5.133669.com
33669.com 时间:2021-05-13 阅读:()
ReviewMicrotubule-targetedagents:WhenmitochondriabecomeessentialtochemotherapyA.
Rovinia,A.
Savrya,b,D.
Braguera,b,M.
Carréa,aINSERMUMR911,CentredeRechercheenOncologiebiologiqueetenOncopharmacologie,UniversitéAix-Marseille,FacultédePharmacie,27BoulevardJeanMoulin,13385MarseilleCedex5,FrancebAssistancePublique-HpitauxdeMarseille,FranceabstractarticleinfoArticlehistory:Received22July2010Receivedinrevisedform2January2011Accepted4January2011Availableonline7January2011Keywords:AnticancerdrugMicrotubuleMitochondriaApoptosisPharmacologyMicrotubule-TargetingAgents(MTAs)constituteaclassofdrugslargelyusedforcancertreatmentinadultsandchildren.
Incancercells,theysuppressmicrotubuledynamics,andinducecelldeathviathemitochondrialintrinsicpathway.
Todate,linksbetweenmitochondriaandmicrotubulenetworkdisturbanceinMTAsmechanismofactionarenotobvious.
Theaimofthepresentcontributionistoprovideelementsthatcouldanswertothequestion:howfararemitochondriaessentialtoanticancerchemotherapythattargetsthemicrotubulecytoskeletonWereviewthemainmolecularcandidatestolinkmicrotubulealterationwiththeapoptoticmitochondrialpathwaycontrol.
InvolvementofdirecttargetingofmitochondriainMTAefcacyisalsodiscussed.
Furthermore,welineupcurrentevidenceandemergingconceptsontheparticipationofbothmitochondriaandmicrotubuleinMTAneurotoxicsideeffects.
Todeciphertheinterconnectionsbetweenthemitochondrialandthemicrotubulenetworksmayhelptoimprovecancercellresponsetochemotherapy.
ThisarticleispartofaSpecialIssueentitled:BioenergeticsofCancer.
2011ElsevierB.
V.
Allrightsreserved.
1.
IntroductionImprovementofanticancertherapeuticstrategiesisoftenlimitedbyapoorknowledgeofmolecularmechanismsunderlyingcarcinogenesisandcellresponsetotreatment.
Althoughcarcinogenesisisaverycomplexprocess,itcanbedividedintotwocrucialsteps:1)appearanceofoncogenemutationsinagroupofcells,leadingto2)disorderlycellproliferation.
Thisuncontrolledcelldivision,joinedtoneo-angiogenesisinduction,isresponsiblefortumorformation,growthandspreading.
Asthemicrotubulenetworkishighlyinvolvedincellproliferation,itappearedtobeapreferentialtargetforcancertherapy.
Forthatmatter,greateffortshavebeendevotedtodiscoverdrugsthataffectmicrotubules.
Nowadays,theso-calledMicrotubule-TargetedAgents(MTAs)constituteaclassofanticancerdrugslargelyusedintheclinics.
Amongthem,taxanesandVincaalkaloidsarepowerfulinhibitorsofmicrotubuledynamicsandapoptosisinducers,usedtotreatsolidtumorsandmalignanthemopathies.
ThetherapeuticsuccessofMTAsaccountsforthedevelopmentofnewmicrotubule-targetingcom-poundsbypharmaceuticalcompanies,whichhasbeen–andisstill–intenseandfruitful.
Theaimofthepresentcontributionistoanswerthequestion:howfararemitochondriaessentialtoanticancerchemotherapythattargetsthemicrotubulecytoskeletonFirst,webrieysummarizedthecellulareffectsofMTAsonmicrotubuledynamics,andtheirfunctionalconsequences.
Then,asMTAanticancereffectivenesshasbeenrelatedtotheapoptoticmitochondrialpathway,welinedupthemainmolecularcandidatestolinkmicrotubulealterationwithapoptosiscontrol;andwediscussthedirecteffectsofMTAsonmitochondria.
WealsoreviewedcurrentevidenceandemergingconceptsofbothmitochondriaandmicrotubuleroleinMTAneurotoxicsideeffects.
Lastly,weconsideredMTAsastoolstostudytheinuenceofmicrotubuledynamicsonmitochondrialdynamics.
2.
MTAfamilyofmolecules,areferenceinanticancerchemotherapyMicrotubulesarecytoskeletalhollowlamentspresentinmosteukaryoticcellsthatresultfrompolymerizationofα/βtubulinpolymers.
Inmammaliancells,microtubulesarepolarizedstructuresnucleatedatthecentrosomewheretheminusendisanchored.
Theplusendgrowstothecellperipheryandconstantlyexploresthecytoplasm,makingmicrotubulehighlydynamicpolymers.
Indeed,afundamentalpropertyofmicrotubulesistoexhibitadynamicinstability,whichischaracterizedbyasuccessionofslowpolymerizationandrapiddepolymerizationphases.
Theswitchfrommicrotubulegrowthorpausetoshrinkageisknownas"catastrophe",andtheswitchfromshrinkagetogrowthorpauseisnamed"rescue"[1].
Thisdynamicbehaviorisofparticularimportanceintheregulationofmanycellularfunctionsbythemicrotubulecytoskeleton,asbeingthesupportforcelldivision,shapechanges,motilityandcelldifferentiationsuchasBiochimicaetBiophysicaActa1807(2011)679–688ThisarticleispartofaSpecialIssueentitled:BioenergeticsofCancer.
Correspondingauthor.
INSERMUMR911-CR02,FacultedePharmacie,27BdJeanMoulin,13385MarseilleCedex5,France.
Tel.
:+33491835626;fax:+33491835635.
E-mailaddress:manon.
carre@univmed.
fr(M.
Carré).
0005-2728/$–seefrontmatter2011ElsevierB.
V.
Allrightsreserved.
doi:10.
1016/j.
bbabio.
2011.
01.
001ContentslistsavailableatScienceDirectBiochimicaetBiophysicaActajournalhomepage:www.
elsevier.
com/locate/bbabioformationofneuronaloutgrowths.
Thus,farfrombeingconsideredasmerearchitecturalelements,microtubulesarekeydeterminantsofcellulareventsandfunctionalities.
Onlyafewmicrotubule-governedcellularprocessesrequireanoverallremodelingofthecytoskeletonnetwork,butalldependonatightlyregulatedmicrotubuledynamics.
Microtubule-TargetedAgents(MTAs)remainbenchmarkclinicaltreatmentsdisplayinghighcytotoxicefciencyandarestillwidelyusedagainstabroadspectrumofchildren'sandadult'stumors.
Theyrecentlyreceivedarevivalofinterestaspotentanti-angiogenicandvascular-disruptingagents[2].
Researchanddevelopmentarestillinprogresstodiscovermoreactiveandlesstoxiccompounds(forextensivereviews,see[3,4]).
Attemptstoimprovetheintracellulardrugconcentrationandamorespecictargetingoftumorcellsareespeciallyunderintenseinvestigations,andtheemergingeldofnanotechnologiesactivelyparticipatestothisquest[5].
TheMTAfamilyiscomposedofmorethan30compounds,historicallyclassiedindestabilizingandstabilizingagents,accordingtotheirbindingsiteontubulinormicrotubules[3,6].
Destabilizingagentsinhibitmicrotubulepolymerizationinvitro;theyincludetheVincaalkaloidssuchasvinblastineorvincristinethatbindtotheso-called"Vinca"tubulindomain,aswellasnocodazoleorcombretastatinsthatbindtothe"colchicine"tubulindomain.
Stabilizingagentsenhancetubulinpolymerizationandmicrotubulestabilization;theyincludetaxanes,suchaspaclitaxelordocetaxel,andEpothilones.
Althoughthedistinctionindestabilizingandstabilizingagentsisusefulforstructure–activitystudies,itisnomoreusedincellularandinvivostudiessincebothclasseshavebeenshowntocommonlydisturbmicrotubuledynamics,withoutchangingtheoverallmicrotubulemassinalargerangeofconcentrations[7].
Itisnowlargelyacceptedthatcytotoxic(i.
e.
pro-apoptotic)concentrationsofMTAssuppressmicrotubuledynamics.
[8–11].
Anextensivedecreaseinmicrotubuledynamicspreventsthenormalalignmentofchromosomes,activatesthespindleassemblycheckpoint,whichresultsinthecellblockadeinmitosis[12].
Itshouldherebenoticedthatamoderatedsuppressionofmicrotubuledynamics,whichdidnotallowtheaccumulationofcellsinmitosis,isalsoassociatedwithapoptosisinductionintumorcells[13–15].
Thus,itremainsunclearwhetherandhowthemitoticarrestiscoupledtotheactivationoftheapoptoticmachinery[16,17].
Elsewhere,microtubuledynamicssup-pressioncorrelateswithcelllocomotionalteration,asdescribedinbroblaststreatedwithpaclitaxelandnocodazole[18,19].
Surprisingly,theincreaseinmicrotubuledynamicsbylowconcentrationsofvinunineandpaclitaxelhasbeenalsoshowntoinhibitendothelialcellmigration,resultinginMTAanti-angiogenicactivities[20].
Thiseffectwascorrelatedwiththeinhibitionofinterphasemicrotubulefunctions,resultingininhibitionofadhesionsitedynamicsandformationoflong-livedstressbers[21].
Interestingly,whatevertheconcentrationstudied,MTAsdisorganizethenetworkofmicrotubule+endtrackingproteins(+TIPs).
Amongthem,theendbinding(EB)familyofproteinsspecicallyformscomet-likeaccumulationattheendsofgrowingmicrotubules.
Astheyensuremicrotubulegrowth,EBproteinsarecrucialregulatorofmicrotubuledynamics[22].
Taxanes,VincaalkaloidsandEpothilonescommonlydisruptEBproteindistributionincancer,endothelialandneuronalcells[21,23](andpersonaldata),whichmayaccountforMTAs'maineffectsoninterphaseandmitoticmicrotubuledynamics.
3.
AnticancereffectivenessofMTAs:mitochondriacomeintoplayMTAs,includingthenewestinclinicaltrials,haveshownahighabilitytoinduceapoptosis[24–28].
Thisprogrammedandtightlyregulatedcelldeathwasrstidentiedin1993,byBhallaetal.
[29],asthemechanismresponsiblefortheanti-tumorcytotoxiceffectsofpaclitaxelinhumanmyeloidleukemiacells[14].
Sincethen,theireffectiveness,evenintheclinics,hasbeenwellcorrelatedtoapoptosisextentinalltumorcells.
Itshouldbenotedthat,eventhoughMTAscaninducecelldeathinendothelialcells,itisdispensablefortheiranti-angiogenicandanti-vascularpropertiesatlowdoses[30,31].
ThissectionwillthusbefocusedonapoptoticactorsnecessarytoMTAanticanceractivityintumorthemselves,givingcluestounderstandhowcanmicrotubuledysfunctionbeanecessarystepinapoptosisinduction.
3.
1.
IntrinsicpathwayinductionbyMTAs:whattolearnforonco-pharmacologyFromthenumerousintracellularapoptoticsignals,twomajorroutescanbediscerned:themitochondrialpathway,knownastheintrinsicpathway,andthedeathreceptorpathway,so-calledextrinsicpathway.
AlthoughMTAshavebeenshowntomodifyexpressionlevelsofdeathreceptorsandtheirligands,theextrinsicpathwayisgenerallyexcludedfromMTA-inducedapoptosis[32–34].
MTAeffectivenessislargelyacceptedasaconsequenceofcaspaseactivationthroughtheintrinsicapoptoticpathway[35].
Ofnote,theanticanceractivityofnovelimprovedpharmacologicalfeaturesofMTAs,suchashydrophilicpaclitaxelderivativeorpaclitaxelloadedpoly(L-lacticacid)microparticles,isevaluatedbymeasuringtheirimpactonmitochondria[36,37].
Itpointsouthowtheseorganellesarecrucialintheappraisalofmicrotubule-targetedchemotherapysuccess.
3.
1.
1.
ThelethalcascadeMitochondrialmembranepermeabilizationisthecentralgateinturningon/offapoptosis,asitallowsthereleaseofalargepanelofpro-apoptoticproteins[38,39],thatactivatesdownstreamsignalingcascadesandleadstothenalexecutionofcelldeath.
AnearlyandtransienthyperpolarizationofthemitochondrionhasbeenreportedwithMTAs,whichis,intumorcells,followedbytheΔΨmcollapseandthereleaseofpro-apoptoticfactors[17,30].
Thesubsequentactivationofthecaspasecascadeisthenon-returnpointtocellbiochemicaldestructionandphenotypicchangesinMTA-inducedapoptosis[35,40–43].
Inresponsetopaclitaxel-orepothilone-treatment,themassivecytochromecreleasefrommitochondriatriggerstheformationofthemulti-factorcomplexapoptosome,whichleadstoanearlyincreaseincaspase-9activity.
Accordingly,thecaspase-9specicinhibitor(z-LEHD-fmk)effectivelyprotectscellsfromMTA-mediatedapoptosis[42–44].
OverexpressionofApaf-1,theadaptormoleculeoftheapoptosome,hasbeenshowntoenhancepaclitaxel-inducedapoptosis[45].
Insituationswhereactivationofcaspase-9isdisturbed,overexpressionofthedownstreameffectorcaspase-3restoressensitivitytoMTAsinresistantcancercells[46].
Smac/DiabloandOmi/Htra2peptidesarealsoreleasedfrommitochondriaduringtheirpermeabilization,andfavorcaspaseactivitybypreventingactionoftheinhibitorofapoptosisproteins(IAPs)[39,47].
InhibitionofIAPscan,invitro,modulatetheefcacyofantineoplasticagents.
Smac/DIABLOpeptideenhancedtheinductionofapoptosisandlongtermantiproliferativeeffectsofpaclitaxelinbreastcancercells[48,49].
CombinationofpaclitaxelwitharecombinantadenovirusencodingSmac/DIABLOalsoproducedgreaterlevelsofapoptosisinovariancarcinomacells[50].
Inaddition,ectopicSmac/DIABLOsensitizeddrug-resistantepithelialovariancancercellstopaclitaxel-inducedapoptosis[51].
Thus,anincreaseinSmac/DIABLOactivityseemstobeapromisingstrategytoimproveMTAtreatment,includingindrug-resistantcancer,butaclinicalapproachisstilllacking.
3.
1.
2.
Bcl-2familymembersMuchefforthasbeendirectedtowardelucidatingthemechanismofmitochondrialmembranepermeabilization,and,whilestilldiscussed,itisnowlargelyacceptedthatthisprocessisunderthecontroloftheBcl-2familymembers.
TheBcl-2familyiscomposedofupto30proteinsthatcanbedividedinto3groups:oneofBcl-2-likesurvivalfactorsandtwoothersofcelldeathpromotingfactorsnamedBax-likeandBH3-only[52].
Therelativelevelsofanti-andpro-apoptoticclansinmitochondrial680A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688membranesarbitratecelllifeordeathdecision.
MTAsmodulatebothexpressionlevelsandactivityofpro-apoptoticmembersoftheBcl-2family.
Up-regulationoftheBad,PUMA,Baxand/orBakhasbeenobservedaftertreatmentwithpaclitaxel,epothiloneBaswellaswithVincaalkaloids[15,53–55].
Asexpected,Bcl-XS,BaxorBadover-expressionsensitizescancercellstopaclitaxelandvincristine[56–58].
Inaddition,MTAstriggerBaxactivationthroughitsconformationalchange[54,59]thatallowsN-terminaldomainexhibitionandBaxstableinsertionintotheoutermitochondrialmembrane[60,61].
MTAsalsoinitiateBimtranslocationfrommicrotubulestomitochondria,asdiscussedinthenextsection.
Lastly,thelatecleavageofBidintoafunctionalfragment(tBid)hasbeenproposedtobeasignalamplica-tionloopwhichcouldberequiredforanoptimalreleaseofmitochondrialfactorsfollowingMTAtreatment[62,63].
Inparallel,Bcl-2-likeanti-apoptoticproteinscanbepost-transla-tionallyinactivatedbyhyperphosphorylationinducedbyalargepanelofMTAs[15,64–68].
Ithasinitiallybeenthoughttobeamarkerofmitosisratherthananapoptosis-relatedsignal,butboththeextentandthedurationofthemitosis-associatedBcl-2hyperphosphorylationislikelytodistinguishapre-apoptoticcellfromonedestinedtodivide[69].
TreatmentswithMTAsalsodecreaseexpressionlevelsofBcl-XLandBcl-2toactivatetheintrinsicpathway[70–72].
Overexpressionoftheanti-apoptoticproteinsBcl-2andBcl-XLareinvolvedinresistanceofcancercellstomicrotubule-targetedchemotherapy[73–76].
Thus,Bcl-2and/orBcl-XLantisensestrategieshavebeendeveloped,andwererstreportedtomediateanincreaseindocetaxel-andpaclitaxel-sensitivityinvitroandinmicexenografts[33,77–79].
ABT-737,aBH3-mimeticthatantagonizesBcl-2,Bcl-XL,andBcl-w,increasedMTApro-apoptoticeffectsinavarietyoftumorcelllines,includingbreastcancercellswithacquiredresistancetopaclitaxel[80–82].
Similarly,A-385358,asmallmoleculewithrelativeselectivityforbindingtoBcl-XLpotentiatedtheactivityofpaclitaxelinnon-small-celllungcancercells,invitroandinvivo[83].
In2003,aphaseIIclinicaltrialofoblimersen(antisenseoligonucleotidestargetingBcl-2)incombinationwithdocetaxelvalidatedprogressionintophaseIIIforpatientswithadvancedhormonerefractoryprostatecancer.
However,Bcl-2inhibitiondidnotalwayssucceedinenhancingtreatmenteffectiveness[84–87].
Furthermore,itshouldbeusedwithcautionincombinationwithMTAssinceworksshowedthatBcl-2down-regulationisresponsibleforanunexpectedresistancetopaclitaxelandvinunineinovariancancercells[17,88].
Accordingly,Bcl-2overexpressioncanincreasenon-smallcelllungcancersensitivitytodocetaxel[89].
Similarly,prostatecancercellscanadapttoantisenseRNAtargetingBcl-xL,leadingtoaparadoxalresistancetodocetaxelandvinblatine[90].
Thisdualroleof"prosurvi-val"mitochondrialproteinspointsouttheneedforfurtherinvestigationtoelucidatetheirrealcontributioninMTAtreatmenteffectivenessandtheirpotentialastargetforclinicalantisensestrategies.
3.
2.
Proteinsreleasedfromthemicrotubuletomitochondria:atthedoorstepsofMTAeffectivenessWhilethemaineffectsofMTAsonboththeintrinsicapoptoticsignalingcascadeandthemicrotubulenetworkarenowquitewellunderstood,clearlinksarestilllackingbetweenthetwoevents.
Sinceapoptosisandproliferationarecloselyrelated[91],effectsofMTAsonproteinsthatcontrolbothphenomenahavebeenstudiedforyears.
Cellcycleisatightlyregulatedprocess,anditsdisturbancebyMTAsmayparticipateinthemitochondrialapoptoticpathwayinitiation.
Therefore,studiesonhowcellcyclecheckpointsmodulatetheintrinsicpathwayareofmajorinterest,andextensivelyreviewed[27].
Thepresentsectionwillbefocusedonproteincandidatesthatcouldbuildmolecularbridgesbetweenmicrotubulesandtheapoptoticmachinery.
Indeed,microtubulesserveasscaffoldsfordifferentsignalingmolecules,extendingthelistofbiologicalprocess-esregulatedbythemicrotubulenetworkincells.
Amongmicrotubule-linkedcomponents,regulatorsoftheapoptoticprocesssuchasp53andBimmaybereleasedfrommicrotubulestowardsmitochondria.
SincepolymerizinganddepolymerizingMTAsdisplaythecommonpropertyofsuppressingmicrotubuledynamics,itprobablyexplainswhytheinvolvementofthemicrotubule-transportedfactorsinapoptosisissimilaramongsttheseanticancerdrugs.
3.
2.
1.
p53,themultifacetedmoleculeDifferentlinesofevidenceindicatethatp53up-regulationandactivationarerequiredformaximalcellsensitivitytoTaxanes,VincaalkaloidsandEpothilones[15,54,71,92–96].
Theroleofp53inapoptosismediatedbymicrotubuledisturbanceisreinforcedbyrecentdatashowingthatoverexpressionofthemicrotubule-associatedproteinTaurenderedneuroblastomacellsresistanttoapoptosisbymechanismsinvolvingreductionofp53level[97].
MTAconcentrationseemstobecritical,aslowdosesofpaclitaxelincreasep53proteinlevels,whereashighdosesdonotaffectoreveninhibittheselevels[72,98,99].
Similarly,lowdosesofvinunineup-regulatep53,whilehighdosesincreasep53toalowerextent[17].
Theconcentration-dependentactivationofthistranscriptionfactormayresultfromitsmicrotubule-governedtrans-port.
Indeed,itsdynein-dependenttransporttothenucleusisthoughttobeaconsequenceofmicrotubuledynamicssuppressionbybothstabilizinganddepolymerizingagents[92,95,100,101].
Highconcentra-tionsofMTAsprobablyinducetooextensivedamagestomicrotubules,whichcannomoreserveastracksforp53trafcking.
Onceinthenucleus,p53transcriptionalpropertiesareactivated,leadingtomodulationingenetargetssuchasp53itselforp21and,moreinterestingly,membersoftheBcl-2family[36,102,103].
UnderMTAtreatment,p53inductionhasbeenshowntodown-regulateBcl-2andup-regulateBax[15,71,104,105].
Recently,weidentiedanovelbindingsiteofp53ontheBcl-2promoter,responsibleforthetranscriptionaldown-regulationofBcl-2byvinorelbineinbreastcarcinomacells[104].
TheBH3-onlymembersPUMAandNoxacanalsobeup-regulatedfollowingp53induction[54,106,107],buttheroleofMTA-mediateddisturbanceofmicrotubuledynamicsinthisprocessremainstobecharacterized.
Besidesitsabilitytomodulatethepro-apoptotic/anti-apoptoticratioinfavorofapoptosis,p53inducesapoptosisthroughatranscription-independentmechanism.
Inresponsetovariouspro-apoptoticstimuli,includingMTAs,p53rapidlymovestothemitochondriaincellularandmicemodels[15,93,108–111].
Onceatthemitochondrion,p53primarilyassociatedwiththeoutermitochondrialmembrane,butasmallsubfractionmaybelocatedwithinthemitochondrialmatrix[108,111–113].
Themitochondrialp53participatesintheapoptoticcascadebyinducingthemitochondrialoutermembranepermeabiliza-tionthroughdirectactivationofBax-likeandBH3-onlyproteins,andbyforminginhibitorycomplexeswithBcl-2-likemembers[106,114].
Thep53-targeteddrugpithrin-μ,whichblockstheinteractionofp53withBcl-XL[115],inhibitsapartofvinorelbine-inducedapoptosis[104],supportingaroleforthemitochondrialp53inMTApro-apoptoticproperties.
Whilesomekeystepshavebeeninvokedtoexplainhowp53translocationfrommicrotubulescanbetriggered[74,115],theexactroleofMTA-induceddamagesofmicrotubuledynamicsisstillanunexploredeld.
3.
2.
2.
BimgoesforawalktomitochondriaBH3-onlymemberoftheBcl-2family,Bimisinvolvedininhibitionofmetastasisformationandinthepro-apoptoticresponseoftumorcellstochemotherapy[116–121].
Bimexpressionlevelincreasesdramaticallyafterpaclitaxeltreatment[99,122]andgenesilencingexperimentsshowedthatitsup-regulationisacriticalregulatorofapoptosisincancercells[122–124].
WhileBimcanbelocalizedatmitochondriawithoutcelldeathstimuliinsomecellularmodels,numberofstudiesshowedthatitissequesteredbymicrotubulesviaitsinteractionwiththedyneinlightchainDLC1/LC8ofthemotorcomplexorviaadirectinteractionwithmicrotubules[125,126].
Interestingly,Bim-decientlymphocytesarelesssensitivetopaclitaxel-mediatedperturbationsof681A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688themicrotubulenetwork,whichunderscorestheimportanceofBiminmediatingapoptosisinducedbyagentsthattargetmicrotubules[127].
Duringtreatmentwithpaclitaxel,Bimmayactasasensorofcytoskeletonintegrity,sinceitisunleashedfrommicrotubulesandtranslocatestomitochondria[123].
Insupporttothishypothesis,theplanttoxincalledpersinhasbeenshowntoinduceBimreleasebyactingasamicrotubule-stabilizingagentinbreastcancercells[128].
Similarly,theHIV-1TatproteinactivatedapoptosisinhostcellsbytriggeringBimtranslocationtomitochondriainresponsetomicrotubulestabilization[127].
WealsoshowedthatepothiloneBinducedBimaccumulationtomitochondria,leadingtoapoptosisinhumanneuroblastomacells[93].
Sincethen,workshaveconrmedthatMTAsfreedBimfrommicro-tubulesandenrichedmitochondriainthispro-apoptoticprotein[129,130].
BimsequestrationfromdyneinmaybereleasedthroughitsphosphorylationbyJNK,asdescribedwithUVtreatment[116,131].
SuchakinasecanbeactivatedbyMTAs[27].
However,itmayalsobearguedthatBimtranslocationtriggeredbyMTAsresultsfromdisturbanceofmicrotubuledynamicsorstructure.
Indeed,microtu-buledestabilizationbyGadd45aledtoBimreleasewithoutactivationofJNK[132].
Amongthedifferenthypotheses,theenhancedgenerationofmitochondrialreactiveoxygenspecies(ROS)byMTAs[35,41,133–136]hasbeenshowntoberesponsibleforBimaccumu-lationtomitochondriainneuroblastomacells[93].
Thus,themitochondrialcompartmentitselfcaninitiatethesignalingdialogwiththemicrotubulenetwork,whichresultsinapoptosisandthusparticipatesinMTAefcacy.
4.
DirecteffectsofMTAsonmitochondria:wheredowestandInparallelwiththeireffectsonthemicrotubulenetwork,MTAscanalsoactivatetheapoptoticpathwaythroughadirectactiononmitochondria.
IncubationofmitochondriaisolatedfromtumorcellswitheitherTaxanes,VincaalkaloidsorEpothilonesprovokescyto-chromecrelease[137].
Incontrast,otherclassesofanticancerdrugssuchas5FUordoxorubicinwerenotabletopermeabilizemembranesfromisolatedmitochondria[138](andpersonaldata).
MTAsinduceanearlyΔΨmcollapseandasubsequentlargeamplitudeswellingofisolatedmitochondria[88,136,138,139].
Themitochondrialmembranepermeabilizationisinhibitablebycyclosporine[138],consistentlywiththepermeabilitypore-dependentCa2+lossfromisolatedmitochondriainducedbypaclitaxelandnocodazole[139,140].
Paclitaxelhasalsobeenshowntosignicantlyincreasethecytochromeoxidase-mediatedROSproductionbypuriedmitochondria[136].
Interestingly,thepro-apoptoticeffectsofpaclitaxelcanbeenhancedbyimprovingitsspecicdeliverytomitochondriausingamitochondria-specicnanocarriersystem(DQAsomes)[141].
Then,itraisesthequestionofthepotentialtarget(s)ofMTAsinmitochondria.
Tubulin,thatwasfoundtobestronglyassociatedwithmitochondrialmembranesinbothpuriedorganellesandwholecells[139,142,143],wastherstcandidateproposedtoexplainthespeciceffectofMTAsonisolatedmitochondria.
ThemitochondrialtubulinsubfractionisenrichedinclassIIIβ-tubulin(TUBB3),but,incontrastwiththecytoskeletalform,itsoverexpressiondoesnotcorrelatewithcellresistancetoMTAs[144].
AnassociationhasbeenreportedbetweenthemitochondrialtubulinandVDAC[142,145],themajoroutermembraneporethatislikelyinvolvedinthereleaseofpro-apoptoticfactorsbyMTAsfromtheintermembranespacetocytosol.
ThecurrentknowledgeontheMTA-inducedintrinsicpathwayhasrejuvenatedthestudyfromEvtodienkoetal.
[146],whichsuggestedtheinvolvementofeithermitochondria-boundtubulinperseand/orcontactsbetweenmitochondriaandmicrotubulesinregulationofmitochondrialmembranepermeability[146].
Morethan10yearslater,theC-terminaltailoftubulinhasbeenproposedtomodulatetheVDACopeningandthemitochondrialrespirationrate[145].
Bcl-2hasalsobeenidentiedasapotentialtargetforpaclitaxelbyphagedisplayandachemicalapproach[147,148].
Recently,Ferlinietal.
revealedthat,inovariancancercells,paclitaxeldirectlytargetedBcl-2intheloopdomain[149].
Asaresult,paclitaxelchangedtheroleofBcl-2frominhibitortoenhancerofthemitochondrialmembranepermeabilization,facilitatingapoptosis.
Thisprocessmayexplainwhythedown-regulationofBcl-2isresponsibleforanunexpectedresistancetoMTAsindifferenttumorcelltypesandcancerpatients[17,66,88,89].
Finally,thetwohypotheseshavejoinedwhentheassociationbetweenthetubulinandBcl-2hasbeenrevealed,byco-immunoprecipitationfrommitochondriallysatesandwithpuriedproteins[17,88,150].
Suchacomplexthatgatherstubulin,Bcl-2andVDACcouldbebothadirecttargetforMTAsandaregulatorofthemitochondrialmembranepermeability.
Thus,whilethemitochondri-altargetsofMTAsareprobablynotalldened,itisreasonabletothinkthattheseanti-tumoragentsdisplaycrucialanti-mitochondrialpropertiesinvolvedintheirefcacy.
Therelevanceofthisphenomenonremainsdifculttoproveinwholecells,sincethedirecteffectsofMTAsonthemitochondrialnetworkareusuallyundistinguishablefromthoseresultingfrommicrotubulemodications.
Moreover,resultsshowingthatinterfer-enceofpaclitaxelwiththemitochondrialsignalingcascadeoccurredupstreamofmicrotubuleorganizationalteration[140]shouldbereevaluatedbymeasuringmicrotubuledynamics,ahighlymoresensitiveparameterthanmicrotubulearchitecture.
Nevertheless,weshowedthattheearlyproductionofROSfrommitochondriawasnecessarytoBimtranslocationtowardsmitochondria,whichinturntriggeredapoptosisinhumanneuroblastomacells[93].
ThesedatastronglysuggestthatsomeofthemostrapideffectsofMTAsontheintrinsicapoptoticpathwaymaybeinitiatedthroughadirectactiononmitochondrialintegrity.
TheinvolvementofMTA-mediatedROSgenerationfrommitochondrianeedstobereconsideredinprocessessuchasEBproteincometdisruptionbyMTAs(seesection2),whichisthoughttoresultfromtargetingofthemicrotubulesystem,butwhichhasbeenrecentlyshowntobetriggeredbyH2O2[151].
Then,itiseasytospeculatethatsomealterationsofthemicrotubuledynamicsinducedbyMTAscouldbe,atleastinpart,linkedtothedrugs'anti-mitochondrialproperties.
5.
Mitochondria–microtubulepair:MTAsstirupthetroubleinneuronalsystemChemotherapy-inducedperipheralneuropathy(CIPN)isthemaindoselimitingsideofalargepanelofMTAs[152,153].
Mostofthetime,peripheralneuropathyreversesifthetreatmentisstopped.
However,insomecases,recoveryfromsymptomsisincompleteandalongperiodofregenerationisrequiredtorestorefunction[154].
Thisneurotoxicsideeffectisstillanunsolvedclinicalissue,sothewaysthatcytoskeletonandorganellesinterplayandhowMTAsaltertheserelationshipsinneuronalmodelsareofhighimportance.
5.
1.
Animalmodelsofchemotherapy-inducedneuropathy:slippingfrommicrotubuletomitochondrialinvolvementDespiteintensiveeffortsinthedevelopmentofneuroprotectiveagents(recentlyreviewedin[155]),todate,therearenoapprovedtherapiesforpreventionortreatmentofneuropathiestriggeredbyMTAchemotherapy[152,153,156].
ThisispartlyduetothepoorunderstandingofmechanismsunderlyingMTA-inducedneurotoxic-ity,andthustoalackofavaluablemethodofstandardizationintheclinicalmeasurementofCIPN.
Asapostulate,ithasoftenbeendeclaredthatMTAssimilarlyaffectthemicrotubulenetworkincancerandneuronalcells,butevidencewasnotalwayssustained.
For20yearsnow,animalmodelsofCIPNhavebeendeveloped(listedin[157]),attemptingtoinvestigateMTAsmechanismofactionontheperipheralnervoussystem.
Mostofthesemodelsareessentially682A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688focusedonthereportofpain-relatedbehaviorandonlyafewgoonfurtheronneurophysiologicalexperiments.
Whileratmodelsofvincristine-inducedperipheralneuropathydescribedabnormalmi-crotubuleassembliesanddensitiesasmaindamages[158,159],thesemicrotubuleproleswerenotobservedwithpaclitaxelinratmodels[160].
IthasthusbeenhypothesizedthatthemicrotubulenetworkwaslikelynottheonlytargetofMTAstobeinvolvedinneurondysfunctions.
Inparallel,thedecipheringofMTAactionprogressivelyslippedconsiderationsfrommicrotubulestomitochondria.
Invitrodatausingpaclitaxelreportedanterogradeandretrogradeaxonaltransportblockadeinratdorsalrootganglia(DRG)andhippocampalneurons.
Then,invivostudiesshowedasignicantincreaseintheincidenceofswollenmitochondriainaxonsafterpaclitaxeltreatment[160,161].
SimilardatawereobtainedwithinvitrocultureofDRG,inwhichtheinductionofatypicalmitochondriabypaclitaxelwasassociatedwithasignicantreductionoftheirfunctioningandthelossofmitochondrialmembranepotential[162].
Nevertheless,inthesestudies,thelinkbetweenmicrotubuledensityandinhibitionofneuronalorganelledistributionremainedcontroversialduetovariablepaclitaxelinjectionsmodesanddifferentadministeredconcentrationsoverthetime[163,164].
5.
2.
InhibitionofMTA'sneurotoxiceffects:domitochondrianeedtobeprotectedManyworksareattemptingtondclinicallyefcientneuropro-tectorsabletoenhanceneuronalcellsurvival.
Todate,severalneuroprotectiveagentslikethiols,neurotrophicfactorsandantiox-idantshavebeentestedinpreclinicalmodelsandclinicaltrialsfortheirabilitytopreventCIPN[165,166].
Althoughseveralofthesecompoundswereidentiedasneuroprotectivemoleculesofinterest,clinicaldataarestilldiscussed.
Mitochondrialdysfunctionandoxidativestressarewidelybelievedtounderliethepathogenesisofvariousneurodegenerativediseases[167].
Theinvestigationofneuroprotectiveantioxidantswasthusrationalizedasapromisingstrategytopreventoralleviatemitochondrialdamages.
Amongthem,efcacyofacetyl-LcarnitineandmorerecentlyalphalipoicacidtoexertneuroprotectiveeffectsagainstMTAs,invitro,wasassociatedwithareducedincidenceofswollenandvacuolatedmitochondriainratC-ber[161]aswellasinsensoryDRGneurons[162].
Moreover,alphalipoicacidpreventedtheearlylossofmembranepotentialdifferentialinmitochondriaexposedtopaclitaxel,thuspreventingneuronsfrommitochondrialenergeticfailureprobablythroughanti-oxidantactivities.
Anattractivestrategy,themitochondrialprotectionmightbelimitedinpreventingMTA-mediatedCIPNwhichinvolvesalterationofothertargetssuchasthemicrotubulecytoskeleton.
Inthiscontext,olesoxime(TRO19622)appearedasapromisingdrugcandidatetotreattheneurotoxicsideeffectsofmicrotubule-targetedchemotherapy[168].
ThisnewmoleculeprotectedneuronalcellsfromMTA-inducedneuriteshrinkagebyrestoringbothmicrotubuledynamics–throughEBproteincometsmaintainingatmicrotubuleends–andthemicrotubule-governedmitochondrialtrafcking[23].
Thus,compoundslikeolesoximethatareabletojointhesetwopropertieswouldholdpromisetobetterpreventandcurepatientssufferingfromneurodegenerativedisordersinwhichmicrotubule-associatedaxonaltransportisdefective.
TheirstudymayalsobringadditionalfundamentalinsightsintothemolecularmechanismsunderlyingneurotoxicpropertiesofMTAs.
6.
Inuenceofmicrotubuledynamicsperturbationonmitochondrialdynamics:aneweldofinvestigationShortlyaftertheirsuccessfuluseintheclinics,MTAshavebeenextensivelyemployedinfundamentalresearch.
Bymodulatingmicro-tubulearchitectureanddynamics,theyareappropriatepharmacolog-icaltoolstoprobethemitochondria–cytoskeletoninteractions.
6.
1.
HowcanMTAsmodulatethemitochondrialmotilityMTAeffectshavebeenespeciallystudiedinneuronalcells,inwhichmitochondriamovethroughouttheneuronalprocessestocontributetosynapticmaintenance.
Appropriatepositioningofthemitochondrialnetworkensuresorganellefunctionandisnecessarytocellsurvivalandfunctionality.
Assoonas1978,ChanKYandBuntAHusedvinblastinetoformparacrystalstructuresandtohighlighttheinterconnectedspatialorganizationofmicrotubulesandmitochon-driainsynaptosomesandaxonterminalsofratcerebralcortex[169].
Afewyearslater,axonalorganelletransporthasbeenshownnottobetotallysuppressedaftermicrotubuledisruption[170],andtobepartiallyinhibitedbytheintroductionofagentsthatspecicallydisruptactinmicrolaments[171–173].
Inparallel,thediscoveryandcharacterizationofmicrotubule-basedmotorskinesinanddyneinallowedtobetterenvisagetheaxonaltransportsystem.
Complemen-tarydata,intendingtodeciphertheimportanceofmicrotubule-governedtransportamongothercytoskeleta,usedamodelofneuronsgrownwithvinblastine.
Resultsshowedthatthewholemitochondrialcompartmentconcentratedintothecellbody,suggestingthatmicrotubuleswerenecessaryandsufcientforthetransportofmitochondriainaxons[174].
Consideringtheuncontestedroleofmicrotubulesastracksfortheintracellulartrafckingofmitochondria,itcanbearguedthatmitochondrialtransportdefectscouldresultfrommicrotubuledynam-icsalteration.
Insupporttothis,theparkinsoniantoxin(MPP+)hasbeenrecentlyshowntoinduceanearlyalterationofmicrotubuledynamicsandorientation,andasubsequentmitochondrialtransportimpairment[175].
Worksintumorcellsshowedthatpaclitaxelincreasedthespeedofmitochondrialmovement,whereascolchicineandnocodazoleretardedit[123,176],suggestingthatmicrotubulestabilizationcouldbenecessarytoorganelletrafcking.
Uptonow,themajorhypothesisexploredwasthechangesinmolecularmotorbindingtothemicrotubulerailways,throughtubulinpost-translationalmod-ications(PTMs).
Indeed,bindingofthemotorproteinkinesin-1,thatmostlyensuresanterogrademitochondrialtransportinaxons,isincreasedbymicrotubuledetyrosinationandacetylation[177,178].
ThesetwomajorPTMscorrelatewithmicrotubulestabilizationandareinducedbypaclitaxelandixabepiloneincancercells[179,180].
Thesedataweresupportedbyobservationsofpaclitaxelinhibitoryeffectsonfastretrogradetransportinratperipheralnerves[163,181].
However,resultsarestillcontroversialsincearecentworkshowedthatpaclitaxelabolishedkinesin-1translocationinpolarizedneuronsbyincreasingtheoveralllevelsoftubulinacetylation,detyrosinationandpolyglutamyla-tion[182].
Oneexplanationcouldbethat,whilemicrotubulestabiliza-tionisnecessaryforthemitochondrialtransport,itsover-stabilizationcompromisestheintracellulartrafcking.
Elsewhere,microtubuleassociatedprotein(MAPs)bindingtomicrotubulescanalsoinuencemotor-basedaxonaltransport,mainlybyaffectingtheattachmentanddetachmentcycleofthemotors.
Inneurons,tauandMAP4cancontroltheintracellulartrafckingbyreducingtheattachmentofkinesintomicrotubules[183,184].
MorerecentlySeitzetal.
showedadecreaseinrun-lengthforbothkinesinordyneinwhenMAP2candtauwereoverexpressedincells,combinedwithasignicantdecreaseinkinesinattachmentfrequencyontaxol-stabilizedmicrotubules[185].
Then,aspaclitaxelhasbeenshowntoincreaseMAP2afnityformicrotubule[186],itcouldthuseasilybethoughtthatpaclitaxelbyregulatingMAPsbindingcouldmodulateorganelletrafcking.
Lastly,thep150Gluedsubunitofdynactinisa+TIP(cfpart2)that,inassociationwithdynein,participatestoorganelleretrogradetransport.
Interestingly,p150GluedinteractionwithEB1atmicrotubuleplus-endsseemstobecentralinthedynein/dynactinfunction[187].
Thus,bysignicantlydisturbingEB1localization[21,23],MTAsmaycausethelossofbothmicrotubuledynamicsandmitochondrialtransport,whichtogethermightleadtocancercelldeath.
Sameobservationscouldbe683A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688transposedtotheneuronalmodelasEBfamilymembersarecrucialforneuritegrowthandmaintenance,andaretoolsofchoicetopreciselymeasureplus-endmicrotubuledynamicsbylivemicroscopy[188].
Interestingly,neurotoxicconcentrationsofpaclitaxelhavebeenshowntoinduceadecreaseinthenumberandlengthofEB3comettailsinAplysianeurons[189].
Moreover,paclitaxelsignicantlydisturbedthemicrotubulepolarorientation,byreducingthepercentageofmicro-tubuleswithplusendsfacingtheaxontipandincreasingthosewithplusendsfacingthecellbody[189].
Allthesemicrotubulemodicationswereassociatedwithaseverelyimpairedmitochondrialtransport.
Inagreementwiththesedata,weshowedthatpaclitaxelandvincristinesuppressedEB1andEB3accumulationatmicrotubuleplus-ends,andsignicantlyreducedthemitochondrialmotilityinhumandifferentiat-edneuronalcells[23].
6.
2.
CanMTAsdisruptthession/fusionequilibriumRecently,withtheemergenceoftheneuropathologyeldofresearch,studieshaveourishedsuggestingthatmitochondrialdysfunctionsareearlyandcausaleventsinmanyneurodegenerativediseases[190]suchasamyotrophiclateralsclerosis,Alzheimer's,Huntington'sorParkinson'sdiseases.
Onepotentialcauseofmitochon-drialdysfunctionisthedisruptionofthehighlycontrolledequilibriumbetweenmitochondrialssionandfusion.
Excessivessionordefectsinfusionaltercellfunctionsandviabilitythroughimpairmentofmitochondrialmotility,decreaseenergyproduction,andincreaseoftheoxidativestress[191].
Examplesaregivenwithstudiesusingtaxolatconcentrationsresponsibleformicrotubulestrongstabilizationandleadingtodisruptionofmitochondrialssion/fusionbalance[192]aswellastheirabilitytofastlymoveanddistributetowardshighenergydemandsubcellularlocations[193].
Inthesestudies,MTAshavebeenemployedathighconcentrationsduringveryshorttimeofexposure(lessthan24h)toinducemicrotubulemodicationsandthustoanalyzemitochondrialdynamicityparameters.
Thereisnowacrucialneedinreconsideringtheconcentrationsemployed.
Indeed,lowerconcentrationsmaygivecomplementarycuestountanglemitochondriaandmicrotubuleinterconnectionsandmayhelptodecipherhowtheanti-microtubulepropertiesofMTAsleadtodisturbancesinmitochon-drialdynamics.
Intumorcells,suchmoderatedconcentrationsofMTAssignicantlyaltermicrotubuledynamicsandinducethemitochondrialnetworkfragmentation,asanearlyprocessassociatedwiththeirpro-apoptotic,anti-angiogenicandneurotoxicactivities[23].
AspreviouslyshownwithBH3-onlypeptides[194],ourrecentworkssuggestedthatthisprocesscouldresultfromBimaccumulationinmitochondrialmembranes(Savryetal,submitted),byamolecularmechanismthatshouldbeinvestigated.
7.
ConclusionToconclude,itclearlyappearsthatMTAsarebothanticancerdrugswithahighclinicalvalueandveryusefultoolstoanalyzetherolesplayedbythemicrotubulenetworkinphysio/pathologicalprocesses.
TodecipherthetangleofMTA-inducedapoptoticsignalsisatrickyexerciseand,todate,itisstilldifculttodeterminewhetherbiochemicaleventsthatleadtoapoptosisareactivateddownstreamorupstreaminhibitionofmicrotubuledynamicsandfunctions.
However,itbecomesclearthatcrucialmolecularlinksareestablishedbetweenthemicrotubulenetworkandtheapoptoticmachinery,toensurethesuccessofthecelldeathprogram.
Inthatsense,analysisofmechanismsresponsiblefortumorcellresistancetoMTAswouldalsoprovidekeyinformationaboutthecloseconnectionsbetweenmicrotubulesandtheapoptoticmachinery.
ThecoexistenceofmodicationsinthemicrotubulesystemandthemitochondrialsignalingcascadeincellsresistanttoMTAs[17,179,195]strengthenstheneedfornovelinsightsintointerconnectionsbetweenthetwocompartmentstohelpcircumventingthisclinicalproblem.
ItalsoconrmedthatthemitochondrionisstillapromisingtherapeutictargetthatcouldimprovecombinatorialtherapywithMTAsandprovidecrucialarmstohelptreatingcancers.
AcknowledgementsWethankDr.
StéphaneHonoréandDr.
VéroniqueBourgarel-Reyforhelpfulcommentsonthemanuscript.
ARreceivedafellowshipfromtheRégionProvenceAlpesCted'Azur(France)andASfromtheAssistancePubliquedesHopitauxdeMarseille.
References[1]T.
Mitchison,M.
Kirschner,Dynamicinstabilityofmicrotubulegrowth,Nature312(1984)237–242.
[2]E.
Pasquier,N.
Andre,D.
Braguer,Targetingmicrotubulestoinhibitangiogenesisanddisrupttumourvasculature:implicationsforcancertreatment,Curr.
CancerDrugTargets7(2007)566–581.
[3]D.
Calligaris,P.
Verdier-Pinard,F.
Devred,C.
Villard,D.
Braguer,D.
Latte,Microtubuletargetingagents:frombiophysicstoproteomics,Cell.
Mol.
LifeSci.
67(2010)1089–1104.
[4]S.
M.
Chen,L.
H.
Meng,J.
Ding,Newmicrotubule-inhibitinganticanceragents,ExpertOpin.
Investig.
Drugs19(2010)329–343.
[5]F.
Petrelli,K.
Borgonovo,S.
Barni,Targeteddeliveryforbreastcancertherapy:thehistoryofnanoparticle-albumin-boundpaclitaxel,ExpertOpin.
Pharmacother.
11(2010)1413–1432.
[6]E.
A.
Perez,Microtubuleinhibitors:differentiatingtubulin-inhibitingagentsbasedonmechanismsofaction,clinicalactivity,andresistance,Mol.
CancerTher.
8(2009)2086–2095.
[7]L.
Wilson,M.
A.
Jordan,Newmicrotubule/tubulin-targetedanticancerdrugsandnovelchemotherapeuticstrategies,J.
Chemother.
16(Suppl4)(2004)83–85.
[8]S.
Honore,K.
Kamath,D.
Braguer,L.
Wilson,C.
Briand,M.
A.
Jordan,Suppressionofmicrotubuledynamicsbydiscodermolidebyanovelmechanismisassociatedwithmitoticarrestandinhibitionoftumorcellproliferation,Mol.
CancerTher.
2(2003)1303–1311.
[9]S.
Honore,E.
Pasquier,D.
Braguer,Understandingmicrotubuledynamicsforimprovedcancertherapy,Cell.
Mol.
LifeSci.
62(2005)3039–3056.
[10]K.
Kamath,M.
A.
Jordan,SuppressionofmicrotubuledynamicsbyepothiloneBisassociatedwithmitoticarrest,CancerRes.
63(2003)6026–6031.
[11]B.
Pourroy,S.
Honore,E.
Pasquier,V.
Bourgarel-Rey,A.
Kruczynski,C.
Briand,D.
Braguer,Antiangiogenicconcentrationsofvinunineincreasetheinterphasemicrotubuledynamicsanddecreasethemotilityofendothelialcells,CancerRes.
66(2006)3256–3263.
[12]C.
L.
Rieder,H.
Maiato,Stuckindivisionorpassingthrough:whathappenswhencellscannotsatisfythespindleassemblycheckpoint,Dev.
Cell7(2004)637–651.
[13]S.
Honore,K.
Kamath,D.
Braguer,S.
B.
Horwitz,L.
Wilson,C.
Briand,M.
A.
Jordan,Synergisticsuppressionofmicrotubuledynamicsbydiscodermolideandpaclitaxelinnon-smallcelllungcarcinomacells,CancerRes.
64(2004)4957–4964.
[14]V.
K.
Ngan,K.
Bellman,B.
T.
Hill,L.
Wilson,M.
A.
Jordan,MechanismofmitoticblockandinhibitionofcellproliferationbythesemisyntheticVincaalkaloidsvinorelbineanditsnewerderivativevinunine,Mol.
Pharmacol.
60(2001)225–232.
[15]B.
Pourroy,M.
Carre,S.
Honore,V.
Bourgarel-Rey,A.
Kruczynski,C.
Briand,D.
Braguer,LowconcentrationsofvinunineinduceapoptosisinhumanSK-N-SHneuroblastomacellsthroughapostmitoticG1arrestandamitochondrialpathway,Mol.
Pharmacol.
66(2004)580–591.
[16]M.
E.
Bekier,R.
Fischbach,J.
Lee,W.
R.
Taylor,Lengthofmitoticarrestinducedbymicrotubule-stabilizingdrugsdeterminescelldeathaftermitoticexit,Mol.
CancerTher.
8(2009)1646–1654.
[17]M.
A.
Esteve,M.
Carre,V.
Bourgarel-Rey,A.
Kruczynski,G.
Raspaglio,C.
Ferlini,D.
Braguer,Bcl-2down-regulationandtubulinsubtypecompositionareinvolvedinresistanceofovariancancercellstovinunine,Mol.
CancerTher.
5(2006)2824–2833.
[18]G.
Liao,T.
Nagasaki,G.
G.
Gundersen,Lowconcentrationsofnocodazoleinterferewithbroblastlocomotionwithoutsignicantlyaffectingmicrotubulelevel:implicationsfortheroleofdynamicmicrotubulesincelllocomotion,J.
CellSci.
108(Pt11)(1995)3473–3483.
[19]A.
Mikhailov,G.
G.
Gundersen,Relationshipbetweenmicrotubuledynamicsandlamellipodiumformationrevealedbydirectimagingofmicrotubulesincellstreatedwithnocodazoleortaxol,CellMotil.
Cytoskeleton41(1998)325–340.
[20]E.
Pasquier,S.
Honore,B.
Pourroy,M.
A.
Jordan,M.
Lehmann,C.
Briand,D.
Braguer,Antiangiogenicconcentrationsofpaclitaxelinduceanincreaseinmicrotubuledynamicsinendothelialcellsbutnotincancercells,CancerRes.
65(2005)2433–2440.
[21]S.
Honore,A.
Pagano,G.
Gauthier,V.
Bourgarel-Rey,P.
Verdier-Pinard,K.
Civiletti,A.
Kruczynski,D.
Braguer,AntiangiogenicvinunineaffectsEB1localizationandmicrotubuletargetingtoadhesionsites,Mol.
CancerTher.
7(2008)2080–2089.
[22]A.
Akhmanova,M.
O.
Steinmetz,Trackingtheends:adynamicproteinnetworkcontrolsthefateofmicrotubuletips,Nat.
Rev.
Mol.
CellBiol.
9(2008)309–322.
[23]A.
Rovini,M.
Carre,T.
Bordet,R.
M.
Pruss,D.
Braguer,Olesoximepreventsmicrotubule-targetingdrugneurotoxicity:selectivepreservationofEB684A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688cometsindifferentiatedneuronalcells,Biochem.
Pharmacol.
80(2010)884–894.
[24]D.
Simoni,R.
Romagnoli,R.
Baruchello,R.
Rondanin,M.
Rizzi,M.
G.
Pavani,D.
Alloatti,G.
Giannini,M.
Marcellini,T.
Riccioni,M.
Castorina,M.
B.
Guglielmi,F.
Bucci,P.
Carminati,C.
Pisano,Novelcombretastatinanaloguesendowedwithantitumoractivity,J.
Med.
Chem.
49(2006)3143–3152.
[25]K.
D.
Wu,Y.
S.
Cho,J.
Katz,V.
Ponomarev,S.
Chen-Kiang,S.
J.
Danishefsky,M.
A.
Moore,Investigationofantitumoreffectsofsyntheticepothiloneanalogsinhumanmyelomamodelsinvitroandinvivo,Proc.
NatlAcad.
Sci.
USA102(2005)10640–10645.
[26]C.
Rappl,P.
Barbier,V.
Bourgarel-Rey,C.
Gregoire,R.
Gilli,M.
Carre,S.
Combes,J.
P.
Finet,V.
Peyrot,Interactionof4-arylcoumarinanaloguesofcombretastatinswithmicrotubulenetworkofHBL100cellsandbindingtotubulin,Biochemistry45(2006)9210–9218.
[27]M.
A.
Esteve,M.
Carre,D.
Braguer,Microtubulesinapoptosisinduction:aretheynecessaryCurr.
CancerDrugTargets7(2007)713–729.
[28]B.
Lin,L.
Catley,R.
LeBlanc,C.
Mitsiades,R.
Burger,Y.
T.
Tai,K.
Podar,M.
Wartmann,D.
Chauhan,J.
D.
Grifn,K.
C.
Anderson,Patupilone(epothiloneB)inhibitsgrowthandsurvivalofmultiplemyelomacellsinvitroandinvivo,Blood105(2005)350–357.
[29]K.
Bhalla,A.
M.
Ibrado,E.
Tourkina,C.
Tang,M.
E.
Mahoney,Y.
Huang,TaxolinducesinternucleosomalDNAfragmentationassociatedwithprogrammedcelldeathinhumanmyeloidleukemiacells,Leukemia7(1993)563–568.
[30]E.
Pasquier,M.
Carre,B.
Pourroy,L.
Camoin,O.
Rebai,C.
Briand,D.
Braguer,Antiangiogenicactivityofpaclitaxelisassociatedwithitscytostaticeffect,mediatedbytheinitiationbutnotcompletionofamitochondrialapoptoticsignalingpathway,Mol.
CancerTher.
3(2004)1301–1310.
[31]S.
E.
Holwell,B.
T.
Hill,M.
C.
Bibby,Anti-vasculareffectsofvinunineintheMAC15Atransplantableadenocarcinomamodel,Br.
J.
Cancer84(2001)290–295.
[32]A.
Goncalves,D.
Braguer,G.
Carles,N.
Andre,C.
Prevot,C.
Briand,Caspase-8activationindependentofCD95/CD95-Linteractionduringpaclitaxel-inducedapoptosisinhumancoloncancercells(HT29-D4),Biochem.
Pharmacol.
60(2000)1579–1584.
[33]R.
Kim,K.
Tanabe,M.
Emi,Y.
Uchida,T.
Toge,Deathreceptor-dependentand-independentpathwaysinanticancerdrug-inducedapoptosisofbreastcancercells,Oncol.
Rep.
10(2003)1925–1930.
[34]S.
Fulda,K.
M.
Debatin,Extrinsicversusintrinsicapoptosispathwaysinanticancerchemotherapy,Oncogene25(2006)4798–4811.
[35]N.
Andre,M.
Carre,G.
Brasseur,B.
Pourroy,H.
Kovacic,C.
Briand,D.
Braguer,Paclitaxeltargetsmitochondriaupstreamofcaspaseactivationinintacthumanneuroblastomacells,FEBSLett.
532(2002)256–260.
[36]S.
Jiang,Y.
Zu,Y.
Fu,Y.
Zhang,T.
Efferth,Activationofthemitochondria-drivenpathwayofapoptosisinhumanPC-3prostatecancercellsbyanovelhydrophilicpaclitaxelderivative,7-xylosyl-10-deacetylpaclitaxel,Int.
J.
Oncol.
33(2008)103–111.
[37]Y.
Kang,J.
Wu,G.
Yin,Z.
Huang,X.
Liao,Y.
Yao,P.
Ouyang,H.
Wang,Q.
Yang,Characterizationandbiologicalevaluationofpaclitaxel-loadedpoly(L-lacticacid)microparticlespreparedbysupercriticalCO2,Langmuir24(2008)7432–7441.
[38]S.
D.
Patterson,C.
S.
Spahr,E.
Daugas,S.
A.
Susin,T.
Irinopoulou,C.
Koehler,G.
Kroemer,Massspectrometricidenticationofproteinsreleasedfrommitochondriaundergoingpermeabilitytransition,CellDeathDiffer.
7(2000)137–144.
[39]G.
VanLoo,H.
Demol,M.
vanGurp,B.
Hoorelbeke,P.
Schotte,R.
Beyaert,B.
Zhivotovsky,K.
Gevaert,W.
Declercq,J.
Vandekerckhove,P.
Vandenabeele,Amatrix-assistedlaserdesorptionionizationpost-sourcedecay(MALDI-PSD)analysisofproteinsreleasedfromisolatedlivermitochondriatreatedwithrecombinanttruncatedBid,CellDeathDiffer.
9(2002)301–308.
[40]A.
Kruczynski,C.
Etievant,D.
Perrin,N.
Chansard,A.
Duos,B.
T.
Hill,Characterizationofcelldeathinducedbyvinunine,themostrecentVincaalkaloidinclinicaldevelopment,Br.
J.
Cancer86(2002)143–150.
[41]S.
J.
Park,C.
H.
Wu,J.
D.
Gordon,X.
Zhong,A.
Emami,A.
R.
Safa,Taxolinducescaspase-10-dependentapoptosis,J.
Biol.
Chem.
279(2004)51057–51067.
[42]C.
L.
Perkins,G.
Fang,C.
N.
Kim,K.
N.
Bhalla,TheroleofApaf-1,caspase-9,andbidproteinsinetoposide-orpaclitaxel-inducedmitochondrialeventsduringapoptosis,CancerRes.
60(2000)1645–1653.
[43]S.
Y.
Yuan,S.
L.
Hsu,K.
J.
Tsai,C.
R.
Yang,InvolvementofmitochondrialpathwayinTaxol-inducedapoptosisofhumanT24bladdercancercells,Urol.
Res.
30(2002)282–288.
[44]D.
Uyar,N.
Takigawa,T.
Mekhail,D.
Grabowski,M.
Markman,F.
Lee,R.
Canetta,R.
Peck,R.
Bukowski,R.
Ganapathi,ApoptoticpathwaysofepothiloneBMS310705,Gynecol.
Oncol.
91(2003)173–178.
[45]C.
Perkins,C.
N.
Kim,G.
Fang,K.
N.
Bhalla,OverexpressionofApaf-1promotesapoptosisofuntreatedandpaclitaxel-oretoposide-treatedHL-60cells,CancerRes.
58(1998)4561–4566.
[46]K.
Friedrich,T.
Wieder,C.
VonHaefen,S.
Radetzki,R.
Janicke,K.
Schulze-Osthoff,B.
Dorken,P.
T.
Daniel,Overexpressionofcaspase-3restoressensitivityfordrug-inducedapoptosisinbreastcancercelllineswithacquireddrugresistance,Oncogene20(2001)2749–2760.
[47]J.
Chai,C.
Du,J.
W.
Wu,S.
Kyin,X.
Wang,Y.
Shi,StructuralandbiochemicalbasisofapoptoticactivationbySmac/DIABLO,Nature406(2000)855–862.
[48]T.
E.
Fandy,S.
Shankar,R.
K.
Srivastava,Smac/DIABLOenhancesthetherapeuticpotentialofchemotherapeuticdrugsandirradiation,andsensitizesTRAIL-resistantbreastcancercells,Mol.
Cancer7(2008)60.
[49]C.
R.
Arnt,M.
V.
Chiorean,M.
P.
Heldebrant,G.
J.
Gores,S.
H.
Kaufmann,SyntheticSmac/DIABLOpeptidesenhancetheeffectsofchemotherapeuticagentsbybindingXIAPandcIAP1insitu,J.
Biol.
Chem.
277(2002)44236–44243.
[50]I.
A.
McNeish,S.
Bell,T.
McKay,T.
Tenev,M.
Marani,N.
R.
Lemoine,ExpressionofSmac/DIABLOinovariancarcinomacellsinducesapoptosisviaacaspase-9-mediatedpathway,Exp.
CellRes.
286(2003)186–198.
[51]H.
L.
Mao,P.
S.
Liu,J.
F.
Zheng,P.
H.
Zhang,L.
G.
Zhou,G.
Xin,C.
Liu,TransfectionofSmac/DIABLOsensitizesdrug-resistanttumorcellstoTRAILorpaclitaxel-inducedapoptosisinvitro,Pharmacol.
Res.
56(2007)483–492.
[52]L.
D.
Walensky,BCL-2inthecrosshairs:tippingthebalanceoflifeanddeath,CellDeathDiffer.
13(2006)1339–1350.
[53]N.
A.
Jones,J.
Turner,A.
J.
McIlwrath,R.
Brown,C.
Dive,Cisplatin-andpaclitaxel-inducedapoptosisofovariancarcinomacellsandtherelationshipbetweenbaxandbakup-regulationandthefunctionalstatusofp53,Mol.
Pharmacol.
53(1998)819–826.
[54]H.
Yamaguchi,J.
Chen,K.
Bhalla,H.
G.
Wang,RegulationofBaxactivationandapoptoticresponsetomicrotubule-damagingagentsbyp53transcription-dependentand-independentpathways,J.
Biol.
Chem.
279(2004)39431–39437.
[55]G.
Tudor,A.
Aguilera,D.
O.
Halverson,N.
D.
Laing,E.
A.
Sausville,Susceptibilitytodrug-inducedapoptosiscorrelateswithdifferentialmodulationofBad,Bcl-2andBcl-xLproteinlevels,CellDeathDiffer.
7(2000)574–586.
[56]T.
Strobel,Y.
T.
Tai,S.
Korsmeyer,S.
A.
Cannistra,BADpartlyreversespaclitaxelresistanceinhumanovariancancercells,Oncogene17(1998)2419–2427.
[57]H.
Sawa,T.
Kobayashi,K.
Mukai,W.
Zhang,H.
Shiku,BaxoverexpressionenhancescytochromecreleasefrommitochondriaandsensitizesKATOIIIgastriccancercellstochemotherapeuticagent-inducedapoptosis,Int.
J.
Oncol.
16(2000)745–749.
[58]V.
N.
Sumantran,M.
W.
Ealovega,G.
Nunez,M.
F.
Clarke,M.
S.
Wicha,Over-expressionofBcl-XSsensitizesMCF-7cellstochemotherapy-inducedapoptosis,CancerRes.
55(1995)2507–2510.
[59]G.
W.
Makin,B.
M.
Corfe,G.
J.
Grifths,A.
Thistlethwaite,J.
A.
Hickman,C.
Dive,Damage-inducedBaxN-terminalchange,translocationtomitochondriaandformationofBaxdimers/complexesoccurregardlessofcellfate,EMBOJ.
20(2001)6306–6315.
[60]B.
Antonsson,S.
Montessuit,B.
Sanchez,J.
C.
Martinou,Baxispresentasahighmolecularweightoligomer/complexinthemitochondrialmembraneofapoptoticcells,J.
Biol.
Chem.
276(2001)11615–11623.
[61]P.
F.
Cartron,M.
Priault,L.
Oliver,K.
Meah,S.
Manon,F.
M.
Vallette,TheN-terminalendofBaxcontainsamitochondrial-targetingsignal,J.
Biol.
Chem.
278(2003)11633–11641.
[62]D.
Grifn,S.
Wittmann,F.
Guo,R.
Nimmanapalli,P.
Bali,H.
G.
Wang,K.
Bhalla,MoleculardeterminantsofepothiloneBderivative(BMS247550)andApo-2L/TRAIL-inducedapoptosisofhumanovariancancercells,Gynecol.
Oncol.
89(2003)37–47.
[63]C.
Huisman,C.
G.
Ferreira,L.
E.
Broker,J.
A.
Rodriguez,E.
F.
Smit,P.
E.
Postmus,F.
A.
Kruyt,G.
Giaccone,Paclitaxeltriggerscelldeathprimarilyviacaspase-independentroutesinthenon-smallcelllungcancercelllineNCI-H460,Clin.
CancerRes.
8(2002)596–606.
[64]L.
Du,C.
S.
Lyle,T.
C.
Chambers,Characterizationofvinblastine-inducedBcl-xLandBcl-2phosphorylation:evidenceforanovelproteinkinaseandacoordinatedphosphorylation/dephosphorylationcycleassociatedwithapoptosisinduction,Oncogene24(2005)107–117.
[65]N.
Pathan,C.
Aime-Sempe,S.
Kitada,S.
Haldar,J.
C.
Reed,Microtubule-targetingdrugsinduceBcl-2phosphorylationandassociationwithPin1,Neoplasia3(2001)70–79.
[66]S.
Haldar,J.
Chintapalli,C.
M.
Croce,Taxolinducesbcl-2phosphorylationanddeathofprostatecancercells,CancerRes.
56(1996)1253–1255.
[67]L.
Brichese,A.
Valette,PP1phosphataseisinvolvedinBcl-2dephosphorylationafterprolongedmitoticarrestinducedbypaclitaxel,Biochem.
Biophys.
Res.
Commun.
294(2002)504–508.
[68]C.
D.
Scatena,Z.
A.
Stewart,D.
Mays,L.
J.
Tang,C.
J.
Keefer,S.
D.
Leach,J.
A.
Pietenpol,MitoticphosphorylationofBcl-2duringnormalcellcycleprogressionandTaxol-inducedgrowtharrest,J.
Biol.
Chem.
273(1998)30777–30784.
[69]M.
Fan,L.
Du,A.
A.
Stone,K.
M.
Gilbert,T.
C.
Chambers,Modulationofmitogen-activatedproteinkinasesandphosphorylationofBcl-2byvinblastinerepresentpersistentformsofnormaluctuationsatG2-M1,CancerRes.
60(2000)6403–6407.
[70]T.
A.
Buchholz,D.
W.
Davis,D.
J.
McConkey,W.
F.
Symmans,V.
Valero,A.
Jhingran,S.
L.
Tucker,L.
Pusztai,M.
Cristofanilli,F.
J.
Esteva,G.
N.
Hortobagyi,A.
A.
Sahin,Chemotherapy-inducedapoptosisandBcl-2levelscorrelatewithbreastcancerresponsetochemotherapy,CancerJ.
9(2003)33–41.
[71]C.
Bressin,V.
Bourgarel-Rey,M.
Carre,B.
Pourroy,D.
Arango,D.
Braguer,Y.
Barra,Decreaseinc-Mycactivityenhancescancercellsensitivitytovinblastine,AnticancerDrugs17(2006)181–187.
[72]M.
L.
Panno,F.
Giordano,F.
Mastroianni,C.
Morelli,E.
Brunelli,M.
G.
Palma,M.
Pellegrino,S.
Aquila,A.
Miglietta,L.
Mauro,D.
Bonoglio,S.
Ando,EvidencethatlowdosesofTaxolenhancethefunctionaltransactivatorypropertiesofp53onp21wafpromoterinMCF-7breastcancercells,FEBSLett.
580(2006)2371–2380.
[73]T.
Yoshino,H.
Shiina,S.
Urakami,N.
Kikuno,T.
Yoneda,K.
Shigeno,M.
Igawa,Bcl-2expressionasapredictivemarkerofhormone-refractoryprostatecancertreatedwithtaxane-basedchemotherapy,Clin.
CancerRes.
12(2006)6116–6124.
[74]I.
Lebedeva,R.
Rando,J.
Ojwang,P.
Cossum,C.
A.
Stein,Bcl-xLinprostatecancercells:effectsofoverexpressionanddown-regulationonchemosensitivity,CancerRes.
60(2000)6052–6060.
[75]X.
Tang,Y.
Zhu,L.
Han,A.
L.
Kim,L.
Kopelovich,D.
R.
Bickers,M.
Athar,CP-31398restoresmutantp53tumorsuppressorfunctionandinhibitsUVB-inducedskincarcinogenesisinmice,J.
Clin.
Invest.
117(2007)3753–3764.
685A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[76]J.
Hoffmann,I.
Vitale,B.
Buchmann,L.
Galluzzi,W.
Schwede,L.
Senovilla,W.
Skuballa,S.
Vivet,R.
B.
Lichtner,J.
M.
Vicencio,T.
Panaretakis,G.
Siemeister,H.
Lage,L.
Nanty,S.
Hammer,K.
Mittelstaedt,S.
Winsel,J.
Eschenbrenner,M.
Castedo,C.
Demarche,U.
Klar,G.
Kroemer,Improvedcellularpharmacokineticsandpharmacodynamicsunderliethewideanticanceractivityofsagopilone,CancerRes.
68(2008)5301–5308.
[77]C.
Leonetti,A.
Biroccio,C.
D'Angelo,S.
C.
Semple,M.
Scarsella,G.
Zupi,Therapeuticintegrationofc-mycandbcl-2antisensemoleculeswithdocetaxelinapreclinicalmodelofhormone-refractoryprostatecancer,Prostate67(2007)1475–1485.
[78]K.
Tanabe,R.
Kim,H.
Inoue,M.
Emi,Y.
Uchida,T.
Toge,AntisenseBcl-2andHER-2oligonucleotidetreatmentofbreastcancercellsenhancestheirsensitivitytoanticancerdrugs,Int.
J.
Oncol.
22(2003)875–881.
[79]K.
Yamanaka,P.
Rocchi,H.
Miyake,L.
Fazli,A.
So,U.
Zangemeister-Wittke,M.
E.
Gleave,InductionofapoptosisandenhancementofchemosensitivityinhumanprostatecancerLNCaPcellsusingbispecicantisenseoligonucleotidetargetingBcl-2andBcl-xLgenes,BJUInt.
97(2006)1300–1308.
[80]O.
Kutuk,A.
Letai,AlterationofthemitochondrialapoptoticpathwayiskeytoacquiredpaclitaxelresistanceandcanbereversedbyABT-737,CancerRes.
68(2008)7985–7994.
[81]T.
Oltersdorf,S.
W.
Elmore,A.
R.
Shoemaker,R.
C.
Armstrong,D.
J.
Augeri,B.
A.
Belli,M.
Bruncko,T.
L.
Deckwerth,J.
Dinges,P.
J.
Hajduk,M.
K.
Joseph,S.
Kitada,S.
J.
Korsmeyer,A.
R.
Kunzer,A.
Letai,C.
Li,M.
J.
Mitten,D.
G.
Nettesheim,S.
Ng,P.
M.
Nimmer,J.
M.
O'Connor,A.
Oleksijew,A.
M.
Petros,J.
C.
Reed,W.
Shen,S.
K.
Tahir,C.
B.
Thompson,K.
J.
Tomaselli,B.
Wang,M.
D.
Wendt,H.
Zhang,S.
W.
Fesik,S.
H.
Rosenberg,AninhibitorofBcl-2familyproteinsinducesregressionofsolidtumours,Nature435(2005)677–681.
[82]H.
Zall,A.
Weber,R.
Besch,N.
Zantl,G.
Hacker,ChemotherapeuticdrugssensitizehumanrenalcellcarcinomacellstoABT-737byamechanisminvolvingtheNoxa-dependentinactivationofMcl-1orA1,Mol.
Cancer9(2010)164.
[83]A.
R.
Shoemaker,A.
Oleksijew,J.
Bauch,B.
A.
Belli,T.
Borre,M.
Bruncko,T.
Deckwirth,D.
J.
Frost,K.
Jarvis,M.
K.
Joseph,K.
Marsh,W.
McClellan,H.
Nellans,S.
Ng,P.
Nimmer,J.
M.
O'Connor,T.
Oltersdorf,W.
Qing,W.
Shen,J.
Stavropoulos,S.
K.
Tahir,B.
Wang,R.
Warner,H.
Zhang,S.
W.
Fesik,S.
H.
Rosenberg,S.
W.
Elmore,Asmall-moleculeinhibitorofBcl-XLpotentiatestheactivityofcytotoxicdrugsinvitroandinvivo,CancerRes.
66(2006)8731–8739.
[84]K.
Bray,H.
Y.
Chen,C.
M.
Karp,M.
May,S.
Ganesan,V.
Karantza-Wadsworth,R.
S.
DiPaola,E.
White,Bcl-2modulationtoactivateapoptosisinprostatecancer,Mol.
CancerRes.
7(2009)1487–1496.
[85]A.
A.
Chanan-Khan,R.
Niesvizky,R.
J.
Hohl,T.
M.
Zimmerman,N.
P.
Christiansen,G.
J.
Schiller,N.
Callander,J.
Lister,M.
Oken,S.
Jagannath,PhaseIIIrandomisedstudyofdexamethasonewithorwithoutoblimersensodiumforpatientswithadvancedmultiplemyeloma,Leuk.
Lymphoma50(2009)559–565.
[86]S.
O'Brien,J.
O.
Moore,T.
E.
Boyd,L.
M.
Larratt,A.
Skotnicki,B.
Koziner,A.
A.
Chanan-Khan,J.
F.
Seymour,R.
G.
Bociek,S.
Pavletic,K.
R.
Rai,RandomizedphaseIIItrialofudarabinepluscyclophosphamidewithorwithoutoblimersensodium(Bcl-2antisense)inpatientswithrelapsedorrefractorychroniclymphocyticleukemia,J.
Clin.
Oncol.
25(2007)1114–1120.
[87]E.
Wesarg,S.
Hoffarth,R.
Wiewrodt,M.
Kroll,S.
Biesterfeld,C.
Huber,M.
Schuler,TargetingBCL-2familyproteinstoovercomedrugresistanceinnon-smallcelllungcancer,Int.
J.
Cancer121(2007)2387–2394.
[88]C.
Ferlini,G.
Raspaglio,S.
Mozzetti,M.
Distefano,F.
Filippetti,E.
Martinelli,G.
Ferrandina,D.
Gallo,F.
O.
Ranelletti,G.
Scambia,Bcl-2down-regulationisanovelmechanismofpaclitaxelresistance,Mol.
Pharmacol.
64(2003)51–58.
[89]Y.
Inoue,M.
Gika,T.
Abiko,T.
Oyama,Y.
Saitoh,H.
Yamazaki,M.
Nakamura,Y.
Abe,M.
Kawamura,K.
Kobayashi,Bcl-2overexpressionenhancesinvitrosensitivityagainstdocetaxelinnon-smallcelllungcancer,Oncol.
Rep.
13(2005)259–264.
[90]M.
Vilenchik,A.
J.
Raffo,L.
Benimetskaya,D.
Shames,C.
A.
Stein,AntisenseRNAdown-regulationofbcl-xLexpressioninprostatecancercellsleadstodiminishedratesofcellularproliferationandresistancetocytotoxicchemo-therapeuticagents,CancerRes.
62(2002)2175–2183.
[91]K.
Vermeulen,Z.
N.
Berneman,D.
R.
VanBockstaele,Cellcycleandapoptosis,CellProlif.
36(2003)165–175.
[92]P.
Giannakakou,D.
L.
Sackett,Y.
Ward,K.
R.
Webster,M.
V.
Blagosklonny,T.
Fojo,p53isassociatedwithcellularmicrotubulesandistransportedtothenucleusbydynein,Nat.
CellBiol.
2(2000)709–717.
[93]N.
R.
Khawaja,M.
Carre,H.
Kovacic,M.
A.
Esteve,D.
Braguer,Patupilone-inducedapoptosisismediatedbymitochondrialreactiveoxygenspeciesthroughBimrelocalizationtomitochondria,Mol.
Pharmacol.
74(2008)1072–1083.
[94]X.
M.
Liu,J.
D.
Jiang,A.
C.
Ferrari,D.
R.
Budman,L.
G.
Wang,Uniqueinductionofp21(WAF1/CIP1)expressionbyvinorelbineinandrogen-independentprostatecancercells,Br.
J.
Cancer89(2003)1566–1573.
[95]K.
Rathinasamy,B.
Jindal,J.
Asthana,P.
Singh,P.
V.
Balaji,D.
Panda,Griseofulvinstabilizesmicrotubuledynamics,activatesp53andinhibitstheproliferationofMCF-7cellssynergisticallywithvinblastine,BMCCancer10(2010)213.
[96]R.
B.
Tishler,D.
M.
Lamppu,S.
Park,B.
D.
Price,Microtubule-activedrugstaxol,vinblastine,andnocodazoleincreasethelevelsoftranscriptionallyactivep53,CancerRes.
55(1995)6021–6025.
[97]H.
H.
Wang,H.
L.
Li,R.
Liu,Y.
Zhang,K.
Liao,Q.
Wang,J.
Z.
Wang,S.
J.
Liu,Tauoverexpressioninhibitscellapoptosiswiththemechanismsinvolvingmultipleviability-relatedfactors,J.
AlzheimersDis.
(2010)167–179.
[98]R.
Drago-Ferrante,A.
Santulli,R.
DiFiore,M.
Giuliano,G.
Calvaruso,G.
Tesoriere,R.
Vento,LowdosesofpaclitaxelpotentlyinduceapoptosisinhumanretinoblastomaY79cellsbyup-regulatingE2F1,Int.
J.
Oncol.
33(2008)677–687.
[99]T.
T.
Tan,K.
Degenhardt,D.
A.
Nelson,B.
Beaudoin,W.
Nieves-Neira,P.
Bouillet,A.
Villunger,J.
M.
Adams,E.
White,KeyrolesofBIM-drivenapoptosisinepithelialtumorsandrationalchemotherapy,CancerCell7(2005)227–238.
[100]M.
D.
Galigniana,J.
M.
Harrell,H.
M.
O'Hagen,M.
Ljungman,W.
B.
Pratt,Hsp90-bindingimmunophilinslinkp53todyneinduringp53transporttothenucleus,J.
Biol.
Chem.
279(2004)22483–22489.
[101]K.
Rathinasamy,D.
Panda,Kineticstabilizationofmicrotubuledynamicinstabilitybybenomylincreasesthenucleartransportofp53,Biochem.
Pharmacol.
76(2008)1669–1680.
[102]A.
Basu,S.
Haldar,TherelationshipbetweenBcI2,Baxandp53:consequencesforcellcycleprogressionandcelldeath,Mol.
Hum.
Reprod.
4(1998)1099–1109.
[103]Y.
Wu,J.
W.
Mehew,C.
A.
Heckman,M.
Arcinas,L.
M.
Boxer,Negativeregulationofbcl-2expressionbyp53inhematopoieticcells,Oncogene20(2001)240–251.
[104]V.
Bourgarel-Rey,A.
Savry,G.
Hua,M.
Carre,C.
Bressin,C.
Chacon,J.
Imbert,D.
Braguer,Y.
Barra,Transcriptionaldown-regulationofBcl-2byvinorelbine:identicationofanovelbindingsiteofp53onBcl-2promoter,Biochem.
Pharmacol.
78(2009)1148–1156.
[105]P.
Giannakakou,R.
Robey,T.
Fojo,M.
V.
Blagosklonny,Lowconcentrationsofpaclitaxelinducecelltype-dependentp53,p21andG1/G2arrestinsteadofmitoticarrest:moleculardeterminantsofpaclitaxel-inducedcytotoxicity,Oncogene20(2001)3806–3813.
[106]J.
Han,C.
Flemington,A.
B.
Houghton,Z.
Gu,G.
P.
Zambetti,R.
J.
Lutz,L.
Zhu,T.
Chittenden,Expressionofbbc3,apro-apoptoticBH3-onlygene,isregulatedbydiversecelldeathandsurvivalsignals,Proc.
NatlAcad.
Sci.
USA98(2001)11318–11323.
[107]K.
Nakano,K.
H.
Vousden,PUMA,anovelproapoptoticgene,isinducedbyp53,Mol.
Cell7(2001)683–694.
[108]G.
Achanta,R.
Sasaki,L.
Feng,J.
S.
Carew,W.
Lu,H.
Pelicano,M.
J.
Keating,P.
Huang,Novelroleofp53inmaintainingmitochondrialgeneticstabilitythroughinteractionwithDNAPolgamma,EMBOJ.
24(2005)3482–3492.
[109]S.
Erster,U.
M.
Moll,Stress-inducedp53runsadirectmitochondrialdeathprogram:itsroleinphysiologicandpathophysiologicstressresponsesinvivo,CellCycle3(2004)1492–1495.
[110]M.
Mihara,S.
Erster,A.
Zaika,O.
Petrenko,T.
Chittenden,P.
Pancoska,U.
M.
Moll,p53hasadirectapoptogenicroleatthemitochondria,Mol.
Cell11(2003)577–590.
[111]C.
Sansome,A.
Zaika,N.
D.
Marchenko,U.
M.
Moll,Hypoxiadeathstimulusinducestranslocationofp53proteintomitochondria.
Detectionbyimmunou-orescenceonwholecells,FEBSLett.
488(2001)110–115.
[112]N.
D.
Marchenko,A.
Zaika,U.
M.
Moll,Deathsignal-inducedlocalizationofp53proteintomitochondria.
Apotentialroleinapoptoticsignaling,J.
Biol.
Chem.
275(2000)16202–16212.
[113]Y.
Zhao,L.
Chaiswing,J.
M.
Velez,I.
Batinic-Haberle,N.
H.
Colburn,T.
D.
Oberley,D.
K.
StClair,p53translocationtomitochondriaprecedesitsnucleartranslocationandtargetsmitochondrialoxidativedefenseprotein-manganesesuperoxidedismutase,CancerRes.
65(2005)3745–3750.
[114]D.
R.
Green,G.
Kroemer,Cytoplasmicfunctionsofthetumoursuppressorp53,Nature458(2009)1127–1130.
[115]E.
Strom,S.
Sathe,P.
G.
Komarov,O.
B.
Chernova,I.
Pavlovska,I.
Shyshynova,D.
A.
Bosykh,L.
G.
Burdelya,R.
M.
Macklis,R.
Skaliter,E.
A.
Komarova,A.
V.
Gudkov,Small-moleculeinhibitorofp53bindingtomitochondriaprotectsmicefromgammaradiation,Nat.
Chem.
Biol.
2(2006)474–479.
[116]N.
Corazza,S.
Jakob,C.
Schaer,S.
Frese,A.
Keogh,D.
Stroka,D.
Kassahn,R.
Torgler,C.
Mueller,P.
Schneider,T.
Brunner,TRAILreceptor-mediatedJNKactivationandBimphosphorylationcriticallyregulateFas-mediatedliverdamageandlethality,J.
Clin.
Invest.
116(2006)2493–2499.
[117]D.
B.
Costa,B.
Halmos,A.
Kumar,S.
T.
Schumer,M.
S.
Huberman,T.
J.
Boggon,D.
G.
Tenen,S.
Kobayashi,BIMmediatesEGFRtyrosinekinaseinhibitor-inducedapoptosisinlungcancerswithoncogenicEGFRmutations,PLoSMed.
4(2007)1669–16798discussion1680.
[118]J.
Kuroda,H.
Puthalakath,M.
S.
Cragg,P.
N.
Kelly,P.
Bouillet,D.
C.
Huang,S.
Kimura,O.
G.
Ottmann,B.
J.
Druker,A.
Villunger,A.
W.
Roberts,A.
Strasser,BimandBadmediateimatinib-inducedkillingofBcr/Abl+leukemiccells,andresistanceduetotheirlossisovercomebyaBH3mimetic,Proc.
NatlAcad.
Sci.
USA103(2006)14907–14912.
[119]J.
Lu,B.
Quearry,H.
Harada,p38-MAPkinaseactivationfollowedbyBIMinductionisessentialforglucocorticoid-inducedapoptosisinlymphoblasticleukemiacells,FEBSLett.
580(2006)3539–3544.
[120]A.
Nordigarden,M.
Kraft,P.
Eliasson,V.
Labi,E.
W.
Lam,A.
Villunger,J.
I.
Jonsson,BH3-onlyproteinBimmorecriticalthanPumaintyrosinekinaseinhibitor-inducedapoptosisofhumanleukemiccellsandtransducedhematopoieticprogenitorscarryingoncogenicFLT3,Blood113(2009)2302–2311.
[121]S.
N.
Willis,J.
M.
Adams,Lifeinthebalance:howBH3-onlyproteinsinduceapoptosis,Curr.
Opin.
CellBiol.
17(2005)617–625.
[122]A.
Sunters,S.
FernandezdeMattos,M.
Stahl,J.
J.
Brosens,G.
Zoumpoulidou,C.
A.
Saunders,P.
J.
Coffer,R.
H.
Medema,R.
C.
Coombes,E.
W.
Lam,FoxO3atranscrip-tionalregulationofBimcontrolsapoptosisinpaclitaxel-treatedbreastcancercelllines,J.
Biol.
Chem.
278(2003)49795–49805.
[123]D.
Li,M.
B.
Sewer,RhoAanddiaphanous-relatedhomolog1mediateadrenocor-ticotropin-stimulatedcortisolbiosynthesisbyregulatingmitochondrialtrafck-ing,Endocrinology(2010)247–258.
[124]P.
Bouillet,D.
Metcalf,D.
C.
Huang,D.
M.
Tarlinton,T.
W.
Kay,F.
Kontgen,J.
M.
Adams,A.
Strasser,ProapoptoticBcl-2relativeBimrequiredforcertainapoptoticresponses,leukocytehomeostasis,andtoprecludeautoimmunity,Science286(1999)1735–1738.
[125]D.
Chen,Q.
Zhou,CaspasecleavageofBimELtriggersapositivefeedbackamplicationofapoptoticsignaling,Proc.
NatlAcad.
Sci.
USA101(2004)1235–1240.
686A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[126]H.
Puthalakath,D.
C.
Huang,L.
A.
O'Reilly,S.
M.
King,A.
Strasser,TheproapoptoticactivityoftheBcl-2familymemberBimisregulatedbyinteractionwiththedyneinmotorcomplex,Mol.
Cell3(1999)287–296.
[127]D.
Chen,M.
Wang,S.
Zhou,Q.
Zhou,HIV-1Tattargetsmicrotubulestoinduceapoptosis,aprocesspromotedbythepro-apoptoticBcl-2relativeBim,EMBOJ.
21(2002)6801–6810.
[128]A.
J.
Butt,C.
G.
Roberts,A.
A.
Seawright,P.
B.
Oelrichs,J.
K.
Macleod,T.
Y.
Liaw,M.
Kavallaris,T.
J.
Somers-Edgar,G.
M.
Lehrbach,C.
K.
Watts,R.
L.
Sutherland,Anovelplanttoxin,persin,withinvivoactivityinthemammarygland,inducesBim-dependentapoptosisinhumanbreastcancercells,Mol.
CancerTher.
5(2006)2300–2309.
[129]C.
Cenciarelli,C.
Tanzarella,I.
Vitale,C.
Pisano,P.
Crateri,S.
Meschini,G.
Arancia,A.
Antoccia,Thetubulin-depolymerisingagentcombretastatin-4inducesectopicasterassemblyandmitoticcatastropheinlungcancercellsH460,Apoptosis13(2008)659–669.
[130]U.
M.
Schneiders,L.
Schyschka,A.
Rudy,A.
M.
Vollmar,BH3-onlyproteinsMcl-1andBimaswellasendonucleaseGaretargetedinspongistatin1-inducedapoptosisinbreastcancercells,Mol.
CancerTher.
8(2009)2914–2925.
[131]K.
Lei,R.
J.
Davis,JNKphosphorylationofBim-relatedmembersoftheBcl2familyinducesBax-dependentapoptosis,Proc.
NatlAcad.
Sci.
USA100(2003)2432–2437.
[132]T.
Tong,J.
Ji,S.
Jin,X.
Li,W.
Fan,Y.
Song,M.
Wang,Z.
Liu,M.
Wu,Q.
Zhan,Gadd45aexpressioninducesBimdissociationfromthecytoskeletonandtranslocationtomitochondria,Mol.
Cell.
Biol.
25(2005)4488–4500.
[133]J.
Alexandre,F.
Batteux,C.
Nicco,C.
Chereau,A.
Laurent,L.
Guillevin,B.
Weill,F.
Goldwasser,Accumulationofhydrogenperoxideisanearlyandcrucialstepforpaclitaxel-inducedcancercelldeathbothinvitroandinvivo,Int.
J.
Cancer119(2006)41–48.
[134]H.
Fawcett,J.
S.
Mader,M.
Robichaud,C.
Giacomantonio,D.
W.
Hoskin,Contributionofreactiveoxygenspeciesandcaspase-3toapoptosisandattenuatedICAM-1expressionbypaclitaxel-treatedMDA-MB-435breastcarcinomacells,Int.
J.
Oncol.
27(2005)1717–1726.
[135]H.
L.
Lin,T.
Y.
Liu,G.
Y.
Chau,W.
Y.
Lui,C.
W.
Chi,Comparisonof2-methoxyestra-diol-induced,docetaxel-induced,andpaclitaxel-inducedapoptosisinhepatomacellsanditscorrelationwithreactiveoxygenspecies,Cancer89(2000)983–994.
[136]G.
Varbiro,B.
Veres,F.
GallyasJr.
,B.
Sumegi,DirecteffectofTaxolonfreeradicalformationandmitochondrialpermeabilitytransition,FreeRadic.
Biol.
Med.
31(2001)548–558.
[137]C.
M.
KhawajaNR,B.
Pourroy,H.
Kovacic,D.
Braguer,Highpotencyofepothilonesinneuroblastomacellsmayinvolvemitochondria,AmericanAssociationforCancerResearch(AACR)MeetingAbstracts,2006.
[138]N.
Andre,D.
Braguer,G.
Brasseur,A.
Goncalves,D.
Lemesle-Meunier,S.
Guise,M.
A.
Jordan,C.
Briand,Paclitaxelinducesreleaseofcytochromecfrommitochondriaisolatedfromhumanneuroblastomacells,CancerRes.
60(2000)5349–5353.
[139]S.
L.
Mironov,M.
V.
Ivannikov,M.
Johansson,[Ca2+]isignalingbetweenmitochondriaandendoplasmicreticuluminneuronsisregulatedbymicro-tubules.
FrommitochondrialpermeabilitytransitionporetoCa2+-inducedCa2+release,J.
Biol.
Chem.
280(2005)715–721.
[140]J.
F.
Kidd,M.
F.
Pilkington,M.
J.
Schell,K.
E.
Fogarty,J.
N.
Skepper,C.
W.
Taylor,P.
Thorn,Paclitaxelaffectscytosoliccalciumsignalsbyopeningthemitochondrialpermeabilitytransitionpore,J.
Biol.
Chem.
277(2002)6504–6510.
[141]G.
G.
D'Souza,S.
M.
Cheng,S.
V.
Boddapati,R.
W.
Horobin,V.
Weissig,Nanocarrier-assistedsub-cellulartargetingtothesiteofmitochondriaimprovesthepro-apoptoticactivityofpaclitaxel,J.
DrugTarget.
16(2008)578–585.
[142]M.
Carre,N.
Andre,G.
Carles,H.
Borghi,L.
Brichese,C.
Briand,D.
Braguer,Tubulinisaninherentcomponentofmitochondrialmembranesthatinteractswiththevoltage-dependentanionchannel,J.
Biol.
Chem.
277(2002)33664–33669.
[143]M.
Carre,G.
Carles,N.
Andre,S.
Douillard,J.
Ciccolini,C.
Briand,D.
Braguer,Involvementofmicrotubulesandmitochondriaintheantagonismofarsenictrioxideonpaclitaxel-inducedapoptosis,Biochem.
Pharmacol.
63(2002)1831–1842.
[144]L.
Cicchillitti,R.
Penci,M.
DiMichele,F.
Filippetti,D.
Rotilio,M.
B.
Donati,G.
Scambia,C.
Ferlini,ProteomiccharacterizationofcytoskeletalandmitochondrialclassIIIbeta-tubulin,Mol.
CancerTher.
7(2008)2070–2079.
[145]T.
K.
Rostovtseva,K.
L.
Sheldon,E.
Hassanzadeh,C.
Monge,V.
Saks,S.
M.
Bezrukov,D.
L.
Sackett,Tubulinbindingblocksmitochondrialvoltage-dependentanionchannelandregulatesrespiration,Proc.
NatlAcad.
Sci.
USA105(2008)18746–18751.
[146]Y.
V.
Evtodienko,V.
V.
Teplova,S.
S.
Sidash,F.
Ichas,J.
P.
Mazat,Microtubule-activedrugssuppresstheclosureofthepermeabilitytransitionporeintumourmitochondria,FEBSLett.
393(1996)86–88.
[147]D.
J.
Rodi,R.
W.
Janes,H.
J.
Sanganee,R.
A.
Holton,B.
A.
Wallace,L.
Makowski,Screeningofalibraryofphage-displayedpeptidesidentieshumanbcl-2asataxol-bindingprotein,J.
Mol.
Biol.
285(1999)197–203.
[148]J.
H.
Wu,G.
Batist,L.
O.
Zamir,AmodelfortheinteractionofpaclitaxelwiththeBcl-2loopdomain:achemicalapproachtoinduceconformation-dependentphosphorylation,AnticancerDrugDes.
15(2000)441–446.
[149]C.
Ferlini,L.
Cicchillitti,G.
Raspaglio,S.
Bartollino,S.
Cimitan,C.
Bertucci,S.
Mozzetti,D.
Gallo,M.
Persico,C.
Fattorusso,G.
Campiani,G.
Scambia,PaclitaxeldirectlybindstoBcl-2andfunctionallymimicsactivityofNur77,CancerRes.
69(2009)6906–6914.
[150]L.
Knipling,J.
Wolff,DirectinteractionofBcl-2proteinswithtubulin,Biochem.
Biophys.
Res.
Commun.
341(2006)433–439.
[151]J.
W.
Smyth,T.
T.
Hong,D.
Gao,J.
M.
Vogan,B.
C.
Jensen,T.
S.
Fong,P.
C.
Simpson,D.
Y.
Stainier,N.
C.
Chi,R.
M.
Shaw,Limitedforwardtrafckingofconnexin43reducescell–cellcouplinginstressedhumanandmousemyocardium,J.
Clin.
Invest.
120(2010)266–279.
[152]C.
Sioka,A.
P.
Kyritsis,Centralandperipheralnervoussystemtoxicityofcommonchemotherapeuticagents,CancerChemother.
Pharmacol.
63(2009)761–767.
[153]S.
Wolf,D.
Barton,L.
Kottschade,A.
Grothey,C.
Loprinzi,Chemotherapy-inducedperipheralneuropathy:preventionandtreatmentstrategies,Eur.
J.
Cancer44(2008)1507–1515.
[154]F.
H.
Hausheer,R.
L.
Schilsky,S.
Bain,E.
J.
Berghorn,F.
Lieberman,Diagnosis,management,andevaluationofchemotherapy-inducedperipheralneuropathy,Semin.
Oncol.
33(2006)15–49.
[155]T.
Bordet,R.
M.
Pruss,Targetingneuroprotectionasanalternativeapproachtopreventingandtreatingneuropathicpain,Neurotherapeutics6(2009)648–662.
[156]T.
J.
Kaley,L.
M.
Deangelis,Therapyofchemotherapy-inducedperipheralneuropathy,Br.
J.
Haematol.
145(2009)3–14.
[157]N.
Authier,D.
Balayssac,F.
Marchand,B.
Ling,A.
Zangarelli,J.
Descoeur,F.
Coudore,E.
Bourinet,A.
Eschalier,Animalmodelsofchemotherapy-evokedpainfulperipheralneuropathies,Neurotherapeutics6(2009)620–629.
[158]K.
D.
Tanner,J.
D.
Levine,K.
S.
Topp,Microtubuledisorientationandaxonalswellinginunmyelinatedsensoryaxonsduringvincristine-inducedpainfulneuropathyinrat,J.
Comp.
Neurol.
395(1998)481–492.
[159]K.
S.
Topp,K.
D.
Tanner,J.
D.
Levine,Damagetothecytoskeletonoflargediametersensoryneuronsandmyelinatedaxonsinvincristine-inducedpainfulperipheralneuropathyintherat,J.
Comp.
Neurol.
424(2000)563–576.
[160]S.
J.
Flatters,G.
J.
Bennett,Studiesofperipheralsensorynervesinpaclitaxel-inducedpainfulperipheralneuropathy:evidenceformitochondrialdysfunction,Pain122(2006)245–257.
[161]H.
W.
Jin,S.
J.
Flatters,W.
H.
Xiao,H.
L.
Mulhern,G.
J.
Bennett,Preventionofpaclitaxel-evokedpainfulperipheralneuropathybyacetyl-L-carnitine:effectsonaxonalmitochondria,sensorynerveberterminalarbors,andcutaneousLangerhanscells,Exp.
Neurol.
210(2008)229–237.
[162]G.
Melli,M.
Taiana,F.
Camozzi,D.
Triolo,P.
Podini,A.
Quattrini,F.
Taroni,G.
Lauria,Alpha-lipoicacidpreventsmitochondrialdamageandneurotox-icityinexperimentalchemotherapyneuropathy,Exp.
Neurol.
214(2008)276–284.
[163]T.
Nakata,H.
Yorifuji,Morphologicalevidenceoftheinhibitoryeffectoftaxolonthefastaxonaltransport,Neurosci.
Res.
35(1999)113–122.
[164]C.
Theiss,K.
Meller,Taxolimpairsanterogradeaxonaltransportofmicroinjectedhorseradishperoxidaseindorsalrootganglianeuronsinvitro,CellTissueRes.
299(2000)213–224.
[165]S.
C.
Apfel,Managingtheneurotoxicityofpaclitaxel(Taxol)anddocetaxel(Taxotere)withneurotrophicfactors,CancerInvestig.
18(2000)564–573.
[166]S.
Quasthoff,H.
P.
Hartung,Chemotherapy-inducedperipheralneuropathy,J.
Neurol.
249(2002)9–17.
[167]J.
K.
Andersen,Oxidativestressinneurodegeneration:causeorconsequenceNat.
Med.
10(Suppl)(2004)S18–S25.
[168]W.
H.
Xiao,F.
Y.
Zheng,G.
J.
Bennett,T.
Bordet,R.
M.
Pruss,Olesoxime(cholest-4-en-3-one,oxime):analgesicandneuroprotectiveeffectsinaratmodelofpainfulperipheralneuropathyproducedbythechemotherapeuticagent,paclitaxel,Pain147(2009)202–209.
[169]K.
Y.
Chan,A.
H.
Bunt,Anassociationbetweenmitochondriaandmicrotubulesinsynaptosomesandaxonterminalsofcerebralcortex,J.
Neurocytol.
7(1978)137–143.
[170]S.
T.
Brady,S.
D.
Crothers,C.
Nosal,W.
O.
McClure,FastaxonaltransportinthepresenceofhighCa2+:evidencethatmicrotubulesarenotrequired,Proc.
NatlAcad.
Sci.
USA77(1980)5909–5913.
[171]S.
T.
Brady,R.
J.
Lasek,R.
D.
Allen,H.
L.
Yin,T.
P.
Stossel,Gelsolininhibitionoffastaxonaltransportindicatesarequirementforactinmicrolaments,Nature310(1984)56–58.
[172]D.
J.
Goldberg,Microinjectionintoanidentiedaxontostudythemechanismoffastaxonaltransport,Proc.
NatlAcad.
Sci.
USA79(1982)4818–4822.
[173]D.
J.
Goldberg,D.
A.
Harris,B.
W.
Lubit,J.
H.
Schwartz,Analysisofthemechanismoffastaxonaltransportbyintracellularinjectionofpotentiallyinhibitorymacromolecules:evidenceforapossibleroleofactinlaments,Proc.
NatlAcad.
Sci.
USA77(1980)7448–7452.
[174]R.
L.
Morris,P.
J.
Hollenbeck,AxonaltransportofmitochondriaalongmicrotubulesandF-actininlivingvertebrateneurons,J.
CellBiol.
131(1995)1315–1326.
[175]D.
Cartelli,C.
Ronchi,M.
G.
Maggioni,S.
Rodighiero,E.
Giavini,G.
Cappelletti,MicrotubuledysfunctionprecedestransportimpairmentandmitochondriadamageinMPP+-inducedneurodegeneration,J.
Neurochem.
115(2010)247–258.
[176]M.
Zheng,Q.
Wang,Y.
Teng,X.
Wang,F.
Wang,T.
Chen,J.
Samaj,J.
Lin,D.
C.
Logan,ThespeedofmitochondrialmovementisregulatedbythecytoskeletonandmyosininPiceawilsoniipollentubes,Planta231(2010)779–791.
[177]J.
P.
Dompierre,J.
D.
Godin,B.
C.
Charrin,F.
P.
Cordelieres,S.
J.
King,S.
Humbert,F.
Saudou,Histonedeacetylase6inhibitioncompensatesforthetransportdecitinHuntington'sdiseasebyincreasingtubulinacetylation,J.
Neurosci.
27(2007)3571–3583.
[178]N.
A.
Reed,D.
Cai,T.
L.
Blasius,G.
T.
Jih,E.
Meyhofer,J.
Gaertig,K.
J.
Verhey,Microtubuleacetylationpromoteskinesin-1bindingandtransport,Curr.
Biol.
16(2006)2166–2172.
[179]J.
Zhou,M.
Liu,R.
Aneja,R.
Chandra,H.
C.
Joshi,Enhancementofpaclitaxel-inducedmicrotubulestabilization,mitoticarrest,andapoptosisbythemicrotubule-targetingagentEM012,Biochem.
Pharmacol.
68(2004)2435–2441.
[180]S.
H.
Zhuang,Y.
E.
Hung,L.
Hung,R.
W.
Robey,D.
L.
Sackett,W.
M.
Linehan,S.
E.
Bates,T.
Fojo,M.
S.
Poruchynsky,Evidenceformicrotubuletargetengagementintumorsofpatientsreceivingixabepilone,Clin.
CancerRes.
13(2007)7480–7486.
687A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[181]I.
Nennesmo,F.
P.
Reinholt,Effectsofintraneuralinjectionoftaxolonretrogradeaxonaltransportandmorphologyofcorrespondingnervecellbodies,VirchowsArch.
BCellPathol.
Incl.
Mol.
Pathol.
55(1988)241–246.
[182]J.
W.
Hammond,C.
F.
Huang,S.
Kaech,C.
Jacobson,G.
Banker,K.
J.
Verhey,Posttranslationalmodicationsoftubulinandthepolarizedtransportofkinesin-1inneurons,Mol.
Biol.
Cell21(2010)572–583.
[183]B.
Trinczek,A.
Ebneth,E.
M.
Mandelkow,E.
Mandelkow,Tauregulatestheattachment/detachmentbutnotthespeedofmotorsinmicrotubule-dependenttransportofsinglevesiclesandorganelles,J.
CellSci.
112(Pt14)(1999)2355–2367.
[184]J.
C.
Bulinski,T.
E.
McGraw,D.
Gruber,H.
L.
Nguyen,M.
P.
Sheetz,OverexpressionofMAP4inhibitsorganellemotilityandtrafckinginvivo,J.
CellSci.
110(Pt24)(1997)3055–3064.
[185]A.
Seitz,H.
Kojima,K.
Oiwa,E.
M.
Mandelkow,Y.
H.
Song,E.
Mandelkow,Single-moleculeinvestigationoftheinterferencebetweenkinesin,tauandMAP2c,EMBOJ.
21(2002)4896–4905.
[186]K.
Nishio,H.
Arioka,T.
Ishida,H.
Fukumoto,H.
Kurokawa,M.
Sata,M.
Ohata,N.
Saijo,Enhancedinteractionbetweentubulinandmicrotubule-associatedprotein2viainhibitionofMAPkinaseandCDC2kinasebypaclitaxel,Int.
J.
Cancer63(1995)688–693.
[187]J.
R.
Levy,C.
J.
Sumner,J.
P.
Caviston,M.
K.
Tokito,S.
Ranganathan,L.
A.
Ligon,K.
E.
Wallace,B.
H.
LaMonte,G.
G.
Harmison,I.
Puls,K.
H.
Fischbeck,E.
L.
Holzbaur,Amotorneurondisease-associatedmutationinp150Gluedperturbsdynactinfunctionandinducesproteinaggregation,J.
CellBiol.
172(2006)733–745.
[188]J.
Jaworski,L.
C.
Kapitein,S.
M.
Gouveia,B.
R.
Dortland,P.
S.
Wulf,I.
Grigoriev,P.
Camera,S.
A.
Spangler,P.
DiStefano,J.
Demmers,H.
Krugers,P.
Delippi,A.
Akhmanova,C.
C.
Hoogenraad,Dynamicmicrotubulesregulatedendriticspinemorphologyandsynapticplasticity,Neuron61(2009)85–100.
[189]O.
A.
Shemesh,M.
E.
Spira,Paclitaxelinducesaxonalmicrotubulespolarrecong-urationandimpairedorganelletransport:implicationsforthepathogenesisofpaclitaxel-inducedpolyneuropathy,ActaNeuropathol.
119(2010)235–248.
[190]A.
B.
Knott,G.
Perkins,R.
Schwarzenbacher,E.
Bossy-Wetzel,Mitochondrialfragmentationinneurodegeneration,Nat.
Rev.
Neurosci.
9(2008)505–518.
[191]A.
B.
Knott,E.
Bossy-Wetzel,Impairingthemitochondrialssionandfusionbalance:anewmechanismofneurodegeneration,Ann.
NYAcad.
Sci.
1147(2008)283–292.
[192]V.
Voccoli,L.
Colombaioni,Mitochondrialremodelingindifferentiatingneuro-blasts,BrainRes.
1252(2009)15–29.
[193]T.
Shprung,I.
Gozes,Anovelmethodforanalyzingmitochondrialmovement:inhibitionbypaclitaxelinapheochromocytomacellmodel,J.
Mol.
Neurosci.
37(2009)254–262.
[194]R.
Yamaguchi,L.
Lartigue,G.
Perkins,R.
T.
Scott,A.
Dixit,Y.
Kushnareva,T.
Kuwana,M.
H.
Ellisman,D.
D.
Newmeyer,Opa1-mediatedcristaeopeningisBax/BakandBH3dependent,requiredforapoptosis,andindependentofBakoligomerization,Mol.
Cell31(2008)557–569.
[195]M.
S.
Poruchynsky,P.
Giannakakou,Y.
Ward,J.
C.
Bulinski,W.
G.
Telford,R.
W.
Robey,T.
Fojo,Accompanyingproteinalterationsinmalignantcellswithamicrotubule-polymerizingdrug-resistancephenotypeandaprimaryresistancemechanism,Biochem.
Pharmacol.
62(2001)1469–1480.
688A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688
Rovinia,A.
Savrya,b,D.
Braguera,b,M.
Carréa,aINSERMUMR911,CentredeRechercheenOncologiebiologiqueetenOncopharmacologie,UniversitéAix-Marseille,FacultédePharmacie,27BoulevardJeanMoulin,13385MarseilleCedex5,FrancebAssistancePublique-HpitauxdeMarseille,FranceabstractarticleinfoArticlehistory:Received22July2010Receivedinrevisedform2January2011Accepted4January2011Availableonline7January2011Keywords:AnticancerdrugMicrotubuleMitochondriaApoptosisPharmacologyMicrotubule-TargetingAgents(MTAs)constituteaclassofdrugslargelyusedforcancertreatmentinadultsandchildren.
Incancercells,theysuppressmicrotubuledynamics,andinducecelldeathviathemitochondrialintrinsicpathway.
Todate,linksbetweenmitochondriaandmicrotubulenetworkdisturbanceinMTAsmechanismofactionarenotobvious.
Theaimofthepresentcontributionistoprovideelementsthatcouldanswertothequestion:howfararemitochondriaessentialtoanticancerchemotherapythattargetsthemicrotubulecytoskeletonWereviewthemainmolecularcandidatestolinkmicrotubulealterationwiththeapoptoticmitochondrialpathwaycontrol.
InvolvementofdirecttargetingofmitochondriainMTAefcacyisalsodiscussed.
Furthermore,welineupcurrentevidenceandemergingconceptsontheparticipationofbothmitochondriaandmicrotubuleinMTAneurotoxicsideeffects.
Todeciphertheinterconnectionsbetweenthemitochondrialandthemicrotubulenetworksmayhelptoimprovecancercellresponsetochemotherapy.
ThisarticleispartofaSpecialIssueentitled:BioenergeticsofCancer.
2011ElsevierB.
V.
Allrightsreserved.
1.
IntroductionImprovementofanticancertherapeuticstrategiesisoftenlimitedbyapoorknowledgeofmolecularmechanismsunderlyingcarcinogenesisandcellresponsetotreatment.
Althoughcarcinogenesisisaverycomplexprocess,itcanbedividedintotwocrucialsteps:1)appearanceofoncogenemutationsinagroupofcells,leadingto2)disorderlycellproliferation.
Thisuncontrolledcelldivision,joinedtoneo-angiogenesisinduction,isresponsiblefortumorformation,growthandspreading.
Asthemicrotubulenetworkishighlyinvolvedincellproliferation,itappearedtobeapreferentialtargetforcancertherapy.
Forthatmatter,greateffortshavebeendevotedtodiscoverdrugsthataffectmicrotubules.
Nowadays,theso-calledMicrotubule-TargetedAgents(MTAs)constituteaclassofanticancerdrugslargelyusedintheclinics.
Amongthem,taxanesandVincaalkaloidsarepowerfulinhibitorsofmicrotubuledynamicsandapoptosisinducers,usedtotreatsolidtumorsandmalignanthemopathies.
ThetherapeuticsuccessofMTAsaccountsforthedevelopmentofnewmicrotubule-targetingcom-poundsbypharmaceuticalcompanies,whichhasbeen–andisstill–intenseandfruitful.
Theaimofthepresentcontributionistoanswerthequestion:howfararemitochondriaessentialtoanticancerchemotherapythattargetsthemicrotubulecytoskeletonFirst,webrieysummarizedthecellulareffectsofMTAsonmicrotubuledynamics,andtheirfunctionalconsequences.
Then,asMTAanticancereffectivenesshasbeenrelatedtotheapoptoticmitochondrialpathway,welinedupthemainmolecularcandidatestolinkmicrotubulealterationwithapoptosiscontrol;andwediscussthedirecteffectsofMTAsonmitochondria.
WealsoreviewedcurrentevidenceandemergingconceptsofbothmitochondriaandmicrotubuleroleinMTAneurotoxicsideeffects.
Lastly,weconsideredMTAsastoolstostudytheinuenceofmicrotubuledynamicsonmitochondrialdynamics.
2.
MTAfamilyofmolecules,areferenceinanticancerchemotherapyMicrotubulesarecytoskeletalhollowlamentspresentinmosteukaryoticcellsthatresultfrompolymerizationofα/βtubulinpolymers.
Inmammaliancells,microtubulesarepolarizedstructuresnucleatedatthecentrosomewheretheminusendisanchored.
Theplusendgrowstothecellperipheryandconstantlyexploresthecytoplasm,makingmicrotubulehighlydynamicpolymers.
Indeed,afundamentalpropertyofmicrotubulesistoexhibitadynamicinstability,whichischaracterizedbyasuccessionofslowpolymerizationandrapiddepolymerizationphases.
Theswitchfrommicrotubulegrowthorpausetoshrinkageisknownas"catastrophe",andtheswitchfromshrinkagetogrowthorpauseisnamed"rescue"[1].
Thisdynamicbehaviorisofparticularimportanceintheregulationofmanycellularfunctionsbythemicrotubulecytoskeleton,asbeingthesupportforcelldivision,shapechanges,motilityandcelldifferentiationsuchasBiochimicaetBiophysicaActa1807(2011)679–688ThisarticleispartofaSpecialIssueentitled:BioenergeticsofCancer.
Correspondingauthor.
INSERMUMR911-CR02,FacultedePharmacie,27BdJeanMoulin,13385MarseilleCedex5,France.
Tel.
:+33491835626;fax:+33491835635.
E-mailaddress:manon.
carre@univmed.
fr(M.
Carré).
0005-2728/$–seefrontmatter2011ElsevierB.
V.
Allrightsreserved.
doi:10.
1016/j.
bbabio.
2011.
01.
001ContentslistsavailableatScienceDirectBiochimicaetBiophysicaActajournalhomepage:www.
elsevier.
com/locate/bbabioformationofneuronaloutgrowths.
Thus,farfrombeingconsideredasmerearchitecturalelements,microtubulesarekeydeterminantsofcellulareventsandfunctionalities.
Onlyafewmicrotubule-governedcellularprocessesrequireanoverallremodelingofthecytoskeletonnetwork,butalldependonatightlyregulatedmicrotubuledynamics.
Microtubule-TargetedAgents(MTAs)remainbenchmarkclinicaltreatmentsdisplayinghighcytotoxicefciencyandarestillwidelyusedagainstabroadspectrumofchildren'sandadult'stumors.
Theyrecentlyreceivedarevivalofinterestaspotentanti-angiogenicandvascular-disruptingagents[2].
Researchanddevelopmentarestillinprogresstodiscovermoreactiveandlesstoxiccompounds(forextensivereviews,see[3,4]).
Attemptstoimprovetheintracellulardrugconcentrationandamorespecictargetingoftumorcellsareespeciallyunderintenseinvestigations,andtheemergingeldofnanotechnologiesactivelyparticipatestothisquest[5].
TheMTAfamilyiscomposedofmorethan30compounds,historicallyclassiedindestabilizingandstabilizingagents,accordingtotheirbindingsiteontubulinormicrotubules[3,6].
Destabilizingagentsinhibitmicrotubulepolymerizationinvitro;theyincludetheVincaalkaloidssuchasvinblastineorvincristinethatbindtotheso-called"Vinca"tubulindomain,aswellasnocodazoleorcombretastatinsthatbindtothe"colchicine"tubulindomain.
Stabilizingagentsenhancetubulinpolymerizationandmicrotubulestabilization;theyincludetaxanes,suchaspaclitaxelordocetaxel,andEpothilones.
Althoughthedistinctionindestabilizingandstabilizingagentsisusefulforstructure–activitystudies,itisnomoreusedincellularandinvivostudiessincebothclasseshavebeenshowntocommonlydisturbmicrotubuledynamics,withoutchangingtheoverallmicrotubulemassinalargerangeofconcentrations[7].
Itisnowlargelyacceptedthatcytotoxic(i.
e.
pro-apoptotic)concentrationsofMTAssuppressmicrotubuledynamics.
[8–11].
Anextensivedecreaseinmicrotubuledynamicspreventsthenormalalignmentofchromosomes,activatesthespindleassemblycheckpoint,whichresultsinthecellblockadeinmitosis[12].
Itshouldherebenoticedthatamoderatedsuppressionofmicrotubuledynamics,whichdidnotallowtheaccumulationofcellsinmitosis,isalsoassociatedwithapoptosisinductionintumorcells[13–15].
Thus,itremainsunclearwhetherandhowthemitoticarrestiscoupledtotheactivationoftheapoptoticmachinery[16,17].
Elsewhere,microtubuledynamicssup-pressioncorrelateswithcelllocomotionalteration,asdescribedinbroblaststreatedwithpaclitaxelandnocodazole[18,19].
Surprisingly,theincreaseinmicrotubuledynamicsbylowconcentrationsofvinunineandpaclitaxelhasbeenalsoshowntoinhibitendothelialcellmigration,resultinginMTAanti-angiogenicactivities[20].
Thiseffectwascorrelatedwiththeinhibitionofinterphasemicrotubulefunctions,resultingininhibitionofadhesionsitedynamicsandformationoflong-livedstressbers[21].
Interestingly,whatevertheconcentrationstudied,MTAsdisorganizethenetworkofmicrotubule+endtrackingproteins(+TIPs).
Amongthem,theendbinding(EB)familyofproteinsspecicallyformscomet-likeaccumulationattheendsofgrowingmicrotubules.
Astheyensuremicrotubulegrowth,EBproteinsarecrucialregulatorofmicrotubuledynamics[22].
Taxanes,VincaalkaloidsandEpothilonescommonlydisruptEBproteindistributionincancer,endothelialandneuronalcells[21,23](andpersonaldata),whichmayaccountforMTAs'maineffectsoninterphaseandmitoticmicrotubuledynamics.
3.
AnticancereffectivenessofMTAs:mitochondriacomeintoplayMTAs,includingthenewestinclinicaltrials,haveshownahighabilitytoinduceapoptosis[24–28].
Thisprogrammedandtightlyregulatedcelldeathwasrstidentiedin1993,byBhallaetal.
[29],asthemechanismresponsiblefortheanti-tumorcytotoxiceffectsofpaclitaxelinhumanmyeloidleukemiacells[14].
Sincethen,theireffectiveness,evenintheclinics,hasbeenwellcorrelatedtoapoptosisextentinalltumorcells.
Itshouldbenotedthat,eventhoughMTAscaninducecelldeathinendothelialcells,itisdispensablefortheiranti-angiogenicandanti-vascularpropertiesatlowdoses[30,31].
ThissectionwillthusbefocusedonapoptoticactorsnecessarytoMTAanticanceractivityintumorthemselves,givingcluestounderstandhowcanmicrotubuledysfunctionbeanecessarystepinapoptosisinduction.
3.
1.
IntrinsicpathwayinductionbyMTAs:whattolearnforonco-pharmacologyFromthenumerousintracellularapoptoticsignals,twomajorroutescanbediscerned:themitochondrialpathway,knownastheintrinsicpathway,andthedeathreceptorpathway,so-calledextrinsicpathway.
AlthoughMTAshavebeenshowntomodifyexpressionlevelsofdeathreceptorsandtheirligands,theextrinsicpathwayisgenerallyexcludedfromMTA-inducedapoptosis[32–34].
MTAeffectivenessislargelyacceptedasaconsequenceofcaspaseactivationthroughtheintrinsicapoptoticpathway[35].
Ofnote,theanticanceractivityofnovelimprovedpharmacologicalfeaturesofMTAs,suchashydrophilicpaclitaxelderivativeorpaclitaxelloadedpoly(L-lacticacid)microparticles,isevaluatedbymeasuringtheirimpactonmitochondria[36,37].
Itpointsouthowtheseorganellesarecrucialintheappraisalofmicrotubule-targetedchemotherapysuccess.
3.
1.
1.
ThelethalcascadeMitochondrialmembranepermeabilizationisthecentralgateinturningon/offapoptosis,asitallowsthereleaseofalargepanelofpro-apoptoticproteins[38,39],thatactivatesdownstreamsignalingcascadesandleadstothenalexecutionofcelldeath.
AnearlyandtransienthyperpolarizationofthemitochondrionhasbeenreportedwithMTAs,whichis,intumorcells,followedbytheΔΨmcollapseandthereleaseofpro-apoptoticfactors[17,30].
Thesubsequentactivationofthecaspasecascadeisthenon-returnpointtocellbiochemicaldestructionandphenotypicchangesinMTA-inducedapoptosis[35,40–43].
Inresponsetopaclitaxel-orepothilone-treatment,themassivecytochromecreleasefrommitochondriatriggerstheformationofthemulti-factorcomplexapoptosome,whichleadstoanearlyincreaseincaspase-9activity.
Accordingly,thecaspase-9specicinhibitor(z-LEHD-fmk)effectivelyprotectscellsfromMTA-mediatedapoptosis[42–44].
OverexpressionofApaf-1,theadaptormoleculeoftheapoptosome,hasbeenshowntoenhancepaclitaxel-inducedapoptosis[45].
Insituationswhereactivationofcaspase-9isdisturbed,overexpressionofthedownstreameffectorcaspase-3restoressensitivitytoMTAsinresistantcancercells[46].
Smac/DiabloandOmi/Htra2peptidesarealsoreleasedfrommitochondriaduringtheirpermeabilization,andfavorcaspaseactivitybypreventingactionoftheinhibitorofapoptosisproteins(IAPs)[39,47].
InhibitionofIAPscan,invitro,modulatetheefcacyofantineoplasticagents.
Smac/DIABLOpeptideenhancedtheinductionofapoptosisandlongtermantiproliferativeeffectsofpaclitaxelinbreastcancercells[48,49].
CombinationofpaclitaxelwitharecombinantadenovirusencodingSmac/DIABLOalsoproducedgreaterlevelsofapoptosisinovariancarcinomacells[50].
Inaddition,ectopicSmac/DIABLOsensitizeddrug-resistantepithelialovariancancercellstopaclitaxel-inducedapoptosis[51].
Thus,anincreaseinSmac/DIABLOactivityseemstobeapromisingstrategytoimproveMTAtreatment,includingindrug-resistantcancer,butaclinicalapproachisstilllacking.
3.
1.
2.
Bcl-2familymembersMuchefforthasbeendirectedtowardelucidatingthemechanismofmitochondrialmembranepermeabilization,and,whilestilldiscussed,itisnowlargelyacceptedthatthisprocessisunderthecontroloftheBcl-2familymembers.
TheBcl-2familyiscomposedofupto30proteinsthatcanbedividedinto3groups:oneofBcl-2-likesurvivalfactorsandtwoothersofcelldeathpromotingfactorsnamedBax-likeandBH3-only[52].
Therelativelevelsofanti-andpro-apoptoticclansinmitochondrial680A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688membranesarbitratecelllifeordeathdecision.
MTAsmodulatebothexpressionlevelsandactivityofpro-apoptoticmembersoftheBcl-2family.
Up-regulationoftheBad,PUMA,Baxand/orBakhasbeenobservedaftertreatmentwithpaclitaxel,epothiloneBaswellaswithVincaalkaloids[15,53–55].
Asexpected,Bcl-XS,BaxorBadover-expressionsensitizescancercellstopaclitaxelandvincristine[56–58].
Inaddition,MTAstriggerBaxactivationthroughitsconformationalchange[54,59]thatallowsN-terminaldomainexhibitionandBaxstableinsertionintotheoutermitochondrialmembrane[60,61].
MTAsalsoinitiateBimtranslocationfrommicrotubulestomitochondria,asdiscussedinthenextsection.
Lastly,thelatecleavageofBidintoafunctionalfragment(tBid)hasbeenproposedtobeasignalamplica-tionloopwhichcouldberequiredforanoptimalreleaseofmitochondrialfactorsfollowingMTAtreatment[62,63].
Inparallel,Bcl-2-likeanti-apoptoticproteinscanbepost-transla-tionallyinactivatedbyhyperphosphorylationinducedbyalargepanelofMTAs[15,64–68].
Ithasinitiallybeenthoughttobeamarkerofmitosisratherthananapoptosis-relatedsignal,butboththeextentandthedurationofthemitosis-associatedBcl-2hyperphosphorylationislikelytodistinguishapre-apoptoticcellfromonedestinedtodivide[69].
TreatmentswithMTAsalsodecreaseexpressionlevelsofBcl-XLandBcl-2toactivatetheintrinsicpathway[70–72].
Overexpressionoftheanti-apoptoticproteinsBcl-2andBcl-XLareinvolvedinresistanceofcancercellstomicrotubule-targetedchemotherapy[73–76].
Thus,Bcl-2and/orBcl-XLantisensestrategieshavebeendeveloped,andwererstreportedtomediateanincreaseindocetaxel-andpaclitaxel-sensitivityinvitroandinmicexenografts[33,77–79].
ABT-737,aBH3-mimeticthatantagonizesBcl-2,Bcl-XL,andBcl-w,increasedMTApro-apoptoticeffectsinavarietyoftumorcelllines,includingbreastcancercellswithacquiredresistancetopaclitaxel[80–82].
Similarly,A-385358,asmallmoleculewithrelativeselectivityforbindingtoBcl-XLpotentiatedtheactivityofpaclitaxelinnon-small-celllungcancercells,invitroandinvivo[83].
In2003,aphaseIIclinicaltrialofoblimersen(antisenseoligonucleotidestargetingBcl-2)incombinationwithdocetaxelvalidatedprogressionintophaseIIIforpatientswithadvancedhormonerefractoryprostatecancer.
However,Bcl-2inhibitiondidnotalwayssucceedinenhancingtreatmenteffectiveness[84–87].
Furthermore,itshouldbeusedwithcautionincombinationwithMTAssinceworksshowedthatBcl-2down-regulationisresponsibleforanunexpectedresistancetopaclitaxelandvinunineinovariancancercells[17,88].
Accordingly,Bcl-2overexpressioncanincreasenon-smallcelllungcancersensitivitytodocetaxel[89].
Similarly,prostatecancercellscanadapttoantisenseRNAtargetingBcl-xL,leadingtoaparadoxalresistancetodocetaxelandvinblatine[90].
Thisdualroleof"prosurvi-val"mitochondrialproteinspointsouttheneedforfurtherinvestigationtoelucidatetheirrealcontributioninMTAtreatmenteffectivenessandtheirpotentialastargetforclinicalantisensestrategies.
3.
2.
Proteinsreleasedfromthemicrotubuletomitochondria:atthedoorstepsofMTAeffectivenessWhilethemaineffectsofMTAsonboththeintrinsicapoptoticsignalingcascadeandthemicrotubulenetworkarenowquitewellunderstood,clearlinksarestilllackingbetweenthetwoevents.
Sinceapoptosisandproliferationarecloselyrelated[91],effectsofMTAsonproteinsthatcontrolbothphenomenahavebeenstudiedforyears.
Cellcycleisatightlyregulatedprocess,anditsdisturbancebyMTAsmayparticipateinthemitochondrialapoptoticpathwayinitiation.
Therefore,studiesonhowcellcyclecheckpointsmodulatetheintrinsicpathwayareofmajorinterest,andextensivelyreviewed[27].
Thepresentsectionwillbefocusedonproteincandidatesthatcouldbuildmolecularbridgesbetweenmicrotubulesandtheapoptoticmachinery.
Indeed,microtubulesserveasscaffoldsfordifferentsignalingmolecules,extendingthelistofbiologicalprocess-esregulatedbythemicrotubulenetworkincells.
Amongmicrotubule-linkedcomponents,regulatorsoftheapoptoticprocesssuchasp53andBimmaybereleasedfrommicrotubulestowardsmitochondria.
SincepolymerizinganddepolymerizingMTAsdisplaythecommonpropertyofsuppressingmicrotubuledynamics,itprobablyexplainswhytheinvolvementofthemicrotubule-transportedfactorsinapoptosisissimilaramongsttheseanticancerdrugs.
3.
2.
1.
p53,themultifacetedmoleculeDifferentlinesofevidenceindicatethatp53up-regulationandactivationarerequiredformaximalcellsensitivitytoTaxanes,VincaalkaloidsandEpothilones[15,54,71,92–96].
Theroleofp53inapoptosismediatedbymicrotubuledisturbanceisreinforcedbyrecentdatashowingthatoverexpressionofthemicrotubule-associatedproteinTaurenderedneuroblastomacellsresistanttoapoptosisbymechanismsinvolvingreductionofp53level[97].
MTAconcentrationseemstobecritical,aslowdosesofpaclitaxelincreasep53proteinlevels,whereashighdosesdonotaffectoreveninhibittheselevels[72,98,99].
Similarly,lowdosesofvinunineup-regulatep53,whilehighdosesincreasep53toalowerextent[17].
Theconcentration-dependentactivationofthistranscriptionfactormayresultfromitsmicrotubule-governedtrans-port.
Indeed,itsdynein-dependenttransporttothenucleusisthoughttobeaconsequenceofmicrotubuledynamicssuppressionbybothstabilizinganddepolymerizingagents[92,95,100,101].
Highconcentra-tionsofMTAsprobablyinducetooextensivedamagestomicrotubules,whichcannomoreserveastracksforp53trafcking.
Onceinthenucleus,p53transcriptionalpropertiesareactivated,leadingtomodulationingenetargetssuchasp53itselforp21and,moreinterestingly,membersoftheBcl-2family[36,102,103].
UnderMTAtreatment,p53inductionhasbeenshowntodown-regulateBcl-2andup-regulateBax[15,71,104,105].
Recently,weidentiedanovelbindingsiteofp53ontheBcl-2promoter,responsibleforthetranscriptionaldown-regulationofBcl-2byvinorelbineinbreastcarcinomacells[104].
TheBH3-onlymembersPUMAandNoxacanalsobeup-regulatedfollowingp53induction[54,106,107],buttheroleofMTA-mediateddisturbanceofmicrotubuledynamicsinthisprocessremainstobecharacterized.
Besidesitsabilitytomodulatethepro-apoptotic/anti-apoptoticratioinfavorofapoptosis,p53inducesapoptosisthroughatranscription-independentmechanism.
Inresponsetovariouspro-apoptoticstimuli,includingMTAs,p53rapidlymovestothemitochondriaincellularandmicemodels[15,93,108–111].
Onceatthemitochondrion,p53primarilyassociatedwiththeoutermitochondrialmembrane,butasmallsubfractionmaybelocatedwithinthemitochondrialmatrix[108,111–113].
Themitochondrialp53participatesintheapoptoticcascadebyinducingthemitochondrialoutermembranepermeabiliza-tionthroughdirectactivationofBax-likeandBH3-onlyproteins,andbyforminginhibitorycomplexeswithBcl-2-likemembers[106,114].
Thep53-targeteddrugpithrin-μ,whichblockstheinteractionofp53withBcl-XL[115],inhibitsapartofvinorelbine-inducedapoptosis[104],supportingaroleforthemitochondrialp53inMTApro-apoptoticproperties.
Whilesomekeystepshavebeeninvokedtoexplainhowp53translocationfrommicrotubulescanbetriggered[74,115],theexactroleofMTA-induceddamagesofmicrotubuledynamicsisstillanunexploredeld.
3.
2.
2.
BimgoesforawalktomitochondriaBH3-onlymemberoftheBcl-2family,Bimisinvolvedininhibitionofmetastasisformationandinthepro-apoptoticresponseoftumorcellstochemotherapy[116–121].
Bimexpressionlevelincreasesdramaticallyafterpaclitaxeltreatment[99,122]andgenesilencingexperimentsshowedthatitsup-regulationisacriticalregulatorofapoptosisincancercells[122–124].
WhileBimcanbelocalizedatmitochondriawithoutcelldeathstimuliinsomecellularmodels,numberofstudiesshowedthatitissequesteredbymicrotubulesviaitsinteractionwiththedyneinlightchainDLC1/LC8ofthemotorcomplexorviaadirectinteractionwithmicrotubules[125,126].
Interestingly,Bim-decientlymphocytesarelesssensitivetopaclitaxel-mediatedperturbationsof681A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688themicrotubulenetwork,whichunderscorestheimportanceofBiminmediatingapoptosisinducedbyagentsthattargetmicrotubules[127].
Duringtreatmentwithpaclitaxel,Bimmayactasasensorofcytoskeletonintegrity,sinceitisunleashedfrommicrotubulesandtranslocatestomitochondria[123].
Insupporttothishypothesis,theplanttoxincalledpersinhasbeenshowntoinduceBimreleasebyactingasamicrotubule-stabilizingagentinbreastcancercells[128].
Similarly,theHIV-1TatproteinactivatedapoptosisinhostcellsbytriggeringBimtranslocationtomitochondriainresponsetomicrotubulestabilization[127].
WealsoshowedthatepothiloneBinducedBimaccumulationtomitochondria,leadingtoapoptosisinhumanneuroblastomacells[93].
Sincethen,workshaveconrmedthatMTAsfreedBimfrommicro-tubulesandenrichedmitochondriainthispro-apoptoticprotein[129,130].
BimsequestrationfromdyneinmaybereleasedthroughitsphosphorylationbyJNK,asdescribedwithUVtreatment[116,131].
SuchakinasecanbeactivatedbyMTAs[27].
However,itmayalsobearguedthatBimtranslocationtriggeredbyMTAsresultsfromdisturbanceofmicrotubuledynamicsorstructure.
Indeed,microtu-buledestabilizationbyGadd45aledtoBimreleasewithoutactivationofJNK[132].
Amongthedifferenthypotheses,theenhancedgenerationofmitochondrialreactiveoxygenspecies(ROS)byMTAs[35,41,133–136]hasbeenshowntoberesponsibleforBimaccumu-lationtomitochondriainneuroblastomacells[93].
Thus,themitochondrialcompartmentitselfcaninitiatethesignalingdialogwiththemicrotubulenetwork,whichresultsinapoptosisandthusparticipatesinMTAefcacy.
4.
DirecteffectsofMTAsonmitochondria:wheredowestandInparallelwiththeireffectsonthemicrotubulenetwork,MTAscanalsoactivatetheapoptoticpathwaythroughadirectactiononmitochondria.
IncubationofmitochondriaisolatedfromtumorcellswitheitherTaxanes,VincaalkaloidsorEpothilonesprovokescyto-chromecrelease[137].
Incontrast,otherclassesofanticancerdrugssuchas5FUordoxorubicinwerenotabletopermeabilizemembranesfromisolatedmitochondria[138](andpersonaldata).
MTAsinduceanearlyΔΨmcollapseandasubsequentlargeamplitudeswellingofisolatedmitochondria[88,136,138,139].
Themitochondrialmembranepermeabilizationisinhibitablebycyclosporine[138],consistentlywiththepermeabilitypore-dependentCa2+lossfromisolatedmitochondriainducedbypaclitaxelandnocodazole[139,140].
Paclitaxelhasalsobeenshowntosignicantlyincreasethecytochromeoxidase-mediatedROSproductionbypuriedmitochondria[136].
Interestingly,thepro-apoptoticeffectsofpaclitaxelcanbeenhancedbyimprovingitsspecicdeliverytomitochondriausingamitochondria-specicnanocarriersystem(DQAsomes)[141].
Then,itraisesthequestionofthepotentialtarget(s)ofMTAsinmitochondria.
Tubulin,thatwasfoundtobestronglyassociatedwithmitochondrialmembranesinbothpuriedorganellesandwholecells[139,142,143],wastherstcandidateproposedtoexplainthespeciceffectofMTAsonisolatedmitochondria.
ThemitochondrialtubulinsubfractionisenrichedinclassIIIβ-tubulin(TUBB3),but,incontrastwiththecytoskeletalform,itsoverexpressiondoesnotcorrelatewithcellresistancetoMTAs[144].
AnassociationhasbeenreportedbetweenthemitochondrialtubulinandVDAC[142,145],themajoroutermembraneporethatislikelyinvolvedinthereleaseofpro-apoptoticfactorsbyMTAsfromtheintermembranespacetocytosol.
ThecurrentknowledgeontheMTA-inducedintrinsicpathwayhasrejuvenatedthestudyfromEvtodienkoetal.
[146],whichsuggestedtheinvolvementofeithermitochondria-boundtubulinperseand/orcontactsbetweenmitochondriaandmicrotubulesinregulationofmitochondrialmembranepermeability[146].
Morethan10yearslater,theC-terminaltailoftubulinhasbeenproposedtomodulatetheVDACopeningandthemitochondrialrespirationrate[145].
Bcl-2hasalsobeenidentiedasapotentialtargetforpaclitaxelbyphagedisplayandachemicalapproach[147,148].
Recently,Ferlinietal.
revealedthat,inovariancancercells,paclitaxeldirectlytargetedBcl-2intheloopdomain[149].
Asaresult,paclitaxelchangedtheroleofBcl-2frominhibitortoenhancerofthemitochondrialmembranepermeabilization,facilitatingapoptosis.
Thisprocessmayexplainwhythedown-regulationofBcl-2isresponsibleforanunexpectedresistancetoMTAsindifferenttumorcelltypesandcancerpatients[17,66,88,89].
Finally,thetwohypotheseshavejoinedwhentheassociationbetweenthetubulinandBcl-2hasbeenrevealed,byco-immunoprecipitationfrommitochondriallysatesandwithpuriedproteins[17,88,150].
Suchacomplexthatgatherstubulin,Bcl-2andVDACcouldbebothadirecttargetforMTAsandaregulatorofthemitochondrialmembranepermeability.
Thus,whilethemitochondri-altargetsofMTAsareprobablynotalldened,itisreasonabletothinkthattheseanti-tumoragentsdisplaycrucialanti-mitochondrialpropertiesinvolvedintheirefcacy.
Therelevanceofthisphenomenonremainsdifculttoproveinwholecells,sincethedirecteffectsofMTAsonthemitochondrialnetworkareusuallyundistinguishablefromthoseresultingfrommicrotubulemodications.
Moreover,resultsshowingthatinterfer-enceofpaclitaxelwiththemitochondrialsignalingcascadeoccurredupstreamofmicrotubuleorganizationalteration[140]shouldbereevaluatedbymeasuringmicrotubuledynamics,ahighlymoresensitiveparameterthanmicrotubulearchitecture.
Nevertheless,weshowedthattheearlyproductionofROSfrommitochondriawasnecessarytoBimtranslocationtowardsmitochondria,whichinturntriggeredapoptosisinhumanneuroblastomacells[93].
ThesedatastronglysuggestthatsomeofthemostrapideffectsofMTAsontheintrinsicapoptoticpathwaymaybeinitiatedthroughadirectactiononmitochondrialintegrity.
TheinvolvementofMTA-mediatedROSgenerationfrommitochondrianeedstobereconsideredinprocessessuchasEBproteincometdisruptionbyMTAs(seesection2),whichisthoughttoresultfromtargetingofthemicrotubulesystem,butwhichhasbeenrecentlyshowntobetriggeredbyH2O2[151].
Then,itiseasytospeculatethatsomealterationsofthemicrotubuledynamicsinducedbyMTAscouldbe,atleastinpart,linkedtothedrugs'anti-mitochondrialproperties.
5.
Mitochondria–microtubulepair:MTAsstirupthetroubleinneuronalsystemChemotherapy-inducedperipheralneuropathy(CIPN)isthemaindoselimitingsideofalargepanelofMTAs[152,153].
Mostofthetime,peripheralneuropathyreversesifthetreatmentisstopped.
However,insomecases,recoveryfromsymptomsisincompleteandalongperiodofregenerationisrequiredtorestorefunction[154].
Thisneurotoxicsideeffectisstillanunsolvedclinicalissue,sothewaysthatcytoskeletonandorganellesinterplayandhowMTAsaltertheserelationshipsinneuronalmodelsareofhighimportance.
5.
1.
Animalmodelsofchemotherapy-inducedneuropathy:slippingfrommicrotubuletomitochondrialinvolvementDespiteintensiveeffortsinthedevelopmentofneuroprotectiveagents(recentlyreviewedin[155]),todate,therearenoapprovedtherapiesforpreventionortreatmentofneuropathiestriggeredbyMTAchemotherapy[152,153,156].
ThisispartlyduetothepoorunderstandingofmechanismsunderlyingMTA-inducedneurotoxic-ity,andthustoalackofavaluablemethodofstandardizationintheclinicalmeasurementofCIPN.
Asapostulate,ithasoftenbeendeclaredthatMTAssimilarlyaffectthemicrotubulenetworkincancerandneuronalcells,butevidencewasnotalwayssustained.
For20yearsnow,animalmodelsofCIPNhavebeendeveloped(listedin[157]),attemptingtoinvestigateMTAsmechanismofactionontheperipheralnervoussystem.
Mostofthesemodelsareessentially682A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688focusedonthereportofpain-relatedbehaviorandonlyafewgoonfurtheronneurophysiologicalexperiments.
Whileratmodelsofvincristine-inducedperipheralneuropathydescribedabnormalmi-crotubuleassembliesanddensitiesasmaindamages[158,159],thesemicrotubuleproleswerenotobservedwithpaclitaxelinratmodels[160].
IthasthusbeenhypothesizedthatthemicrotubulenetworkwaslikelynottheonlytargetofMTAstobeinvolvedinneurondysfunctions.
Inparallel,thedecipheringofMTAactionprogressivelyslippedconsiderationsfrommicrotubulestomitochondria.
Invitrodatausingpaclitaxelreportedanterogradeandretrogradeaxonaltransportblockadeinratdorsalrootganglia(DRG)andhippocampalneurons.
Then,invivostudiesshowedasignicantincreaseintheincidenceofswollenmitochondriainaxonsafterpaclitaxeltreatment[160,161].
SimilardatawereobtainedwithinvitrocultureofDRG,inwhichtheinductionofatypicalmitochondriabypaclitaxelwasassociatedwithasignicantreductionoftheirfunctioningandthelossofmitochondrialmembranepotential[162].
Nevertheless,inthesestudies,thelinkbetweenmicrotubuledensityandinhibitionofneuronalorganelledistributionremainedcontroversialduetovariablepaclitaxelinjectionsmodesanddifferentadministeredconcentrationsoverthetime[163,164].
5.
2.
InhibitionofMTA'sneurotoxiceffects:domitochondrianeedtobeprotectedManyworksareattemptingtondclinicallyefcientneuropro-tectorsabletoenhanceneuronalcellsurvival.
Todate,severalneuroprotectiveagentslikethiols,neurotrophicfactorsandantiox-idantshavebeentestedinpreclinicalmodelsandclinicaltrialsfortheirabilitytopreventCIPN[165,166].
Althoughseveralofthesecompoundswereidentiedasneuroprotectivemoleculesofinterest,clinicaldataarestilldiscussed.
Mitochondrialdysfunctionandoxidativestressarewidelybelievedtounderliethepathogenesisofvariousneurodegenerativediseases[167].
Theinvestigationofneuroprotectiveantioxidantswasthusrationalizedasapromisingstrategytopreventoralleviatemitochondrialdamages.
Amongthem,efcacyofacetyl-LcarnitineandmorerecentlyalphalipoicacidtoexertneuroprotectiveeffectsagainstMTAs,invitro,wasassociatedwithareducedincidenceofswollenandvacuolatedmitochondriainratC-ber[161]aswellasinsensoryDRGneurons[162].
Moreover,alphalipoicacidpreventedtheearlylossofmembranepotentialdifferentialinmitochondriaexposedtopaclitaxel,thuspreventingneuronsfrommitochondrialenergeticfailureprobablythroughanti-oxidantactivities.
Anattractivestrategy,themitochondrialprotectionmightbelimitedinpreventingMTA-mediatedCIPNwhichinvolvesalterationofothertargetssuchasthemicrotubulecytoskeleton.
Inthiscontext,olesoxime(TRO19622)appearedasapromisingdrugcandidatetotreattheneurotoxicsideeffectsofmicrotubule-targetedchemotherapy[168].
ThisnewmoleculeprotectedneuronalcellsfromMTA-inducedneuriteshrinkagebyrestoringbothmicrotubuledynamics–throughEBproteincometsmaintainingatmicrotubuleends–andthemicrotubule-governedmitochondrialtrafcking[23].
Thus,compoundslikeolesoximethatareabletojointhesetwopropertieswouldholdpromisetobetterpreventandcurepatientssufferingfromneurodegenerativedisordersinwhichmicrotubule-associatedaxonaltransportisdefective.
TheirstudymayalsobringadditionalfundamentalinsightsintothemolecularmechanismsunderlyingneurotoxicpropertiesofMTAs.
6.
Inuenceofmicrotubuledynamicsperturbationonmitochondrialdynamics:aneweldofinvestigationShortlyaftertheirsuccessfuluseintheclinics,MTAshavebeenextensivelyemployedinfundamentalresearch.
Bymodulatingmicro-tubulearchitectureanddynamics,theyareappropriatepharmacolog-icaltoolstoprobethemitochondria–cytoskeletoninteractions.
6.
1.
HowcanMTAsmodulatethemitochondrialmotilityMTAeffectshavebeenespeciallystudiedinneuronalcells,inwhichmitochondriamovethroughouttheneuronalprocessestocontributetosynapticmaintenance.
Appropriatepositioningofthemitochondrialnetworkensuresorganellefunctionandisnecessarytocellsurvivalandfunctionality.
Assoonas1978,ChanKYandBuntAHusedvinblastinetoformparacrystalstructuresandtohighlighttheinterconnectedspatialorganizationofmicrotubulesandmitochon-driainsynaptosomesandaxonterminalsofratcerebralcortex[169].
Afewyearslater,axonalorganelletransporthasbeenshownnottobetotallysuppressedaftermicrotubuledisruption[170],andtobepartiallyinhibitedbytheintroductionofagentsthatspecicallydisruptactinmicrolaments[171–173].
Inparallel,thediscoveryandcharacterizationofmicrotubule-basedmotorskinesinanddyneinallowedtobetterenvisagetheaxonaltransportsystem.
Complemen-tarydata,intendingtodeciphertheimportanceofmicrotubule-governedtransportamongothercytoskeleta,usedamodelofneuronsgrownwithvinblastine.
Resultsshowedthatthewholemitochondrialcompartmentconcentratedintothecellbody,suggestingthatmicrotubuleswerenecessaryandsufcientforthetransportofmitochondriainaxons[174].
Consideringtheuncontestedroleofmicrotubulesastracksfortheintracellulartrafckingofmitochondria,itcanbearguedthatmitochondrialtransportdefectscouldresultfrommicrotubuledynam-icsalteration.
Insupporttothis,theparkinsoniantoxin(MPP+)hasbeenrecentlyshowntoinduceanearlyalterationofmicrotubuledynamicsandorientation,andasubsequentmitochondrialtransportimpairment[175].
Worksintumorcellsshowedthatpaclitaxelincreasedthespeedofmitochondrialmovement,whereascolchicineandnocodazoleretardedit[123,176],suggestingthatmicrotubulestabilizationcouldbenecessarytoorganelletrafcking.
Uptonow,themajorhypothesisexploredwasthechangesinmolecularmotorbindingtothemicrotubulerailways,throughtubulinpost-translationalmod-ications(PTMs).
Indeed,bindingofthemotorproteinkinesin-1,thatmostlyensuresanterogrademitochondrialtransportinaxons,isincreasedbymicrotubuledetyrosinationandacetylation[177,178].
ThesetwomajorPTMscorrelatewithmicrotubulestabilizationandareinducedbypaclitaxelandixabepiloneincancercells[179,180].
Thesedataweresupportedbyobservationsofpaclitaxelinhibitoryeffectsonfastretrogradetransportinratperipheralnerves[163,181].
However,resultsarestillcontroversialsincearecentworkshowedthatpaclitaxelabolishedkinesin-1translocationinpolarizedneuronsbyincreasingtheoveralllevelsoftubulinacetylation,detyrosinationandpolyglutamyla-tion[182].
Oneexplanationcouldbethat,whilemicrotubulestabiliza-tionisnecessaryforthemitochondrialtransport,itsover-stabilizationcompromisestheintracellulartrafcking.
Elsewhere,microtubuleassociatedprotein(MAPs)bindingtomicrotubulescanalsoinuencemotor-basedaxonaltransport,mainlybyaffectingtheattachmentanddetachmentcycleofthemotors.
Inneurons,tauandMAP4cancontroltheintracellulartrafckingbyreducingtheattachmentofkinesintomicrotubules[183,184].
MorerecentlySeitzetal.
showedadecreaseinrun-lengthforbothkinesinordyneinwhenMAP2candtauwereoverexpressedincells,combinedwithasignicantdecreaseinkinesinattachmentfrequencyontaxol-stabilizedmicrotubules[185].
Then,aspaclitaxelhasbeenshowntoincreaseMAP2afnityformicrotubule[186],itcouldthuseasilybethoughtthatpaclitaxelbyregulatingMAPsbindingcouldmodulateorganelletrafcking.
Lastly,thep150Gluedsubunitofdynactinisa+TIP(cfpart2)that,inassociationwithdynein,participatestoorganelleretrogradetransport.
Interestingly,p150GluedinteractionwithEB1atmicrotubuleplus-endsseemstobecentralinthedynein/dynactinfunction[187].
Thus,bysignicantlydisturbingEB1localization[21,23],MTAsmaycausethelossofbothmicrotubuledynamicsandmitochondrialtransport,whichtogethermightleadtocancercelldeath.
Sameobservationscouldbe683A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688transposedtotheneuronalmodelasEBfamilymembersarecrucialforneuritegrowthandmaintenance,andaretoolsofchoicetopreciselymeasureplus-endmicrotubuledynamicsbylivemicroscopy[188].
Interestingly,neurotoxicconcentrationsofpaclitaxelhavebeenshowntoinduceadecreaseinthenumberandlengthofEB3comettailsinAplysianeurons[189].
Moreover,paclitaxelsignicantlydisturbedthemicrotubulepolarorientation,byreducingthepercentageofmicro-tubuleswithplusendsfacingtheaxontipandincreasingthosewithplusendsfacingthecellbody[189].
Allthesemicrotubulemodicationswereassociatedwithaseverelyimpairedmitochondrialtransport.
Inagreementwiththesedata,weshowedthatpaclitaxelandvincristinesuppressedEB1andEB3accumulationatmicrotubuleplus-ends,andsignicantlyreducedthemitochondrialmotilityinhumandifferentiat-edneuronalcells[23].
6.
2.
CanMTAsdisruptthession/fusionequilibriumRecently,withtheemergenceoftheneuropathologyeldofresearch,studieshaveourishedsuggestingthatmitochondrialdysfunctionsareearlyandcausaleventsinmanyneurodegenerativediseases[190]suchasamyotrophiclateralsclerosis,Alzheimer's,Huntington'sorParkinson'sdiseases.
Onepotentialcauseofmitochon-drialdysfunctionisthedisruptionofthehighlycontrolledequilibriumbetweenmitochondrialssionandfusion.
Excessivessionordefectsinfusionaltercellfunctionsandviabilitythroughimpairmentofmitochondrialmotility,decreaseenergyproduction,andincreaseoftheoxidativestress[191].
Examplesaregivenwithstudiesusingtaxolatconcentrationsresponsibleformicrotubulestrongstabilizationandleadingtodisruptionofmitochondrialssion/fusionbalance[192]aswellastheirabilitytofastlymoveanddistributetowardshighenergydemandsubcellularlocations[193].
Inthesestudies,MTAshavebeenemployedathighconcentrationsduringveryshorttimeofexposure(lessthan24h)toinducemicrotubulemodicationsandthustoanalyzemitochondrialdynamicityparameters.
Thereisnowacrucialneedinreconsideringtheconcentrationsemployed.
Indeed,lowerconcentrationsmaygivecomplementarycuestountanglemitochondriaandmicrotubuleinterconnectionsandmayhelptodecipherhowtheanti-microtubulepropertiesofMTAsleadtodisturbancesinmitochon-drialdynamics.
Intumorcells,suchmoderatedconcentrationsofMTAssignicantlyaltermicrotubuledynamicsandinducethemitochondrialnetworkfragmentation,asanearlyprocessassociatedwiththeirpro-apoptotic,anti-angiogenicandneurotoxicactivities[23].
AspreviouslyshownwithBH3-onlypeptides[194],ourrecentworkssuggestedthatthisprocesscouldresultfromBimaccumulationinmitochondrialmembranes(Savryetal,submitted),byamolecularmechanismthatshouldbeinvestigated.
7.
ConclusionToconclude,itclearlyappearsthatMTAsarebothanticancerdrugswithahighclinicalvalueandveryusefultoolstoanalyzetherolesplayedbythemicrotubulenetworkinphysio/pathologicalprocesses.
TodecipherthetangleofMTA-inducedapoptoticsignalsisatrickyexerciseand,todate,itisstilldifculttodeterminewhetherbiochemicaleventsthatleadtoapoptosisareactivateddownstreamorupstreaminhibitionofmicrotubuledynamicsandfunctions.
However,itbecomesclearthatcrucialmolecularlinksareestablishedbetweenthemicrotubulenetworkandtheapoptoticmachinery,toensurethesuccessofthecelldeathprogram.
Inthatsense,analysisofmechanismsresponsiblefortumorcellresistancetoMTAswouldalsoprovidekeyinformationaboutthecloseconnectionsbetweenmicrotubulesandtheapoptoticmachinery.
ThecoexistenceofmodicationsinthemicrotubulesystemandthemitochondrialsignalingcascadeincellsresistanttoMTAs[17,179,195]strengthenstheneedfornovelinsightsintointerconnectionsbetweenthetwocompartmentstohelpcircumventingthisclinicalproblem.
ItalsoconrmedthatthemitochondrionisstillapromisingtherapeutictargetthatcouldimprovecombinatorialtherapywithMTAsandprovidecrucialarmstohelptreatingcancers.
AcknowledgementsWethankDr.
StéphaneHonoréandDr.
VéroniqueBourgarel-Reyforhelpfulcommentsonthemanuscript.
ARreceivedafellowshipfromtheRégionProvenceAlpesCted'Azur(France)andASfromtheAssistancePubliquedesHopitauxdeMarseille.
References[1]T.
Mitchison,M.
Kirschner,Dynamicinstabilityofmicrotubulegrowth,Nature312(1984)237–242.
[2]E.
Pasquier,N.
Andre,D.
Braguer,Targetingmicrotubulestoinhibitangiogenesisanddisrupttumourvasculature:implicationsforcancertreatment,Curr.
CancerDrugTargets7(2007)566–581.
[3]D.
Calligaris,P.
Verdier-Pinard,F.
Devred,C.
Villard,D.
Braguer,D.
Latte,Microtubuletargetingagents:frombiophysicstoproteomics,Cell.
Mol.
LifeSci.
67(2010)1089–1104.
[4]S.
M.
Chen,L.
H.
Meng,J.
Ding,Newmicrotubule-inhibitinganticanceragents,ExpertOpin.
Investig.
Drugs19(2010)329–343.
[5]F.
Petrelli,K.
Borgonovo,S.
Barni,Targeteddeliveryforbreastcancertherapy:thehistoryofnanoparticle-albumin-boundpaclitaxel,ExpertOpin.
Pharmacother.
11(2010)1413–1432.
[6]E.
A.
Perez,Microtubuleinhibitors:differentiatingtubulin-inhibitingagentsbasedonmechanismsofaction,clinicalactivity,andresistance,Mol.
CancerTher.
8(2009)2086–2095.
[7]L.
Wilson,M.
A.
Jordan,Newmicrotubule/tubulin-targetedanticancerdrugsandnovelchemotherapeuticstrategies,J.
Chemother.
16(Suppl4)(2004)83–85.
[8]S.
Honore,K.
Kamath,D.
Braguer,L.
Wilson,C.
Briand,M.
A.
Jordan,Suppressionofmicrotubuledynamicsbydiscodermolidebyanovelmechanismisassociatedwithmitoticarrestandinhibitionoftumorcellproliferation,Mol.
CancerTher.
2(2003)1303–1311.
[9]S.
Honore,E.
Pasquier,D.
Braguer,Understandingmicrotubuledynamicsforimprovedcancertherapy,Cell.
Mol.
LifeSci.
62(2005)3039–3056.
[10]K.
Kamath,M.
A.
Jordan,SuppressionofmicrotubuledynamicsbyepothiloneBisassociatedwithmitoticarrest,CancerRes.
63(2003)6026–6031.
[11]B.
Pourroy,S.
Honore,E.
Pasquier,V.
Bourgarel-Rey,A.
Kruczynski,C.
Briand,D.
Braguer,Antiangiogenicconcentrationsofvinunineincreasetheinterphasemicrotubuledynamicsanddecreasethemotilityofendothelialcells,CancerRes.
66(2006)3256–3263.
[12]C.
L.
Rieder,H.
Maiato,Stuckindivisionorpassingthrough:whathappenswhencellscannotsatisfythespindleassemblycheckpoint,Dev.
Cell7(2004)637–651.
[13]S.
Honore,K.
Kamath,D.
Braguer,S.
B.
Horwitz,L.
Wilson,C.
Briand,M.
A.
Jordan,Synergisticsuppressionofmicrotubuledynamicsbydiscodermolideandpaclitaxelinnon-smallcelllungcarcinomacells,CancerRes.
64(2004)4957–4964.
[14]V.
K.
Ngan,K.
Bellman,B.
T.
Hill,L.
Wilson,M.
A.
Jordan,MechanismofmitoticblockandinhibitionofcellproliferationbythesemisyntheticVincaalkaloidsvinorelbineanditsnewerderivativevinunine,Mol.
Pharmacol.
60(2001)225–232.
[15]B.
Pourroy,M.
Carre,S.
Honore,V.
Bourgarel-Rey,A.
Kruczynski,C.
Briand,D.
Braguer,LowconcentrationsofvinunineinduceapoptosisinhumanSK-N-SHneuroblastomacellsthroughapostmitoticG1arrestandamitochondrialpathway,Mol.
Pharmacol.
66(2004)580–591.
[16]M.
E.
Bekier,R.
Fischbach,J.
Lee,W.
R.
Taylor,Lengthofmitoticarrestinducedbymicrotubule-stabilizingdrugsdeterminescelldeathaftermitoticexit,Mol.
CancerTher.
8(2009)1646–1654.
[17]M.
A.
Esteve,M.
Carre,V.
Bourgarel-Rey,A.
Kruczynski,G.
Raspaglio,C.
Ferlini,D.
Braguer,Bcl-2down-regulationandtubulinsubtypecompositionareinvolvedinresistanceofovariancancercellstovinunine,Mol.
CancerTher.
5(2006)2824–2833.
[18]G.
Liao,T.
Nagasaki,G.
G.
Gundersen,Lowconcentrationsofnocodazoleinterferewithbroblastlocomotionwithoutsignicantlyaffectingmicrotubulelevel:implicationsfortheroleofdynamicmicrotubulesincelllocomotion,J.
CellSci.
108(Pt11)(1995)3473–3483.
[19]A.
Mikhailov,G.
G.
Gundersen,Relationshipbetweenmicrotubuledynamicsandlamellipodiumformationrevealedbydirectimagingofmicrotubulesincellstreatedwithnocodazoleortaxol,CellMotil.
Cytoskeleton41(1998)325–340.
[20]E.
Pasquier,S.
Honore,B.
Pourroy,M.
A.
Jordan,M.
Lehmann,C.
Briand,D.
Braguer,Antiangiogenicconcentrationsofpaclitaxelinduceanincreaseinmicrotubuledynamicsinendothelialcellsbutnotincancercells,CancerRes.
65(2005)2433–2440.
[21]S.
Honore,A.
Pagano,G.
Gauthier,V.
Bourgarel-Rey,P.
Verdier-Pinard,K.
Civiletti,A.
Kruczynski,D.
Braguer,AntiangiogenicvinunineaffectsEB1localizationandmicrotubuletargetingtoadhesionsites,Mol.
CancerTher.
7(2008)2080–2089.
[22]A.
Akhmanova,M.
O.
Steinmetz,Trackingtheends:adynamicproteinnetworkcontrolsthefateofmicrotubuletips,Nat.
Rev.
Mol.
CellBiol.
9(2008)309–322.
[23]A.
Rovini,M.
Carre,T.
Bordet,R.
M.
Pruss,D.
Braguer,Olesoximepreventsmicrotubule-targetingdrugneurotoxicity:selectivepreservationofEB684A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688cometsindifferentiatedneuronalcells,Biochem.
Pharmacol.
80(2010)884–894.
[24]D.
Simoni,R.
Romagnoli,R.
Baruchello,R.
Rondanin,M.
Rizzi,M.
G.
Pavani,D.
Alloatti,G.
Giannini,M.
Marcellini,T.
Riccioni,M.
Castorina,M.
B.
Guglielmi,F.
Bucci,P.
Carminati,C.
Pisano,Novelcombretastatinanaloguesendowedwithantitumoractivity,J.
Med.
Chem.
49(2006)3143–3152.
[25]K.
D.
Wu,Y.
S.
Cho,J.
Katz,V.
Ponomarev,S.
Chen-Kiang,S.
J.
Danishefsky,M.
A.
Moore,Investigationofantitumoreffectsofsyntheticepothiloneanalogsinhumanmyelomamodelsinvitroandinvivo,Proc.
NatlAcad.
Sci.
USA102(2005)10640–10645.
[26]C.
Rappl,P.
Barbier,V.
Bourgarel-Rey,C.
Gregoire,R.
Gilli,M.
Carre,S.
Combes,J.
P.
Finet,V.
Peyrot,Interactionof4-arylcoumarinanaloguesofcombretastatinswithmicrotubulenetworkofHBL100cellsandbindingtotubulin,Biochemistry45(2006)9210–9218.
[27]M.
A.
Esteve,M.
Carre,D.
Braguer,Microtubulesinapoptosisinduction:aretheynecessaryCurr.
CancerDrugTargets7(2007)713–729.
[28]B.
Lin,L.
Catley,R.
LeBlanc,C.
Mitsiades,R.
Burger,Y.
T.
Tai,K.
Podar,M.
Wartmann,D.
Chauhan,J.
D.
Grifn,K.
C.
Anderson,Patupilone(epothiloneB)inhibitsgrowthandsurvivalofmultiplemyelomacellsinvitroandinvivo,Blood105(2005)350–357.
[29]K.
Bhalla,A.
M.
Ibrado,E.
Tourkina,C.
Tang,M.
E.
Mahoney,Y.
Huang,TaxolinducesinternucleosomalDNAfragmentationassociatedwithprogrammedcelldeathinhumanmyeloidleukemiacells,Leukemia7(1993)563–568.
[30]E.
Pasquier,M.
Carre,B.
Pourroy,L.
Camoin,O.
Rebai,C.
Briand,D.
Braguer,Antiangiogenicactivityofpaclitaxelisassociatedwithitscytostaticeffect,mediatedbytheinitiationbutnotcompletionofamitochondrialapoptoticsignalingpathway,Mol.
CancerTher.
3(2004)1301–1310.
[31]S.
E.
Holwell,B.
T.
Hill,M.
C.
Bibby,Anti-vasculareffectsofvinunineintheMAC15Atransplantableadenocarcinomamodel,Br.
J.
Cancer84(2001)290–295.
[32]A.
Goncalves,D.
Braguer,G.
Carles,N.
Andre,C.
Prevot,C.
Briand,Caspase-8activationindependentofCD95/CD95-Linteractionduringpaclitaxel-inducedapoptosisinhumancoloncancercells(HT29-D4),Biochem.
Pharmacol.
60(2000)1579–1584.
[33]R.
Kim,K.
Tanabe,M.
Emi,Y.
Uchida,T.
Toge,Deathreceptor-dependentand-independentpathwaysinanticancerdrug-inducedapoptosisofbreastcancercells,Oncol.
Rep.
10(2003)1925–1930.
[34]S.
Fulda,K.
M.
Debatin,Extrinsicversusintrinsicapoptosispathwaysinanticancerchemotherapy,Oncogene25(2006)4798–4811.
[35]N.
Andre,M.
Carre,G.
Brasseur,B.
Pourroy,H.
Kovacic,C.
Briand,D.
Braguer,Paclitaxeltargetsmitochondriaupstreamofcaspaseactivationinintacthumanneuroblastomacells,FEBSLett.
532(2002)256–260.
[36]S.
Jiang,Y.
Zu,Y.
Fu,Y.
Zhang,T.
Efferth,Activationofthemitochondria-drivenpathwayofapoptosisinhumanPC-3prostatecancercellsbyanovelhydrophilicpaclitaxelderivative,7-xylosyl-10-deacetylpaclitaxel,Int.
J.
Oncol.
33(2008)103–111.
[37]Y.
Kang,J.
Wu,G.
Yin,Z.
Huang,X.
Liao,Y.
Yao,P.
Ouyang,H.
Wang,Q.
Yang,Characterizationandbiologicalevaluationofpaclitaxel-loadedpoly(L-lacticacid)microparticlespreparedbysupercriticalCO2,Langmuir24(2008)7432–7441.
[38]S.
D.
Patterson,C.
S.
Spahr,E.
Daugas,S.
A.
Susin,T.
Irinopoulou,C.
Koehler,G.
Kroemer,Massspectrometricidenticationofproteinsreleasedfrommitochondriaundergoingpermeabilitytransition,CellDeathDiffer.
7(2000)137–144.
[39]G.
VanLoo,H.
Demol,M.
vanGurp,B.
Hoorelbeke,P.
Schotte,R.
Beyaert,B.
Zhivotovsky,K.
Gevaert,W.
Declercq,J.
Vandekerckhove,P.
Vandenabeele,Amatrix-assistedlaserdesorptionionizationpost-sourcedecay(MALDI-PSD)analysisofproteinsreleasedfromisolatedlivermitochondriatreatedwithrecombinanttruncatedBid,CellDeathDiffer.
9(2002)301–308.
[40]A.
Kruczynski,C.
Etievant,D.
Perrin,N.
Chansard,A.
Duos,B.
T.
Hill,Characterizationofcelldeathinducedbyvinunine,themostrecentVincaalkaloidinclinicaldevelopment,Br.
J.
Cancer86(2002)143–150.
[41]S.
J.
Park,C.
H.
Wu,J.
D.
Gordon,X.
Zhong,A.
Emami,A.
R.
Safa,Taxolinducescaspase-10-dependentapoptosis,J.
Biol.
Chem.
279(2004)51057–51067.
[42]C.
L.
Perkins,G.
Fang,C.
N.
Kim,K.
N.
Bhalla,TheroleofApaf-1,caspase-9,andbidproteinsinetoposide-orpaclitaxel-inducedmitochondrialeventsduringapoptosis,CancerRes.
60(2000)1645–1653.
[43]S.
Y.
Yuan,S.
L.
Hsu,K.
J.
Tsai,C.
R.
Yang,InvolvementofmitochondrialpathwayinTaxol-inducedapoptosisofhumanT24bladdercancercells,Urol.
Res.
30(2002)282–288.
[44]D.
Uyar,N.
Takigawa,T.
Mekhail,D.
Grabowski,M.
Markman,F.
Lee,R.
Canetta,R.
Peck,R.
Bukowski,R.
Ganapathi,ApoptoticpathwaysofepothiloneBMS310705,Gynecol.
Oncol.
91(2003)173–178.
[45]C.
Perkins,C.
N.
Kim,G.
Fang,K.
N.
Bhalla,OverexpressionofApaf-1promotesapoptosisofuntreatedandpaclitaxel-oretoposide-treatedHL-60cells,CancerRes.
58(1998)4561–4566.
[46]K.
Friedrich,T.
Wieder,C.
VonHaefen,S.
Radetzki,R.
Janicke,K.
Schulze-Osthoff,B.
Dorken,P.
T.
Daniel,Overexpressionofcaspase-3restoressensitivityfordrug-inducedapoptosisinbreastcancercelllineswithacquireddrugresistance,Oncogene20(2001)2749–2760.
[47]J.
Chai,C.
Du,J.
W.
Wu,S.
Kyin,X.
Wang,Y.
Shi,StructuralandbiochemicalbasisofapoptoticactivationbySmac/DIABLO,Nature406(2000)855–862.
[48]T.
E.
Fandy,S.
Shankar,R.
K.
Srivastava,Smac/DIABLOenhancesthetherapeuticpotentialofchemotherapeuticdrugsandirradiation,andsensitizesTRAIL-resistantbreastcancercells,Mol.
Cancer7(2008)60.
[49]C.
R.
Arnt,M.
V.
Chiorean,M.
P.
Heldebrant,G.
J.
Gores,S.
H.
Kaufmann,SyntheticSmac/DIABLOpeptidesenhancetheeffectsofchemotherapeuticagentsbybindingXIAPandcIAP1insitu,J.
Biol.
Chem.
277(2002)44236–44243.
[50]I.
A.
McNeish,S.
Bell,T.
McKay,T.
Tenev,M.
Marani,N.
R.
Lemoine,ExpressionofSmac/DIABLOinovariancarcinomacellsinducesapoptosisviaacaspase-9-mediatedpathway,Exp.
CellRes.
286(2003)186–198.
[51]H.
L.
Mao,P.
S.
Liu,J.
F.
Zheng,P.
H.
Zhang,L.
G.
Zhou,G.
Xin,C.
Liu,TransfectionofSmac/DIABLOsensitizesdrug-resistanttumorcellstoTRAILorpaclitaxel-inducedapoptosisinvitro,Pharmacol.
Res.
56(2007)483–492.
[52]L.
D.
Walensky,BCL-2inthecrosshairs:tippingthebalanceoflifeanddeath,CellDeathDiffer.
13(2006)1339–1350.
[53]N.
A.
Jones,J.
Turner,A.
J.
McIlwrath,R.
Brown,C.
Dive,Cisplatin-andpaclitaxel-inducedapoptosisofovariancarcinomacellsandtherelationshipbetweenbaxandbakup-regulationandthefunctionalstatusofp53,Mol.
Pharmacol.
53(1998)819–826.
[54]H.
Yamaguchi,J.
Chen,K.
Bhalla,H.
G.
Wang,RegulationofBaxactivationandapoptoticresponsetomicrotubule-damagingagentsbyp53transcription-dependentand-independentpathways,J.
Biol.
Chem.
279(2004)39431–39437.
[55]G.
Tudor,A.
Aguilera,D.
O.
Halverson,N.
D.
Laing,E.
A.
Sausville,Susceptibilitytodrug-inducedapoptosiscorrelateswithdifferentialmodulationofBad,Bcl-2andBcl-xLproteinlevels,CellDeathDiffer.
7(2000)574–586.
[56]T.
Strobel,Y.
T.
Tai,S.
Korsmeyer,S.
A.
Cannistra,BADpartlyreversespaclitaxelresistanceinhumanovariancancercells,Oncogene17(1998)2419–2427.
[57]H.
Sawa,T.
Kobayashi,K.
Mukai,W.
Zhang,H.
Shiku,BaxoverexpressionenhancescytochromecreleasefrommitochondriaandsensitizesKATOIIIgastriccancercellstochemotherapeuticagent-inducedapoptosis,Int.
J.
Oncol.
16(2000)745–749.
[58]V.
N.
Sumantran,M.
W.
Ealovega,G.
Nunez,M.
F.
Clarke,M.
S.
Wicha,Over-expressionofBcl-XSsensitizesMCF-7cellstochemotherapy-inducedapoptosis,CancerRes.
55(1995)2507–2510.
[59]G.
W.
Makin,B.
M.
Corfe,G.
J.
Grifths,A.
Thistlethwaite,J.
A.
Hickman,C.
Dive,Damage-inducedBaxN-terminalchange,translocationtomitochondriaandformationofBaxdimers/complexesoccurregardlessofcellfate,EMBOJ.
20(2001)6306–6315.
[60]B.
Antonsson,S.
Montessuit,B.
Sanchez,J.
C.
Martinou,Baxispresentasahighmolecularweightoligomer/complexinthemitochondrialmembraneofapoptoticcells,J.
Biol.
Chem.
276(2001)11615–11623.
[61]P.
F.
Cartron,M.
Priault,L.
Oliver,K.
Meah,S.
Manon,F.
M.
Vallette,TheN-terminalendofBaxcontainsamitochondrial-targetingsignal,J.
Biol.
Chem.
278(2003)11633–11641.
[62]D.
Grifn,S.
Wittmann,F.
Guo,R.
Nimmanapalli,P.
Bali,H.
G.
Wang,K.
Bhalla,MoleculardeterminantsofepothiloneBderivative(BMS247550)andApo-2L/TRAIL-inducedapoptosisofhumanovariancancercells,Gynecol.
Oncol.
89(2003)37–47.
[63]C.
Huisman,C.
G.
Ferreira,L.
E.
Broker,J.
A.
Rodriguez,E.
F.
Smit,P.
E.
Postmus,F.
A.
Kruyt,G.
Giaccone,Paclitaxeltriggerscelldeathprimarilyviacaspase-independentroutesinthenon-smallcelllungcancercelllineNCI-H460,Clin.
CancerRes.
8(2002)596–606.
[64]L.
Du,C.
S.
Lyle,T.
C.
Chambers,Characterizationofvinblastine-inducedBcl-xLandBcl-2phosphorylation:evidenceforanovelproteinkinaseandacoordinatedphosphorylation/dephosphorylationcycleassociatedwithapoptosisinduction,Oncogene24(2005)107–117.
[65]N.
Pathan,C.
Aime-Sempe,S.
Kitada,S.
Haldar,J.
C.
Reed,Microtubule-targetingdrugsinduceBcl-2phosphorylationandassociationwithPin1,Neoplasia3(2001)70–79.
[66]S.
Haldar,J.
Chintapalli,C.
M.
Croce,Taxolinducesbcl-2phosphorylationanddeathofprostatecancercells,CancerRes.
56(1996)1253–1255.
[67]L.
Brichese,A.
Valette,PP1phosphataseisinvolvedinBcl-2dephosphorylationafterprolongedmitoticarrestinducedbypaclitaxel,Biochem.
Biophys.
Res.
Commun.
294(2002)504–508.
[68]C.
D.
Scatena,Z.
A.
Stewart,D.
Mays,L.
J.
Tang,C.
J.
Keefer,S.
D.
Leach,J.
A.
Pietenpol,MitoticphosphorylationofBcl-2duringnormalcellcycleprogressionandTaxol-inducedgrowtharrest,J.
Biol.
Chem.
273(1998)30777–30784.
[69]M.
Fan,L.
Du,A.
A.
Stone,K.
M.
Gilbert,T.
C.
Chambers,Modulationofmitogen-activatedproteinkinasesandphosphorylationofBcl-2byvinblastinerepresentpersistentformsofnormaluctuationsatG2-M1,CancerRes.
60(2000)6403–6407.
[70]T.
A.
Buchholz,D.
W.
Davis,D.
J.
McConkey,W.
F.
Symmans,V.
Valero,A.
Jhingran,S.
L.
Tucker,L.
Pusztai,M.
Cristofanilli,F.
J.
Esteva,G.
N.
Hortobagyi,A.
A.
Sahin,Chemotherapy-inducedapoptosisandBcl-2levelscorrelatewithbreastcancerresponsetochemotherapy,CancerJ.
9(2003)33–41.
[71]C.
Bressin,V.
Bourgarel-Rey,M.
Carre,B.
Pourroy,D.
Arango,D.
Braguer,Y.
Barra,Decreaseinc-Mycactivityenhancescancercellsensitivitytovinblastine,AnticancerDrugs17(2006)181–187.
[72]M.
L.
Panno,F.
Giordano,F.
Mastroianni,C.
Morelli,E.
Brunelli,M.
G.
Palma,M.
Pellegrino,S.
Aquila,A.
Miglietta,L.
Mauro,D.
Bonoglio,S.
Ando,EvidencethatlowdosesofTaxolenhancethefunctionaltransactivatorypropertiesofp53onp21wafpromoterinMCF-7breastcancercells,FEBSLett.
580(2006)2371–2380.
[73]T.
Yoshino,H.
Shiina,S.
Urakami,N.
Kikuno,T.
Yoneda,K.
Shigeno,M.
Igawa,Bcl-2expressionasapredictivemarkerofhormone-refractoryprostatecancertreatedwithtaxane-basedchemotherapy,Clin.
CancerRes.
12(2006)6116–6124.
[74]I.
Lebedeva,R.
Rando,J.
Ojwang,P.
Cossum,C.
A.
Stein,Bcl-xLinprostatecancercells:effectsofoverexpressionanddown-regulationonchemosensitivity,CancerRes.
60(2000)6052–6060.
[75]X.
Tang,Y.
Zhu,L.
Han,A.
L.
Kim,L.
Kopelovich,D.
R.
Bickers,M.
Athar,CP-31398restoresmutantp53tumorsuppressorfunctionandinhibitsUVB-inducedskincarcinogenesisinmice,J.
Clin.
Invest.
117(2007)3753–3764.
685A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[76]J.
Hoffmann,I.
Vitale,B.
Buchmann,L.
Galluzzi,W.
Schwede,L.
Senovilla,W.
Skuballa,S.
Vivet,R.
B.
Lichtner,J.
M.
Vicencio,T.
Panaretakis,G.
Siemeister,H.
Lage,L.
Nanty,S.
Hammer,K.
Mittelstaedt,S.
Winsel,J.
Eschenbrenner,M.
Castedo,C.
Demarche,U.
Klar,G.
Kroemer,Improvedcellularpharmacokineticsandpharmacodynamicsunderliethewideanticanceractivityofsagopilone,CancerRes.
68(2008)5301–5308.
[77]C.
Leonetti,A.
Biroccio,C.
D'Angelo,S.
C.
Semple,M.
Scarsella,G.
Zupi,Therapeuticintegrationofc-mycandbcl-2antisensemoleculeswithdocetaxelinapreclinicalmodelofhormone-refractoryprostatecancer,Prostate67(2007)1475–1485.
[78]K.
Tanabe,R.
Kim,H.
Inoue,M.
Emi,Y.
Uchida,T.
Toge,AntisenseBcl-2andHER-2oligonucleotidetreatmentofbreastcancercellsenhancestheirsensitivitytoanticancerdrugs,Int.
J.
Oncol.
22(2003)875–881.
[79]K.
Yamanaka,P.
Rocchi,H.
Miyake,L.
Fazli,A.
So,U.
Zangemeister-Wittke,M.
E.
Gleave,InductionofapoptosisandenhancementofchemosensitivityinhumanprostatecancerLNCaPcellsusingbispecicantisenseoligonucleotidetargetingBcl-2andBcl-xLgenes,BJUInt.
97(2006)1300–1308.
[80]O.
Kutuk,A.
Letai,AlterationofthemitochondrialapoptoticpathwayiskeytoacquiredpaclitaxelresistanceandcanbereversedbyABT-737,CancerRes.
68(2008)7985–7994.
[81]T.
Oltersdorf,S.
W.
Elmore,A.
R.
Shoemaker,R.
C.
Armstrong,D.
J.
Augeri,B.
A.
Belli,M.
Bruncko,T.
L.
Deckwerth,J.
Dinges,P.
J.
Hajduk,M.
K.
Joseph,S.
Kitada,S.
J.
Korsmeyer,A.
R.
Kunzer,A.
Letai,C.
Li,M.
J.
Mitten,D.
G.
Nettesheim,S.
Ng,P.
M.
Nimmer,J.
M.
O'Connor,A.
Oleksijew,A.
M.
Petros,J.
C.
Reed,W.
Shen,S.
K.
Tahir,C.
B.
Thompson,K.
J.
Tomaselli,B.
Wang,M.
D.
Wendt,H.
Zhang,S.
W.
Fesik,S.
H.
Rosenberg,AninhibitorofBcl-2familyproteinsinducesregressionofsolidtumours,Nature435(2005)677–681.
[82]H.
Zall,A.
Weber,R.
Besch,N.
Zantl,G.
Hacker,ChemotherapeuticdrugssensitizehumanrenalcellcarcinomacellstoABT-737byamechanisminvolvingtheNoxa-dependentinactivationofMcl-1orA1,Mol.
Cancer9(2010)164.
[83]A.
R.
Shoemaker,A.
Oleksijew,J.
Bauch,B.
A.
Belli,T.
Borre,M.
Bruncko,T.
Deckwirth,D.
J.
Frost,K.
Jarvis,M.
K.
Joseph,K.
Marsh,W.
McClellan,H.
Nellans,S.
Ng,P.
Nimmer,J.
M.
O'Connor,T.
Oltersdorf,W.
Qing,W.
Shen,J.
Stavropoulos,S.
K.
Tahir,B.
Wang,R.
Warner,H.
Zhang,S.
W.
Fesik,S.
H.
Rosenberg,S.
W.
Elmore,Asmall-moleculeinhibitorofBcl-XLpotentiatestheactivityofcytotoxicdrugsinvitroandinvivo,CancerRes.
66(2006)8731–8739.
[84]K.
Bray,H.
Y.
Chen,C.
M.
Karp,M.
May,S.
Ganesan,V.
Karantza-Wadsworth,R.
S.
DiPaola,E.
White,Bcl-2modulationtoactivateapoptosisinprostatecancer,Mol.
CancerRes.
7(2009)1487–1496.
[85]A.
A.
Chanan-Khan,R.
Niesvizky,R.
J.
Hohl,T.
M.
Zimmerman,N.
P.
Christiansen,G.
J.
Schiller,N.
Callander,J.
Lister,M.
Oken,S.
Jagannath,PhaseIIIrandomisedstudyofdexamethasonewithorwithoutoblimersensodiumforpatientswithadvancedmultiplemyeloma,Leuk.
Lymphoma50(2009)559–565.
[86]S.
O'Brien,J.
O.
Moore,T.
E.
Boyd,L.
M.
Larratt,A.
Skotnicki,B.
Koziner,A.
A.
Chanan-Khan,J.
F.
Seymour,R.
G.
Bociek,S.
Pavletic,K.
R.
Rai,RandomizedphaseIIItrialofudarabinepluscyclophosphamidewithorwithoutoblimersensodium(Bcl-2antisense)inpatientswithrelapsedorrefractorychroniclymphocyticleukemia,J.
Clin.
Oncol.
25(2007)1114–1120.
[87]E.
Wesarg,S.
Hoffarth,R.
Wiewrodt,M.
Kroll,S.
Biesterfeld,C.
Huber,M.
Schuler,TargetingBCL-2familyproteinstoovercomedrugresistanceinnon-smallcelllungcancer,Int.
J.
Cancer121(2007)2387–2394.
[88]C.
Ferlini,G.
Raspaglio,S.
Mozzetti,M.
Distefano,F.
Filippetti,E.
Martinelli,G.
Ferrandina,D.
Gallo,F.
O.
Ranelletti,G.
Scambia,Bcl-2down-regulationisanovelmechanismofpaclitaxelresistance,Mol.
Pharmacol.
64(2003)51–58.
[89]Y.
Inoue,M.
Gika,T.
Abiko,T.
Oyama,Y.
Saitoh,H.
Yamazaki,M.
Nakamura,Y.
Abe,M.
Kawamura,K.
Kobayashi,Bcl-2overexpressionenhancesinvitrosensitivityagainstdocetaxelinnon-smallcelllungcancer,Oncol.
Rep.
13(2005)259–264.
[90]M.
Vilenchik,A.
J.
Raffo,L.
Benimetskaya,D.
Shames,C.
A.
Stein,AntisenseRNAdown-regulationofbcl-xLexpressioninprostatecancercellsleadstodiminishedratesofcellularproliferationandresistancetocytotoxicchemo-therapeuticagents,CancerRes.
62(2002)2175–2183.
[91]K.
Vermeulen,Z.
N.
Berneman,D.
R.
VanBockstaele,Cellcycleandapoptosis,CellProlif.
36(2003)165–175.
[92]P.
Giannakakou,D.
L.
Sackett,Y.
Ward,K.
R.
Webster,M.
V.
Blagosklonny,T.
Fojo,p53isassociatedwithcellularmicrotubulesandistransportedtothenucleusbydynein,Nat.
CellBiol.
2(2000)709–717.
[93]N.
R.
Khawaja,M.
Carre,H.
Kovacic,M.
A.
Esteve,D.
Braguer,Patupilone-inducedapoptosisismediatedbymitochondrialreactiveoxygenspeciesthroughBimrelocalizationtomitochondria,Mol.
Pharmacol.
74(2008)1072–1083.
[94]X.
M.
Liu,J.
D.
Jiang,A.
C.
Ferrari,D.
R.
Budman,L.
G.
Wang,Uniqueinductionofp21(WAF1/CIP1)expressionbyvinorelbineinandrogen-independentprostatecancercells,Br.
J.
Cancer89(2003)1566–1573.
[95]K.
Rathinasamy,B.
Jindal,J.
Asthana,P.
Singh,P.
V.
Balaji,D.
Panda,Griseofulvinstabilizesmicrotubuledynamics,activatesp53andinhibitstheproliferationofMCF-7cellssynergisticallywithvinblastine,BMCCancer10(2010)213.
[96]R.
B.
Tishler,D.
M.
Lamppu,S.
Park,B.
D.
Price,Microtubule-activedrugstaxol,vinblastine,andnocodazoleincreasethelevelsoftranscriptionallyactivep53,CancerRes.
55(1995)6021–6025.
[97]H.
H.
Wang,H.
L.
Li,R.
Liu,Y.
Zhang,K.
Liao,Q.
Wang,J.
Z.
Wang,S.
J.
Liu,Tauoverexpressioninhibitscellapoptosiswiththemechanismsinvolvingmultipleviability-relatedfactors,J.
AlzheimersDis.
(2010)167–179.
[98]R.
Drago-Ferrante,A.
Santulli,R.
DiFiore,M.
Giuliano,G.
Calvaruso,G.
Tesoriere,R.
Vento,LowdosesofpaclitaxelpotentlyinduceapoptosisinhumanretinoblastomaY79cellsbyup-regulatingE2F1,Int.
J.
Oncol.
33(2008)677–687.
[99]T.
T.
Tan,K.
Degenhardt,D.
A.
Nelson,B.
Beaudoin,W.
Nieves-Neira,P.
Bouillet,A.
Villunger,J.
M.
Adams,E.
White,KeyrolesofBIM-drivenapoptosisinepithelialtumorsandrationalchemotherapy,CancerCell7(2005)227–238.
[100]M.
D.
Galigniana,J.
M.
Harrell,H.
M.
O'Hagen,M.
Ljungman,W.
B.
Pratt,Hsp90-bindingimmunophilinslinkp53todyneinduringp53transporttothenucleus,J.
Biol.
Chem.
279(2004)22483–22489.
[101]K.
Rathinasamy,D.
Panda,Kineticstabilizationofmicrotubuledynamicinstabilitybybenomylincreasesthenucleartransportofp53,Biochem.
Pharmacol.
76(2008)1669–1680.
[102]A.
Basu,S.
Haldar,TherelationshipbetweenBcI2,Baxandp53:consequencesforcellcycleprogressionandcelldeath,Mol.
Hum.
Reprod.
4(1998)1099–1109.
[103]Y.
Wu,J.
W.
Mehew,C.
A.
Heckman,M.
Arcinas,L.
M.
Boxer,Negativeregulationofbcl-2expressionbyp53inhematopoieticcells,Oncogene20(2001)240–251.
[104]V.
Bourgarel-Rey,A.
Savry,G.
Hua,M.
Carre,C.
Bressin,C.
Chacon,J.
Imbert,D.
Braguer,Y.
Barra,Transcriptionaldown-regulationofBcl-2byvinorelbine:identicationofanovelbindingsiteofp53onBcl-2promoter,Biochem.
Pharmacol.
78(2009)1148–1156.
[105]P.
Giannakakou,R.
Robey,T.
Fojo,M.
V.
Blagosklonny,Lowconcentrationsofpaclitaxelinducecelltype-dependentp53,p21andG1/G2arrestinsteadofmitoticarrest:moleculardeterminantsofpaclitaxel-inducedcytotoxicity,Oncogene20(2001)3806–3813.
[106]J.
Han,C.
Flemington,A.
B.
Houghton,Z.
Gu,G.
P.
Zambetti,R.
J.
Lutz,L.
Zhu,T.
Chittenden,Expressionofbbc3,apro-apoptoticBH3-onlygene,isregulatedbydiversecelldeathandsurvivalsignals,Proc.
NatlAcad.
Sci.
USA98(2001)11318–11323.
[107]K.
Nakano,K.
H.
Vousden,PUMA,anovelproapoptoticgene,isinducedbyp53,Mol.
Cell7(2001)683–694.
[108]G.
Achanta,R.
Sasaki,L.
Feng,J.
S.
Carew,W.
Lu,H.
Pelicano,M.
J.
Keating,P.
Huang,Novelroleofp53inmaintainingmitochondrialgeneticstabilitythroughinteractionwithDNAPolgamma,EMBOJ.
24(2005)3482–3492.
[109]S.
Erster,U.
M.
Moll,Stress-inducedp53runsadirectmitochondrialdeathprogram:itsroleinphysiologicandpathophysiologicstressresponsesinvivo,CellCycle3(2004)1492–1495.
[110]M.
Mihara,S.
Erster,A.
Zaika,O.
Petrenko,T.
Chittenden,P.
Pancoska,U.
M.
Moll,p53hasadirectapoptogenicroleatthemitochondria,Mol.
Cell11(2003)577–590.
[111]C.
Sansome,A.
Zaika,N.
D.
Marchenko,U.
M.
Moll,Hypoxiadeathstimulusinducestranslocationofp53proteintomitochondria.
Detectionbyimmunou-orescenceonwholecells,FEBSLett.
488(2001)110–115.
[112]N.
D.
Marchenko,A.
Zaika,U.
M.
Moll,Deathsignal-inducedlocalizationofp53proteintomitochondria.
Apotentialroleinapoptoticsignaling,J.
Biol.
Chem.
275(2000)16202–16212.
[113]Y.
Zhao,L.
Chaiswing,J.
M.
Velez,I.
Batinic-Haberle,N.
H.
Colburn,T.
D.
Oberley,D.
K.
StClair,p53translocationtomitochondriaprecedesitsnucleartranslocationandtargetsmitochondrialoxidativedefenseprotein-manganesesuperoxidedismutase,CancerRes.
65(2005)3745–3750.
[114]D.
R.
Green,G.
Kroemer,Cytoplasmicfunctionsofthetumoursuppressorp53,Nature458(2009)1127–1130.
[115]E.
Strom,S.
Sathe,P.
G.
Komarov,O.
B.
Chernova,I.
Pavlovska,I.
Shyshynova,D.
A.
Bosykh,L.
G.
Burdelya,R.
M.
Macklis,R.
Skaliter,E.
A.
Komarova,A.
V.
Gudkov,Small-moleculeinhibitorofp53bindingtomitochondriaprotectsmicefromgammaradiation,Nat.
Chem.
Biol.
2(2006)474–479.
[116]N.
Corazza,S.
Jakob,C.
Schaer,S.
Frese,A.
Keogh,D.
Stroka,D.
Kassahn,R.
Torgler,C.
Mueller,P.
Schneider,T.
Brunner,TRAILreceptor-mediatedJNKactivationandBimphosphorylationcriticallyregulateFas-mediatedliverdamageandlethality,J.
Clin.
Invest.
116(2006)2493–2499.
[117]D.
B.
Costa,B.
Halmos,A.
Kumar,S.
T.
Schumer,M.
S.
Huberman,T.
J.
Boggon,D.
G.
Tenen,S.
Kobayashi,BIMmediatesEGFRtyrosinekinaseinhibitor-inducedapoptosisinlungcancerswithoncogenicEGFRmutations,PLoSMed.
4(2007)1669–16798discussion1680.
[118]J.
Kuroda,H.
Puthalakath,M.
S.
Cragg,P.
N.
Kelly,P.
Bouillet,D.
C.
Huang,S.
Kimura,O.
G.
Ottmann,B.
J.
Druker,A.
Villunger,A.
W.
Roberts,A.
Strasser,BimandBadmediateimatinib-inducedkillingofBcr/Abl+leukemiccells,andresistanceduetotheirlossisovercomebyaBH3mimetic,Proc.
NatlAcad.
Sci.
USA103(2006)14907–14912.
[119]J.
Lu,B.
Quearry,H.
Harada,p38-MAPkinaseactivationfollowedbyBIMinductionisessentialforglucocorticoid-inducedapoptosisinlymphoblasticleukemiacells,FEBSLett.
580(2006)3539–3544.
[120]A.
Nordigarden,M.
Kraft,P.
Eliasson,V.
Labi,E.
W.
Lam,A.
Villunger,J.
I.
Jonsson,BH3-onlyproteinBimmorecriticalthanPumaintyrosinekinaseinhibitor-inducedapoptosisofhumanleukemiccellsandtransducedhematopoieticprogenitorscarryingoncogenicFLT3,Blood113(2009)2302–2311.
[121]S.
N.
Willis,J.
M.
Adams,Lifeinthebalance:howBH3-onlyproteinsinduceapoptosis,Curr.
Opin.
CellBiol.
17(2005)617–625.
[122]A.
Sunters,S.
FernandezdeMattos,M.
Stahl,J.
J.
Brosens,G.
Zoumpoulidou,C.
A.
Saunders,P.
J.
Coffer,R.
H.
Medema,R.
C.
Coombes,E.
W.
Lam,FoxO3atranscrip-tionalregulationofBimcontrolsapoptosisinpaclitaxel-treatedbreastcancercelllines,J.
Biol.
Chem.
278(2003)49795–49805.
[123]D.
Li,M.
B.
Sewer,RhoAanddiaphanous-relatedhomolog1mediateadrenocor-ticotropin-stimulatedcortisolbiosynthesisbyregulatingmitochondrialtrafck-ing,Endocrinology(2010)247–258.
[124]P.
Bouillet,D.
Metcalf,D.
C.
Huang,D.
M.
Tarlinton,T.
W.
Kay,F.
Kontgen,J.
M.
Adams,A.
Strasser,ProapoptoticBcl-2relativeBimrequiredforcertainapoptoticresponses,leukocytehomeostasis,andtoprecludeautoimmunity,Science286(1999)1735–1738.
[125]D.
Chen,Q.
Zhou,CaspasecleavageofBimELtriggersapositivefeedbackamplicationofapoptoticsignaling,Proc.
NatlAcad.
Sci.
USA101(2004)1235–1240.
686A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[126]H.
Puthalakath,D.
C.
Huang,L.
A.
O'Reilly,S.
M.
King,A.
Strasser,TheproapoptoticactivityoftheBcl-2familymemberBimisregulatedbyinteractionwiththedyneinmotorcomplex,Mol.
Cell3(1999)287–296.
[127]D.
Chen,M.
Wang,S.
Zhou,Q.
Zhou,HIV-1Tattargetsmicrotubulestoinduceapoptosis,aprocesspromotedbythepro-apoptoticBcl-2relativeBim,EMBOJ.
21(2002)6801–6810.
[128]A.
J.
Butt,C.
G.
Roberts,A.
A.
Seawright,P.
B.
Oelrichs,J.
K.
Macleod,T.
Y.
Liaw,M.
Kavallaris,T.
J.
Somers-Edgar,G.
M.
Lehrbach,C.
K.
Watts,R.
L.
Sutherland,Anovelplanttoxin,persin,withinvivoactivityinthemammarygland,inducesBim-dependentapoptosisinhumanbreastcancercells,Mol.
CancerTher.
5(2006)2300–2309.
[129]C.
Cenciarelli,C.
Tanzarella,I.
Vitale,C.
Pisano,P.
Crateri,S.
Meschini,G.
Arancia,A.
Antoccia,Thetubulin-depolymerisingagentcombretastatin-4inducesectopicasterassemblyandmitoticcatastropheinlungcancercellsH460,Apoptosis13(2008)659–669.
[130]U.
M.
Schneiders,L.
Schyschka,A.
Rudy,A.
M.
Vollmar,BH3-onlyproteinsMcl-1andBimaswellasendonucleaseGaretargetedinspongistatin1-inducedapoptosisinbreastcancercells,Mol.
CancerTher.
8(2009)2914–2925.
[131]K.
Lei,R.
J.
Davis,JNKphosphorylationofBim-relatedmembersoftheBcl2familyinducesBax-dependentapoptosis,Proc.
NatlAcad.
Sci.
USA100(2003)2432–2437.
[132]T.
Tong,J.
Ji,S.
Jin,X.
Li,W.
Fan,Y.
Song,M.
Wang,Z.
Liu,M.
Wu,Q.
Zhan,Gadd45aexpressioninducesBimdissociationfromthecytoskeletonandtranslocationtomitochondria,Mol.
Cell.
Biol.
25(2005)4488–4500.
[133]J.
Alexandre,F.
Batteux,C.
Nicco,C.
Chereau,A.
Laurent,L.
Guillevin,B.
Weill,F.
Goldwasser,Accumulationofhydrogenperoxideisanearlyandcrucialstepforpaclitaxel-inducedcancercelldeathbothinvitroandinvivo,Int.
J.
Cancer119(2006)41–48.
[134]H.
Fawcett,J.
S.
Mader,M.
Robichaud,C.
Giacomantonio,D.
W.
Hoskin,Contributionofreactiveoxygenspeciesandcaspase-3toapoptosisandattenuatedICAM-1expressionbypaclitaxel-treatedMDA-MB-435breastcarcinomacells,Int.
J.
Oncol.
27(2005)1717–1726.
[135]H.
L.
Lin,T.
Y.
Liu,G.
Y.
Chau,W.
Y.
Lui,C.
W.
Chi,Comparisonof2-methoxyestra-diol-induced,docetaxel-induced,andpaclitaxel-inducedapoptosisinhepatomacellsanditscorrelationwithreactiveoxygenspecies,Cancer89(2000)983–994.
[136]G.
Varbiro,B.
Veres,F.
GallyasJr.
,B.
Sumegi,DirecteffectofTaxolonfreeradicalformationandmitochondrialpermeabilitytransition,FreeRadic.
Biol.
Med.
31(2001)548–558.
[137]C.
M.
KhawajaNR,B.
Pourroy,H.
Kovacic,D.
Braguer,Highpotencyofepothilonesinneuroblastomacellsmayinvolvemitochondria,AmericanAssociationforCancerResearch(AACR)MeetingAbstracts,2006.
[138]N.
Andre,D.
Braguer,G.
Brasseur,A.
Goncalves,D.
Lemesle-Meunier,S.
Guise,M.
A.
Jordan,C.
Briand,Paclitaxelinducesreleaseofcytochromecfrommitochondriaisolatedfromhumanneuroblastomacells,CancerRes.
60(2000)5349–5353.
[139]S.
L.
Mironov,M.
V.
Ivannikov,M.
Johansson,[Ca2+]isignalingbetweenmitochondriaandendoplasmicreticuluminneuronsisregulatedbymicro-tubules.
FrommitochondrialpermeabilitytransitionporetoCa2+-inducedCa2+release,J.
Biol.
Chem.
280(2005)715–721.
[140]J.
F.
Kidd,M.
F.
Pilkington,M.
J.
Schell,K.
E.
Fogarty,J.
N.
Skepper,C.
W.
Taylor,P.
Thorn,Paclitaxelaffectscytosoliccalciumsignalsbyopeningthemitochondrialpermeabilitytransitionpore,J.
Biol.
Chem.
277(2002)6504–6510.
[141]G.
G.
D'Souza,S.
M.
Cheng,S.
V.
Boddapati,R.
W.
Horobin,V.
Weissig,Nanocarrier-assistedsub-cellulartargetingtothesiteofmitochondriaimprovesthepro-apoptoticactivityofpaclitaxel,J.
DrugTarget.
16(2008)578–585.
[142]M.
Carre,N.
Andre,G.
Carles,H.
Borghi,L.
Brichese,C.
Briand,D.
Braguer,Tubulinisaninherentcomponentofmitochondrialmembranesthatinteractswiththevoltage-dependentanionchannel,J.
Biol.
Chem.
277(2002)33664–33669.
[143]M.
Carre,G.
Carles,N.
Andre,S.
Douillard,J.
Ciccolini,C.
Briand,D.
Braguer,Involvementofmicrotubulesandmitochondriaintheantagonismofarsenictrioxideonpaclitaxel-inducedapoptosis,Biochem.
Pharmacol.
63(2002)1831–1842.
[144]L.
Cicchillitti,R.
Penci,M.
DiMichele,F.
Filippetti,D.
Rotilio,M.
B.
Donati,G.
Scambia,C.
Ferlini,ProteomiccharacterizationofcytoskeletalandmitochondrialclassIIIbeta-tubulin,Mol.
CancerTher.
7(2008)2070–2079.
[145]T.
K.
Rostovtseva,K.
L.
Sheldon,E.
Hassanzadeh,C.
Monge,V.
Saks,S.
M.
Bezrukov,D.
L.
Sackett,Tubulinbindingblocksmitochondrialvoltage-dependentanionchannelandregulatesrespiration,Proc.
NatlAcad.
Sci.
USA105(2008)18746–18751.
[146]Y.
V.
Evtodienko,V.
V.
Teplova,S.
S.
Sidash,F.
Ichas,J.
P.
Mazat,Microtubule-activedrugssuppresstheclosureofthepermeabilitytransitionporeintumourmitochondria,FEBSLett.
393(1996)86–88.
[147]D.
J.
Rodi,R.
W.
Janes,H.
J.
Sanganee,R.
A.
Holton,B.
A.
Wallace,L.
Makowski,Screeningofalibraryofphage-displayedpeptidesidentieshumanbcl-2asataxol-bindingprotein,J.
Mol.
Biol.
285(1999)197–203.
[148]J.
H.
Wu,G.
Batist,L.
O.
Zamir,AmodelfortheinteractionofpaclitaxelwiththeBcl-2loopdomain:achemicalapproachtoinduceconformation-dependentphosphorylation,AnticancerDrugDes.
15(2000)441–446.
[149]C.
Ferlini,L.
Cicchillitti,G.
Raspaglio,S.
Bartollino,S.
Cimitan,C.
Bertucci,S.
Mozzetti,D.
Gallo,M.
Persico,C.
Fattorusso,G.
Campiani,G.
Scambia,PaclitaxeldirectlybindstoBcl-2andfunctionallymimicsactivityofNur77,CancerRes.
69(2009)6906–6914.
[150]L.
Knipling,J.
Wolff,DirectinteractionofBcl-2proteinswithtubulin,Biochem.
Biophys.
Res.
Commun.
341(2006)433–439.
[151]J.
W.
Smyth,T.
T.
Hong,D.
Gao,J.
M.
Vogan,B.
C.
Jensen,T.
S.
Fong,P.
C.
Simpson,D.
Y.
Stainier,N.
C.
Chi,R.
M.
Shaw,Limitedforwardtrafckingofconnexin43reducescell–cellcouplinginstressedhumanandmousemyocardium,J.
Clin.
Invest.
120(2010)266–279.
[152]C.
Sioka,A.
P.
Kyritsis,Centralandperipheralnervoussystemtoxicityofcommonchemotherapeuticagents,CancerChemother.
Pharmacol.
63(2009)761–767.
[153]S.
Wolf,D.
Barton,L.
Kottschade,A.
Grothey,C.
Loprinzi,Chemotherapy-inducedperipheralneuropathy:preventionandtreatmentstrategies,Eur.
J.
Cancer44(2008)1507–1515.
[154]F.
H.
Hausheer,R.
L.
Schilsky,S.
Bain,E.
J.
Berghorn,F.
Lieberman,Diagnosis,management,andevaluationofchemotherapy-inducedperipheralneuropathy,Semin.
Oncol.
33(2006)15–49.
[155]T.
Bordet,R.
M.
Pruss,Targetingneuroprotectionasanalternativeapproachtopreventingandtreatingneuropathicpain,Neurotherapeutics6(2009)648–662.
[156]T.
J.
Kaley,L.
M.
Deangelis,Therapyofchemotherapy-inducedperipheralneuropathy,Br.
J.
Haematol.
145(2009)3–14.
[157]N.
Authier,D.
Balayssac,F.
Marchand,B.
Ling,A.
Zangarelli,J.
Descoeur,F.
Coudore,E.
Bourinet,A.
Eschalier,Animalmodelsofchemotherapy-evokedpainfulperipheralneuropathies,Neurotherapeutics6(2009)620–629.
[158]K.
D.
Tanner,J.
D.
Levine,K.
S.
Topp,Microtubuledisorientationandaxonalswellinginunmyelinatedsensoryaxonsduringvincristine-inducedpainfulneuropathyinrat,J.
Comp.
Neurol.
395(1998)481–492.
[159]K.
S.
Topp,K.
D.
Tanner,J.
D.
Levine,Damagetothecytoskeletonoflargediametersensoryneuronsandmyelinatedaxonsinvincristine-inducedpainfulperipheralneuropathyintherat,J.
Comp.
Neurol.
424(2000)563–576.
[160]S.
J.
Flatters,G.
J.
Bennett,Studiesofperipheralsensorynervesinpaclitaxel-inducedpainfulperipheralneuropathy:evidenceformitochondrialdysfunction,Pain122(2006)245–257.
[161]H.
W.
Jin,S.
J.
Flatters,W.
H.
Xiao,H.
L.
Mulhern,G.
J.
Bennett,Preventionofpaclitaxel-evokedpainfulperipheralneuropathybyacetyl-L-carnitine:effectsonaxonalmitochondria,sensorynerveberterminalarbors,andcutaneousLangerhanscells,Exp.
Neurol.
210(2008)229–237.
[162]G.
Melli,M.
Taiana,F.
Camozzi,D.
Triolo,P.
Podini,A.
Quattrini,F.
Taroni,G.
Lauria,Alpha-lipoicacidpreventsmitochondrialdamageandneurotox-icityinexperimentalchemotherapyneuropathy,Exp.
Neurol.
214(2008)276–284.
[163]T.
Nakata,H.
Yorifuji,Morphologicalevidenceoftheinhibitoryeffectoftaxolonthefastaxonaltransport,Neurosci.
Res.
35(1999)113–122.
[164]C.
Theiss,K.
Meller,Taxolimpairsanterogradeaxonaltransportofmicroinjectedhorseradishperoxidaseindorsalrootganglianeuronsinvitro,CellTissueRes.
299(2000)213–224.
[165]S.
C.
Apfel,Managingtheneurotoxicityofpaclitaxel(Taxol)anddocetaxel(Taxotere)withneurotrophicfactors,CancerInvestig.
18(2000)564–573.
[166]S.
Quasthoff,H.
P.
Hartung,Chemotherapy-inducedperipheralneuropathy,J.
Neurol.
249(2002)9–17.
[167]J.
K.
Andersen,Oxidativestressinneurodegeneration:causeorconsequenceNat.
Med.
10(Suppl)(2004)S18–S25.
[168]W.
H.
Xiao,F.
Y.
Zheng,G.
J.
Bennett,T.
Bordet,R.
M.
Pruss,Olesoxime(cholest-4-en-3-one,oxime):analgesicandneuroprotectiveeffectsinaratmodelofpainfulperipheralneuropathyproducedbythechemotherapeuticagent,paclitaxel,Pain147(2009)202–209.
[169]K.
Y.
Chan,A.
H.
Bunt,Anassociationbetweenmitochondriaandmicrotubulesinsynaptosomesandaxonterminalsofcerebralcortex,J.
Neurocytol.
7(1978)137–143.
[170]S.
T.
Brady,S.
D.
Crothers,C.
Nosal,W.
O.
McClure,FastaxonaltransportinthepresenceofhighCa2+:evidencethatmicrotubulesarenotrequired,Proc.
NatlAcad.
Sci.
USA77(1980)5909–5913.
[171]S.
T.
Brady,R.
J.
Lasek,R.
D.
Allen,H.
L.
Yin,T.
P.
Stossel,Gelsolininhibitionoffastaxonaltransportindicatesarequirementforactinmicrolaments,Nature310(1984)56–58.
[172]D.
J.
Goldberg,Microinjectionintoanidentiedaxontostudythemechanismoffastaxonaltransport,Proc.
NatlAcad.
Sci.
USA79(1982)4818–4822.
[173]D.
J.
Goldberg,D.
A.
Harris,B.
W.
Lubit,J.
H.
Schwartz,Analysisofthemechanismoffastaxonaltransportbyintracellularinjectionofpotentiallyinhibitorymacromolecules:evidenceforapossibleroleofactinlaments,Proc.
NatlAcad.
Sci.
USA77(1980)7448–7452.
[174]R.
L.
Morris,P.
J.
Hollenbeck,AxonaltransportofmitochondriaalongmicrotubulesandF-actininlivingvertebrateneurons,J.
CellBiol.
131(1995)1315–1326.
[175]D.
Cartelli,C.
Ronchi,M.
G.
Maggioni,S.
Rodighiero,E.
Giavini,G.
Cappelletti,MicrotubuledysfunctionprecedestransportimpairmentandmitochondriadamageinMPP+-inducedneurodegeneration,J.
Neurochem.
115(2010)247–258.
[176]M.
Zheng,Q.
Wang,Y.
Teng,X.
Wang,F.
Wang,T.
Chen,J.
Samaj,J.
Lin,D.
C.
Logan,ThespeedofmitochondrialmovementisregulatedbythecytoskeletonandmyosininPiceawilsoniipollentubes,Planta231(2010)779–791.
[177]J.
P.
Dompierre,J.
D.
Godin,B.
C.
Charrin,F.
P.
Cordelieres,S.
J.
King,S.
Humbert,F.
Saudou,Histonedeacetylase6inhibitioncompensatesforthetransportdecitinHuntington'sdiseasebyincreasingtubulinacetylation,J.
Neurosci.
27(2007)3571–3583.
[178]N.
A.
Reed,D.
Cai,T.
L.
Blasius,G.
T.
Jih,E.
Meyhofer,J.
Gaertig,K.
J.
Verhey,Microtubuleacetylationpromoteskinesin-1bindingandtransport,Curr.
Biol.
16(2006)2166–2172.
[179]J.
Zhou,M.
Liu,R.
Aneja,R.
Chandra,H.
C.
Joshi,Enhancementofpaclitaxel-inducedmicrotubulestabilization,mitoticarrest,andapoptosisbythemicrotubule-targetingagentEM012,Biochem.
Pharmacol.
68(2004)2435–2441.
[180]S.
H.
Zhuang,Y.
E.
Hung,L.
Hung,R.
W.
Robey,D.
L.
Sackett,W.
M.
Linehan,S.
E.
Bates,T.
Fojo,M.
S.
Poruchynsky,Evidenceformicrotubuletargetengagementintumorsofpatientsreceivingixabepilone,Clin.
CancerRes.
13(2007)7480–7486.
687A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688[181]I.
Nennesmo,F.
P.
Reinholt,Effectsofintraneuralinjectionoftaxolonretrogradeaxonaltransportandmorphologyofcorrespondingnervecellbodies,VirchowsArch.
BCellPathol.
Incl.
Mol.
Pathol.
55(1988)241–246.
[182]J.
W.
Hammond,C.
F.
Huang,S.
Kaech,C.
Jacobson,G.
Banker,K.
J.
Verhey,Posttranslationalmodicationsoftubulinandthepolarizedtransportofkinesin-1inneurons,Mol.
Biol.
Cell21(2010)572–583.
[183]B.
Trinczek,A.
Ebneth,E.
M.
Mandelkow,E.
Mandelkow,Tauregulatestheattachment/detachmentbutnotthespeedofmotorsinmicrotubule-dependenttransportofsinglevesiclesandorganelles,J.
CellSci.
112(Pt14)(1999)2355–2367.
[184]J.
C.
Bulinski,T.
E.
McGraw,D.
Gruber,H.
L.
Nguyen,M.
P.
Sheetz,OverexpressionofMAP4inhibitsorganellemotilityandtrafckinginvivo,J.
CellSci.
110(Pt24)(1997)3055–3064.
[185]A.
Seitz,H.
Kojima,K.
Oiwa,E.
M.
Mandelkow,Y.
H.
Song,E.
Mandelkow,Single-moleculeinvestigationoftheinterferencebetweenkinesin,tauandMAP2c,EMBOJ.
21(2002)4896–4905.
[186]K.
Nishio,H.
Arioka,T.
Ishida,H.
Fukumoto,H.
Kurokawa,M.
Sata,M.
Ohata,N.
Saijo,Enhancedinteractionbetweentubulinandmicrotubule-associatedprotein2viainhibitionofMAPkinaseandCDC2kinasebypaclitaxel,Int.
J.
Cancer63(1995)688–693.
[187]J.
R.
Levy,C.
J.
Sumner,J.
P.
Caviston,M.
K.
Tokito,S.
Ranganathan,L.
A.
Ligon,K.
E.
Wallace,B.
H.
LaMonte,G.
G.
Harmison,I.
Puls,K.
H.
Fischbeck,E.
L.
Holzbaur,Amotorneurondisease-associatedmutationinp150Gluedperturbsdynactinfunctionandinducesproteinaggregation,J.
CellBiol.
172(2006)733–745.
[188]J.
Jaworski,L.
C.
Kapitein,S.
M.
Gouveia,B.
R.
Dortland,P.
S.
Wulf,I.
Grigoriev,P.
Camera,S.
A.
Spangler,P.
DiStefano,J.
Demmers,H.
Krugers,P.
Delippi,A.
Akhmanova,C.
C.
Hoogenraad,Dynamicmicrotubulesregulatedendriticspinemorphologyandsynapticplasticity,Neuron61(2009)85–100.
[189]O.
A.
Shemesh,M.
E.
Spira,Paclitaxelinducesaxonalmicrotubulespolarrecong-urationandimpairedorganelletransport:implicationsforthepathogenesisofpaclitaxel-inducedpolyneuropathy,ActaNeuropathol.
119(2010)235–248.
[190]A.
B.
Knott,G.
Perkins,R.
Schwarzenbacher,E.
Bossy-Wetzel,Mitochondrialfragmentationinneurodegeneration,Nat.
Rev.
Neurosci.
9(2008)505–518.
[191]A.
B.
Knott,E.
Bossy-Wetzel,Impairingthemitochondrialssionandfusionbalance:anewmechanismofneurodegeneration,Ann.
NYAcad.
Sci.
1147(2008)283–292.
[192]V.
Voccoli,L.
Colombaioni,Mitochondrialremodelingindifferentiatingneuro-blasts,BrainRes.
1252(2009)15–29.
[193]T.
Shprung,I.
Gozes,Anovelmethodforanalyzingmitochondrialmovement:inhibitionbypaclitaxelinapheochromocytomacellmodel,J.
Mol.
Neurosci.
37(2009)254–262.
[194]R.
Yamaguchi,L.
Lartigue,G.
Perkins,R.
T.
Scott,A.
Dixit,Y.
Kushnareva,T.
Kuwana,M.
H.
Ellisman,D.
D.
Newmeyer,Opa1-mediatedcristaeopeningisBax/BakandBH3dependent,requiredforapoptosis,andindependentofBakoligomerization,Mol.
Cell31(2008)557–569.
[195]M.
S.
Poruchynsky,P.
Giannakakou,Y.
Ward,J.
C.
Bulinski,W.
G.
Telford,R.
W.
Robey,T.
Fojo,Accompanyingproteinalterationsinmalignantcellswithamicrotubule-polymerizingdrug-resistancephenotypeandaprimaryresistancemechanism,Biochem.
Pharmacol.
62(2001)1469–1480.
688A.
Rovinietal.
/BiochimicaetBiophysicaActa1807(2011)679–688
美国高防云服务器 1核 1G 10M 38元/月 百纵科技
百纵科技:美国云服务器活动重磅来袭,洛杉矶C3机房 带金盾高防,会员后台可自助管理防火墙,添加黑白名单 CC策略开启低中高.CPU全系列E52680v3 DDR4内存 三星固态盘列阵。另有高防清洗!百纵科技官网:https://www.baizon.cn/联系QQ:3005827206美国洛杉矶 CN2 云服务器CPU内存带宽数据盘防御价格活动活动地址1核1G10M10G10G38/月续费同价点击...

亚洲云Asiayu,成都云服务器 4核4G 30M 120元一月
点击进入亚云官方网站(www.asiayun.com)公司名:上海玥悠悠云计算有限公司成都铂金宿主机IO测试图亚洲云Asiayun怎么样?亚洲云Asiayun好不好?亚云由亚云团队运营,拥有ICP/ISP/IDC/CDN等资质,亚云团队成立于2018年,经过多次品牌升级。主要销售主VPS服务器,提供云服务器和物理服务器,机房有成都、美国CERA、中国香港安畅和电信,香港提供CN2 GIA线路,CE...
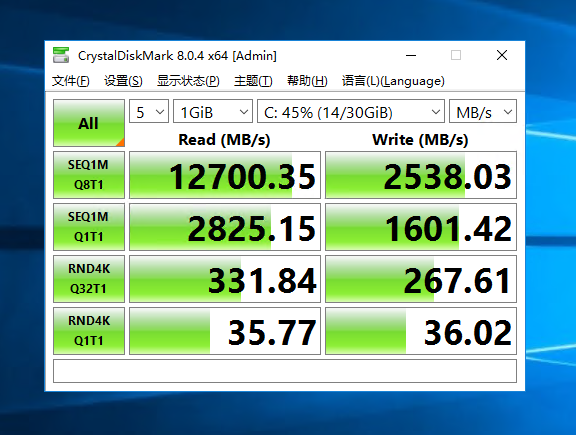
tmhhost:全场VPS低至6.4折,香港BGP200M日本软银美国cn2 gia 200G高防美国三网cn2 gia韩国CN2
tmhhost放出了2021年的端午佳节+618年中大促的优惠活动:日本软银、洛杉矶200G高防cn2 gia、洛杉矶三网cn2 gia、香港200M直连BGP、韩国cn2,全都是高端优化线路,所有这些VPS直接8折,部分已经做了季付8折然后再在此基础上继续8折(也就是6.4折)。 官方网站:https://www.tmhhost.com 香港BGP线路VPS ,200M带宽 200M带...
33669.com为你推荐
-
supplementedroutecontentcss支持ipadipad连不上wifiiPad 连不上Wifi,显示无互联网连接360chrome360的chrome浏览器进程有点多哦???360chrome使用360急速浏览器,360chrome进程结束不了联通iphone4联通iphone4好用吗谷歌sbgoogle一下"SB",虽然显示的是baidu排第一,链接的不是baidu.win7关闭135端口win7下怎么关135和8909端口css3按钮HTML中,怎么表示一个图片按钮