preparedwww.55fang.com
www.55fang.com 时间:2021-04-08 阅读:()
2010WILEY-VCHVerlagGmbH&Co.
KGaA,Weinheim4814www.
advmat.
dewww.
MaterialsViews.
comCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818ByYuhuaXue,HongxiaWang,YanZhao,LimingDai,LianfangFeng,XungaiWang,andTongLin*MagneticLiquidMarbles:A"Precise"MiniatureReactor[*]Dr.
Y.
Xue,Dr.
H.
Wang,Dr.
Y.
Zhao,Prof.
X.
Wang,Prof.
T.
LinCentreforMaterialandFibreInnovationDeakinUniversityGeelong,VIC3217(Australia)E-mail:tong.
lin@deakin.
edu.
auProf.
L.
DaiDepartmentofChemicalEngineeringCaseWesternReserveUniversityCleveland,Ohio44106(USA)Prof.
L.
FengStateKeyLaboratoryofChemicalEngineeringDepartmentofChemicalandBiologicalEngineeringZhejiangUniversityHangzhou310027(P.
R.
China)DOI:10.
1002/adma.
201001898Miniaturizedchemicalprocesseshavemanyadvantages,suchasreduceduseofchemicalreagentsandsolvents,preciselycontrolledreactionconditions,muchshortenedreactiontime,andtheabilitytointegrateintoadigitaldevice.
[1–4]Theyareveryusefulforhigh-throughputanalysesandpuricationsinchemicalandbiologicalprocesses,suchasdrugdiscovery,[5]DNAanalysis,[6,7]proteincrystallization,[8]andthesynthesisofmoleculesorparticles.
[9–11]Indeed,considerableeffortshavebeenmadetominiaturizechemicalprocessesusingvariousprinciples.
Forexample,oil/wateremulsionshavebeenusedtoperformchemicalreactionsinparallelinalargenumberofemulsiedtinydroplets.
[10]Microuidic"lab-on-a-chip"deviceshavebeendesignedtomanipulatechemicalprocesseswithinmicro-channelseitherincontinuousuidordisconnecteduidsegmentsseparatedbyanimmiscibleuidorgas.
[10,12]Althoughthechannel-basedmicro-reactorsshowgreatpoten-tialinhigh-throughputreactionandintegratabilitytoexternalanalysisfacilities,thereaction-specicdevicepreparations,thechannel-associatedcross-contaminations,andtheneedforexternalpumpstoactuatetheuidmotionmakethemneitheruniversalnorminiature.
Also,microuidicsishardtousetohandleasingledroplet.
Recently,freeaqueousdropletsinanimmiscibleuid(channel-freemicrouidics)[9]hasalsobeenproposedasanalternativestrategy,butcross-contaminationstillremainsaconcern.
Inlivingsystems,cellsarewell-knownasthebasicstructuralandfunctionalunits[13]thatmediatevariousbiochemicalreac-tions(e.
g.
,catabolismandanabolism)forsustainingthegrowthanddivisionofcellsandhencelivingsystems.
Althoughtheirstructurelooksverysimple,cellscommunicatewitheachotherinahighlyintelligentway,throughoutthewholelivingsystem.
Inthisregard,howtomimiclivingcells,bothinstructureandfunction,istheultimatechallengetowardthedevelopmentofarticialminiaturereactors.
Liquidmarbles,liquiddropsencapsulatedwithsolidpowderattheliquid–gasinterface,haverecentlybeenreportedasanewapproachtomanipulatingliquid,[14]whichcouldbeusedasminiaturereactors.
Theyarenon-wettingtoanysolidsur-facesandcouldbemanipulatedbyexternalforces,suchasgravity,[14]electrical,[15]andmagnetic,[16]dependingontheircomponents.
[14–19]However,mostliquidmarblesreportedsofarhavebeenbasedonaqueousliquid.
Althoughafewpapers[19]havereportedtheencapsulationoforganicliquidsintoliquidmarbles,theliquidswereconnedtothosehavingahighsur-facetension.
[19]Theformationofstableliquidmarblesfromawiderangeoforganicsolvents,especiallythosethathavealowsurfacetension,isimportantforchemicalreactions,butstillremainsachallenge.
Inthisstudy,wedemonstratetheincredibleabilityofauor-inateddecylpolyhedraloligomericsilsesquioxane(FD-POSS,structureshowninFigure1a)anditscombinationwithhydro-phobicmagneticnanoparticlestoactasencapsulatingagentstopreparestableliquidmarblesfrombothaqueoussolutionandorganicliquidswithasurfacetensionaslowas20.
1dynecm1,andthefeasibilityofusingthemagneticliquidmarblesasuni-versal"smart"miniaturereactors.
FD-POSSwassynthesizedbythereportedmethod[20,21]andaneFD-POSSpowderwaspreparedbyagrindingtechnique.
TheFD-POSS/Fe3O4nanoparticlecompositepowdersofdif-ferentratios(1:10to1:1,w/w)werepreparedbyco-dispersingtheneFD-POSSpowderwithhydrophobicFe3O4nanopar-ticlesinethanol.
AsshowninthephotographsinFigure1b,FD-POSSpowdersarewhiteandhaveacertaintransparencytovisiblelightbutarenotresponsivetoamagneticeld.
Inethanol,FD-POSSandFe3O4nanoparticleshadastrongafnity,andtheytendedtoaggregateintopowderswhichshowedasuperparamagneticproperty(SupportingInfor-mation).
TheopticalmicrographsinFigure1cindicatethatFD-POSSandFD-POSS/Fe3O4compositepowdersarelessthan70μm,andtheFD-POSSandFe3O4nanoparticlesformedarandomlyblendedcompositestructure.
Whenathinbedofthepowderswerecoatedonasolidsurface,thesurfacebecamehighlyrepellenttoliquids.
Water,dimethylsulfoxide(DMSO),toluene,hexadecane,andethanolallformedrounddropletsonthepowdercoatedsurfacewithoverallcontactanglesof171.
1°,166.
2°,155.
7°,154.
4°,and142.
8°,respectively(Figure1d).
Thepowderlayershowedstrongrepellencetootherorganicsolvents(SupportingInformation).
ThecompositepowdersinthestudiedFD-POSS/Fe3O4ratiorangeshowedaverysimilaruidrepellencytothepureFD-POSSgrains.
Liquidmarbleswerepreparedsimplybydroppingasmallvolumeofliquidontothepowdersurfaceandsubsequentlyshakingthedropletgentlysothatthepowderscoveredtheentiredropletsurface.
Theas-preparedpowders,bothFD-POSS4815www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818Severalwaterdropletswereaddedtotheopenedmarble(10μL)toformawater-in-oilbicomponentliquidmarble.
Oil-in-waterbicomponentliquidmarblescanalsobeproducedinasimilarway.
Thesebicomponentliquidmarblesshowtheabilitytopre-ventthecoreliquidfromrapidevaporation.
Todemonstratetheabilitytomediatechemicalreactions,weusedtwodifferentwaystoundertakeachemiluminescencereaction.
[22,23]Onemethodusedtwomagneticliquidmarblestocarrydifferentreactiveagents,oneforhydrogenperoxideandtheotherforbis(2,4,6-trichlorophenyl)oxalateanddye.
AsshowninFigure2f(toppanel),thetwoliquidmarbleswereactuatedunderamagneticeldtomovetogetherandtheythencoalescedintoalargerliquidmarble,initiatingthechemilumi-nescencereactionwhichcanbeprovenbyanimmediatelightemissionfromthecoalescedliquidmarble.
Anothermethodinvolvedusingabicomponentliquidmarbletohosttwocoredroplets,onefortheoxidantandtheotherfortheuorescer,inanimmiscibleuid.
Undergentlemovementactuatedbyamagnetbar,thetwocoredropletsjointedtogethertriggeringthechemiluminescencereaction.
TheimageinFigure2f(bottompanel)clearlyindicatesthelightemittingjustfromthecombinedcoredroplet.
Besidesthechemiluminescencereaction,otherchemicalreactionssuchasphotochemicalpolymerization,nanoparticlesynthesis,andanacid–basereactionhavealsobeentrialedandproventobesuccessful(SupportingInformation).
Oneimportantaspectforminiaturechemicalreactorsistheabilitytointegratewithpuricationandanalysisfacilities.
SincethemagneticliquidmarblescanbeopenedandclosedreversiblyandFD-POSS/Fe3O4composite,canencapsulatenotonlywaterbutalsoorganicsolventstoformstableliquidmarbles.
Figure2ashowsphotosofliquidmarblespreparedfromwater,DMSO,toluene,ethanol,andoctane(3μL).
Manyotherorganicsol-ventshavealsobeentestedsofarandthepowderswereabletoencapsulatealiquidofsurfacetensionaslowas20.
1dynescm1(SupportingInformation).
Theliquidmarblesareverystableandtheycanoatnotonlyonwaterbutalsoonauidoflowsurfacetension(Figure2b).
ThesuperparamagneticpropertyoftheFe3O4nanoparticlesimpartstheFD-POSS/Fe3O4powder-encapsulatedliquidmar-bleswithamagnetresponsiveability.
Asaresult,theliquidmarblescanbeactuatedtomoveindifferentdirections,oropenedandclosedreversibly,underamagneticeld.
Figure2cshowsmagnetopenedliquidmarblesfromdifferentliquids.
Theopenedliquidmarbleallowsittobecoveredwithanothertypeofhydrophobicmaterial.
Todemonstratethis,weusedpureFD-POSSpowdertocovertheopenedliquidsurfaceofamagneticliquidmarble,andthecoveredpartshowedadifferentcolortothemagneticpowdercoveredsurface.
Consequently,themotionofliquidcanbetracedeasily.
Figure2dillustratesthemotionofawhiteFD-POSS-stainedFD-POSS/Fe3O4hexa-decanemarbleunderamagneticeld.
Itcanbeclearlyseenthatthemarblerotatesonasolidsurface(toppanel),butshiftssidewaysonaliquidsurface(bottompanel).
Themagnetopenedliquidmarblealsoallowstheliquidtobetakenfromthemarbleorthemarbletobereplenishedwithotherliquids.
Figure2edemonstratestheadditionofcolorfulwaterdropletsintoamagnetopenedhexadecanemarble.
Figure1.
a)StructureofFD-POSS.
b)ProcesstomaketheFD-POSS/Fe3O4nanoparticlecomposite.
Theinsertimagetotherightshowsthesuspen-sionsofDP-POSSandtheFD-POSS/Fe3O4nanoparticlecompositepowdersinsolventandtheFD-POSS/Fe3O4underamagneticeld.
c)OpticalmicroscopyimagesofFD-POSSandFD-POSS/Fe3O4powders(FD-POSS:Fe3O4=1:1,w/w).
d)Water,dimethylsulfoxide(DMSO),toluene,hexade-cane,andethanoldropletsontheFD-POSS/Fe3O4powder-coatedsiliconsurface.
4816www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818thatpushestheliquidoutofthepowderlayer.
Whetheraliquidmarblecanremainstableonasolidsurfaceornotisdeterminedbytheabilityofthenon-wettingpowderlayertopreventtheliquidbreakthrough(i.
e.
,breakthroughpressure,Pbreakthrough)andtheactualliquidpressuregeneratedbytheliquiddroplet(Pdroplet).
IfPbreakthroughPdroplet,theactualpressuregeneratedbytheliquiddropletisnotenoughtoforcethroughthepowderlayerhencetheliquidmarbleisstable.
WhenPdropletisclosetoPbreakthrough,itiseasyfortheliquidtobreakthepowderlayer,whichleadstobreakageoftheliquidmarble.
Basedonthecapillaryforceandtheliquidpressurethatforcesliquidtosagintothepowderypores(Figure3b,c),theratiobetweenthebreakthroughpressureandthemaximumliquidpressureformedbythedroplet(Pmaxdroplet=Dgl,whereρisthedensityoftheliquid,gistheconstantduetogravity,andl=γlv/ρgiscapillarylengthoftheliquid),PbreakthroughPmaxdroplet/,canbeestimatedas:PbreakthroughPmaxdroplet=A=H.
TH+T/(1)byamagneticeld,thereactionliquidcanberemovedeasilybyacapillarydeviceforfurtheranalysesordirectlytestedbyanopticalspectrometer(e.
g.
,UVanduorescentspectrom-eters).
Figure2galsoshowsthedirectpuricationofareac-tionproductthroughanarrowchromatographicaluminasheetpluggedintotheopenedliquidmarble.
Theabilityofahydrophobicpowdertorepelliquiduidismainlydeterminedbythesurfacechemistryandphysicaltopologyofthepowdermaterial,thesurfacetensionofaliquid,andthecontactmodebetweentheliquidandpowder.
TheFD-POSSmoleculecompriseseightuorinateddecylgroupswhichinclude136uorineatomssurroundingthePOSScage,makingthemoleculeextremelylowinsurfacefreeenergy.
Theroughsurfaceattributabletotheparticulatestructurefacilitatestheformationofanon-wettingpowderysurface.
[24–26]Inthestaticstate,theencapsulatingpowdersbetweenthedropletandthesolidsupport(Figure3a)receivethelargestliquidpressure.
Becauseofthenon-wettingfeature,theliquidonapowderyporouslayerreceivesanegativecapillarypressureFigure2.
a)DigitalgraphicimagesofliquidmarblesproducedfromdifferentliquidsandFD-POSSpowder(dropletsize3μL).
Foreasyobservation,asmallamountofdyewasaddedtotheliquidandtheexistenceofthedyehasnoinuenceonthestabilityoftheliquidmarbles.
b)Liquidmarblesoatingonwaterandhexadecanesurfaces(dropletsize3μL).
c)Magnetopenedliquidmarbles(dropletsize7μL).
d)Magnet-drivenmotionsofaFD-POSS-stainedFD-POSS/Fe3O4hexadecanemarbleonaglassslide(top)andwatersurface(bottom)(dropletsize7μL).
e)Magnetopenedhexa-decanemarbleswithdifferentnumbersofcoloredwaterdropletsadded(overalldropletsize10μL).
f)Achemiluminescencereactionthatoccursasaresultofcoalescingoftwomagneticliquidmarblesthatcontaindifferentreagents(top),andthesamechemiluminescencereactionhappeningwithinasingleliquidmarble(bottom)(dropletsize10μL).
g)Chromatographicanalysisofliquidintheopenedliquidmarble(dropletsize10μL).
Scalebar:1mm.
4817www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818liquids.
Magneticmanipulationenablestheliquidinthemarbletocommunicatewiththeoutsideenvironmentondemand,whichprovidesasimplewaytointegratewithportablepurica-tionandanalysisfacilities.
Allthesefeaturescouldleadtonewuniversal"lab-on-chip"reactorsystemswithexcellentintegrationabilitytoincorporatesamplefabrication,actuation,purication,andanalysisintoasingleelectronicchipfordiverseapplications.
ExperimentalSectionPreparationofFD-POSS/Fe3O4NanoparticleCompositePowders:(1H,1H,2H,2H-heptadecauorodecyl)8Si8O12(FD-POSS),synthesizedaccordingtotheliteraturemethod,[20]andhydrophobicFe3O4nanoparticles,preparedbyaliteraturemethod,[16]weredispersedin20mLofethanolandcontinuouslystirredfor24h.
Themixturewasthenisolatedfromthesolutionwithabarmagnetanddriedundervacuumat60°C.
PreparationandMagneticManipulationofLiquidMarbles:LiquidmarbleswerepreparedbydroppingliquiddropletsontoabedofFD-POSSorFD-POSS/Fe3O4nanoparticlegrains.
Upongentleshakingofthedroplet,thesolidpowdersbecamestucktothedropletsurfaceformingapowder-encapsulatedliquiddroplet(i.
e.
,liquidmarble).
Magneticmanipulationoftheliquidmarbleswasperformedusinganeodymiumcylindermagnet(diameter10mmandlength12mm).
Thedirectedmovementoftheliquidmarbleswasrecordedusingadigitalmicroscope(Dino-LiteAM313).
SupportingInformationSupportingInformationisavailablefromtheWileyOnlineLibraryorfromtheauthor.
H=2(1cosθ)lcapD(2√3D2ππ)(2)T=Blcapsin(2Rmin)R(2√3DB)(3)HandTaretherobustnessheightandangledeterminedbytheliquid–gassurfacetension(γlv),theparticleradius(R),theinterparticledistance(2D),andtheapparentcontactangleofliquidonthepowdermaterial(θ)(fordetailsseetheSupportingInformation).
ThedependencyofPbreakthroughPmaxdroplet/onthepar-ticleradiusandthehalfinter-particledistanceforthepowderarrangementisgiveninFigure3d(thecalculationontheotherparticlearrangementisgivenintheSupportingInformation).
ItisinterestingtonotethatamaximumPbreakthroughPmaxdroplet/ratiooccurswhenthepowderradiusisabout35μmandtheinter-powderdistanceissmall.
Basedontheactualpowderdimen-sionandsurfaceproperties,thePbreakthroughPmaxdroplet/Dforthreestudiedliquidsystemswascomputed(Figure3e).
Whenthisinter-powderdistanceislessthan160μm(D<80μm),thePbreakthroughPmaxdroplet/islargerthanunityeveniftheliquidsurfacetensionisverylow,suggestingtheformationofaverystableliquidmarble.
Insummary,wehavedemonstratedthepotentialofusinguorinatedPOSSandmagneticnanoparticlesasauniversalencapsulatingagenttoprepareliquidmarblesfromvariousFigure3.
a)Idealizedliquidmarblewithasinglelayerofpowderscoveringthedropletsurface.
b)Fluidsaggingintotwoparticlesandthedimen-sionsforcalculationofthebreakthroughpressure.
c)Onepowderarrangementusedforcalculationofbreakthroughpressure.
d)DependenciesofPbreakthroughPmax/dropletratioonRandDforoctane(θ=55°).
e)PbreakthroughPmax/dropletDdependencycalculatedbasedontheactualpowderdimensionforwater(θ=120°),hexadecane(θ=80°),andoctane.
4818www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818AcknowledgementsTheauthorsacknowledgethefundingsupportfromDeakinUniversityundertheAlfredDeakinPostdoctoralFellowshipschemeandStateKeyLaboratoryofChemicalEngineering(SKL-ChE-09D05).
Received:May22,2010Publishedonline:September5,2010[1]K.
Jensen,Nature1998,393,735.
[2]A.
J.
deMello,Nature2006,442,394.
[3]S.
-Y.
Teh,R.
Lin,L.
-H.
Hung,A.
P.
Lee,LabChip2008,8,198.
[4]M.
Abdelgawad,A.
R.
Wheeler,Adv.
Mater.
2009,21,920.
[5]P.
S.
Dittrich,A.
Manz,Nat.
Rev.
DrugDiscov.
2006,5,210.
[6]R.
H.
Liu,J.
Yang,R.
Lenigk,J.
Bonanno,P.
Grodzinski,Anal.
Chem.
2004,79,1824.
[7]M.
A.
Burns,B.
N.
Johnson,S.
N.
Brahmasandra,K.
Handique,J.
R.
Webster,M.
Krishnan,T.
S.
Sammarco,P.
M.
Man,D.
Jones,D.
Heldsinger,C.
H.
Mastrangelo,D.
T.
Burke,Science1998,282,484.
[8]B.
Zheng,L.
S.
Roach,R.
F.
Ismagilov,J.
Am.
Chem.
Soc.
2003,125,11170.
[9]O.
D.
Velev,B.
G.
Prevo,K.
H.
Bhatt,Nature2003,426,515.
[10]M.
Joanicot,A.
Ajdari,Science2005,309,887.
[11]H.
Song,D.
L.
Chen,R.
F.
Ismagilov,Angew.
Chem.
Int.
Ed.
2006,45,7336.
[12]A.
Günther,M.
Jhunjhunwala,M.
Thalmann,M.
A.
Schmidt,K.
F.
Jensen,Langmuir2005,21,1547.
[13]B.
Alberts,A.
Johnson,J.
Lewis,M.
Raff,K.
Roberts,P.
Walter,MolecularBiologyoftheCell,GarlandScience,NewYork2002.
[14]P.
Aussillous,D.
Quere,Nature2001,411,924.
[15]P.
Aussillous,D.
Quere,Proc.
R.
Soc.
A2006,462,973.
[16]Y.
Zhao,J.
Fang,H.
Wang,X.
Wang,T.
Lin,Adv.
Mater.
2010,22,707.
[17]A.
V.
Rao,M.
M.
Kulkarni,S.
D.
Bhagat,J.
ColloidInterfaceSci.
2005,285,413.
[18]G.
McHale,D.
L.
Herbertson,S.
J.
Elliott,N.
J.
Shirtcliffe,M.
I.
Newton,Langmuir2007,23,918.
[19]L.
Gao,T.
J.
Mccarthy,Langmuir2007,23,10445.
[20]J.
M.
Mabry,A.
Vij,S.
T.
Iacono,B.
D.
Viers,Angew.
Chem.
Int.
Ed.
2008,47,4137.
[21]A.
Tuteja,w.
Choi,M.
Ma,J.
M.
Mabry,S.
A.
Mazzella,G.
C.
Rutledge,G.
H.
Mckinley,R.
E.
Cohen,Science2007,318,1618.
[22]R.
B.
Thompson,S.
E.
S.
McBee,Langmuir1988,4,106.
[23]R.
E.
Milofsky,J.
W.
Birks,J.
Am.
Chem.
Soc.
1991,113,9715.
[24]X.
Feng,L.
Jiang,Adv.
Mater.
2006,18,3063.
[25]P.
Roach,N.
J.
Shirtcliffe,M.
I.
Newton,SoftMatter2008,4,224.
[26]X.
Zhang,F.
Shi,J.
Niu,Y.
Jiang,Z.
Wang,J.
Chem.
Mater.
2008,18,621.
KGaA,Weinheim4814www.
advmat.
dewww.
MaterialsViews.
comCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818ByYuhuaXue,HongxiaWang,YanZhao,LimingDai,LianfangFeng,XungaiWang,andTongLin*MagneticLiquidMarbles:A"Precise"MiniatureReactor[*]Dr.
Y.
Xue,Dr.
H.
Wang,Dr.
Y.
Zhao,Prof.
X.
Wang,Prof.
T.
LinCentreforMaterialandFibreInnovationDeakinUniversityGeelong,VIC3217(Australia)E-mail:tong.
lin@deakin.
edu.
auProf.
L.
DaiDepartmentofChemicalEngineeringCaseWesternReserveUniversityCleveland,Ohio44106(USA)Prof.
L.
FengStateKeyLaboratoryofChemicalEngineeringDepartmentofChemicalandBiologicalEngineeringZhejiangUniversityHangzhou310027(P.
R.
China)DOI:10.
1002/adma.
201001898Miniaturizedchemicalprocesseshavemanyadvantages,suchasreduceduseofchemicalreagentsandsolvents,preciselycontrolledreactionconditions,muchshortenedreactiontime,andtheabilitytointegrateintoadigitaldevice.
[1–4]Theyareveryusefulforhigh-throughputanalysesandpuricationsinchemicalandbiologicalprocesses,suchasdrugdiscovery,[5]DNAanalysis,[6,7]proteincrystallization,[8]andthesynthesisofmoleculesorparticles.
[9–11]Indeed,considerableeffortshavebeenmadetominiaturizechemicalprocessesusingvariousprinciples.
Forexample,oil/wateremulsionshavebeenusedtoperformchemicalreactionsinparallelinalargenumberofemulsiedtinydroplets.
[10]Microuidic"lab-on-a-chip"deviceshavebeendesignedtomanipulatechemicalprocesseswithinmicro-channelseitherincontinuousuidordisconnecteduidsegmentsseparatedbyanimmiscibleuidorgas.
[10,12]Althoughthechannel-basedmicro-reactorsshowgreatpoten-tialinhigh-throughputreactionandintegratabilitytoexternalanalysisfacilities,thereaction-specicdevicepreparations,thechannel-associatedcross-contaminations,andtheneedforexternalpumpstoactuatetheuidmotionmakethemneitheruniversalnorminiature.
Also,microuidicsishardtousetohandleasingledroplet.
Recently,freeaqueousdropletsinanimmiscibleuid(channel-freemicrouidics)[9]hasalsobeenproposedasanalternativestrategy,butcross-contaminationstillremainsaconcern.
Inlivingsystems,cellsarewell-knownasthebasicstructuralandfunctionalunits[13]thatmediatevariousbiochemicalreac-tions(e.
g.
,catabolismandanabolism)forsustainingthegrowthanddivisionofcellsandhencelivingsystems.
Althoughtheirstructurelooksverysimple,cellscommunicatewitheachotherinahighlyintelligentway,throughoutthewholelivingsystem.
Inthisregard,howtomimiclivingcells,bothinstructureandfunction,istheultimatechallengetowardthedevelopmentofarticialminiaturereactors.
Liquidmarbles,liquiddropsencapsulatedwithsolidpowderattheliquid–gasinterface,haverecentlybeenreportedasanewapproachtomanipulatingliquid,[14]whichcouldbeusedasminiaturereactors.
Theyarenon-wettingtoanysolidsur-facesandcouldbemanipulatedbyexternalforces,suchasgravity,[14]electrical,[15]andmagnetic,[16]dependingontheircomponents.
[14–19]However,mostliquidmarblesreportedsofarhavebeenbasedonaqueousliquid.
Althoughafewpapers[19]havereportedtheencapsulationoforganicliquidsintoliquidmarbles,theliquidswereconnedtothosehavingahighsur-facetension.
[19]Theformationofstableliquidmarblesfromawiderangeoforganicsolvents,especiallythosethathavealowsurfacetension,isimportantforchemicalreactions,butstillremainsachallenge.
Inthisstudy,wedemonstratetheincredibleabilityofauor-inateddecylpolyhedraloligomericsilsesquioxane(FD-POSS,structureshowninFigure1a)anditscombinationwithhydro-phobicmagneticnanoparticlestoactasencapsulatingagentstopreparestableliquidmarblesfrombothaqueoussolutionandorganicliquidswithasurfacetensionaslowas20.
1dynecm1,andthefeasibilityofusingthemagneticliquidmarblesasuni-versal"smart"miniaturereactors.
FD-POSSwassynthesizedbythereportedmethod[20,21]andaneFD-POSSpowderwaspreparedbyagrindingtechnique.
TheFD-POSS/Fe3O4nanoparticlecompositepowdersofdif-ferentratios(1:10to1:1,w/w)werepreparedbyco-dispersingtheneFD-POSSpowderwithhydrophobicFe3O4nanopar-ticlesinethanol.
AsshowninthephotographsinFigure1b,FD-POSSpowdersarewhiteandhaveacertaintransparencytovisiblelightbutarenotresponsivetoamagneticeld.
Inethanol,FD-POSSandFe3O4nanoparticleshadastrongafnity,andtheytendedtoaggregateintopowderswhichshowedasuperparamagneticproperty(SupportingInfor-mation).
TheopticalmicrographsinFigure1cindicatethatFD-POSSandFD-POSS/Fe3O4compositepowdersarelessthan70μm,andtheFD-POSSandFe3O4nanoparticlesformedarandomlyblendedcompositestructure.
Whenathinbedofthepowderswerecoatedonasolidsurface,thesurfacebecamehighlyrepellenttoliquids.
Water,dimethylsulfoxide(DMSO),toluene,hexadecane,andethanolallformedrounddropletsonthepowdercoatedsurfacewithoverallcontactanglesof171.
1°,166.
2°,155.
7°,154.
4°,and142.
8°,respectively(Figure1d).
Thepowderlayershowedstrongrepellencetootherorganicsolvents(SupportingInformation).
ThecompositepowdersinthestudiedFD-POSS/Fe3O4ratiorangeshowedaverysimilaruidrepellencytothepureFD-POSSgrains.
Liquidmarbleswerepreparedsimplybydroppingasmallvolumeofliquidontothepowdersurfaceandsubsequentlyshakingthedropletgentlysothatthepowderscoveredtheentiredropletsurface.
Theas-preparedpowders,bothFD-POSS4815www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818Severalwaterdropletswereaddedtotheopenedmarble(10μL)toformawater-in-oilbicomponentliquidmarble.
Oil-in-waterbicomponentliquidmarblescanalsobeproducedinasimilarway.
Thesebicomponentliquidmarblesshowtheabilitytopre-ventthecoreliquidfromrapidevaporation.
Todemonstratetheabilitytomediatechemicalreactions,weusedtwodifferentwaystoundertakeachemiluminescencereaction.
[22,23]Onemethodusedtwomagneticliquidmarblestocarrydifferentreactiveagents,oneforhydrogenperoxideandtheotherforbis(2,4,6-trichlorophenyl)oxalateanddye.
AsshowninFigure2f(toppanel),thetwoliquidmarbleswereactuatedunderamagneticeldtomovetogetherandtheythencoalescedintoalargerliquidmarble,initiatingthechemilumi-nescencereactionwhichcanbeprovenbyanimmediatelightemissionfromthecoalescedliquidmarble.
Anothermethodinvolvedusingabicomponentliquidmarbletohosttwocoredroplets,onefortheoxidantandtheotherfortheuorescer,inanimmiscibleuid.
Undergentlemovementactuatedbyamagnetbar,thetwocoredropletsjointedtogethertriggeringthechemiluminescencereaction.
TheimageinFigure2f(bottompanel)clearlyindicatesthelightemittingjustfromthecombinedcoredroplet.
Besidesthechemiluminescencereaction,otherchemicalreactionssuchasphotochemicalpolymerization,nanoparticlesynthesis,andanacid–basereactionhavealsobeentrialedandproventobesuccessful(SupportingInformation).
Oneimportantaspectforminiaturechemicalreactorsistheabilitytointegratewithpuricationandanalysisfacilities.
SincethemagneticliquidmarblescanbeopenedandclosedreversiblyandFD-POSS/Fe3O4composite,canencapsulatenotonlywaterbutalsoorganicsolventstoformstableliquidmarbles.
Figure2ashowsphotosofliquidmarblespreparedfromwater,DMSO,toluene,ethanol,andoctane(3μL).
Manyotherorganicsol-ventshavealsobeentestedsofarandthepowderswereabletoencapsulatealiquidofsurfacetensionaslowas20.
1dynescm1(SupportingInformation).
Theliquidmarblesareverystableandtheycanoatnotonlyonwaterbutalsoonauidoflowsurfacetension(Figure2b).
ThesuperparamagneticpropertyoftheFe3O4nanoparticlesimpartstheFD-POSS/Fe3O4powder-encapsulatedliquidmar-bleswithamagnetresponsiveability.
Asaresult,theliquidmarblescanbeactuatedtomoveindifferentdirections,oropenedandclosedreversibly,underamagneticeld.
Figure2cshowsmagnetopenedliquidmarblesfromdifferentliquids.
Theopenedliquidmarbleallowsittobecoveredwithanothertypeofhydrophobicmaterial.
Todemonstratethis,weusedpureFD-POSSpowdertocovertheopenedliquidsurfaceofamagneticliquidmarble,andthecoveredpartshowedadifferentcolortothemagneticpowdercoveredsurface.
Consequently,themotionofliquidcanbetracedeasily.
Figure2dillustratesthemotionofawhiteFD-POSS-stainedFD-POSS/Fe3O4hexa-decanemarbleunderamagneticeld.
Itcanbeclearlyseenthatthemarblerotatesonasolidsurface(toppanel),butshiftssidewaysonaliquidsurface(bottompanel).
Themagnetopenedliquidmarblealsoallowstheliquidtobetakenfromthemarbleorthemarbletobereplenishedwithotherliquids.
Figure2edemonstratestheadditionofcolorfulwaterdropletsintoamagnetopenedhexadecanemarble.
Figure1.
a)StructureofFD-POSS.
b)ProcesstomaketheFD-POSS/Fe3O4nanoparticlecomposite.
Theinsertimagetotherightshowsthesuspen-sionsofDP-POSSandtheFD-POSS/Fe3O4nanoparticlecompositepowdersinsolventandtheFD-POSS/Fe3O4underamagneticeld.
c)OpticalmicroscopyimagesofFD-POSSandFD-POSS/Fe3O4powders(FD-POSS:Fe3O4=1:1,w/w).
d)Water,dimethylsulfoxide(DMSO),toluene,hexade-cane,andethanoldropletsontheFD-POSS/Fe3O4powder-coatedsiliconsurface.
4816www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818thatpushestheliquidoutofthepowderlayer.
Whetheraliquidmarblecanremainstableonasolidsurfaceornotisdeterminedbytheabilityofthenon-wettingpowderlayertopreventtheliquidbreakthrough(i.
e.
,breakthroughpressure,Pbreakthrough)andtheactualliquidpressuregeneratedbytheliquiddroplet(Pdroplet).
IfPbreakthroughPdroplet,theactualpressuregeneratedbytheliquiddropletisnotenoughtoforcethroughthepowderlayerhencetheliquidmarbleisstable.
WhenPdropletisclosetoPbreakthrough,itiseasyfortheliquidtobreakthepowderlayer,whichleadstobreakageoftheliquidmarble.
Basedonthecapillaryforceandtheliquidpressurethatforcesliquidtosagintothepowderypores(Figure3b,c),theratiobetweenthebreakthroughpressureandthemaximumliquidpressureformedbythedroplet(Pmaxdroplet=Dgl,whereρisthedensityoftheliquid,gistheconstantduetogravity,andl=γlv/ρgiscapillarylengthoftheliquid),PbreakthroughPmaxdroplet/,canbeestimatedas:PbreakthroughPmaxdroplet=A=H.
TH+T/(1)byamagneticeld,thereactionliquidcanberemovedeasilybyacapillarydeviceforfurtheranalysesordirectlytestedbyanopticalspectrometer(e.
g.
,UVanduorescentspectrom-eters).
Figure2galsoshowsthedirectpuricationofareac-tionproductthroughanarrowchromatographicaluminasheetpluggedintotheopenedliquidmarble.
Theabilityofahydrophobicpowdertorepelliquiduidismainlydeterminedbythesurfacechemistryandphysicaltopologyofthepowdermaterial,thesurfacetensionofaliquid,andthecontactmodebetweentheliquidandpowder.
TheFD-POSSmoleculecompriseseightuorinateddecylgroupswhichinclude136uorineatomssurroundingthePOSScage,makingthemoleculeextremelylowinsurfacefreeenergy.
Theroughsurfaceattributabletotheparticulatestructurefacilitatestheformationofanon-wettingpowderysurface.
[24–26]Inthestaticstate,theencapsulatingpowdersbetweenthedropletandthesolidsupport(Figure3a)receivethelargestliquidpressure.
Becauseofthenon-wettingfeature,theliquidonapowderyporouslayerreceivesanegativecapillarypressureFigure2.
a)DigitalgraphicimagesofliquidmarblesproducedfromdifferentliquidsandFD-POSSpowder(dropletsize3μL).
Foreasyobservation,asmallamountofdyewasaddedtotheliquidandtheexistenceofthedyehasnoinuenceonthestabilityoftheliquidmarbles.
b)Liquidmarblesoatingonwaterandhexadecanesurfaces(dropletsize3μL).
c)Magnetopenedliquidmarbles(dropletsize7μL).
d)Magnet-drivenmotionsofaFD-POSS-stainedFD-POSS/Fe3O4hexadecanemarbleonaglassslide(top)andwatersurface(bottom)(dropletsize7μL).
e)Magnetopenedhexa-decanemarbleswithdifferentnumbersofcoloredwaterdropletsadded(overalldropletsize10μL).
f)Achemiluminescencereactionthatoccursasaresultofcoalescingoftwomagneticliquidmarblesthatcontaindifferentreagents(top),andthesamechemiluminescencereactionhappeningwithinasingleliquidmarble(bottom)(dropletsize10μL).
g)Chromatographicanalysisofliquidintheopenedliquidmarble(dropletsize10μL).
Scalebar:1mm.
4817www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818liquids.
Magneticmanipulationenablestheliquidinthemarbletocommunicatewiththeoutsideenvironmentondemand,whichprovidesasimplewaytointegratewithportablepurica-tionandanalysisfacilities.
Allthesefeaturescouldleadtonewuniversal"lab-on-chip"reactorsystemswithexcellentintegrationabilitytoincorporatesamplefabrication,actuation,purication,andanalysisintoasingleelectronicchipfordiverseapplications.
ExperimentalSectionPreparationofFD-POSS/Fe3O4NanoparticleCompositePowders:(1H,1H,2H,2H-heptadecauorodecyl)8Si8O12(FD-POSS),synthesizedaccordingtotheliteraturemethod,[20]andhydrophobicFe3O4nanoparticles,preparedbyaliteraturemethod,[16]weredispersedin20mLofethanolandcontinuouslystirredfor24h.
Themixturewasthenisolatedfromthesolutionwithabarmagnetanddriedundervacuumat60°C.
PreparationandMagneticManipulationofLiquidMarbles:LiquidmarbleswerepreparedbydroppingliquiddropletsontoabedofFD-POSSorFD-POSS/Fe3O4nanoparticlegrains.
Upongentleshakingofthedroplet,thesolidpowdersbecamestucktothedropletsurfaceformingapowder-encapsulatedliquiddroplet(i.
e.
,liquidmarble).
Magneticmanipulationoftheliquidmarbleswasperformedusinganeodymiumcylindermagnet(diameter10mmandlength12mm).
Thedirectedmovementoftheliquidmarbleswasrecordedusingadigitalmicroscope(Dino-LiteAM313).
SupportingInformationSupportingInformationisavailablefromtheWileyOnlineLibraryorfromtheauthor.
H=2(1cosθ)lcapD(2√3D2ππ)(2)T=Blcapsin(2Rmin)R(2√3DB)(3)HandTaretherobustnessheightandangledeterminedbytheliquid–gassurfacetension(γlv),theparticleradius(R),theinterparticledistance(2D),andtheapparentcontactangleofliquidonthepowdermaterial(θ)(fordetailsseetheSupportingInformation).
ThedependencyofPbreakthroughPmaxdroplet/onthepar-ticleradiusandthehalfinter-particledistanceforthepowderarrangementisgiveninFigure3d(thecalculationontheotherparticlearrangementisgivenintheSupportingInformation).
ItisinterestingtonotethatamaximumPbreakthroughPmaxdroplet/ratiooccurswhenthepowderradiusisabout35μmandtheinter-powderdistanceissmall.
Basedontheactualpowderdimen-sionandsurfaceproperties,thePbreakthroughPmaxdroplet/Dforthreestudiedliquidsystemswascomputed(Figure3e).
Whenthisinter-powderdistanceislessthan160μm(D<80μm),thePbreakthroughPmaxdroplet/islargerthanunityeveniftheliquidsurfacetensionisverylow,suggestingtheformationofaverystableliquidmarble.
Insummary,wehavedemonstratedthepotentialofusinguorinatedPOSSandmagneticnanoparticlesasauniversalencapsulatingagenttoprepareliquidmarblesfromvariousFigure3.
a)Idealizedliquidmarblewithasinglelayerofpowderscoveringthedropletsurface.
b)Fluidsaggingintotwoparticlesandthedimen-sionsforcalculationofthebreakthroughpressure.
c)Onepowderarrangementusedforcalculationofbreakthroughpressure.
d)DependenciesofPbreakthroughPmax/dropletratioonRandDforoctane(θ=55°).
e)PbreakthroughPmax/dropletDdependencycalculatedbasedontheactualpowderdimensionforwater(θ=120°),hexadecane(θ=80°),andoctane.
4818www.
advmat.
dewww.
MaterialsViews.
com2010WILEY-VCHVerlagGmbH&Co.
KGaA,WeinheimCOMMUNICATIONwileyonlinelibrary.
comAdv.
Mater.
2010,22,4814–4818AcknowledgementsTheauthorsacknowledgethefundingsupportfromDeakinUniversityundertheAlfredDeakinPostdoctoralFellowshipschemeandStateKeyLaboratoryofChemicalEngineering(SKL-ChE-09D05).
Received:May22,2010Publishedonline:September5,2010[1]K.
Jensen,Nature1998,393,735.
[2]A.
J.
deMello,Nature2006,442,394.
[3]S.
-Y.
Teh,R.
Lin,L.
-H.
Hung,A.
P.
Lee,LabChip2008,8,198.
[4]M.
Abdelgawad,A.
R.
Wheeler,Adv.
Mater.
2009,21,920.
[5]P.
S.
Dittrich,A.
Manz,Nat.
Rev.
DrugDiscov.
2006,5,210.
[6]R.
H.
Liu,J.
Yang,R.
Lenigk,J.
Bonanno,P.
Grodzinski,Anal.
Chem.
2004,79,1824.
[7]M.
A.
Burns,B.
N.
Johnson,S.
N.
Brahmasandra,K.
Handique,J.
R.
Webster,M.
Krishnan,T.
S.
Sammarco,P.
M.
Man,D.
Jones,D.
Heldsinger,C.
H.
Mastrangelo,D.
T.
Burke,Science1998,282,484.
[8]B.
Zheng,L.
S.
Roach,R.
F.
Ismagilov,J.
Am.
Chem.
Soc.
2003,125,11170.
[9]O.
D.
Velev,B.
G.
Prevo,K.
H.
Bhatt,Nature2003,426,515.
[10]M.
Joanicot,A.
Ajdari,Science2005,309,887.
[11]H.
Song,D.
L.
Chen,R.
F.
Ismagilov,Angew.
Chem.
Int.
Ed.
2006,45,7336.
[12]A.
Günther,M.
Jhunjhunwala,M.
Thalmann,M.
A.
Schmidt,K.
F.
Jensen,Langmuir2005,21,1547.
[13]B.
Alberts,A.
Johnson,J.
Lewis,M.
Raff,K.
Roberts,P.
Walter,MolecularBiologyoftheCell,GarlandScience,NewYork2002.
[14]P.
Aussillous,D.
Quere,Nature2001,411,924.
[15]P.
Aussillous,D.
Quere,Proc.
R.
Soc.
A2006,462,973.
[16]Y.
Zhao,J.
Fang,H.
Wang,X.
Wang,T.
Lin,Adv.
Mater.
2010,22,707.
[17]A.
V.
Rao,M.
M.
Kulkarni,S.
D.
Bhagat,J.
ColloidInterfaceSci.
2005,285,413.
[18]G.
McHale,D.
L.
Herbertson,S.
J.
Elliott,N.
J.
Shirtcliffe,M.
I.
Newton,Langmuir2007,23,918.
[19]L.
Gao,T.
J.
Mccarthy,Langmuir2007,23,10445.
[20]J.
M.
Mabry,A.
Vij,S.
T.
Iacono,B.
D.
Viers,Angew.
Chem.
Int.
Ed.
2008,47,4137.
[21]A.
Tuteja,w.
Choi,M.
Ma,J.
M.
Mabry,S.
A.
Mazzella,G.
C.
Rutledge,G.
H.
Mckinley,R.
E.
Cohen,Science2007,318,1618.
[22]R.
B.
Thompson,S.
E.
S.
McBee,Langmuir1988,4,106.
[23]R.
E.
Milofsky,J.
W.
Birks,J.
Am.
Chem.
Soc.
1991,113,9715.
[24]X.
Feng,L.
Jiang,Adv.
Mater.
2006,18,3063.
[25]P.
Roach,N.
J.
Shirtcliffe,M.
I.
Newton,SoftMatter2008,4,224.
[26]X.
Zhang,F.
Shi,J.
Niu,Y.
Jiang,Z.
Wang,J.
Chem.
Mater.
2008,18,621.
- preparedwww.55fang.com相关文档
- 深圳市www.55fang.com
- boardwww.55fang.com
- analyzedwww.55fang.com
- fullywww.55fang.com
- 随着知识资本流经消息发送系统以及员工的移动性越来越高,企业面临
- leachingwww.55fang.com
搬瓦工:香港PCCW机房即将关闭;可免费升级至香港CN2 GIA;2核2G/1Gbps大带宽高端线路,89美元/年
搬瓦工怎么样?这几天收到搬瓦工发来的邮件,告知香港pccw机房(HKHK_1)即将关闭,这也不算是什么出乎意料的事情,反而他不关闭我倒觉得奇怪。因为目前搬瓦工香港cn2 GIA 机房和香港pccw机房价格、配置都一样,可以互相迁移,但是不管是速度还是延迟还是丢包率,搬瓦工香港PCCW机房都比不上香港cn2 gia 机房,所以不知道香港 PCCW 机房存在还有什么意义?关闭也是理所当然的事情。点击进...
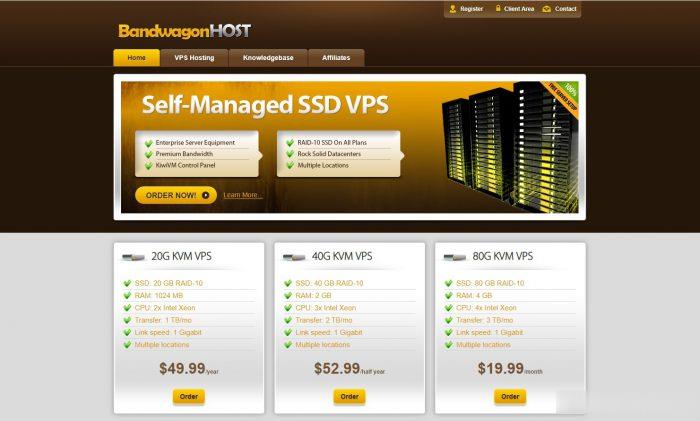
VPS云服务器GT线路,KVM虚vps消息CloudCone美国洛杉矶便宜年付VPS云服务器补货14美元/年
近日CloudCone发布了最新的补货消息,针对此前新年闪购年付便宜VPS云服务器计划方案进行了少量补货,KVM虚拟架构,美国洛杉矶CN2 GT线路,1Gbps带宽,最低3TB流量,仅需14美元/年,有需要国外便宜美国洛杉矶VPS云服务器的朋友可以尝试一下。CloudCone怎么样?CloudCone服务器好不好?CloudCone值不值得购买?CloudCone是一家成立于2017年的美国服务器...
10gbiz:香港/洛杉矶CN2直连线路VPS四折优惠,直连香港/香港/洛杉矶CN2四折
10gbiz怎么样?10gbiz在本站也多次分享过,是一家成立于2020的国人主机商家,主要销售VPS和独立服务器,机房目前有中国香港和美国洛杉矶、硅谷等地,线路都非常不错,香港为三网直连,电信走CN2,洛杉矶线路为三网回程CN2 GIA,10gbiz商家七月连续推出各种优惠活动,除了延续之前的VPS产品4折优惠,目前增加了美国硅谷独立服务器首月半价的活动,有需要的朋友可以看看。10gbiz优惠码...
www.55fang.com为你推荐
-
美国互联网瘫痪网络中断会对美国军力造成什么影响小度商城小度智能音箱1s上面的黄圈不熄灭怎么回事,第一天还能熄灭云计算什么是云计算?关键字关键词标签里写多少个关键词为最好百度关键词价格查询百度关键词排名价格是多少地陷裂口天上顿时露出一个大窟窿地上也裂开了,一到黑幽幽的深沟可以用什么四字词语来?网站检测如何进行网站全面诊断m.2828dy.combabady为啥打不开了,大家帮我提供几个看电影的网址www.zjs.com.cn我的信用卡已经申请成功了,显示正在寄卡,怎么查询寄卡信息?lcoc.top日本Ni-TOP是什么意思?