waterstablehost
stablehost 时间:2021-01-03 阅读:()
SHORTCOMMUNICATIONSThreonineinCollagenTriple-helicalStructureNatthaJIRAVANICHANUN,1;2KazunoriMIZUNO,3HansPeterBA¨CHINGER,3andKenjiOKUYAMA1;y1DepartmentofMacromolecularScience,GraduateSchoolofScience,OsakaUniversity,Toyonaka560-0043,Japan2DepartmentofBiotechnologyandLifeScience,GraduateSchoolofEngineering,TokyoUniversityofAgricultureandTechnology,Koganei184-8588,Japan3DepartmentofBiochemistryandMolecularBiology,OregonHealth&ScienceUniversity,andShrinersHospitalforChildren,ResearchDepartment,Portland,Oregon97239,USA(ReceivedOctober4,2005;AcceptedDecember5,2005;PublishedApril15,2006)KEYWORDSThreonine/Collagen/Triple-helicalStructure/Host-guestPeptide/Side-chainConformation/HydrationPattern/[DOI10.
1295/polymj.
38.
400]Collagenisthemostabundantproteinsfoundintheextracellularmatrixofmulticellularanimals,andhasauniquetriple-helicalstructure,whichiscomposedofthethreepolypeptides.
Thethreechainsformaright-handedsupercoiledtriple-helix.
Eachpolypep-tidechainrequiresGlyateverythirdresidue,whichgenerates-Xaa-Yaa-Gly-repeatingsequence.
Theglycineresiduesineverythirdpositionarepackedinthecenterofthetriple-helix.
TheresiduesintheXaaandtheYaapositionsareexposedtothemolecu-larsurface.
HighcontentsofiminoacidsintheXaaandtheYaapositionsarerequiredtothestabilityofthestructure.
Collagenfamilyincludesmorethanthirtyproteinsinvertebrates.
1,2Similarcollagensandmuchmorediversecollagenproteinsarealsopresent-edthroughoutinvertebratesincludingafewgiantmoleculesfoundinthecuticlesofseveralwormspe-cies.
3,4Forexample,theRiftiapachyptilacuticlecol-lagenhasalowHypcontentbuttheThrcontentismuchhigherthanthosefoundinothercollagens.
5Themechanismofthestabilityofthecollagenhelixisstillunknown.
TheThrofthecollagenishighlygly-cosylated.
4Severalmodelpeptidesweresynthesizedtoanalyzeitsthermalstabilityandproperty.
5–9TheO-galactosylationofThrincreasesthethermalstability(thehelix-coiltransitiontemperature)ofAc-(Gly-Pro/4(R)Hyp-Thr)10-NH2peptides.
6TheCDexperimentsofAc-(Gly-4(R)Hyp-Yaa)10-NH2peptideswithvari-ousaminoacidsintheYaaposition(Thr,Ser,Val,Ala,andalloThr)suggestedthatthemethylgroup,hydroxylgroupandstereocongurationofThrareim-portantforthestability.
9ThemethylgroupofThrwashypothesizedtoshieldtheinter-chainhydrogenbondbetweentheamideofGlyandcarbonylofXaaresi-duesfromwatermoleculesbyenergy-minimizationmethod.
9Althoughseveralstudieshavechallengedtorationalizetheexperimentaldata,themechanismofthestabilityincuticlecollagenisstillambiguous.
InordertounderstandthestabilizationmechanismofThrintheYaaposition,weattemptedtocrystallizethepeptidesAc-(Gly-4(R)Hyp-Thr)10-NH2andH-(Gly-4(R)Hyp-Thr)10-OH.
Despitetheirabilitytoformatriple-helicalstructure,6wecouldnotsucceedintheformationofthesinglecrystalsofthesepeptidesyet.
Thehost-guestpeptidesystemisanalternativewaytogetsinglecrystalsofthepeptidewithinterest-ingsequence.
Therefore,4(R)Hyp-Thr-Glytripeptideunitwasinsertedintothestablehostpeptide(Pro-Pro-Gly)9.
10Thehost-guestpeptideH-(Pro-Pro-Gly)4-(4(R)Hyp-Thr-Gly)-(Pro-Pro-Gly)4-OH(OTG)con-tains4(R)Hyp-Thr-GlytripeptideunitthatisabundantintheRiftiapachyptilacuticlecollagen.
ThesinglecrystalanalysisoftheOTGpeptideprovidedtherstinsightintotheunique4(R)Hyp-Thr-Glytripeptideunitconformation.
Here,theThrconformationandtheobservedhydrationpatternsaroundThrresidueintriple-helicalstructurewererevealed.
EXPERIMENTALCrystallizationwasperformedbyhanging-dropdif-fusionmethodat4C.
Samplesolutionwaspreparedatconcentrated10mgmL1.
Reservoirsolutioncon-tained0.
1MHepesbuerpH7.
5and23%(w/v)PEG1000.
Dropmixturemadeupofsamplesolution2mlandreservoirsolution2ml.
Rod-likecrystalsap-pearedinabout3weeks.
Asinglecrystalwasmeas-uredat100KonthebeamlineBL6AatthePhotonFactoryinTsukuba.
IntensitydatawasprocessedbyCrystalClear.
11CrystalbelongstomonoclinicspaceyTowhomcorrespondenceshouldbeaddressed(Tel:+81-66-850-5455,Fax:+81-66-850-5455,E-mail:okuyamak@chem.
sci.
osaka-u.
ac.
jp).
400PolymerJournal,Vol.
38,No.
4,pp.
400–403(2006)groupP21withunitcellparametersa26:0,b26:5,c80:2A,90:4.
ThestructureofOTGwasdeterminedbymolecularreplacementmethodusing(Pro-Pro-Gly)9peptide(PDBcode1ITT)12asasearchmodel.
PositionalrenementwasperformedbyX-PLOR13andstructurerenementwascarriedoutbySHELX-L.
14RESULTSANDDISCUSSIONThrresiduesareatthecentraltripeptideunitofthemolecule.
Todescribeside-chainconformationofThr,1dihedralangleisdenedbyN-C-C-O1orN-C-C-C2.
Thedierentconformationsoftheside-chainasafunctionof1valuesof60,180,and60arereferredtogauche,trans,andgauche,respectively.
Thus,Thrside-chaininOTGstructure,theO1takesgaucheconformation,whereastheC2takestransconformationtoamidegroup(Figure1).
BoththeO1andtheC2aredirectedtowardadja-centchains.
ThiskindofThrside-chainconformationisthesameastwooutofthreeThrinT3-785pep-tide.
15Theaveragemain-chaindihedralangles(=)ofThrinthisstudyare61and145,whicharecon-sistentwiththosevaluesintheYaapositionofcolla-gen-likepeptides.
10,16InT3-785structure,15water-mediatedhydrogenbondwasreportedbetweentheThrOHgroupandtheGlycarbonylinthesamechainviaonewatermolecule.
ForThr,notonlytheabovewater-mediatedpattern,butalsodiversewater-medi-atedpatternsareobservedintheOTGstructureinFigure2a.
Intherstcase,twowatermoleculesmakehydrogenbondswiththeOHgroupofThr114andthegauche+NOγ1transCγ2Figure1.
gauche/transconformationofThrintheOTGstructure.
Gly1AOGly2BOGly112OThr314O1γHyp3AOThr114NHyp4BOδδδThr114O1Hyp5COHyp4BOThr114OThr214O1γγHyp5COPro317OHyp6AO(a)(b)Figure2.
(a)CentralregionoftheOTGmoleculeshowsthreecasesofwater-mediatedhydrogenbondsatOHgroupofThr.
Case1:Thr114O1connectsthroughtwowatermoleculestoGly112Ointhesamechain.
Case2:Thr114O1connectsthroughtwowatermole-culestoThr114Nwithinthesameresidue.
Case3:Thr314O1connectsthroughtwowatermoleculestoThr114OofadjacentchainandThr214O1connectsthroughtwowatermoleculestoPro317Oofadjacentchain.
Intheresiduename,therstdigitcorrespondstoachainnumberandthenexttwodigitscorrespondtoaresiduenumber.
(b)HydrationpatternsinvolvingOHgroupof4(R)Hypandcarbonylgroupsin(Pro-4(R)Hyp-Gly)11structure.
16Threechainsinamoleculeareshownbydierentshedding;light-,middle-anddark-gray.
Spheresarewatermolecules.
Intra-chainandinter-chainwater-mediatednetworksareshowninbrokenandsolidlines,respectively.
ThreonineinCollagenTriple-helicalStructurePolym.
J.
,Vol.
38,No.
4,2006401carbonylgroupofGly112inthesamechain(brokenlines).
Inthesecondcase,twowatermoleculesinter-actbetweentheOHandtheamidegroupswithinthesameThr114residue(brokenlines).
Andthethirdcaseoccursattwopositions;oneistwowatermole-culesarelinkedbetweentheOHgroupofThr314andthecarbonylgroupofThr114ofneighboringchainandanotheristhesimilarpatternbetweentheOHgroupofThr214andthecarbonylgroupofPro317(solidlines).
Theaveragehydrogenbonddis-tanceinthesethreewater-mediatedpatternsis2.
95A.
Thehydrationpatterninthesecondcasecouldnotbefoundat4(R)Hypduetothelackofhydrogenattheamidegroup.
However,hydrationpatternsintherstandthethirdcases,whichareinter-andintra-chainhydrogenbondnetworks,aregenerallyobservedat4(R)HypintheYaapositioninthepeptidesincludingPro-4(R)Hyp-Glytripeptideunit.
16,17Theseinter-andintra-chainhydrationnetworksoccurrepeatedlyalongthetriple-helicalmolecule,forexample,in(Pro-4(R)Hyp-Gly)11structure16asshowninFigure2b.
Theyarethedominantfeatureintherepetitivepat-ternsof4(R)HypinpeptideshavingPro-4(R)Hyp-Glyrepeatingsequence.
16,17Thus,thisresultindicatesthattheOHgroupofThrintheYaapositionpartici-patesinwater-mediatedinter-andintra-chainhydro-genbondsinthesimilarwaytotheOHgroupof4(R)Hyp.
Moreover,thepositionoftheOHgroupofThrislocatedclosetothatof4(R)HypwhenThrintheOTGstructureissuperimposedoverthe4(R)Hypinthe(Pro-4(R)Hyp-Gly)11structure(Figure3).
Thedistancebetweenbothhydroxyloxygenatomsisabout1A.
ThecloselocationoftheOHgroupsofThrto4(R)Hypcouldcontributetothesimilarforma-tionofwater-mediatedhydrogenbonds.
Therefore,theOTGstructuredemonstratesthatThrcouldactlike4(R)HypatOHgroupside-chaintomakesimilarwater-mediatednetworks.
ThethermalstabilityofAc-(Gly-4(R)Hyp-Yaa)10-NH2peptidescontainingtheThrishigherthanthosecontainingtheSer,alloThr,AlaandVal,9whichsuggeststheimportanceoftheside-chainconformationinthetriple-helicalstructure.
SofaronlytheT3-78515peptidehasareportedstructureofThrinatriple-helicalstructure.
TheX-raydeterminationoftheOTGpeptideprovidesinsightintodetailedstructureoffrequentlyobservedresiduesinRiftiapachytilacuticlecollagen.
Althoughthestabi-lizationmechanismoftheOTGpeptideisnotclearlyunderstood,thenestructureoftheOTGpeptidepro-videsvaluableinformationofThrconformationin-cludingdiversityofwater-mediatedhydrogenbondsaroundThrinthetriple-helicalstructure.
Interestingly,theobservedhydrationpatternsofThraresimilartothoseof4(R)Hypandmoreover,OHgroupside-chaincharacteristicofThrand4(R)Hypissimilaraswell.
REFERENCES1.
J.
MyllyharjuandK.
I.
Kivirikko,TrendsGenet.
,20,33(2004).
2.
C.
M.
KieltyandM.
E.
Grant,'Connectivetissueanditsheritabledisorders.
MolecularGeneticsinMedicalAs-pects,'2nded.
,WileyLiss,NewYork,2002,pp159–221.
3.
R.
Har-ElandM.
L.
Tanzer,FASEBJ.
,7,1115(1993).
4.
F.
Gaill,K.
Mann,H.
Wiedemann,J.
Engel,andR.
Timpl,J.
Mol.
Biol.
,246,284(1995).
5.
K.
Mann,D.
E.
Mechling,H.
P.
Ba¨chinger,C.
Eckerskorn,F.
Gaill,andR.
Timpl,J.
Mol.
Biol.
,261,255(1996).
6.
J.
G.
BannandH.
P.
Ba¨chinger,J.
Biol.
Chem.
,275,24466(2000).
7.
J.
G.
Bann,D.
H.
Peyton,andH.
P.
Ba¨chinger,FEBSLett.
,473,237(2000).
8.
J.
G.
Bann,H.
P.
Ba¨chinger,andD.
H.
Peyton,Biochemis-try,42,4042(2003).
9.
K.
Mizuno,T.
Hayashi,andH.
P.
Ba¨chinger,J.
Biol.
Chem.
,278,32373(2003).
10.
C.
Hongo,K.
Noguchi,K.
Okuyama,Y.
Tanaka,andN.
Nishino,J.
Biochem.
,138,135(2005).
11.
CrystalClear(Rigaku)MolecularStructureCorporation,TheWoodlands,Texas,USA,1999.
12.
C.
Hongo,V.
Nagarajan,K.
Noguchi,S.
Kamitori,K.
Okuyama,Y.
Tanaka,andN.
Nishino,Polym.
J.
,33,812(2001).
13.
A.
T.
Brunger,'X-PLORVersion3.
1SystemforX-rayCrystallographyandNMR,'YaleUniversityPress,NewHaven:CT,1992.
1Figure3.
SuperimpositionofThrintheOTGpeptide(dark-gray)onthe(Pro-4(R)Hyp-Gly)11triple-helix(light-gray).
16ThedistancebetweenOHgroupsofThrand4(R)HypintheYpositionoftwopeptidesisabout1Aandbothpeptidesshowthesimilarhydrationpattern.
Gly-4(R)Hyp-ThrintheOTGandGly-Pro-4(R)Hypinthe(Pro-4(R)Hyp-Gly)11areshowninballandstickdiagram.
N.
JIRAVANICHANUNetal.
402Polym.
J.
,Vol.
38,No.
4,200614.
G.
M.
SheldricandT.
R.
Schneidern,MethodsEnzymol.
,277,319(1997).
15.
R.
Z.
Kramer,J.
Bella,P.
Mayville,B.
Brodsky,andH.
M.
Berman,Nat.
Struct.
Biol.
,6,454(1999).
16.
K.
Okuyama,C.
Hongo,R.
Fukushima,G.
Wu,H.
Narita,K.
Noguchi,Y.
Tanaka,andN.
Nishino,Biopolymers,76,367(2004).
17.
J.
Bella,B.
Brodsky,andH.
M.
Berman,Structure,3,893(1995).
4(R)Hyp:4(R)-hydroxyprolineO:4(R)-hydroxyprolineGal:galactoseT3-785peptide:(Pro-Hyp-Gly)3-Ile-Thr-Gly-Ala-Arg-Gly-Leu-Ala-Gly-Pro-Hyp-Gly-(Pro-Hyp-Gly)3ThreonineinCollagenTriple-helicalStructurePolym.
J.
,Vol.
38,No.
4,2006403
1295/polymj.
38.
400]Collagenisthemostabundantproteinsfoundintheextracellularmatrixofmulticellularanimals,andhasauniquetriple-helicalstructure,whichiscomposedofthethreepolypeptides.
Thethreechainsformaright-handedsupercoiledtriple-helix.
Eachpolypep-tidechainrequiresGlyateverythirdresidue,whichgenerates-Xaa-Yaa-Gly-repeatingsequence.
Theglycineresiduesineverythirdpositionarepackedinthecenterofthetriple-helix.
TheresiduesintheXaaandtheYaapositionsareexposedtothemolecu-larsurface.
HighcontentsofiminoacidsintheXaaandtheYaapositionsarerequiredtothestabilityofthestructure.
Collagenfamilyincludesmorethanthirtyproteinsinvertebrates.
1,2Similarcollagensandmuchmorediversecollagenproteinsarealsopresent-edthroughoutinvertebratesincludingafewgiantmoleculesfoundinthecuticlesofseveralwormspe-cies.
3,4Forexample,theRiftiapachyptilacuticlecol-lagenhasalowHypcontentbuttheThrcontentismuchhigherthanthosefoundinothercollagens.
5Themechanismofthestabilityofthecollagenhelixisstillunknown.
TheThrofthecollagenishighlygly-cosylated.
4Severalmodelpeptidesweresynthesizedtoanalyzeitsthermalstabilityandproperty.
5–9TheO-galactosylationofThrincreasesthethermalstability(thehelix-coiltransitiontemperature)ofAc-(Gly-Pro/4(R)Hyp-Thr)10-NH2peptides.
6TheCDexperimentsofAc-(Gly-4(R)Hyp-Yaa)10-NH2peptideswithvari-ousaminoacidsintheYaaposition(Thr,Ser,Val,Ala,andalloThr)suggestedthatthemethylgroup,hydroxylgroupandstereocongurationofThrareim-portantforthestability.
9ThemethylgroupofThrwashypothesizedtoshieldtheinter-chainhydrogenbondbetweentheamideofGlyandcarbonylofXaaresi-duesfromwatermoleculesbyenergy-minimizationmethod.
9Althoughseveralstudieshavechallengedtorationalizetheexperimentaldata,themechanismofthestabilityincuticlecollagenisstillambiguous.
InordertounderstandthestabilizationmechanismofThrintheYaaposition,weattemptedtocrystallizethepeptidesAc-(Gly-4(R)Hyp-Thr)10-NH2andH-(Gly-4(R)Hyp-Thr)10-OH.
Despitetheirabilitytoformatriple-helicalstructure,6wecouldnotsucceedintheformationofthesinglecrystalsofthesepeptidesyet.
Thehost-guestpeptidesystemisanalternativewaytogetsinglecrystalsofthepeptidewithinterest-ingsequence.
Therefore,4(R)Hyp-Thr-Glytripeptideunitwasinsertedintothestablehostpeptide(Pro-Pro-Gly)9.
10Thehost-guestpeptideH-(Pro-Pro-Gly)4-(4(R)Hyp-Thr-Gly)-(Pro-Pro-Gly)4-OH(OTG)con-tains4(R)Hyp-Thr-GlytripeptideunitthatisabundantintheRiftiapachyptilacuticlecollagen.
ThesinglecrystalanalysisoftheOTGpeptideprovidedtherstinsightintotheunique4(R)Hyp-Thr-Glytripeptideunitconformation.
Here,theThrconformationandtheobservedhydrationpatternsaroundThrresidueintriple-helicalstructurewererevealed.
EXPERIMENTALCrystallizationwasperformedbyhanging-dropdif-fusionmethodat4C.
Samplesolutionwaspreparedatconcentrated10mgmL1.
Reservoirsolutioncon-tained0.
1MHepesbuerpH7.
5and23%(w/v)PEG1000.
Dropmixturemadeupofsamplesolution2mlandreservoirsolution2ml.
Rod-likecrystalsap-pearedinabout3weeks.
Asinglecrystalwasmeas-uredat100KonthebeamlineBL6AatthePhotonFactoryinTsukuba.
IntensitydatawasprocessedbyCrystalClear.
11CrystalbelongstomonoclinicspaceyTowhomcorrespondenceshouldbeaddressed(Tel:+81-66-850-5455,Fax:+81-66-850-5455,E-mail:okuyamak@chem.
sci.
osaka-u.
ac.
jp).
400PolymerJournal,Vol.
38,No.
4,pp.
400–403(2006)groupP21withunitcellparametersa26:0,b26:5,c80:2A,90:4.
ThestructureofOTGwasdeterminedbymolecularreplacementmethodusing(Pro-Pro-Gly)9peptide(PDBcode1ITT)12asasearchmodel.
PositionalrenementwasperformedbyX-PLOR13andstructurerenementwascarriedoutbySHELX-L.
14RESULTSANDDISCUSSIONThrresiduesareatthecentraltripeptideunitofthemolecule.
Todescribeside-chainconformationofThr,1dihedralangleisdenedbyN-C-C-O1orN-C-C-C2.
Thedierentconformationsoftheside-chainasafunctionof1valuesof60,180,and60arereferredtogauche,trans,andgauche,respectively.
Thus,Thrside-chaininOTGstructure,theO1takesgaucheconformation,whereastheC2takestransconformationtoamidegroup(Figure1).
BoththeO1andtheC2aredirectedtowardadja-centchains.
ThiskindofThrside-chainconformationisthesameastwooutofthreeThrinT3-785pep-tide.
15Theaveragemain-chaindihedralangles(=)ofThrinthisstudyare61and145,whicharecon-sistentwiththosevaluesintheYaapositionofcolla-gen-likepeptides.
10,16InT3-785structure,15water-mediatedhydrogenbondwasreportedbetweentheThrOHgroupandtheGlycarbonylinthesamechainviaonewatermolecule.
ForThr,notonlytheabovewater-mediatedpattern,butalsodiversewater-medi-atedpatternsareobservedintheOTGstructureinFigure2a.
Intherstcase,twowatermoleculesmakehydrogenbondswiththeOHgroupofThr114andthegauche+NOγ1transCγ2Figure1.
gauche/transconformationofThrintheOTGstructure.
Gly1AOGly2BOGly112OThr314O1γHyp3AOThr114NHyp4BOδδδThr114O1Hyp5COHyp4BOThr114OThr214O1γγHyp5COPro317OHyp6AO(a)(b)Figure2.
(a)CentralregionoftheOTGmoleculeshowsthreecasesofwater-mediatedhydrogenbondsatOHgroupofThr.
Case1:Thr114O1connectsthroughtwowatermoleculestoGly112Ointhesamechain.
Case2:Thr114O1connectsthroughtwowatermole-culestoThr114Nwithinthesameresidue.
Case3:Thr314O1connectsthroughtwowatermoleculestoThr114OofadjacentchainandThr214O1connectsthroughtwowatermoleculestoPro317Oofadjacentchain.
Intheresiduename,therstdigitcorrespondstoachainnumberandthenexttwodigitscorrespondtoaresiduenumber.
(b)HydrationpatternsinvolvingOHgroupof4(R)Hypandcarbonylgroupsin(Pro-4(R)Hyp-Gly)11structure.
16Threechainsinamoleculeareshownbydierentshedding;light-,middle-anddark-gray.
Spheresarewatermolecules.
Intra-chainandinter-chainwater-mediatednetworksareshowninbrokenandsolidlines,respectively.
ThreonineinCollagenTriple-helicalStructurePolym.
J.
,Vol.
38,No.
4,2006401carbonylgroupofGly112inthesamechain(brokenlines).
Inthesecondcase,twowatermoleculesinter-actbetweentheOHandtheamidegroupswithinthesameThr114residue(brokenlines).
Andthethirdcaseoccursattwopositions;oneistwowatermole-culesarelinkedbetweentheOHgroupofThr314andthecarbonylgroupofThr114ofneighboringchainandanotheristhesimilarpatternbetweentheOHgroupofThr214andthecarbonylgroupofPro317(solidlines).
Theaveragehydrogenbonddis-tanceinthesethreewater-mediatedpatternsis2.
95A.
Thehydrationpatterninthesecondcasecouldnotbefoundat4(R)Hypduetothelackofhydrogenattheamidegroup.
However,hydrationpatternsintherstandthethirdcases,whichareinter-andintra-chainhydrogenbondnetworks,aregenerallyobservedat4(R)HypintheYaapositioninthepeptidesincludingPro-4(R)Hyp-Glytripeptideunit.
16,17Theseinter-andintra-chainhydrationnetworksoccurrepeatedlyalongthetriple-helicalmolecule,forexample,in(Pro-4(R)Hyp-Gly)11structure16asshowninFigure2b.
Theyarethedominantfeatureintherepetitivepat-ternsof4(R)HypinpeptideshavingPro-4(R)Hyp-Glyrepeatingsequence.
16,17Thus,thisresultindicatesthattheOHgroupofThrintheYaapositionpartici-patesinwater-mediatedinter-andintra-chainhydro-genbondsinthesimilarwaytotheOHgroupof4(R)Hyp.
Moreover,thepositionoftheOHgroupofThrislocatedclosetothatof4(R)HypwhenThrintheOTGstructureissuperimposedoverthe4(R)Hypinthe(Pro-4(R)Hyp-Gly)11structure(Figure3).
Thedistancebetweenbothhydroxyloxygenatomsisabout1A.
ThecloselocationoftheOHgroupsofThrto4(R)Hypcouldcontributetothesimilarforma-tionofwater-mediatedhydrogenbonds.
Therefore,theOTGstructuredemonstratesthatThrcouldactlike4(R)HypatOHgroupside-chaintomakesimilarwater-mediatednetworks.
ThethermalstabilityofAc-(Gly-4(R)Hyp-Yaa)10-NH2peptidescontainingtheThrishigherthanthosecontainingtheSer,alloThr,AlaandVal,9whichsuggeststheimportanceoftheside-chainconformationinthetriple-helicalstructure.
SofaronlytheT3-78515peptidehasareportedstructureofThrinatriple-helicalstructure.
TheX-raydeterminationoftheOTGpeptideprovidesinsightintodetailedstructureoffrequentlyobservedresiduesinRiftiapachytilacuticlecollagen.
Althoughthestabi-lizationmechanismoftheOTGpeptideisnotclearlyunderstood,thenestructureoftheOTGpeptidepro-videsvaluableinformationofThrconformationin-cludingdiversityofwater-mediatedhydrogenbondsaroundThrinthetriple-helicalstructure.
Interestingly,theobservedhydrationpatternsofThraresimilartothoseof4(R)Hypandmoreover,OHgroupside-chaincharacteristicofThrand4(R)Hypissimilaraswell.
REFERENCES1.
J.
MyllyharjuandK.
I.
Kivirikko,TrendsGenet.
,20,33(2004).
2.
C.
M.
KieltyandM.
E.
Grant,'Connectivetissueanditsheritabledisorders.
MolecularGeneticsinMedicalAs-pects,'2nded.
,WileyLiss,NewYork,2002,pp159–221.
3.
R.
Har-ElandM.
L.
Tanzer,FASEBJ.
,7,1115(1993).
4.
F.
Gaill,K.
Mann,H.
Wiedemann,J.
Engel,andR.
Timpl,J.
Mol.
Biol.
,246,284(1995).
5.
K.
Mann,D.
E.
Mechling,H.
P.
Ba¨chinger,C.
Eckerskorn,F.
Gaill,andR.
Timpl,J.
Mol.
Biol.
,261,255(1996).
6.
J.
G.
BannandH.
P.
Ba¨chinger,J.
Biol.
Chem.
,275,24466(2000).
7.
J.
G.
Bann,D.
H.
Peyton,andH.
P.
Ba¨chinger,FEBSLett.
,473,237(2000).
8.
J.
G.
Bann,H.
P.
Ba¨chinger,andD.
H.
Peyton,Biochemis-try,42,4042(2003).
9.
K.
Mizuno,T.
Hayashi,andH.
P.
Ba¨chinger,J.
Biol.
Chem.
,278,32373(2003).
10.
C.
Hongo,K.
Noguchi,K.
Okuyama,Y.
Tanaka,andN.
Nishino,J.
Biochem.
,138,135(2005).
11.
CrystalClear(Rigaku)MolecularStructureCorporation,TheWoodlands,Texas,USA,1999.
12.
C.
Hongo,V.
Nagarajan,K.
Noguchi,S.
Kamitori,K.
Okuyama,Y.
Tanaka,andN.
Nishino,Polym.
J.
,33,812(2001).
13.
A.
T.
Brunger,'X-PLORVersion3.
1SystemforX-rayCrystallographyandNMR,'YaleUniversityPress,NewHaven:CT,1992.
1Figure3.
SuperimpositionofThrintheOTGpeptide(dark-gray)onthe(Pro-4(R)Hyp-Gly)11triple-helix(light-gray).
16ThedistancebetweenOHgroupsofThrand4(R)HypintheYpositionoftwopeptidesisabout1Aandbothpeptidesshowthesimilarhydrationpattern.
Gly-4(R)Hyp-ThrintheOTGandGly-Pro-4(R)Hypinthe(Pro-4(R)Hyp-Gly)11areshowninballandstickdiagram.
N.
JIRAVANICHANUNetal.
402Polym.
J.
,Vol.
38,No.
4,200614.
G.
M.
SheldricandT.
R.
Schneidern,MethodsEnzymol.
,277,319(1997).
15.
R.
Z.
Kramer,J.
Bella,P.
Mayville,B.
Brodsky,andH.
M.
Berman,Nat.
Struct.
Biol.
,6,454(1999).
16.
K.
Okuyama,C.
Hongo,R.
Fukushima,G.
Wu,H.
Narita,K.
Noguchi,Y.
Tanaka,andN.
Nishino,Biopolymers,76,367(2004).
17.
J.
Bella,B.
Brodsky,andH.
M.
Berman,Structure,3,893(1995).
4(R)Hyp:4(R)-hydroxyprolineO:4(R)-hydroxyprolineGal:galactoseT3-785peptide:(Pro-Hyp-Gly)3-Ile-Thr-Gly-Ala-Arg-Gly-Leu-Ala-Gly-Pro-Hyp-Gly-(Pro-Hyp-Gly)3ThreonineinCollagenTriple-helicalStructurePolym.
J.
,Vol.
38,No.
4,2006403
- waterstablehost相关文档
- Dendriticstablehost
- 化学stablehost
- 主机StableHost主机购买图文教程
- 主机stablehost主机50%优惠码 终身5折
- 优惠Stablehost主机注意事项
- 主机StableHost主机介绍
Hostwinds:免费更换IP/优惠码美元VPS免费更换IP4.99,7月最新优惠码西雅图直连VPS
hostwinds怎么样?2021年7月最新 hostwinds 优惠码整理,Hostwinds 优惠套餐整理,Hostwinds 西雅图机房直连线路 VPS 推荐,目前最低仅需 $4.99 月付,并且可以免费更换 IP 地址。本文分享整理一下最新的 Hostwinds 优惠套餐,包括托管型 VPS、无托管型 VPS、Linux VPS、Windows VPS 等多种套餐。目前 Hostwinds...
Letbox(35美元/年),美国洛杉矶VPS终身7折
Letbox 云服务商在前面的文章中其实也有多次介绍,这个服务商其实也算是比较老牌的海外服务商,几年前我也一直有使用过他们家的VPS主机,早年那时候低至年付15-35美元左右的VPS算式比较稀缺的。后来由于服务商确实比较多,而且也没有太多的网站需要用到,所以就没有续费,最近这个服务商好像有点活动就躁动的发布希望引起他人注意。这不有看到所谓的家中有喜事,应该是团队中有生宝宝了,所以也有借此来发布一些...
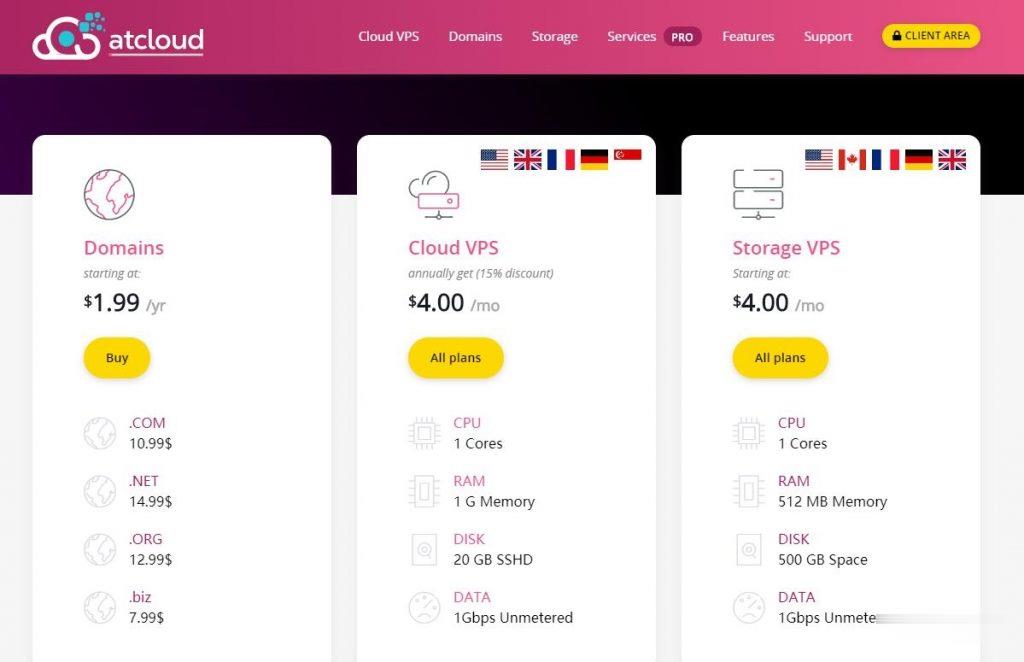
ATCLOUD-KVM架构的VPS产品$4.5,杜绝DDoS攻击
ATCLOUD.NET怎么样?ATCLOUD.NET主要提供KVM架构的VPS产品、LXC容器化产品、权威DNS智能解析、域名注册、SSL证书等海外网站建设服务。 其大部分数据中心是由OVH机房提供,其节点包括美国(俄勒冈、弗吉尼亚)、加拿大、英国、法国、德国以及新加坡。 提供超过480Gbps的DDoS高防保护,杜绝DDoS攻击骚扰,比较适合海外建站等业务。官方网站:点击访问ATCLOUD官网活...
stablehost为你推荐
-
免费虚拟主机求免费的虚拟主机,一定要推荐自己用过了的好东东!域名注册查询如何知道域名注册信息?域名服务域名系统主要是什么?海外域名求国外域名商列表免费域名空间哪个免费空间的域名最好云服务器租用云服务器租用费用是多少jsp虚拟空间java虚拟主机空间怎么选择,国内jsp虚拟主机比较稳定java项目做好后需要推荐一下吧什么是虚拟主机什么是“虚拟主机”?请解释祥细些!100m虚拟主机100M的虚拟主机都能做些什么虚拟主机控制面板虚拟主机管理面板与网站后台有什么区别?