sorbentsjufy
jufy 时间:2021-03-03 阅读:()
JournalofChromatographicScience,Vol.
27,July1989FundamentalsandApplicationsofSupercriticalFluidExtractioninChromatographicScienceJerryW.
KingNorthernRegionalResearchCenter,AgriculturalResearchService,UnitedStatesDepartmentofAgriculture,1815NorthUniversityStreet,Peoria,Illinois61604[Abstract/Theuniquepropertiesofsupercriticalfluidshavepromptedtheiruseforavarietyofapplicationsinthefieldofanalyticaichemistry.
Perhapsthemostwidelyciteduseofthesecompressedfluidshasbeeninthefieldofchromatography,eitherasmobilephaseeluentsorasextractionsolvents.
Thisstudyexaminesthevariousmodesinwhichsupercriticalfluidextraction(SFE)canbeemployedbythechromatographer.
Extraction,solubilization,andfractionationconditionsarepredictedbytheapplicationofwell-knownsolutionthermodynamicprinciples.
Experimentalresultsarereportedfortheremovaloflipidphasesfromnaturalproductsandthecoextractionofpe,sticidemoieties.
Finally,amethodofpredictingtherequiredmobilephasepressuresforsolubilizingandfractionatingoligomericmixturesinsupercriticalfluidchromatographyiscomparedwithliteraturedata.
IntroductionSupercriticalfluids(SF)arefindingwideacceptanceinanumberofanalyticaldisciplinesasuniquesolvationmedia.
Byfarthelargestnumberofapplicationsoccurinthefieldofchromatography,wherethesedensegasesareemployedasex-tractionsolventsandinteractivemobilephases.
Historically,supercriticalfluidchromatography(SFC)hasitsoriginsinthemid-l960s(l-3),whileitsextractionanaloguehasonlyrecentlyseenapplicationinthefieldofanalyticalchemistry.
Intruth,supercriticalfluidextraction(SFE)playsamechanisticroleinSFC,whereitcontributestotheseparationofthesolutesthatareinjectedintothechromatographicsystem.
Themajorityofreportedanalyticalchromatographicapplica-tionsofSFEtodatehavebeenconcernedwiththecouplingofSFE,usingverysmallextractioncells,withcapillary(4,5)andpackedcolumn(6,7)SFCinstrumentstoeffectsequentialSFE-SFCseparationschemes,Examplesoftheseapplicationsarereportedbyothercontributorstothisvolumeandwillnotbediscussedhere.
Thepurposeofthispaperistoprovideamoregeneralguidelinefortheapplicationofsupercriticalfluidstoavarietyofproblemsfacingthechromatographer.
Thesewillincludesamplepreparationpriortochromatography,theselec-tionofphysicalconditionsforextraction,andthespecificationofchromatographicconditionsneededforfractionatingoligomericmixtures.
ThereareanumberofmodesbywhichSFEcantieappliedtothepreparationofsamplesforchromatography.
ThesemethodsareillustratedinFigures1and2,whereananalyteiscontainedinamatrixofinterferingcomponents.
PerhapsthesimplestformofSFEisshowninFigurela,wherebytheanalyteofinterestisseparatedfromtheinterferingmatrixcomponents.
Suchanextractioncaseisrelativelyrareforreasonsthatwillbeexplainedlater.
Infact,SFEisbasicallynotaveryselectiveextractionmethod,exceptincaseswhereselectivesolubilizationofcomponentscanbeeffectedoveraverynarrowpressurerangeorwherethesolutestobeseparateddiffersignificantlyintheirrespectivephysicalproperties(molecularweight,polarity).
Figure1bisperhapsmoregenerallyencounteredinapplyingSFEtosamplematrices.
Heretheanalyteofinterestiscoex-tractedwithanumberofinterferingcomponents.
Initially,thismethodmaynotseemtobeanattractivechoice;however,theuseofanontoxiccompressedgasasanextractionsolventoffersmanyadvantagesoverconventionalliquidorganicsolventsintermsofdisposalandexposureoflaboratorypersonneltotheextractingmedium.
Anexampleofthisapproachinpesticideresidueanalysiswillbecitedlater.
AnextensionofFigurelbisshowninFigurelc.
Heretheanalyteofinterestandtheinterferingcomponentsareextractedbyasupercriticalfluidandtheanalyteissubsequentlyanalyzedbyanappropriateinstrumentaltechnique.
Theanalysismaybeoff-lineoron-line,dependingonthechosenanalyticalmethod.
Severalexamplesofon-lineSFEcoupledwithSFC(8-lo),gaschromatography(11,12),orhigh-performanceliquidchromatog-raphy(13)havebeenreported.
MoreelaboratemethodsareshowninFigure2,whereSFEiscoupledwithsorbenttechnology.
Figure2aproposesSFEextrac-tionofbothanalyteandinterferingcomponentsfollowedbyfractionationoftheanalytefrominterferingsolutes.
Applicationofsorbentcolumnsfortheseseparationsmaybebytraditionalmethodsafterthehighpressureextractionsteporbyswitchingthesupercritical-fluid-derivedextracton-linetoasorbentcol-umnheldatanelevatedpressure.
Retentioncharacteristicsofcompoundsonselectedsorbentsinthepresenceofsupercriticalcarbondioxidehavebeenreportedbytheauthor(14)andmayserveasabasisforchoosingconditionstoisolatespecificanalytes.
Reproduction(photocopying)otedilorialcontentofthispurnalLSprohibitedwithoutpublisher'spermission.
355AnalternativetoFigure2aisillustratedinFigure2b,wherebytheinterferingcoextractedcomponentsfromtheSFEsteparepermanentlyisolatedonthesorbentcartridge.
Suchaschemecanbeeffectedwithasupercriticalfluidmediumthroughoutboththeextractionandisolationsteps.
Theanalytecanthenbedirectlyintroducedintothechoseninstrumentaltechnique.
Suchamethodhasbeenrecentlyreportedforfractionatingcar-bamatepesticidesfromcoextractedlipidcomponents(15).
Finally,itshouldberecognizedthatsupercritical-fluid-basedanalyticaltechniques,suchaschromatography,offertheanalystexcitingpossibilitiesforeliminatingsampleworkuptechniquescompletely.
Theauthorhasreportedseveralexamplesofdirectinjectionofcomplexmatricesintoasupercriticalfluidchro-matograph,therebyeffectingtheseparationoftheanalytefromtheinterferingcomponentswithoutresortingtoanyformalex-tractionstepbeforeanalysis(16).
Suchamethod,Figure2c,makesuseofSFEasaninsitustepduringtheSFCprocess.
AnexampleofFigure2cwillbeprovidedlater.
TheoryManytheoreticalapproachesforpredictingthesolubilityandphaseequilibriaofsolutesinsupercriticalfluidsolventshavebeenreported(17-21).
Thesetheoriesrequireanarrayofphysi-cochemicaldataandconsiderabletimetoyieldinformationthatispertinenttooptimizingextractionconditions.
Suchmethodsareoflimitedvaluetothechromatographerfacedwithday-to-dayanalyticaldecisionsanddonotlendthemselvestopredict-ingtheextractionparametersrequiredforSFEorSFCofstruc-turallycomplexsolutes.
Wehavefoundthataknowledgeoffourbasicparametersofsupercriticalfluidextractionareextremelyhelpfulinunderstandingsolutebehaviorincompressedgasmedia.
Thefirstoftheseparametersisthemiscibilitypressure,whichisthepressureatwhichthesolutestartstodissolveinthesupercriticalfluid.
Thisparameterwastermedthe"thresholdpressure"byGiddings(22)andcorrespondstothecriticallociofmixingbe-tweenthedissolvedsoluteandthesolventgas.
Asnotedbytheauthor(23),themiscibilitypressureistechnique-dependentandAnalytetMatrixComponentsExtractionExtractionExtractionAnalyte(4AnalyteInter&eweAnalytefnterflrence(b)InstrumentalAnalysis63Figure1.
GeneralizedSFmethodsforextractionandanalysis.
JournalofChromatographicScience,Vol.
27,July,989willvaryslightly,dependingonthesensitivityoftheanalyticalmethodthatischosentomonitorthesoluteconcentrationinthesupercriticalfluidphase.
Nonetheless,anapproximateknowledgeofthispressure(orcorrespondingdensity)isveryuseful,foritpermitstheanalysttochooseastartingpressureforsupercritical-fluid-basedfractionationprocesses.
Anotherusefulparameterforspecifyingsupercriticalfluidextractionconditionsisthepressureatwhichthesoluteattainsitsmaximumsolubilityinthecompressedfluid.
ThisconditioncanbeapproximatedbyGiddings'equationwhichrelatesthesolubilityparameterofthegastoitscriticalandreducedstateproperties(3).
Whenthesolubilityparameteroftheextractingfluid(gas)isequivalenttothatofthesolute,maximumsoiubilityshouldbeattained.
Solubilitymaximaforsupercriticalfluid-solutesystemshavebeenrecordedbyanumberofinvestigators(24-27)andcorrelatedbythebasictenetsoftheregularsolutiontheorybyKing(28,29).
Thethirdparameter,thepressureregionbetweenthemisci-bilityandsolubilitymaximumpressures,isthefractionationpressurerangeinwhichasolute'ssolubilitywillrangebetweenzeroanditsmaximumvalueinthesupercriticalgas.
Inthisin-terval,itbecomespossibletoregulatethesolubilityofonesoluterelativetoanotherinthesupercriticalfluid.
Enrichmentofonecomponentoveranotherispossiblebyemployingthevariableofpressure,butitisextremelyrareinSFEexperimentstoisolateonecomponentfromtheotherwithoutresortingtoanauxiliarytechnique(thermalgradients,chromatography,etc.
).
Afrac-tionationbetweensolutesismaximizedinthispressureregionbydifferencesinthephysicalpropertiesofthedissolvedsolutesandbykeepingtheirconcentrationslowinthecompressedfluid.
Finally,aknowledgeofthesolute'sphysicalpropertiesiscriticaltooptimizinganSFE.
ThemeltingpointofthesoluteisaparticularlygermaneparameterinSFE,becausemostsolutesaredissolvedtoagreaterextentinthesupercriticalfluidmediumwhenintheirliquidstate.
IncreasingtheextractiontemperaturemayalsocauseenhancedsolutesolubilityintheSFbecauseofadecreaseinthesolute'scohesiveenergydensity,P,andthereforeitssolubilityparameter,6.
Hence,increasingtheex-Analyte+MatrixComponents/11ExtractionExtractionExtraction111AnalyteAnalyteAnalyteInterflrenceInterfirencelnterfirence1i(4SorbentSorbenti---JiAnalyteInterferenceAnalyte(4iInstrumentalAnalysis(b)Figure2.
GeneralizedSFmethodsforextractionandanalysisinvolvingseparationofinterferingcomponents.
JournalofChromatographicScience,Vol.
27,July1989tractiontemperaturewillnotnecessarilyresultinalowersolutesolubilityinthecriticalfluid.
TheuseofthesolubilityparametertheorycoupledwiththeFlory-Hugginsinteractionparameterconceptexplainsmanyofthephenomenaencounteredinsupercriticalfluidextraction(30-32).
Thisapproachwasfirstutilizedinpolymerchemistrytopredictphasemiscibilityrelationshipsbetweenpolymersdissolvedindensegases,suchasethylene(33,34).
Thedatare-quiredbytheabovetheoriesconsistsofcriticalpropertydataandsoluteorsolventsolubilityparameters.
Suchdataisusuallyavailableorcanbeestimatedfromcorrespondingstatestheory,groupcontributionmethods,ornomographs.
Withtheseparametersinhand,onecanusethefollowingequationtopredictthepressureatwhichmaximumsolutesolubilitywillbeattainedinthesupercriticalfluid:2=x,,+xs=v,(c%-6J2/RT+xsEq.
Iwherexisthetotalinteractionparameter;x,,andxsaretheenthalpicandentropicinteractionparameters,respectively;6,isthesolubilityparameterofthegasasf'(T,P);&isthesolubilityparameterofthesoluteasJ(7',P);7,isthemolarvolumeofthegasasf(T,P)=M,/Q,;M,isthemolecularweightofthegas;andQ,isthedensityofthegas.
Assumingxshasacon-stantvaluedefinedbythelatticecoordinationnumber,themax-imuminsolubilityshouldbeachievedwhen6,equals6,.
Plotsof2versuspressurearehyperbolic,theminimumoccuringatavalueequaltoxs.
Solubilityparametersforthecompressedgasarecalculatedby6,=1.
25PC'JUfY198927.
J.
P.
CalameandFt.
Steiner.
CO2extractionintheflavorandper-44.
AC.
Eldridge,J.
PFriedrich,K.
Warner,andW.
F.
Kwolek.
PrePara-fumeryindustries.
Chem.
/no.
No.
12:399-402(1982).
tionandevaluationofsupercriticalcarbondioxidedefattedsoy-28.
J.
W.
King.
Applicationsofthesolubilityparameterconcepttobeanflakes.
J.
food.
Sci.
51:584-87(1986j.
criticalfluidextraction.
Presentedatthe16thGreatLakesRegional45.
ChemistryLaboratoryGuidebook.
FoodSafety&InspectionSer-ACSMeeting,Normal,Illinois,June9,1982.
vice.
USDA,Washington,DC,1986,pp.
5.
1-5.
11.
29.
J.
W.
King.
Supercriticalfluidextractionofpolymersandsolvents:46.
J.
W.
King.
GeneralizedextractionconditionsforthecriticalfluidUtilizationofthesolubilityparameterconcept.
Polym.
Materialsprocessingofoilsandoleophiliccompounds.
J.
Am.
OilChem.
SC;.
Preprints51:707-12(1984).
SOC.
80:711(1983).
30.
J.
W.
KingandJ.
PFriedrich.
PredictionofthethresholdpressureinsupercriticalfluidextractionutilizingtheFlory-Hugginsinterac-tionparameter.
Presentedatthe20thAnnualGreatLakesRegionalACSMeeting,Milwaukee,Wisconsin,June2,1986.
31.
D.
C.
Bonner.
Solubilityofsupercriticalgasesinpolymers-Areview.
/%/ym.
Eng.
Sci17:65-72(1977).
32.
D.
H.
ZigerandC.
A.
Eckert.
Correlationandpredictionofsolidsupercriticalfluidphaseequilibria.
/no.
Eng.
ProcessDes.
Dev.
22:582-88(1983).
47.
E.
Stahl,E.
Schutz,andH.
K.
Mangold.
Extractionofseedoilswithliquidandsupercriticalcarbondioxide.
J.
Agric.
FoodChem.
28:1153-57(1980).
33.
E.
M.
CerniaandC.
Mancini.
Athermodynamicapproachtophaseequilibria.
Investigationofthepolyethylene-ethylenesystemathighpressures.
KobunshiKagaku22:797-803(1965).
34.
D.
P.
MaloneyandJ.
M.
Prausnitz.
Solubilityofethyleneinliquid,low-densitypolyethyleneatindustrial-separationpressures.
fnd.
Eng.
Chem.
ProcessDes.
Dev.
15:216-20(1976).
35.
A.
F.
M.
Barton.
CRCHandbookofSokubilityParametersandOtherCohesionalParameters.
CRCPress,BocaRaton,Florida,1983.
48.
E.
Stahl,W.
Schilz,E.
Shutz,andE.
Willing.
Aquackmethodforthemicroanalyticalevaluationofthedissolvingpowerofsuper-criticalgases.
Angew.
cbem.
/ntEd.
Engl.
17:731-38(1978).
49.
J.
H.
HildebrandandR.
L.
Scott.
TheSo/ubi/ityofNonelectrolytes,3rded.
DoverPublications,Inc.
,NewYork,1964,pp.
361-67.
50.
V.
J.
Krukonis,PhasexCorporation,personalcommunication,1988.
51.
K.
S.
Nam,S.
Kapila,G.
Pieczonka,T.
E.
Clevenger,A.
F.
Yanders,D.
S.
Viswanath,B.
Maliu.
ProceedingsofthelntemationalSym-posiumonSupercriticalFluids-Volume2.
InstituteNationalPolytechniquedeLorraine,France,1988,pp.
743-50.
52.
S.
KennedyandR.
J.
Wall.
Electron-capturedetectionofagro-chemicalsbysupercriticalfluidchromatography.
LC-GC6:930-31(1988).
36.
RFFedors.
Amethodforestimatingboththesolubilityparametersandmolarvolumesofliquids.
Polym.
Eng.
Sci.
14:147-54(1974).
37.
H.
AhmandandM.
Yaseen.
Estimationofthesolubilityparametersoflowmolecularweightcompoundsbyachemicalgroupcon-tributiontechnique.
J.
OilCo/ourChem.
Assoc.
60:99-103(1977).
53.
M.
R.
Andersen,N.
L.
Porter,E.
R.
Campbell,andB.
E.
Richter.
Theanalysisofenvironmentalresiduesusingsupercriticalfluidex-tractionasaninjectionmethodinsupercriticalfluidchroma-tography.
Presentedatthe30thRockyMountainConference,Denver,Colorado,August2,1988.
54.
E.
KlesperandW.
Hartmann.
Parametersinsupercriticalfluidchromatographyofstyreneoligomers.
J.
Polym.
Sci.
,folym.
Left.
Ed.
15:707-12(1977).
38.
A.
JayasnandM.
Yaseen.
Nomogramsforsolubilityparameter.
J.
CoatingsTechnol.
52:41-45(1980).
39.
G.
DiPaola-BaranyiandJ.
Guillet.
Estimationofpolymersolubilityparametersbygaschromatography.
Macromolecules11:228-35(1978).
40.
J.
W.
King,G.
R.
List,andJ.
PFriedrich.
Characterizationofsolute-solventinteractionsinsoybeanoilbyinversegaschromatography.
J.
Am.
OilChem.
Sot.
65:500(1988).
41.
P.
J.
Flory.
PrinciplesofPolymerChemistry,CornellUniversityPress,Ithaca,NewYork,1953,p.
544.
42.
JPFriedrich,G.
R.
List,andA.
J.
Heakin.
Petroleum-freeextractionofoilfromsoybeanswithsupercriticalCO*.
J.
Am.
OilChem.
Sot.
59:288-92(1982).
55.
W.
PJackson,B.
E.
Richter,J.
C.
Fjeldsted,R.
C.
Kong,andM.
L.
Lee.
Highresolutionsupercnticalfluidchromatography.
ACSSymp.
Sel:250:121-33(1984).
56.
J.
C.
FjeldstedandM.
L.
Lee.
Capillarysupercriticalfluidchro-matography.
Anal.
Chem.
619A-628A(1984).
57.
Siloxanes.
SuprexCorporationApplicationNote.
SuprexCorp.
,Pittsburgh,Pennsylvania.
58.
Surfacfants.
SuprexCorporationApplicationNote.
SuprexCorp.
,Pittsburgh,Pennsylvania.
59.
T.
L.
Chester.
Capillarysupercritical-fluidchromatographywithflameionizationdetection:Reductionofdetectionartifactsandextensionofdetectablemolecularweightrange.
J.
Chromatogr.
299:424-31(1984).
43.
J.
P.
FriedrichandE.
H.
Pryde.
SupercriticalCOextractionoflipid-bearingmaterialsandcharacterizationoftheproducts.
J.
Am.
OilCbem.
Sot.
61:223-28(1984).
ManuscriptreceivedMarch31,1989;revisionreceivedApril27,1989.
4364
27,July1989FundamentalsandApplicationsofSupercriticalFluidExtractioninChromatographicScienceJerryW.
KingNorthernRegionalResearchCenter,AgriculturalResearchService,UnitedStatesDepartmentofAgriculture,1815NorthUniversityStreet,Peoria,Illinois61604[Abstract/Theuniquepropertiesofsupercriticalfluidshavepromptedtheiruseforavarietyofapplicationsinthefieldofanalyticaichemistry.
Perhapsthemostwidelyciteduseofthesecompressedfluidshasbeeninthefieldofchromatography,eitherasmobilephaseeluentsorasextractionsolvents.
Thisstudyexaminesthevariousmodesinwhichsupercriticalfluidextraction(SFE)canbeemployedbythechromatographer.
Extraction,solubilization,andfractionationconditionsarepredictedbytheapplicationofwell-knownsolutionthermodynamicprinciples.
Experimentalresultsarereportedfortheremovaloflipidphasesfromnaturalproductsandthecoextractionofpe,sticidemoieties.
Finally,amethodofpredictingtherequiredmobilephasepressuresforsolubilizingandfractionatingoligomericmixturesinsupercriticalfluidchromatographyiscomparedwithliteraturedata.
IntroductionSupercriticalfluids(SF)arefindingwideacceptanceinanumberofanalyticaldisciplinesasuniquesolvationmedia.
Byfarthelargestnumberofapplicationsoccurinthefieldofchromatography,wherethesedensegasesareemployedasex-tractionsolventsandinteractivemobilephases.
Historically,supercriticalfluidchromatography(SFC)hasitsoriginsinthemid-l960s(l-3),whileitsextractionanaloguehasonlyrecentlyseenapplicationinthefieldofanalyticalchemistry.
Intruth,supercriticalfluidextraction(SFE)playsamechanisticroleinSFC,whereitcontributestotheseparationofthesolutesthatareinjectedintothechromatographicsystem.
Themajorityofreportedanalyticalchromatographicapplica-tionsofSFEtodatehavebeenconcernedwiththecouplingofSFE,usingverysmallextractioncells,withcapillary(4,5)andpackedcolumn(6,7)SFCinstrumentstoeffectsequentialSFE-SFCseparationschemes,Examplesoftheseapplicationsarereportedbyothercontributorstothisvolumeandwillnotbediscussedhere.
Thepurposeofthispaperistoprovideamoregeneralguidelinefortheapplicationofsupercriticalfluidstoavarietyofproblemsfacingthechromatographer.
Thesewillincludesamplepreparationpriortochromatography,theselec-tionofphysicalconditionsforextraction,andthespecificationofchromatographicconditionsneededforfractionatingoligomericmixtures.
ThereareanumberofmodesbywhichSFEcantieappliedtothepreparationofsamplesforchromatography.
ThesemethodsareillustratedinFigures1and2,whereananalyteiscontainedinamatrixofinterferingcomponents.
PerhapsthesimplestformofSFEisshowninFigurela,wherebytheanalyteofinterestisseparatedfromtheinterferingmatrixcomponents.
Suchanextractioncaseisrelativelyrareforreasonsthatwillbeexplainedlater.
Infact,SFEisbasicallynotaveryselectiveextractionmethod,exceptincaseswhereselectivesolubilizationofcomponentscanbeeffectedoveraverynarrowpressurerangeorwherethesolutestobeseparateddiffersignificantlyintheirrespectivephysicalproperties(molecularweight,polarity).
Figure1bisperhapsmoregenerallyencounteredinapplyingSFEtosamplematrices.
Heretheanalyteofinterestiscoex-tractedwithanumberofinterferingcomponents.
Initially,thismethodmaynotseemtobeanattractivechoice;however,theuseofanontoxiccompressedgasasanextractionsolventoffersmanyadvantagesoverconventionalliquidorganicsolventsintermsofdisposalandexposureoflaboratorypersonneltotheextractingmedium.
Anexampleofthisapproachinpesticideresidueanalysiswillbecitedlater.
AnextensionofFigurelbisshowninFigurelc.
Heretheanalyteofinterestandtheinterferingcomponentsareextractedbyasupercriticalfluidandtheanalyteissubsequentlyanalyzedbyanappropriateinstrumentaltechnique.
Theanalysismaybeoff-lineoron-line,dependingonthechosenanalyticalmethod.
Severalexamplesofon-lineSFEcoupledwithSFC(8-lo),gaschromatography(11,12),orhigh-performanceliquidchromatog-raphy(13)havebeenreported.
MoreelaboratemethodsareshowninFigure2,whereSFEiscoupledwithsorbenttechnology.
Figure2aproposesSFEextrac-tionofbothanalyteandinterferingcomponentsfollowedbyfractionationoftheanalytefrominterferingsolutes.
Applicationofsorbentcolumnsfortheseseparationsmaybebytraditionalmethodsafterthehighpressureextractionsteporbyswitchingthesupercritical-fluid-derivedextracton-linetoasorbentcol-umnheldatanelevatedpressure.
Retentioncharacteristicsofcompoundsonselectedsorbentsinthepresenceofsupercriticalcarbondioxidehavebeenreportedbytheauthor(14)andmayserveasabasisforchoosingconditionstoisolatespecificanalytes.
Reproduction(photocopying)otedilorialcontentofthispurnalLSprohibitedwithoutpublisher'spermission.
355AnalternativetoFigure2aisillustratedinFigure2b,wherebytheinterferingcoextractedcomponentsfromtheSFEsteparepermanentlyisolatedonthesorbentcartridge.
Suchaschemecanbeeffectedwithasupercriticalfluidmediumthroughoutboththeextractionandisolationsteps.
Theanalytecanthenbedirectlyintroducedintothechoseninstrumentaltechnique.
Suchamethodhasbeenrecentlyreportedforfractionatingcar-bamatepesticidesfromcoextractedlipidcomponents(15).
Finally,itshouldberecognizedthatsupercritical-fluid-basedanalyticaltechniques,suchaschromatography,offertheanalystexcitingpossibilitiesforeliminatingsampleworkuptechniquescompletely.
Theauthorhasreportedseveralexamplesofdirectinjectionofcomplexmatricesintoasupercriticalfluidchro-matograph,therebyeffectingtheseparationoftheanalytefromtheinterferingcomponentswithoutresortingtoanyformalex-tractionstepbeforeanalysis(16).
Suchamethod,Figure2c,makesuseofSFEasaninsitustepduringtheSFCprocess.
AnexampleofFigure2cwillbeprovidedlater.
TheoryManytheoreticalapproachesforpredictingthesolubilityandphaseequilibriaofsolutesinsupercriticalfluidsolventshavebeenreported(17-21).
Thesetheoriesrequireanarrayofphysi-cochemicaldataandconsiderabletimetoyieldinformationthatispertinenttooptimizingextractionconditions.
Suchmethodsareoflimitedvaluetothechromatographerfacedwithday-to-dayanalyticaldecisionsanddonotlendthemselvestopredict-ingtheextractionparametersrequiredforSFEorSFCofstruc-turallycomplexsolutes.
Wehavefoundthataknowledgeoffourbasicparametersofsupercriticalfluidextractionareextremelyhelpfulinunderstandingsolutebehaviorincompressedgasmedia.
Thefirstoftheseparametersisthemiscibilitypressure,whichisthepressureatwhichthesolutestartstodissolveinthesupercriticalfluid.
Thisparameterwastermedthe"thresholdpressure"byGiddings(22)andcorrespondstothecriticallociofmixingbe-tweenthedissolvedsoluteandthesolventgas.
Asnotedbytheauthor(23),themiscibilitypressureistechnique-dependentandAnalytetMatrixComponentsExtractionExtractionExtractionAnalyte(4AnalyteInter&eweAnalytefnterflrence(b)InstrumentalAnalysis63Figure1.
GeneralizedSFmethodsforextractionandanalysis.
JournalofChromatographicScience,Vol.
27,July,989willvaryslightly,dependingonthesensitivityoftheanalyticalmethodthatischosentomonitorthesoluteconcentrationinthesupercriticalfluidphase.
Nonetheless,anapproximateknowledgeofthispressure(orcorrespondingdensity)isveryuseful,foritpermitstheanalysttochooseastartingpressureforsupercritical-fluid-basedfractionationprocesses.
Anotherusefulparameterforspecifyingsupercriticalfluidextractionconditionsisthepressureatwhichthesoluteattainsitsmaximumsolubilityinthecompressedfluid.
ThisconditioncanbeapproximatedbyGiddings'equationwhichrelatesthesolubilityparameterofthegastoitscriticalandreducedstateproperties(3).
Whenthesolubilityparameteroftheextractingfluid(gas)isequivalenttothatofthesolute,maximumsoiubilityshouldbeattained.
Solubilitymaximaforsupercriticalfluid-solutesystemshavebeenrecordedbyanumberofinvestigators(24-27)andcorrelatedbythebasictenetsoftheregularsolutiontheorybyKing(28,29).
Thethirdparameter,thepressureregionbetweenthemisci-bilityandsolubilitymaximumpressures,isthefractionationpressurerangeinwhichasolute'ssolubilitywillrangebetweenzeroanditsmaximumvalueinthesupercriticalgas.
Inthisin-terval,itbecomespossibletoregulatethesolubilityofonesoluterelativetoanotherinthesupercriticalfluid.
Enrichmentofonecomponentoveranotherispossiblebyemployingthevariableofpressure,butitisextremelyrareinSFEexperimentstoisolateonecomponentfromtheotherwithoutresortingtoanauxiliarytechnique(thermalgradients,chromatography,etc.
).
Afrac-tionationbetweensolutesismaximizedinthispressureregionbydifferencesinthephysicalpropertiesofthedissolvedsolutesandbykeepingtheirconcentrationslowinthecompressedfluid.
Finally,aknowledgeofthesolute'sphysicalpropertiesiscriticaltooptimizinganSFE.
ThemeltingpointofthesoluteisaparticularlygermaneparameterinSFE,becausemostsolutesaredissolvedtoagreaterextentinthesupercriticalfluidmediumwhenintheirliquidstate.
IncreasingtheextractiontemperaturemayalsocauseenhancedsolutesolubilityintheSFbecauseofadecreaseinthesolute'scohesiveenergydensity,P,andthereforeitssolubilityparameter,6.
Hence,increasingtheex-Analyte+MatrixComponents/11ExtractionExtractionExtraction111AnalyteAnalyteAnalyteInterflrenceInterfirencelnterfirence1i(4SorbentSorbenti---JiAnalyteInterferenceAnalyte(4iInstrumentalAnalysis(b)Figure2.
GeneralizedSFmethodsforextractionandanalysisinvolvingseparationofinterferingcomponents.
JournalofChromatographicScience,Vol.
27,July1989tractiontemperaturewillnotnecessarilyresultinalowersolutesolubilityinthecriticalfluid.
TheuseofthesolubilityparametertheorycoupledwiththeFlory-Hugginsinteractionparameterconceptexplainsmanyofthephenomenaencounteredinsupercriticalfluidextraction(30-32).
Thisapproachwasfirstutilizedinpolymerchemistrytopredictphasemiscibilityrelationshipsbetweenpolymersdissolvedindensegases,suchasethylene(33,34).
Thedatare-quiredbytheabovetheoriesconsistsofcriticalpropertydataandsoluteorsolventsolubilityparameters.
Suchdataisusuallyavailableorcanbeestimatedfromcorrespondingstatestheory,groupcontributionmethods,ornomographs.
Withtheseparametersinhand,onecanusethefollowingequationtopredictthepressureatwhichmaximumsolutesolubilitywillbeattainedinthesupercriticalfluid:2=x,,+xs=v,(c%-6J2/RT+xsEq.
Iwherexisthetotalinteractionparameter;x,,andxsaretheenthalpicandentropicinteractionparameters,respectively;6,isthesolubilityparameterofthegasasf'(T,P);&isthesolubilityparameterofthesoluteasJ(7',P);7,isthemolarvolumeofthegasasf(T,P)=M,/Q,;M,isthemolecularweightofthegas;andQ,isthedensityofthegas.
Assumingxshasacon-stantvaluedefinedbythelatticecoordinationnumber,themax-imuminsolubilityshouldbeachievedwhen6,equals6,.
Plotsof2versuspressurearehyperbolic,theminimumoccuringatavalueequaltoxs.
Solubilityparametersforthecompressedgasarecalculatedby6,=1.
25PC'JUfY198927.
J.
P.
CalameandFt.
Steiner.
CO2extractionintheflavorandper-44.
AC.
Eldridge,J.
PFriedrich,K.
Warner,andW.
F.
Kwolek.
PrePara-fumeryindustries.
Chem.
/no.
No.
12:399-402(1982).
tionandevaluationofsupercriticalcarbondioxidedefattedsoy-28.
J.
W.
King.
Applicationsofthesolubilityparameterconcepttobeanflakes.
J.
food.
Sci.
51:584-87(1986j.
criticalfluidextraction.
Presentedatthe16thGreatLakesRegional45.
ChemistryLaboratoryGuidebook.
FoodSafety&InspectionSer-ACSMeeting,Normal,Illinois,June9,1982.
vice.
USDA,Washington,DC,1986,pp.
5.
1-5.
11.
29.
J.
W.
King.
Supercriticalfluidextractionofpolymersandsolvents:46.
J.
W.
King.
GeneralizedextractionconditionsforthecriticalfluidUtilizationofthesolubilityparameterconcept.
Polym.
Materialsprocessingofoilsandoleophiliccompounds.
J.
Am.
OilChem.
SC;.
Preprints51:707-12(1984).
SOC.
80:711(1983).
30.
J.
W.
KingandJ.
PFriedrich.
PredictionofthethresholdpressureinsupercriticalfluidextractionutilizingtheFlory-Hugginsinterac-tionparameter.
Presentedatthe20thAnnualGreatLakesRegionalACSMeeting,Milwaukee,Wisconsin,June2,1986.
31.
D.
C.
Bonner.
Solubilityofsupercriticalgasesinpolymers-Areview.
/%/ym.
Eng.
Sci17:65-72(1977).
32.
D.
H.
ZigerandC.
A.
Eckert.
Correlationandpredictionofsolidsupercriticalfluidphaseequilibria.
/no.
Eng.
ProcessDes.
Dev.
22:582-88(1983).
47.
E.
Stahl,E.
Schutz,andH.
K.
Mangold.
Extractionofseedoilswithliquidandsupercriticalcarbondioxide.
J.
Agric.
FoodChem.
28:1153-57(1980).
33.
E.
M.
CerniaandC.
Mancini.
Athermodynamicapproachtophaseequilibria.
Investigationofthepolyethylene-ethylenesystemathighpressures.
KobunshiKagaku22:797-803(1965).
34.
D.
P.
MaloneyandJ.
M.
Prausnitz.
Solubilityofethyleneinliquid,low-densitypolyethyleneatindustrial-separationpressures.
fnd.
Eng.
Chem.
ProcessDes.
Dev.
15:216-20(1976).
35.
A.
F.
M.
Barton.
CRCHandbookofSokubilityParametersandOtherCohesionalParameters.
CRCPress,BocaRaton,Florida,1983.
48.
E.
Stahl,W.
Schilz,E.
Shutz,andE.
Willing.
Aquackmethodforthemicroanalyticalevaluationofthedissolvingpowerofsuper-criticalgases.
Angew.
cbem.
/ntEd.
Engl.
17:731-38(1978).
49.
J.
H.
HildebrandandR.
L.
Scott.
TheSo/ubi/ityofNonelectrolytes,3rded.
DoverPublications,Inc.
,NewYork,1964,pp.
361-67.
50.
V.
J.
Krukonis,PhasexCorporation,personalcommunication,1988.
51.
K.
S.
Nam,S.
Kapila,G.
Pieczonka,T.
E.
Clevenger,A.
F.
Yanders,D.
S.
Viswanath,B.
Maliu.
ProceedingsofthelntemationalSym-posiumonSupercriticalFluids-Volume2.
InstituteNationalPolytechniquedeLorraine,France,1988,pp.
743-50.
52.
S.
KennedyandR.
J.
Wall.
Electron-capturedetectionofagro-chemicalsbysupercriticalfluidchromatography.
LC-GC6:930-31(1988).
36.
RFFedors.
Amethodforestimatingboththesolubilityparametersandmolarvolumesofliquids.
Polym.
Eng.
Sci.
14:147-54(1974).
37.
H.
AhmandandM.
Yaseen.
Estimationofthesolubilityparametersoflowmolecularweightcompoundsbyachemicalgroupcon-tributiontechnique.
J.
OilCo/ourChem.
Assoc.
60:99-103(1977).
53.
M.
R.
Andersen,N.
L.
Porter,E.
R.
Campbell,andB.
E.
Richter.
Theanalysisofenvironmentalresiduesusingsupercriticalfluidex-tractionasaninjectionmethodinsupercriticalfluidchroma-tography.
Presentedatthe30thRockyMountainConference,Denver,Colorado,August2,1988.
54.
E.
KlesperandW.
Hartmann.
Parametersinsupercriticalfluidchromatographyofstyreneoligomers.
J.
Polym.
Sci.
,folym.
Left.
Ed.
15:707-12(1977).
38.
A.
JayasnandM.
Yaseen.
Nomogramsforsolubilityparameter.
J.
CoatingsTechnol.
52:41-45(1980).
39.
G.
DiPaola-BaranyiandJ.
Guillet.
Estimationofpolymersolubilityparametersbygaschromatography.
Macromolecules11:228-35(1978).
40.
J.
W.
King,G.
R.
List,andJ.
PFriedrich.
Characterizationofsolute-solventinteractionsinsoybeanoilbyinversegaschromatography.
J.
Am.
OilChem.
Sot.
65:500(1988).
41.
P.
J.
Flory.
PrinciplesofPolymerChemistry,CornellUniversityPress,Ithaca,NewYork,1953,p.
544.
42.
JPFriedrich,G.
R.
List,andA.
J.
Heakin.
Petroleum-freeextractionofoilfromsoybeanswithsupercriticalCO*.
J.
Am.
OilChem.
Sot.
59:288-92(1982).
55.
W.
PJackson,B.
E.
Richter,J.
C.
Fjeldsted,R.
C.
Kong,andM.
L.
Lee.
Highresolutionsupercnticalfluidchromatography.
ACSSymp.
Sel:250:121-33(1984).
56.
J.
C.
FjeldstedandM.
L.
Lee.
Capillarysupercriticalfluidchro-matography.
Anal.
Chem.
619A-628A(1984).
57.
Siloxanes.
SuprexCorporationApplicationNote.
SuprexCorp.
,Pittsburgh,Pennsylvania.
58.
Surfacfants.
SuprexCorporationApplicationNote.
SuprexCorp.
,Pittsburgh,Pennsylvania.
59.
T.
L.
Chester.
Capillarysupercritical-fluidchromatographywithflameionizationdetection:Reductionofdetectionartifactsandextensionofdetectablemolecularweightrange.
J.
Chromatogr.
299:424-31(1984).
43.
J.
P.
FriedrichandE.
H.
Pryde.
SupercriticalCOextractionoflipid-bearingmaterialsandcharacterizationoftheproducts.
J.
Am.
OilCbem.
Sot.
61:223-28(1984).
ManuscriptreceivedMarch31,1989;revisionreceivedApril27,1989.
4364
- sorbentsjufy相关文档
- Tiszatajjufy
- 1994.jufy
- Excaliburjufy
- LIVIAjufy
- melpomenejufy
- fieldjufy
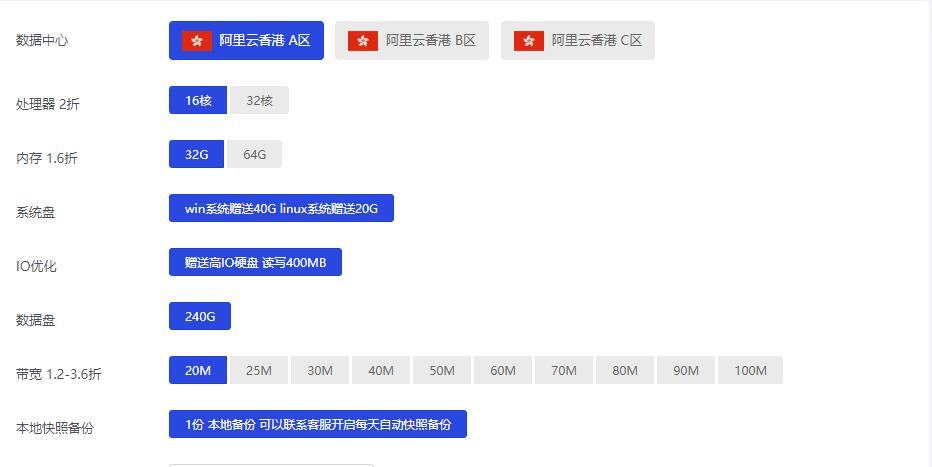
阿里云香港 16核32G 20M 999元/月
阿里云香港配置图提速啦是成立于2012年的十分老牌的一个商家这次给大家评测的是 阿里云香港 16核32G 20M 这款产品,单单说价格上就是十分的离谱原价8631元/月的现价只要 999元 而且还有个8折循环优惠。废话不多说直接进入正题。优惠时间 2021年8月20日-2021年9月20日 优惠码 wn789 8折优惠阿里云香港BGP专线 16核32G 10M带宽 优惠购买 399元购买链接阿里云...
火数云-618限时活动,国内云服务器大连3折,限量50台,九江7折 限量30台!
官方网站:点击访问火数云活动官网活动方案:CPU内存硬盘带宽流量架构IP机房价格购买地址4核4G50G 高效云盘20Mbps独享不限openstack1个九江287元/月立即抢购4核8G50G 高效云盘20Mbps独享不限openstack1个九江329元/月立即抢购2核2G50G 高效云盘5Mbps独享不限openstack1个大连15.9元/月立即抢购2核4G50G 高效云盘5Mbps独享不限...

10gbiz七月活动首月半价$2.36/月: 香港/洛杉矶CN2 GIA VPS
10gbiz怎么样?10gbiz 美国万兆带宽供应商,主打美国直连大带宽,真实硬防。除美国外还提供线路非常优质的香港、日本等数据中心可供选择,全部机房均支持增加独立硬防。洛杉矶特色线路去程三网直连(电信、联通、移动)回程CN2 GIA优化,全天低延迟。中国大陆访问质量优秀,最多可增加至600G硬防。香港七星级网络,去程回程均为电信CN2 GIA+联通+移动,大陆访问相较其他香港GIA线路平均速度更...
jufy为你推荐
-
推广方法推广方案怎么写绵阳电信绵阳电信营业厅哪家最大手机最全邮箱打不开怎么办163邮箱突然打不开了怎么办ps抠图技巧ps中怎么抠图?安卓应用平台哪个手机应用平台的软件比较正版,安全?安装迅雷看看播放器迅雷看看播放器安装mate8价格华为麦特八多少价格ios系统ios系统的手机有哪些?虚拟专用网虚拟专用网 有什么用处?小米手柄买了个小米蓝牙手柄,游戏是可以玩但是按键位置不舒服,怎么可以改按键