Ankylosingselected
selected 时间:2021-05-21 阅读:()
Item4.
InformationontheCompany1SelecteddevelopmentprojectsYearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseABL001asciminibBCRABLinhibitorChronicmyeloidleukemia,3rdlineOncologyOral20162021/IIIACZ885canakinumabAntiinterleukin1beta2ndlinenonsmallcelllungcancerOncologySubcutaneousinjection20172021/IIImonoclonalantibody1stlinenonsmallcelllungcancerOncologySubcutaneousinjection20172021/IIIAdjuvantnonsmallcelllungcancerOncologySubcutaneousinjection20172022/IIIAVXS1011onasemno-SurvivalmotorneuronSpinalmuscularatrophyNeuroscienceIntravenousinfusion2018USapprovedgeneabepar-(SMN)gene(IVformulation)EUregistrationvovecreplacementtherapySpinalmuscularatrophyNeuroscienceIntrathecalinjection20182020/I(ITformulation)2AVXS201TBDMethylCpGbindingRettsyndromeNeuroscienceIntrathecalinjection20182023/Iprotein2(MECP2)genereplacementtherapyBYL7193alpelisibPI3KalphainhibitorPIK3CAmutanthormonereceptorpositiveOncologyOral2018USapproved(HR+)/humanepidermalgrowthfactorEUregistrationreceptor2negative(HER2)postmenopausaladvancedbreastcancer,2ndline(+fulvestrant)PIK3CA-relatedovergrowthspectrumOncologyOral20192020/IIITriplenegativebreastcancerOncologyOral20192023/IIIHormonereceptor-negative(HR-)/humanOncologyOral20192023/IIIepidermalgrowthfactorreceptor2-positive(HER2+)advancedbreastcancerOvariancancerOncologyOral20192023/IIIHeadandnecksquamouscellcarcinomaOncologyOral2019≥2024/IIICEE321TBDPan-JAKinhibitorAtopicdermatitisImmunology,Topical2019≥2024/IIHepatologyandDermatologyCFZ533iscalimabBlocking,nondepleting,SolidorgantransplantationImmunology,Intravenousinfusion20172023/IIantiCD40monoclonalHepatologyandantibodyDermatologySjgren'ssyndromeImmunology,Intravenousinfusion2018≥2024/IIHepatologyandDermatologyCosentyxsecukinumabAntiinterleukin17NonradiographicaxialspondyloarthritisImmunology,Subcutaneousinjection2015US/EUmonoclonalantibodyHepatologyandregistrationDermatologyPsoriaticarthritisheadtoheadstudyImmunology,Subcutaneousinjection20152020/IIIversusHumira(adalimumab)HepatologyandDermatologyAnkylosingspondylitisheadtoheadstudyImmunology,Subcutaneousinjection20152022/IIIversusSandozbiosimilarHyrimozHepatologyand(adalimumab)DermatologyHidradenitissuppurativaImmunology,Intravenousinfusion20172022/IIIHepatologyandDermatologyGiantcellarteritisImmunology,Intravenousinfusion2018≥2024/IIHepatologyandDermatologyLichenplanusImmunology,Intravenousinfusion2019≥2024/IIHepatologyandDermatologyCSJ117TBDAntithymicstromalSevereasthmaRespiratoryInhalation20182023/IIlymphopoietinmonoclonalantibodyfragmentECF843TBDBoundarylubricantDryeyeOphthalmologyEyedrops20172022/IIEntrestovalsartanandAngiotensinreceptor/ChronicheartfailurewithpreservedCardiovascular,Oral20122020/IIIsacubitrilneprilysininhibitorejectionfractionRenal(assodiumandMetabolismsaltcomplex)PostacutemyocardialinfarctionCardiovascular,Oral20152021/IIIRenalandMetabolism1ApprovedintheUSasZolgensmaforspinalmuscularatrophy(IVformulation)2TheFDAhasplacedapartialclinicalholdonAVXS-101intrathecaltrialsforspinalmuscularatrophypatientsbasedonfindingsinasmallpreclinicalanimalstudy.
3ApprovedintheUSasPiqrayforPIK3CAmutantHR+/HER2-postmenopausaladvancedbreastcancer,2ndline(+fulvestrant)Item4.
InformationontheCompany2YearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseINC280capmatinibc-METinhibitorNonsmallcelllungcancerOncologyOral2014USregistrationSolidtumorsOncologyOral2019≥2024/IIJakaviruxolitinibJAK1/2inhibitorAcutegraftversushostdiseaseOncologyOral20162021/IIIChronicgraftversushostdiseaseOncologyOral20162021/IIIKAE609cipargaminPfATP4inhibitorMalariaEstablishedOral2012≥2024/IIMedicinesSeveremalariaEstablishedOral2019≥2024/IIMedicinesKAF156ganaplacideImidazolopiperazinesMalariaEstablishedOral2014≥2024/IIderivativeMedicinesKisqaliribociclibCDK4/6inhibitorHR+/HER2breastcancer(adjuvant)OncologyOral20182022/IIIKJX839inclisiranSmall-interferingRNAHyperlipidemiaCardiovascular,Subcutaneousinjection2019US/EU(PCSK9)RenalregistrationandMetabolismSecondarypreventionofcardiovascularCardiovascular,Subcutaneousinjection2019≥2024/IIIeventsinpatientswithelevatedlevelsRenalofLDL-CandMetabolismKymriahtisagen-CD19targetedchimericRelapsed/refractoryfollicularlymphomaOncologyIntravenousinfusion20172021/IIlecleucelantigenreceptorTcellimmunotherapyRelapsed/refractorydiffuselargeBcellOncologyIntravenousinfusion20182021/IIIlymphomain1strelapseRelapsed/refractorydiffuselargeBcellOncologyIntravenousinfusion2017≥2024/IIlymphoma(+pembrolizumab)LAM320clofazimineMycobacterialMultidrugresistanttuberculosisEstablishedOral20162021/IIIDNAbindingMedicinesLJC242tropifexor,FXRagonistandNonalcoholicsteatohepatitisImmunology,Oral2017≥2024/IIcenicrivirocCCR2/5inhibitorHepatologyand(infixed-doseDermatologycombination)LJN452tropifexorFXRagonistNonalcoholicsteatohepatitisImmunology,Oral2015≥2024/IIHepatologyandDermatologyLMI070branaplamSMN2RNAsplicingSpinalmuscularatrophyNeuroscienceOral2017≥2024/IImodulatorLNP023TBDFactorBinhibitorIgAnephropathyCardiovascular,Oral20182023/IIRenalandMetabolismC3glomerulopathyCardiovascular,Oral20182023/IIRenalandMetabolismParoxysmalnocturnalhemoglobinuriaCardiovascular,Oral20192023/IIRenalandMetabolismMembranousnephropathyCardiovascular,Oral2018≥2024/IIRenalandMetabolismLOU064TBDBTKinhibitorChronicspontaneousurticariaImmunology,Oral20172023/IIHepatologyandDermatology177Lu-TBDTargetedDNAMetastaticcastration-resistantOncologyIntravenousinfusion20182020/IIIPSMA-617destructionviaprostatecancerbeta-particleradiationLXE408TBDKinetoplastidVisceralleishmaniasisEstablishedOral2019≥2024/IIproteasomeinhibitorMedicinesMBG453TBDTIM-3antagonistMyelodysplasticsyndromeOncologyIntravenousinfusion20182021/IIAcutemyeloidleukemiaOncologyIntravenousinfusion2019≥2024/IIOMB157ofatumumabAntiCD20monoclonalRelapsingmultiplesclerosisNeuroscienceSubcutaneousinjection2015US/EUantibodyregistrationPDR001spartalizumabAntiPD1monoclonalMetastaticBRAFV600+OncologyIntravenousinfusion20172020/IIIantibodymelanoma(w/Tafinlar+Mekinist)Metastaticmelanoma(combo)OncologyIntravenousinfusion20172023/IIQBW251TBDCFTRpotentiatorChronicobstructivepulmonarydiseaseRespiratoryOral2017≥2024/IIQGE031ligelizumabHighaffinityantiIgEChronicspontaneousurticaria/Immunology,Subcutaneousinjection20172021/IIImonoclonalantibodychronicidiopathicurticariaHepatologyandDermatologyQMF149indacaterol,Longactingbeta2AsthmaRespiratoryInhalation2019EUregistrationmometasoneadrenergicagonistandfuroateinhaledcorticosteroid(infixeddosecombination)Item4.
InformationontheCompany3YearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseQVM149indacaterol,Longactingbeta2AsthmaRespiratoryInhalation2019EUregistrationmometasoneadrenergicagonist,furoate,longactingmuscarinicglyco-antagonistandinhaledpyrroniumcorticosteroidbromide(infixeddosecombination)RTH2584brolucizumabAntiVEGFsinglechainNeovascular(wet)agerelatedmacularOphthalmologyIntravitrealinjection2019USapprovedantibodyfragmentdegenerationEUregistrationDiabeticmacularedemaOphthalmologyIntravitrealinjection20172021/IIIRetinalveinocclusionOphthalmologyIntravitrealinjection20182023/IIIProliferativediabeticretinopathyOphthalmologyIntravitrealinjection20192023/IIISAF312TBDTRPV1antagonistChronicocularsurfacepainOphthalmologyTopical2019≥2024/IISEG1015crizanlizumabPselectininhibitorSicklecelldiseaseOncologyIntravenousinfusion2019USapprovedEUregistrationTQJ230TBDAnti-apo(a)antisenseSecondarypreventionofcardiovascularCardiovascular,Subcutaneousinjection2018≥2024/IIIoligonucleotideeventsinpatientswithelevatedlevelsRenalandoflipoprotein(a)MetabolismUNR844TBDReductionofPresbyopiaCardiovascular,Eyedrops2017≥2024/IIdisulfidebondsRenalandMetabolismVAY736ianalumabAntiBAFF(BcellAutoimmunehepatitisImmunology,Subcutaneousinjection2016≥2024/IIactivatingfactor)HepatologyandmonoclonalantibodyDermatologyPrimarySjgren'ssyndromeImmunology,Subcutaneousinjection2015≥2024/IIHepatologyandDermatologyVPM087TBDInterleukin-1betaColorectalcancer,1stline;OncologyIntravenousinfusion2018≥2024/Ineutralizationrenalcellcarcinoma,1stlinemonoclonalantibodyXolairomalizumabAntiIgEmonoclonalNasalpolypsRespiratorySubcutaneousinjection2017US/EUantibodyregistrationFoodallergyRespiratorySubcutaneousinjection20192021/IIIZPL389adriforantHistamineH4receptorAtopicdermatitisImmunology,Oral2017≥2024/IIantagonistHepatologyandDermatology4ApprovedintheUSasBeovuforneovascular(wet)agerelatedmaculardegeneration5ApprovedintheUSasAdakveoforsicklecelldisease
InformationontheCompany1SelecteddevelopmentprojectsYearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseABL001asciminibBCRABLinhibitorChronicmyeloidleukemia,3rdlineOncologyOral20162021/IIIACZ885canakinumabAntiinterleukin1beta2ndlinenonsmallcelllungcancerOncologySubcutaneousinjection20172021/IIImonoclonalantibody1stlinenonsmallcelllungcancerOncologySubcutaneousinjection20172021/IIIAdjuvantnonsmallcelllungcancerOncologySubcutaneousinjection20172022/IIIAVXS1011onasemno-SurvivalmotorneuronSpinalmuscularatrophyNeuroscienceIntravenousinfusion2018USapprovedgeneabepar-(SMN)gene(IVformulation)EUregistrationvovecreplacementtherapySpinalmuscularatrophyNeuroscienceIntrathecalinjection20182020/I(ITformulation)2AVXS201TBDMethylCpGbindingRettsyndromeNeuroscienceIntrathecalinjection20182023/Iprotein2(MECP2)genereplacementtherapyBYL7193alpelisibPI3KalphainhibitorPIK3CAmutanthormonereceptorpositiveOncologyOral2018USapproved(HR+)/humanepidermalgrowthfactorEUregistrationreceptor2negative(HER2)postmenopausaladvancedbreastcancer,2ndline(+fulvestrant)PIK3CA-relatedovergrowthspectrumOncologyOral20192020/IIITriplenegativebreastcancerOncologyOral20192023/IIIHormonereceptor-negative(HR-)/humanOncologyOral20192023/IIIepidermalgrowthfactorreceptor2-positive(HER2+)advancedbreastcancerOvariancancerOncologyOral20192023/IIIHeadandnecksquamouscellcarcinomaOncologyOral2019≥2024/IIICEE321TBDPan-JAKinhibitorAtopicdermatitisImmunology,Topical2019≥2024/IIHepatologyandDermatologyCFZ533iscalimabBlocking,nondepleting,SolidorgantransplantationImmunology,Intravenousinfusion20172023/IIantiCD40monoclonalHepatologyandantibodyDermatologySjgren'ssyndromeImmunology,Intravenousinfusion2018≥2024/IIHepatologyandDermatologyCosentyxsecukinumabAntiinterleukin17NonradiographicaxialspondyloarthritisImmunology,Subcutaneousinjection2015US/EUmonoclonalantibodyHepatologyandregistrationDermatologyPsoriaticarthritisheadtoheadstudyImmunology,Subcutaneousinjection20152020/IIIversusHumira(adalimumab)HepatologyandDermatologyAnkylosingspondylitisheadtoheadstudyImmunology,Subcutaneousinjection20152022/IIIversusSandozbiosimilarHyrimozHepatologyand(adalimumab)DermatologyHidradenitissuppurativaImmunology,Intravenousinfusion20172022/IIIHepatologyandDermatologyGiantcellarteritisImmunology,Intravenousinfusion2018≥2024/IIHepatologyandDermatologyLichenplanusImmunology,Intravenousinfusion2019≥2024/IIHepatologyandDermatologyCSJ117TBDAntithymicstromalSevereasthmaRespiratoryInhalation20182023/IIlymphopoietinmonoclonalantibodyfragmentECF843TBDBoundarylubricantDryeyeOphthalmologyEyedrops20172022/IIEntrestovalsartanandAngiotensinreceptor/ChronicheartfailurewithpreservedCardiovascular,Oral20122020/IIIsacubitrilneprilysininhibitorejectionfractionRenal(assodiumandMetabolismsaltcomplex)PostacutemyocardialinfarctionCardiovascular,Oral20152021/IIIRenalandMetabolism1ApprovedintheUSasZolgensmaforspinalmuscularatrophy(IVformulation)2TheFDAhasplacedapartialclinicalholdonAVXS-101intrathecaltrialsforspinalmuscularatrophypatientsbasedonfindingsinasmallpreclinicalanimalstudy.
3ApprovedintheUSasPiqrayforPIK3CAmutantHR+/HER2-postmenopausaladvancedbreastcancer,2ndline(+fulvestrant)Item4.
InformationontheCompany2YearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseINC280capmatinibc-METinhibitorNonsmallcelllungcancerOncologyOral2014USregistrationSolidtumorsOncologyOral2019≥2024/IIJakaviruxolitinibJAK1/2inhibitorAcutegraftversushostdiseaseOncologyOral20162021/IIIChronicgraftversushostdiseaseOncologyOral20162021/IIIKAE609cipargaminPfATP4inhibitorMalariaEstablishedOral2012≥2024/IIMedicinesSeveremalariaEstablishedOral2019≥2024/IIMedicinesKAF156ganaplacideImidazolopiperazinesMalariaEstablishedOral2014≥2024/IIderivativeMedicinesKisqaliribociclibCDK4/6inhibitorHR+/HER2breastcancer(adjuvant)OncologyOral20182022/IIIKJX839inclisiranSmall-interferingRNAHyperlipidemiaCardiovascular,Subcutaneousinjection2019US/EU(PCSK9)RenalregistrationandMetabolismSecondarypreventionofcardiovascularCardiovascular,Subcutaneousinjection2019≥2024/IIIeventsinpatientswithelevatedlevelsRenalofLDL-CandMetabolismKymriahtisagen-CD19targetedchimericRelapsed/refractoryfollicularlymphomaOncologyIntravenousinfusion20172021/IIlecleucelantigenreceptorTcellimmunotherapyRelapsed/refractorydiffuselargeBcellOncologyIntravenousinfusion20182021/IIIlymphomain1strelapseRelapsed/refractorydiffuselargeBcellOncologyIntravenousinfusion2017≥2024/IIlymphoma(+pembrolizumab)LAM320clofazimineMycobacterialMultidrugresistanttuberculosisEstablishedOral20162021/IIIDNAbindingMedicinesLJC242tropifexor,FXRagonistandNonalcoholicsteatohepatitisImmunology,Oral2017≥2024/IIcenicrivirocCCR2/5inhibitorHepatologyand(infixed-doseDermatologycombination)LJN452tropifexorFXRagonistNonalcoholicsteatohepatitisImmunology,Oral2015≥2024/IIHepatologyandDermatologyLMI070branaplamSMN2RNAsplicingSpinalmuscularatrophyNeuroscienceOral2017≥2024/IImodulatorLNP023TBDFactorBinhibitorIgAnephropathyCardiovascular,Oral20182023/IIRenalandMetabolismC3glomerulopathyCardiovascular,Oral20182023/IIRenalandMetabolismParoxysmalnocturnalhemoglobinuriaCardiovascular,Oral20192023/IIRenalandMetabolismMembranousnephropathyCardiovascular,Oral2018≥2024/IIRenalandMetabolismLOU064TBDBTKinhibitorChronicspontaneousurticariaImmunology,Oral20172023/IIHepatologyandDermatology177Lu-TBDTargetedDNAMetastaticcastration-resistantOncologyIntravenousinfusion20182020/IIIPSMA-617destructionviaprostatecancerbeta-particleradiationLXE408TBDKinetoplastidVisceralleishmaniasisEstablishedOral2019≥2024/IIproteasomeinhibitorMedicinesMBG453TBDTIM-3antagonistMyelodysplasticsyndromeOncologyIntravenousinfusion20182021/IIAcutemyeloidleukemiaOncologyIntravenousinfusion2019≥2024/IIOMB157ofatumumabAntiCD20monoclonalRelapsingmultiplesclerosisNeuroscienceSubcutaneousinjection2015US/EUantibodyregistrationPDR001spartalizumabAntiPD1monoclonalMetastaticBRAFV600+OncologyIntravenousinfusion20172020/IIIantibodymelanoma(w/Tafinlar+Mekinist)Metastaticmelanoma(combo)OncologyIntravenousinfusion20172023/IIQBW251TBDCFTRpotentiatorChronicobstructivepulmonarydiseaseRespiratoryOral2017≥2024/IIQGE031ligelizumabHighaffinityantiIgEChronicspontaneousurticaria/Immunology,Subcutaneousinjection20172021/IIImonoclonalantibodychronicidiopathicurticariaHepatologyandDermatologyQMF149indacaterol,Longactingbeta2AsthmaRespiratoryInhalation2019EUregistrationmometasoneadrenergicagonistandfuroateinhaledcorticosteroid(infixeddosecombination)Item4.
InformationontheCompany3YearprojectenteredFormulation/currentPlannedfilingProject/CommonMechanismBusinessrouteofdevelopmentdates/currentproductnameofactionPotentialindicationfranchiseadministrationphasephaseQVM149indacaterol,Longactingbeta2AsthmaRespiratoryInhalation2019EUregistrationmometasoneadrenergicagonist,furoate,longactingmuscarinicglyco-antagonistandinhaledpyrroniumcorticosteroidbromide(infixeddosecombination)RTH2584brolucizumabAntiVEGFsinglechainNeovascular(wet)agerelatedmacularOphthalmologyIntravitrealinjection2019USapprovedantibodyfragmentdegenerationEUregistrationDiabeticmacularedemaOphthalmologyIntravitrealinjection20172021/IIIRetinalveinocclusionOphthalmologyIntravitrealinjection20182023/IIIProliferativediabeticretinopathyOphthalmologyIntravitrealinjection20192023/IIISAF312TBDTRPV1antagonistChronicocularsurfacepainOphthalmologyTopical2019≥2024/IISEG1015crizanlizumabPselectininhibitorSicklecelldiseaseOncologyIntravenousinfusion2019USapprovedEUregistrationTQJ230TBDAnti-apo(a)antisenseSecondarypreventionofcardiovascularCardiovascular,Subcutaneousinjection2018≥2024/IIIoligonucleotideeventsinpatientswithelevatedlevelsRenalandoflipoprotein(a)MetabolismUNR844TBDReductionofPresbyopiaCardiovascular,Eyedrops2017≥2024/IIdisulfidebondsRenalandMetabolismVAY736ianalumabAntiBAFF(BcellAutoimmunehepatitisImmunology,Subcutaneousinjection2016≥2024/IIactivatingfactor)HepatologyandmonoclonalantibodyDermatologyPrimarySjgren'ssyndromeImmunology,Subcutaneousinjection2015≥2024/IIHepatologyandDermatologyVPM087TBDInterleukin-1betaColorectalcancer,1stline;OncologyIntravenousinfusion2018≥2024/Ineutralizationrenalcellcarcinoma,1stlinemonoclonalantibodyXolairomalizumabAntiIgEmonoclonalNasalpolypsRespiratorySubcutaneousinjection2017US/EUantibodyregistrationFoodallergyRespiratorySubcutaneousinjection20192021/IIIZPL389adriforantHistamineH4receptorAtopicdermatitisImmunology,Oral2017≥2024/IIantagonistHepatologyandDermatology4ApprovedintheUSasBeovuforneovascular(wet)agerelatedmaculardegeneration5ApprovedintheUSasAdakveoforsicklecelldisease
- Ankylosingselected相关文档
- frequencyselected
- mesonselected
- lowestselected
- approachselected
- schemesselected
- detailedselected
RackNerd:便宜vps补货/1核/768M内存/12G SSD/2T流量/1G带宽,可选机房圣何塞/芝加哥/达拉斯/亚特拉大/荷兰/$9.49/年
RackNerd今天补货了3款便宜vps,最便宜的仅$9.49/年, 硬盘是SSD RAID-10 Storage,共享G口带宽,最低配给的流量也有2T,注意,这3款补货的便宜vps是intel平台。官方网站便宜VPS套餐机型均为KVM虚拟,SolusVM Control Panel ,硬盘是SSD RAID-10 Storage,共享G口带宽,大流量。CPU:1核心内存:768 MB硬盘:12 ...
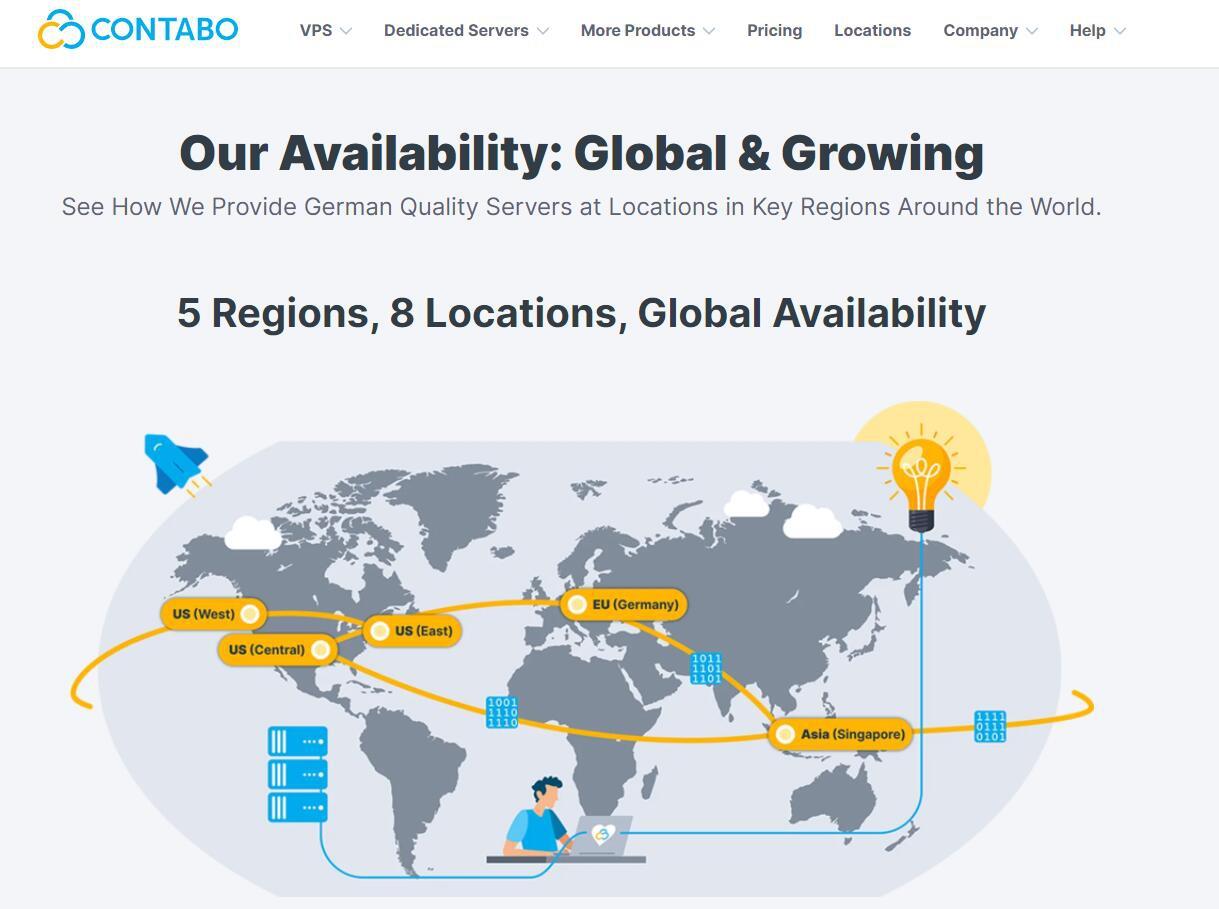
Contabo美国独立日促销,独立服7月€3.99/月
Contabo自4月份在新加坡增设数据中心以后,这才短短的过去不到3个月,现在同时新增了美国纽约和西雅图数据中心。可见Contabo加速了全球布局,目前可选的数据中心包括:德国本土、美国东部(纽约)、美国西部(西雅图)、美国中部(圣路易斯)和亚洲的新加坡数据中心。为了庆祝美国独立日和新增数据中心,自7月4日开始,购买美国地区的VPS、VDS和独立服务器均免设置费。Contabo是德国的老牌服务商,...
香港服务器多少钱一个月?香港云服务器最便宜价格
香港服务器多少钱一个月?香港服务器租用配置价格一个月多少,现在很多中小型企业在建站时都会租用香港服务器,租用香港服务器可以使网站访问更流畅、稳定性更好,安全性会更高等等。香港服务器的租用和其他地区的服务器租用配置元素都是一样的,那么为什么香港服务器那么受欢迎呢,香港云服务器最便宜价格多少钱一个月呢?阿里云轻量应用服务器最便宜的是1核1G峰值带宽30Mbps,24元/月,288元/年。不过我们一般选...

selected为你推荐
-
路由routepqqgraph支持ipad支付appleeaccelerator开启eAccelerator内存优化就各种毛病,DZ到底用哪个内存优化比较好。。。ipad连不上wifiipad无法加入网络怎么回事canvas2动漫cv井口裕香,都有哪些作品?ms17-010win10pybaen.10.的硬币是哪国的再中国至多少钱ms17-010win10华为 slatl10是什么型号google中国地图怎样用GOOLE搜中国地图用卫星看的那一种(可以看到城市和房子的)