www.scholarsresearchlibrary.com
http://www.qvod.com/ 时间:2021-03-05 阅读:()
tAvailableonlineaScholarsResearchLibraryArchivesofAppliedScienceResearch,2015,7(1):22-27(http://scholarsresearchlibrary.
com/archive.
html)ISSN0975-508XCODEN(USA)AASRC922ScholarsResearchLibraryPlantregenerationofA.
lakoochafromencapsulatednodalexplantsShivKumarVerma*,DhirajKumarChoudhary,Ashish,AnnandKumarandMotiLalSchoolofBiochemicalEngineering,IIT(BHU),VaranasiABSTRACTOneofthealternativemethodsadoptedinrecentyearsistousebiotechnologicalapproachesforimprovingthetreespecies.
AproficientprotocolforencapsulationofnodalsegmentsofArtocarpuslakoochaRoxbhasbeendevelopedforplantregenerationthroughnon-embryogenicsyntheticseeds.
Concentrationsofsodiumalginateandcalciumchloridegreatlyaffectedmorphologyandtextureencapsulatinggel.
3%sodiumalginatewith100mMCaCl2hasbeenfoundtobebestpossibleconcentrationfortheproductionofidenticalsyntheticseeds.
25daysoldinvivoplantletofA.
lakoochawasusedforobtainingnodalsegments,usedinallexperiments.
FiveexperimentswereperformedusingMSmedium(agarsolidifiedandliquid)supplementedwith1,3and5mg/lBAP.
Concentrationof1mg/lBAPwasobservedtobemoreeffectiveforachievinghighestregenerationfrequencyincomparisontootherconcentrationofBAP(3and5mg/l).
InthirdexperimentonlydifferentstrengthofMSmediumwasused,inlasttwoexperimentsnodalsegmentspriorencapsulationandencapsulatednodalsegmentsweregivenpulsetreatmentwith(IBA)for48hoursandconcentrationof3mg/lBAPwasobservedtobemosteffectiveforsyntheticseedregeneration.
Datawererecordedafter4weeksofculture.
Keywords:Pulsetreatment,MSmedium,BAP,IBA,Acclimatization,syntheticseeds.
INTRODUCTIONThereisworldwidesusceptibilityofplantgeneticdiversityduetounmatchedperturbations,habitatlossanddestructionrates.
Variousspeciesaredescribedasrareorendangered,andasaresultintegratedprogramsarerequiredtodefendandconservebiodiversityofcurrentlyavailablespecies[1].
ArtocarpuslakoochaRoxb,aspeciesoffamilyMoraceae,isanimportanttropicalmedicinaltreespeciesnativetoIndiaandusedforvariousimportantpurposeslike;fruit,furniture,timber,andfeed.
Ripfruitsoflakoochaaregenerallyeatenfresh.
Eachfruitgenerallycontains20–30seedsthatarefleshywiththinseedcoat.
Theediblefruitpulpisbelievedtocontainessentialingredientswhichplaykeyroleinfunctioningiftheliver.
lkoochaseedsandmilkylatexarepurgativeinnature.
Seedscontainartocarpins(ALAIandALAII),theisolectinswhichexhibithighhaemagglutinationactivity(Wongkham1995).
However,theagglutinin(ALA)fromArtocarpuslakoochaisnotorganspecificintheplant.
Moreover,thehaemagglutinationactivityofALAwasdemonstratedinvariousorgansoftheplantexceptfruitflesh[2].
Traditionallytheplantispropagatedthroughseedbutseedpropagationisnotrapidandseasondependent.
Extensiveimprovementhasbeenmadeinthepropagationofthisplantthroughplantcell,tissueandorganculture[3-4].
However,inordertocompletethedemandofpharmaceuticalindustries,continuoussupplyofplantthroughoutyear,thereisneedtodevelopamethodofconservationandtransportofhealthyplant.
Inthiscontext,forrapidpropagation,encapsulationtechnologymaybeanalternativemethodofconservationandgermplasmexchangeofthismedicinallyimportantplant[3].
Inrecentyears,syntheticseedtechnologyusingencapsulationofinvitro-derivednon-embryogenicpropaguleshasbecomeanimportantassettomicropropagation(Naiketal.
2006).
InShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2723ScholarsResearchLibraryaddition,syntheticseedtechnologycouldbeusefulingermplasmconservationofelite,endangeredandcommerciallyimportantplantsbyusingappropriatestoragetechniqueaswellasexchangeofaxenicplantmaterialbetweenlaboratoriesandpharmaceuticalindustries[5]thatcanbeusedifstockplantsorproliferationculturesbecomeinfestedwithbacteria,fungi,orarthropods.
Successfulcasesofsyntheticseedproductionandplantletregenerationhavebeenreportedforawiderangeofplantsincludingcereals,vegetables,fruits,ornamentals,medicinalplantsandforesttree,[7].
Someotherpotentialadvantagesofsyntheticseedtechnologyareeaseinhandlingduetosmallsizeofcapsules,reductionincosts,geneticuniformityofplantsanddirectdeliverytothefield[8]Anartificialseedpreparedbycoatingasomaticembryousingapolymermatrixisatrueseedanalog.
Animmobilizedsomaticembryocangerminateundersuitablegrowthconditionsandbecomeacompleteplant.
Syntheticseedtechnologyusingencapsulationofvegetativepropagulesofwoodyplantspecieshasbecomeapotentiallycosteffectiveclonalpropagationsystem.
Successfulplantregenerationfromsyntheticseedshasbeenreportedinseveralplantspecies.
However,inmostofthecases,theembryogenicpropagulessuchassomaticembryoswereusedforsyntheticseedsproduction.
Thereareonlyfewreportsonencapsulationofvegetativepropagules[9-14].
Encapsulationofvegetativepropagulescouldbeusedformassclonalpropagationatareasonablecost[14].
Therehasbeennoreportonutilizingsomaticembryostoproducesyntheticseeds.
Thedevelopmentofsyntheticseedsfromsomaticembryoscouldofferapracticalmeansformasspropagationofbanana.
Inthisstudy,encapsulationofsomaticembryosdevelopingfromembryogenicsuspensionculturesinanimportantIndiancultivarofbanana,Rasthali(AABwithtwoAAandoneBgenome),andconversionoftheencapsulatedembryosintoplantsarereported(Ganpatietal.
2001).
Amongseveralnon-embryogenicpropagules,shoottipexplantsaremoreresponsivethanotherexplantsbecauseofgreatermitoticactivityinthemeristem[15].
Althoughtherearemanyreportsonencapsulationofshoottipsobtainedfrominvivoraisedplants[7,16].
Inmanyoftheworks,somaticembryoshavealreadybeenusedintheencapsulationprocess.
However,encapsulationofsomaticembryoswererestrictedmostlytoplantsinwhichsomaticembryogenesishasbeendocumented.
But,incurrenttime,useofnon-embryogenicvegetativepropaguleslikeapicalshootbuds,axillarybuds,nodalsegments,etc.
,fortheencapsulationhavealsobeenextendedasasuitablealternativetosomaticembryos[6,9,11,14,17,18,20,21].
ThepresentstudywasaimedtoinvestigateencapsulationofsomaticembryosofArtocarpuslakoochinsodiumalginatebeadsandconversionofencapsulatedsomaticembryosintoplantlets.
EffectofdifferentconcentrationofBAP,wasalsostudiedtoexplorethemorphogeneticresponsesofencapsulatedshoottips.
Validationofthepossibilitytostoretheencapsulatedshoottipsfortimeperiodsufficientforexchangesanddistributionofgermplasmwasdiscussed.
Inthepresentinvestigation,wedescribertheencapsulationofshoottipsforthedevelopmentofsyntheticseedinA.
lakoocha.
MATERIALSANDMETHODS2.
1.
PreparationofexplantsandcultureconditionsSeedsofripenfruitsofA.
lakoochawereobtainedfromagriculturefieldofChandraShekharAjadAgricultureUniversity,Kanpur,India.
SeedsweregrownonagarsolidifiedMSmedium.
25daysoldplantletswereusedformakingnodalsegments.
Nodalsegments(0.
5cm)ofA.
lakoochawereprepared.
Initiallytheexplantswerewashedinrunningtapwaterfor20–30mintominimizemicrobialconcentrationonthesurfaceofnodalsegments.
Afterthatsurfacesterilizedinlaminarairhoodwith70%ethanolfor30-40sfollowedby0.
05%mercuricchloride(Hi-Media,India),for3–4min,andrinsed4–5timeswithsteriledoubledistilledwater[imp].
Forshootmultiplication,nodalsegmentswereculturedonMS,medium[22]supplementedwithsucrose(30g/l).
Themediaweresolidifiedwith0.
8%(w/v)agar(Hi-Media,India).
ThemediawereadjustedtopH5.
7using1NNaOHor0.
1NHClbeforeautoclavingat1210Cfor15min.
Culturesweremaintainedat25±20Cwitha16hphotoperiodataphotonfluxdensityof50–70mmolm-2s-1fromcoolwhitefluorescenttubes(Philips,India).
Nodalsegmentsexcisedfrominvitroproliferatedplantletswereusedasexplantsforthesyntheticseedproduction.
2.
2.
EncapsulationofnodalsegmentsandpreparationofsodiumalginatebeadsForencapsulationofnodalsegments,sodiumalginate(Himedia,india)waspreparedintherangeof2.
0,3.
0,4.
0,or5.
0%(w/v),whereascalciumchloride(CaCl2.
2H2O)solutionwaspreparedin100,mM(w/v)indouble-distilledwaterorliquidMS(MurashigeandSkoog,1962)mediumwithoutanyplantgrowthregulator.
Boththegelmatrixandcomplexingagentwereautoclavedat121°Cfor15min.
Encapsulationwasaccomplishedbymixingthenodalexplantintothesodiumalginatesolutionanddroppingtheseexplantsintothecalciumchloridesolution.
Thebeadscontainingthesomaticembryoswereheldfor20–30mininthecalciumchloridesolutionandafterhardeningoftheShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2724ScholarsResearchLibrarybeads,encapsulatedsomaticembryoswerewashedwithsterilizeddistilledwatertwotimestotakeawaytracesofcalciumchloride.
(A)(B)(C)(D)(E)Figure1-SyntheticseedpreparedindifferentsodiumalginateandcalciumchloridecompositionPlantletregenerationinagarsolidifiedMSmedium(D)andMSmediumsupplementedwith1mg/lBAP(E)3.
ExperimentsDifferentfactorsaffectingconversionwereevaluatedandthefollowingexperimentswereperformed.
Tomaintaintheculture,samelightandtemperatureconditionswereusedinthegrowthroomasdescribedpreviously.
Dataforconversionfrequencyandshootlengthwasrecordedafter3weeksofculture.
3.
1EffectofBAPinagarsolidifiedMSfullstrengthMediumSyntheticseedswerepreparedusinginsodiumalginate(3%w/v)and100mMCaCl2,seedswereinoculatedinsolidifiedMSmediumsupplementedwithBAPattheconcentrationof1,3and5mgl-1.
AfterthreeweekspercentseedgerminationwasrecordedinallthreesupplementofBAP.
3.
2EffectofBAPinliquidMSfullstrengthMediumInthisexperimentsamecompositionofsyntheticseedandsameconcentrationofBAPwasused.
LiquidMSmediumwasused.
AfterthreeweeksinoculationpercentseedgerminationwasrecordedinallthreesupplementofBAP.
3.
3EffectofCompositionofEncapsulatingGelSodiumalginatebeadscomplexedwithnodalexplantswereinoculatedinMSfullstrength,1/2MSand1/4MSstrength.
Percentseedgerminationwasrecordedafterthreeweeksofinoculation.
ShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2725ScholarsResearchLibrary3.
4EffectofPulsetreatmentofIBA(IndoleButyricAcid)BeforeEncapsulationofnodalExplantNodalexplantswerepulsetreatedfor48hours,withIBA(3mgl-1),beforepreparationofsyntheticseeds.
Aftersyntheticseedpreparation,seedswereinoculatedinfullstrengthliquidMSmediumsupplementedwith,3and5mgl_1BAP.
3.
5EffectofPulsetreatmentofIBA(IndoleButyricAcid)AfterEncapsulationofnodalExplantInthisexperimentafterencapsulation,nodalexplantsweresubjectedto48hourspulsetreatmentwithsamegrowthregulatorandsameconcentration.
SeedswereinoculatedinfullstrengthliquidMSmediumsupplementedwith,3and5mgl-1BAP.
3.
6AcclimatizationofplantsinsoilArtificiallygrownplantletsregeneratedfromencapsulatednodalsegmentsweretransferredtoplasticpotscontaining3:1(w/w)mixtureofsterilesandandsoilmoistenedwithliquidMSmedium.
Plantletswerecoveredwithpolyethylenebagstomaintainhighhumidityandirrigatedwithtapwater.
Potswithplantletswerekeptunderlaboratoriesconditionat250Cinartificiallight(irradianceof60mmolm-2s-1)providedbycoolwhitefluorescenttubesfor4Weeksandthenthepotsweretransferredtofieldlevels.
RESULTSANDDISCUSSIONAtpresentdaysnewrouteinsyntheticseedtechnologyhasbeenreportedwiththeuseofnonembryogenicplantpropagulesviz;rootstemleaves(StandardiandPiccioni,1998).
Themostimportantbenefitofusingvegetativepropagulesforthepreparationofsyntheticseedswouldbeinthosecaseswheresomaticembryogenesisisnotwellestablishedorsomaticembryosdonotgerminateintocompleteplantlets(Raietal.
,2009).
Insuchcases,syntheticseedscanbeproducedfromshoottipsforcost-effectivemassclonalpropagation,potentiallong-termgermplasmstorage,anddeliveryoftissue-culturedplants.
Inthepresentexploration,nodalsegmentsexcisedfrominvivoproliferatedsamallplantletswereusedasanexplantforthedevelopmentofsyntheticseedinA.
lakoocha.
inmanyexperimentalstudiesSimilarobservationswerealsomadeinDalbergiasissooRoxb(Chandetal.
2004),Ceropegiabulbosavar.
bulbosa(Dhiretal.
2013),VitexnegundoL.
(Ahmadetal.
2010).
EvaluationofconcentrationofsodiumalginateandcalciumchloridewhichareeffectiveforthegellingpropertiesofthematrixandeventuallyfortheexcellenceofcalciumalginatebeadsisanimportantaspectforthesuccessfulinvitropropagationofplantsthroughencapsulationmethodsConcentrationofsodiumalginateandcalciumchloridegreatlyaffectedtheencapsulationofnodalsegmentsdifferedqualitativelywithrespecttotexture,shape,andtransparency.
Table1.
EffectofBAPinagarsolidifiedMSfullstrengthMediumConcentrationofBAP(mgl-1)+MS(S)PercentresponseofsyntheticSeedGerminationDays1+MS(S)91213+MS(S)81215+MS(S)7321Inourstudy3.
0%sodiumalginateand100mMCaCl2.
2H2Owasfoundmostsuitableforformationofidealcalciumalginatebeads.
ConcentrationofBAPalongwithMS(MurashigandSkoog1962.
)mediumgreatlyaffectedthepercentconversionfrequencyofencapsulatednodalsegmentsofA.
lakoocha,highestconversionfrequency(91%)wasobservedonsolidMSmediumsupplementedwith1mg/lBAP(6-BenzyleAminoPurine)followedby3and5mg/lBAPwithpercentsyntheticseedgerminationof89%and72%respectively(Table1).
Table2.
EffectofBAPinliquidMSfullstrengthMediumConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeedGerminationDays1+MS(L)96213+MS(L)84215+MS(L)7821WehaveobservedinourexperimentthatwhensameexperimentwasperformedwithliquidMSmediumwithsameconcentrationofBAP,percentconversionfrequencywasobservedhigherthantheearlierexperiment.
Itwas96%onliquidMSmediumsupplementedwith1mg/lBAP.
LiquidMSmediumsupplementedwith3and5mg/lBAPshowedconversionfrequencyof84%and78%respectively(Table2).
thisdifferenceofconversionfrequencyintwoconditionsofmediummaybeduetofactthatinliquidMSmediumnutrientcomponentsaremoreeaslyandrapidlyShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2726ScholarsResearchLibraryabsorbedthroughthesurfaceofsodiumalginatebeadswhileinsolidMSmediumnutrientcomponentsareslowlyabsorbedwhichisresponsibleforslowregenerationofnodalsegments.
Table3.
EffectofCompositionofEncapsulatingGelStrengthofMS(L)PercentresponseofsyntheticSeeGerminationDaysMS(FS)8721MS(1/2S)5421MS(1/4S)4821WhenonlydifferentstrengthofliquidMSmedium(MSFS,MS1/2SandMS1/4S)wasuseditwasobservedthatsyntheticseedregenerationfrequencyoffullstrengthliquidMSwashighest(87)amongthreedifferentstrengthofliquidMSmedium.
Possiblereasonofthisdifferenceofregenerationfrequencymaybeduetoinsufficientconcentrationofallnutrientcomponents,becauseinsideaplantcellforabiochemicalreactionparticularconcentrationofnutrientcomponent(metalionsetc.
)isneeded.
DuetothisfactinMS1/2SandMS1/4S)regenerationfrequencywasobservedtobe54%and48%respectively(Table3).
Table4.
EffectofPulsetreatmentofIBA(IndoleButyricAcid)BeforeEncapsulationofnodalExplantsConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeeGerminationDays1+MS(L)81213+MS(L)87215+MS(L)7821When,beforeencapsulation,nodalsegmentswerepulsetreatedwithIBA(IndoleButyricAcid)for48hrsandthenencapsulatedinsodiumalginatebeadsandinoculatedinmediumcompositionasdescribedintable2syntheticseedregenerationfrequencyobservedwasdifferent.
InthisexperimentwasobservedthatliquidMSmediumsupplementedwith3mg/lBAPshowedhighestregenerationfrequencyof87%incomparisontoMSmediumsupplementedwith1and5mg/lBAPwhichshowedregenerationfrequency81%and78%respectively(Table4).
Inourlastexperimentafterencapsulation,beadswerepulsetreatedwithIBAforsametimeperiodasinexperimentTable5.
EffectofPulsetreatmentofIBA(IndoleButyricAcid)AfterEncapsulationofnodalExplantsConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeedGerminationDays1+MS(L)81213+MS(L)87215+MS(L)6821Samepatternofresultswereobtainedwithdifferenceinregenerationfrequencyvalue.
Thesevalueswere81%,87%and68%onMSmediumsupplementedwith1,3and5mg/lBAPrespectively(Table5).
PossiblereasonofeffectofPulsetreatmenttoaffectregenerationfrequencyisnotknown.
Allexperimentswereperformedintriplicatetominimizeerrorduringdatacalculation.
CONCLUSIONTheartificialseedtechnologyprovidesanoptionalmethodofmicropropagationforawidearrayofmedicinalplants,especiallydesirableelitegenotypes.
However,successfulplantretrievalfromencapsulatedvegetativemicropropagulesfollowingshort-termstorageismostlydependsonplantspecies,matrixcompositionandperiodofstorage.
Treesgenerallyhavealonggenerationtimeandaremostlyheterozygous.
Duetothesereasons,geneticdevelopmentinthesespecieshasbeenamostimportanthindrance.
Largescaleclonalpropagationofsuperiorclonesalongwithacceleratedtreeimprovementprogramsisnecessaryforsuccessfulandrapidexplorationoftreeplant.
Itispossibletouseeasy-to-obtainsomaticfragmentsoftheplantsbynodalsegmentcuttingsbyapplyingartificialseedtechnologyinA.
lakoocha.
Oneoftheadvantagesofsyntheticseedtechnologyisthattheriskofsomaclonalvariationwouldalsobereducedbecausemutationsareknowntooccurmorefrequentlywhenthede-andre-differentiationprocessesoccurduringinitialgrowth.
However,there-growthabilityoftheunipolarpropagulesafterencapsulationhinderedbyfactorssuchasprecociousgrowthandlackofataproot.
Encapsulatednodalsegmentsorapical/auxiliaryshootbudsareconsideredtobemoreeffectivecomeintoviewifwemaketherelativeeaseandextensibilityofthetechnique,aswellasthepossibilityofundamagedanduninfectedmovingsmall-sizedmicroShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2727ScholarsResearchLibrarypropagatedplantmaterialbetweenlaboratoriesandcountries,whilereducingphytosanitaryandquarantineproblems(Bapat,1993.
,Mathuretal.
,1989;HasanandTakagi,1995;Maruyamaetal.
,1997b.
,PiccioniandStandardi,1995).
Theresultspresentedinthispapersupportthefeasibilityofthiscost-effectivemethod,foralargescalepropagationofthisecologicallyandmedicinallypotentplantspecies.
Oneofthedrawbackofsyntheticseedtechnologyisthatonstorageofseeds,percentregenerationfrequencyisgreatlyreducedTherefore,forlarge-scaleapplicationofthissyntheticseedtechnology,furtherexperimentsarenecessary;toaccomplishahigherpercentageofconversionmakethemviableevenafterlongtimestorageofencapsulatednodalsegmentsofA.
lakoocha.
Further,encapsulatedsomaticembryoscouldofferanimportantsystemforthetransportationandexchangeofgermplasminasafeandeconomicalmanner.
REFERENCES[1]RDhir;GSShekhawat,IndustrialCropsandProducts2013,47139–144.
[2]NJoshee;DRBastola;VPAgrawal;A.
K.
Yadav,Lakoocha:AMultipurposeTreeofWarmClimateTrendsinnewcropsandnewuses.
2002.
J.
JanickandA.
Whipkey(eds.
).
ASHSPress,Alexandria,VA.
[3]DPBhatt;GFassuliotis,ZeitschriftfurPflanzenphysiologie,1981,104,481-489[4]MKHusain;MAnis;AShahzad,InVitroCell.
Dev.
Biol.
Plant,2007,43,59–64.
[5]SKVerma;MKRai;PAsthana;VSJaiswal;UJaiswal,ScientiaHorticulturae,2010,124,517–521[6]SKNaik;PKChand,Nutrient-alginateencapsulationofinvitronodalsegmentsofpomegranate(PunicagranatumL.
)forgermplasmdistributionandexchange2006,103,3,247–252.
[7]SKSingh;MKRai;PAsthana;SPandey;VSJaiswal;UJaiswal,ActaPhysiol.
2009.
[8]EMaruyama;IKinoshita;KIshi;HShigenaga;KOhba;ASaito,PlantCellReproduction.
1997b,16,393–396.
[9]SMathur;SKumar,JMedAromPlantSci1998,20,1056–1059.
[10]VABapat;Studiesonsyntheticseedsofsandalwood(SantalumalbumL.
)andmulberry(MorusindicaL)-In:Redenbough,K.
(ed.
),Synseeds,ApplicationsofSyntheticSeedstoCropImprovement.
CRCPress,BocaRaton1993,Pp.
381-407.
[11]TRGanapathi;LSrinivas;PSuprasanna;VABapat,InVitroCellDevelopmentBiology,2001,37,178-181.
[12]PSharma;MVRajam,J.
Exptl.
Bot.
,1995,46,135-141.
[13]EPiccioni;AStandardi,PlantCellTissueOrgan.
Culture,1995,42,221–226.
[14]ChandS.
,SinghA.
K.
,.
JournalofPlantPhysiology2004,161,237–243.
[15]BallesterA.
,JaneiroL.
V.
,VietezA.
M.
,.
ScientiaHorticulturae,1997,71.
67-78.
[16]AKSingh;MSharmaRVarshney;SSAgarwal,KCBansal,InVitroCellular&DevelopmentalBiology,2008,42,109-113.
[17]HAra;UJaiswal;VSJaiswal,CurrentScience,2000,78,1438-1444[18]BBMandal,RKTyagi,RPandey;NSharma;AAgarwal(2000),Invitroconservationofgermplasmofagri-horticulturalcropsatNBPGR:anoverview.
In:RazdanMK,CockingEC(eds)Conservationofplantgenetic[19]SChand;AKSingh.
JournalofPlantPhysiology2004,161,237–243.
[20]MMicheli;IAHafiz.
AStandard,ScientiaHorticulturae,2007,113,286–292[21]TMurashige;Skoog.
Physiol.
Plant.
1962,15,473-494.
com/archive.
html)ISSN0975-508XCODEN(USA)AASRC922ScholarsResearchLibraryPlantregenerationofA.
lakoochafromencapsulatednodalexplantsShivKumarVerma*,DhirajKumarChoudhary,Ashish,AnnandKumarandMotiLalSchoolofBiochemicalEngineering,IIT(BHU),VaranasiABSTRACTOneofthealternativemethodsadoptedinrecentyearsistousebiotechnologicalapproachesforimprovingthetreespecies.
AproficientprotocolforencapsulationofnodalsegmentsofArtocarpuslakoochaRoxbhasbeendevelopedforplantregenerationthroughnon-embryogenicsyntheticseeds.
Concentrationsofsodiumalginateandcalciumchloridegreatlyaffectedmorphologyandtextureencapsulatinggel.
3%sodiumalginatewith100mMCaCl2hasbeenfoundtobebestpossibleconcentrationfortheproductionofidenticalsyntheticseeds.
25daysoldinvivoplantletofA.
lakoochawasusedforobtainingnodalsegments,usedinallexperiments.
FiveexperimentswereperformedusingMSmedium(agarsolidifiedandliquid)supplementedwith1,3and5mg/lBAP.
Concentrationof1mg/lBAPwasobservedtobemoreeffectiveforachievinghighestregenerationfrequencyincomparisontootherconcentrationofBAP(3and5mg/l).
InthirdexperimentonlydifferentstrengthofMSmediumwasused,inlasttwoexperimentsnodalsegmentspriorencapsulationandencapsulatednodalsegmentsweregivenpulsetreatmentwith(IBA)for48hoursandconcentrationof3mg/lBAPwasobservedtobemosteffectiveforsyntheticseedregeneration.
Datawererecordedafter4weeksofculture.
Keywords:Pulsetreatment,MSmedium,BAP,IBA,Acclimatization,syntheticseeds.
INTRODUCTIONThereisworldwidesusceptibilityofplantgeneticdiversityduetounmatchedperturbations,habitatlossanddestructionrates.
Variousspeciesaredescribedasrareorendangered,andasaresultintegratedprogramsarerequiredtodefendandconservebiodiversityofcurrentlyavailablespecies[1].
ArtocarpuslakoochaRoxb,aspeciesoffamilyMoraceae,isanimportanttropicalmedicinaltreespeciesnativetoIndiaandusedforvariousimportantpurposeslike;fruit,furniture,timber,andfeed.
Ripfruitsoflakoochaaregenerallyeatenfresh.
Eachfruitgenerallycontains20–30seedsthatarefleshywiththinseedcoat.
Theediblefruitpulpisbelievedtocontainessentialingredientswhichplaykeyroleinfunctioningiftheliver.
lkoochaseedsandmilkylatexarepurgativeinnature.
Seedscontainartocarpins(ALAIandALAII),theisolectinswhichexhibithighhaemagglutinationactivity(Wongkham1995).
However,theagglutinin(ALA)fromArtocarpuslakoochaisnotorganspecificintheplant.
Moreover,thehaemagglutinationactivityofALAwasdemonstratedinvariousorgansoftheplantexceptfruitflesh[2].
Traditionallytheplantispropagatedthroughseedbutseedpropagationisnotrapidandseasondependent.
Extensiveimprovementhasbeenmadeinthepropagationofthisplantthroughplantcell,tissueandorganculture[3-4].
However,inordertocompletethedemandofpharmaceuticalindustries,continuoussupplyofplantthroughoutyear,thereisneedtodevelopamethodofconservationandtransportofhealthyplant.
Inthiscontext,forrapidpropagation,encapsulationtechnologymaybeanalternativemethodofconservationandgermplasmexchangeofthismedicinallyimportantplant[3].
Inrecentyears,syntheticseedtechnologyusingencapsulationofinvitro-derivednon-embryogenicpropaguleshasbecomeanimportantassettomicropropagation(Naiketal.
2006).
InShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2723ScholarsResearchLibraryaddition,syntheticseedtechnologycouldbeusefulingermplasmconservationofelite,endangeredandcommerciallyimportantplantsbyusingappropriatestoragetechniqueaswellasexchangeofaxenicplantmaterialbetweenlaboratoriesandpharmaceuticalindustries[5]thatcanbeusedifstockplantsorproliferationculturesbecomeinfestedwithbacteria,fungi,orarthropods.
Successfulcasesofsyntheticseedproductionandplantletregenerationhavebeenreportedforawiderangeofplantsincludingcereals,vegetables,fruits,ornamentals,medicinalplantsandforesttree,[7].
Someotherpotentialadvantagesofsyntheticseedtechnologyareeaseinhandlingduetosmallsizeofcapsules,reductionincosts,geneticuniformityofplantsanddirectdeliverytothefield[8]Anartificialseedpreparedbycoatingasomaticembryousingapolymermatrixisatrueseedanalog.
Animmobilizedsomaticembryocangerminateundersuitablegrowthconditionsandbecomeacompleteplant.
Syntheticseedtechnologyusingencapsulationofvegetativepropagulesofwoodyplantspecieshasbecomeapotentiallycosteffectiveclonalpropagationsystem.
Successfulplantregenerationfromsyntheticseedshasbeenreportedinseveralplantspecies.
However,inmostofthecases,theembryogenicpropagulessuchassomaticembryoswereusedforsyntheticseedsproduction.
Thereareonlyfewreportsonencapsulationofvegetativepropagules[9-14].
Encapsulationofvegetativepropagulescouldbeusedformassclonalpropagationatareasonablecost[14].
Therehasbeennoreportonutilizingsomaticembryostoproducesyntheticseeds.
Thedevelopmentofsyntheticseedsfromsomaticembryoscouldofferapracticalmeansformasspropagationofbanana.
Inthisstudy,encapsulationofsomaticembryosdevelopingfromembryogenicsuspensionculturesinanimportantIndiancultivarofbanana,Rasthali(AABwithtwoAAandoneBgenome),andconversionoftheencapsulatedembryosintoplantsarereported(Ganpatietal.
2001).
Amongseveralnon-embryogenicpropagules,shoottipexplantsaremoreresponsivethanotherexplantsbecauseofgreatermitoticactivityinthemeristem[15].
Althoughtherearemanyreportsonencapsulationofshoottipsobtainedfrominvivoraisedplants[7,16].
Inmanyoftheworks,somaticembryoshavealreadybeenusedintheencapsulationprocess.
However,encapsulationofsomaticembryoswererestrictedmostlytoplantsinwhichsomaticembryogenesishasbeendocumented.
But,incurrenttime,useofnon-embryogenicvegetativepropaguleslikeapicalshootbuds,axillarybuds,nodalsegments,etc.
,fortheencapsulationhavealsobeenextendedasasuitablealternativetosomaticembryos[6,9,11,14,17,18,20,21].
ThepresentstudywasaimedtoinvestigateencapsulationofsomaticembryosofArtocarpuslakoochinsodiumalginatebeadsandconversionofencapsulatedsomaticembryosintoplantlets.
EffectofdifferentconcentrationofBAP,wasalsostudiedtoexplorethemorphogeneticresponsesofencapsulatedshoottips.
Validationofthepossibilitytostoretheencapsulatedshoottipsfortimeperiodsufficientforexchangesanddistributionofgermplasmwasdiscussed.
Inthepresentinvestigation,wedescribertheencapsulationofshoottipsforthedevelopmentofsyntheticseedinA.
lakoocha.
MATERIALSANDMETHODS2.
1.
PreparationofexplantsandcultureconditionsSeedsofripenfruitsofA.
lakoochawereobtainedfromagriculturefieldofChandraShekharAjadAgricultureUniversity,Kanpur,India.
SeedsweregrownonagarsolidifiedMSmedium.
25daysoldplantletswereusedformakingnodalsegments.
Nodalsegments(0.
5cm)ofA.
lakoochawereprepared.
Initiallytheexplantswerewashedinrunningtapwaterfor20–30mintominimizemicrobialconcentrationonthesurfaceofnodalsegments.
Afterthatsurfacesterilizedinlaminarairhoodwith70%ethanolfor30-40sfollowedby0.
05%mercuricchloride(Hi-Media,India),for3–4min,andrinsed4–5timeswithsteriledoubledistilledwater[imp].
Forshootmultiplication,nodalsegmentswereculturedonMS,medium[22]supplementedwithsucrose(30g/l).
Themediaweresolidifiedwith0.
8%(w/v)agar(Hi-Media,India).
ThemediawereadjustedtopH5.
7using1NNaOHor0.
1NHClbeforeautoclavingat1210Cfor15min.
Culturesweremaintainedat25±20Cwitha16hphotoperiodataphotonfluxdensityof50–70mmolm-2s-1fromcoolwhitefluorescenttubes(Philips,India).
Nodalsegmentsexcisedfrominvitroproliferatedplantletswereusedasexplantsforthesyntheticseedproduction.
2.
2.
EncapsulationofnodalsegmentsandpreparationofsodiumalginatebeadsForencapsulationofnodalsegments,sodiumalginate(Himedia,india)waspreparedintherangeof2.
0,3.
0,4.
0,or5.
0%(w/v),whereascalciumchloride(CaCl2.
2H2O)solutionwaspreparedin100,mM(w/v)indouble-distilledwaterorliquidMS(MurashigeandSkoog,1962)mediumwithoutanyplantgrowthregulator.
Boththegelmatrixandcomplexingagentwereautoclavedat121°Cfor15min.
Encapsulationwasaccomplishedbymixingthenodalexplantintothesodiumalginatesolutionanddroppingtheseexplantsintothecalciumchloridesolution.
Thebeadscontainingthesomaticembryoswereheldfor20–30mininthecalciumchloridesolutionandafterhardeningoftheShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2724ScholarsResearchLibrarybeads,encapsulatedsomaticembryoswerewashedwithsterilizeddistilledwatertwotimestotakeawaytracesofcalciumchloride.
(A)(B)(C)(D)(E)Figure1-SyntheticseedpreparedindifferentsodiumalginateandcalciumchloridecompositionPlantletregenerationinagarsolidifiedMSmedium(D)andMSmediumsupplementedwith1mg/lBAP(E)3.
ExperimentsDifferentfactorsaffectingconversionwereevaluatedandthefollowingexperimentswereperformed.
Tomaintaintheculture,samelightandtemperatureconditionswereusedinthegrowthroomasdescribedpreviously.
Dataforconversionfrequencyandshootlengthwasrecordedafter3weeksofculture.
3.
1EffectofBAPinagarsolidifiedMSfullstrengthMediumSyntheticseedswerepreparedusinginsodiumalginate(3%w/v)and100mMCaCl2,seedswereinoculatedinsolidifiedMSmediumsupplementedwithBAPattheconcentrationof1,3and5mgl-1.
AfterthreeweekspercentseedgerminationwasrecordedinallthreesupplementofBAP.
3.
2EffectofBAPinliquidMSfullstrengthMediumInthisexperimentsamecompositionofsyntheticseedandsameconcentrationofBAPwasused.
LiquidMSmediumwasused.
AfterthreeweeksinoculationpercentseedgerminationwasrecordedinallthreesupplementofBAP.
3.
3EffectofCompositionofEncapsulatingGelSodiumalginatebeadscomplexedwithnodalexplantswereinoculatedinMSfullstrength,1/2MSand1/4MSstrength.
Percentseedgerminationwasrecordedafterthreeweeksofinoculation.
ShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2725ScholarsResearchLibrary3.
4EffectofPulsetreatmentofIBA(IndoleButyricAcid)BeforeEncapsulationofnodalExplantNodalexplantswerepulsetreatedfor48hours,withIBA(3mgl-1),beforepreparationofsyntheticseeds.
Aftersyntheticseedpreparation,seedswereinoculatedinfullstrengthliquidMSmediumsupplementedwith,3and5mgl_1BAP.
3.
5EffectofPulsetreatmentofIBA(IndoleButyricAcid)AfterEncapsulationofnodalExplantInthisexperimentafterencapsulation,nodalexplantsweresubjectedto48hourspulsetreatmentwithsamegrowthregulatorandsameconcentration.
SeedswereinoculatedinfullstrengthliquidMSmediumsupplementedwith,3and5mgl-1BAP.
3.
6AcclimatizationofplantsinsoilArtificiallygrownplantletsregeneratedfromencapsulatednodalsegmentsweretransferredtoplasticpotscontaining3:1(w/w)mixtureofsterilesandandsoilmoistenedwithliquidMSmedium.
Plantletswerecoveredwithpolyethylenebagstomaintainhighhumidityandirrigatedwithtapwater.
Potswithplantletswerekeptunderlaboratoriesconditionat250Cinartificiallight(irradianceof60mmolm-2s-1)providedbycoolwhitefluorescenttubesfor4Weeksandthenthepotsweretransferredtofieldlevels.
RESULTSANDDISCUSSIONAtpresentdaysnewrouteinsyntheticseedtechnologyhasbeenreportedwiththeuseofnonembryogenicplantpropagulesviz;rootstemleaves(StandardiandPiccioni,1998).
Themostimportantbenefitofusingvegetativepropagulesforthepreparationofsyntheticseedswouldbeinthosecaseswheresomaticembryogenesisisnotwellestablishedorsomaticembryosdonotgerminateintocompleteplantlets(Raietal.
,2009).
Insuchcases,syntheticseedscanbeproducedfromshoottipsforcost-effectivemassclonalpropagation,potentiallong-termgermplasmstorage,anddeliveryoftissue-culturedplants.
Inthepresentexploration,nodalsegmentsexcisedfrominvivoproliferatedsamallplantletswereusedasanexplantforthedevelopmentofsyntheticseedinA.
lakoocha.
inmanyexperimentalstudiesSimilarobservationswerealsomadeinDalbergiasissooRoxb(Chandetal.
2004),Ceropegiabulbosavar.
bulbosa(Dhiretal.
2013),VitexnegundoL.
(Ahmadetal.
2010).
EvaluationofconcentrationofsodiumalginateandcalciumchloridewhichareeffectiveforthegellingpropertiesofthematrixandeventuallyfortheexcellenceofcalciumalginatebeadsisanimportantaspectforthesuccessfulinvitropropagationofplantsthroughencapsulationmethodsConcentrationofsodiumalginateandcalciumchloridegreatlyaffectedtheencapsulationofnodalsegmentsdifferedqualitativelywithrespecttotexture,shape,andtransparency.
Table1.
EffectofBAPinagarsolidifiedMSfullstrengthMediumConcentrationofBAP(mgl-1)+MS(S)PercentresponseofsyntheticSeedGerminationDays1+MS(S)91213+MS(S)81215+MS(S)7321Inourstudy3.
0%sodiumalginateand100mMCaCl2.
2H2Owasfoundmostsuitableforformationofidealcalciumalginatebeads.
ConcentrationofBAPalongwithMS(MurashigandSkoog1962.
)mediumgreatlyaffectedthepercentconversionfrequencyofencapsulatednodalsegmentsofA.
lakoocha,highestconversionfrequency(91%)wasobservedonsolidMSmediumsupplementedwith1mg/lBAP(6-BenzyleAminoPurine)followedby3and5mg/lBAPwithpercentsyntheticseedgerminationof89%and72%respectively(Table1).
Table2.
EffectofBAPinliquidMSfullstrengthMediumConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeedGerminationDays1+MS(L)96213+MS(L)84215+MS(L)7821WehaveobservedinourexperimentthatwhensameexperimentwasperformedwithliquidMSmediumwithsameconcentrationofBAP,percentconversionfrequencywasobservedhigherthantheearlierexperiment.
Itwas96%onliquidMSmediumsupplementedwith1mg/lBAP.
LiquidMSmediumsupplementedwith3and5mg/lBAPshowedconversionfrequencyof84%and78%respectively(Table2).
thisdifferenceofconversionfrequencyintwoconditionsofmediummaybeduetofactthatinliquidMSmediumnutrientcomponentsaremoreeaslyandrapidlyShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2726ScholarsResearchLibraryabsorbedthroughthesurfaceofsodiumalginatebeadswhileinsolidMSmediumnutrientcomponentsareslowlyabsorbedwhichisresponsibleforslowregenerationofnodalsegments.
Table3.
EffectofCompositionofEncapsulatingGelStrengthofMS(L)PercentresponseofsyntheticSeeGerminationDaysMS(FS)8721MS(1/2S)5421MS(1/4S)4821WhenonlydifferentstrengthofliquidMSmedium(MSFS,MS1/2SandMS1/4S)wasuseditwasobservedthatsyntheticseedregenerationfrequencyoffullstrengthliquidMSwashighest(87)amongthreedifferentstrengthofliquidMSmedium.
Possiblereasonofthisdifferenceofregenerationfrequencymaybeduetoinsufficientconcentrationofallnutrientcomponents,becauseinsideaplantcellforabiochemicalreactionparticularconcentrationofnutrientcomponent(metalionsetc.
)isneeded.
DuetothisfactinMS1/2SandMS1/4S)regenerationfrequencywasobservedtobe54%and48%respectively(Table3).
Table4.
EffectofPulsetreatmentofIBA(IndoleButyricAcid)BeforeEncapsulationofnodalExplantsConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeeGerminationDays1+MS(L)81213+MS(L)87215+MS(L)7821When,beforeencapsulation,nodalsegmentswerepulsetreatedwithIBA(IndoleButyricAcid)for48hrsandthenencapsulatedinsodiumalginatebeadsandinoculatedinmediumcompositionasdescribedintable2syntheticseedregenerationfrequencyobservedwasdifferent.
InthisexperimentwasobservedthatliquidMSmediumsupplementedwith3mg/lBAPshowedhighestregenerationfrequencyof87%incomparisontoMSmediumsupplementedwith1and5mg/lBAPwhichshowedregenerationfrequency81%and78%respectively(Table4).
Inourlastexperimentafterencapsulation,beadswerepulsetreatedwithIBAforsametimeperiodasinexperimentTable5.
EffectofPulsetreatmentofIBA(IndoleButyricAcid)AfterEncapsulationofnodalExplantsConcentrationofBAP(mgl-1)+MS(L)PercentresponseofsyntheticSeedGerminationDays1+MS(L)81213+MS(L)87215+MS(L)6821Samepatternofresultswereobtainedwithdifferenceinregenerationfrequencyvalue.
Thesevalueswere81%,87%and68%onMSmediumsupplementedwith1,3and5mg/lBAPrespectively(Table5).
PossiblereasonofeffectofPulsetreatmenttoaffectregenerationfrequencyisnotknown.
Allexperimentswereperformedintriplicatetominimizeerrorduringdatacalculation.
CONCLUSIONTheartificialseedtechnologyprovidesanoptionalmethodofmicropropagationforawidearrayofmedicinalplants,especiallydesirableelitegenotypes.
However,successfulplantretrievalfromencapsulatedvegetativemicropropagulesfollowingshort-termstorageismostlydependsonplantspecies,matrixcompositionandperiodofstorage.
Treesgenerallyhavealonggenerationtimeandaremostlyheterozygous.
Duetothesereasons,geneticdevelopmentinthesespecieshasbeenamostimportanthindrance.
Largescaleclonalpropagationofsuperiorclonesalongwithacceleratedtreeimprovementprogramsisnecessaryforsuccessfulandrapidexplorationoftreeplant.
Itispossibletouseeasy-to-obtainsomaticfragmentsoftheplantsbynodalsegmentcuttingsbyapplyingartificialseedtechnologyinA.
lakoocha.
Oneoftheadvantagesofsyntheticseedtechnologyisthattheriskofsomaclonalvariationwouldalsobereducedbecausemutationsareknowntooccurmorefrequentlywhenthede-andre-differentiationprocessesoccurduringinitialgrowth.
However,there-growthabilityoftheunipolarpropagulesafterencapsulationhinderedbyfactorssuchasprecociousgrowthandlackofataproot.
Encapsulatednodalsegmentsorapical/auxiliaryshootbudsareconsideredtobemoreeffectivecomeintoviewifwemaketherelativeeaseandextensibilityofthetechnique,aswellasthepossibilityofundamagedanduninfectedmovingsmall-sizedmicroShivKumarVermaetalArch.
Appl.
Sci.
Res.
,2015,7(1):22-2727ScholarsResearchLibrarypropagatedplantmaterialbetweenlaboratoriesandcountries,whilereducingphytosanitaryandquarantineproblems(Bapat,1993.
,Mathuretal.
,1989;HasanandTakagi,1995;Maruyamaetal.
,1997b.
,PiccioniandStandardi,1995).
Theresultspresentedinthispapersupportthefeasibilityofthiscost-effectivemethod,foralargescalepropagationofthisecologicallyandmedicinallypotentplantspecies.
Oneofthedrawbackofsyntheticseedtechnologyisthatonstorageofseeds,percentregenerationfrequencyisgreatlyreducedTherefore,forlarge-scaleapplicationofthissyntheticseedtechnology,furtherexperimentsarenecessary;toaccomplishahigherpercentageofconversionmakethemviableevenafterlongtimestorageofencapsulatednodalsegmentsofA.
lakoocha.
Further,encapsulatedsomaticembryoscouldofferanimportantsystemforthetransportationandexchangeofgermplasminasafeandeconomicalmanner.
REFERENCES[1]RDhir;GSShekhawat,IndustrialCropsandProducts2013,47139–144.
[2]NJoshee;DRBastola;VPAgrawal;A.
K.
Yadav,Lakoocha:AMultipurposeTreeofWarmClimateTrendsinnewcropsandnewuses.
2002.
J.
JanickandA.
Whipkey(eds.
).
ASHSPress,Alexandria,VA.
[3]DPBhatt;GFassuliotis,ZeitschriftfurPflanzenphysiologie,1981,104,481-489[4]MKHusain;MAnis;AShahzad,InVitroCell.
Dev.
Biol.
Plant,2007,43,59–64.
[5]SKVerma;MKRai;PAsthana;VSJaiswal;UJaiswal,ScientiaHorticulturae,2010,124,517–521[6]SKNaik;PKChand,Nutrient-alginateencapsulationofinvitronodalsegmentsofpomegranate(PunicagranatumL.
)forgermplasmdistributionandexchange2006,103,3,247–252.
[7]SKSingh;MKRai;PAsthana;SPandey;VSJaiswal;UJaiswal,ActaPhysiol.
2009.
[8]EMaruyama;IKinoshita;KIshi;HShigenaga;KOhba;ASaito,PlantCellReproduction.
1997b,16,393–396.
[9]SMathur;SKumar,JMedAromPlantSci1998,20,1056–1059.
[10]VABapat;Studiesonsyntheticseedsofsandalwood(SantalumalbumL.
)andmulberry(MorusindicaL)-In:Redenbough,K.
(ed.
),Synseeds,ApplicationsofSyntheticSeedstoCropImprovement.
CRCPress,BocaRaton1993,Pp.
381-407.
[11]TRGanapathi;LSrinivas;PSuprasanna;VABapat,InVitroCellDevelopmentBiology,2001,37,178-181.
[12]PSharma;MVRajam,J.
Exptl.
Bot.
,1995,46,135-141.
[13]EPiccioni;AStandardi,PlantCellTissueOrgan.
Culture,1995,42,221–226.
[14]ChandS.
,SinghA.
K.
,.
JournalofPlantPhysiology2004,161,237–243.
[15]BallesterA.
,JaneiroL.
V.
,VietezA.
M.
,.
ScientiaHorticulturae,1997,71.
67-78.
[16]AKSingh;MSharmaRVarshney;SSAgarwal,KCBansal,InVitroCellular&DevelopmentalBiology,2008,42,109-113.
[17]HAra;UJaiswal;VSJaiswal,CurrentScience,2000,78,1438-1444[18]BBMandal,RKTyagi,RPandey;NSharma;AAgarwal(2000),Invitroconservationofgermplasmofagri-horticulturalcropsatNBPGR:anoverview.
In:RazdanMK,CockingEC(eds)Conservationofplantgenetic[19]SChand;AKSingh.
JournalofPlantPhysiology2004,161,237–243.
[20]MMicheli;IAHafiz.
AStandard,ScientiaHorticulturae,2007,113,286–292[21]TMurashige;Skoog.
Physiol.
Plant.
1962,15,473-494.
域名注册需要哪些条件(新手注册域名考虑的问题)
今天下午遇到一个网友聊到他昨天新注册的一个域名,今天在去使用的时候发现域名居然不见。开始怀疑他昨天是否付款扣费,以及是否有实名认证过,毕竟我们在国内域名注册平台注册域名是需要实名认证的,大概3-5天内如果不验证那是不可以使用的。但是如果注册完毕的域名找不到那也是奇怪。同时我也有怀疑他是不是忘记记错账户。毕竟我们有很多朋友在某个商家注册很多账户,有时候自己都忘记是用哪个账户的。但是我们去找账户也不办...
特网云-新上线香港五区补货资源充足限时抢 虚拟主机6折,低至38元!
官方网站:点击访问特网云官网活动方案:===========================香港云限时购==============================支持Linux和Windows操作系统,配置都是可以自选的,非常的灵活,宽带充足新老客户活动期间新购活动款产品都可以享受续费折扣(只限在活动期间购买活动款产品才可享受续费折扣 优惠码:AADE01),购买折扣与续费折扣不叠加,都是在原价...

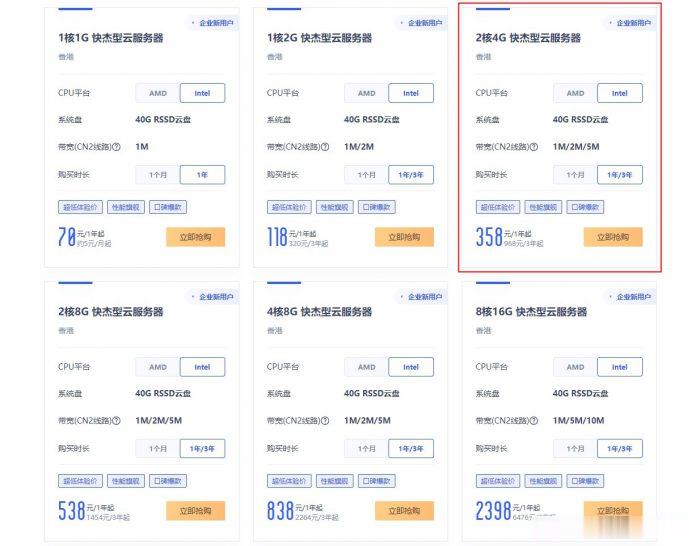
ucloud香港服务器优惠活动:香港2核4G云服务器低至358元/年,968元/3年
ucloud香港服务器优惠降价活动开始了!此前,ucloud官方全球云大促活动的香港云服务器一度上涨至2核4G配置752元/年,2031元/3年。让很多想购买ucloud香港云服务器的新用户望而却步!不过,目前,ucloud官方下调了香港服务器价格,此前2核4G香港云服务器752元/年,现在降至358元/年,968元/3年,价格降了快一半了!UCloud活动路子和阿里云、腾讯云不同,活动一步到位,...
http://www.qvod.com/为你推荐
-
小度商城小度智能音箱1s上面的黄圈不熄灭怎么回事,第一天还能熄灭中老铁路中长铁路的铁路的新中国历史xyq.163.cbg.com梦幻西游里,CBG是什么?在那里,能帮忙详细说一下吗钟神发战旗TV ID:新年快乐丶未央不见是哪个主播www.zjs.com.cn请问宅急送客服电话号码是多少?avtt4.comwww.5c5c.com怎么进入www.bbb551.combbb是什么意思yinrentangweichentang产品功效好不好?www.toutoulu.comSEO行业外链怎么做?www.gogo.com哪种丰胸产品是不含激素的?