Methodswww.qqq147.com
www.qqq147.com 时间:2021-03-20 阅读:()
ThisisanAcceptedManuscript,whichhasbeenthroughtheRoyalSocietyofChemistrypeerreviewprocessandhasbeenacceptedforpublication.
AcceptedManuscriptsarepublishedonlineshortlyafteracceptance,beforetechnicalediting,formattingandproofreading.
Usingthisfreeservice,authorscanmaketheirresultsavailabletothecommunity,incitableform,beforewepublishtheeditedarticle.
WewillreplacethisAcceptedManuscriptwiththeeditedandformattedAdvanceArticleassoonasitisavailable.
YoucanfindmoreinformationaboutAcceptedManuscriptsintheInformationforAuthors.
Pleasenotethattechnicaleditingmayintroduceminorchangestothetextand/orgraphics,whichmayaltercontent.
Thejournal'sstandardTerms&ConditionsandtheEthicalguidelinesstillapply.
InnoeventshalltheRoyalSocietyofChemistrybeheldresponsibleforanyerrorsoromissionsinthisAcceptedManuscriptoranyconsequencesarisingfromtheuseofanyinformationitcontains.
AcceptedManuscriptFood&Functionwww.
rsc.
org/foodfunction1ChemicalcomponentsfromtheHaulmofArtemisiaSelengensisandthe1InhibitoryEffectonGlycationofβ-lactoglobulin23XiaomingLi,YonglinLu,RonghuaDeng,TiesongZheng,LishuangLv*45DepartmentofFoodScienceandTechnology,GinlingCollege,NanjingNormal6University,122#NinghaiRoad,Nanjing,210097,P.
R.
China789101112Correspondingauthor:LishuangLv13Tel.
:+862583598286;fax:+862583707623.
14E-mailaddress:lishuanglv@126.
com;lulishuang@njnu.
edu.
cn1516Page1of23Food&Function2ABSTRACT17Artemisiaselengensis(AS)hasbeentraditionallyusedasbothfoodand18medicineforthousandsofyearsinChina.
Inourstudies,L-tryptophanwasfirstly19isolatedfromthehaulmofAStogetherwithluteolin,rutin,and20kaempferol-3-O-glucuronide.
Theirstructureswereelucidatedbyspectroscopic21methodsincludingHRMS,1Dand2DNMR.
Threeflavonoidcompoundsshowed22satisfactorysuppressioneffectsonformationofadvancedglycationendproducts23(AGEs)inβ-lactoglobulin-Lactose/MGO/GOmodelsystems,andtheiranti-glycation24activitiesexhibitedadose-dependentmanner.
Amongthosecompounds,25kaempferol-3-O-glucuronidewasdemonstratedthestrongestinhibitoragainst26formationofAGEs.
27KEYWORDS:ArtemisiaSelengensishaulm;β-lactoglobulin;advancedglycation28endproducts(AGEs).
29Kaempferol-3-O-glucuronideCID:44258914;RutinCID:5280805;30LuteolinCID:5280445;3132Page2of23Food&Function31.
Introduction33Artemisiaselengensis(AS)isanherbaceousperennialplantoftheCompositae34family,localizedatthewater'sedgeonbanksorinswamps,withwildspecieswidely35distributedovertheNortheast,North,andcentralChinasincetheMingdynasty.
36They'realsofoundinMongolia,Russia,andKorea.
InCompendiumofMateria37Medica,itisrecordedthatAShasdiversebiologicalactivities,e.
g.
hemostasia,38anti-inflammation,relievingcough,reducingsputum,andtreatmentofacute39infectioushepatitis,duetoAS'svariousactivecompounds.
Beingavaluablenatural40product,ASisbeneficialtohuman'shealth.
Someclinicaltherapiesarealsoreported41intheliterature1-3.
42InNanjing,earlythe1990's,peoplestartedplantingAS.
Nowadays,AShas43becomeverypopularasahealthyfood,beingdeliciousandnutritious,foritstender44stemisavegetablewithuniqueflavor,beingfragrant,fresh,andcrisp.
However,the45olderhaulmofASisusuallydiscardedbecauseofit'sinedible.
Todate,someofits46majorbiologicallyactivecomponentshavebeenisolated,suchasflavonoids4-6,47polysaccharides7,phenolicacids8guaianolides9andfattyacids10.
Flavonoidsinthe48haulmofAScomprisemorethan1%byweight,buthavehithertobeenlargely49ignoredinthefieldoffoodsciences.
Ourpreviousstudyshowedthattheflavonoid50contentofASincreasedwiththegrowthofASstems.
Withthisstudy,weinvestigate51thechemicalcompositionandbiologicalactivitiesoftheAShaulmtopromotethe52utilizationofASinfoodprocessing.
53Methylglyoxal(MGO)andglyoxal(GO),twomajorα-dicarbonylcompounds54Page3of23Food&Function4formedfrombothglycoxidationandlipoxidation11,arethepivotalintermediatesin55theformationofadvancedglycationendproducts(AGEs)invivo12.
Infood56processing,theα-dicarbonylcompoundsaregeneratedthroughroasting,baking,57broiling,andfryingduetocaramelization,theMaillardreaction,andlipidoxidation13.
58Duringsuchreactions,MGOandGOglycateproteinsfasterthansugars,causing59inter-andintramolecularcross-linksofproteins,thustheamountofAGEssharply60increases14.
61β-lactoglobulin(β-lg)isahighqualityproteinfoundinavarietyoffoodstuffs,62includinginfantformulas,bakedproducts,andbeverages.
TheeffectsoftheMaillard63reactionandglycationonthisproteinduringheatingarewellknownandreplicable,as64milkcontainshighamountsoflactoseandlowerquantitiesofotherreducingsugars.
65Thisreplicabilitymakesitagoodmodelwithrealworld-applicationstotestthe66anti-AGEspropertiesofourcompounds.
67Flavonoidsarecommondietarycomponentsofplant-derivedfoods.
Several68flavonoids:(-)-epigallocatechin3-gallate(EGCG)fromtea15,phloretinfromapple16,69genisteinfromsoybean17,proanthocyanidinsandanthocyaninfromberries18,are70knowntobescavengersofAGEsbytrappingreactivedicarbonylcompounds.
71Inthepresentstudy,weinvestigatechemicalcomponentsfromAShaulmand72theirinhibitoryactivitiesagainsttheformationofAGEsusingthe73β-lg-lactose/MGO/GOmodelsystems,tofindfood-derivedinhibitorsofAGEs.
We74expectflavonoidstopreventproteinglycationduringfoodthermalprocessing.
752.
MaterialsandMethods76Page4of23Food&Function52.
1.
Materials77Theair-driedhaulmofArtemisiaSelengensiswasobtainedfromthe"Baguazhou"78districtinNanjing.
β-lactoglobulin(≥92%)waspurifiedinourlab(Nanjing,79Jiangsu,China).
Thechemicalstandardkaempferol-3-O-glucuronide,rutin,and80luteolin;methylglyoxal(MGO,40%inwater),glyoxal(GO,40%inwater);D2O,81CD3ODandDMSO-d6wereobtainedfromSigma-AldrichCo.
(St.
Louis,MO,USA).
82HPLC-gradesolventsandotherreagentswereobtainedfromShanghaiSinopharm83ChemicalReagentCo.
,Ltd(Shanghai,China).
HPLC-gradewaterwaspreparedusing84aMilliporeMilli-Qpurificationsystem(Bedford,MA,USA).
852.
2.
Extractionandpurificationprocedure86TheextractionandpurificationprocedureareshowninSupplementalFigure1.
87ThedriedpowderofAShaulm(1kg)wasextractedwith75%ethanol(7.
5L)at8890°Cfor60min.
Thesolventwasremovedbyfiltration,andthenfreshsolventwas89addedtotheresidue.
Theextractionprocesswastwicerepeated.
Thecombined90filtrateswereconcentratedunderreducedpressureat40°Cbyarotaryevaporator91(TokyoRikakikaiCo.
,Tokyo,Japan).
92Toremovethepigment,thethicksolutionwasputintoaseparatingfunnelfor93furtherextraction.
Petroleumetherwasusedasextractantata1:3ratio.
These94water-solublefractionswerelaterevaporatedandlyophilized.
95DriedAScrudeextractwasre-suspendedin200mLdistilledwaterandloadedonto96aglasscolumn(5.
8*55cm)packedwithAB-8macroporousadsorptionresin.
After97thesamplewasloaded,elutionwashaltedfor10mintofacilitateadsorptionof98Page5of23Food&Function6chemicalsonresinbeads.
Fivefractions(AS-01~AS-05)werecollectedbyelutingthe99columnwith1.
5Lof0,10%,30%,50%,and70%ethanolsequentially.
TheAS-02100fraction(10%ethanolelutedportion)wassubjectedtoanODScolumn,elutedwitha101gradientsystem:10%,20%,30%,40%,and100%MeOH(500mLforeachgradient102system).
Thetwofractionsyielded,AS-02-1(10%MeOHeluted)andAS-02-2(20%103MeOHeluted),werefurtherseparatedonaSephadexLH-20columnrespectively,and104elutedwithethanol.
TwocompoundsASF-1(fromAS-02-1)andASF-2(from105AS-02-2)wereobtained.
TheAS-03fraction(30%ethanolelutedportionfromAB-8106column)wasseparatedbyanODScolumnusingthesameprocedureastheAS-02107fractiontoobtainASF-3.
TheAS-04fraction(50%ethanolelutedportionfromAB-8108column)waschromatographedonsilicagelcolumnandelutedwithCH3Cl3/MeOH109byagradientof98:2,20:1,10:1,8:1,6:1,2:1,and0:1toaffordASF-4.
Theamount110ofAS-05fraction(70%ethanolelutedportionfromAB-8column)wastoosmallto111abandon.
1122.
3.
AnalysisHPLC-MSprocedure113TheseparationofallfractionsexceptASF-1andofflinedatacollectionforHPLC114basedactivityprofilingwerecarriedoutwithaseriesAgilent1200HPLCsystem,115whichconsistedofadegasser,abinpump,acolumnoven,adiodearraydetector,and116aQQQmassdetector(Agilent,SantaClara,CA,USA)incorporatedwithelectrospray117ionization(ESI)interfaces.
AHPLCZORBAXEclipseXDB-C18(250*4.
6mmi.
d.
,5118m)wasusedforseparationataflowrateof0.
6mL/min.
Themobilephasefulfilled119thefollowingrequirements:(1)CompoundASF-2:Waterwith0.
5%formicacid(A)120Page6of23Food&Function7andAcetonitrilewith0.
1%formicacid(B)wereusedassolventsfora40min121program:Thecolumnwaselutedwith12%solventBfor10min,followedbylinear122increasesinBto20%within10to30min,thento30%from30to40min.
TheUV123detectorwassetat350nm.
Compound2hadanintensepeekatRT=37.
22min;(2)124CompoundASF-3:Waterwith0.
01%aceticacid(A)andAcetonitrile(B)wereused125assolventsfora25minprogram:Thecolumnwaselutedwith90%solventAfor10126min,followedbylinearincreasesinBto60%from10to20min,andthenwith60%127Bfrom21to25min.
TheUVdetectorwassetat350nm.
Compound3hadanintense128peekatRT=15.
17min.
(3)CompoundASF-4:Waterwith0.
5%formicacid(A)129andAcetonitrilewith0.
1%formicacid(B)wereusedassolventsfora55min130program:Thecolumnwaselutedwith88%solventAfor10min,followedbylinear131increasesinBto20%from10to30min,to30%from30to40min,to60%from40132to48min,andthenre-equilibratedwith90%Bfor6minfrom49to55min.
TheUV133detectorwassetat254nm.
Compound4hadanintensepeekatRT=47.
33min.
For134MS:ThenegativeionpolaritymodewassetforESIionsource.
Thetypicaloperating135parameterswereasfollows:sprayneedlevoltage,5kV;nitrogensheathgas,45136(arbitraryunits);auxiliarygas,5(arbitraryunits).
137Incontrast,fractionASF-1wascarriedoutwithaseriesAgilent1290Infinity6224138TOFsystem(Agilent,SantaClara,CA,USA).
AHPLCZORBAXEclipseXDB-C18139(250*4.
6mmi.
d.
,5m)wasusedforseparationataflowrateof0.
8mL/min.
The140columnwaselutedwith40%solventB(Acetonitrile)and60%solventA(waterwith1410.
01%aceticacid)fora15min.
TheUVdetectorwassetat280nm.
Compound1had142Page7of23Food&Function8anintensepeekatRT=3.
77min.
ForMS:Thenegativeionpolaritymodewassetfor143ESIionsource.
Thetypicaloperatingparameterswereasfollows:sprayneedle144voltage,5kV;nitrogensheathgas,45(arbitraryunits);auxiliarygas,5(arbitrary145units).
146Thestructuralinformationofcompounds1-4wasobtainedbytandemmass147spectrometry(MS/MS)throughcollision-induceddissociation(CID)witharelative148collisionenergysettingas35%.
DataacquisitionwasperformedwithQualitative149AnalysisofMasshunter(Agilent,SantaClara,CA,USA).
1502.
4.
NMRAnalysis151NMR1H(400MHz),13C(100MHz),and2DNMRspectrawereobtainedonan152AVANCE400(BrukerDaltonicsCo.
,Bremen,Germany)spectrometerwithTMSas153internalreference.
1542.
5.
InhibitionofCompounds(ASF-2~ASF-4)againstformationofAGEsin155β-lactoglobulinglycationsystems156β-lactoglobulin(0.
083mmol/L)wasincubatedwithlactose(0.
083mol/L)or157MGO/GO(1.
5mmol/L)atchosenratios(1:1000,1:18)inthepresenceorabsenceof158Compounds1-4(0.
05,0.
1,0.
5mmol/L)inphosphatebuffer(pH6.
5)at85°C.
Then,159thesamplewascollectedatselectedtimepoints(0,15,30,45,60,90,120min)and160storedat-80°C.
Amultimodemicroplatereader(BioTek,Winooski,VT)wasusedfor161thequantificationofAGEs.
The%inhibitionofAGEsformation=[1-(fluorescenceof162thetestgroup/fluorescenceofthecontrolgroup)]*100%19.
Eachsamplewas163Page8of23Food&Function9performedintriplicateandtheexperimentwasdonethreetimeswithcomparable164results.
1653.
Results1663.
1Structureelucidation167AnalysisoftheethanolextractobtainedfromthehaulmofASbyrepeatedcolumn168chromatography(AB-8,ODS,andSephadexLH-20orsilicagel)ledtotheisolation169andidentificationonecompound(ASF-1),alongwiththreeflavonoidcompounds170(ASF-2~ASF-4).
171Thethreeflavonoids,kaempferol-3-O-glucuronide(ASF-2)20,172rutin(ASF-3)21andluteolin(ASF-4)22,wereidentifiedbycomparisonoftheir173spectroscopicdatawiththosereportedintheliterature.
174CompoundASF-1hadamolecularformulaofC11H12N2O2asdeterminedby175ESI-HRMS,NMR1H,13C,HMBC,HMQCdata(SupplementalFigure2-3).
The176negativeESI-MSofcompoundASF-1showedamolecularionpeakatm/z203.
1000177[M-H]-,indicatingamolecularweightof204.
The1HNMRofdata(Table1)revealed178fouraromaticH-atoms,whosesignalfromoneprotonindownfieldwasobservedatd1797.
62(d,J=8.
0Hz),wasidentifiedasH-4,whileoneprotonatd7.
42(d,J=8.
1Hz)180wasevidencedtobeH-7;andthereweretwoothersignals,oneprotonatd7.
17(d,1818.
1),theotheratd7.
09(t,J=7.
7Hz),whichwereassignedtoH-5andH-6,182respectively.
Oneprotonresonancemultipletindicativeofamethinegroupwas183observedatδH3.
93(m),andtwoone-protondoubledoubletsasmethylenegroups184attachedtocarbonylmoieties[δH3.
18(dd,J=7.
2,8.
0Hz);δH3.
37(dd,J=10.
6,4.
7185Page9of23Food&Function10Hz)].
The13CNMRspectrumofASF-1(Table1)indicatedthepresenceofacarbonyl186group(δC174.
4),anun-substitutedaromaticring(δC111.
9,119.
4,122.
1,118.
4),a187tetra-substitutedaromaticring(δC136.
3,126.
6),amethylenegroup(δC26.
3),a188methinegroup(δC55.
8),aswellastwoolefincarbons(δC125.
0,107.
5).
This189spectroscopicinformationwasusedtoestablishitscorestructureasanaromaticring190fusedtoaheterocyclicring,whichwasconfirmedbytheHMBCcorrelationdata191(Figure2):H-11[δH3.
93(m)]andH-10[δH3.
18(dd,J=7.
2,8.
0Hz);δH3.
37(dd,J=1924.
7,10.
6Hz)]werecorrelatedwithC-12(δC174.
5)andthequaternarycarbonatδC193107.
5(C-3),whichconfirmedtheconnectionofCH-CH2andC=Omoieties.
194Additionally,H-7[δH7.
42(d,8.
1)]wascorrelatedwithC-9(δC126.
6)andC-6(δC195119.
4);H-4[δH7.
62(d,J=8.
0Hz)]wascorrelatedwithC-5(δC122.
1)andC-9(δC196126.
3).
ThissuggestedthatC-7andC-4atthearomaticringconnectedwitha197heterocyclicring.
Thus,ASF-1wasdeterminedasL-tryptophan(Figure1).
1983.
2.
InhibitoryEffectsofCompounds(ASF-2~ASF-4)ontheFormationofAGEs199Theflavonoidcompounds(ASF-2~ASF-4)showedsatisfactorysuppressionagainst200theformationofAGEsintheβ-lg-lactose/MGO/GOmodelsystems(Figure2).
201Increasingtheconcentration(0.
05-0.
5mmol/mL)ofeachcompoundgradually202augmentedtheinhibitoryactivitiesagainsttheformationofAGEs.
Amongthese203compounds,kaempferol-3-O-glucuronideexhibitedthestrongestanti-glycation204capacityatthesameconcentrationinthreedifferentβ-lactoglobulinsystems.
The205abilitiesofthefourcompoundstoinhibitAGEswere:kaempferol-3-O-glucuronide>206luteolin>rutin.
207Page10of23Food&Function11Intheβ-lg-lactosesystem,therespectiveinhibitionratiosof208kaempferol-3-O-glucuronide,rutin,orluteolin(0.
05mmol/L)againstAGEswere20933.
56%,23.
75%,and22.
38%at120min(Figure2),asAGEswereslowlygenerated210throughoutthereaction.
Withanincreaseto0.
5mmol/L,theinhibitionratios211increasedto60.
35%,54.
32%and33.
56%respectively.
Thissuggeststhatthe212anti-glycationactivitiesofthesecompoundshaveapositivecorrelationwith213concentration.
214Intheβ-lg-MGO/GOsystems(Figures3and4),thesethreecompoundsalso215showedsignificantinhibitionat0.
05mmol/L,althoughhighlyactiveMGOorGOled216tomoreAGEsthanlactose.
Ofthesecompounds,kaempferol-3-O-glucuronideisthe217mostefficientAGEsinhibitorindifferentβ-lactoglobulinsystems.
For60%inhibition,2180.
5mmol/Lkaempferol-3-O-glucuronidewasneededintheβ-lg-lactosesystemat21985°Cfor120min;whileonly0.
1mmol/Lkaempferol-3-O-glucuronideneededinthe220β-lg-GOsystemandonly0.
05mmol/Lwasneededtheinβ-lg-MGOsystem(Figures2212B,3B,and4B).
2224.
Discussion223Inthisstudy,acompoundL-tryptophanwasfirstlyisolatedfromAShaulm224togetherwiththreeflavonoidcompounds.
ThestructureofL-tryptophanwas225determinedbyHRMSspectra,1Dand2DNMRdata.
Theflavonoidsshowed226powerfulinhibitoryeffectsagainsttheformationofAGEsinβ-lactoglobulin227glycation.
228Recentstudieshaveshownthattheflavonoids,e.
g.
,flavanol(EGCG),chalcone229Page11of23Food&Function12(phloretinandphloredzin),andisoflavone(genistein),rapidlytrapsMGOatC-6,C-8230unsubstitutedcarbonsattheAringandformmono-anddi-MGOadducts,thus231inhibitingtheformationofAGEs15-17.
232Thestructuresofthesecompoundscanaccountforthedifferenceintheiractivities.
233Kaempferol-3-O-glucuronideistheglycosideoftheflavonolkaemoferol.
Rutinisthe234glycosidebetweenflavonolquercetinanddisacchariderutinose.
Asflavonols,they235bothhavethesamearomaticAring,Bring,andaheterocyclicCring.
Wefoundthat236kaempferol-3-O-glucuronideexhibitshigheractivityintheanti-glycationof237β-lactoglobulin,althoughrutinhasonemore-OHthankaempferol-3-O-glucuronide238atC-5ontheBring.
However,ifflavonoidspossessthesameAandCrings,a239differenceinthenumberofhydroxylgroupsontheBringdoesnotplayasignificant240roleontrappingefficacy23.
Thegreaterefficiencyofkaempferil-3-O-glucuronideis241likelyduetolesssterichindrancefromitssingleglycosideversusrutin'sdisaccharide,242rutinose.
Thedifferencebetweenrutinandluteolinisthatluteolinisanaglyconeof243flavone,andtherearenohydroxylgroupsorglucosidesatC-3onluteolin'sCring.
244Andourresultsindicatethatluteolinhashigherinhibitoryeffectsthanrutin.
Thisis245consistentwithcurrentliteraturethatluteolinandrutinexhibitsignificantinhibitory246effectsof82.
2%and77.
7%intheBSAglycationsystematpH7.
4and37°C.
We247predictthatthereasonforthisphenomenonisthatluteolinlackssterichindrancein248thetrappingreaction.
249Ourdatahighlighttheeffectsofthesecompoundsonglycationofβ-lactoglobulin250viaα-dicarbonylcompounds.
α-dicarbonylcompoundsarisefromfreesugar,the251Page12of23Food&Function13initialSchiffbases,Amadoriandotherintermediates24-26.
Sinceα-dicarbonyl252compoundsandglucosecanpotentiallydamagedifferentsubsetsofproteins,253experimentaloutcomesmaybedifferent.
Whileintheβ-lg-lactosesystem,these254flavonoidcompoundsalsoexhibitedsignificantinhibitionagainstglycationof255β-lactoglobulin.
Themechanismofinhibitionofglucose-mediatedglycationneeds256furtherdiscussion.
257Inaddition,thehighpercentageofL-tryptophaninAShaulmcouldalsocontribute258totheinhibitoryeffectontheglycationofβ-lactoglobulininβ-lg-MGO/GO/lactose259systemsviathefreeaminogrouporiminogroupreactingwiththereactivecarbonyl.
260Recently,flavonoidshaveshownmoreandmorepotentialasstronginhibitors261againsttheformationofAGEsinvivo,butthereonlyafewstudiesthatinvolved262flavonoidsintheanti-glycationoffoodproteinsathightemperatures.
Wesuggestthat263theflavonoidsfromvariousfoodsandnaturalplantscanbeusedaseffectiveAGEs264inhibitorsinfoodthermalprocessing.
Ourresultsinthisstudypavethewayfor265furtherinvestigationofthesecompoundsfromAShaulminrealfoodmatricesto266confirmtheinhibitoryactivitiesagainsttheformationofAGEs.
267Appendix268SupplementalFigure1.
Procedureofextractionandpurificationofflavonesfrom269ArtemisiaSelengensishaulm270SupplementalFigure2.
TheHPLC-MSspectraofcompoundASF-1.
(A)271HPLCDADchromatogramsspectrum,(B)HRMSspectrum272Page13of23Food&Function14SupplementalFigure3.
The1HNMRspectraofcompoundASF-1.
(A).
1HNMR273spectrum;(B).
13CNMRspectrum;(C).
HMQCspectrum;(D).
HMBCspectrum274Acknowledgements275ThisworkwassupportedbyNSFofJiangsuprovinceofChina(Project276BK2012850)andNaturalScienceFoundationofZhejiangprovinceofChina(Project277LY12C15001).
OurthankstoAaronYerkefromNorthCarolinaAgriculturaland278TechnicalUniversityforhissuggestionsonrevisionsandediting.
279Conflictofinterest280Thereisnoconflictofinterestforallauthors.
281282Page14of23Food&Function15References2831.
C.
Deng,X.
Xu,N.
Yao,N.
LiandX.
Zhang,AnalyticaChimicaActa,2006,556,289-294.
2842.
L.
Peng,Y.
Wang,H.
ZhuandQ.
Chen,FoodChemistry,2011,125,1064-1071.
2853.
Y.
ZhuandM.
Qin,ChineseArchivesOfTraditionalChineseMedicine,2006,24,1749-1786.
2864.
L.
Peng,X.
Jia,Y.
Wang,H.
ZhuandQ.
Chen,FoodAnalyticalMethods,2009,3,261-268.
2875.
J.
ZhangandL.
Kong,ChineseTraditionalandHerbalDrugs,2008,39,23-26.
2886.
J.
Zhang,Y.
LinandL.
Kong,ChineseTraditionalandHerbalDrugs,2004,35,979-980.
2897.
K.
A.
Koo,Kwak,J.
H.
,Lee,K.
R.
,Zee,O.
P.
,Woo,E.
R.
,Park,H.
K.
,Youn,290H.
J.
,ArchivesofPharmacalResearch1994,17,371-374.
2918.
Z.
Tu,L.
Zhang,H.
Wang,Y.
YeandW.
Liu,ScienceandTechnologyofFoodIndustry,2012,29233,239-242.
2939.
X.
F.
JinfengHu,ChineseChemicalLetters,1998,9,829-832.
29410.
L.
Zhang,Z.
-c.
Tu,T.
Yuan,H.
Wang,Z.
-f.
Fu,Q.
-h.
WenandX.
-q.
Wang,IndustrialCrops295andProducts,2014,56,223-230.
29611.
E.
B.
Frye,T.
P.
Degenhardt,S.
R.
ThorpeandJ.
W.
Baynes,JournalofBiologicalChemistry,2971998,273,18714-18719.
29812.
P.
Beisswenger,S.
Howell,R.
Nelson,M.
MauerandB.
Szwergold,BiochemicalSociety299Transactions,2003,31,1358-1363.
30013.
J.
Degen,M.
HellwigandT.
Henle,JournalofAgriculturalandFoodChemistry,2012,60,3017071-7079.
30214.
N.
RabbaniandP.
J.
Thornalley,BiochemicalSocietyTransactions,2008,36,1045-1050.
30315.
S.
Sang,X.
Shao,N.
Bai,C.
-Y.
Lo,C.
S.
YangandC.
-T.
Ho,Chemicalresearchintoxicology,3042007,20,1862-1870.
30516.
X.
Shao,N.
Bai,K.
He,C.
-T.
Ho,C.
S.
YangandS.
Sang,Chemicalresearchintoxicology,3062008,21,2042-2050.
30717.
L.
Lv,X.
Shao,H.
Chen,C.
T.
HoandS.
Sang,Chemicalresearchintoxicology,2011,24,308579-586.
30918.
W.
Wang,Y.
Yagiz,T.
J.
Buran,C.
d.
N.
NunesandL.
Gu,FoodResearchInternational,2011,31044,2666-2673.
31119.
A.
J.
Furth,Analyticalbiochemistry,1988,175,347-360.
31220.
N.
Castillo-Muoz,S.
Gómez-Alonso,E.
García-Romero,M.
V.
Gómez,A.
H.
VeldersandI.
313Hermosín-Gutiérrez,JournalofAgriculturalandFoodChemistry,2008,57,209-219.
31421.
F.
Fathiazad,A.
Delazar,R.
AmiriandS.
D.
Sarker,IranianJournalofPharmaceutical315Research,2006,3,222-227.
31622.
S.
J.
Kellam,K.
A.
Mitchell,J.
W.
Blunt,M.
H.
MunroandJ.
R.
Walker,Phytochemistry,3171993,33,867-869.
31823.
X.
Shao,H.
Chen,Y.
Zhu,R.
Sedighi,C.
-T.
HoandS.
Sang,JournalofAgriculturalandFood319Chemistry,2014,62,3202-3210.
32024.
E.
Fujimori,BiochimBiophysActa,1989,998,105-110.
32125.
S.
P.
WolffandR.
T.
Dean,BiochemicalJournal,1987,245,243-250.
32226.
J.
V.
Hunt,R.
T.
DeanandS.
P.
Wolff,BiochemicalJournal,1988,256,205-212.
323324325Page15of23Food&Function16FigureCaption326Figure1.
StructuresofcompoundsASF-1~ASF-4.
A.
L-tryptophan(compound327ASF-1);B.
kaempferol-3-O-glucuronide(compoundASF-2);C.
rutin(compound328ASF-3);D.
luteolin(compoundASF-4).
329Figure2.
KeyHMBCcorrelationsofcompoundASF-1.
330Figure3.
InhibitoryeffectsofCompoundsASF-2~ASF-4onAGEs'formationin331β-lg-MGOmodelundertheratio1:18at85°Cfor120min.
Dataarepresentedasthe332means±SDofthreereplications.
A.
kaempferol-3-O-glucuronide(compoundASF-2);333B.
rutin(compoundASF-3);C.
luteolin(compoundASF-4).
Dataarepresentedasthe334means±SDofthreereplications.
335Figure4.
InhibitoryeffectsofCompoundsASF-2~ASF-4onAGEs'formationin336β-lg-GOmodelundertheration1:18at85°Cfor120min.
A.
337kaempferol-3-O-glucuronide(compoundASF-2);B.
rutin(compoundASF-3);C.
338luteolin(compoundASF-4).
Dataarepresentedasthemeans±SDofthree339replications.
340Figure5.
InhibitoryeffectsofCompoundsASF-2~ASF-4(A-D)onAGEs'formation341inβ-lg-Lactosemodelundertheratio1:1000at85°Cfor120min.
A.
342kaempferol-3-O-glucuronide(compoundASF-2);B.
rutin(compoundASF-3);C.
343luteolin(compoundASF-4).
Dataarepresentedasthemeans±SDofthree344replications.
345346Page16of23Food&Function17Table13471Hand13CNMRchemicalshiftdataaofASF-1,13C-1Hlong-rangecorrelationsignals348intheHMBCspectra(δinppm,JinHz).
349δH(,m,JHz)δCHMBC(1H→13C)27.
20(s)125.
0C-3,C-9,C-10,C-113107.
447.
62(d,8.
0)118.
4C-5,C-9,C-3,C-857.
17(d,8.
1)122.
1C-4,C-9,C-367.
09(t,7.
7)119.
4C-777.
42(d,8.
1)111.
9C-9,C-68136.
39126.
6103.
18(dd,7.
2,8.
0);3.
37(dd,4.
7,10.
6)26.
3C-3,C-11,C-12,C-2,C-9113.
93(m)55.
0C-10,C-3,C-1212174.
4aDatawasrecordedinD2O.
350351Page17of23Food&Function18SupplementalFigureCaption352SupplementalFigure1Procedureofpurification353SupplementalFigure2HPLC-HRMSSpectrumofASF-1354SupplementalFigure3-A1H-NMRSpectrumofASF-1355SupplementalFigure3-B13C-NMRSpectrumofASF-1356SupplementalFigure3-CHMBCSpectrumofASF-1357SupplementalFigure3-DHMQCSpectrumofASF-1358359Page18of23Food&Function254x190mm(96x96DPI)Page19of23Food&Function73x41mm(300x300DPI)Page20of23Food&Function254x190mm(96x96DPI)Page21of23Food&Function254x190mm(96x96DPI)Page22of23Food&Function254x190mm(96x96DPI)Page23of23Food&Function
AcceptedManuscriptsarepublishedonlineshortlyafteracceptance,beforetechnicalediting,formattingandproofreading.
Usingthisfreeservice,authorscanmaketheirresultsavailabletothecommunity,incitableform,beforewepublishtheeditedarticle.
WewillreplacethisAcceptedManuscriptwiththeeditedandformattedAdvanceArticleassoonasitisavailable.
YoucanfindmoreinformationaboutAcceptedManuscriptsintheInformationforAuthors.
Pleasenotethattechnicaleditingmayintroduceminorchangestothetextand/orgraphics,whichmayaltercontent.
Thejournal'sstandardTerms&ConditionsandtheEthicalguidelinesstillapply.
InnoeventshalltheRoyalSocietyofChemistrybeheldresponsibleforanyerrorsoromissionsinthisAcceptedManuscriptoranyconsequencesarisingfromtheuseofanyinformationitcontains.
AcceptedManuscriptFood&Functionwww.
rsc.
org/foodfunction1ChemicalcomponentsfromtheHaulmofArtemisiaSelengensisandthe1InhibitoryEffectonGlycationofβ-lactoglobulin23XiaomingLi,YonglinLu,RonghuaDeng,TiesongZheng,LishuangLv*45DepartmentofFoodScienceandTechnology,GinlingCollege,NanjingNormal6University,122#NinghaiRoad,Nanjing,210097,P.
R.
China789101112Correspondingauthor:LishuangLv13Tel.
:+862583598286;fax:+862583707623.
14E-mailaddress:lishuanglv@126.
com;lulishuang@njnu.
edu.
cn1516Page1of23Food&Function2ABSTRACT17Artemisiaselengensis(AS)hasbeentraditionallyusedasbothfoodand18medicineforthousandsofyearsinChina.
Inourstudies,L-tryptophanwasfirstly19isolatedfromthehaulmofAStogetherwithluteolin,rutin,and20kaempferol-3-O-glucuronide.
Theirstructureswereelucidatedbyspectroscopic21methodsincludingHRMS,1Dand2DNMR.
Threeflavonoidcompoundsshowed22satisfactorysuppressioneffectsonformationofadvancedglycationendproducts23(AGEs)inβ-lactoglobulin-Lactose/MGO/GOmodelsystems,andtheiranti-glycation24activitiesexhibitedadose-dependentmanner.
Amongthosecompounds,25kaempferol-3-O-glucuronidewasdemonstratedthestrongestinhibitoragainst26formationofAGEs.
27KEYWORDS:ArtemisiaSelengensishaulm;β-lactoglobulin;advancedglycation28endproducts(AGEs).
29Kaempferol-3-O-glucuronideCID:44258914;RutinCID:5280805;30LuteolinCID:5280445;3132Page2of23Food&Function31.
Introduction33Artemisiaselengensis(AS)isanherbaceousperennialplantoftheCompositae34family,localizedatthewater'sedgeonbanksorinswamps,withwildspecieswidely35distributedovertheNortheast,North,andcentralChinasincetheMingdynasty.
36They'realsofoundinMongolia,Russia,andKorea.
InCompendiumofMateria37Medica,itisrecordedthatAShasdiversebiologicalactivities,e.
g.
hemostasia,38anti-inflammation,relievingcough,reducingsputum,andtreatmentofacute39infectioushepatitis,duetoAS'svariousactivecompounds.
Beingavaluablenatural40product,ASisbeneficialtohuman'shealth.
Someclinicaltherapiesarealsoreported41intheliterature1-3.
42InNanjing,earlythe1990's,peoplestartedplantingAS.
Nowadays,AShas43becomeverypopularasahealthyfood,beingdeliciousandnutritious,foritstender44stemisavegetablewithuniqueflavor,beingfragrant,fresh,andcrisp.
However,the45olderhaulmofASisusuallydiscardedbecauseofit'sinedible.
Todate,someofits46majorbiologicallyactivecomponentshavebeenisolated,suchasflavonoids4-6,47polysaccharides7,phenolicacids8guaianolides9andfattyacids10.
Flavonoidsinthe48haulmofAScomprisemorethan1%byweight,buthavehithertobeenlargely49ignoredinthefieldoffoodsciences.
Ourpreviousstudyshowedthattheflavonoid50contentofASincreasedwiththegrowthofASstems.
Withthisstudy,weinvestigate51thechemicalcompositionandbiologicalactivitiesoftheAShaulmtopromotethe52utilizationofASinfoodprocessing.
53Methylglyoxal(MGO)andglyoxal(GO),twomajorα-dicarbonylcompounds54Page3of23Food&Function4formedfrombothglycoxidationandlipoxidation11,arethepivotalintermediatesin55theformationofadvancedglycationendproducts(AGEs)invivo12.
Infood56processing,theα-dicarbonylcompoundsaregeneratedthroughroasting,baking,57broiling,andfryingduetocaramelization,theMaillardreaction,andlipidoxidation13.
58Duringsuchreactions,MGOandGOglycateproteinsfasterthansugars,causing59inter-andintramolecularcross-linksofproteins,thustheamountofAGEssharply60increases14.
61β-lactoglobulin(β-lg)isahighqualityproteinfoundinavarietyoffoodstuffs,62includinginfantformulas,bakedproducts,andbeverages.
TheeffectsoftheMaillard63reactionandglycationonthisproteinduringheatingarewellknownandreplicable,as64milkcontainshighamountsoflactoseandlowerquantitiesofotherreducingsugars.
65Thisreplicabilitymakesitagoodmodelwithrealworld-applicationstotestthe66anti-AGEspropertiesofourcompounds.
67Flavonoidsarecommondietarycomponentsofplant-derivedfoods.
Several68flavonoids:(-)-epigallocatechin3-gallate(EGCG)fromtea15,phloretinfromapple16,69genisteinfromsoybean17,proanthocyanidinsandanthocyaninfromberries18,are70knowntobescavengersofAGEsbytrappingreactivedicarbonylcompounds.
71Inthepresentstudy,weinvestigatechemicalcomponentsfromAShaulmand72theirinhibitoryactivitiesagainsttheformationofAGEsusingthe73β-lg-lactose/MGO/GOmodelsystems,tofindfood-derivedinhibitorsofAGEs.
We74expectflavonoidstopreventproteinglycationduringfoodthermalprocessing.
752.
MaterialsandMethods76Page4of23Food&Function52.
1.
Materials77Theair-driedhaulmofArtemisiaSelengensiswasobtainedfromthe"Baguazhou"78districtinNanjing.
β-lactoglobulin(≥92%)waspurifiedinourlab(Nanjing,79Jiangsu,China).
Thechemicalstandardkaempferol-3-O-glucuronide,rutin,and80luteolin;methylglyoxal(MGO,40%inwater),glyoxal(GO,40%inwater);D2O,81CD3ODandDMSO-d6wereobtainedfromSigma-AldrichCo.
(St.
Louis,MO,USA).
82HPLC-gradesolventsandotherreagentswereobtainedfromShanghaiSinopharm83ChemicalReagentCo.
,Ltd(Shanghai,China).
HPLC-gradewaterwaspreparedusing84aMilliporeMilli-Qpurificationsystem(Bedford,MA,USA).
852.
2.
Extractionandpurificationprocedure86TheextractionandpurificationprocedureareshowninSupplementalFigure1.
87ThedriedpowderofAShaulm(1kg)wasextractedwith75%ethanol(7.
5L)at8890°Cfor60min.
Thesolventwasremovedbyfiltration,andthenfreshsolventwas89addedtotheresidue.
Theextractionprocesswastwicerepeated.
Thecombined90filtrateswereconcentratedunderreducedpressureat40°Cbyarotaryevaporator91(TokyoRikakikaiCo.
,Tokyo,Japan).
92Toremovethepigment,thethicksolutionwasputintoaseparatingfunnelfor93furtherextraction.
Petroleumetherwasusedasextractantata1:3ratio.
These94water-solublefractionswerelaterevaporatedandlyophilized.
95DriedAScrudeextractwasre-suspendedin200mLdistilledwaterandloadedonto96aglasscolumn(5.
8*55cm)packedwithAB-8macroporousadsorptionresin.
After97thesamplewasloaded,elutionwashaltedfor10mintofacilitateadsorptionof98Page5of23Food&Function6chemicalsonresinbeads.
Fivefractions(AS-01~AS-05)werecollectedbyelutingthe99columnwith1.
5Lof0,10%,30%,50%,and70%ethanolsequentially.
TheAS-02100fraction(10%ethanolelutedportion)wassubjectedtoanODScolumn,elutedwitha101gradientsystem:10%,20%,30%,40%,and100%MeOH(500mLforeachgradient102system).
Thetwofractionsyielded,AS-02-1(10%MeOHeluted)andAS-02-2(20%103MeOHeluted),werefurtherseparatedonaSephadexLH-20columnrespectively,and104elutedwithethanol.
TwocompoundsASF-1(fromAS-02-1)andASF-2(from105AS-02-2)wereobtained.
TheAS-03fraction(30%ethanolelutedportionfromAB-8106column)wasseparatedbyanODScolumnusingthesameprocedureastheAS-02107fractiontoobtainASF-3.
TheAS-04fraction(50%ethanolelutedportionfromAB-8108column)waschromatographedonsilicagelcolumnandelutedwithCH3Cl3/MeOH109byagradientof98:2,20:1,10:1,8:1,6:1,2:1,and0:1toaffordASF-4.
Theamount110ofAS-05fraction(70%ethanolelutedportionfromAB-8column)wastoosmallto111abandon.
1122.
3.
AnalysisHPLC-MSprocedure113TheseparationofallfractionsexceptASF-1andofflinedatacollectionforHPLC114basedactivityprofilingwerecarriedoutwithaseriesAgilent1200HPLCsystem,115whichconsistedofadegasser,abinpump,acolumnoven,adiodearraydetector,and116aQQQmassdetector(Agilent,SantaClara,CA,USA)incorporatedwithelectrospray117ionization(ESI)interfaces.
AHPLCZORBAXEclipseXDB-C18(250*4.
6mmi.
d.
,5118m)wasusedforseparationataflowrateof0.
6mL/min.
Themobilephasefulfilled119thefollowingrequirements:(1)CompoundASF-2:Waterwith0.
5%formicacid(A)120Page6of23Food&Function7andAcetonitrilewith0.
1%formicacid(B)wereusedassolventsfora40min121program:Thecolumnwaselutedwith12%solventBfor10min,followedbylinear122increasesinBto20%within10to30min,thento30%from30to40min.
TheUV123detectorwassetat350nm.
Compound2hadanintensepeekatRT=37.
22min;(2)124CompoundASF-3:Waterwith0.
01%aceticacid(A)andAcetonitrile(B)wereused125assolventsfora25minprogram:Thecolumnwaselutedwith90%solventAfor10126min,followedbylinearincreasesinBto60%from10to20min,andthenwith60%127Bfrom21to25min.
TheUVdetectorwassetat350nm.
Compound3hadanintense128peekatRT=15.
17min.
(3)CompoundASF-4:Waterwith0.
5%formicacid(A)129andAcetonitrilewith0.
1%formicacid(B)wereusedassolventsfora55min130program:Thecolumnwaselutedwith88%solventAfor10min,followedbylinear131increasesinBto20%from10to30min,to30%from30to40min,to60%from40132to48min,andthenre-equilibratedwith90%Bfor6minfrom49to55min.
TheUV133detectorwassetat254nm.
Compound4hadanintensepeekatRT=47.
33min.
For134MS:ThenegativeionpolaritymodewassetforESIionsource.
Thetypicaloperating135parameterswereasfollows:sprayneedlevoltage,5kV;nitrogensheathgas,45136(arbitraryunits);auxiliarygas,5(arbitraryunits).
137Incontrast,fractionASF-1wascarriedoutwithaseriesAgilent1290Infinity6224138TOFsystem(Agilent,SantaClara,CA,USA).
AHPLCZORBAXEclipseXDB-C18139(250*4.
6mmi.
d.
,5m)wasusedforseparationataflowrateof0.
8mL/min.
The140columnwaselutedwith40%solventB(Acetonitrile)and60%solventA(waterwith1410.
01%aceticacid)fora15min.
TheUVdetectorwassetat280nm.
Compound1had142Page7of23Food&Function8anintensepeekatRT=3.
77min.
ForMS:Thenegativeionpolaritymodewassetfor143ESIionsource.
Thetypicaloperatingparameterswereasfollows:sprayneedle144voltage,5kV;nitrogensheathgas,45(arbitraryunits);auxiliarygas,5(arbitrary145units).
146Thestructuralinformationofcompounds1-4wasobtainedbytandemmass147spectrometry(MS/MS)throughcollision-induceddissociation(CID)witharelative148collisionenergysettingas35%.
DataacquisitionwasperformedwithQualitative149AnalysisofMasshunter(Agilent,SantaClara,CA,USA).
1502.
4.
NMRAnalysis151NMR1H(400MHz),13C(100MHz),and2DNMRspectrawereobtainedonan152AVANCE400(BrukerDaltonicsCo.
,Bremen,Germany)spectrometerwithTMSas153internalreference.
1542.
5.
InhibitionofCompounds(ASF-2~ASF-4)againstformationofAGEsin155β-lactoglobulinglycationsystems156β-lactoglobulin(0.
083mmol/L)wasincubatedwithlactose(0.
083mol/L)or157MGO/GO(1.
5mmol/L)atchosenratios(1:1000,1:18)inthepresenceorabsenceof158Compounds1-4(0.
05,0.
1,0.
5mmol/L)inphosphatebuffer(pH6.
5)at85°C.
Then,159thesamplewascollectedatselectedtimepoints(0,15,30,45,60,90,120min)and160storedat-80°C.
Amultimodemicroplatereader(BioTek,Winooski,VT)wasusedfor161thequantificationofAGEs.
The%inhibitionofAGEsformation=[1-(fluorescenceof162thetestgroup/fluorescenceofthecontrolgroup)]*100%19.
Eachsamplewas163Page8of23Food&Function9performedintriplicateandtheexperimentwasdonethreetimeswithcomparable164results.
1653.
Results1663.
1Structureelucidation167AnalysisoftheethanolextractobtainedfromthehaulmofASbyrepeatedcolumn168chromatography(AB-8,ODS,andSephadexLH-20orsilicagel)ledtotheisolation169andidentificationonecompound(ASF-1),alongwiththreeflavonoidcompounds170(ASF-2~ASF-4).
171Thethreeflavonoids,kaempferol-3-O-glucuronide(ASF-2)20,172rutin(ASF-3)21andluteolin(ASF-4)22,wereidentifiedbycomparisonoftheir173spectroscopicdatawiththosereportedintheliterature.
174CompoundASF-1hadamolecularformulaofC11H12N2O2asdeterminedby175ESI-HRMS,NMR1H,13C,HMBC,HMQCdata(SupplementalFigure2-3).
The176negativeESI-MSofcompoundASF-1showedamolecularionpeakatm/z203.
1000177[M-H]-,indicatingamolecularweightof204.
The1HNMRofdata(Table1)revealed178fouraromaticH-atoms,whosesignalfromoneprotonindownfieldwasobservedatd1797.
62(d,J=8.
0Hz),wasidentifiedasH-4,whileoneprotonatd7.
42(d,J=8.
1Hz)180wasevidencedtobeH-7;andthereweretwoothersignals,oneprotonatd7.
17(d,1818.
1),theotheratd7.
09(t,J=7.
7Hz),whichwereassignedtoH-5andH-6,182respectively.
Oneprotonresonancemultipletindicativeofamethinegroupwas183observedatδH3.
93(m),andtwoone-protondoubledoubletsasmethylenegroups184attachedtocarbonylmoieties[δH3.
18(dd,J=7.
2,8.
0Hz);δH3.
37(dd,J=10.
6,4.
7185Page9of23Food&Function10Hz)].
The13CNMRspectrumofASF-1(Table1)indicatedthepresenceofacarbonyl186group(δC174.
4),anun-substitutedaromaticring(δC111.
9,119.
4,122.
1,118.
4),a187tetra-substitutedaromaticring(δC136.
3,126.
6),amethylenegroup(δC26.
3),a188methinegroup(δC55.
8),aswellastwoolefincarbons(δC125.
0,107.
5).
This189spectroscopicinformationwasusedtoestablishitscorestructureasanaromaticring190fusedtoaheterocyclicring,whichwasconfirmedbytheHMBCcorrelationdata191(Figure2):H-11[δH3.
93(m)]andH-10[δH3.
18(dd,J=7.
2,8.
0Hz);δH3.
37(dd,J=1924.
7,10.
6Hz)]werecorrelatedwithC-12(δC174.
5)andthequaternarycarbonatδC193107.
5(C-3),whichconfirmedtheconnectionofCH-CH2andC=Omoieties.
194Additionally,H-7[δH7.
42(d,8.
1)]wascorrelatedwithC-9(δC126.
6)andC-6(δC195119.
4);H-4[δH7.
62(d,J=8.
0Hz)]wascorrelatedwithC-5(δC122.
1)andC-9(δC196126.
3).
ThissuggestedthatC-7andC-4atthearomaticringconnectedwitha197heterocyclicring.
Thus,ASF-1wasdeterminedasL-tryptophan(Figure1).
1983.
2.
InhibitoryEffectsofCompounds(ASF-2~ASF-4)ontheFormationofAGEs199Theflavonoidcompounds(ASF-2~ASF-4)showedsatisfactorysuppressionagainst200theformationofAGEsintheβ-lg-lactose/MGO/GOmodelsystems(Figure2).
201Increasingtheconcentration(0.
05-0.
5mmol/mL)ofeachcompoundgradually202augmentedtheinhibitoryactivitiesagainsttheformationofAGEs.
Amongthese203compounds,kaempferol-3-O-glucuronideexhibitedthestrongestanti-glycation204capacityatthesameconcentrationinthreedifferentβ-lactoglobulinsystems.
The205abilitiesofthefourcompoundstoinhibitAGEswere:kaempferol-3-O-glucuronide>206luteolin>rutin.
207Page10of23Food&Function11Intheβ-lg-lactosesystem,therespectiveinhibitionratiosof208kaempferol-3-O-glucuronide,rutin,orluteolin(0.
05mmol/L)againstAGEswere20933.
56%,23.
75%,and22.
38%at120min(Figure2),asAGEswereslowlygenerated210throughoutthereaction.
Withanincreaseto0.
5mmol/L,theinhibitionratios211increasedto60.
35%,54.
32%and33.
56%respectively.
Thissuggeststhatthe212anti-glycationactivitiesofthesecompoundshaveapositivecorrelationwith213concentration.
214Intheβ-lg-MGO/GOsystems(Figures3and4),thesethreecompoundsalso215showedsignificantinhibitionat0.
05mmol/L,althoughhighlyactiveMGOorGOled216tomoreAGEsthanlactose.
Ofthesecompounds,kaempferol-3-O-glucuronideisthe217mostefficientAGEsinhibitorindifferentβ-lactoglobulinsystems.
For60%inhibition,2180.
5mmol/Lkaempferol-3-O-glucuronidewasneededintheβ-lg-lactosesystemat21985°Cfor120min;whileonly0.
1mmol/Lkaempferol-3-O-glucuronideneededinthe220β-lg-GOsystemandonly0.
05mmol/Lwasneededtheinβ-lg-MGOsystem(Figures2212B,3B,and4B).
2224.
Discussion223Inthisstudy,acompoundL-tryptophanwasfirstlyisolatedfromAShaulm224togetherwiththreeflavonoidcompounds.
ThestructureofL-tryptophanwas225determinedbyHRMSspectra,1Dand2DNMRdata.
Theflavonoidsshowed226powerfulinhibitoryeffectsagainsttheformationofAGEsinβ-lactoglobulin227glycation.
228Recentstudieshaveshownthattheflavonoids,e.
g.
,flavanol(EGCG),chalcone229Page11of23Food&Function12(phloretinandphloredzin),andisoflavone(genistein),rapidlytrapsMGOatC-6,C-8230unsubstitutedcarbonsattheAringandformmono-anddi-MGOadducts,thus231inhibitingtheformationofAGEs15-17.
232Thestructuresofthesecompoundscanaccountforthedifferenceintheiractivities.
233Kaempferol-3-O-glucuronideistheglycosideoftheflavonolkaemoferol.
Rutinisthe234glycosidebetweenflavonolquercetinanddisacchariderutinose.
Asflavonols,they235bothhavethesamearomaticAring,Bring,andaheterocyclicCring.
Wefoundthat236kaempferol-3-O-glucuronideexhibitshigheractivityintheanti-glycationof237β-lactoglobulin,althoughrutinhasonemore-OHthankaempferol-3-O-glucuronide238atC-5ontheBring.
However,ifflavonoidspossessthesameAandCrings,a239differenceinthenumberofhydroxylgroupsontheBringdoesnotplayasignificant240roleontrappingefficacy23.
Thegreaterefficiencyofkaempferil-3-O-glucuronideis241likelyduetolesssterichindrancefromitssingleglycosideversusrutin'sdisaccharide,242rutinose.
Thedifferencebetweenrutinandluteolinisthatluteolinisanaglyconeof243flavone,andtherearenohydroxylgroupsorglucosidesatC-3onluteolin'sCring.
244Andourresultsindicatethatluteolinhashigherinhibitoryeffectsthanrutin.
Thisis245consistentwithcurrentliteraturethatluteolinandrutinexhibitsignificantinhibitory246effectsof82.
2%and77.
7%intheBSAglycationsystematpH7.
4and37°C.
We247predictthatthereasonforthisphenomenonisthatluteolinlackssterichindrancein248thetrappingreaction.
249Ourdatahighlighttheeffectsofthesecompoundsonglycationofβ-lactoglobulin250viaα-dicarbonylcompounds.
α-dicarbonylcompoundsarisefromfreesugar,the251Page12of23Food&Function13initialSchiffbases,Amadoriandotherintermediates24-26.
Sinceα-dicarbonyl252compoundsandglucosecanpotentiallydamagedifferentsubsetsofproteins,253experimentaloutcomesmaybedifferent.
Whileintheβ-lg-lactosesystem,these254flavonoidcompoundsalsoexhibitedsignificantinhibitionagainstglycationof255β-lactoglobulin.
Themechanismofinhibitionofglucose-mediatedglycationneeds256furtherdiscussion.
257Inaddition,thehighpercentageofL-tryptophaninAShaulmcouldalsocontribute258totheinhibitoryeffectontheglycationofβ-lactoglobulininβ-lg-MGO/GO/lactose259systemsviathefreeaminogrouporiminogroupreactingwiththereactivecarbonyl.
260Recently,flavonoidshaveshownmoreandmorepotentialasstronginhibitors261againsttheformationofAGEsinvivo,butthereonlyafewstudiesthatinvolved262flavonoidsintheanti-glycationoffoodproteinsathightemperatures.
Wesuggestthat263theflavonoidsfromvariousfoodsandnaturalplantscanbeusedaseffectiveAGEs264inhibitorsinfoodthermalprocessing.
Ourresultsinthisstudypavethewayfor265furtherinvestigationofthesecompoundsfromAShaulminrealfoodmatricesto266confirmtheinhibitoryactivitiesagainsttheformationofAGEs.
267Appendix268SupplementalFigure1.
Procedureofextractionandpurificationofflavonesfrom269ArtemisiaSelengensishaulm270SupplementalFigure2.
TheHPLC-MSspectraofcompoundASF-1.
(A)271HPLCDADchromatogramsspectrum,(B)HRMSspectrum272Page13of23Food&Function14SupplementalFigure3.
The1HNMRspectraofcompoundASF-1.
(A).
1HNMR273spectrum;(B).
13CNMRspectrum;(C).
HMQCspectrum;(D).
HMBCspectrum274Acknowledgements275ThisworkwassupportedbyNSFofJiangsuprovinceofChina(Project276BK2012850)andNaturalScienceFoundationofZhejiangprovinceofChina(Project277LY12C15001).
OurthankstoAaronYerkefromNorthCarolinaAgriculturaland278TechnicalUniversityforhissuggestionsonrevisionsandediting.
279Conflictofinterest280Thereisnoconflictofinterestforallauthors.
281282Page14of23Food&Function15References2831.
C.
Deng,X.
Xu,N.
Yao,N.
LiandX.
Zhang,AnalyticaChimicaActa,2006,556,289-294.
2842.
L.
Peng,Y.
Wang,H.
ZhuandQ.
Chen,FoodChemistry,2011,125,1064-1071.
2853.
Y.
ZhuandM.
Qin,ChineseArchivesOfTraditionalChineseMedicine,2006,24,1749-1786.
2864.
L.
Peng,X.
Jia,Y.
Wang,H.
ZhuandQ.
Chen,FoodAnalyticalMethods,2009,3,261-268.
2875.
J.
ZhangandL.
Kong,ChineseTraditionalandHerbalDrugs,2008,39,23-26.
2886.
J.
Zhang,Y.
LinandL.
Kong,ChineseTraditionalandHerbalDrugs,2004,35,979-980.
2897.
K.
A.
Koo,Kwak,J.
H.
,Lee,K.
R.
,Zee,O.
P.
,Woo,E.
R.
,Park,H.
K.
,Youn,290H.
J.
,ArchivesofPharmacalResearch1994,17,371-374.
2918.
Z.
Tu,L.
Zhang,H.
Wang,Y.
YeandW.
Liu,ScienceandTechnologyofFoodIndustry,2012,29233,239-242.
2939.
X.
F.
JinfengHu,ChineseChemicalLetters,1998,9,829-832.
29410.
L.
Zhang,Z.
-c.
Tu,T.
Yuan,H.
Wang,Z.
-f.
Fu,Q.
-h.
WenandX.
-q.
Wang,IndustrialCrops295andProducts,2014,56,223-230.
29611.
E.
B.
Frye,T.
P.
Degenhardt,S.
R.
ThorpeandJ.
W.
Baynes,JournalofBiologicalChemistry,2971998,273,18714-18719.
29812.
P.
Beisswenger,S.
Howell,R.
Nelson,M.
MauerandB.
Szwergold,BiochemicalSociety299Transactions,2003,31,1358-1363.
30013.
J.
Degen,M.
HellwigandT.
Henle,JournalofAgriculturalandFoodChemistry,2012,60,3017071-7079.
30214.
N.
RabbaniandP.
J.
Thornalley,BiochemicalSocietyTransactions,2008,36,1045-1050.
30315.
S.
Sang,X.
Shao,N.
Bai,C.
-Y.
Lo,C.
S.
YangandC.
-T.
Ho,Chemicalresearchintoxicology,3042007,20,1862-1870.
30516.
X.
Shao,N.
Bai,K.
He,C.
-T.
Ho,C.
S.
YangandS.
Sang,Chemicalresearchintoxicology,3062008,21,2042-2050.
30717.
L.
Lv,X.
Shao,H.
Chen,C.
T.
HoandS.
Sang,Chemicalresearchintoxicology,2011,24,308579-586.
30918.
W.
Wang,Y.
Yagiz,T.
J.
Buran,C.
d.
N.
NunesandL.
Gu,FoodResearchInternational,2011,31044,2666-2673.
31119.
A.
J.
Furth,Analyticalbiochemistry,1988,175,347-360.
31220.
N.
Castillo-Muoz,S.
Gómez-Alonso,E.
García-Romero,M.
V.
Gómez,A.
H.
VeldersandI.
313Hermosín-Gutiérrez,JournalofAgriculturalandFoodChemistry,2008,57,209-219.
31421.
F.
Fathiazad,A.
Delazar,R.
AmiriandS.
D.
Sarker,IranianJournalofPharmaceutical315Research,2006,3,222-227.
31622.
S.
J.
Kellam,K.
A.
Mitchell,J.
W.
Blunt,M.
H.
MunroandJ.
R.
Walker,Phytochemistry,3171993,33,867-869.
31823.
X.
Shao,H.
Chen,Y.
Zhu,R.
Sedighi,C.
-T.
HoandS.
Sang,JournalofAgriculturalandFood319Chemistry,2014,62,3202-3210.
32024.
E.
Fujimori,BiochimBiophysActa,1989,998,105-110.
32125.
S.
P.
WolffandR.
T.
Dean,BiochemicalJournal,1987,245,243-250.
32226.
J.
V.
Hunt,R.
T.
DeanandS.
P.
Wolff,BiochemicalJournal,1988,256,205-212.
323324325Page15of23Food&Function16FigureCaption326Figure1.
StructuresofcompoundsASF-1~ASF-4.
A.
L-tryptophan(compound327ASF-1);B.
kaempferol-3-O-glucuronide(compoundASF-2);C.
rutin(compound328ASF-3);D.
luteolin(compoundASF-4).
329Figure2.
KeyHMBCcorrelationsofcompoundASF-1.
330Figure3.
InhibitoryeffectsofCompoundsASF-2~ASF-4onAGEs'formationin331β-lg-MGOmodelundertheratio1:18at85°Cfor120min.
Dataarepresentedasthe332means±SDofthreereplications.
A.
kaempferol-3-O-glucuronide(compoundASF-2);333B.
rutin(compoundASF-3);C.
luteolin(compoundASF-4).
Dataarepresentedasthe334means±SDofthreereplications.
335Figure4.
InhibitoryeffectsofCompoundsASF-2~ASF-4onAGEs'formationin336β-lg-GOmodelundertheration1:18at85°Cfor120min.
A.
337kaempferol-3-O-glucuronide(compoundASF-2);B.
rutin(compoundASF-3);C.
338luteolin(compoundASF-4).
Dataarepresentedasthemeans±SDofthree339replications.
340Figure5.
InhibitoryeffectsofCompoundsASF-2~ASF-4(A-D)onAGEs'formation341inβ-lg-Lactosemodelundertheratio1:1000at85°Cfor120min.
A.
342kaempferol-3-O-glucuronide(compoundASF-2);B.
rutin(compoundASF-3);C.
343luteolin(compoundASF-4).
Dataarepresentedasthemeans±SDofthree344replications.
345346Page16of23Food&Function17Table13471Hand13CNMRchemicalshiftdataaofASF-1,13C-1Hlong-rangecorrelationsignals348intheHMBCspectra(δinppm,JinHz).
349δH(,m,JHz)δCHMBC(1H→13C)27.
20(s)125.
0C-3,C-9,C-10,C-113107.
447.
62(d,8.
0)118.
4C-5,C-9,C-3,C-857.
17(d,8.
1)122.
1C-4,C-9,C-367.
09(t,7.
7)119.
4C-777.
42(d,8.
1)111.
9C-9,C-68136.
39126.
6103.
18(dd,7.
2,8.
0);3.
37(dd,4.
7,10.
6)26.
3C-3,C-11,C-12,C-2,C-9113.
93(m)55.
0C-10,C-3,C-1212174.
4aDatawasrecordedinD2O.
350351Page17of23Food&Function18SupplementalFigureCaption352SupplementalFigure1Procedureofpurification353SupplementalFigure2HPLC-HRMSSpectrumofASF-1354SupplementalFigure3-A1H-NMRSpectrumofASF-1355SupplementalFigure3-B13C-NMRSpectrumofASF-1356SupplementalFigure3-CHMBCSpectrumofASF-1357SupplementalFigure3-DHMQCSpectrumofASF-1358359Page18of23Food&Function254x190mm(96x96DPI)Page19of23Food&Function73x41mm(300x300DPI)Page20of23Food&Function254x190mm(96x96DPI)Page21of23Food&Function254x190mm(96x96DPI)Page22of23Food&Function254x190mm(96x96DPI)Page23of23Food&Function
- Methodswww.qqq147.com相关文档
- 广发海外多元配置证券投资基金(QDII)2019
- meantwww.qqq147.com
- assumingwww.qqq147.com
- 基金www.qqq147.com
- CURRENTwww.qqq147.com
- suceswww.qqq147.com
Linode($5/月),新用户注册送100美元,11个数据中心云服务器
关于Linode,这是一家运营超过18年的VPS云主机商家,产品支持随时删除(按小时计费),可选包括美国、英国、新加坡、日本、印度、加拿大、德国等全球十多个数据中心,最低每月费用5美元($0.0075/小时)起。目前,注册Linode的新用户添加付款方式后可以获得100美元赠送,有效期为60天,让更多新朋友可以体验Linode的产品和服务。Linode的云主机产品分为几类,下面分别列出几款套餐配置...
妮妮云(30元),美国300G防御 2核4G 107.6元,美国高速建站 2核2G
妮妮云的来历妮妮云是 789 陈总 张总 三方共同投资建立的网站 本着“良心 便宜 稳定”的初衷 为小白用户避免被坑妮妮云的市场定位妮妮云主要代理市场稳定速度的云服务器产品,避免新手购买云服务器的时候众多商家不知道如何选择,妮妮云就帮你选择好了产品,无需承担购买风险,不用担心出现被跑路 被诈骗的情况。妮妮云的售后保证妮妮云退款 通过于合作商的友好协商,云服务器提供2天内全额退款,超过2天不退款 物...
简单测评v5.net的美国cn2云服务器:电信双程cn2+联通AS9929+移动直连
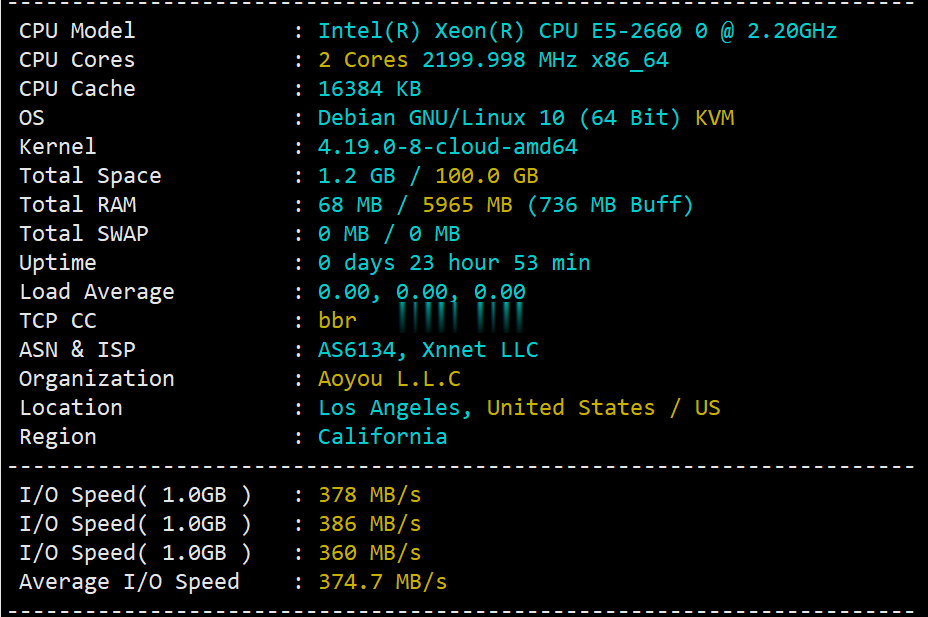
v5.net一直做独立服务器这块儿的,自从推出云服务器(VPS)以来站长一直还没有关注过,在网友的提醒下弄了个6G内存、2核、100G SSD的美国云服务器来写测评,主机测评给大家趟雷,让你知道v5.net的美国云服务器效果怎么样。本次测评数据仅供参考,有兴趣的还是亲自测试吧! 官方网站:https://v5.net/cloud.html 从显示来看CPU是e5-2660(2.2GHz主频),...
www.qqq147.com为你推荐
-
公司网络被攻击网络遭受攻击分为哪几类美国互联网瘫痪2000年美国的互联网危机事件的原因?今日油条天天吃油条,身体会怎么样百度商城百度商城知道在哪个地方,怎么找不到啊75ff.com开机出现www.ami.com是什么?怎么解决啊1377.com真实.女友下载地址谁有国风商讯说下,郑州国风艺考画室有人了解吗?www.38.com俺去也的最新网址是什么?剑影绝杀dnf匕首刺客刷图天诛、弧光闪、迅影、绝杀斩要不要加满,给理由欢颜网欢颜网的信誉怎样?