Statisticalwww.666abcd
www.666abcd 时间:2021-04-09 阅读:()
PHARMACOGENETICSIn-WhaKim&YooJinMoon&EunheeJi&KyungImKim&NayoungHan&SungJuKim&WanGyoonShin&JongwonHa&Jeong-HyunYoon&HyeSukLee&JungMiOhReceived:17October2011/Accepted:21November2011/Publishedonline:20December2011#Springer-Verlag2011AbstractPurposeThepurposeofthisstudywastocharacterizetheeffectsofclinicalandgeneticvariablesonthepharmacoki-neticsandcomplicationsoftacrolimusduringthefirstyearafterkidneytransplantation.
MethodsOnehundredandthirty-twoKoreankidneyrecip-ientswhoreceivedtacrolimusweregenotypedforABCB1(exons12,21,and26)andCYP3A5(intron3).
Tacrolimustroughlevels,dose,ordose-adjustedtroughlevelsandcom-plicationswerecomparedamongpatientsduringtheearlystage(3,7,14,30,and90days)andupto1yearaccordingtothegenotypes.
ResultsAdonorsource-adjustedlinearmixedmodelwithmultilevelanalysisadjustingforage,bodyweight,hemato-crit,andserumcreatinineshowedthatCYP3A5genotypeisassociatedwithdose-adjustedleveloftacrolimus(pT),exon21(2677G>T/A),andexon26(3435C>T).
Thenon-synonymousSNPG2677T>A,andthesynonymousSNPC3435ThavebeenreportedtobecorrelatedwithcellularexpressionlevelsofABCB1[17]andthusmayinfluencethetacrolimuspharma-cokinetics.
However,theeffectoftheseABCB1polymor-phismsontacrolimuspharmacokineticsandclinicaloutcomesstillremainscontroversial[15,18–23]Moreover,littleisknownabouttheassociationofCYP3A5andABCB1genotypeswithtacrolimuspharmacokinetics,especiallywhenstratifiedbydonorsourcesintheearlystageoftrans-plantation.
Tostudytheseassociationswiththerepeatedmeasurementsoftacrolimusthatarelikelypositivelycorre-latedovertime,aflexibleyetversatilemodel,likealinearmixedeffect(LME)modelshouldbeusedfortheanalysis.
Likewise,whenanalysisofthefactorsassociatedwiththeclinicaloutcomesoftacrolimusthataretransientovertimeandcanbecorrelatedwithmanydifferentfactors,aregres-sionmodelsuchasthegeneralizedestimatingequation(GEE)modelisappropriatetoproduceefficientandunbi-asedregressionestimates.
Accordingly,thepurposeofthisstudywastoexplicatethelinearmixedeffectsmodelandGEEmodelasameansoftestingthefactorsthataccountfortheindividualvariationsinthepharmacokineticsandthecomplicationsoftacrolimusduringtheearlystage(3,17,14,30,and90days)andupto1yearaftertransplantationinKoreankidneytransplantrecipients.
MaterialsandmethodsPatientsDatafrom132renalallograftrecipientswhoreceivedkid-neytransplantationfromSeoulNationalUniversityHospi-tal,Seoul,Koreabetween2002and2008werereviewedretrospectively.
Patientsaged>18years,andsingleprimaryorsecondarykidneyrecipientspluscombinedorgantrans-plantationswereeligible.
TheinstitutionalreviewboardoftheClinicalResearchInstituteatSeoulNationalUniversityHospitalapprovedthestudyprotocol(IRBNo.
C-0609-014-182).
Writteninformedconsentwasobtainedfromallsub-jectsbeforebeingenrolledinthisstudy.
ImmunosuppressiveprotocolanddatacollectionAninitialoraldoseof0.
075–0.
1mg/kgtacrolimuswasadministeredtwicedailythedaybeforetransplantationforcadavericdonororgansrecipients,and2hbeforetransplan-tationinthecaseoflivinggrafts,whichwasthenadjustedaccordinglytothetacrolimustherapeuticdrugmonitoring(TDM).
Thedoseoftacrolimuswasadjustedtomaintainthetargetbloodtroughconcentrationof10–12ng/mLduringthefirstmonth.
Theconsequentwhole-bloodtargettroughconcentrationswereadjustedto15–20ng/mLduringthefirst5days,8–12ng/mLupto3months,6–8ng/mLupto6months,and4–6ng/mLthereafter.
Thesteroidswasgivenatastandardintravenousdoseof500mgmethylpredniso-loneatthetimeofsurgery,andwasthenwasgraduallytaperedtoamaintenancedoseof10mgby2weeksaftertransplantation.
Mycophenolatemofetil(MMF)wasadmin-isteredorallyatafixeddoseof1–1.
5g/day.
SomeofthepatientswhoreceivedMMFexperiencedgastrointestinaldisorders,whichledtoachangeintherapytoenteric-coatedmycophenolatesodium.
Otherconcomitantmedica-tionswerescreenedforpossibleCYP3A5andABCB1inter-actions.
Recipientswithcadavericdonorkidneys,positiveresultsonapanelreactiveantibodytest,and/orwhohadmorethanthreemismatchesofhumanleukocyteantigens,wereadministeredtwodoses(20mgeach)ofIVbasilix-imab(Novartis,EastHanover,NJ,USA)1hpreoperativelyandonday4aftertransplantation.
658EurJClinPharmacol(2012)68:657–669TacrolimusmeasurementsTacrolimustroughsamplesweretakenapproximately12hafterthelastdoseondays3,7,14,30,90,180,and360.
Whole-bloodtacrolimusconcentrationsweredeterminedusingamicroparticulateenzymeimmunoassay(IMxana-lyzer;TacrolimusII;AbbottDiagnostics,AbbottPark,IL,USA).
Dose-adjustedtroughconcentrationswerecalculatedbydividingtacrolimustroughconcentrationsbythecorresponding24-hdoseonmg/kgbasis.
Clinicalandsafetyfollow-upAtthetimeoftroughlevelsampling,acompletebiochem-icalbloodanalysiswasperformedtomeasurethelevelsofbloodurinenitrogen,serumcreatinine,electrolytes,glucose,hematologyparameters,lipids,andalbumin.
CreatinineclearancewascalculatedusingtheModificationofDietinRenalDiseaseStudyequation[24]andmeasuredfrom24-hurinecollections.
Patientsunderwentacompletephysicalexamination,includingstandardizedmeasurementofsystol-icanddiastolicbloodpressure,bodyweight,andvitalsigns.
Concomitantmedicationwasnotedandtheuseoflipid-loweringdrugs,antihypertensivedrugs,andanti-diabeticdrugsinparticular,wererecordedcarefully.
Allpatientsunderwentprotocolrenaltransplantbiopsiesattransplanta-tionand10daysaftertransplantation,atwhichtimeTDMcanbecomeeffectiveandthetargettacrolimustroughcon-centrationisachieved.
Recipientswith≥20%elevationsofserumcreatininelevelsunderwentultrasound-guidedpercu-taneousallograftbiopsiesonanydayaftertransplantation.
Acuteallograftrejection,calcineurininhibitor(CNI)-in-ducednephrotoxicityandchronicallograftnephropathywereexclusivelydiagnosedbasedonhistologicalfindingsbyanexperiencedrenalhistopathologistaccordingtothe1997Banffclassificationcriteria[25].
Patientswereconsid-eredtohavearterialhypertensionifsystolicand/ordiastolicbloodpressureexceeded140/90mmHgorwereonantihy-pertensivedrugs.
Recipientswereconsideredtohavehyper-lipidemiaifthefastingtotalcholesterollevelexceeded190mg/dL,low-densitylipoproteincholesterollevelwasabove110mg/dL,and/orthepatientswerereceivinganti-triglyceridedrugs.
Newonsetdiabetesmellitusaftertrans-plantation(NODAT)wasconfirmedthroughastandardoralglucosetolerancetestinpatientswithsuspectedhypergly-cemiaandwasdefinedastheuseofanti-diabeticdrugsformorethan6months[26].
IdentificationofgenotypesGenomicDNAwasisolatedfromwholevenousbloodusingtheQIAampDNAbloodkit(Qiagen,Valencia,CA,USA)accordingtothemanufacturer'sprotocol.
GenotypingforCYP3A5(6986A>G)(rs776746)andABCB1exon12(C1236T)(rs1128503)andexon26(C3435T)(rs1045642)SNPswasperformedusingtheTaqManallelicdiscriminationassaywithanABI7900HT(AppliedBio-systems,FosterCity,CA,USA)[23].
Polymerasechainreaction(PCR)wasprogrammedasfollows:95°Cfor15minfollowedby40cyclesat95°Cfor15sand60°Cfor1min.
TheABCB1exon21(G2677T/A)(rs2032582)regionwasamplifiedusingPCR.
PCRwasprogrammedasfollows:94°Cfor5minand40cyclesat94°Cfor30s,48°Cfor30sand72°Cfor1min,followedby72°Cfor7min.
Amplifiedproductswerepurifiedwith1.
2%agarosegelsusingPCRpurification(PCRquick-spin;iNtRONBiotechnol-ogy,Gyeonggi-do,Korea).
Wholesequencesofamplifiedfragmentswerethendetermined[18].
Boththepatientsandtheinvestigatorswereblindedtothepatients'geneticvariants.
PrimersandprobesweredesignedwithPrimerExpressSoftwareVersion3.
0(AppliedBiosystems)andareshowninTable1.
StatisticalanalysesQuantitativedatawererepresentedasfrequenciesandper-centages,whilequalitativedatawerepresentedasmean±standarddeviation.
TheMann–WhitneyUtestandKruskal–Wallistestwereusedtocomparecontinuousvariablesbe-tweengenotypegroupswithposthoctestingformultiplecomparisons.
CategoricalvariableswereanalyzedusingtheChi-squaredtestandFisher'sexacttest.
Weperformedlinearmixedmodelanalysesonrepeatedmeasuresoftacro-limustroughlevels,doses,anddose-adjustedtroughlevels.
Clinicalcovariatesappliedinthemodelwereage,sex,bodyweight,steroidandMMFdoses,hematocrit,serumcreati-nine,albumin,creatinineclearance,andconcomitantmedi-cations.
Linearmixedmodelanalyseswererepeated,adjustingthemeanfordonorsourceusingamultilevelmodelwithadjustmentsfortheaforementionedcovariates.
Analysisofvariancefollowedbymultipleregressionanal-ysiswasusedtoassessthecontributionofcorticosteroiddoseandotherclinicalcovariatestotacrolimustroughlev-els,dose,anddose-adjustedlevelsatdifferenttimepointsaftertransplantation.
Covariateswithapvalueof5%.
Noneofthesesixhaplotypeswasassociatedwithtacrolimusdose-adjustedtroughlevels.
Combinationsofthethreepredominanthap-lotypesshowedthatonlyonepatientcarrieddiplotypeTTT–TTTofABCB1.
TTThaplotypecarriersdisplayedlowertacrolimusdose-adjustedtroughlevelsthannon-TTTcar-riersonday3post-transplantation(p10ng/mLmustbeachieved[31]bydays2–3aftertransplantationtominimizegraftrejections[7].
PolymorphismsinABCB1andCYP3A5areexpectedtoaffectthisinterindividualvariabilityintacrolimuspharma-cokineticsandclinicaloutcomesintheearlystageoftrans-plantation.
Inourpatients,thetacrolimustroughconcentrationonday3variedwidely,rangingfrom6.
27to32.
00ng/mL.
Morespecifically,12patients(9%)hadTable7Univariateandmultivariatecoxproportionalhazardsanalysesforbiopsy-provenacuterejectionsVariablesUnivariateMultivariateHazardratio(95%CI)pvalueHazardratio(95%CI)pvalueMalegender(vsfemale)1.
196(0.
401~3.
579)0.
748––Age1.
052(1.
005~1.
102)0.
0311.
049(0.
999~1.
102)0.
054Bodyweight1.
010(0.
963~1.
059)0.
693––New–onsetdiabetesaftertransplantation(vsnodiabetes)0.
577(0.
181~1.
840)0.
352––Hypertension(vsnohypertension)0.
723(0.
095~5.
524)0.
754––Hyperlipidemia(vsnohyperlipidemia)0.
917(0.
205~4.
098)0.
910––Deceaseddonor(vslivingdonor)3.
250(1.
139~9.
272)0.
0282.
878(1.
007~8.
226)0.
048ABCB11236CA(vsCC)0.
478(0.
114~2.
001)0.
312––TT(vsCC)0.
962(0.
241~3.
848)0.
957––ABCB12677GA/GT(vsGG)0.
665(0.
183~2.
417)0.
536––AA/AT/TT(vsGG)0.
389(0.
040~3.
740)0.
413––ABCB13435CA(vsCC)0.
794(0.
256~2.
461)0.
689––TT(vsCC)1.
637(0.
330~8.
114)0.
546––CYP3A5*1/*3(vs*1/*1)0.
169(0.
011~2.
696)0.
208––*3/*3(vs*1/*1)1.
122(0.
146~8.
627)0.
912––RelativeriskofacuterejectionforeachvariableisgivenasHR(95%CI)andthepvalueforCoxproportionalhazardsanalysiswithcensoringforpatientdeath,graftfailure,andendoffollow-upTable8Associationbetweenclinicalcovariatesandtacrolimus-inducedcomplicationsaComplicationsVariablesUnivariateMultivariateEstimate95%CIpvalueEstimate95%CIpvalueDiabetes(n022)Tacrolimustroughlevel1.
0950.
998~1.
2020.
054–––Tremor(n014)Age1.
0460.
992~1.
1020.
097–––Hairloss(n034)Tacrolimustroughlevel1.
2431.
146~1.
34715μg/mL[39].
Ithasbeenreportedthattacrolimuscanincreasevery-low-densitylipoproteinandhigh-densitylipo-proteincholesterols[40],buttherewasnoreportsofacorrelationbetweendruglevelandserumlipids.
Inauni-variateanalysis,tacrolimustroughlevelswereexpectedtoincreasetheincidenceofNODATwithborderlinesignifi-cance(p00.
054).
OnecanthinkthatgenotypesofCYP3A5andABCB1maybeassociatedwithriskoftacrolimus-relatedcomplications.
However,nostudytodatehassuc-cessfullydemonstratedtheroleofCYP3A5*3orABCB1genotypesintacrolimus-relatedcomplications[16,41].
Hy-perlipidemiaoccurredapproximately3-foldhigherinCYP3A5*3/*3carrierscomparedwithCYP3A5*1carriersafteradjustingtacrolimustroughlevel(p00.
045).
Ofnote,andtoourknowledge,thisisthefirststudytodemonstratethatclinicalandgeneticfactorsareassociatedwithtacrolimuspharmacokineticsandclinicaloutcomesusingstatisticaltoolslikealinearmixedeffectmodelforrepeatedmeasurementsoftacrolimusovertimeandagen-eralizedestimatingequationmodeltoproduceefficientandunbiasedregressionestimates.
WehaveexplicatedthelinearmixedeffectsmodelandGEEmodelasameansoftestingtheassociationamongthecovariates,thepharmacokinetics,andthecomplicationsoftacrolimusduringtheearlystagesEurJClinPharmacol(2012)68:657–669667andupto1yearaftertransplantationinKoreankidneytransplantrecipients.
ThepossiblelimitationofourstudyisthatwecouldnotstudytheeffectsoftheABCB1TTT-TTThaplotypebecauseonlyonerecipientcarriedthisdiplotypeinourstudy.
More-over,wecouldnotfindadefinitiveassociationbetweenthegenepolymorphismofCYP3A5andcomplications,possiblyowingtotheeffortsofmaintainingsimilartroughlevelsoftacrolimuswithTDMamongstrecipientsofdifferentCYP3A5genotypes.
Additionally,theassayweusedforthedeterminationoftacrolimusmeasurementscouldnotdistinguishbetweenactiveandinactivemetabolites.
Anoth-erpossiblelimitationisthatourstudywasaretrospectivestudy.
AprospectivecontrolledstudywithalargernumberoftransplantrecipientsiswarrantedtofurtherconfirmtheclinicalsignificanceofCYP3A5andABCB1genotypesontransplantationoutcomesinpatientstreatedwithtacrolimus.
Inconclusion,age,bodyweight,hematocrit,serumcre-atininelevels,andCYP3A5genotypeswerefoundtobesignificantfactorsresponsibleforaffectingthetacrolimusdose-adjustedtroughlevels.
Additionally,transplantationofacadavericdonorkidneywasassociatedwithincreasedincidenceofacuterejections,whileatacrolimustroughlevelwasasignificantpredictingfactorfortacrolimus-relatedcomplicationsincludingalopeciaandhyperlipidemia.
Theclinicalandgeneticfactorswehaveidentifiedcouldcon-tributetotheindividualizationoftacrolimustreatmentandenhancedrugsafetyandresponse,helpingtoobtainade-quateimmunosuppressionwithoutincreasingtheriskofcomplicationsinkidneytransplantpatients.
AcknowledgementsThisworkwassupportedbyagrantfromtheNationalResearchFoundationofKorea,fundedbytheKoreangovern-mentMinistryofEducation,ScienceandTechnology(no.
2009–0081414)andbyagrantfromtheKoreaHealthtechnologyR&DProject,MinistryofHealth&Welfare,RepublicofKorea(A090930)andtheClinicalResearchInstitute(CRI),SeoulNationalUniversityHospital(no.
1120070027).
ConflictofinterestTheauthorsdeclarenocompetinginterests.
References1.
KershnerRP,FitzsimmonsWE(1996)RelationshipofFK506wholebloodconcentrationsandefficacyandtoxicityafterliverandkidneytransplantation.
Transplantation62(7):920–9262.
LaskowDA,VincentiF,NeylanJF,MendezR,MatasAJ(1996)Anopen-label,concentration-rangingtrialofFK506inprimarykidneytransplantation:areportoftheUnitedStatesMulticenterFK506KidneyTransplantGroup.
Transplantation62(7):900–9053.
VenkataramananR,SwaminathanA,PrasadT,JainA,ZuckermanS,WartyV,McMichaelJ,LeverJ,BurckartG,StarzlT(1995)Clinicalpharmacokineticsoftacrolimus.
ClinPharmacokinet29(6):404–4304.
ScottLJ,McKeageK,KeamSJ,PloskerGL(2003)Tacrolimus:afurtherupdateofitsuseinthemanagementoforgantransplanta-tion.
Drugs63(12):1247–12975.
VenkataramananR,JainA,WartyVS,Abu-ElmagdK,AlessianiM,LeverJ,KrajakA,FlowersJ,MehtaS,ZuckermanSetal(1991)PharmacokineticsofFK506intransplantpatients.
Trans-plantProc23(6):2736–27406.
NagaseK,IwasakiK,NozakiK,NodaK(1994)DistributionandproteinbindingofFK506,apotentimmunosuppressivemacrolidelactone,inhumanbloodanditsuptakebyerythrocytes.
JPharmPharmacol46(2):113–1177.
UndreNA,vanHooffJ,ChristiaansM,VanrenterghemY,DonckJ,HeemanU,KohnleM,ZankerB,LandW,MoralesJM,AndresA,SchaferA,StevensonP(1999)Lowsystemicexposuretotacrolimuscorrelateswithacuterejection.
TransplantProc31(1–2):296–2988.
MouradM,WallemacqP,DeMeyerM,BrandtD,VanKerkhoveV,MalaiseJ,ChaibEddourD,LisonD,HaufroidV(2006)TheinfluenceofgeneticpolymorphismsofcytochromeP4503A5andABCB1onstartingdose-andweight-standardizedtacrolimustroughconcentrationsafterkidneytransplantationinrelationtorenalfunction.
ClinChemLabMed44(10):1192–11989.
KuypersDR,ClaesK,EvenepoelP,MaesB,CoosemansW,PirenneJ,VanrenterghemY(2004)Time-relatedclinicaldetermi-nantsoflong-termtacrolimuspharmacokineticsincombinationtherapywithmycophenolicacidandcorticosteroids:aprospectivestudyinonehundreddenovorenaltransplantrecipients.
ClinPharmacokinet43(11):741–76210.
MouradM,MouradG,WallemacqP,GarrigueV,VanBellingenC,VanKerckhoveV,DeMeyerM,MalaiseJ,EddourDC,LisonD,SquiffletJP,HaufroidV(2005)SirolimusandtacrolimustroughconcentrationsanddoserequirementsafterkidneytransplantationinrelationtoCYP3A5andMDR1polymorphismsandsteroids.
Transplantation80(7):977–98411.
ChristiansU,JacobsenW,BenetLZ,LampenA(2002)Mecha-nismsofclinicallyrelevantdruginteractionsassociatedwithtacro-limus.
ClinPharmacokinet41(11):813–85112.
SaekiT,UedaK,TanigawaraY,HoriR,KomanoT(1993)HumanP-glycoproteintransportscyclosporinAandFK506.
JBiolChem268(9):6077–608013.
HesselinkDA,vanGelderT,vanSchaikRH(2005)Thepharma-cogeneticsofcalcineurininhibitors:onestepclosertowardindi-vidualizedimmunosuppressionPharmacogenomics6(4):323–33714.
KuehlP,ZhangJ,LinY,LambaJ,AssemM,SchuetzJ,WatkinsPB,DalyA,WrightonSA,HallSD,MaurelP,RellingM,BrimerC,YasudaK,VenkataramananR,StromS,ThummelK,BoguskiMS,SchuetzE(2001)SequencediversityinCYP3ApromotersandcharacterizationofthegeneticbasisofpolymorphicCYP3A5expression.
NatGenet27(4):383–39115.
MacPheeIA,FredericksS,TaiT,SyrrisP,CarterND,JohnstonA,GoldbergL,HoltDW(2004)Theinfluenceofpharmacogeneticsonthetimetoachievetargettacrolimusconcentrationsafterkidneytransplantation.
AmJTransplant4(6):914–91916.
NumakuraK,SatohS,TsuchiyaN,HorikawaY,InoueT,KakinumaH,MatsuuraS,SaitoM,TadaH,SuzukiT,HabuchiT(2005)Clinicalandgeneticriskfactorsforposttransplantdiabetesmellitusinadultrenaltransplantrecipientstreatedwithtacrolimus.
Transplan-tation80(10):1419–142417.
HoffmeyerS,BurkO,vonRichterO,ArnoldHP,BrockmollerJ,JohneA,CascorbiI,GerloffT,RootsI,EichelbaumM,BrinkmannU(2000)Functionalpolymorphismsofthehumanmultidrug-resistancegene:multiplesequencevariationsandcorrelationofoneallelewithP-glycoproteinexpressionandactivityinvivo.
ProcNatlAcadSciUSA97(7):3473–347818.
AnglicheauD,FlamantM,SchlageterMH,MartinezF,CassinatB,BeauneP,LegendreC,ThervetE(2003)Pharmacokinetic668EurJClinPharmacol(2012)68:657–669interactionbetweencorticosteroidsandtacrolimusafterrenaltransplantation.
NephrolDialTransplant18(11):2409–241419.
HaufroidV,MouradM,VanKerckhoveV,WawrzyniakJ,DeMeyerM,EddourDC,MalaiseJ,LisonD,SquiffletJP,WallemacqP(2004)TheeffectofCYP3A5andMDR1(ABCB1)polymor-phismsoncyclosporineandtacrolimusdoserequirementsandtroughbloodlevelsinstablerenaltransplantpatients.
Pharmaco-genetics14(3):147–15420.
WangW,ZhangXD,MaLL,LuYP,HuXP,ZhangP,WangY,GuanDL(2005)RelationshipbetweenMDR1genepolymorphismandbloodconcentrationoftacrolimusinrenaltransplantpatients.
ZhonghuaYiXueZaZhi85(46):3277–328121.
RoyJN,BaramaA,PoirierC,VinetB,RogerM(2006)Cyp3A4,Cyp3A5,andMDR-1geneticinfluencesontacrolimuspharmaco-kineticsinrenaltransplantrecipients.
PharmacogenetGenomics16(9):659–66522.
KuypersDR,deJongeH,NaesensM,LerutE,VerbekeK,VanrenterghemY(2007)CYP3A5andCYP3A4butnotMDR1single-nucleotidepolymorphismsdeterminelong-termtacrolimusdispositionanddrug-relatednephrotoxicityinrenalrecipients.
ClinPharmacolTher82(6):711–72523.
HesselinkDA,vanSchaikRH,vanAgterenM,deFijterJW,HartmannA,ZeierM,BuddeK,KuypersDR,PisarskiP,LeMeurY,MamelokRD,vanGelderT(2008)CYP3A5genotypeisnotassociatedwithahigherriskofacuterejectionintacrolimus-treatedrenaltransplantrecipients.
PharmacogenetGenomics18(4):339–34824.
LeveyAS,BoschJP,LewisJB,GreeneT,RogersN,RothD(1999)Amoreaccuratemethodtoestimateglomerularfiltrationratefromserumcreatinine:anewpredictionequation.
ModificationofDietinRenalDiseaseStudyGroup.
AnnInternMed130(6):461–47025.
RacusenLC,SolezK,ColvinRB,BonsibSM,CastroMC,CavalloT,CrokerBP,DemetrisAJ,DrachenbergCB,FogoAB,FurnessP,GaberLW,GibsonIW,GlotzD,GoldbergJC,GrandeJ,HalloranPF,HansenHE,HartleyB,HayryPJ,HillCM,HoffmanEO,HunsickerLG,LindbladAS,YamaguchiYetal(1999)TheBanff97workingclassificationofrenalallograftpathol-ogy.
KidneyInt55(2):713–72326.
DavidsonJ,WilkinsonA,DantalJ,DottaF,HallerH,HernandezD,KasiskeBL,KiberdB,KrentzA,LegendreC,MarchettiP,MarkellM,vanderWoudeFJ,WheelerDC(2003)New-onsetdiabetesaftertransplantation:2003Internationalconsensusguide-lines.
Proceedingsofaninternationalexpertpanelmeeting.
Barcelona,Spain,19February2003.
Transplantation75(10Suppl):SS3–SS2427.
JunKR,LeeW,JangMS,ChunS,SongGW,ParkKT,LeeSG,HanDJ,KangC,ChoDY,KimJQ,MinWK(2009)TacrolimusconcentrationsinrelationtoCYP3AandABCB1polymorphismsamongsolidorgantransplantrecipientsinKorea.
Transplantation87(8):1225–123128.
HustertE,HaberlM,BurkO,WolboldR,HeYQ,KleinK,NuesslerAC,NeuhausP,KlattigJ,EiseltR,KochI,ZibatA,BrockmollerJ,HalpertJR,ZangerUM,WojnowskiL(2001)ThegeneticdeterminantsoftheCYP3A5polymorphism.
Pharmacogenetics11(9):773–77929.
HesselinkDA,vanGelderT,vanSchaikRH,BalkAH,vanderHeidenIP,vanDamT,vanderWerfM,WeimarW,MathotRA(2004)Populationpharmacokineticsofcyclosporineinkidneyandhearttransplantrecipientsandtheinfluenceofethnicityandge-neticpolymorphismsintheMDR-1,CYP3A4,andCYP3A5genes.
ClinPharmacolTher76(6):545–55630.
BarniehL,MannsBJ,KlarenbachS,McLaughlinK,YilmazS,HemmelgarnBR(2011)Adescriptionofthecostsoflivingandstandardcriteriadeceaseddonorkidneytransplantation.
AmJTransplant11(3):478–48831.
StaatzC,TaylorP,TettS(2001)Lowtacrolimusconcentrationsandincreasedriskofearlyacuterejectioninadultrenaltransplan-tation.
NephrolDialTransplant16(9):1905–190932.
WynneHA,CopeLH,MutchE,RawlinsMD,WoodhouseKW,JamesOF(1989)Theeffectofageuponlivervolumeandapparentliverbloodflowinhealthyman.
Hepatology9(2):297–30133.
SotaniemiEA,ArrantoAJ,PelkonenO,PasanenM(1997)AgeandcytochromeP450-linkeddrugmetabolisminhumans:ananaly-sisof226subjectswithequalhistopathologicconditions.
ClinPhar-macolTher61(3):331–33934.
WijnenRM,EriczonBG,TieboschAT,BeysensAJ,GrothCG,KootstraG(1991)ToxicityofFK506inthecynomolgusmonkey:noncorrelationwithFK506serumlevels.
TransplantProc23(6):3101–310435.
ShirbachehMV,JonesJW,HarralsonTA,EdelsteinJ,TecimerT,BreidenbachWC,JevansAW,MaldonadoC,BarkerJH,GruberSA(1999)Pharmacokineticsofintra-arterialdeliveryoftacrolimustovascularlyisolatedrabbitforelimb.
JPharmacolExpTher289(3):1196–120136.
PerroneRD,MadiasNE,LeveyAS(1992)Serumcreatinineasanindexofrenalfunction:newinsightsintooldconcepts.
ClinChem38(10):1933–195337.
LeblondF,GuevinC,DemersC,PellerinI,Gascon-BarreM,PichetteV(2001)DownregulationofhepaticcytochromeP450inchronicrenalfailure.
JAmSocNephrol12(2):326–33238.
StaatzCE,TettSE(2005)Pharmacokineticconsiderationsrelatingtotacrolimusdosingintheelderly.
DrugsAging22(7):541–55739.
FinkJ,FlasarM,HarringtonJ,WeirM,BartlettS(1998)Alopeciatotalisrelatedtotacrolimususefollowingkidneyandpancreastransplantation.
[abstractpresentedatAST1998].
1998[cited2010May122010];Availablefrom:http://www.
a-s-t.
org/abstracts98/abs661.
htm40.
IchimaruN,TakaharaS,KokadoY,WangJD,HatoriM,KameokaH,InoueT,OkuyamaA(2001)Changesinlipidmetabolismandeffectofsimvastatininrenaltransplantrecipientsinducedbycyclosporineortacrolimus.
Atherosclerosis158(2):417–42341.
YamauchiA,IeiriI,KataokaY,TanabeM,NishizakiT,OishiR,HiguchiS,OtsuboK,SugimachiK(2002)Neurotoxicityinducedbytacrolimusafterlivertransplantation:relationtogeneticpoly-morphismsoftheABCB1(MDR1)gene.
Transplantation74(4):571–572EurJClinPharmacol(2012)68:657–669669
MethodsOnehundredandthirty-twoKoreankidneyrecip-ientswhoreceivedtacrolimusweregenotypedforABCB1(exons12,21,and26)andCYP3A5(intron3).
Tacrolimustroughlevels,dose,ordose-adjustedtroughlevelsandcom-plicationswerecomparedamongpatientsduringtheearlystage(3,7,14,30,and90days)andupto1yearaccordingtothegenotypes.
ResultsAdonorsource-adjustedlinearmixedmodelwithmultilevelanalysisadjustingforage,bodyweight,hemato-crit,andserumcreatinineshowedthatCYP3A5genotypeisassociatedwithdose-adjustedleveloftacrolimus(pT),exon21(2677G>T/A),andexon26(3435C>T).
Thenon-synonymousSNPG2677T>A,andthesynonymousSNPC3435ThavebeenreportedtobecorrelatedwithcellularexpressionlevelsofABCB1[17]andthusmayinfluencethetacrolimuspharma-cokinetics.
However,theeffectoftheseABCB1polymor-phismsontacrolimuspharmacokineticsandclinicaloutcomesstillremainscontroversial[15,18–23]Moreover,littleisknownabouttheassociationofCYP3A5andABCB1genotypeswithtacrolimuspharmacokinetics,especiallywhenstratifiedbydonorsourcesintheearlystageoftrans-plantation.
Tostudytheseassociationswiththerepeatedmeasurementsoftacrolimusthatarelikelypositivelycorre-latedovertime,aflexibleyetversatilemodel,likealinearmixedeffect(LME)modelshouldbeusedfortheanalysis.
Likewise,whenanalysisofthefactorsassociatedwiththeclinicaloutcomesoftacrolimusthataretransientovertimeandcanbecorrelatedwithmanydifferentfactors,aregres-sionmodelsuchasthegeneralizedestimatingequation(GEE)modelisappropriatetoproduceefficientandunbi-asedregressionestimates.
Accordingly,thepurposeofthisstudywastoexplicatethelinearmixedeffectsmodelandGEEmodelasameansoftestingthefactorsthataccountfortheindividualvariationsinthepharmacokineticsandthecomplicationsoftacrolimusduringtheearlystage(3,17,14,30,and90days)andupto1yearaftertransplantationinKoreankidneytransplantrecipients.
MaterialsandmethodsPatientsDatafrom132renalallograftrecipientswhoreceivedkid-neytransplantationfromSeoulNationalUniversityHospi-tal,Seoul,Koreabetween2002and2008werereviewedretrospectively.
Patientsaged>18years,andsingleprimaryorsecondarykidneyrecipientspluscombinedorgantrans-plantationswereeligible.
TheinstitutionalreviewboardoftheClinicalResearchInstituteatSeoulNationalUniversityHospitalapprovedthestudyprotocol(IRBNo.
C-0609-014-182).
Writteninformedconsentwasobtainedfromallsub-jectsbeforebeingenrolledinthisstudy.
ImmunosuppressiveprotocolanddatacollectionAninitialoraldoseof0.
075–0.
1mg/kgtacrolimuswasadministeredtwicedailythedaybeforetransplantationforcadavericdonororgansrecipients,and2hbeforetransplan-tationinthecaseoflivinggrafts,whichwasthenadjustedaccordinglytothetacrolimustherapeuticdrugmonitoring(TDM).
Thedoseoftacrolimuswasadjustedtomaintainthetargetbloodtroughconcentrationof10–12ng/mLduringthefirstmonth.
Theconsequentwhole-bloodtargettroughconcentrationswereadjustedto15–20ng/mLduringthefirst5days,8–12ng/mLupto3months,6–8ng/mLupto6months,and4–6ng/mLthereafter.
Thesteroidswasgivenatastandardintravenousdoseof500mgmethylpredniso-loneatthetimeofsurgery,andwasthenwasgraduallytaperedtoamaintenancedoseof10mgby2weeksaftertransplantation.
Mycophenolatemofetil(MMF)wasadmin-isteredorallyatafixeddoseof1–1.
5g/day.
SomeofthepatientswhoreceivedMMFexperiencedgastrointestinaldisorders,whichledtoachangeintherapytoenteric-coatedmycophenolatesodium.
Otherconcomitantmedica-tionswerescreenedforpossibleCYP3A5andABCB1inter-actions.
Recipientswithcadavericdonorkidneys,positiveresultsonapanelreactiveantibodytest,and/orwhohadmorethanthreemismatchesofhumanleukocyteantigens,wereadministeredtwodoses(20mgeach)ofIVbasilix-imab(Novartis,EastHanover,NJ,USA)1hpreoperativelyandonday4aftertransplantation.
658EurJClinPharmacol(2012)68:657–669TacrolimusmeasurementsTacrolimustroughsamplesweretakenapproximately12hafterthelastdoseondays3,7,14,30,90,180,and360.
Whole-bloodtacrolimusconcentrationsweredeterminedusingamicroparticulateenzymeimmunoassay(IMxana-lyzer;TacrolimusII;AbbottDiagnostics,AbbottPark,IL,USA).
Dose-adjustedtroughconcentrationswerecalculatedbydividingtacrolimustroughconcentrationsbythecorresponding24-hdoseonmg/kgbasis.
Clinicalandsafetyfollow-upAtthetimeoftroughlevelsampling,acompletebiochem-icalbloodanalysiswasperformedtomeasurethelevelsofbloodurinenitrogen,serumcreatinine,electrolytes,glucose,hematologyparameters,lipids,andalbumin.
CreatinineclearancewascalculatedusingtheModificationofDietinRenalDiseaseStudyequation[24]andmeasuredfrom24-hurinecollections.
Patientsunderwentacompletephysicalexamination,includingstandardizedmeasurementofsystol-icanddiastolicbloodpressure,bodyweight,andvitalsigns.
Concomitantmedicationwasnotedandtheuseoflipid-loweringdrugs,antihypertensivedrugs,andanti-diabeticdrugsinparticular,wererecordedcarefully.
Allpatientsunderwentprotocolrenaltransplantbiopsiesattransplanta-tionand10daysaftertransplantation,atwhichtimeTDMcanbecomeeffectiveandthetargettacrolimustroughcon-centrationisachieved.
Recipientswith≥20%elevationsofserumcreatininelevelsunderwentultrasound-guidedpercu-taneousallograftbiopsiesonanydayaftertransplantation.
Acuteallograftrejection,calcineurininhibitor(CNI)-in-ducednephrotoxicityandchronicallograftnephropathywereexclusivelydiagnosedbasedonhistologicalfindingsbyanexperiencedrenalhistopathologistaccordingtothe1997Banffclassificationcriteria[25].
Patientswereconsid-eredtohavearterialhypertensionifsystolicand/ordiastolicbloodpressureexceeded140/90mmHgorwereonantihy-pertensivedrugs.
Recipientswereconsideredtohavehyper-lipidemiaifthefastingtotalcholesterollevelexceeded190mg/dL,low-densitylipoproteincholesterollevelwasabove110mg/dL,and/orthepatientswerereceivinganti-triglyceridedrugs.
Newonsetdiabetesmellitusaftertrans-plantation(NODAT)wasconfirmedthroughastandardoralglucosetolerancetestinpatientswithsuspectedhypergly-cemiaandwasdefinedastheuseofanti-diabeticdrugsformorethan6months[26].
IdentificationofgenotypesGenomicDNAwasisolatedfromwholevenousbloodusingtheQIAampDNAbloodkit(Qiagen,Valencia,CA,USA)accordingtothemanufacturer'sprotocol.
GenotypingforCYP3A5(6986A>G)(rs776746)andABCB1exon12(C1236T)(rs1128503)andexon26(C3435T)(rs1045642)SNPswasperformedusingtheTaqManallelicdiscriminationassaywithanABI7900HT(AppliedBio-systems,FosterCity,CA,USA)[23].
Polymerasechainreaction(PCR)wasprogrammedasfollows:95°Cfor15minfollowedby40cyclesat95°Cfor15sand60°Cfor1min.
TheABCB1exon21(G2677T/A)(rs2032582)regionwasamplifiedusingPCR.
PCRwasprogrammedasfollows:94°Cfor5minand40cyclesat94°Cfor30s,48°Cfor30sand72°Cfor1min,followedby72°Cfor7min.
Amplifiedproductswerepurifiedwith1.
2%agarosegelsusingPCRpurification(PCRquick-spin;iNtRONBiotechnol-ogy,Gyeonggi-do,Korea).
Wholesequencesofamplifiedfragmentswerethendetermined[18].
Boththepatientsandtheinvestigatorswereblindedtothepatients'geneticvariants.
PrimersandprobesweredesignedwithPrimerExpressSoftwareVersion3.
0(AppliedBiosystems)andareshowninTable1.
StatisticalanalysesQuantitativedatawererepresentedasfrequenciesandper-centages,whilequalitativedatawerepresentedasmean±standarddeviation.
TheMann–WhitneyUtestandKruskal–Wallistestwereusedtocomparecontinuousvariablesbe-tweengenotypegroupswithposthoctestingformultiplecomparisons.
CategoricalvariableswereanalyzedusingtheChi-squaredtestandFisher'sexacttest.
Weperformedlinearmixedmodelanalysesonrepeatedmeasuresoftacro-limustroughlevels,doses,anddose-adjustedtroughlevels.
Clinicalcovariatesappliedinthemodelwereage,sex,bodyweight,steroidandMMFdoses,hematocrit,serumcreati-nine,albumin,creatinineclearance,andconcomitantmedi-cations.
Linearmixedmodelanalyseswererepeated,adjustingthemeanfordonorsourceusingamultilevelmodelwithadjustmentsfortheaforementionedcovariates.
Analysisofvariancefollowedbymultipleregressionanal-ysiswasusedtoassessthecontributionofcorticosteroiddoseandotherclinicalcovariatestotacrolimustroughlev-els,dose,anddose-adjustedlevelsatdifferenttimepointsaftertransplantation.
Covariateswithapvalueof5%.
Noneofthesesixhaplotypeswasassociatedwithtacrolimusdose-adjustedtroughlevels.
Combinationsofthethreepredominanthap-lotypesshowedthatonlyonepatientcarrieddiplotypeTTT–TTTofABCB1.
TTThaplotypecarriersdisplayedlowertacrolimusdose-adjustedtroughlevelsthannon-TTTcar-riersonday3post-transplantation(p10ng/mLmustbeachieved[31]bydays2–3aftertransplantationtominimizegraftrejections[7].
PolymorphismsinABCB1andCYP3A5areexpectedtoaffectthisinterindividualvariabilityintacrolimuspharma-cokineticsandclinicaloutcomesintheearlystageoftrans-plantation.
Inourpatients,thetacrolimustroughconcentrationonday3variedwidely,rangingfrom6.
27to32.
00ng/mL.
Morespecifically,12patients(9%)hadTable7Univariateandmultivariatecoxproportionalhazardsanalysesforbiopsy-provenacuterejectionsVariablesUnivariateMultivariateHazardratio(95%CI)pvalueHazardratio(95%CI)pvalueMalegender(vsfemale)1.
196(0.
401~3.
579)0.
748––Age1.
052(1.
005~1.
102)0.
0311.
049(0.
999~1.
102)0.
054Bodyweight1.
010(0.
963~1.
059)0.
693––New–onsetdiabetesaftertransplantation(vsnodiabetes)0.
577(0.
181~1.
840)0.
352––Hypertension(vsnohypertension)0.
723(0.
095~5.
524)0.
754––Hyperlipidemia(vsnohyperlipidemia)0.
917(0.
205~4.
098)0.
910––Deceaseddonor(vslivingdonor)3.
250(1.
139~9.
272)0.
0282.
878(1.
007~8.
226)0.
048ABCB11236CA(vsCC)0.
478(0.
114~2.
001)0.
312––TT(vsCC)0.
962(0.
241~3.
848)0.
957––ABCB12677GA/GT(vsGG)0.
665(0.
183~2.
417)0.
536––AA/AT/TT(vsGG)0.
389(0.
040~3.
740)0.
413––ABCB13435CA(vsCC)0.
794(0.
256~2.
461)0.
689––TT(vsCC)1.
637(0.
330~8.
114)0.
546––CYP3A5*1/*3(vs*1/*1)0.
169(0.
011~2.
696)0.
208––*3/*3(vs*1/*1)1.
122(0.
146~8.
627)0.
912––RelativeriskofacuterejectionforeachvariableisgivenasHR(95%CI)andthepvalueforCoxproportionalhazardsanalysiswithcensoringforpatientdeath,graftfailure,andendoffollow-upTable8Associationbetweenclinicalcovariatesandtacrolimus-inducedcomplicationsaComplicationsVariablesUnivariateMultivariateEstimate95%CIpvalueEstimate95%CIpvalueDiabetes(n022)Tacrolimustroughlevel1.
0950.
998~1.
2020.
054–––Tremor(n014)Age1.
0460.
992~1.
1020.
097–––Hairloss(n034)Tacrolimustroughlevel1.
2431.
146~1.
34715μg/mL[39].
Ithasbeenreportedthattacrolimuscanincreasevery-low-densitylipoproteinandhigh-densitylipo-proteincholesterols[40],buttherewasnoreportsofacorrelationbetweendruglevelandserumlipids.
Inauni-variateanalysis,tacrolimustroughlevelswereexpectedtoincreasetheincidenceofNODATwithborderlinesignifi-cance(p00.
054).
OnecanthinkthatgenotypesofCYP3A5andABCB1maybeassociatedwithriskoftacrolimus-relatedcomplications.
However,nostudytodatehassuc-cessfullydemonstratedtheroleofCYP3A5*3orABCB1genotypesintacrolimus-relatedcomplications[16,41].
Hy-perlipidemiaoccurredapproximately3-foldhigherinCYP3A5*3/*3carrierscomparedwithCYP3A5*1carriersafteradjustingtacrolimustroughlevel(p00.
045).
Ofnote,andtoourknowledge,thisisthefirststudytodemonstratethatclinicalandgeneticfactorsareassociatedwithtacrolimuspharmacokineticsandclinicaloutcomesusingstatisticaltoolslikealinearmixedeffectmodelforrepeatedmeasurementsoftacrolimusovertimeandagen-eralizedestimatingequationmodeltoproduceefficientandunbiasedregressionestimates.
WehaveexplicatedthelinearmixedeffectsmodelandGEEmodelasameansoftestingtheassociationamongthecovariates,thepharmacokinetics,andthecomplicationsoftacrolimusduringtheearlystagesEurJClinPharmacol(2012)68:657–669667andupto1yearaftertransplantationinKoreankidneytransplantrecipients.
ThepossiblelimitationofourstudyisthatwecouldnotstudytheeffectsoftheABCB1TTT-TTThaplotypebecauseonlyonerecipientcarriedthisdiplotypeinourstudy.
More-over,wecouldnotfindadefinitiveassociationbetweenthegenepolymorphismofCYP3A5andcomplications,possiblyowingtotheeffortsofmaintainingsimilartroughlevelsoftacrolimuswithTDMamongstrecipientsofdifferentCYP3A5genotypes.
Additionally,theassayweusedforthedeterminationoftacrolimusmeasurementscouldnotdistinguishbetweenactiveandinactivemetabolites.
Anoth-erpossiblelimitationisthatourstudywasaretrospectivestudy.
AprospectivecontrolledstudywithalargernumberoftransplantrecipientsiswarrantedtofurtherconfirmtheclinicalsignificanceofCYP3A5andABCB1genotypesontransplantationoutcomesinpatientstreatedwithtacrolimus.
Inconclusion,age,bodyweight,hematocrit,serumcre-atininelevels,andCYP3A5genotypeswerefoundtobesignificantfactorsresponsibleforaffectingthetacrolimusdose-adjustedtroughlevels.
Additionally,transplantationofacadavericdonorkidneywasassociatedwithincreasedincidenceofacuterejections,whileatacrolimustroughlevelwasasignificantpredictingfactorfortacrolimus-relatedcomplicationsincludingalopeciaandhyperlipidemia.
Theclinicalandgeneticfactorswehaveidentifiedcouldcon-tributetotheindividualizationoftacrolimustreatmentandenhancedrugsafetyandresponse,helpingtoobtainade-quateimmunosuppressionwithoutincreasingtheriskofcomplicationsinkidneytransplantpatients.
AcknowledgementsThisworkwassupportedbyagrantfromtheNationalResearchFoundationofKorea,fundedbytheKoreangovern-mentMinistryofEducation,ScienceandTechnology(no.
2009–0081414)andbyagrantfromtheKoreaHealthtechnologyR&DProject,MinistryofHealth&Welfare,RepublicofKorea(A090930)andtheClinicalResearchInstitute(CRI),SeoulNationalUniversityHospital(no.
1120070027).
ConflictofinterestTheauthorsdeclarenocompetinginterests.
References1.
KershnerRP,FitzsimmonsWE(1996)RelationshipofFK506wholebloodconcentrationsandefficacyandtoxicityafterliverandkidneytransplantation.
Transplantation62(7):920–9262.
LaskowDA,VincentiF,NeylanJF,MendezR,MatasAJ(1996)Anopen-label,concentration-rangingtrialofFK506inprimarykidneytransplantation:areportoftheUnitedStatesMulticenterFK506KidneyTransplantGroup.
Transplantation62(7):900–9053.
VenkataramananR,SwaminathanA,PrasadT,JainA,ZuckermanS,WartyV,McMichaelJ,LeverJ,BurckartG,StarzlT(1995)Clinicalpharmacokineticsoftacrolimus.
ClinPharmacokinet29(6):404–4304.
ScottLJ,McKeageK,KeamSJ,PloskerGL(2003)Tacrolimus:afurtherupdateofitsuseinthemanagementoforgantransplanta-tion.
Drugs63(12):1247–12975.
VenkataramananR,JainA,WartyVS,Abu-ElmagdK,AlessianiM,LeverJ,KrajakA,FlowersJ,MehtaS,ZuckermanSetal(1991)PharmacokineticsofFK506intransplantpatients.
Trans-plantProc23(6):2736–27406.
NagaseK,IwasakiK,NozakiK,NodaK(1994)DistributionandproteinbindingofFK506,apotentimmunosuppressivemacrolidelactone,inhumanbloodanditsuptakebyerythrocytes.
JPharmPharmacol46(2):113–1177.
UndreNA,vanHooffJ,ChristiaansM,VanrenterghemY,DonckJ,HeemanU,KohnleM,ZankerB,LandW,MoralesJM,AndresA,SchaferA,StevensonP(1999)Lowsystemicexposuretotacrolimuscorrelateswithacuterejection.
TransplantProc31(1–2):296–2988.
MouradM,WallemacqP,DeMeyerM,BrandtD,VanKerkhoveV,MalaiseJ,ChaibEddourD,LisonD,HaufroidV(2006)TheinfluenceofgeneticpolymorphismsofcytochromeP4503A5andABCB1onstartingdose-andweight-standardizedtacrolimustroughconcentrationsafterkidneytransplantationinrelationtorenalfunction.
ClinChemLabMed44(10):1192–11989.
KuypersDR,ClaesK,EvenepoelP,MaesB,CoosemansW,PirenneJ,VanrenterghemY(2004)Time-relatedclinicaldetermi-nantsoflong-termtacrolimuspharmacokineticsincombinationtherapywithmycophenolicacidandcorticosteroids:aprospectivestudyinonehundreddenovorenaltransplantrecipients.
ClinPharmacokinet43(11):741–76210.
MouradM,MouradG,WallemacqP,GarrigueV,VanBellingenC,VanKerckhoveV,DeMeyerM,MalaiseJ,EddourDC,LisonD,SquiffletJP,HaufroidV(2005)SirolimusandtacrolimustroughconcentrationsanddoserequirementsafterkidneytransplantationinrelationtoCYP3A5andMDR1polymorphismsandsteroids.
Transplantation80(7):977–98411.
ChristiansU,JacobsenW,BenetLZ,LampenA(2002)Mecha-nismsofclinicallyrelevantdruginteractionsassociatedwithtacro-limus.
ClinPharmacokinet41(11):813–85112.
SaekiT,UedaK,TanigawaraY,HoriR,KomanoT(1993)HumanP-glycoproteintransportscyclosporinAandFK506.
JBiolChem268(9):6077–608013.
HesselinkDA,vanGelderT,vanSchaikRH(2005)Thepharma-cogeneticsofcalcineurininhibitors:onestepclosertowardindi-vidualizedimmunosuppressionPharmacogenomics6(4):323–33714.
KuehlP,ZhangJ,LinY,LambaJ,AssemM,SchuetzJ,WatkinsPB,DalyA,WrightonSA,HallSD,MaurelP,RellingM,BrimerC,YasudaK,VenkataramananR,StromS,ThummelK,BoguskiMS,SchuetzE(2001)SequencediversityinCYP3ApromotersandcharacterizationofthegeneticbasisofpolymorphicCYP3A5expression.
NatGenet27(4):383–39115.
MacPheeIA,FredericksS,TaiT,SyrrisP,CarterND,JohnstonA,GoldbergL,HoltDW(2004)Theinfluenceofpharmacogeneticsonthetimetoachievetargettacrolimusconcentrationsafterkidneytransplantation.
AmJTransplant4(6):914–91916.
NumakuraK,SatohS,TsuchiyaN,HorikawaY,InoueT,KakinumaH,MatsuuraS,SaitoM,TadaH,SuzukiT,HabuchiT(2005)Clinicalandgeneticriskfactorsforposttransplantdiabetesmellitusinadultrenaltransplantrecipientstreatedwithtacrolimus.
Transplan-tation80(10):1419–142417.
HoffmeyerS,BurkO,vonRichterO,ArnoldHP,BrockmollerJ,JohneA,CascorbiI,GerloffT,RootsI,EichelbaumM,BrinkmannU(2000)Functionalpolymorphismsofthehumanmultidrug-resistancegene:multiplesequencevariationsandcorrelationofoneallelewithP-glycoproteinexpressionandactivityinvivo.
ProcNatlAcadSciUSA97(7):3473–347818.
AnglicheauD,FlamantM,SchlageterMH,MartinezF,CassinatB,BeauneP,LegendreC,ThervetE(2003)Pharmacokinetic668EurJClinPharmacol(2012)68:657–669interactionbetweencorticosteroidsandtacrolimusafterrenaltransplantation.
NephrolDialTransplant18(11):2409–241419.
HaufroidV,MouradM,VanKerckhoveV,WawrzyniakJ,DeMeyerM,EddourDC,MalaiseJ,LisonD,SquiffletJP,WallemacqP(2004)TheeffectofCYP3A5andMDR1(ABCB1)polymor-phismsoncyclosporineandtacrolimusdoserequirementsandtroughbloodlevelsinstablerenaltransplantpatients.
Pharmaco-genetics14(3):147–15420.
WangW,ZhangXD,MaLL,LuYP,HuXP,ZhangP,WangY,GuanDL(2005)RelationshipbetweenMDR1genepolymorphismandbloodconcentrationoftacrolimusinrenaltransplantpatients.
ZhonghuaYiXueZaZhi85(46):3277–328121.
RoyJN,BaramaA,PoirierC,VinetB,RogerM(2006)Cyp3A4,Cyp3A5,andMDR-1geneticinfluencesontacrolimuspharmaco-kineticsinrenaltransplantrecipients.
PharmacogenetGenomics16(9):659–66522.
KuypersDR,deJongeH,NaesensM,LerutE,VerbekeK,VanrenterghemY(2007)CYP3A5andCYP3A4butnotMDR1single-nucleotidepolymorphismsdeterminelong-termtacrolimusdispositionanddrug-relatednephrotoxicityinrenalrecipients.
ClinPharmacolTher82(6):711–72523.
HesselinkDA,vanSchaikRH,vanAgterenM,deFijterJW,HartmannA,ZeierM,BuddeK,KuypersDR,PisarskiP,LeMeurY,MamelokRD,vanGelderT(2008)CYP3A5genotypeisnotassociatedwithahigherriskofacuterejectionintacrolimus-treatedrenaltransplantrecipients.
PharmacogenetGenomics18(4):339–34824.
LeveyAS,BoschJP,LewisJB,GreeneT,RogersN,RothD(1999)Amoreaccuratemethodtoestimateglomerularfiltrationratefromserumcreatinine:anewpredictionequation.
ModificationofDietinRenalDiseaseStudyGroup.
AnnInternMed130(6):461–47025.
RacusenLC,SolezK,ColvinRB,BonsibSM,CastroMC,CavalloT,CrokerBP,DemetrisAJ,DrachenbergCB,FogoAB,FurnessP,GaberLW,GibsonIW,GlotzD,GoldbergJC,GrandeJ,HalloranPF,HansenHE,HartleyB,HayryPJ,HillCM,HoffmanEO,HunsickerLG,LindbladAS,YamaguchiYetal(1999)TheBanff97workingclassificationofrenalallograftpathol-ogy.
KidneyInt55(2):713–72326.
DavidsonJ,WilkinsonA,DantalJ,DottaF,HallerH,HernandezD,KasiskeBL,KiberdB,KrentzA,LegendreC,MarchettiP,MarkellM,vanderWoudeFJ,WheelerDC(2003)New-onsetdiabetesaftertransplantation:2003Internationalconsensusguide-lines.
Proceedingsofaninternationalexpertpanelmeeting.
Barcelona,Spain,19February2003.
Transplantation75(10Suppl):SS3–SS2427.
JunKR,LeeW,JangMS,ChunS,SongGW,ParkKT,LeeSG,HanDJ,KangC,ChoDY,KimJQ,MinWK(2009)TacrolimusconcentrationsinrelationtoCYP3AandABCB1polymorphismsamongsolidorgantransplantrecipientsinKorea.
Transplantation87(8):1225–123128.
HustertE,HaberlM,BurkO,WolboldR,HeYQ,KleinK,NuesslerAC,NeuhausP,KlattigJ,EiseltR,KochI,ZibatA,BrockmollerJ,HalpertJR,ZangerUM,WojnowskiL(2001)ThegeneticdeterminantsoftheCYP3A5polymorphism.
Pharmacogenetics11(9):773–77929.
HesselinkDA,vanGelderT,vanSchaikRH,BalkAH,vanderHeidenIP,vanDamT,vanderWerfM,WeimarW,MathotRA(2004)Populationpharmacokineticsofcyclosporineinkidneyandhearttransplantrecipientsandtheinfluenceofethnicityandge-neticpolymorphismsintheMDR-1,CYP3A4,andCYP3A5genes.
ClinPharmacolTher76(6):545–55630.
BarniehL,MannsBJ,KlarenbachS,McLaughlinK,YilmazS,HemmelgarnBR(2011)Adescriptionofthecostsoflivingandstandardcriteriadeceaseddonorkidneytransplantation.
AmJTransplant11(3):478–48831.
StaatzC,TaylorP,TettS(2001)Lowtacrolimusconcentrationsandincreasedriskofearlyacuterejectioninadultrenaltransplan-tation.
NephrolDialTransplant16(9):1905–190932.
WynneHA,CopeLH,MutchE,RawlinsMD,WoodhouseKW,JamesOF(1989)Theeffectofageuponlivervolumeandapparentliverbloodflowinhealthyman.
Hepatology9(2):297–30133.
SotaniemiEA,ArrantoAJ,PelkonenO,PasanenM(1997)AgeandcytochromeP450-linkeddrugmetabolisminhumans:ananaly-sisof226subjectswithequalhistopathologicconditions.
ClinPhar-macolTher61(3):331–33934.
WijnenRM,EriczonBG,TieboschAT,BeysensAJ,GrothCG,KootstraG(1991)ToxicityofFK506inthecynomolgusmonkey:noncorrelationwithFK506serumlevels.
TransplantProc23(6):3101–310435.
ShirbachehMV,JonesJW,HarralsonTA,EdelsteinJ,TecimerT,BreidenbachWC,JevansAW,MaldonadoC,BarkerJH,GruberSA(1999)Pharmacokineticsofintra-arterialdeliveryoftacrolimustovascularlyisolatedrabbitforelimb.
JPharmacolExpTher289(3):1196–120136.
PerroneRD,MadiasNE,LeveyAS(1992)Serumcreatinineasanindexofrenalfunction:newinsightsintooldconcepts.
ClinChem38(10):1933–195337.
LeblondF,GuevinC,DemersC,PellerinI,Gascon-BarreM,PichetteV(2001)DownregulationofhepaticcytochromeP450inchronicrenalfailure.
JAmSocNephrol12(2):326–33238.
StaatzCE,TettSE(2005)Pharmacokineticconsiderationsrelatingtotacrolimusdosingintheelderly.
DrugsAging22(7):541–55739.
FinkJ,FlasarM,HarringtonJ,WeirM,BartlettS(1998)Alopeciatotalisrelatedtotacrolimususefollowingkidneyandpancreastransplantation.
[abstractpresentedatAST1998].
1998[cited2010May122010];Availablefrom:http://www.
a-s-t.
org/abstracts98/abs661.
htm40.
IchimaruN,TakaharaS,KokadoY,WangJD,HatoriM,KameokaH,InoueT,OkuyamaA(2001)Changesinlipidmetabolismandeffectofsimvastatininrenaltransplantrecipientsinducedbycyclosporineortacrolimus.
Atherosclerosis158(2):417–42341.
YamauchiA,IeiriI,KataokaY,TanabeM,NishizakiT,OishiR,HiguchiS,OtsuboK,SugimachiK(2002)Neurotoxicityinducedbytacrolimusafterlivertransplantation:relationtogeneticpoly-morphismsoftheABCB1(MDR1)gene.
Transplantation74(4):571–572EurJClinPharmacol(2012)68:657–669669
- Statisticalwww.666abcd相关文档
- 中小学www.666abcd
- 中小学www.666abcd
- 数据www.666abcd
- 公司www.666abcd
- inequalitywww.666abcd
- 公司www.666abcd
CloudCone月付$48,MC机房可小时付费
CloudCone商家在前面的文章中也有多次介绍,他们家的VPS主机还是蛮有特点的,和我们熟悉的DO、Linode、VuLTR商家很相似可以采用小时时间计费,如果我们不满意且不需要可以删除机器,这样就不扣费,如果希望用的时候再开通。唯独比较吐槽的就是他们家的产品太过于单一,一来是只有云服务器,而且是机房就唯一的MC机房。CloudCone 这次四周年促销活动期间,商家有新增独立服务器业务。同样的C...

乌云数据(10/月),香港cera 1核1G 10M带宽/美国cera 8核8G10M
乌云数据主营高性价比国内外云服务器,物理机,本着机器为主服务为辅的运营理念,将客户的体验放在第一位,提供性价比最高的云服务器,帮助各位站长上云,同时我们深知新人站长的不易,特此提供永久免费虚拟主机,已提供两年之久,帮助了上万名站长从零上云官网:https://wuvps.cn迎国庆豪礼一多款机型史上最低价,续费不加价 尽在wuvps.cn香港cera机房,香港沙田机房,超低延迟CN2线路地区CPU...
€4.99/月Contabo云服务器,美国高性价比VPS/4核8G内存200G SSD存储
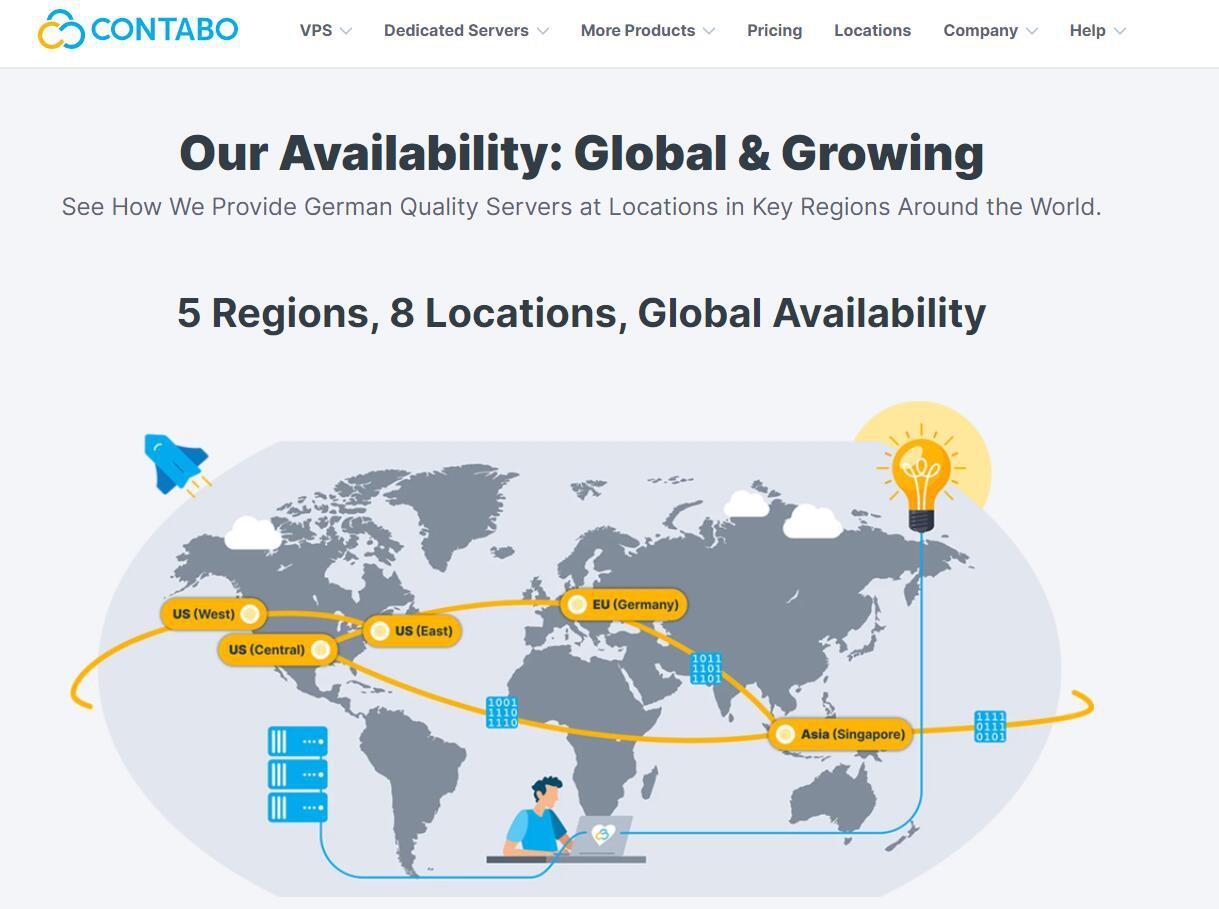
Contabo是一家运营了20多年的欧洲老牌主机商,之前主要是运营德国数据中心,Contabo在今年4月份增设新加坡数据中心,近期同时新增了美国纽约和西雅图数据中心。全球布局基本完成,目前可选的数据中心包括:德国本土、美国东部(纽约)、美国西部(西雅图)、美国中部(圣路易斯)和亚洲的新加坡数据中心。Contabo的之前国外主机测评网站有多次介绍,他们家的特点就是性价比高,而且这个高不是一般的高,是...
www.666abcd为你推荐
-
firetrap牛仔裤的四大品牌是那几个啊?甲骨文不满赔偿工作不满半年被辞退,请问赔偿金是怎么算的?原代码源代码是什么同ip域名同IP网站具体是什么意思,能换独立的吗16668.com香港最快开奖现场直播今晚开dadi.tv智能网络电视smartTV是什么牌子月风随笔关于中秋作文干支论坛天干地支性间道什么是性高潮?或者怎么才算是呢?男女的carlymilobaby milo是什么品牌