8.5www.88ququ.com
www.88ququ.com 时间:2021-03-21 阅读:()
Quetal.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16OpenAccessRESEARCH2010Quetal;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAt-tributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
ResearchAntibioticsusceptibilityofcoagulase-negativestaphylococciisolatedfromverylowbirthweightbabies:comprehensivecomparisonsofbacteriaatdifferentstagesofbiofilmformationYueQu1,AndrewJDaley2,TaghridSIstivan1,SuzanneMGarland2,3,4andMargaretADeighton*1AbstractBackground:Coagulase-negativestaphylococciaremajorcausesofbloodstreaminfectionsinverylowbirthweightbabiescaredforinNeonatalIntensiveCareUnits.
Thevirulenceofthesebacteriaismainlyduetotheirabilitytoformbiofilmsonindwellingmedicaldevices.
Biofilm-relatedinfectionsoftenfailtorespondtoantibioticchemotherapyguidedbyconventionalantibioticsusceptibilitytests.
Methods:Coagulase-negativestaphylococcalbloodcultureisolatesweregrownindifferentphasesrelevanttobiofilmformation:planktoniccellsatmid-logphase,planktoniccellsatstationaryphase,adherentmonolayersandmaturebiofilmsandtheirsusceptibilitiestoconventionalantibioticswereassessed.
Theeffectsofoxacillin,gentamicin,andvancomycinonpreformedbiofilms,atthehighestachievableserumconcentrationswereexamined.
Epifluorescencemicroscopyandconfocallaserscanningmicroscopyincombinationwithbacterialviabilitystainingandpolysaccharidestainingwereusedtoconfirmthestimulatoryeffectsofantibioticsonbiofilms.
Results:Mostcoagulase-negativestaphylococcalclinicalisolateswereresistanttopenicillinG(100%),gentamicin(83.
3%)andoxacillin(91.
7%)andsusceptibletovancomycin(100%),ciprofloxacin(100%),andrifampicin(79.
2%).
Bacteriagrownasadherentmonolayersshowedsimilarsusceptibilitiestotheirplanktoniccounterpartsatmid-logphase.
Isolatesinabiofilmgrowthmodeweremoreresistanttoantibioticsthanbothplanktonicculturesatmid-logphaseandadherentmonolayers;howevertheywereequallyresistantorlessresistantthanplanktoniccellsatstationaryphase.
Moreover,forsomecell-wallactiveantibiotics,concentrationshigherthanconventionalMICswererequiredtopreventtheestablishmentofplanktonicculturesfrombiofilms.
Finally,thebiofilm-growthoftwoS.
capitisisolatescouldbeenhancedbyoxacillinatthehighestachievableserumconcentration.
Conclusion:Weconcludethattheresistanceofcoagulase-negativestaphylococcitomultipleantibioticsinitiallyremainsimilarwhenthebacteriashiftfromaplanktonicgrowthmodeintoanearlyattachedmode,thenincreasesignificantlyastheadherentmodefurtherdevelops.
Furthermore,preformedbiofilmsofsomeCoNSareenhancedbyoxacillininadose-dependentmanner.
BackgroundCoagulase-negativestaphylococci(CoNS),predomi-nantlyStaphylococcusepidermidis,arethemostcommoncausativeagentsofneonatalsepsis[1-3],aconditionwhichhasbeenrelatedtosignificantmorbidityandmor-talityinneonatalintensivecareunits(NICUs)[2].
Thepresenceofacentralvenouscatheterinverylowbirthweight(VLBW)babies(48h),thechoiceiseitherflucloxacillinorvancomycinwithgentamicin.
Ciprofloxacinandrifampicinwerealsoeval-uatedinthisstudyastheefficacyoftheseantibioticsonCoNSbiofilmshasbeenreportedbyotherinvitrostud-ies.
PenicillinGwaspurchasedfromCSLBiotherapies,Parkville,AustraliaandallotherswereobtainedfromSigma-Aldrich,CastleHill,Australia.
EstablishmentofadherentmonolayersBacterialculturesofadherentmonolayerswereestab-lishedfollowingthemethodofMiyakeetal.
(1992),whichinvolvedtheadditionof50μLvolumesofbacterialTable1:Bacterialisolatesandgrowthmediausedforbiofilmformation.
IsolateSpeciesStatusGrowthmediumforbiofilmformationaTSBTSB+1%glucoseTSB+4%NaClicaAicaCicaD1S.
warneriInvasive+---2S.
haemolyticusInvasive----3S.
epidermidisInvasive++++4S.
epidermidisInvasive+---5S.
epidermidisInvasive++++6S.
capitisInvasive+++-7S.
epidermidisInvasive++++8aS.
capitisInvasive+++-8bS.
capitisInvasive+++-9S.
capitisInvasive+++-10S.
epidermidisInvasive+---11S.
epidermidisInvasive++++12S.
epidermidisContaminantw---13S.
epidermidisContaminant++++15S.
capitisContaminantw++-16S.
capitisContaminant+++-17S.
capitisContaminant+++-18S.
capitisContaminant+++-19S.
epidermidisContaminant----20S.
epidermidisContaminant++++21S.
epidermidisContaminant++++22S.
capitisContaminantw+++23S.
epidermidisContaminant++++24S.
epidermidisContaminant++++RP62AS.
epidermidisReference+SP2S.
hominisReference-aSelectionofgrowthmediumwasbasedontheproductionofthehighestbiofilmdensityforeachisolate.
Theamountofbiofilmwasindicatedas:"+",strong(OD600≥0.
24);"w",weak(0.
12≤OD6002*log2inMICorMBCforoxacillin,vancomycin,ciprofloxacinandrifampicin,andanincreaseof>3*log2forMICorMBCforpenicillinandgentamicin,wereconsideredsignificant[38].
Experi-mentstargetingtheeffectsofantibioticsonpreformedbiofilmswererepeatedatleastthreetimesintriplicate.
Onewayanalysisofvariance(ANOVA)orthenon-para-metricMann-Whitneytestwasusedfortwo-setcompar-isonsandap-valueof10241024>102432≤132Biofilm(0.
9-1.
9)*109>1024e>102464>102464>102416>1024d0.
25>10240.
0040.
015Isolate3LogPlanktonic(2.
9-8.
3)*1058160.
250.
250.
250.
25240.
120.
250.
0080.
015Monolayer(5.
0-11.
0)*10532640.
250.
50.
120.
5280.
250.
50.
0020.
004StatPlanktonic(0.
6-2.
2)*109>102432>1024>10241024256Biofilm(0.
3-1.
3)*109>1024>102416160.
25216160.
2520.
54Isolate8aLogPlanktonic(2.
6-5.
5)*105128128323288120.
250.
250.
0080.
03Monolayer(6.
4-11.
1)*105>128>12832646464180.
120.
50.
0040.
015StatPlanktonic(4.
4-4.
9)*108>1024>1024>1024>102410242Biofilm(0.
6-1.
8)*1081024>1024256>1024256>10248>10240.
12>10240.
0044Isolate9LogPlanktonic(3.
1-5.
2)*10532643232816120.
250.
250.
0150.
03Monolayer(4.
6-10.
8)*105>128>12832>1281632280.
250.
50.
0040.
015StatPlanktonic(0.
7-0.
8)*109>1024>1024>1024>1024>1024256Biofilm(1.
1-1.
7)*109>1024>102464>1024>128>10248>10240.
25>10240.
0084Isolate11LogPlanktonic(2.
7-8.
5)*1058864>1283264110.
060.
12>128>128Monolayer(3.
2-17.
8)*1053264>128>1283264120.
060.
12>128>128StatPlanktonic(0.
8-1.
6)*109>1024>1024>1024>10245121024Biofilm(0.
2-0.
6)*109>1024>1024256>1024256>10248>10240.
251664>1024αBiofilm-positiveisolates(OD600>0.
24).
bMICsforCoNSatstationaryphasearenotprovidedasthevaluescouldnotbedeterminedbystandardmethods.
cValuesunderlinedindicateasignificantincreaseintheMICsorMBCsbetweenlog-planktonicandadherentmonolayermodesofgrowth.
dValuesinitalicindicateasignificantchangeintheMBCsbetweenstationary-planktonicandbiofilmmodesofgrowth.
eValuesinboldindicateasignificantincreaseinMICsorMBCsbetweenlog-planktonic/adherentmonolayerandbiofilmmodesofgrowthQuetal.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16Page8of12adherentmonolayers,however,itisalsolikelythatthedifferencewasduetothedifferentkineticsofbiofilmfor-mationbetweenP.
aeruginosaandStaphylococcusspp.
[19].
ConventionalMICshavebeenusedtoguidethetreat-mentofbiofilm-relatedinfectionsatthefebrilestage,basedontheassumptionthatbiofilm-releasedcellsaresimilarintheirsusceptibilitiestocellsintheplanktonicphase[23].
Reporteddifferencesbetweentheconven-tionalMICsandbiofilmMICswereattributedtolackofstandardizationofinitialinoculaandtothepresenceofsmallcolonyvariants[23].
However,inourstudy,wefoundthatMICsofcellwallactiveantibioticswerefre-quentlyhigherforbiofilmgrownbacteriathanplanktoniccultures.
ThisisconsistentwithrecentstudiesbyMoskowitzetal.
(2004)andMelchioretal.
(2006),whoreportedthatbiofilmMICsofβ-lactamantibiotics,butnototherantibiotics,weremuchhigherthantheconven-tionalMICsforP.
aeruginosaandS.
aureusrespectively[21,24].
Theseresultsarenotsurprisinggiventhatcellwallactiveantibioticsmainlyaffectrapidlygrowingbac-teria.
Biofilm-releasedcellsarelikelytobelessactivethandividingplanktoniccellsatmid-logphase,probablybecausetheyhaverecentlyundergoneaswitchfromabiofilmmodeofgrowthtoafree-livingmode,similartocellsatlag-phasegrowth,andrequireachangeingeneexpressiontoadapttothenewenvironment.
TheMBCsofpenicillinG,gentamicin,oxacillin,andvancomycinforCoNSgrowninabiofilmmodeweregen-erally>1024μg/ml,whichiswellbeyondthehighestachievableserumconcentrations.
Althoughsomeoftheseantibioticsatthehighestachievableserumconcen-trationswereeffectiveagainstbacteriagrownplanktoni-callytomid-logphase,theywereinadequatetokillTable3:Antibioticsusceptibilityoffourbiofilm-negativeisolatesagrownindifferentmodes.
IsolateandmodeofgrowthInitialbacterialdensity(CFU/ml)Penicillin(μg/ml)Gentamicin(μg/ml)Oxacillin(μg/ml)Vancomycin(μg/ml)Ciprofloxacin(μg/ml)Rifampicin(μg/ml)MICMBCMICMBCMICMBCMICMBCMICMBCMICMBCSP2LogPlanktonic(1.
2-4.
1)*1050.
50.
5880.
060.
06120.
120.
120.
0080.
03Monolayer(2.
6-4.
7)*10528c16160.
120.
120.
510.
120.
120.
0040.
008StatPlanktonic(3.
5-9.
5)*107b>10241024d2420.
008Biofilm(1.
0-3.
2)*107128>1024e8320.
122240.
1220.
0020.
008Isolate15LogPlanktonic(2.
2-5.
5)*10512812832641632440.
250.
250.
0150.
03Monolayer(5.
2-16.
4)*105>128>12832641664480.
120.
250.
0040.
008StatPlanktonic(3.
7-7.
9)*108>1024>1024>1024>1024>102464Biofilm(0.
7-0.
9)*108>1024>1024256>1024256>102416>10240.
255120.
0040.
008Isolate19LogPlanktonic(2.
9-7.
4)*105>128>128646428220.
250.
250.
0080.
015Monolayer(3.
2-14.
6)*105>128>12864>12828280.
120.
250.
0020.
008StatPlanktonic(0.
7-1.
5)*109>1024>1024>1024>102410242Biofilm(0.
1-0.
2)*1091024>102464>102464>10244>10240.
2540.
0080.
06Isolate22LogPlanktonic(2.
8-5.
6)*105>128>1286464816440.
120.
250.
0150.
03Monolayer(4.
8-16.
3)*105>128>12832>1283232280.
120.
250.
0040.
008StatPlanktonic(0.
5-0.
7)*109>1024>1024>1024>1024>1024256Biofilm0.
2*1091024>1024>128>1024>128>102416>10240.
2510.
0080.
03aBiofilm-negativeisolatesincludedbiofilm-weakproducer(0.
24>OD600≥0.
12)andbiofilm-negativeproducer(OD6002003,88:F89-93.
3.
CheungGY,OttoM:UnderstandingthesignificanceofStaphylococcusepidermidisbacteremiainbabiesandchildren.
CurrOpinInfectDis2010,23:208-216.
4.
Johnson-RobbinsLA,el-MohandesAE,SimmensSJ,KeiserJF:Staphylococcusepidermidissepsisintheintensivecarenursery:acharacterizationofriskassociationsininfants<1,000g.
BiolNeonate1996,69:249-256.
5.
MaasA,FlamentP,PardouA,DeplanoA,DramaixM,StruelensMJ:Centralvenouscatheter-relatedbacteraemiaincriticallyillneonates:riskfactorsandimpactofapreventionprogramme.
JHospInfect1998,40:211-224.
6.
vonEiffC,PetersG,HeilmannC:Pathogenesisofinfectionsduetocoagulase-negativestaphylococci.
LancetInfectDis2002,2:677-685.
7.
KlingenbergC,AaragE,RonnestadA,SollidJE,AbrahamsenTG,KjeldsenG,FlaegstadT:Coagulase-negativestaphylococcalsepsisinneonates.
Associationbetweenantibioticresistance,biofilmformationandthehostinflammatoryresponse.
PediatrInfectDisJ2005,24:817-822.
8.
OttoM:Staphylococcalbiofilms.
CurrTopMicrobiolImmunol2008,322:207-228.
9.
QinZ,OuY,YangL,ZhuY,Tolker-NielsenT,MolinS,QuD:Roleofautolysin-mediatedDNAreleaseinbiofilmformationofStaphylococcusepidermidis.
Microbiology2007,153:2083-2092.
10.
SutherlandIW:Thebiofilmmatrix--animmobilizedbutdynamicmicrobialenvironment.
TrendsMicrobiol2001,9:222-227.
11.
CostertonJW,StewartPS,GreenbergEP:Bacterialbiofilms:acommoncauseofpersistentinfections.
Science1999,284:1318-1322.
12.
Hall-StoodleyL,CostertonJW,StoodleyP:Bacterialbiofilms:fromthenaturalenvironmenttoinfectiousdiseases.
NatRevMicrobiol2004,2:95-108.
13.
MonzonM,OteizaC,LeivaJ,LamataM,AmorenaB:BiofilmtestingofStaphylococcusepidermidisclinicalisolates:lowperformanceofvancomycininrelationtootherantibiotics.
DiagnMicrobiolInfectDis2002,44:319-324.
14.
NishimuraS,TsurumotoT,YonekuraA,AdachiK,ShindoH:AntimicrobialsusceptibilityofStaphylococcusaureusandStaphylococcusepidermidisbiofilmsisolatedfrominfectedtotalhiparthroplastycases.
JOrthopSci2006,11:46-50.
15.
AndersonGG,O'TooleGA:Innateandinducedresistancemechanismsofbacterialbiofilms.
CurrTopMicrobiolImmunol2008,322:85-105.
16.
StewartPS:Mechanismsofantibioticresistanceinbacterialbiofilms.
IntJMedMicrobiol2002,292:107-113.
17.
SpoeringAL,LewisK:BiofilmsandplanktoniccellsofPseudomonasaeruginosahavesimilarresistancetokillingbyantimicrobials.
JBacteriol2001,183:6746-6751.
18.
CercaN,MartinsS,CercaF,JeffersonKK,PierGB,OliveiraR,AzeredoJ:Comparativeassessmentofantibioticsusceptibilityofcoagulase-negativestaphylococciinbiofilmversusplanktoniccultureasassessedbybacterialenumerationorrapidXTTcolorimetry.
JAntimicrobChemother2005,56:331-336.
19.
AaronSD,FerrisW,RamotarK,VandemheenK,ChanF,SaginurR:Singleandcombinationantibioticsusceptibilitiesofplanktonic,adherent,andbiofilm-grownPseudomonasaeruginosaisolatesculturedfromsputaofadultswithcysticfibrosis.
JClinMicrobiol2002,40:4172-4179.
20.
CeriH,OlsonME,StremickC,ReadRR,MorckD,BuretA:TheCalgaryBiofilmDevice:newtechnologyforrapiddeterminationofantibioticsusceptibilitiesofbacterialbiofilms.
JClinMicrobiol1999,37:1771-1776.
21.
MelchiorMB,Fink-GremmelsJ,GaastraW:ComparativeassessmentoftheantimicrobialsusceptibilityofStaphylococcusaureusisolatesfrombovinemastitisinbiofilmversusplanktonicculture.
JVetMedBInfectDisVetPublicHealth2006,53:326-332.
22.
MizunagaS,KamiyamaT,FukudaY,TakahataM,MitsuyamaJ:InfluenceofinoculumsizeofStaphylococcusaureusandPseudomonasaeruginosaoninvitroactivitiesandinvivoefficacyoffluoroquinolonesandcarbapenems.
JAntimicrobChemother2005,56:91-96.
Received:5February2010Accepted:27May2010Published:27May2010Thisarticleisavailablefrom:http://www.
ann-clinmicrob.
com/content/9/1/162010Quetal;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16Quetal.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16Page12of1223.
CeriH,OlsonM,MorckD,StoreyD,ReadR,BuretA,OlsonB:TheMBECAssaySystem:multipleequivalentbiofilmsforantibioticandbiocidesusceptibilitytesting.
MethodsEnzymol2001,337:377-385.
24.
MoskowitzSM,FosterJM,EmersonJ,BurnsJL:ClinicallyfeasiblebiofilmsusceptibilityassayforisolatesofPseudomonasaeruginosafrompatientswithcysticfibrosis.
JClinMicrobiol2004,42:1915-1922.
25.
KumonH:Managementofbiofilminfectionsintheurinarytract.
WorldJSurg2000,24:1193-1196.
26.
LewisK:Persistercellsandtheriddleofbiofilmsurvival.
Biochemistry(Mosc)2005,70:267-274.
27.
LewisK:Multidrugtoleranceofbiofilmsandpersistercells.
CurrtTopMicrobiolImmunol2008,322:107-131.
28.
PettitRK,WeberCA,KeanMJ,HoffmannH,PettitGR,TanR,FranksKS,HortonML:MicroplateAlamarblueassayforStaphylococcusepidermidisbiofilmsusceptibilitytesting.
AntimicrobAgentsChemother2005,49:2612-2617.
29.
QuY,IstivanTS,DaleyAJ,RouchDA,DeightonMA:Comparisonofvariousantimicrobialagentsascatheterlocksolutions:preferenceforethanolineradicationofcoagulase-negativestaphylococcalbiofilms.
JMedMicrobiol2009,58:442-450.
30.
CercaN,MartinsS,SillankorvaS,JeffersonKK,PierGB,OliveiraR,AzeredoJ:EffectsofgrowthinthepresenceofsubinhibitoryconcentrationsofdicloxacillinonStaphylococcusepidermidisandStaphylococcushaemolyticusbiofilms.
ApplEnvironMicrobiol2005,71:8677-8622.
31.
RachidS,OhlsenK,WitteW,HackerJ,ZiebuhrW:Effectofsubinhibitoryantibioticconcentrationsonpolysaccharideintercellularadhesinexpressioninbiofilm-formingStaphylococcusepidermidis.
AntimicrobAgentsChemother2000,44:3357-3363.
32.
RuppME,HamerKE:Effectofsubinhibitoryconcentrationsofvancomycin,cefazolin,ofloxacin,L-ofloxacinandD-ofloxacinonadherencetointravascularcathetersandbiofilmformationbyStaphylococcusepidermidis.
JAntimicrobChemother1998,41:155-161.
33.
BradfordR,AbdulMananR,DaleyAJ,PearceC,RamalingamA,D'MelloD,MuellerY,UahwatanasakulW,QuY,GrandoD,GarlandS,DeightonM:Coagulase-negativestaphylococciinvery-low-birth-weightinfants:inabilityofgeneticmarkerstodistinguishinvasivestrainsfrombloodculturecontaminants.
EurJClinMicrobiolInfectDis2006,25:283-290.
34.
MiyakeY,FujiwaraS,UsuiT,SuginakaH:Simplemethodformeasuringtheantibioticconcentrationrequiredtokilladherentbacteria.
Chemotherapy1992,38:286-290.
35.
DeightonMA,CapstickJ,DomalewskiE,vanNguyenT:MethodsforstudyingbiofilmsproducedbyStaphylococcusepidermidis.
MethodsEnzymol2001,336:177-195.
36.
Villain-GuillotP,GualtieriM,BastideL,LeonettiJP:InvitroactivitiesofdifferentinhibitorsofbacterialtranscriptionagainstStaphylococcusepidermidisbiofilm.
AntimicrobAgentsChemother2007,51:3117-3121.
37.
GualtieriM,BastideL,Villain-GuillotP,Michaux-CharachonS,LatoucheJ,LeonettiJP:InvitroactivityofanewantibacterialrhodaninederivativeagainstStaphylococcusepidermidisbiofilms.
JAntimicrobChemother2006,58:778-783.
38.
ClinicalandLaboratoryStandardsInstitute(CLSI):Methodsfordilutionantimicrobialsusceptibilitytestsforbacteriathatgrowaerobically.
CLSIdocumentM7-A7,approvedstandard.
7thedition.
Wayne,PA;CLSI;2004.
39.
MurrayPR,BaronEJ,JorgensenJH,LandryML,PfallerMA,(ed):ManualofClinicalMicrobiology.
Washington,D.
C.
:ASMPress;2007.
40.
VillariP,SarnataroC,IacuzioL:MolecularepidemiologyofStaphylococcusepidermidisinaneonatalintensivecareunitoverathree-yearperiod.
JClinMicrobiol2008,38:1740-1746.
41.
LabthavikulP,PetersenPJ,BradfordPA:InvitroactivityoftigecyclineagainstStaphylococcusepidermidisgrowinginanadherent-cellbiofilmmodel.
AntimicrobAgentsChemother2003,47:3967-3969.
42.
DonlanRM:Biofilms:microbiallifeonsurfaces.
EmergInfectDis2002,8:881-890.
43.
ChristensenGD,SimpsonWA,YoungerJJ,BaddourLM,BarrettFF,MeltonDM,BeacheyEH:Adherenceofcoagulase-negativestaphylococcitoplastictissuecultureplates:aquantitativemodelfortheadherenceofstaphylococcitomedicaldevices.
JClinMicrobiol1985,22:996-1006.
44.
OttoM:Virulencefactorsofthecoagulase-negativestaphylococci.
FrontBiosci2004,9:841-863.
45.
EdmistonCEJr,GoheenMP,SeabrookGR,JohnsonCP,LewisBD,BrownKR,TowneJB:Impactofselectiveantimicrobialagentsonstaphylococcaladherencetobiomedicaldevices.
AmJSurg2006,192:344-354.
46.
WuJA,KusumaC,MondJJ,Kokai-KunJF:LysostaphindisruptsStaphylococcusaureusandStaphylococcusepidermidisbiofilmsonartificialsurfaces.
AntimicrobAgentsChemother2003,47:3407-3414.
47.
MoretroT,HermansenL,HolckAL,SidhuMS,RudiK,LangsrudS:Biofilmformationandthepresenceoftheintercellularadhesionlocusicaamongstaphylococcifromfoodandfoodprocessingenvironments.
ApplEnvironMicrobiol2003,69:5648-5655.
48.
GollerCC,RomeoT:Environmentalinfluencesonbiofilmdevelopment.
CurrTopMicrobiolImmunol2008,322:37-66.
doi:10.
1186/1476-0711-9-16Citethisarticleas:Quetal.
,Antibioticsusceptibilityofcoagulase-negativestaphylococciisolatedfromverylowbirthweightbabies:comprehensivecomparisonsofbacteriaatdifferentstagesofbiofilmformationAnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16OpenAccessRESEARCH2010Quetal;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAt-tributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
ResearchAntibioticsusceptibilityofcoagulase-negativestaphylococciisolatedfromverylowbirthweightbabies:comprehensivecomparisonsofbacteriaatdifferentstagesofbiofilmformationYueQu1,AndrewJDaley2,TaghridSIstivan1,SuzanneMGarland2,3,4andMargaretADeighton*1AbstractBackground:Coagulase-negativestaphylococciaremajorcausesofbloodstreaminfectionsinverylowbirthweightbabiescaredforinNeonatalIntensiveCareUnits.
Thevirulenceofthesebacteriaismainlyduetotheirabilitytoformbiofilmsonindwellingmedicaldevices.
Biofilm-relatedinfectionsoftenfailtorespondtoantibioticchemotherapyguidedbyconventionalantibioticsusceptibilitytests.
Methods:Coagulase-negativestaphylococcalbloodcultureisolatesweregrownindifferentphasesrelevanttobiofilmformation:planktoniccellsatmid-logphase,planktoniccellsatstationaryphase,adherentmonolayersandmaturebiofilmsandtheirsusceptibilitiestoconventionalantibioticswereassessed.
Theeffectsofoxacillin,gentamicin,andvancomycinonpreformedbiofilms,atthehighestachievableserumconcentrationswereexamined.
Epifluorescencemicroscopyandconfocallaserscanningmicroscopyincombinationwithbacterialviabilitystainingandpolysaccharidestainingwereusedtoconfirmthestimulatoryeffectsofantibioticsonbiofilms.
Results:Mostcoagulase-negativestaphylococcalclinicalisolateswereresistanttopenicillinG(100%),gentamicin(83.
3%)andoxacillin(91.
7%)andsusceptibletovancomycin(100%),ciprofloxacin(100%),andrifampicin(79.
2%).
Bacteriagrownasadherentmonolayersshowedsimilarsusceptibilitiestotheirplanktoniccounterpartsatmid-logphase.
Isolatesinabiofilmgrowthmodeweremoreresistanttoantibioticsthanbothplanktonicculturesatmid-logphaseandadherentmonolayers;howevertheywereequallyresistantorlessresistantthanplanktoniccellsatstationaryphase.
Moreover,forsomecell-wallactiveantibiotics,concentrationshigherthanconventionalMICswererequiredtopreventtheestablishmentofplanktonicculturesfrombiofilms.
Finally,thebiofilm-growthoftwoS.
capitisisolatescouldbeenhancedbyoxacillinatthehighestachievableserumconcentration.
Conclusion:Weconcludethattheresistanceofcoagulase-negativestaphylococcitomultipleantibioticsinitiallyremainsimilarwhenthebacteriashiftfromaplanktonicgrowthmodeintoanearlyattachedmode,thenincreasesignificantlyastheadherentmodefurtherdevelops.
Furthermore,preformedbiofilmsofsomeCoNSareenhancedbyoxacillininadose-dependentmanner.
BackgroundCoagulase-negativestaphylococci(CoNS),predomi-nantlyStaphylococcusepidermidis,arethemostcommoncausativeagentsofneonatalsepsis[1-3],aconditionwhichhasbeenrelatedtosignificantmorbidityandmor-talityinneonatalintensivecareunits(NICUs)[2].
Thepresenceofacentralvenouscatheterinverylowbirthweight(VLBW)babies(48h),thechoiceiseitherflucloxacillinorvancomycinwithgentamicin.
Ciprofloxacinandrifampicinwerealsoeval-uatedinthisstudyastheefficacyoftheseantibioticsonCoNSbiofilmshasbeenreportedbyotherinvitrostud-ies.
PenicillinGwaspurchasedfromCSLBiotherapies,Parkville,AustraliaandallotherswereobtainedfromSigma-Aldrich,CastleHill,Australia.
EstablishmentofadherentmonolayersBacterialculturesofadherentmonolayerswereestab-lishedfollowingthemethodofMiyakeetal.
(1992),whichinvolvedtheadditionof50μLvolumesofbacterialTable1:Bacterialisolatesandgrowthmediausedforbiofilmformation.
IsolateSpeciesStatusGrowthmediumforbiofilmformationaTSBTSB+1%glucoseTSB+4%NaClicaAicaCicaD1S.
warneriInvasive+---2S.
haemolyticusInvasive----3S.
epidermidisInvasive++++4S.
epidermidisInvasive+---5S.
epidermidisInvasive++++6S.
capitisInvasive+++-7S.
epidermidisInvasive++++8aS.
capitisInvasive+++-8bS.
capitisInvasive+++-9S.
capitisInvasive+++-10S.
epidermidisInvasive+---11S.
epidermidisInvasive++++12S.
epidermidisContaminantw---13S.
epidermidisContaminant++++15S.
capitisContaminantw++-16S.
capitisContaminant+++-17S.
capitisContaminant+++-18S.
capitisContaminant+++-19S.
epidermidisContaminant----20S.
epidermidisContaminant++++21S.
epidermidisContaminant++++22S.
capitisContaminantw+++23S.
epidermidisContaminant++++24S.
epidermidisContaminant++++RP62AS.
epidermidisReference+SP2S.
hominisReference-aSelectionofgrowthmediumwasbasedontheproductionofthehighestbiofilmdensityforeachisolate.
Theamountofbiofilmwasindicatedas:"+",strong(OD600≥0.
24);"w",weak(0.
12≤OD6002*log2inMICorMBCforoxacillin,vancomycin,ciprofloxacinandrifampicin,andanincreaseof>3*log2forMICorMBCforpenicillinandgentamicin,wereconsideredsignificant[38].
Experi-mentstargetingtheeffectsofantibioticsonpreformedbiofilmswererepeatedatleastthreetimesintriplicate.
Onewayanalysisofvariance(ANOVA)orthenon-para-metricMann-Whitneytestwasusedfortwo-setcompar-isonsandap-valueof10241024>102432≤132Biofilm(0.
9-1.
9)*109>1024e>102464>102464>102416>1024d0.
25>10240.
0040.
015Isolate3LogPlanktonic(2.
9-8.
3)*1058160.
250.
250.
250.
25240.
120.
250.
0080.
015Monolayer(5.
0-11.
0)*10532640.
250.
50.
120.
5280.
250.
50.
0020.
004StatPlanktonic(0.
6-2.
2)*109>102432>1024>10241024256Biofilm(0.
3-1.
3)*109>1024>102416160.
25216160.
2520.
54Isolate8aLogPlanktonic(2.
6-5.
5)*105128128323288120.
250.
250.
0080.
03Monolayer(6.
4-11.
1)*105>128>12832646464180.
120.
50.
0040.
015StatPlanktonic(4.
4-4.
9)*108>1024>1024>1024>102410242Biofilm(0.
6-1.
8)*1081024>1024256>1024256>10248>10240.
12>10240.
0044Isolate9LogPlanktonic(3.
1-5.
2)*10532643232816120.
250.
250.
0150.
03Monolayer(4.
6-10.
8)*105>128>12832>1281632280.
250.
50.
0040.
015StatPlanktonic(0.
7-0.
8)*109>1024>1024>1024>1024>1024256Biofilm(1.
1-1.
7)*109>1024>102464>1024>128>10248>10240.
25>10240.
0084Isolate11LogPlanktonic(2.
7-8.
5)*1058864>1283264110.
060.
12>128>128Monolayer(3.
2-17.
8)*1053264>128>1283264120.
060.
12>128>128StatPlanktonic(0.
8-1.
6)*109>1024>1024>1024>10245121024Biofilm(0.
2-0.
6)*109>1024>1024256>1024256>10248>10240.
251664>1024αBiofilm-positiveisolates(OD600>0.
24).
bMICsforCoNSatstationaryphasearenotprovidedasthevaluescouldnotbedeterminedbystandardmethods.
cValuesunderlinedindicateasignificantincreaseintheMICsorMBCsbetweenlog-planktonicandadherentmonolayermodesofgrowth.
dValuesinitalicindicateasignificantchangeintheMBCsbetweenstationary-planktonicandbiofilmmodesofgrowth.
eValuesinboldindicateasignificantincreaseinMICsorMBCsbetweenlog-planktonic/adherentmonolayerandbiofilmmodesofgrowthQuetal.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16Page8of12adherentmonolayers,however,itisalsolikelythatthedifferencewasduetothedifferentkineticsofbiofilmfor-mationbetweenP.
aeruginosaandStaphylococcusspp.
[19].
ConventionalMICshavebeenusedtoguidethetreat-mentofbiofilm-relatedinfectionsatthefebrilestage,basedontheassumptionthatbiofilm-releasedcellsaresimilarintheirsusceptibilitiestocellsintheplanktonicphase[23].
Reporteddifferencesbetweentheconven-tionalMICsandbiofilmMICswereattributedtolackofstandardizationofinitialinoculaandtothepresenceofsmallcolonyvariants[23].
However,inourstudy,wefoundthatMICsofcellwallactiveantibioticswerefre-quentlyhigherforbiofilmgrownbacteriathanplanktoniccultures.
ThisisconsistentwithrecentstudiesbyMoskowitzetal.
(2004)andMelchioretal.
(2006),whoreportedthatbiofilmMICsofβ-lactamantibiotics,butnototherantibiotics,weremuchhigherthantheconven-tionalMICsforP.
aeruginosaandS.
aureusrespectively[21,24].
Theseresultsarenotsurprisinggiventhatcellwallactiveantibioticsmainlyaffectrapidlygrowingbac-teria.
Biofilm-releasedcellsarelikelytobelessactivethandividingplanktoniccellsatmid-logphase,probablybecausetheyhaverecentlyundergoneaswitchfromabiofilmmodeofgrowthtoafree-livingmode,similartocellsatlag-phasegrowth,andrequireachangeingeneexpressiontoadapttothenewenvironment.
TheMBCsofpenicillinG,gentamicin,oxacillin,andvancomycinforCoNSgrowninabiofilmmodeweregen-erally>1024μg/ml,whichiswellbeyondthehighestachievableserumconcentrations.
Althoughsomeoftheseantibioticsatthehighestachievableserumconcen-trationswereeffectiveagainstbacteriagrownplanktoni-callytomid-logphase,theywereinadequatetokillTable3:Antibioticsusceptibilityoffourbiofilm-negativeisolatesagrownindifferentmodes.
IsolateandmodeofgrowthInitialbacterialdensity(CFU/ml)Penicillin(μg/ml)Gentamicin(μg/ml)Oxacillin(μg/ml)Vancomycin(μg/ml)Ciprofloxacin(μg/ml)Rifampicin(μg/ml)MICMBCMICMBCMICMBCMICMBCMICMBCMICMBCSP2LogPlanktonic(1.
2-4.
1)*1050.
50.
5880.
060.
06120.
120.
120.
0080.
03Monolayer(2.
6-4.
7)*10528c16160.
120.
120.
510.
120.
120.
0040.
008StatPlanktonic(3.
5-9.
5)*107b>10241024d2420.
008Biofilm(1.
0-3.
2)*107128>1024e8320.
122240.
1220.
0020.
008Isolate15LogPlanktonic(2.
2-5.
5)*10512812832641632440.
250.
250.
0150.
03Monolayer(5.
2-16.
4)*105>128>12832641664480.
120.
250.
0040.
008StatPlanktonic(3.
7-7.
9)*108>1024>1024>1024>1024>102464Biofilm(0.
7-0.
9)*108>1024>1024256>1024256>102416>10240.
255120.
0040.
008Isolate19LogPlanktonic(2.
9-7.
4)*105>128>128646428220.
250.
250.
0080.
015Monolayer(3.
2-14.
6)*105>128>12864>12828280.
120.
250.
0020.
008StatPlanktonic(0.
7-1.
5)*109>1024>1024>1024>102410242Biofilm(0.
1-0.
2)*1091024>102464>102464>10244>10240.
2540.
0080.
06Isolate22LogPlanktonic(2.
8-5.
6)*105>128>1286464816440.
120.
250.
0150.
03Monolayer(4.
8-16.
3)*105>128>12832>1283232280.
120.
250.
0040.
008StatPlanktonic(0.
5-0.
7)*109>1024>1024>1024>1024>1024256Biofilm0.
2*1091024>1024>128>1024>128>102416>10240.
2510.
0080.
03aBiofilm-negativeisolatesincludedbiofilm-weakproducer(0.
24>OD600≥0.
12)andbiofilm-negativeproducer(OD6002003,88:F89-93.
3.
CheungGY,OttoM:UnderstandingthesignificanceofStaphylococcusepidermidisbacteremiainbabiesandchildren.
CurrOpinInfectDis2010,23:208-216.
4.
Johnson-RobbinsLA,el-MohandesAE,SimmensSJ,KeiserJF:Staphylococcusepidermidissepsisintheintensivecarenursery:acharacterizationofriskassociationsininfants<1,000g.
BiolNeonate1996,69:249-256.
5.
MaasA,FlamentP,PardouA,DeplanoA,DramaixM,StruelensMJ:Centralvenouscatheter-relatedbacteraemiaincriticallyillneonates:riskfactorsandimpactofapreventionprogramme.
JHospInfect1998,40:211-224.
6.
vonEiffC,PetersG,HeilmannC:Pathogenesisofinfectionsduetocoagulase-negativestaphylococci.
LancetInfectDis2002,2:677-685.
7.
KlingenbergC,AaragE,RonnestadA,SollidJE,AbrahamsenTG,KjeldsenG,FlaegstadT:Coagulase-negativestaphylococcalsepsisinneonates.
Associationbetweenantibioticresistance,biofilmformationandthehostinflammatoryresponse.
PediatrInfectDisJ2005,24:817-822.
8.
OttoM:Staphylococcalbiofilms.
CurrTopMicrobiolImmunol2008,322:207-228.
9.
QinZ,OuY,YangL,ZhuY,Tolker-NielsenT,MolinS,QuD:Roleofautolysin-mediatedDNAreleaseinbiofilmformationofStaphylococcusepidermidis.
Microbiology2007,153:2083-2092.
10.
SutherlandIW:Thebiofilmmatrix--animmobilizedbutdynamicmicrobialenvironment.
TrendsMicrobiol2001,9:222-227.
11.
CostertonJW,StewartPS,GreenbergEP:Bacterialbiofilms:acommoncauseofpersistentinfections.
Science1999,284:1318-1322.
12.
Hall-StoodleyL,CostertonJW,StoodleyP:Bacterialbiofilms:fromthenaturalenvironmenttoinfectiousdiseases.
NatRevMicrobiol2004,2:95-108.
13.
MonzonM,OteizaC,LeivaJ,LamataM,AmorenaB:BiofilmtestingofStaphylococcusepidermidisclinicalisolates:lowperformanceofvancomycininrelationtootherantibiotics.
DiagnMicrobiolInfectDis2002,44:319-324.
14.
NishimuraS,TsurumotoT,YonekuraA,AdachiK,ShindoH:AntimicrobialsusceptibilityofStaphylococcusaureusandStaphylococcusepidermidisbiofilmsisolatedfrominfectedtotalhiparthroplastycases.
JOrthopSci2006,11:46-50.
15.
AndersonGG,O'TooleGA:Innateandinducedresistancemechanismsofbacterialbiofilms.
CurrTopMicrobiolImmunol2008,322:85-105.
16.
StewartPS:Mechanismsofantibioticresistanceinbacterialbiofilms.
IntJMedMicrobiol2002,292:107-113.
17.
SpoeringAL,LewisK:BiofilmsandplanktoniccellsofPseudomonasaeruginosahavesimilarresistancetokillingbyantimicrobials.
JBacteriol2001,183:6746-6751.
18.
CercaN,MartinsS,CercaF,JeffersonKK,PierGB,OliveiraR,AzeredoJ:Comparativeassessmentofantibioticsusceptibilityofcoagulase-negativestaphylococciinbiofilmversusplanktoniccultureasassessedbybacterialenumerationorrapidXTTcolorimetry.
JAntimicrobChemother2005,56:331-336.
19.
AaronSD,FerrisW,RamotarK,VandemheenK,ChanF,SaginurR:Singleandcombinationantibioticsusceptibilitiesofplanktonic,adherent,andbiofilm-grownPseudomonasaeruginosaisolatesculturedfromsputaofadultswithcysticfibrosis.
JClinMicrobiol2002,40:4172-4179.
20.
CeriH,OlsonME,StremickC,ReadRR,MorckD,BuretA:TheCalgaryBiofilmDevice:newtechnologyforrapiddeterminationofantibioticsusceptibilitiesofbacterialbiofilms.
JClinMicrobiol1999,37:1771-1776.
21.
MelchiorMB,Fink-GremmelsJ,GaastraW:ComparativeassessmentoftheantimicrobialsusceptibilityofStaphylococcusaureusisolatesfrombovinemastitisinbiofilmversusplanktonicculture.
JVetMedBInfectDisVetPublicHealth2006,53:326-332.
22.
MizunagaS,KamiyamaT,FukudaY,TakahataM,MitsuyamaJ:InfluenceofinoculumsizeofStaphylococcusaureusandPseudomonasaeruginosaoninvitroactivitiesandinvivoefficacyoffluoroquinolonesandcarbapenems.
JAntimicrobChemother2005,56:91-96.
Received:5February2010Accepted:27May2010Published:27May2010Thisarticleisavailablefrom:http://www.
ann-clinmicrob.
com/content/9/1/162010Quetal;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16Quetal.
AnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16http://www.
ann-clinmicrob.
com/content/9/1/16Page12of1223.
CeriH,OlsonM,MorckD,StoreyD,ReadR,BuretA,OlsonB:TheMBECAssaySystem:multipleequivalentbiofilmsforantibioticandbiocidesusceptibilitytesting.
MethodsEnzymol2001,337:377-385.
24.
MoskowitzSM,FosterJM,EmersonJ,BurnsJL:ClinicallyfeasiblebiofilmsusceptibilityassayforisolatesofPseudomonasaeruginosafrompatientswithcysticfibrosis.
JClinMicrobiol2004,42:1915-1922.
25.
KumonH:Managementofbiofilminfectionsintheurinarytract.
WorldJSurg2000,24:1193-1196.
26.
LewisK:Persistercellsandtheriddleofbiofilmsurvival.
Biochemistry(Mosc)2005,70:267-274.
27.
LewisK:Multidrugtoleranceofbiofilmsandpersistercells.
CurrtTopMicrobiolImmunol2008,322:107-131.
28.
PettitRK,WeberCA,KeanMJ,HoffmannH,PettitGR,TanR,FranksKS,HortonML:MicroplateAlamarblueassayforStaphylococcusepidermidisbiofilmsusceptibilitytesting.
AntimicrobAgentsChemother2005,49:2612-2617.
29.
QuY,IstivanTS,DaleyAJ,RouchDA,DeightonMA:Comparisonofvariousantimicrobialagentsascatheterlocksolutions:preferenceforethanolineradicationofcoagulase-negativestaphylococcalbiofilms.
JMedMicrobiol2009,58:442-450.
30.
CercaN,MartinsS,SillankorvaS,JeffersonKK,PierGB,OliveiraR,AzeredoJ:EffectsofgrowthinthepresenceofsubinhibitoryconcentrationsofdicloxacillinonStaphylococcusepidermidisandStaphylococcushaemolyticusbiofilms.
ApplEnvironMicrobiol2005,71:8677-8622.
31.
RachidS,OhlsenK,WitteW,HackerJ,ZiebuhrW:Effectofsubinhibitoryantibioticconcentrationsonpolysaccharideintercellularadhesinexpressioninbiofilm-formingStaphylococcusepidermidis.
AntimicrobAgentsChemother2000,44:3357-3363.
32.
RuppME,HamerKE:Effectofsubinhibitoryconcentrationsofvancomycin,cefazolin,ofloxacin,L-ofloxacinandD-ofloxacinonadherencetointravascularcathetersandbiofilmformationbyStaphylococcusepidermidis.
JAntimicrobChemother1998,41:155-161.
33.
BradfordR,AbdulMananR,DaleyAJ,PearceC,RamalingamA,D'MelloD,MuellerY,UahwatanasakulW,QuY,GrandoD,GarlandS,DeightonM:Coagulase-negativestaphylococciinvery-low-birth-weightinfants:inabilityofgeneticmarkerstodistinguishinvasivestrainsfrombloodculturecontaminants.
EurJClinMicrobiolInfectDis2006,25:283-290.
34.
MiyakeY,FujiwaraS,UsuiT,SuginakaH:Simplemethodformeasuringtheantibioticconcentrationrequiredtokilladherentbacteria.
Chemotherapy1992,38:286-290.
35.
DeightonMA,CapstickJ,DomalewskiE,vanNguyenT:MethodsforstudyingbiofilmsproducedbyStaphylococcusepidermidis.
MethodsEnzymol2001,336:177-195.
36.
Villain-GuillotP,GualtieriM,BastideL,LeonettiJP:InvitroactivitiesofdifferentinhibitorsofbacterialtranscriptionagainstStaphylococcusepidermidisbiofilm.
AntimicrobAgentsChemother2007,51:3117-3121.
37.
GualtieriM,BastideL,Villain-GuillotP,Michaux-CharachonS,LatoucheJ,LeonettiJP:InvitroactivityofanewantibacterialrhodaninederivativeagainstStaphylococcusepidermidisbiofilms.
JAntimicrobChemother2006,58:778-783.
38.
ClinicalandLaboratoryStandardsInstitute(CLSI):Methodsfordilutionantimicrobialsusceptibilitytestsforbacteriathatgrowaerobically.
CLSIdocumentM7-A7,approvedstandard.
7thedition.
Wayne,PA;CLSI;2004.
39.
MurrayPR,BaronEJ,JorgensenJH,LandryML,PfallerMA,(ed):ManualofClinicalMicrobiology.
Washington,D.
C.
:ASMPress;2007.
40.
VillariP,SarnataroC,IacuzioL:MolecularepidemiologyofStaphylococcusepidermidisinaneonatalintensivecareunitoverathree-yearperiod.
JClinMicrobiol2008,38:1740-1746.
41.
LabthavikulP,PetersenPJ,BradfordPA:InvitroactivityoftigecyclineagainstStaphylococcusepidermidisgrowinginanadherent-cellbiofilmmodel.
AntimicrobAgentsChemother2003,47:3967-3969.
42.
DonlanRM:Biofilms:microbiallifeonsurfaces.
EmergInfectDis2002,8:881-890.
43.
ChristensenGD,SimpsonWA,YoungerJJ,BaddourLM,BarrettFF,MeltonDM,BeacheyEH:Adherenceofcoagulase-negativestaphylococcitoplastictissuecultureplates:aquantitativemodelfortheadherenceofstaphylococcitomedicaldevices.
JClinMicrobiol1985,22:996-1006.
44.
OttoM:Virulencefactorsofthecoagulase-negativestaphylococci.
FrontBiosci2004,9:841-863.
45.
EdmistonCEJr,GoheenMP,SeabrookGR,JohnsonCP,LewisBD,BrownKR,TowneJB:Impactofselectiveantimicrobialagentsonstaphylococcaladherencetobiomedicaldevices.
AmJSurg2006,192:344-354.
46.
WuJA,KusumaC,MondJJ,Kokai-KunJF:LysostaphindisruptsStaphylococcusaureusandStaphylococcusepidermidisbiofilmsonartificialsurfaces.
AntimicrobAgentsChemother2003,47:3407-3414.
47.
MoretroT,HermansenL,HolckAL,SidhuMS,RudiK,LangsrudS:Biofilmformationandthepresenceoftheintercellularadhesionlocusicaamongstaphylococcifromfoodandfoodprocessingenvironments.
ApplEnvironMicrobiol2003,69:5648-5655.
48.
GollerCC,RomeoT:Environmentalinfluencesonbiofilmdevelopment.
CurrTopMicrobiolImmunol2008,322:37-66.
doi:10.
1186/1476-0711-9-16Citethisarticleas:Quetal.
,Antibioticsusceptibilityofcoagulase-negativestaphylococciisolatedfromverylowbirthweightbabies:comprehensivecomparisonsofbacteriaatdifferentstagesofbiofilmformationAnnalsofClinicalMicrobiologyandAntimicrobials2010,9:16
- 8.5www.88ququ.com相关文档
- Versionwww.88ququ.com
- apwww.88ququ.com
- recordedwww.88ququ.com
- stractionwww.88ququ.com
- Websterwww.88ququ.com
- componentswww.88ququ.com
酷番云-618云上秒杀,香港1核2M 29/月,高防服务器20M 147/月 50M 450/月,续费同价!
官方网站:点击访问酷番云官网活动方案:优惠方案一(限时秒杀专场)有需要海外的可以看看,比较划算29月,建议年付划算,月付续费不同价,这个专区。国内节点可以看看,性能高IO为主, 比较少见。平常一般就100IO 左右。优惠方案二(高防专场)高防专区主要以高防为主,节点有宿迁,绍兴,成都,宁波等,节点挺多,都支持防火墙自助控制。续费同价以下专场。 优惠方案三(精选物理机)西南地区节点比较划算,赠送5...

Sharktech10Gbps带宽,不限制流量,自带5个IPv4,100G防御
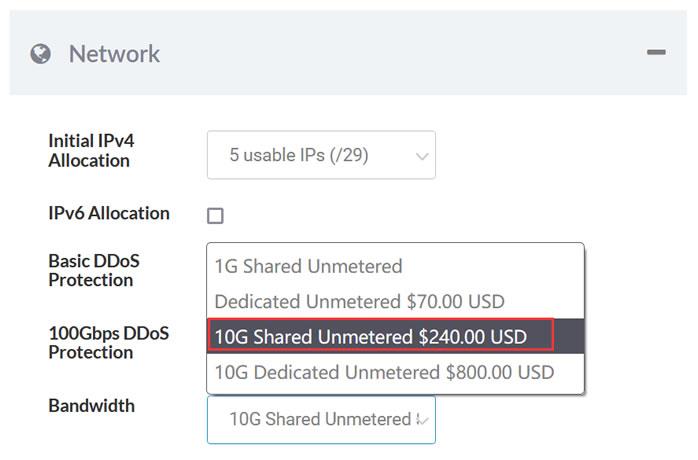
Sharktech荷兰10G带宽的独立服务器月付319美元起,10Gbps共享带宽,不限制流量,自带5个IPv4,免费60Gbps的 DDoS防御,可加到100G防御。CPU内存HDD价格购买地址E3-1270v216G2T$319/月链接E3-1270v516G2T$329/月链接2*E5-2670v232G2T$389/月链接2*E5-2678v364G2T$409/月链接这里我们需要注意,默...
UCloud优刻得,新增1核1G内存AMD快杰云机型,服务器2元/首月,47元/年
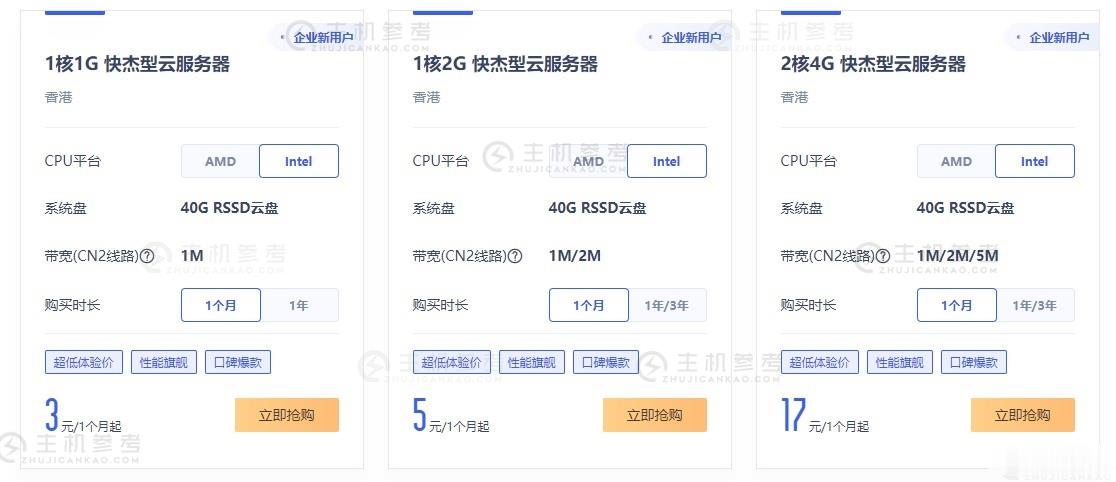
UCloud优刻得近日针对全球大促活动进行了一次改版,这次改版更加优惠了,要比之前的优惠价格还要低一些,并且新增了1核心1G内存的快杰云服务器,2元/首年,47元/年,这个价格应该是目前市面上最低最便宜的云服务器产品了,有需要国内外便宜VPS云服务器的朋友可以关注一下。UCloud好不好,UCloud服务器怎么样?UCloud服务器值不值得购买UCloud是优刻得科技股份有限公司旗下拥有的云计算服...
www.88ququ.com为你推荐
-
brandoff国际大牌包包都有哪些呐?蓝色骨头手机蓝色骨头为什么还没上映陈嘉垣马德钟狼吻案事件是怎么回事铂金血痕手上出现这种血痕是什么情况。有谁知道能告诉下吗? 怎么治疗!www.stockstar.com股票分析软件哪个好用?用过的介绍一些莱姿蔓蕊姿蔓是什么样的牌子来的雀嘴鳝专家教下怎么才能饲养好一条雀鳝鱼?长房娇女人蛮好脾气好很乖巧很听话?娇身惯养,父亲长得好看大眼睛高鼻梁樱桃小嘴瓜子脸女儿也是眼睛大大的皮肤chudian365经常看到“防触电保护Ⅰ类”,这个是什么意思?这些类又是怎么分的啊?请指教◎弗雷德疯哈利波特与死亡圣器前面的两首诗是什么含义啊?