number789se.com
789se.com 时间:2021-03-19 阅读:()
Availableonlineatwww.
sciencedirect.
comChemicalmutagenesis:selectivepost-expressioninterconversionofproteinaminoacidresiduesJustinMChalkerandBenjaminGDavisTheabilitytoalterproteinstructurebysite-directedmutagenesishasrevolutionizedbiochemicalresearch.
ControlledmutationsattheDNAlevel,beforeproteintranslation,arenowroutine.
Thesetechniquesallowspecic,highdelityinterconversionlargelybetween20natural,proteinogenicaminoacids.
Nonetheless,thereisaneedtoincorporateotheraminoacids,bothnaturalandunnatural,thatarenotaccessibleusingstandardsite-directedmutagenesisandexpressionsystems.
Post-translationalchemistryoffersaccesstothesesidechains.
Nearlyhalfacenturyago,theideaofa'chemicalmutation'wasproposedandtheinterconversionbetweenaminoacidsidechainswasdemonstratedonselectproteins.
Intheseisolatedexamples,apowerfulproof-of-conceptwasdemonstrated.
Here,werevivetheideaofchemicalmutagenesisanddiscusstheprospectofitsgeneralapplicationinproteinscience.
Inparticular,weconsideraminoacidsthatarechemicalprecursorstoafunctionalsetofothersidechains.
Amongthese,dehydroalaninehasmuchpotential.
Therearemultiplemethodsavailablefordehydroalanineincorporationintoproteinsandthisresidueisanacceptorforavarietyofnucleophiles.
Whenusedinconjunctionwithstandardgenetictechniques,chemicalmutagenesismayallowaccesstonatural,modied,andunnaturalaminoresiduesontranslated,foldedproteins.
AddressDepartmentofChemistry,UniversityofOxford,ChemistryResearchLaboratory,ManseldRoad,OxfordOX13TA,UnitedKingdomCorrespondingauthor:Davis,BenjaminG(ben.
davis@chem.
ox.
ac.
uk)CurrentOpinioninChemicalBiology2010,14:781–789ThisreviewcomesfromathemedissueonMethodsforBiomolecularSynthesisandModicationEditedbyMattFrancisandIsaacCarrico1367-5931/$–seefrontmatter#2010ElsevierLtd.
Allrightsreserved.
DOI10.
1016/j.
cbpa.
2010.
10.
007IntroductionThedevelopmentofsite-directedmutagenesishasrevo-lutionizedproteinscience[1,2].
Specic,highdelitymutationsattheDNAlevelarenowroutineandrecombi-nantexpressionsystemsenablesite-specicincorporationandvariationofaminoacidresiduesinthenalprotein.
Standardsite-directedmutagenesis,however,islargelylimitedto20natural,proteinogenicaminoacidresidues.
Asbiochemicalendeavorsandgoalshaveadvanced,aneedtoincorporatenon-naturalormodiedaminoacidsidechainshasemerged.
Unnaturalaminoacidscanbetargetedforfurtherlabelingaswellasproteintrackingandanalysis[3,4].
Aminoacidsidechainsarealsonaturallymodied—phosphorylation,methylation,acylation,glycosylationareafewsuchexamples—andaccesstothesesidechainsishighlydesirableifwearetoexploretheirrolesmoreprecisely[5].
Ambercodonsuppressionandreprogrammedgeneticcodesaretwostrategiesforincorporatingsomeunnaturalormodiedaminoacidsnotaccessiblethroughnormaltranslation[6–9].
Semi-synthesisbynativechemi-calligationisanotheralternativeandthroughthismethodthetotalsynthesisofproteinshasbecomereality[10].
Anotherstrategyistousechemistryonanexpressedandfoldedproteintoinstalladesiredsidechainatadesiredsite.
Itisthisnalstrategythatisthefocusofthisreview.
Ourintentionisnottoprovideacomprehensivereviewofproteinlabelingmethods.
Rather,wewishtoexplore—andindeedrevive—theconceptof'chemicalmutagen-esis'putforthnearlyhalfacenturyagobyDanielE.
Koshland,Jr.
[11].
VisionarycontributionsfromtheKosh-land[11]andBender[12]laboratoriesdescribed,forthersttime,thechemicalconversionofoneaminoacidsidechaintoanother—achemicalmutation.
Asgenetictech-nology,expressionsystems,andaqueouschemistryhavedeveloped,itisworthwhiletorevisittheideaofachemicalmutation.
Wersttracetheinceptionofthisconceptandthenconsideritsplaceincontemporarychemistryandbiologyasageneralmethodtoalterproteinstructureprecisely.
Chemicalmutagenesis:aseminalconceptinproteinscienceIn1966,thelaboratoriesofDanielE.
Koshland,Jr.
andMyronL.
Benderindependentlyreportedtherstpointmutationofanenzyme[11,12].
Inbothreports,theserineproteasesubtilisinwaschemicallyconvertedtoacysteineprotease.
ThetransformationisdepictedinFigure1.
Theactivesiteserinewasrstselectivelyconvertedintoaleavinggroupbytreatmentwithphe-nylmethanesulfonyluoride(PMSF)andthendisplacedbytheattackofthioacetate.
Theresultingthioacyl-enzymewashydrolyzedthroughtheinnateactivityoftheprotease,providingthethiol-subtilisinproduct.
Themutantenzymewascharacterizedbothchemically(thecysteineproteasecontainsasingle,easilydetectedcysteine)andthroughkineticanalysisofproteaseactivity.
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789TheSertoCysmutationprovidedanear-isostericmutantforstudyoftheproteaseactive-siteandmechanism.
Itshouldbepointedoutthatatthetimeofthesediscov-eries,nomethodsyetexistedforsite-specicmutagenesisattheDNAlevelandthetotalchemicalsynthesisofproteinswasnotyetfeasible.
Remarkably,KoshlandandBenderbothanticipatedtheuseofgeneticmodicationstospecicallycontrolproteinstructurebutuntilthistechnologywasavailable,theyproposedamoreimmedi-atelyaccessiblemethodofmutationperformedonthetranslatedproteinthroughtheuseofchemistry—suchmodicationswerereferredtoas'chemicalmutations'byKoshland[11]and'simulatedmutations'byBender[13].
Thenomenclatureforthesetransformationscanbeamatterofcontentionandconfusion,soafewpointsregardingtheconceptof'chemicalmutation'areperhapsinorder.
Inthisreview,werefertoa'chemicalmutation'asaprocessthatconvertsanaminoacidresidueonatranslatedproteintoanotheraminoacidresidue.
Chemi-calmutationinthiscontextdoesnotrefertochemicalalterationofDNAanditdoesnotrefertocovalentlabelingofproteinswithsyntheticprobes,cofactors,andotherbiochemicaltools.
ToavoidanyconfusionwithDNAmodication,weproposetheterm'post-expressionmutagenesis'forchemicalalterationofthetranslated,foldedprotein[14].
Sinceinthisreviewweconsideronlymutationsonproteins,wewilluse'chemicalmutation'and'post-expressionmutation'interchangeably.
Theproof-of-principlesetforthbyKoshlandandBenderwasalandmarkinproteinscience.
Theconversionofactivesiteserinestocysteineshasbeenappliedtootherproteasesubstratessuchastrypsin[15]andrelatedpro-tocolsforconversiontoselenocysteine[16–19]andeventellurocysteine[20]havebeenreported.
Despitetheseadvances,theserinetocysteinemutationwasonlypossibleinthesecasesbecauseoftheuniquechemicalreactivityofactive-siteserine.
AdifferentapproachwasreportednotlongafterKoshlandandBender'sdisclosures.
Laskowskidescribedan'enzy-maticmutation'onsoybeantrypsininhibitorthatreliedontrypsinandcaboxypeptidaseBtoexiseanaminoacidfromtheproteinandre-ligateanotheraminoacidatthesiteofmutation[21].
Themutationiscarriedoutonthenativeprotein,post-expression,inthesamespiritasthechemicalmutationdescribedbyKoshlandandBender,butLas-kowski'smethoddiffersconceptuallysincetheproteinbackboneisaltered,ratherthansidechain.
Moreover,Laskowskihimselfconcededthatthismethodrelieson'abitofluck'thatallthenecessaryenzymescanco-existunderthereactionconditionswithoutdeleteriousproteol-ysis[21].
Nevertheless,thisconceptwasfurtherdevelopedbyTschesche,whodescribedsequentialaminoacidexci-sion,chemicalcoupling,andelastaseligationasamethodtomutatesoybeantrypsininhibitor[22,23].
Tschescheconsideredtheseaminoacidexchangesaformof'chemicalmutation'[23].
Importantly,theseexamplesillustratethatsomealterationscanbeaccomplishedenzymaticallyandthatsuchamutationdoesnotnecessarilyrelysolelyonselectivesmall-moleculechemistry.
Collectively,themutationsdescribedbyKoshland,Bender,andLaskowskiwerethemostprecisemanipulationsofproteinstructurethatprecededthegeneticandbiotechnologicalrevolution.
Chemicalmutagenesis:expandingscopefornaturalresiduesAdecadeaftertherstexamplesofchemicalmutagenesiswereputforthbyKoshlandandBender,PeterClarkandGordonLowereportedtheuseofcysteineasaprecursortomultipleaminoacidsidechains.
Their'chemicalmutationsofpapain'canbeconsideredmoregeneralthanpreviouseffortsinchemicalmutagenesissincethecysteineresidueneednotbeinaprotease,cysteineresidueneednotbeactivatedbyitspresenceinanactivesite,thoughinthiscasetheresiduewasindeedthenucleophileofthecysteineproteasepapain[24,25].
Inthesereports,cysteinewasalkylatedwithaphenacylbromide(Figure2).
Photolysisofthisintermediateledto782MethodsforBiomolecularSynthesisandModificationFigure1ThefirstpointmutationofanenzymewasreportedindependentlybyKoshlandandBenderin1966[11,12].
Theserine-to-cysteinemutationwasaccomplishedchemically.
Themorereactiveserineoftheactivesiteofsubtilisinisselectivelyconvertedintoaleavinggroupusingasulfonylfluoride(BnSO2F).
Displacementbythioacetateprovidesathioacyl-enzymeintermediatethatishydrolyzedtothefreethiolofcysteine.
CurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
comirreversibleNorrishtypeIIcleavage,providingthethioal-dehydedehydrocysteine.
HydrationofthethiocarbonylandlossofH2Sresultsintheformationofformylglycine.
FormylglycinewasinturnreducedwithNaBH4topro-videserine.
Inthisway,theoveralltransformationisamutationfromcysteinetoserine.
Moreover,formylgly-cinecanalsobeconvertedtoglycineafterprolongedincubationatpH9.
0(Figure2).
Lowe'sextensionoftheconceptofchemicalmutagenesiswasthereforeuniqueinthatitcouldprovidemultiplemutationsfromasingle,commonprecursorand,inprinciple,couldbeappliedtoanyfreecysteine.
SinceLowe'sreport,therehavebeennotablyfewpub-licationsconcernedwithchemicalmutations.
Geneticandrecombinanttechnologywasmovingforwardatastrikingrateandaminoacidinterconversionusingpost-translationalchemistrymayhaveseemedobsolete.
Someillustrativereportsofchemicalmutationshavenonethe-lessappearedthatindicatestrategicadvantagesofchemi-calmutations.
Forinstance,Venkateshshowedthatglutamineandasparagineresiduescanbehydrolyzedwithacidto'chemicallymutate'thesidechaintoglu-tamicandasparticacid,respectively.
ItwasshownthatthesemutationsinuencedthekineticsoffoldinginRNaseA[26].
Inthereversemutation,Imotoshowedthatamidationofglutamateandaspartateresiduescouldbeusedinthestudyoftheseresiduesascatalyticsidechains[27].
Themutationinthiscaseisfromglutamateandaspartatetoglutamineandasparagine,respectively.
Inthesereports,multi-sitemutationsareaccomplished.
Inthecaseofamidation,thesemutationsarecarriedoutonthefoldedprotein.
Itisconceivablethatifthesemutationswereintroducedatthegeneticleveltheproteinwouldnotfoldproperlyandsothisexamplehighlightsastrategicadvantageofpost-expressionmuta-genesiswheredifferencesinactivitybetweentheproteinmutantscanbeattributedtothemutationanddecoupledfrommisfoldingduringexpression.
Otherexamplesofchemicalmutationshavebeentheproductsofeffortstogeneralizenativechemicalligation(NCL)andovercomeitsinherentrelianceoncysteine[10].
AnumberofcysteinedesulfurizatonprocesseshavebeenputforththatallowtheuseofNCLinthesynthesisofpeptidesandproteinsthatdonotcontaincysteine.
DawsonhasdisclosedamethodtoreducecysteinetoalanineusingpalladiumorRaneynickel[28].
Relatedtransformationshavesincebeenreportedtoprovidephenylalanine[29]andvaline[30,31]atthesiteofligation.
OfnoteisDanishefsky'smild,radicalbasedmethodforthedesulfurizationofcysteine[32].
Intheseexamplestheapplicationofchemicalmutagenesisinnativechemicalligationisapparent:achemicalmutationofcysteinetoanotherresidueconstitutesaligation,intheformalsense,atthenalresidue.
ChemicalmutationtoanaminoacidanalogChemicalmutagenesisinitspurestformconstitutestheconversionofsomeprecursorsidechaintoanaturalChemicalmutagenesisChalkerandDavis783Figure2ClarkandLowe's'ChemicalMutationsofPapain.
'Cysteinewasusedasacommonprecursortomultipleaminoacids:formylglycine,glycine,andserine[24,25].
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789residue.
Insomecases,however,thenaturalresiduemaynotbeeasilyobtainedbychemicalmethods.
Intheseinstances,amimicoranalogmaysufce[33].
Indeed,someoftheearliestexamplesofchemicalproteinmodi-cationweretheconversionoflysinetohomoarginineandtheconversionofcysteinetothialysine.
Asearlyas1949,itwasshownthattreatmentofhumanserumalbuminwithO-methylisoureaprovidedhomoarginineanalogsthatfacilitatedproteincrystallization[34].
Suchatransformationhasalsobeenusedtoinvestigateactive-sitearginineresidues[35,36].
Inthe1950s,aminoethyla-tionofcysteinewasusedtoinstallasyntheticsiteofproteolysisfortrypsin,whichrecognizeslysineresidues[37–39].
Althoughthistechniquewasusedmainlyfortrypticanalysisandsequencingproteins,theunderlyingenablingtechnologyistheconversionofcysteinetothialysine.
Althoughthispseudo-mutationdoesnotpro-videanaturalresidue,thesimilaritytolysinehassincebeenusedmanytimesintheinvestigationofcatalyticlysineresidues,highlightingtheclearfunctionalutilityoftheseanalogs[40–44].
Similaralkylationsofcysteinehavealsoprovidedarginineanalogs[45].
Recentinterestincysteineaminoethylationhasintensiedsinceaccesstomethylatedlysineresiduesisrequired,especiallyonhistoneproteins[46].
Thispseudo-mutationhasallowedanincreasedunderstandingoftheeffectsofhistonemodicationandtheoverarchingepigeneticcode[47–49].
Otherpseudo-mutationshaveallowedaccesstodifferentpost-translationalmimics,suchassulfatedandphosphorylatedtyrosine[3,50].
Althoughtheseaminoacidanalogshaveprovenusefulinanumberofcontexts,theyarestillonlyanalogs,indicatingperhapsasmuchabouttheexibilityofthesystemthatrecognizesthem.
Flexiblemethodstoaccesstheresidue,modiedorotherwise,initsnaturalstateremainsanongoingchal-lengeinchemicalmutagenesisandinproteinscienceingeneral.
Wenowturntothechallengesandrecenteffortsthathaveadvancedthenotionofachemicalmutation.
TowardsageneralchemicalmutagenesisForchemicalmutagenesistobeageneralstrategyforproteinaminoacidinterconversion,itisdesirabletouseaprecursor,naturalorunnatural,thatcanprovideaccesstoafunctionalsetofsidechains.
Ideally,thisuniversalprecursorwouldprovidedivergentaccesstothe20mostcommonnaturalsidechains,alongwithmanyunnaturalresidues.
Forexample,intheworkofKoshland[11]andBender[12]suchaprecursorwastheactiveserineofsubtilisin.
Thisresiduecanbeselectivelyconvertedintoaleavinggroupanddisplacedwithanucleophile.
Intheircasetheconversiontocysteinewastheirtarget,yieldinganearisostericmutanttoinvestigatethecatalyticactivesiteoftheseproteases.
Yetonecanimagineothernucleo-philesthatcoulddisplacethesulfonylatedserineofsubtilisin.
Suchatransformationcouldprovideotherchemicalmutants,andindeedhassincebeenextendedtoSe-andTellurocysteinevariants[16,20].
Nonetheless,whensuchtransformationsarepossible,theyrelyontheinnatereactivityoftheproteasetorenderthehydroxylmoietyoftheserinealeavinggroup.
Themethodcouldnotbeeasilyextendedtootherproteinsubstrates.
Forgeneralapplication,adifferenthandleisneeded.
ArecentexampleofaprecursortootheraminoacidswasreportedbytheSchultzgroup.
Althoughtheirmethodwasnotpresentedinthecontextofchemicalmutagenesis,theoveralltransformationscouldbeconsideredchemicalmutations.
Theincorporationofp-boronophenylalanineinresponsetotheamberstopcodoncreatedaproteinwithanunnaturalaminoacidthatcanbeconvertedtophenyl-alanineandtyrosinewhenreducedoroxidized,respect-ively(Figure3)[51].
Toaidthiswork,theauthorselegantlytookadvantageoftheboronicacidasanafnitytagsinceitbindstopolyhydroxylatedresin.
Elutionwithoxidantorreductantprovidedthenativeprotein,freeoftag.
Fullconversionswereobservedintheoxidationafter2hourswhileovernightincubationwasrequiredforthereduction.
Whilethemainapplicationofthistechnologyistracelessafnitypurication,thechemicalconversionofacommonprecursortootheraminoacidscanbeconsideredachemicalmutation.
Moreover,thechemicalhandleformutation(theboronicacid)canbeincorporatedatapre-determinedsiteoftheproteininresponsetoauniquecodonanddoesnotrelyontheinnatefunctionalityorsequenceoftheproteinofinterest.
Theprecedingexampleprovidesaccesstophenylalanineandtyrosinefromacommonprecursor.
Adifferentpre-cursorisneededforaliphaticsidechains.
Koshlandagainprovidesinspirationforsuchanaminoacidprecursor.
Inaproteasesystemsimilartotheonewherehereportedtherstpointmutationofanenzyme,Koshlandshowedthatthesulfonylatedserineofchymotrypsincanbeelimi-natedunderbasicconditionstogivedehydroalanine[52,53].
Thisenamideindehydroalaninecanbecon-sideredtobeanelectrophile,andvariousthiolswereaddedtothisresidue.
Koshlandrecognizedthatthisresiduecould,inprinciple,beaprecursortootheraminoacidsidechains[53].
AdditionofH2S,forinstance,wouldprovidecysteine.
Indeedtheadditionofthiolstodehydroalaninehassinceprovidedaccesstomultiplecysteinederivativesandaminoacidanalogs.
Theseexampleshavereliedlargelyonthemultiplemethodsthathavebecomeavailablefortheincorporationofdehy-droalanineintoproteins.
Forinstance,theSchultzgrouphasdescribedtheuseofphenylselenocysteineasaprecursortodehydroalanineonproteins[54].
Oxidativeeliminationusinghydrogenper-oxideprovideddehydroalanine.
AdditionofglycosylthiolsandaliphaticthiolsprovidedS-hexadecylcysteineandS-mannosylcysteine.
OurownlabhasreportedanovelchemicalmethodtooxidativelyeliminatecysteinedirectlytodehydroalanineusingO-mesitylenesulfonylhy-784MethodsforBiomolecularSynthesisandModificationCurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
comdroxylamine(MSH)[55].
Inthisreportitwasshownthatdehydroalanineisaprecursortoglycosylcysteines,phos-phocysteine,andfarnesylcysteine—allresiduesthatarefoundinnature.
Wealsoreportedaccesstomethylatedlysineanalogs;thisuseofDhaasaprecursortolysineanalogsisacomplementarymethodtothemorecommonaminoethylationprotocol[46].
Similarly,theSchultzgrouplateruseddehydroalanineasaprecursortomethylandacyllysineanalogsonhistoneH3[56].
Intheirreport,theydemonstratedtherstuseofN-acetyl-thialysineasasub-strateforhistonedeacetylaseenzymes.
Inthisway,dehydroalanineisausefulprecursortocysteinederivatives,includingnaturalresiduessuchasglycosyl-,phosphoryl-,andfarnesylcysteine.
Thialysineanalogs,however,areonlymimicsofthenaturalsidechain.
Whilemimickingthisfunctionalitymaysufceinmanycases,itisdesirabletoaccessnativestructures.
Withthedevelopmentofahostofaqueousandasym-metrictransformations,thepotentialofdehydroalanineasageneralhandleinchemicalmutagenesisisnotoutofthequestionandwemayspeculateonfuturedirections.
Forinstance,arylsidechainscanbeaccessedfromdehydroa-laninebytheRh(I)catalyzedconjugateadditionofarylboronicacids.
Thisreactioniscompatiblewithwater,hasseveralasymmetricvariants[57],anditsuseonpeptidesubstrateshasalsobeeninvestigated[58,59].
Foraliphaticsidechains,radicaladditionstounsaturatedsystemsarepossibleinwater[60].
Thedeploymentofsuchtrans-formationsatdehydroalaninecan,inprinciple,provideaccesstomanydiversesidechains.
Applyingasimilarretrosyntheticanalysistoothersidechainsrevealsthatasignicantnumberofresiduescanbeconsideredacces-siblefromadehydroalanineprecursor.
Alanine,forinstancecanbeaccessedbycatalytic,andperhapsasym-metric,hydrogenation.
Asimilarexerciseinretrosynth-esismayrevealothertransformationsthatwillconstitutea'chemicalmutation.
'AproposalforseveralsidechainsisdepictedinFigure4.
Itisworthnotingthediversefunctionalityaccessiblefromdehydroalanine.
Aromatic,aliphatic,acidic,basic,andpost-translationallymodiedsidechainsareallaccessible,inprinciple,fromthisprecursor.
Figure4onlydepictsnaturalresidues.
Itisclearthatmanyothercomplementaryunnaturalaminoacidsshouldbeaccessiblethroughasimilarroute.
Dehydroalanineasahandleforchemicalmutagenesisisnotwithoutpotentialpitfallsandcautionarynotes.
Therstistherequirementfordehydroalanine.
Althoughsev-eralmethodsforitsincorporationintoproteinsareknown,noneisentirelygeneral.
Asimple,selective(orbetteryet,specic)installationofdehydroalanineisrequired.
Second,thetransformationsproposedinFigure4havenotbeenreportedonanyproteinsubstrates,muchlessfragile,pHsensitiveproteinsamples.
Thechallengeofwatercompa-tible,chemoselectivemutationsofDhatootherresiduesisthesecondoutstandingproblem.
Inaddition,manyoftheseproposedtransformationsmayrequireanasymmetricvariantorsomeunderstandingofsubstratecontrolofthediastereoselectivity.
Theveryanalysisofthechemicalandstereochemicaloutcomeoftheproposedtransformationisalsonosmallfeat.
ChemicalmutagenesisChalkerandDavis785Figure3Schultz'sboronicacid:achemicalprecursortoPheandTyrmutantproteins[51].
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789Whenthesechallengesaremetusingdiverseanddiver-gentintermediatessuchasdehydroalanine,weenvisionmanyadvantagesandapplicationsofchemicalmutagen-esis.
Forinstance,asinglemutantderivedfromroutinesite-directedmutagenesiscanbeachemicalprecursorformultiplemutantproteins.
Thus,asingleroundofexpres-sionandpuricationcouldyieldadiverselibraryofmutantproteinsafterchemicalmutation.
Furthermore,thenalchemicalmutationcouldbecarriedoutonthefoldedprotein,soproteinactivitycanbeattributedtothe786MethodsforBiomolecularSynthesisandModificationFigure4XX=SH,SePhEliminationDehydroalanine"RH"RRH=nucleophileChemicalMutantNHONHONHOOHNHOHNNHONHNNHONHOSMeNHOOHONHOOH2NNHONH2NHONHNHH2NNHONHAcNHONHnMe(3-n)n=0-2NHONHNNMeMe+NHONHNHnMe(2-n)H2N+HHn=0,1AromaticHydrophobiccisaBcidicAAcylatedandMethylatedNHORR,reductant(R=aliphatic)R-Rh(I)Ln(R=Ar)ChemicalMutantDehydroalanineorPolarCurrentOpinioninChemicalBiologyAproposal:dehydroalanineasageneralchemicalmutagenesishandle.
Arylandaliphaticnucleophilesmayallowaccesstoadiversefunctionalsetofaminoacidsidechains.
CurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
commutationitselfandnotmisfoldingduringexpression.
Anothersignicantapplicationwouldbeaccesstounna-turalaminoacidsandmodiednaturalaminoacidsthatarenotyetaccessibleinstandardexpressionsystems.
Ultimately,chemicalmutagenesismayresolvesupplyissuesforproteinsbearingthesepost-translationalmodi-cations,severalexamplesofwhichareshowninFigure4.
Finally,effortsinchemicalmutagenesisareattractivechemicalchallengesthatmayinspirenewchemistrythatismildandcompatiblewithbiologicalsystems.
ThesechallengesandpotentialapplicationsareguidingourcurrenteffortswhilewerevisitKoshland'sideaofchemicalmutations.
ConclusionsTheconceptofchemicalmutagenesisdatesbacktotherstpointmutationofanenzymebyKoshlandandBenderin1966.
However,asDNAandrecombinantexpressiontechnologydeveloped,theuseofchemistryinaminoacidconversionwaslargelyreplaced.
Wehaverevisitedtheideaofchemicalmutagenesisagaininanefforttoreviveitsuseinproteinscience.
Wearenotinanywaypittingchemistryagainstbiologybutratherencourageadualandreinforcinguseofcurrentmethodsinbiosyntheticincorporationofnaturalandunnaturalaminoacidsandtheirpost-expression'mutation'usingchemistry.
Itisourhypothesisthatmutationoftranslated,foldedproteinsusingchemistrycanallowrapidaccesstonatural,unnatural,andmodiednaturalsidechains.
Thechemistryandbiologyrequiredforageneral'chemicalmutagenesis'isrifewithopportunity.
Meetingthechal-lengesdescribedabovewillhelpbuildtheconceptualandpracticalfoundationforageneralmethodofproteinconstructionfreeofmanyofthecurrentlimitsofpurelybiologicalstrategies.
AcknowledgementsTheauthorsthanktheirgeneroussourcesofsupport.
JustinM.
ChalkerisaRhodesScholarandNationalScienceFoundationGraduateResearchFellow.
BenjaminG.
DavisisarecipientofaRoyalSocietyWolfsonResearchMeritAwardandissupportedbyanEngineeringandPhysicalSciencesResearchCouncilLifeSciencesInterfacePlatformGrant.
ReferencesandrecommendedreadingPapersofparticularinterest,publishedwithintheperiodofreview,havebeenhighlightedas:ofspecialinterestofoutstandinginterest1.
HutchisonCAIII,PhillipsS,EdgellMH,GillamS,JahnkeP,SmithM:MutagenesisataspecicpositioninaDNAsequence.
JBiolChem1978,253:6551-6560.
2.
SmithM:Site-directedmutagenesis.
PhilosTransRSocLondA1986,317:295-304.
3.
HackenbergerCPR,SchwarzerD:Chemoselectiveligationandmodicationstrategiesforpeptidesandproteins.
AngewChemIntEd2008,47:10030-10074.
4.
deGraafAJ,KooijmanM,HenninkWE,MastrobattistaE:Nonnaturalaminoacidsforsite-specicproteinconjugation.
BioconjugateChem2009,20:1281-1295.
5.
WalshCT,Garneau-TsodikovaS,GattoGJJr:Proteinposttranslationalmodications:thechemistryofproteomediversications.
AngewChemIntEd2005,44:7342-7372.
6.
DoughertyDA:Unnaturalaminoacidsasprobesofproteinstructureandfunction.
CurrOpinChemBiol2000,4:645-652.
7.
LinkAJ,MockML,TirrellDA:Non-canonicalaminoacidsinproteinengineering.
CurrOpinBiotechnol2003,14:603-609.
8.
XieJ,SchultzPG:Achemicaltoolkitforproteins—anexpandedgeneticcode.
NatRevMolCellBiol2006,7:775-782.
9.
OhtaA,YamagishiY,SugaH:Synthesisofbiopolymersusinggeneticcodereprogramming.
CurrOpinChemBiol2008,12:159-167.
10.
DawsonPE,KentSBH:Synthesisofnativeproteinsbychemicalligation.
AnnuRevBiochem2000,69:923-960.
11.
NeetKE,KoshlandDEJr:Theconversionofserineattheactivesiteofsubtilisintocysteine:a''chemicalmutation''.
ProcNatlAcadSciUSA1966,56:1606-1611.
Theconceptof'chemicalmutation'isintroducedinKoshland'sseminalpaper.
Theconversionoftheactivesiteserinetocysteineofsubtilisinwastherstreportofapointmutationofanenzyme.
12.
PolgarL,BenderML:Anewenzymecontainingasyntheticallyformedactivesite,thiol-subtilisin.
JAmChemSoc1966,88:3153-3154.
Theactivesiteserineofsubtilisinwaschemicallyconvertedtocysteine.
Thisreport,disclosedatthesametimeasKoshland's'chemicalmuta-tion,'wastherstpointmutationofanenzyme.
13.
PolgarL,BenderML:Simulatedmutationattheactivesiteofbiologicallyactiveproteins.
InAdvancesinEnzymologyandRelatedAreasofMolecularBiology.
EditedbyNordFF.
IntersciencePublishers;1970:381-400.
14.
DavisBG:Sugarsandproteins:newstrategiesinsyntheticbiology.
PureApplChem2009,81:285-298.
15.
YokosawaH,OjimaS,IshiiS-I:Thioltrypsin:chemicaltransformationoftheactive-aiteserineresidueofStreptomycesgriseustrypsintoacysteineresidue.
JBiochem1977,82:869-876.
16.
WuZ-P,HilvertD:Conversionofaproteaseintoanacyltransferase:selenolsubtilisin.
JAmChemSoc1989,111:4513-4514.
17.
LiuJ-Q,ShiC-B,LuoG-M,LiuZ,ZhangG-L,MaW,ShenJ-C:AnFvcatalyticantibodywithhighglutathioneperoxidaseactivity.
MaterSciEngC1999,10:131-134.
18.
LiuJ-Q,JiangM-S,LuoG-M,YanG-L,ShenJ-C:Conversionoftrypsinintoaselenium-containingenzymebyusingchemicalmutation.
BiotechnolLett1998,20:693-696.
19.
LianG,DingL,ChenM,LiuL,ZhaoD,NiJ:Aselenium-containingcatalyticantibodywithtypeIdeiodinaseactivity.
BiochemBiophysResCommun2001,283:1007-1012.
20.
MaoS,DongZ,LiuJ,LiX,LiuX,LuoG,ShenJ:Semisynthetictellurosubtilisinwithglutathioneperoxidaseactivity.
JAmChemSoc2005,127:11588-11589.
21.
SealockRW,LaskowskiMJr:Enzymicreplacementofthearginylbyalysylresidueinthereactivesiteofsoybeantrypsininhibitor.
Biochemistry1969,8:3703-3710.
22.
JeringH,TschescheH:Replacementoflysinebyarginine,phenylalanineandtryptophaninthereactivesiteofthebovinetrypsin-kallikreininhibitor(Kunitz)andchangeoftheinhibitoryproperties.
EurJBiochem1976,61:453-463.
23.
WenzelHR,TschescheH:''Chemicalmutation''byaminoacidexchangeinthereactivesiteofaproteinaseinhibitorandalterationofitsinhibitorspecity.
AngewChemIntEdEngl1981,20:295-296.
24.
ClarkPI,LoweG:Chemicalmutationsofpapain.
ThepreparationofSer25-andGly25-papain.
JChemSocChemCommun1977:923-924.
ChemicalmutagenesisChalkerandDavis787www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789Lowetakesadvantageoftheuniquereactivityofcysteineandchemicallyconvertstheactivesitecysteineofpapaintoformylglycine,serine,andglycine.
Thisreportfeaturesaselectivealkylationandbio-orthogonalphotochemistry.
Itisapowerfuldemonstrationofasingleaminoacidasadivergentchemicalprecursortomultipleresidues.
25.
ClarkPI,LoweG:Conversionoftheactive-sitecysteineresidueofpapainintoadehydro-serine,aserineandaglycineresidue.
EurJBiochem1978,84:293-299.
ThispublicationisLowe'sfullaccountofchemicalmutationsonpapain.
Again,cysteineisusedasachemicalprecursortomultipleaminoacidsidechains.
26.
VenkateshYP,VithayathilPJ:Inuenceofdeamidation(s)inthe67–74regionofribonucleaseonitsrefolding.
IntJPeptProteinRes1985,25:27-32.
27.
KurokiR,YamadaH,MoriyamaT,ImotoT:Chemicalmutationsofthecatalyticcarboxylgroupsinlysozymetothecorrespondingamides.
JBiolChem1986,261:13571-13574.
28.
YanLZ,DawsonPE:Synthesisofpeptidesandproteinswithoutcysteineresiduesbynativechemicalligationcombinedwithdesulfurization.
JAmChemSoc2001,123:526-533.
29.
CrichD,BanerjeeA:Nativechemicalligationatphenylalanine.
JAmChemSoc2007,129:10064-10065.
30.
HaaseC,RohdeH,SeitzO:Nativechemicalligationatvaline.
AngewChemIntEd2008,47:6807-6810.
31.
ChenJ,WanQ,YuanY,ZhuJ,DanishefskySJ:Nativechemicalligationatvaline:acontributiontopeptideandglycopeptidesynthesis.
AngewChemIntEd2008,47:8521-8524.
32.
WanQ,DanishefskySJ:Free-radical-based,specicdesulfurizationofcysteine:apowerfuladvanceinthesynthesisofpolypeptidesandglycopolypeptides.
AngewChemIntEd2007,46:9248-9252.
33.
DavisBG:Mimickingposttranslationalmodicationsofproteins.
Science2004,303:480-482.
34.
HughesWLJr,SaroffHA,CarneyAL:Preparationandpropertiesofserumandplasmaproteins.
XXII.
Acrystallizableguanidinatedderivativeofhumanserumalbumin.
JAmChemSoc1949,71:2476-2480.
35.
BeyerWF,FridovichI,MullenbachGT,HallewellR:Examinationoftheroleofarginine-143inthehumancopperandzincsuperoxidedismutasebysite-specicmutagenesis.
JBiolChem1987,262:11182-11187.
36.
WhitePW,KirschJF:Sequentialsite-directedmutagenesisandchemicalmodicationtoconverttheactivesitearginine292ofaspartateaminotransferasetohomoarginine.
JAmChemSoc1992,114:3567-3568.
37.
LindleyH:Anewsyntheticsubstratefortrypsinanditsapplicationtothedeterminationoftheamino-acidsequenceofproteins.
Nature1956,178:647-648.
38.
TietzeF,GladnerJA,FolkJE:ReleaseofC-terminalS-(b-aminoethyl)-cysteineresiduesbycarboxypeptidase-B.
BiochimBiophysActa1957,26:659.
39.
RafteryMA,ColeRD:Trypticcleavageatcysteinylpeptidebonds.
BiochemBiophysResCommun1963,10:467-472.
40.
SmithHB,HartmanFC:Restorationofactivitytocatalyticallydecientmutantsofribulosebisphosphatecarboxylase/oxygenasebyaminoethylation.
JBiolChem1988,263:4921-4925.
41.
SmithHB,LarimerFW,HartmanFC:Subtlealterationoftheactivesiteofribulosebisphosphatecarboxylase/oxygenasebyconcertedsite-directedmutagenesisandchemicalmodication.
BiochemBiophysResCommun1988,152:579-584.
42.
YoshimuraT,MatsushimaY,TanizawaK,SungM-H,YamauchiT,WakayamaM,EsakiN,SodaK:SubstitutionofS-(b-aminoethyl)-cysteineforactive-sitelysineofthermostableaspartateaminotransferase.
JBiochem1990,108:699-700.
43.
PlanasA,KirschJF:Reengineeringthecatalyticlysineofaspartateaminotransferasebychemicalelaborationofageneticallyintroducedcysteine.
Biochemistry1991,30:8268-8276.
44.
ZhengR,DamTK,BrewerCF,BlanchardJS:ActivesiteresiduesinMycobacteriumtuberculosispantothenatesynthetaserequiredintheformationandstabilizationoftheadenylateintermediate.
Biochemistry2004,43:7171-7178.
45.
DhallaAM,LiB,AlibhaiMF,YostKJ,HemmingsenJM,AtkinsWM,SchinellerJ,VillafrancaJJ:Regenerationofcatalyticactivityofglutaminesynthetasemutantsbychemicalactivation:explorationoftheroleofarginines339and359inactivity.
ProteinSci1994,3:476-481.
46.
SimonMD,ChuF,RackiLR,delaCruzCC,BurlingameAL,PanningB,NarlikarGJ,ShokatKM:Thesite-specicinstallationofmethyl-lysineanalogsintorecombinanthistones.
Cell2007,128:1003-1012.
47.
LuX,SimonMD,ChodaparambilJV,HansenJC,ShokatKM,LugerK:TheeffectofH3K79dimethylationandH4K20trimethylationonnucleosomeandchromatinstructure.
NatStructMolBiol2008,15:1122-1124.
48.
XuC,CuiG,BotuyanMV,MerG:StructuralbasisfortherecognitionofmethylatedhistoneH3K36bytheEaf3subunitofhistonedeacetylasecomplexRpd3S.
Structure2008,16:1740-1750.
49.
LiB,JacksonJ,SimonMD,FlehartyB,GogolM,SeidelC,WorkmanJL,ShilatifardA:HistoneH3lysine36dimethylation(H3K36me2)issufcienttorecruittheRpd3shistonedeacetylasecomplexandtorepressspurioustranscription.
JBiolChem2009,284:7970-7976.
50.
vanKasterenSI,KramerHB,JensenHH,CampbellSJ,KirkpatrickJ,OldhamNJ,AnthonyDC,DavisBG:Expandingthediversityofchemicalproteinmodicationallowspost-translationalmimicry.
Nature2007,446:1105-1109.
51.
BrustadE,BusheyML,LeeJW,GroffD,LiuW,SchultzPG:Ageneticallyencodedboronate-containingaminoacid.
AngewChemIntEd2008,47:8220-8223.
Theauthorsdescribethegeneticincorporationofp-boronophenylalanineintotheZ-domainofstaphylococcalproteinA.
Thisunnaturalresidueisusedasanafnitytagandalsoasasiteforproteinlabeling.
Thep-bornophenylalaninecanbeoxidizedtotyrosineorreducedtophenyla-lanine.
Thischemicalmutationrenderstheafnitytagtraceless.
52.
StrumeyerDH,WhiteWN,KoshlandDEJr:Roleofserineinchymotrypsinaction.
Conversionoftheactiveserinetodehydroalanine.
ProcNatlAcadSciUSA1963,50:931-935.
53.
WeinerH,WhiteWN,HoareDG,KoshlandDEJr:Theformationofanhydrochymotrypsinbyremovingtheelementsofwaterfromtheserineattheactivesite.
JAmChemSoc1966,88:3851-3859.
Theauthorsdescribethechemicalconversionoftheactivesiteserineofchymotrypsintodehydroalanine.
Koshlandproposesthatdehydroala-ninecanserveasaprecursortootheraminoacidsidechains.
54.
WangJ,SchillerSM,SchultzPG:Abiosyntheticroutetodehydroalanine-containingproteins.
AngewChemIntEd2007,46:6849-6851.
Thegeneticincorporationofphenylselenocysteineintoproteinback-bonesanditschemicalconversiontodehydroalanineisdescribed.
Thedehydroalanineresiduewasconvertedtohexadecylcysteineandmannosylcysteinebytheadditionofthecorrespondingthiol.
55.
BernardesGJL,ChalkerJM,ErreyJC,DavisBG:Facileconversionofcysteineandalkylcysteinestodehydroalanineonproteinsurfaces:versatileandswitchableaccesstofunctionalizedproteins.
JAmChemSoc2008,130:5052-5053.
Anoveloxidationofcysteinetodehydroalanineisdescribed.
Thedehy-droalanineresiduewasconvertedtoglycocysteines,phosphocysteine,farnesylcysteine,andmethyllysineanalogsbytheadditionofanappro-priatethiol.
56.
GuoJ,WangJ,LeeJS,SchultzPG:Site-specicincorporationofmethyl-andacetyl-lysineanaloguesintorecombinantproteins.
AngewChemIntEd2008,47:6399-6401.
57.
HayashiT,YamasakiK:Rhodium-catalyzedasymmetric1,4-additionanditsrelatedasymmetricreactions.
ChemRev2003,103:2829-2844.
788MethodsforBiomolecularSynthesisandModificationCurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
com58.
ChapmanCJ,MatsunoA,FrostCG,WillisMC:Site-selectivemodicationofpeptidesusingrhodiumandpalladiumcatalysis:complementaryelectrophilicandnucleophilicarylation.
ChemCommun2007:3903-3905.
59.
ChapmanCJ,HargraveJD,BishG,FrostCG:Peptidemodicationthroughsite-selectiveresidueinterconversion:applicationoftherhodium-catalysed1,4-additionofarylsiloxanesandboronates.
Tetrahedron2008,64:9528-9539.
60.
LiC-J:Organicreactionsinaqueousmediawithafocusoncarbon–carbonbondformations:adecadeupdate.
ChemRev2005,105:3095-3165.
ChemicalmutagenesisChalkerandDavis789www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789
sciencedirect.
comChemicalmutagenesis:selectivepost-expressioninterconversionofproteinaminoacidresiduesJustinMChalkerandBenjaminGDavisTheabilitytoalterproteinstructurebysite-directedmutagenesishasrevolutionizedbiochemicalresearch.
ControlledmutationsattheDNAlevel,beforeproteintranslation,arenowroutine.
Thesetechniquesallowspecic,highdelityinterconversionlargelybetween20natural,proteinogenicaminoacids.
Nonetheless,thereisaneedtoincorporateotheraminoacids,bothnaturalandunnatural,thatarenotaccessibleusingstandardsite-directedmutagenesisandexpressionsystems.
Post-translationalchemistryoffersaccesstothesesidechains.
Nearlyhalfacenturyago,theideaofa'chemicalmutation'wasproposedandtheinterconversionbetweenaminoacidsidechainswasdemonstratedonselectproteins.
Intheseisolatedexamples,apowerfulproof-of-conceptwasdemonstrated.
Here,werevivetheideaofchemicalmutagenesisanddiscusstheprospectofitsgeneralapplicationinproteinscience.
Inparticular,weconsideraminoacidsthatarechemicalprecursorstoafunctionalsetofothersidechains.
Amongthese,dehydroalaninehasmuchpotential.
Therearemultiplemethodsavailablefordehydroalanineincorporationintoproteinsandthisresidueisanacceptorforavarietyofnucleophiles.
Whenusedinconjunctionwithstandardgenetictechniques,chemicalmutagenesismayallowaccesstonatural,modied,andunnaturalaminoresiduesontranslated,foldedproteins.
AddressDepartmentofChemistry,UniversityofOxford,ChemistryResearchLaboratory,ManseldRoad,OxfordOX13TA,UnitedKingdomCorrespondingauthor:Davis,BenjaminG(ben.
davis@chem.
ox.
ac.
uk)CurrentOpinioninChemicalBiology2010,14:781–789ThisreviewcomesfromathemedissueonMethodsforBiomolecularSynthesisandModicationEditedbyMattFrancisandIsaacCarrico1367-5931/$–seefrontmatter#2010ElsevierLtd.
Allrightsreserved.
DOI10.
1016/j.
cbpa.
2010.
10.
007IntroductionThedevelopmentofsite-directedmutagenesishasrevo-lutionizedproteinscience[1,2].
Specic,highdelitymutationsattheDNAlevelarenowroutineandrecombi-nantexpressionsystemsenablesite-specicincorporationandvariationofaminoacidresiduesinthenalprotein.
Standardsite-directedmutagenesis,however,islargelylimitedto20natural,proteinogenicaminoacidresidues.
Asbiochemicalendeavorsandgoalshaveadvanced,aneedtoincorporatenon-naturalormodiedaminoacidsidechainshasemerged.
Unnaturalaminoacidscanbetargetedforfurtherlabelingaswellasproteintrackingandanalysis[3,4].
Aminoacidsidechainsarealsonaturallymodied—phosphorylation,methylation,acylation,glycosylationareafewsuchexamples—andaccesstothesesidechainsishighlydesirableifwearetoexploretheirrolesmoreprecisely[5].
Ambercodonsuppressionandreprogrammedgeneticcodesaretwostrategiesforincorporatingsomeunnaturalormodiedaminoacidsnotaccessiblethroughnormaltranslation[6–9].
Semi-synthesisbynativechemi-calligationisanotheralternativeandthroughthismethodthetotalsynthesisofproteinshasbecomereality[10].
Anotherstrategyistousechemistryonanexpressedandfoldedproteintoinstalladesiredsidechainatadesiredsite.
Itisthisnalstrategythatisthefocusofthisreview.
Ourintentionisnottoprovideacomprehensivereviewofproteinlabelingmethods.
Rather,wewishtoexplore—andindeedrevive—theconceptof'chemicalmutagen-esis'putforthnearlyhalfacenturyagobyDanielE.
Koshland,Jr.
[11].
VisionarycontributionsfromtheKosh-land[11]andBender[12]laboratoriesdescribed,forthersttime,thechemicalconversionofoneaminoacidsidechaintoanother—achemicalmutation.
Asgenetictech-nology,expressionsystems,andaqueouschemistryhavedeveloped,itisworthwhiletorevisittheideaofachemicalmutation.
Wersttracetheinceptionofthisconceptandthenconsideritsplaceincontemporarychemistryandbiologyasageneralmethodtoalterproteinstructureprecisely.
Chemicalmutagenesis:aseminalconceptinproteinscienceIn1966,thelaboratoriesofDanielE.
Koshland,Jr.
andMyronL.
Benderindependentlyreportedtherstpointmutationofanenzyme[11,12].
Inbothreports,theserineproteasesubtilisinwaschemicallyconvertedtoacysteineprotease.
ThetransformationisdepictedinFigure1.
Theactivesiteserinewasrstselectivelyconvertedintoaleavinggroupbytreatmentwithphe-nylmethanesulfonyluoride(PMSF)andthendisplacedbytheattackofthioacetate.
Theresultingthioacyl-enzymewashydrolyzedthroughtheinnateactivityoftheprotease,providingthethiol-subtilisinproduct.
Themutantenzymewascharacterizedbothchemically(thecysteineproteasecontainsasingle,easilydetectedcysteine)andthroughkineticanalysisofproteaseactivity.
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789TheSertoCysmutationprovidedanear-isostericmutantforstudyoftheproteaseactive-siteandmechanism.
Itshouldbepointedoutthatatthetimeofthesediscov-eries,nomethodsyetexistedforsite-specicmutagenesisattheDNAlevelandthetotalchemicalsynthesisofproteinswasnotyetfeasible.
Remarkably,KoshlandandBenderbothanticipatedtheuseofgeneticmodicationstospecicallycontrolproteinstructurebutuntilthistechnologywasavailable,theyproposedamoreimmedi-atelyaccessiblemethodofmutationperformedonthetranslatedproteinthroughtheuseofchemistry—suchmodicationswerereferredtoas'chemicalmutations'byKoshland[11]and'simulatedmutations'byBender[13].
Thenomenclatureforthesetransformationscanbeamatterofcontentionandconfusion,soafewpointsregardingtheconceptof'chemicalmutation'areperhapsinorder.
Inthisreview,werefertoa'chemicalmutation'asaprocessthatconvertsanaminoacidresidueonatranslatedproteintoanotheraminoacidresidue.
Chemi-calmutationinthiscontextdoesnotrefertochemicalalterationofDNAanditdoesnotrefertocovalentlabelingofproteinswithsyntheticprobes,cofactors,andotherbiochemicaltools.
ToavoidanyconfusionwithDNAmodication,weproposetheterm'post-expressionmutagenesis'forchemicalalterationofthetranslated,foldedprotein[14].
Sinceinthisreviewweconsideronlymutationsonproteins,wewilluse'chemicalmutation'and'post-expressionmutation'interchangeably.
Theproof-of-principlesetforthbyKoshlandandBenderwasalandmarkinproteinscience.
Theconversionofactivesiteserinestocysteineshasbeenappliedtootherproteasesubstratessuchastrypsin[15]andrelatedpro-tocolsforconversiontoselenocysteine[16–19]andeventellurocysteine[20]havebeenreported.
Despitetheseadvances,theserinetocysteinemutationwasonlypossibleinthesecasesbecauseoftheuniquechemicalreactivityofactive-siteserine.
AdifferentapproachwasreportednotlongafterKoshlandandBender'sdisclosures.
Laskowskidescribedan'enzy-maticmutation'onsoybeantrypsininhibitorthatreliedontrypsinandcaboxypeptidaseBtoexiseanaminoacidfromtheproteinandre-ligateanotheraminoacidatthesiteofmutation[21].
Themutationiscarriedoutonthenativeprotein,post-expression,inthesamespiritasthechemicalmutationdescribedbyKoshlandandBender,butLas-kowski'smethoddiffersconceptuallysincetheproteinbackboneisaltered,ratherthansidechain.
Moreover,Laskowskihimselfconcededthatthismethodrelieson'abitofluck'thatallthenecessaryenzymescanco-existunderthereactionconditionswithoutdeleteriousproteol-ysis[21].
Nevertheless,thisconceptwasfurtherdevelopedbyTschesche,whodescribedsequentialaminoacidexci-sion,chemicalcoupling,andelastaseligationasamethodtomutatesoybeantrypsininhibitor[22,23].
Tschescheconsideredtheseaminoacidexchangesaformof'chemicalmutation'[23].
Importantly,theseexamplesillustratethatsomealterationscanbeaccomplishedenzymaticallyandthatsuchamutationdoesnotnecessarilyrelysolelyonselectivesmall-moleculechemistry.
Collectively,themutationsdescribedbyKoshland,Bender,andLaskowskiwerethemostprecisemanipulationsofproteinstructurethatprecededthegeneticandbiotechnologicalrevolution.
Chemicalmutagenesis:expandingscopefornaturalresiduesAdecadeaftertherstexamplesofchemicalmutagenesiswereputforthbyKoshlandandBender,PeterClarkandGordonLowereportedtheuseofcysteineasaprecursortomultipleaminoacidsidechains.
Their'chemicalmutationsofpapain'canbeconsideredmoregeneralthanpreviouseffortsinchemicalmutagenesissincethecysteineresidueneednotbeinaprotease,cysteineresidueneednotbeactivatedbyitspresenceinanactivesite,thoughinthiscasetheresiduewasindeedthenucleophileofthecysteineproteasepapain[24,25].
Inthesereports,cysteinewasalkylatedwithaphenacylbromide(Figure2).
Photolysisofthisintermediateledto782MethodsforBiomolecularSynthesisandModificationFigure1ThefirstpointmutationofanenzymewasreportedindependentlybyKoshlandandBenderin1966[11,12].
Theserine-to-cysteinemutationwasaccomplishedchemically.
Themorereactiveserineoftheactivesiteofsubtilisinisselectivelyconvertedintoaleavinggroupusingasulfonylfluoride(BnSO2F).
Displacementbythioacetateprovidesathioacyl-enzymeintermediatethatishydrolyzedtothefreethiolofcysteine.
CurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
comirreversibleNorrishtypeIIcleavage,providingthethioal-dehydedehydrocysteine.
HydrationofthethiocarbonylandlossofH2Sresultsintheformationofformylglycine.
FormylglycinewasinturnreducedwithNaBH4topro-videserine.
Inthisway,theoveralltransformationisamutationfromcysteinetoserine.
Moreover,formylgly-cinecanalsobeconvertedtoglycineafterprolongedincubationatpH9.
0(Figure2).
Lowe'sextensionoftheconceptofchemicalmutagenesiswasthereforeuniqueinthatitcouldprovidemultiplemutationsfromasingle,commonprecursorand,inprinciple,couldbeappliedtoanyfreecysteine.
SinceLowe'sreport,therehavebeennotablyfewpub-licationsconcernedwithchemicalmutations.
Geneticandrecombinanttechnologywasmovingforwardatastrikingrateandaminoacidinterconversionusingpost-translationalchemistrymayhaveseemedobsolete.
Someillustrativereportsofchemicalmutationshavenonethe-lessappearedthatindicatestrategicadvantagesofchemi-calmutations.
Forinstance,Venkateshshowedthatglutamineandasparagineresiduescanbehydrolyzedwithacidto'chemicallymutate'thesidechaintoglu-tamicandasparticacid,respectively.
ItwasshownthatthesemutationsinuencedthekineticsoffoldinginRNaseA[26].
Inthereversemutation,Imotoshowedthatamidationofglutamateandaspartateresiduescouldbeusedinthestudyoftheseresiduesascatalyticsidechains[27].
Themutationinthiscaseisfromglutamateandaspartatetoglutamineandasparagine,respectively.
Inthesereports,multi-sitemutationsareaccomplished.
Inthecaseofamidation,thesemutationsarecarriedoutonthefoldedprotein.
Itisconceivablethatifthesemutationswereintroducedatthegeneticleveltheproteinwouldnotfoldproperlyandsothisexamplehighlightsastrategicadvantageofpost-expressionmuta-genesiswheredifferencesinactivitybetweentheproteinmutantscanbeattributedtothemutationanddecoupledfrommisfoldingduringexpression.
Otherexamplesofchemicalmutationshavebeentheproductsofeffortstogeneralizenativechemicalligation(NCL)andovercomeitsinherentrelianceoncysteine[10].
AnumberofcysteinedesulfurizatonprocesseshavebeenputforththatallowtheuseofNCLinthesynthesisofpeptidesandproteinsthatdonotcontaincysteine.
DawsonhasdisclosedamethodtoreducecysteinetoalanineusingpalladiumorRaneynickel[28].
Relatedtransformationshavesincebeenreportedtoprovidephenylalanine[29]andvaline[30,31]atthesiteofligation.
OfnoteisDanishefsky'smild,radicalbasedmethodforthedesulfurizationofcysteine[32].
Intheseexamplestheapplicationofchemicalmutagenesisinnativechemicalligationisapparent:achemicalmutationofcysteinetoanotherresidueconstitutesaligation,intheformalsense,atthenalresidue.
ChemicalmutationtoanaminoacidanalogChemicalmutagenesisinitspurestformconstitutestheconversionofsomeprecursorsidechaintoanaturalChemicalmutagenesisChalkerandDavis783Figure2ClarkandLowe's'ChemicalMutationsofPapain.
'Cysteinewasusedasacommonprecursortomultipleaminoacids:formylglycine,glycine,andserine[24,25].
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789residue.
Insomecases,however,thenaturalresiduemaynotbeeasilyobtainedbychemicalmethods.
Intheseinstances,amimicoranalogmaysufce[33].
Indeed,someoftheearliestexamplesofchemicalproteinmodi-cationweretheconversionoflysinetohomoarginineandtheconversionofcysteinetothialysine.
Asearlyas1949,itwasshownthattreatmentofhumanserumalbuminwithO-methylisoureaprovidedhomoarginineanalogsthatfacilitatedproteincrystallization[34].
Suchatransformationhasalsobeenusedtoinvestigateactive-sitearginineresidues[35,36].
Inthe1950s,aminoethyla-tionofcysteinewasusedtoinstallasyntheticsiteofproteolysisfortrypsin,whichrecognizeslysineresidues[37–39].
Althoughthistechniquewasusedmainlyfortrypticanalysisandsequencingproteins,theunderlyingenablingtechnologyistheconversionofcysteinetothialysine.
Althoughthispseudo-mutationdoesnotpro-videanaturalresidue,thesimilaritytolysinehassincebeenusedmanytimesintheinvestigationofcatalyticlysineresidues,highlightingtheclearfunctionalutilityoftheseanalogs[40–44].
Similaralkylationsofcysteinehavealsoprovidedarginineanalogs[45].
Recentinterestincysteineaminoethylationhasintensiedsinceaccesstomethylatedlysineresiduesisrequired,especiallyonhistoneproteins[46].
Thispseudo-mutationhasallowedanincreasedunderstandingoftheeffectsofhistonemodicationandtheoverarchingepigeneticcode[47–49].
Otherpseudo-mutationshaveallowedaccesstodifferentpost-translationalmimics,suchassulfatedandphosphorylatedtyrosine[3,50].
Althoughtheseaminoacidanalogshaveprovenusefulinanumberofcontexts,theyarestillonlyanalogs,indicatingperhapsasmuchabouttheexibilityofthesystemthatrecognizesthem.
Flexiblemethodstoaccesstheresidue,modiedorotherwise,initsnaturalstateremainsanongoingchal-lengeinchemicalmutagenesisandinproteinscienceingeneral.
Wenowturntothechallengesandrecenteffortsthathaveadvancedthenotionofachemicalmutation.
TowardsageneralchemicalmutagenesisForchemicalmutagenesistobeageneralstrategyforproteinaminoacidinterconversion,itisdesirabletouseaprecursor,naturalorunnatural,thatcanprovideaccesstoafunctionalsetofsidechains.
Ideally,thisuniversalprecursorwouldprovidedivergentaccesstothe20mostcommonnaturalsidechains,alongwithmanyunnaturalresidues.
Forexample,intheworkofKoshland[11]andBender[12]suchaprecursorwastheactiveserineofsubtilisin.
Thisresiduecanbeselectivelyconvertedintoaleavinggroupanddisplacedwithanucleophile.
Intheircasetheconversiontocysteinewastheirtarget,yieldinganearisostericmutanttoinvestigatethecatalyticactivesiteoftheseproteases.
Yetonecanimagineothernucleo-philesthatcoulddisplacethesulfonylatedserineofsubtilisin.
Suchatransformationcouldprovideotherchemicalmutants,andindeedhassincebeenextendedtoSe-andTellurocysteinevariants[16,20].
Nonetheless,whensuchtransformationsarepossible,theyrelyontheinnatereactivityoftheproteasetorenderthehydroxylmoietyoftheserinealeavinggroup.
Themethodcouldnotbeeasilyextendedtootherproteinsubstrates.
Forgeneralapplication,adifferenthandleisneeded.
ArecentexampleofaprecursortootheraminoacidswasreportedbytheSchultzgroup.
Althoughtheirmethodwasnotpresentedinthecontextofchemicalmutagenesis,theoveralltransformationscouldbeconsideredchemicalmutations.
Theincorporationofp-boronophenylalanineinresponsetotheamberstopcodoncreatedaproteinwithanunnaturalaminoacidthatcanbeconvertedtophenyl-alanineandtyrosinewhenreducedoroxidized,respect-ively(Figure3)[51].
Toaidthiswork,theauthorselegantlytookadvantageoftheboronicacidasanafnitytagsinceitbindstopolyhydroxylatedresin.
Elutionwithoxidantorreductantprovidedthenativeprotein,freeoftag.
Fullconversionswereobservedintheoxidationafter2hourswhileovernightincubationwasrequiredforthereduction.
Whilethemainapplicationofthistechnologyistracelessafnitypurication,thechemicalconversionofacommonprecursortootheraminoacidscanbeconsideredachemicalmutation.
Moreover,thechemicalhandleformutation(theboronicacid)canbeincorporatedatapre-determinedsiteoftheproteininresponsetoauniquecodonanddoesnotrelyontheinnatefunctionalityorsequenceoftheproteinofinterest.
Theprecedingexampleprovidesaccesstophenylalanineandtyrosinefromacommonprecursor.
Adifferentpre-cursorisneededforaliphaticsidechains.
Koshlandagainprovidesinspirationforsuchanaminoacidprecursor.
Inaproteasesystemsimilartotheonewherehereportedtherstpointmutationofanenzyme,Koshlandshowedthatthesulfonylatedserineofchymotrypsincanbeelimi-natedunderbasicconditionstogivedehydroalanine[52,53].
Thisenamideindehydroalaninecanbecon-sideredtobeanelectrophile,andvariousthiolswereaddedtothisresidue.
Koshlandrecognizedthatthisresiduecould,inprinciple,beaprecursortootheraminoacidsidechains[53].
AdditionofH2S,forinstance,wouldprovidecysteine.
Indeedtheadditionofthiolstodehydroalaninehassinceprovidedaccesstomultiplecysteinederivativesandaminoacidanalogs.
Theseexampleshavereliedlargelyonthemultiplemethodsthathavebecomeavailablefortheincorporationofdehy-droalanineintoproteins.
Forinstance,theSchultzgrouphasdescribedtheuseofphenylselenocysteineasaprecursortodehydroalanineonproteins[54].
Oxidativeeliminationusinghydrogenper-oxideprovideddehydroalanine.
AdditionofglycosylthiolsandaliphaticthiolsprovidedS-hexadecylcysteineandS-mannosylcysteine.
OurownlabhasreportedanovelchemicalmethodtooxidativelyeliminatecysteinedirectlytodehydroalanineusingO-mesitylenesulfonylhy-784MethodsforBiomolecularSynthesisandModificationCurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
comdroxylamine(MSH)[55].
Inthisreportitwasshownthatdehydroalanineisaprecursortoglycosylcysteines,phos-phocysteine,andfarnesylcysteine—allresiduesthatarefoundinnature.
Wealsoreportedaccesstomethylatedlysineanalogs;thisuseofDhaasaprecursortolysineanalogsisacomplementarymethodtothemorecommonaminoethylationprotocol[46].
Similarly,theSchultzgrouplateruseddehydroalanineasaprecursortomethylandacyllysineanalogsonhistoneH3[56].
Intheirreport,theydemonstratedtherstuseofN-acetyl-thialysineasasub-strateforhistonedeacetylaseenzymes.
Inthisway,dehydroalanineisausefulprecursortocysteinederivatives,includingnaturalresiduessuchasglycosyl-,phosphoryl-,andfarnesylcysteine.
Thialysineanalogs,however,areonlymimicsofthenaturalsidechain.
Whilemimickingthisfunctionalitymaysufceinmanycases,itisdesirabletoaccessnativestructures.
Withthedevelopmentofahostofaqueousandasym-metrictransformations,thepotentialofdehydroalanineasageneralhandleinchemicalmutagenesisisnotoutofthequestionandwemayspeculateonfuturedirections.
Forinstance,arylsidechainscanbeaccessedfromdehydroa-laninebytheRh(I)catalyzedconjugateadditionofarylboronicacids.
Thisreactioniscompatiblewithwater,hasseveralasymmetricvariants[57],anditsuseonpeptidesubstrateshasalsobeeninvestigated[58,59].
Foraliphaticsidechains,radicaladditionstounsaturatedsystemsarepossibleinwater[60].
Thedeploymentofsuchtrans-formationsatdehydroalaninecan,inprinciple,provideaccesstomanydiversesidechains.
Applyingasimilarretrosyntheticanalysistoothersidechainsrevealsthatasignicantnumberofresiduescanbeconsideredacces-siblefromadehydroalanineprecursor.
Alanine,forinstancecanbeaccessedbycatalytic,andperhapsasym-metric,hydrogenation.
Asimilarexerciseinretrosynth-esismayrevealothertransformationsthatwillconstitutea'chemicalmutation.
'AproposalforseveralsidechainsisdepictedinFigure4.
Itisworthnotingthediversefunctionalityaccessiblefromdehydroalanine.
Aromatic,aliphatic,acidic,basic,andpost-translationallymodiedsidechainsareallaccessible,inprinciple,fromthisprecursor.
Figure4onlydepictsnaturalresidues.
Itisclearthatmanyothercomplementaryunnaturalaminoacidsshouldbeaccessiblethroughasimilarroute.
Dehydroalanineasahandleforchemicalmutagenesisisnotwithoutpotentialpitfallsandcautionarynotes.
Therstistherequirementfordehydroalanine.
Althoughsev-eralmethodsforitsincorporationintoproteinsareknown,noneisentirelygeneral.
Asimple,selective(orbetteryet,specic)installationofdehydroalanineisrequired.
Second,thetransformationsproposedinFigure4havenotbeenreportedonanyproteinsubstrates,muchlessfragile,pHsensitiveproteinsamples.
Thechallengeofwatercompa-tible,chemoselectivemutationsofDhatootherresiduesisthesecondoutstandingproblem.
Inaddition,manyoftheseproposedtransformationsmayrequireanasymmetricvariantorsomeunderstandingofsubstratecontrolofthediastereoselectivity.
Theveryanalysisofthechemicalandstereochemicaloutcomeoftheproposedtransformationisalsonosmallfeat.
ChemicalmutagenesisChalkerandDavis785Figure3Schultz'sboronicacid:achemicalprecursortoPheandTyrmutantproteins[51].
www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789Whenthesechallengesaremetusingdiverseanddiver-gentintermediatessuchasdehydroalanine,weenvisionmanyadvantagesandapplicationsofchemicalmutagen-esis.
Forinstance,asinglemutantderivedfromroutinesite-directedmutagenesiscanbeachemicalprecursorformultiplemutantproteins.
Thus,asingleroundofexpres-sionandpuricationcouldyieldadiverselibraryofmutantproteinsafterchemicalmutation.
Furthermore,thenalchemicalmutationcouldbecarriedoutonthefoldedprotein,soproteinactivitycanbeattributedtothe786MethodsforBiomolecularSynthesisandModificationFigure4XX=SH,SePhEliminationDehydroalanine"RH"RRH=nucleophileChemicalMutantNHONHONHOOHNHOHNNHONHNNHONHOSMeNHOOHONHOOH2NNHONH2NHONHNHH2NNHONHAcNHONHnMe(3-n)n=0-2NHONHNNMeMe+NHONHNHnMe(2-n)H2N+HHn=0,1AromaticHydrophobiccisaBcidicAAcylatedandMethylatedNHORR,reductant(R=aliphatic)R-Rh(I)Ln(R=Ar)ChemicalMutantDehydroalanineorPolarCurrentOpinioninChemicalBiologyAproposal:dehydroalanineasageneralchemicalmutagenesishandle.
Arylandaliphaticnucleophilesmayallowaccesstoadiversefunctionalsetofaminoacidsidechains.
CurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
commutationitselfandnotmisfoldingduringexpression.
Anothersignicantapplicationwouldbeaccesstounna-turalaminoacidsandmodiednaturalaminoacidsthatarenotyetaccessibleinstandardexpressionsystems.
Ultimately,chemicalmutagenesismayresolvesupplyissuesforproteinsbearingthesepost-translationalmodi-cations,severalexamplesofwhichareshowninFigure4.
Finally,effortsinchemicalmutagenesisareattractivechemicalchallengesthatmayinspirenewchemistrythatismildandcompatiblewithbiologicalsystems.
ThesechallengesandpotentialapplicationsareguidingourcurrenteffortswhilewerevisitKoshland'sideaofchemicalmutations.
ConclusionsTheconceptofchemicalmutagenesisdatesbacktotherstpointmutationofanenzymebyKoshlandandBenderin1966.
However,asDNAandrecombinantexpressiontechnologydeveloped,theuseofchemistryinaminoacidconversionwaslargelyreplaced.
Wehaverevisitedtheideaofchemicalmutagenesisagaininanefforttoreviveitsuseinproteinscience.
Wearenotinanywaypittingchemistryagainstbiologybutratherencourageadualandreinforcinguseofcurrentmethodsinbiosyntheticincorporationofnaturalandunnaturalaminoacidsandtheirpost-expression'mutation'usingchemistry.
Itisourhypothesisthatmutationoftranslated,foldedproteinsusingchemistrycanallowrapidaccesstonatural,unnatural,andmodiednaturalsidechains.
Thechemistryandbiologyrequiredforageneral'chemicalmutagenesis'isrifewithopportunity.
Meetingthechal-lengesdescribedabovewillhelpbuildtheconceptualandpracticalfoundationforageneralmethodofproteinconstructionfreeofmanyofthecurrentlimitsofpurelybiologicalstrategies.
AcknowledgementsTheauthorsthanktheirgeneroussourcesofsupport.
JustinM.
ChalkerisaRhodesScholarandNationalScienceFoundationGraduateResearchFellow.
BenjaminG.
DavisisarecipientofaRoyalSocietyWolfsonResearchMeritAwardandissupportedbyanEngineeringandPhysicalSciencesResearchCouncilLifeSciencesInterfacePlatformGrant.
ReferencesandrecommendedreadingPapersofparticularinterest,publishedwithintheperiodofreview,havebeenhighlightedas:ofspecialinterestofoutstandinginterest1.
HutchisonCAIII,PhillipsS,EdgellMH,GillamS,JahnkeP,SmithM:MutagenesisataspecicpositioninaDNAsequence.
JBiolChem1978,253:6551-6560.
2.
SmithM:Site-directedmutagenesis.
PhilosTransRSocLondA1986,317:295-304.
3.
HackenbergerCPR,SchwarzerD:Chemoselectiveligationandmodicationstrategiesforpeptidesandproteins.
AngewChemIntEd2008,47:10030-10074.
4.
deGraafAJ,KooijmanM,HenninkWE,MastrobattistaE:Nonnaturalaminoacidsforsite-specicproteinconjugation.
BioconjugateChem2009,20:1281-1295.
5.
WalshCT,Garneau-TsodikovaS,GattoGJJr:Proteinposttranslationalmodications:thechemistryofproteomediversications.
AngewChemIntEd2005,44:7342-7372.
6.
DoughertyDA:Unnaturalaminoacidsasprobesofproteinstructureandfunction.
CurrOpinChemBiol2000,4:645-652.
7.
LinkAJ,MockML,TirrellDA:Non-canonicalaminoacidsinproteinengineering.
CurrOpinBiotechnol2003,14:603-609.
8.
XieJ,SchultzPG:Achemicaltoolkitforproteins—anexpandedgeneticcode.
NatRevMolCellBiol2006,7:775-782.
9.
OhtaA,YamagishiY,SugaH:Synthesisofbiopolymersusinggeneticcodereprogramming.
CurrOpinChemBiol2008,12:159-167.
10.
DawsonPE,KentSBH:Synthesisofnativeproteinsbychemicalligation.
AnnuRevBiochem2000,69:923-960.
11.
NeetKE,KoshlandDEJr:Theconversionofserineattheactivesiteofsubtilisintocysteine:a''chemicalmutation''.
ProcNatlAcadSciUSA1966,56:1606-1611.
Theconceptof'chemicalmutation'isintroducedinKoshland'sseminalpaper.
Theconversionoftheactivesiteserinetocysteineofsubtilisinwastherstreportofapointmutationofanenzyme.
12.
PolgarL,BenderML:Anewenzymecontainingasyntheticallyformedactivesite,thiol-subtilisin.
JAmChemSoc1966,88:3153-3154.
Theactivesiteserineofsubtilisinwaschemicallyconvertedtocysteine.
Thisreport,disclosedatthesametimeasKoshland's'chemicalmuta-tion,'wastherstpointmutationofanenzyme.
13.
PolgarL,BenderML:Simulatedmutationattheactivesiteofbiologicallyactiveproteins.
InAdvancesinEnzymologyandRelatedAreasofMolecularBiology.
EditedbyNordFF.
IntersciencePublishers;1970:381-400.
14.
DavisBG:Sugarsandproteins:newstrategiesinsyntheticbiology.
PureApplChem2009,81:285-298.
15.
YokosawaH,OjimaS,IshiiS-I:Thioltrypsin:chemicaltransformationoftheactive-aiteserineresidueofStreptomycesgriseustrypsintoacysteineresidue.
JBiochem1977,82:869-876.
16.
WuZ-P,HilvertD:Conversionofaproteaseintoanacyltransferase:selenolsubtilisin.
JAmChemSoc1989,111:4513-4514.
17.
LiuJ-Q,ShiC-B,LuoG-M,LiuZ,ZhangG-L,MaW,ShenJ-C:AnFvcatalyticantibodywithhighglutathioneperoxidaseactivity.
MaterSciEngC1999,10:131-134.
18.
LiuJ-Q,JiangM-S,LuoG-M,YanG-L,ShenJ-C:Conversionoftrypsinintoaselenium-containingenzymebyusingchemicalmutation.
BiotechnolLett1998,20:693-696.
19.
LianG,DingL,ChenM,LiuL,ZhaoD,NiJ:Aselenium-containingcatalyticantibodywithtypeIdeiodinaseactivity.
BiochemBiophysResCommun2001,283:1007-1012.
20.
MaoS,DongZ,LiuJ,LiX,LiuX,LuoG,ShenJ:Semisynthetictellurosubtilisinwithglutathioneperoxidaseactivity.
JAmChemSoc2005,127:11588-11589.
21.
SealockRW,LaskowskiMJr:Enzymicreplacementofthearginylbyalysylresidueinthereactivesiteofsoybeantrypsininhibitor.
Biochemistry1969,8:3703-3710.
22.
JeringH,TschescheH:Replacementoflysinebyarginine,phenylalanineandtryptophaninthereactivesiteofthebovinetrypsin-kallikreininhibitor(Kunitz)andchangeoftheinhibitoryproperties.
EurJBiochem1976,61:453-463.
23.
WenzelHR,TschescheH:''Chemicalmutation''byaminoacidexchangeinthereactivesiteofaproteinaseinhibitorandalterationofitsinhibitorspecity.
AngewChemIntEdEngl1981,20:295-296.
24.
ClarkPI,LoweG:Chemicalmutationsofpapain.
ThepreparationofSer25-andGly25-papain.
JChemSocChemCommun1977:923-924.
ChemicalmutagenesisChalkerandDavis787www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789Lowetakesadvantageoftheuniquereactivityofcysteineandchemicallyconvertstheactivesitecysteineofpapaintoformylglycine,serine,andglycine.
Thisreportfeaturesaselectivealkylationandbio-orthogonalphotochemistry.
Itisapowerfuldemonstrationofasingleaminoacidasadivergentchemicalprecursortomultipleresidues.
25.
ClarkPI,LoweG:Conversionoftheactive-sitecysteineresidueofpapainintoadehydro-serine,aserineandaglycineresidue.
EurJBiochem1978,84:293-299.
ThispublicationisLowe'sfullaccountofchemicalmutationsonpapain.
Again,cysteineisusedasachemicalprecursortomultipleaminoacidsidechains.
26.
VenkateshYP,VithayathilPJ:Inuenceofdeamidation(s)inthe67–74regionofribonucleaseonitsrefolding.
IntJPeptProteinRes1985,25:27-32.
27.
KurokiR,YamadaH,MoriyamaT,ImotoT:Chemicalmutationsofthecatalyticcarboxylgroupsinlysozymetothecorrespondingamides.
JBiolChem1986,261:13571-13574.
28.
YanLZ,DawsonPE:Synthesisofpeptidesandproteinswithoutcysteineresiduesbynativechemicalligationcombinedwithdesulfurization.
JAmChemSoc2001,123:526-533.
29.
CrichD,BanerjeeA:Nativechemicalligationatphenylalanine.
JAmChemSoc2007,129:10064-10065.
30.
HaaseC,RohdeH,SeitzO:Nativechemicalligationatvaline.
AngewChemIntEd2008,47:6807-6810.
31.
ChenJ,WanQ,YuanY,ZhuJ,DanishefskySJ:Nativechemicalligationatvaline:acontributiontopeptideandglycopeptidesynthesis.
AngewChemIntEd2008,47:8521-8524.
32.
WanQ,DanishefskySJ:Free-radical-based,specicdesulfurizationofcysteine:apowerfuladvanceinthesynthesisofpolypeptidesandglycopolypeptides.
AngewChemIntEd2007,46:9248-9252.
33.
DavisBG:Mimickingposttranslationalmodicationsofproteins.
Science2004,303:480-482.
34.
HughesWLJr,SaroffHA,CarneyAL:Preparationandpropertiesofserumandplasmaproteins.
XXII.
Acrystallizableguanidinatedderivativeofhumanserumalbumin.
JAmChemSoc1949,71:2476-2480.
35.
BeyerWF,FridovichI,MullenbachGT,HallewellR:Examinationoftheroleofarginine-143inthehumancopperandzincsuperoxidedismutasebysite-specicmutagenesis.
JBiolChem1987,262:11182-11187.
36.
WhitePW,KirschJF:Sequentialsite-directedmutagenesisandchemicalmodicationtoconverttheactivesitearginine292ofaspartateaminotransferasetohomoarginine.
JAmChemSoc1992,114:3567-3568.
37.
LindleyH:Anewsyntheticsubstratefortrypsinanditsapplicationtothedeterminationoftheamino-acidsequenceofproteins.
Nature1956,178:647-648.
38.
TietzeF,GladnerJA,FolkJE:ReleaseofC-terminalS-(b-aminoethyl)-cysteineresiduesbycarboxypeptidase-B.
BiochimBiophysActa1957,26:659.
39.
RafteryMA,ColeRD:Trypticcleavageatcysteinylpeptidebonds.
BiochemBiophysResCommun1963,10:467-472.
40.
SmithHB,HartmanFC:Restorationofactivitytocatalyticallydecientmutantsofribulosebisphosphatecarboxylase/oxygenasebyaminoethylation.
JBiolChem1988,263:4921-4925.
41.
SmithHB,LarimerFW,HartmanFC:Subtlealterationoftheactivesiteofribulosebisphosphatecarboxylase/oxygenasebyconcertedsite-directedmutagenesisandchemicalmodication.
BiochemBiophysResCommun1988,152:579-584.
42.
YoshimuraT,MatsushimaY,TanizawaK,SungM-H,YamauchiT,WakayamaM,EsakiN,SodaK:SubstitutionofS-(b-aminoethyl)-cysteineforactive-sitelysineofthermostableaspartateaminotransferase.
JBiochem1990,108:699-700.
43.
PlanasA,KirschJF:Reengineeringthecatalyticlysineofaspartateaminotransferasebychemicalelaborationofageneticallyintroducedcysteine.
Biochemistry1991,30:8268-8276.
44.
ZhengR,DamTK,BrewerCF,BlanchardJS:ActivesiteresiduesinMycobacteriumtuberculosispantothenatesynthetaserequiredintheformationandstabilizationoftheadenylateintermediate.
Biochemistry2004,43:7171-7178.
45.
DhallaAM,LiB,AlibhaiMF,YostKJ,HemmingsenJM,AtkinsWM,SchinellerJ,VillafrancaJJ:Regenerationofcatalyticactivityofglutaminesynthetasemutantsbychemicalactivation:explorationoftheroleofarginines339and359inactivity.
ProteinSci1994,3:476-481.
46.
SimonMD,ChuF,RackiLR,delaCruzCC,BurlingameAL,PanningB,NarlikarGJ,ShokatKM:Thesite-specicinstallationofmethyl-lysineanalogsintorecombinanthistones.
Cell2007,128:1003-1012.
47.
LuX,SimonMD,ChodaparambilJV,HansenJC,ShokatKM,LugerK:TheeffectofH3K79dimethylationandH4K20trimethylationonnucleosomeandchromatinstructure.
NatStructMolBiol2008,15:1122-1124.
48.
XuC,CuiG,BotuyanMV,MerG:StructuralbasisfortherecognitionofmethylatedhistoneH3K36bytheEaf3subunitofhistonedeacetylasecomplexRpd3S.
Structure2008,16:1740-1750.
49.
LiB,JacksonJ,SimonMD,FlehartyB,GogolM,SeidelC,WorkmanJL,ShilatifardA:HistoneH3lysine36dimethylation(H3K36me2)issufcienttorecruittheRpd3shistonedeacetylasecomplexandtorepressspurioustranscription.
JBiolChem2009,284:7970-7976.
50.
vanKasterenSI,KramerHB,JensenHH,CampbellSJ,KirkpatrickJ,OldhamNJ,AnthonyDC,DavisBG:Expandingthediversityofchemicalproteinmodicationallowspost-translationalmimicry.
Nature2007,446:1105-1109.
51.
BrustadE,BusheyML,LeeJW,GroffD,LiuW,SchultzPG:Ageneticallyencodedboronate-containingaminoacid.
AngewChemIntEd2008,47:8220-8223.
Theauthorsdescribethegeneticincorporationofp-boronophenylalanineintotheZ-domainofstaphylococcalproteinA.
Thisunnaturalresidueisusedasanafnitytagandalsoasasiteforproteinlabeling.
Thep-bornophenylalaninecanbeoxidizedtotyrosineorreducedtophenyla-lanine.
Thischemicalmutationrenderstheafnitytagtraceless.
52.
StrumeyerDH,WhiteWN,KoshlandDEJr:Roleofserineinchymotrypsinaction.
Conversionoftheactiveserinetodehydroalanine.
ProcNatlAcadSciUSA1963,50:931-935.
53.
WeinerH,WhiteWN,HoareDG,KoshlandDEJr:Theformationofanhydrochymotrypsinbyremovingtheelementsofwaterfromtheserineattheactivesite.
JAmChemSoc1966,88:3851-3859.
Theauthorsdescribethechemicalconversionoftheactivesiteserineofchymotrypsintodehydroalanine.
Koshlandproposesthatdehydroala-ninecanserveasaprecursortootheraminoacidsidechains.
54.
WangJ,SchillerSM,SchultzPG:Abiosyntheticroutetodehydroalanine-containingproteins.
AngewChemIntEd2007,46:6849-6851.
Thegeneticincorporationofphenylselenocysteineintoproteinback-bonesanditschemicalconversiontodehydroalanineisdescribed.
Thedehydroalanineresiduewasconvertedtohexadecylcysteineandmannosylcysteinebytheadditionofthecorrespondingthiol.
55.
BernardesGJL,ChalkerJM,ErreyJC,DavisBG:Facileconversionofcysteineandalkylcysteinestodehydroalanineonproteinsurfaces:versatileandswitchableaccesstofunctionalizedproteins.
JAmChemSoc2008,130:5052-5053.
Anoveloxidationofcysteinetodehydroalanineisdescribed.
Thedehy-droalanineresiduewasconvertedtoglycocysteines,phosphocysteine,farnesylcysteine,andmethyllysineanalogsbytheadditionofanappro-priatethiol.
56.
GuoJ,WangJ,LeeJS,SchultzPG:Site-specicincorporationofmethyl-andacetyl-lysineanaloguesintorecombinantproteins.
AngewChemIntEd2008,47:6399-6401.
57.
HayashiT,YamasakiK:Rhodium-catalyzedasymmetric1,4-additionanditsrelatedasymmetricreactions.
ChemRev2003,103:2829-2844.
788MethodsforBiomolecularSynthesisandModificationCurrentOpinioninChemicalBiology2010,14:781–789www.
sciencedirect.
com58.
ChapmanCJ,MatsunoA,FrostCG,WillisMC:Site-selectivemodicationofpeptidesusingrhodiumandpalladiumcatalysis:complementaryelectrophilicandnucleophilicarylation.
ChemCommun2007:3903-3905.
59.
ChapmanCJ,HargraveJD,BishG,FrostCG:Peptidemodicationthroughsite-selectiveresidueinterconversion:applicationoftherhodium-catalysed1,4-additionofarylsiloxanesandboronates.
Tetrahedron2008,64:9528-9539.
60.
LiC-J:Organicreactionsinaqueousmediawithafocusoncarbon–carbonbondformations:adecadeupdate.
ChemRev2005,105:3095-3165.
ChemicalmutagenesisChalkerandDavis789www.
sciencedirect.
comCurrentOpinioninChemicalBiology2010,14:781–789
- number789se.com相关文档
- plete789se.com
- 1.0789se.com
- RF789se.com
- ARIL789se.com
- inici789se.com
- limiting789se.com
瓜云互联-美国洛杉矶高防CN2高防云服务器,新老用户均可9折促销!低至32.4元/月!
瓜云互联一直主打超高性价比的海外vps产品,主要以美国cn2、香港cn2线路为主,100M以内高宽带,非常适合个人使用、企业等等!安全防护体系 弹性灵活,能为提供简单、 高效、智能、快速、低成本的云防护,帮助个人、企业从实现网络攻击防御,同时也承诺产品24H支持退换,不喜欢可以找客服退现,诚信自由交易!官方网站:点击访问瓜云互联官网活动方案:打折优惠策略:新老用户购买服务器统统9折优惠预存返款活动...

云步云72.5元/月起云服务器,香港安畅/葵湾/将军澳/沙田/大浦CN2机房,2核2G5M
云步云怎么样?云步云是创建于2021年的品牌,主要从事出售香港vps、美国VPS、日本VPS、香港独立服务器、香港站群服务器等,机房有香港、美国、日本东京等机房,目前在售VPS线路有CN2+BGP、CN2 GIA,香港的线路也是CN2直连大陆,该公司旗下产品均采用KVM虚拟化架构。目前,云步云提供香港安畅、沙田、大浦、葵湾、将军澳、新世界等CN2机房云服务器,2核2G5M仅72.5元/月起。点击进...

.asia域名是否适合做个人网站及.asia域名注册和续费成本
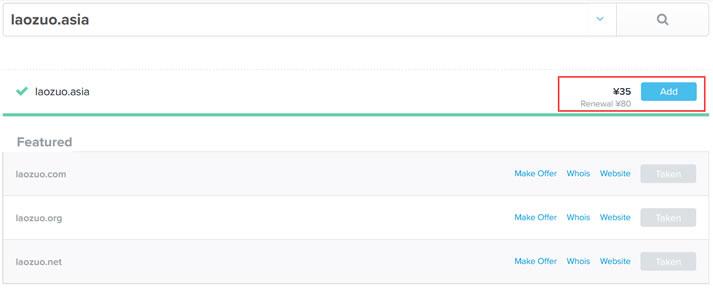
今天看到群里的老秦同学在布局自己的网站项目,这个同学还是比较奇怪的,他就喜欢用这些奇怪的域名。比如前几天看到有用.in域名,个人网站他用的.me域名不奇怪,这个还是常见的。今天看到他在做的一个范文网站的域名,居然用的是 .asia 后缀。问到其理由,是有不错好记的前缀。这里简单的搜索到.ASIA域名的新注册价格是有促销的,大约35元首年左右,续费大约是80元左右,这个成本算的话,比COM域名还贵。...
789se.com为你推荐
-
淘宝门户分析淘宝网、三大门户网站、易趣、阿里巴巴属于哪种电子商务模式京沪高铁上市首秀我能买京沪高铁股票吗摩根币摩根币是怎么骗人的?网红名字被抢注想用的微博名被人抢注了 而且是个死号 没发博没粉丝 该怎么办西部妈妈网烟台分类妈妈网 分类妈妈网的前2个字什么?百花百游百花百游的五滴自游进程www.544qq.COM跪求:天时达T092怎么下载QQ杨丽晓博客杨丽晓今年高考了吗?ww.66bobo.com谁知道11qqq com被换成哪个网站www.175qq.com这表情是什么?