationstratiedbyGFRcategory.
DISCUSSIONUsingdatareleasedpubliclyforusebyhealth-careproviders[6],thisanalysisfoundthattherateofadverserenaleventswasnotsignicantlydifferentfortheLES200mggroupandtheplacebogroup.
WhiletheincidenceofincreasedsCRshowedamodestincreasefortheLES200mggroupascomparedtotheplacebogroup,theincidenceratesofrenaloutcomesotherthanincreasedserumcreatinine(sCri:renalfailureandnephrolithiasis)werenumeri-callylowerfortheLES200mgarmthanfortheplaceboarm,withnoneofthesedifferencesbetweengroupsbeingstatisticallysignicant.
Incontrast,treatmentwiththehigherLES400mgdoseyieldedsignicantlyhigherratesofincreasedsCRcomparedtotheplacebogroup,but—surprisingly—nosignicantlyincreasedriskofrenalfailureofnephrolithiasis.
Ingen-eral,adverserenaleventrateswerelowestamongpatientswithnormalrenalfunctionratherthanamongthosewithdecreasedGFR.
However,weobservedthattherateofadverserenaleventswithincreasedsCRroseatafasterrateintheplaceboarmthanintheLES200mgand400mgarmswithworseningGFR,leadingtoareductionintherelativerisk.
Itshouldbenotedthatwhiletheelevationofserumcreatininefromthebaselinewasmea-suredaspartofasafetyanalysisperformedbythesponsor,clinicaldiagnosesweremadebytheinvestigatorsandcompiledusingpredenedpreferredtermstocompilerenalsafetyreportsandincludedassupplementalmaterialin[3].
Anotherlimitationinherenttothisanalysisisthatitisnotameta-analysis.
Whilesuchameta-analysisincludingveclinicaltrialswasrecentlyreported[13],itdidnotprovideanyfreshper-spectiveontheoptimalclinicalapproachtothesafetyoflesinuradorinsightintopathophysio-logicalmechanisms.
Anotherlimitationofourstudyisthefactthatwecouldonlyusepubliclyavailabledatacompiledfromphase3trials,andthatthenumberofeventswassmall.
Therefore,toevaluatetheriskofadverserenaleventsbetweentheplaceboandactivearms,weemployedtherelativeriskorriskratio,Table1OverallincidenceratesofadverserenaleventsinphaseIIIclinicaltrialscomparinglesinuradplusXOItoplaceboplusXOIXOI1PBON=516XOI1LES200N=511RR(95%CI)NNTpEvents(%)Events(%)IncreasedsCr12(2.
3)22(4.
3)1.
85(0.
93–3.
70)-510.
081Renalfailure11(2.
1)6(1.
2)0.
55(0.
20–1.
48)1040.
236Nephrolithiasis9(1.
7)3(0.
6)0.
34(0.
09–1.
24)860.
101XOI1PBON=516XOI1LES400N=510RR(95%CI)NNTpEvents(%)Events(%)IncreasedsCr12(2.
3)40(7.
8)3.
37(1.
79–6.
35)-18\0.
000Renalfailure11(2.
1)18(3.
5)1.
65(0.
79–3.
47)-720.
182Nephrolithiasis9(1.
7)13(2.
5)1.
46(0.
63–3.
39)-1240.
377IncreasedsCrserumcreatinine[1.
59baselinevalue,RRrelativeriskorriskratio(absoluteriskofaneventintheactivegroup/absoluteriskforeventinthecontrolgroup),NNTnumberneededtotreat(-forharm,forbenet),XOIxanthineoxidaseinhibitor,PBOplacebo,LES200lesinurad200mgonceaday,LES400lesinurad400mgonceaday104RheumatolTher(2019)6:101–108Table2AnalysisofadverserenaleventsinpatientsstratiedbyestimatedglomerularltrationrateCrCl90mL/minXOI1PBON=510XOI1LES200N=509RR(95%CI)NNTpEvents(%)N=180Events(%)N=200IncreasedsCr1(0.
6)6(3.
0)5.
34(0.
65–43.
98)-410.
199Renalfailure03(1.
5)6.
30(0.
32–121.
21)-290.
222CrCl60toNNTpEvents(%)N=229Events(%)N=208IncreasedsCr4(1.
7)8(3.
8)2.
20(0.
67–7.
21)-480.
192Renalfailure4(1.
7)1(0.
5)0.
28(0.
03–2.
44)790.
247CrCl30toNNTpEvents(%)N=101Events(%)N=101IncreasedsCr6(5.
9)7(6.
9)1.
17(0.
40–3.
35)-1010.
775Renalfailure5(5.
0)2(2.
0)0.
40(0.
08–2.
01)490.
267CrCl90mL/minXOI1PBON=510XOI1LES400N=508RR(95%CI)NNTpEvents(%)N=180Events(%)N=203IncreasedsCr1(0.
6)12(5.
9)10.
64(1.
40–81.
03)-190.
022Renalfailure07(3.
4)13.
31(0.
76–231.
40)-290.
0756CrCl60toNNTpEvents(%)N=229Events(%)N=213IncreasedsCr4(1.
7)21(9.
9)5.
64(1.
97–16.
18)-120.
001Renalfailure4(1.
7)7(3.
3)1.
88(0.
59–6.
34)-650.
308CrCl30toNNTpEvents(%)N=101Events(%)N=92IncreasedsCr6(5.
9)10(10.
9)1.
83(0.
69–4.
84)-200.
223Renalfailure5(5.
0)7(4.
2)0.
87(0.
24–3.
17)-1660.
843CrClcreatinineclearance,IncreasedsCrserumcreatininemorethan1.
59baselinevalue,RRrelativeriskorriskratio(absoluteriskfortheeventintheactivegroup/absoluteriskfortheeventinthecontrolgroup),NNTnumberneededtotreat(-forharm,forbenet),XOIxanthineoxidaseinhibitor,PBOplacebo,LES200lesinurad200mgonceaday,LES400lesinurad400mgonceadayRheumatolTher(2019)6:101–108105asrecommendedbytheCochranecollaborationtoevaluateinterventions[12].
Patient-yearexposurewassimilarforallgroups,aswasthenumberofpatientsallocatedtoeachgroup,thusfacilitatingunbiasedestimates.
Ineachanalysis,wefoundthattheRRsforLES200mgvs.
placeboandLES400mgvspla-cebodecreasedsubstantiallywithworseningkidneyfunction,suggestingthattheriskofadverserenaleventsdiminishedorwaselimi-natedinpatientswithmoderatekidneyimpairment.
Iflesinuradexhibiteddirectnephrotoxicity[5],anincreasedriskofadverseeventsinpatientswiththeworstkidneyfunc-tionwouldbeexpected,ashappenswithNSAIDs[14].
Incontrast,weobservedapatternofdecreasingrelativeriskofadverserenaleventswithdecreasingeGFR,suggestingthatchronickidneydisease(CKD)isassociatedwithpathophysiologicalchangesthatmayprotectagainstanypotentialnephrotoxicityassociatedwithlesinurad/XOIcombinations.
Bycontrast,theUS[6]andEU[7]recommendeithernotprescribinginsomepatientswithmoderaterenalimpairment(GFR\45ml/min)ortakingspecialprecautionsifitisprescribed,respectively.
Riskfactorsforadverserenaleventsassoci-atedwiththeuseofuricosuricmedicationsincludethelteredloadofuricacid(theproductoftheserumurateconcentra-tion9GFR),urinarypH(relatedtotheacidi-cationcapacity),andurinaryacidconcentration(relatedtotheconcentrationcapacity).
Indeed,higherclearanceofuricacid(associatedwithbetterrenalfunction)andundissociatedurinaryurate(relatedtobothacidicurinarypHandtheurinaryuricacidconcentration)havebothbeenassociatedwithanincreasedriskofnephrolithiasisduringuricosurictreatmentinmonotherapy[15].
Moreover,theappearanceofalteredurinesediment,namelythepresenceofredcells,whilestartinguricosuricmedicationswasalsoassociatedwithincreasedurinedensityandurinarycreatinineanduricacidconcen-trations[16].
PatientswithsignicantCKDhavereducedeGFR,areducedcapacitytoconcen-trateurine,andadecreasedcapacitytoacidifyurine[17,sotheyshouldbenetfromacollec-tivereductioninmajorriskfactorsforurate-inducedadverseevents.
Moreover,thecon-comitantuseofaXOIwithlesinuradreducestheserumurateconcentrationandthereforethelteredloadofuricacidintheglomerulus[18].
Althoughnotderivedfromthisanalysis,ourempiricalapproachasprecticingclinicianstotherenalsafetyoflesinuradtreatment—untilfurtherandspecicallyderivedevidenceisavailable—istoencourageadherencetotheXOIFig.
1PlotshowingthattheincidencerateofincreasedserumcreatinineincreasedwithdecreasingeGFR,butthattheRRdecreasedwithdecreasingeGFR(becausetheincidencerateofincreasedserumcreatinineincreasesmorerapidlywithdecreasingeGFRinthePBOarmthanintheLESarms).
RRrelativerisk,PBOplacebo,LESlesinurad106RheumatolTher(2019)6:101–108treatmentwhenusedincombinationwithlesinurad,toensurethatadequateuidintakeismaintained,initialevaluationfollowedbyreg-ularsurveillanceorrenalfunctionisuntertakenatleastusingspoturinesamplesforanalysisincludingabnormals,uricacid,creatinine,density,andpHforallpatients,asweusedtodowithotheruricosurics.
CONCLUSIONSTherateofadverserenaleventswassimilarintheplaceboandlesinurad200mggroups,whiletheratesofrenalfailureandnephrolithiasiswerenumericallylowerinthelesinurad200mggroupthanintheplacebogroup.
TheriskratioofsCriwithlesinuradversusplacebodecreasedwithworseningrenalfunction.
ACKNOWLEDGEMENTSFunding.
Thisworkwaspartiallysupportedbyagrant(FPR)fromtheAsociaciondeReu-matologosdelHospitaldeCruces;noothersourcesoffundingwereused.
Nofundingorsponsorshipwasreceivedforthepublicationofthisarticle.
Authorship.
AllnamedauthorsmeettheInternationalCommitteeofMedicalJournalEditors(ICMJE)criteriaforauthorshipforthisarticle,takeresponsibilityfortheintegrityoftheworkasawhole,andhavegiventheirapprovalforthisversiontobepublished.
MedicalWriting,Editorial,andOtherAssistance.
Allauthorssharedthepreliminaryidea.
FernandoPerez-Ruizperformedthesta-tisticalanalysisandwroteadraftpaperwith-outanyexternalsupport.
Allauthorsactivelydiscussedtheresults,signicantlycontributedtothemanuscript,andapprovedthenalversion.
Disclosures.
FernandoPerezRuiz:AdvisororspeakerfeesfromAstellas,Gru¨nenthal,Hori-zon,JapanTobacco,Logarithm,Menarini.
TimL.
Jansen:AdvisororspeakerfeesfromAbbVie,Celgene,Gru¨nenthal,Novartis.
Anne-KathrinTausche:SpeakerfeesfromBer-linChemieMenarini,Gru¨nenthal,Novartis.
ResearchsupportfromArdeaBiosciences/As-traZeneca,BerlinChemieMenarini.
AlexanderK.
So:AdvisoryboardforSOBI,Gru¨nenthal.
SpeakersboardforMenarini,Gru¨nenthalSwitzerland.
PascalRichette:AdvisororspeakerfeesfromGru¨nenthal,IpsenPharma,MenariniFrance,AstraZeneca.
FredericLiote:AdvisororspeakerfeesfromGru¨nenthalFrance,Gru¨nenthalGlobal,IpsenPharma,MenariniFrance,NovartisFrance;unrestrictededucationalgrantsforsupportingannualworkshopsoftheEuropeanCrystalNetwork,Paris:Gru¨nenthalGlobal,IpsenPhar-ma,MenariniFrance,Novartis,SOBI.
AustinStack:AdvisororspeakerfeesfromAstellas,Gru¨nenthal,AstraZeneca,andMenar-ini.
UnrestrictededucationalgrantfromMenar-ini.
CompliancewithEthicsGuidelines.
Datawereretrievedfrompublicresources(fullpre-scribinginformationforZurampic).
Thisarticledoesnotcontainanystudieswithhumanpar-ticipantsoranimalsperformedbyanyoftheauthors.
OpenAccess.
ThisarticleisdistributedunderthetermsoftheCreativeCommonsAttribution-NonCommercial4.
0InternationalLicense(http://creativecommons.
org/licenses/by-nc/4.
0/),whichpermitsanynon-commercialuse,distribution,andreproductioninanymedium,providedyougiveappropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreativeCommonslicense,andindicateifchangesweremade.
REFERENCES1.
JansenTL,Perez-RuizF,TauscheAK,RichetteP.
Internationalpositionpaperontheappropriateuseofuricosuricswiththeintroductionoflesinurad.
ClinRheumatol.
2018;37(12):3159–65.
https://doi.
org/10.
1007/s10067-018-4306-9.
RheumatolTher(2019)6:101–1081072.
MinerJ,TanPK,HyndmanD,LiuS,IversonC,NanavatiP,etal.
Lesinurad,anovel,oralcom-poundforgout,actstodecreaseserumuricacidthroughinhibitionofuratetransportersinthekidney.
ArthritisResTher.
2016;18(1):214.
3.
TerkeltaubR,SaagKG,GoldfarbDS,BaumgartnerS,SchechterBM,ValiyilR,etal.
Integratedsafetystudiesoftheuratereabsorptioninhibitorlesinuradintreatmentofgout.
Rheumatology(Oxford).
2018;58(1):61–9.
https://doi.
org/10.
1093/rheumatology/key245.
4.
TauscheAK,AltenR,DalbethN,KopickoJ,FungM,AdlerS,etal.
Lesinuradmonotherapyingoutpatientsintoleranttoaxanthineoxidaseinhibitor:a6monthphase3clinicaltrialandextensionstudy.
Rheumatology(Oxford).
2017;56(12):2170–8.
5.
Sanchez-NinoMD,Zheng-LinB,Valino-RivasL,SanzAB,RamosAM,LunoJ,etal.
Lesinurad:whatthenephrologistshouldknow.
ClinKidneyJ.
2017;10(5):679–87.
6.
FDA.
Zurampic.
Fullprescribinginformation.
2018.
https://www.
accessdata.
fda.
gov/drugsatfda_docs/label/2015/207988lbl.
pdf.
Accessed14Dec20187.
EMA.
Zurampic:summaryofproductcharacteris-tics.
2018.
https://www.
ema.
europa.
eu/documents/product-information/zurampic-epar-product-information_en.
pdf.
Accessed14Dec20188.
TerkeltaubR,SaagKG,GoldfarbDS,BaumgartnerS,SchechterBM,ValiyilR,etal.
Integratedsafetystudiesoftheuratereabsorptioninhibitorlesinuradintreatmentofgout.
Rheumatology(Oxford).
2019;58:61–9.
9.
BardinT,KeenanRT,KhannaPP,KopickoJ,FungM,BhaktaN,etal.
Lesinuradincombinationwithallopurinol:arandomised,double-blind,placebo-controlledstudyinpatientswithgoutwithinade-quateresponsetostandardofcare(themultina-tionalCLEAR2study).
AnnRheumDis.
2017;76(5):811–20.
10.
DalbethN,JonesG,TerkeltaubR,KhannaD,KopickoJ,BhaktaN,etal.
Lesinurad,aselectiveuricacidreabsorptioninhibitor,incombinationwithfebuxostatinpatientswithtophaceousgout:aphaseIIIclinicaltrial.
ArthritisRheumatol.
2017;69(9):1903–13.
11.
SaagKG,Fitz-PatrickD,KopickoJ,FungM,BhaktaN,AdlerS,etal.
Lesinuradcombinedwithallop-urinol:arandomized,double-blind,placebo-con-trolledstudyingoutpatientswithaninadequateresponsetostandard-of-careallopurinol(aUS-basedstudy).
ArthritisRheumatol.
2017;69(1):203–12.
12.
Cochrane.
Cochranehandbookforsystematicreviewsofinterventions,version5.
1.
0[updatedMarch2011](9.
2.
2.
2).
2011.
https://handbook-5-1.
cochrane.
org/index.
htm#chapter_9/9_8_chapter_information.
htm.
Accessed14Dec201813.
WuJY,ChangYT,LinYC,LeeCH,LohEW,WuMY,etal.
Efcacyandsafetyoflesinuradinpatientswithhyperuricemiaassociatedwithgout:asys-tematicreviewandmeta-analysisofrandomizedcontrolledtrials.
Pharmacotherapy.
2018;38:1106–19.
14.
MoonKW,KimJ,KimJH,SongR,LeeEY,SongYW,etal.
Riskfactorsforacutekidneyinjurybynon-steroidalanti-inammatorydrugsinpatientswithhyperuricaemia.
Rheumatology(Oxford).
2011;50(12):2278–82.
15.
Perez-RuizF,Hernandez-BaldizonS,Herrero-BeitesAM,Gonzalez-GayMA.
Riskfactorsassociatedwithrenallithiasisduringuricosurictreatmentofhype-ruricemiainpatientswithgout.
ArthritisCareRes(Hoboken).
2010;62(9):1299–305.
16.
Perez-RuizF,CalabozoRaluyM,HerreroBeitesA,DurueloJ,RuibalA,GarciaErauskinG.
Utilityofurinespotsamplesforthefollow-upofuricosurictherapy.
RevEspRheumatol.
2001;28:57–61.
17.
HarrisRC,NeilsonEG.
Adaptationofthekidneytorenalinjury.
In:LongoDL,KasperDL,JamesonJL,FauciAS,HauserSL,LoscalzoJ,editors.
Harrison'sprinciplesofinternalmedicine.
18thed.
NewYork:McGraw-Hill;2012.
p.
2289–93.
18.
Perez-RuizF,CalabozoM,Garcia-ErauskinG,RuibalA,Herrero-BeitesAM.
Renalunderexcretionofuricacidispresentinpatientswithapparenthighuri-naryuricacidoutput.
ArthritisRheumatol.
2002;47(6):610–3.
108RheumatolTher(2019)6:101–108
HostKvm($4.25/月),俄罗斯CN2带宽大升级,俄罗斯/香港高防限量5折优惠进行中
HostKvm是一家成立于2013年的国外VPS服务商,产品基于KVM架构,数据中心包括日本、新加坡、韩国、美国、俄罗斯、中国香港等多个地区机房,均为国内直连或优化线路,延迟较低,适合建站或者远程办公等。本月,商家旗下俄罗斯、新加坡、美国、香港等节点带宽进行了大幅度升级,俄罗斯机房国内电信/联通直连,CN2线路,150Mbps(原来30Mbps)带宽起,目前俄罗斯和香港高防节点5折骨折码继续优惠中...
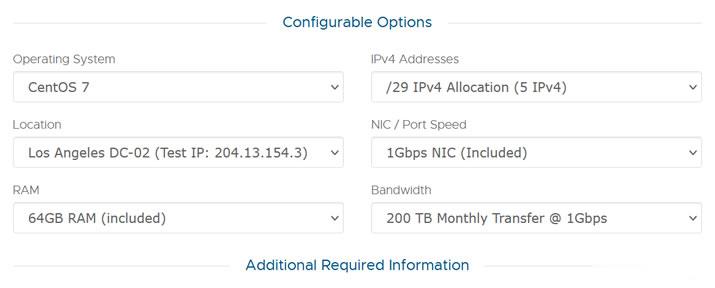
月费$389,RackNerd美国大硬盘独立服务器
这次RackNerd商家提供的美国大硬盘独立服务器,数据中心位于洛杉矶multacom,可选Windows、Linux镜像系统,默认内存是64GB,也可升级至128GB内存,而且硬盘采用的是256G SSD系统盘+10个16TSAS数据盘,端口提供的是1Gbps带宽,每月提供200TB,且包含5个IPv4,如果有需要更多IP,也可以升级增加。CPU核心内存硬盘流量带宽价格选择2XE5-2640V2...
妮妮云,美国cera CN2线路,VPS享3折优惠
近期联通CUVIP的线路(AS4837线路)非常火热,妮妮云也推出了这类线路的套餐以及优惠,目前到国内优质线路排行大致如下:电信CN2 GIA>联通AS9929>联通AS4837>电信CN2 GT>普通线路,AS4837线路比起前两的优势就是带宽比较大,相对便宜一些,所以大家才能看到这个线路的带宽都非常高。妮妮云互联目前云服务器开放抽奖活动,每天开通前10台享3折优惠,另外...
nnt为你推荐
-
海外虚拟主机空间国外虚拟主机和国内空间的差别?域名注册公司一般公司注册的都是什么域名?vps主机什么是vps主机海外主机如何选择优质的海外主机?国内免费空间免费空间哪个好用台湾vps香港vps和台湾vps哪个好用域名备案域名怎么备案网站空间购买哪里买网站空间好?免费网站空间申请哪里有免费申请空间的(网页制作)韩国虚拟主机香港和韩国的虚拟主机哪个比较好?