Servicesrewrite
rewrite 时间:2021-01-25 阅读:()
U.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
03.
08SilverSpring,MD20993www.
fda.
govMarch29,2019SpryHealth,Inc.
℅CraigCoombsConsultantCoombsMedicalDeviceConsulting,Inc.
1193ShermanSt.
Alameda,CA94501Re:K181352Trade/DeviceName:LoopSystemRegulationNumber:21CFR870.
2700RegulationName:OximeterRegulatoryClass:ClassIIProductCode:DQA,BZQDated:March4,2019Received:March5,2019DearCraigCoombs:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
K181352-CraigCoombsPage2PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalstatutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,BramD.
Zuckerman,M.
D.
DirectorDivisionofCardiovascularDevicesOfficeofDeviceEvaluationCenterforDevicesandRadiologicalHealthEnclosureShawnW.
Forrest-S2019.
03.
2910:28:03-04'00'FORMFDA3881(7/17)Page1of1PSCPublishingServices(301)443-6740EFDEPARTMENTOFHEALTHANDHUMANSERVICESFoodandDrugAdministrationIndicationsforUseFormApproved:OMBNo.
0910-0120ExpirationDate:06/30/2020SeePRAStatementbelow.
510(k)Number(ifknown)K181352DeviceNameLoopSystemIndicationsforUse(Describe)TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsTypeofUse(Selectoneorboth,asapplicable)PrescriptionUse(Part21CFR801SubpartD)Over-The-CounterUse(21CFR801SubpartC)CONTINUEONASEPARATEPAGEIFNEEDED.
ThissectionappliesonlytorequirementsofthePaperworkReductionActof1995.
*DONOTSENDYOURCOMPLETEDFORMTOTHEPRASTAFFEMAILADDRESSBELOW.
*Theburdentimeforthiscollectionofinformationisestimatedtoaverage79hoursperresponse,includingthetimetoreviewinstructions,searchexistingdatasources,gatherandmaintainthedataneededandcompleteandreviewthecollectionofinformation.
Sendcommentsregardingthisburdenestimateoranyotheraspectofthisinformationcollection,includingsuggestionsforreducingthisburden,to:DepartmentofHealthandHumanServicesFoodandDrugAdministrationOfficeofChiefInformationOfficerPaperworkReductionAct(PRA)StaffPRAStaff@fda.
hhs.
gov"Anagencymaynotconductorsponsor,andapersonisnotrequiredtorespondto,acollectionofinformationunlessitdisplaysacurrentlyvalidOMBnumber.
"K181352:LoopSystemTraditionalPremarketNotificationPage1Section5:510(k)SummaryA.
DeviceInformationCategoryCommentsSponsor:SpryHealth,Inc235AlmaStPaloAlto,CA94301Tel:(650)352-3429PrimaryCommunicantCraigCoombsCoombsMedicalDeviceConsulting1193ShermanStreetAlameda,CA94501Tel:510-337-0140DeviceCommonName:OximeterBreathingFrequencyMonitorDeviceClassificationNumber:21CFR870.
270021CFR868.
2375DeviceClassification&ProductCode:Class2,DQA&BZQDeviceProprietaryName:LoopSystemPredicateDeviceInformation:PredicateDevice:MightySatRXFingertipPulseOximeterPredicateDeviceManufacturer:MasimoPredicateDeviceCommonName:OximeterBreathingFrequencyMonitorPredicateDevicePremarketNotification#K181956PredicateDeviceClassification:21CFR870.
2700:Oximeter21CFR868.
2375:BreathingFrequencyMonitorPredicateDeviceClass&ProductCode:Class2,DQA&BZQB.
DateSummaryPrepared13March2019C.
DescriptionofDeviceTheLoopSystemisaprescription-onlymedicaldeviceindicatedforusebyadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofresting(i.
e.
,nomotion)physiologicalparameters.
Thedataisderivedfromreflectance-basedphotoplethysmogram(PPG)signalsfromthepatient'swrist,collectedbyLightEmittingDiodesK181352:LoopSystemTraditionalPremarketNotificationPage2(LEDs)ofvaryingwavelengthsandphotodiodessensitivetosaidwavelengthsembeddedintheLoopBand.
AnaccelerometerincorporatedintheLoopBanddetermineswhenthepatientisatrestbyconstantlymonitoringtheactivitylevelofthepatient.
Thedataisrecordedduringthoserestingperiods.
Usingfilteringtechnologyforremovalofnoise(includingambientlight)anddataprocessingalgorithms,theLoopSystemstorestherawdatacollecteduntilitisabletosendtotheSpryServer.
PatientsweartheLoopBandontheirwristforperiodsofupto24hours.
PatientswillthenhavetochargetheLoopBandwithaLoopChargingStationprovidedtothemaspartoftheLoopSystem.
Duringcharging,thedataisuploadedtotheSpryServer.
TheLoopbandcanindependentlyanalyzeanddisplayrestingheartrateandarterialoxygensaturation(SpO2).
ThedatamustbedownloadedandanalyzedbytheSpryServertodeterminerespirationrate.
AllphysiologicalmeasurementscollectedbytheLoopSystemarereportedonacommaseparatedvalue(CSV)fileasatime-stampedseries.
TheCSVcanthenberemotelyaccessedthroughawebinterfaceforreviewandanalysisbyaclinician.
D.
IndicationsforUseTheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsK181352:LoopSystemTraditionalPremarketNotificationPage3E.
ComparisontoPredicateDeviceTheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracy.
PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceIntendedUseIssuesIndicationsforUseTheMasimoMightySatRxFingertipPulseOximeterisintendedforhospitals,hospital-typefacilities,homeenvironments,andtransport.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingoffunctionaloxygensaturationofarterialhemoglobin(SpO2)andpulserate(PR)foradultandpediatricpatientsduringbothnomotionandmotionconditions,andforpatientswhoarewellorpoorlyperfused.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingofrespirationrate(RRp)foradultpatients.
TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarms.
TheapplicationLoopSystemIndicationsforUseincludesasubsetofthepatientsanduseconditionsclearedinthepredicatedevice.
TheLoopSystemandthepredicateareintendedforadultsinahomeenvironmentwhenthewearersarenotmoving.
Sincetheapplicationdeviceisintendedforasubsetoftheapplicationconditionsofthepredicateddevice,nonewtypesofsafetyorefficacyquestionsareraised.
BothdevicesprovideSpO2,HR,andRR.
Neitherdeviceisintendedforcontinuousvitalsignmonitoring.
ProductCodeRegulationDescriptionDQA-OximeterBZQ–BreathingFrequencyMonitorDQA–OximeterBZQ–BreathingFrequencyMonitorArterialOxygensaturationandderivedHRandRRcapabilitiesallfallunderthesameproductcodesforbothdevices.
PatientPopulationHR&SpO2-Adults,pediatricsRespiratoryRate–AdultsNon-criticalcareAdultpatientsforallparametersNon-criticalcareTheapplicationdeviceintendedcohortisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
K181352:LoopSystemTraditionalPremarketNotificationPage4PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceUseEnvironmenthospitals,hospital-typefacilities,homeenvironments,andtransport.
homeenvironmentTheapplicationdeviceuseenvironmentisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
PrescribedPrescriptionuseonlyPrescriptionuseonlyIdenticalParameterSamplingFrequencySpotcheckparametersasdesiredbyusers.
Notdesignedforcontinuouswearing.
DesignedfornearlycontinuousmeasuringandrecordingofphysiologicaldatawhenthepatientisnotinmotionDifferenceinsamplingfrequencydoesnotraisenewsafetyorefficacyquestionsTechnologyassociatedwitheachparameterRespirationRateFundamentalScientificTechnologyRespirationrate(RR)measuredbyanalyzingcyclicvariationsinthephotoplethysmogram.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentRespirationRateRange4–70respirationsperminute(RPM)4-40RPMApplicationdevicehasalowerupperlimitthanpredicate.
SpecificRPMsthatare>40arenotclinicallydifferentiatinginaretrospectivemonitoringcontextRespirationRateAccuracy3RPMARMS3RPMARMSAclinicalstudydemonstratedthattheLoopSystemcouldaccuratelymonitorrespirationrateaswellasthepredicate,withinthesensorrangeArterialhemoglobinoxygensaturation(SpO2)FundamentalScientificTechnologySpO2measuredbyanalyzingreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentK181352:LoopSystemTraditionalPremarketNotificationPage5PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceSpO2Range70–100%70–100%IdenticalSpO2Accuracy2%ARMS,nomotion3%ARMS,nomotionBothcomplywithISO80601-2-61aswellaswithFDAGuidanceforPulseOximeters(2013)HeartRate(HR)FundamentalScientificTechnologyHRmeasuredbyanalyzingcyclicvariationsinreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentHeartRateRange25–240beatsperminute(BPM)25–250beatsperminute(BPM)Clinicallyequivalent.
BothcomplywithISO80601-2-61HeartRateAccuracy3BPMARMS,nomotion3BPMARMS,nomotionClinicallyequivalent.
BothcomplywithISO80601-2-61StandardsComplianceIEC60601-1IEC60601-1-2IEC60601-1-11ISO80601-2-61ISO10993-1IEC60601-1IEC60601-1-2IEC60601-1-11IEC60601-1-6ISO80601-2-61ISO10993-1BothdevicesmeetlatestguidelinesforsafetyandefficacyDesignUserInterfaceFingerclampWristbandBothdevicesareworntoprovidedeviceaccesstoperipheralarteries.
Differenceinparticularuserinterfacedoesnotraisenewquestionsofsafetyorefficacy.
K181352:LoopSystemTraditionalPremarketNotificationPage6PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceWearinglocationandfrequencyForintermittentwearing,aka"spotchecking"Continuouswearing,nearlycontinuousrecordingofalldata.
Differenceinwearinglocationonthebodyandfrequencydoesnotraisenewquestionsofsafetyorefficacy.
Bothlocationsallowformonitoringperipheralarteries.
DisplayOLEDcolordisplayofHRandSpO2whendevicewornLEDcolordisplayofHRandSpO2ondemandSimilarPowersource2AAbatteriesInternalrechargeablebatteriesBothbattery-poweredDataCommunicationWireless(Bluetoothpairing)withmobiledevicessuchassmartphonesWireless(cellularconnection)viachargingstationtoSpryServer.
Bothdevicesaredesignedtotransmittheirdatatoalternatedevicesorsites.
TypeofprotectionUnknownTypeBF–AppliedPartperIEC60601-1ApplicationdevicemeetslatestguidelinesforelectricalsafetyPatientcontractingmaterialsPlasticPlasticSimilarBiocompatibilityComplianttoISO10993-1ComplianttoISO10993-1IdenticalK181352:LoopSystemTraditionalPremarketNotificationPage7F.
SummaryofSupportingDataSpryHealthhasconductedextensivetestingtoensurethattheLoopSystemmetdesignspecifications,functionsasintended,andconformstointernationallyrecognizedstandardsandFDAGuidelines.
BenchTestingAlltestresultsdemonstratetheperformanceofSpryHealthLoopSystemmettherequirementsofitspre-definedacceptancecriteriaandintendeduse.
Theresultsofthebench(non-clinical)testingdemonstratethattheLoopSystemisassafeandeffectiveasthepredicatedevice.
BenchtestingdemonstratedtheaccuracyoftheheartratemonitoringwiththeLoopSystem.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
Additionally,transittestingandone-yearshelflifetestingwasconducted.
ElectricalSafetyandElectromagneticCompatibilityTestingElectricalsafetytestingwasconductedinaccordancewith:IEC60601-1:2005+AM1:2012Medicalelectricalequipment-Part1:GeneralrequirementsforbasicsafetyandessentialperformanceIEC60601-1-2:2014:MedicalElectricalEquipment–Part1-2:GeneralRequirementsforSafety–Collateralstandard:ElectromagneticDisturbances–RequirementsandtestsIEC60601-1-11:2015Medicalelectricalequipment–Part1-11:Generalrequirementsforbasicsafetyandessentialperformance–CollateralStandard:RequirementsformedicalelectricalequipmentandmedicalelectricalsystemsusedinthehomehealthcareenvironmentIEC60601-1-6:2013Medicalelectricalequipment–Part1-6:Generalrequirementsforbasicsafetyandessentialperformance–Collateralstandard:UsabilityTheSpryHealthLoopSystempassedallelectricalsafetyandEMCtesting.
BiocompatibilityTestingBiocompatibilitytestingwasconductedinaccordancewithISO10993-1:2009/AC:2010,Biologicalevaluationofmedicaldevices–Part1:Evaluationandtestingwithinariskmanagementprocess,andFDA'sguidancedocuments,UseofInternationalStandardISO10993-1,"Biologicalevaluationofmedicaldevices-Part1:Evaluationandtestingwithinariskmanagementprocess"issuedJune16,2016.
ThistestingdemonstratesthatthematerialsintheLoopBandwillnotcauseanadversebiocompatibilityreactionwhenusedasintended.
K181352:LoopSystemTraditionalPremarketNotificationPage8ClinicalStudiesTwoclinicalstudieswereconductedtodemonstratetheperformanceoftheLoopSystem.
Oneclinicalstudyinvestigatedtheaccuracyofrespirationratemonitoringin12healthyadultsubjectswitharangeofskintypesandrespiratoryrates.
TheLoopSystemaccuracywasshowntobeequivalenttoagoldstandardmonitoringprocedure.
Anotherclinicalstudyinvestigatedtheaccuracyofthepulseoximetrymonitoringin12healthyadultsubjectswitharangeofskintypes.
TheLoopSystemaccuracywascomparedtothegoldstandard,arterialbloodgasanalysis.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
G.
ConclusionSpryHealthconcludesthattheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracyinmonitoringrespirationrate,heartrateandpulseoximetrysaturation.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
03.
08SilverSpring,MD20993www.
fda.
govMarch29,2019SpryHealth,Inc.
℅CraigCoombsConsultantCoombsMedicalDeviceConsulting,Inc.
1193ShermanSt.
Alameda,CA94501Re:K181352Trade/DeviceName:LoopSystemRegulationNumber:21CFR870.
2700RegulationName:OximeterRegulatoryClass:ClassIIProductCode:DQA,BZQDated:March4,2019Received:March5,2019DearCraigCoombs:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
K181352-CraigCoombsPage2PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalstatutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,BramD.
Zuckerman,M.
D.
DirectorDivisionofCardiovascularDevicesOfficeofDeviceEvaluationCenterforDevicesandRadiologicalHealthEnclosureShawnW.
Forrest-S2019.
03.
2910:28:03-04'00'FORMFDA3881(7/17)Page1of1PSCPublishingServices(301)443-6740EFDEPARTMENTOFHEALTHANDHUMANSERVICESFoodandDrugAdministrationIndicationsforUseFormApproved:OMBNo.
0910-0120ExpirationDate:06/30/2020SeePRAStatementbelow.
510(k)Number(ifknown)K181352DeviceNameLoopSystemIndicationsforUse(Describe)TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsTypeofUse(Selectoneorboth,asapplicable)PrescriptionUse(Part21CFR801SubpartD)Over-The-CounterUse(21CFR801SubpartC)CONTINUEONASEPARATEPAGEIFNEEDED.
ThissectionappliesonlytorequirementsofthePaperworkReductionActof1995.
*DONOTSENDYOURCOMPLETEDFORMTOTHEPRASTAFFEMAILADDRESSBELOW.
*Theburdentimeforthiscollectionofinformationisestimatedtoaverage79hoursperresponse,includingthetimetoreviewinstructions,searchexistingdatasources,gatherandmaintainthedataneededandcompleteandreviewthecollectionofinformation.
Sendcommentsregardingthisburdenestimateoranyotheraspectofthisinformationcollection,includingsuggestionsforreducingthisburden,to:DepartmentofHealthandHumanServicesFoodandDrugAdministrationOfficeofChiefInformationOfficerPaperworkReductionAct(PRA)StaffPRAStaff@fda.
hhs.
gov"Anagencymaynotconductorsponsor,andapersonisnotrequiredtorespondto,acollectionofinformationunlessitdisplaysacurrentlyvalidOMBnumber.
"K181352:LoopSystemTraditionalPremarketNotificationPage1Section5:510(k)SummaryA.
DeviceInformationCategoryCommentsSponsor:SpryHealth,Inc235AlmaStPaloAlto,CA94301Tel:(650)352-3429PrimaryCommunicantCraigCoombsCoombsMedicalDeviceConsulting1193ShermanStreetAlameda,CA94501Tel:510-337-0140DeviceCommonName:OximeterBreathingFrequencyMonitorDeviceClassificationNumber:21CFR870.
270021CFR868.
2375DeviceClassification&ProductCode:Class2,DQA&BZQDeviceProprietaryName:LoopSystemPredicateDeviceInformation:PredicateDevice:MightySatRXFingertipPulseOximeterPredicateDeviceManufacturer:MasimoPredicateDeviceCommonName:OximeterBreathingFrequencyMonitorPredicateDevicePremarketNotification#K181956PredicateDeviceClassification:21CFR870.
2700:Oximeter21CFR868.
2375:BreathingFrequencyMonitorPredicateDeviceClass&ProductCode:Class2,DQA&BZQB.
DateSummaryPrepared13March2019C.
DescriptionofDeviceTheLoopSystemisaprescription-onlymedicaldeviceindicatedforusebyadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofresting(i.
e.
,nomotion)physiologicalparameters.
Thedataisderivedfromreflectance-basedphotoplethysmogram(PPG)signalsfromthepatient'swrist,collectedbyLightEmittingDiodesK181352:LoopSystemTraditionalPremarketNotificationPage2(LEDs)ofvaryingwavelengthsandphotodiodessensitivetosaidwavelengthsembeddedintheLoopBand.
AnaccelerometerincorporatedintheLoopBanddetermineswhenthepatientisatrestbyconstantlymonitoringtheactivitylevelofthepatient.
Thedataisrecordedduringthoserestingperiods.
Usingfilteringtechnologyforremovalofnoise(includingambientlight)anddataprocessingalgorithms,theLoopSystemstorestherawdatacollecteduntilitisabletosendtotheSpryServer.
PatientsweartheLoopBandontheirwristforperiodsofupto24hours.
PatientswillthenhavetochargetheLoopBandwithaLoopChargingStationprovidedtothemaspartoftheLoopSystem.
Duringcharging,thedataisuploadedtotheSpryServer.
TheLoopbandcanindependentlyanalyzeanddisplayrestingheartrateandarterialoxygensaturation(SpO2).
ThedatamustbedownloadedandanalyzedbytheSpryServertodeterminerespirationrate.
AllphysiologicalmeasurementscollectedbytheLoopSystemarereportedonacommaseparatedvalue(CSV)fileasatime-stampedseries.
TheCSVcanthenberemotelyaccessedthroughawebinterfaceforreviewandanalysisbyaclinician.
D.
IndicationsforUseTheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsK181352:LoopSystemTraditionalPremarketNotificationPage3E.
ComparisontoPredicateDeviceTheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracy.
PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceIntendedUseIssuesIndicationsforUseTheMasimoMightySatRxFingertipPulseOximeterisintendedforhospitals,hospital-typefacilities,homeenvironments,andtransport.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingoffunctionaloxygensaturationofarterialhemoglobin(SpO2)andpulserate(PR)foradultandpediatricpatientsduringbothnomotionandmotionconditions,andforpatientswhoarewellorpoorlyperfused.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingofrespirationrate(RRp)foradultpatients.
TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarms.
TheapplicationLoopSystemIndicationsforUseincludesasubsetofthepatientsanduseconditionsclearedinthepredicatedevice.
TheLoopSystemandthepredicateareintendedforadultsinahomeenvironmentwhenthewearersarenotmoving.
Sincetheapplicationdeviceisintendedforasubsetoftheapplicationconditionsofthepredicateddevice,nonewtypesofsafetyorefficacyquestionsareraised.
BothdevicesprovideSpO2,HR,andRR.
Neitherdeviceisintendedforcontinuousvitalsignmonitoring.
ProductCodeRegulationDescriptionDQA-OximeterBZQ–BreathingFrequencyMonitorDQA–OximeterBZQ–BreathingFrequencyMonitorArterialOxygensaturationandderivedHRandRRcapabilitiesallfallunderthesameproductcodesforbothdevices.
PatientPopulationHR&SpO2-Adults,pediatricsRespiratoryRate–AdultsNon-criticalcareAdultpatientsforallparametersNon-criticalcareTheapplicationdeviceintendedcohortisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
K181352:LoopSystemTraditionalPremarketNotificationPage4PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceUseEnvironmenthospitals,hospital-typefacilities,homeenvironments,andtransport.
homeenvironmentTheapplicationdeviceuseenvironmentisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
PrescribedPrescriptionuseonlyPrescriptionuseonlyIdenticalParameterSamplingFrequencySpotcheckparametersasdesiredbyusers.
Notdesignedforcontinuouswearing.
DesignedfornearlycontinuousmeasuringandrecordingofphysiologicaldatawhenthepatientisnotinmotionDifferenceinsamplingfrequencydoesnotraisenewsafetyorefficacyquestionsTechnologyassociatedwitheachparameterRespirationRateFundamentalScientificTechnologyRespirationrate(RR)measuredbyanalyzingcyclicvariationsinthephotoplethysmogram.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentRespirationRateRange4–70respirationsperminute(RPM)4-40RPMApplicationdevicehasalowerupperlimitthanpredicate.
SpecificRPMsthatare>40arenotclinicallydifferentiatinginaretrospectivemonitoringcontextRespirationRateAccuracy3RPMARMS3RPMARMSAclinicalstudydemonstratedthattheLoopSystemcouldaccuratelymonitorrespirationrateaswellasthepredicate,withinthesensorrangeArterialhemoglobinoxygensaturation(SpO2)FundamentalScientificTechnologySpO2measuredbyanalyzingreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentK181352:LoopSystemTraditionalPremarketNotificationPage5PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceSpO2Range70–100%70–100%IdenticalSpO2Accuracy2%ARMS,nomotion3%ARMS,nomotionBothcomplywithISO80601-2-61aswellaswithFDAGuidanceforPulseOximeters(2013)HeartRate(HR)FundamentalScientificTechnologyHRmeasuredbyanalyzingcyclicvariationsinreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentHeartRateRange25–240beatsperminute(BPM)25–250beatsperminute(BPM)Clinicallyequivalent.
BothcomplywithISO80601-2-61HeartRateAccuracy3BPMARMS,nomotion3BPMARMS,nomotionClinicallyequivalent.
BothcomplywithISO80601-2-61StandardsComplianceIEC60601-1IEC60601-1-2IEC60601-1-11ISO80601-2-61ISO10993-1IEC60601-1IEC60601-1-2IEC60601-1-11IEC60601-1-6ISO80601-2-61ISO10993-1BothdevicesmeetlatestguidelinesforsafetyandefficacyDesignUserInterfaceFingerclampWristbandBothdevicesareworntoprovidedeviceaccesstoperipheralarteries.
Differenceinparticularuserinterfacedoesnotraisenewquestionsofsafetyorefficacy.
K181352:LoopSystemTraditionalPremarketNotificationPage6PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceWearinglocationandfrequencyForintermittentwearing,aka"spotchecking"Continuouswearing,nearlycontinuousrecordingofalldata.
Differenceinwearinglocationonthebodyandfrequencydoesnotraisenewquestionsofsafetyorefficacy.
Bothlocationsallowformonitoringperipheralarteries.
DisplayOLEDcolordisplayofHRandSpO2whendevicewornLEDcolordisplayofHRandSpO2ondemandSimilarPowersource2AAbatteriesInternalrechargeablebatteriesBothbattery-poweredDataCommunicationWireless(Bluetoothpairing)withmobiledevicessuchassmartphonesWireless(cellularconnection)viachargingstationtoSpryServer.
Bothdevicesaredesignedtotransmittheirdatatoalternatedevicesorsites.
TypeofprotectionUnknownTypeBF–AppliedPartperIEC60601-1ApplicationdevicemeetslatestguidelinesforelectricalsafetyPatientcontractingmaterialsPlasticPlasticSimilarBiocompatibilityComplianttoISO10993-1ComplianttoISO10993-1IdenticalK181352:LoopSystemTraditionalPremarketNotificationPage7F.
SummaryofSupportingDataSpryHealthhasconductedextensivetestingtoensurethattheLoopSystemmetdesignspecifications,functionsasintended,andconformstointernationallyrecognizedstandardsandFDAGuidelines.
BenchTestingAlltestresultsdemonstratetheperformanceofSpryHealthLoopSystemmettherequirementsofitspre-definedacceptancecriteriaandintendeduse.
Theresultsofthebench(non-clinical)testingdemonstratethattheLoopSystemisassafeandeffectiveasthepredicatedevice.
BenchtestingdemonstratedtheaccuracyoftheheartratemonitoringwiththeLoopSystem.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
Additionally,transittestingandone-yearshelflifetestingwasconducted.
ElectricalSafetyandElectromagneticCompatibilityTestingElectricalsafetytestingwasconductedinaccordancewith:IEC60601-1:2005+AM1:2012Medicalelectricalequipment-Part1:GeneralrequirementsforbasicsafetyandessentialperformanceIEC60601-1-2:2014:MedicalElectricalEquipment–Part1-2:GeneralRequirementsforSafety–Collateralstandard:ElectromagneticDisturbances–RequirementsandtestsIEC60601-1-11:2015Medicalelectricalequipment–Part1-11:Generalrequirementsforbasicsafetyandessentialperformance–CollateralStandard:RequirementsformedicalelectricalequipmentandmedicalelectricalsystemsusedinthehomehealthcareenvironmentIEC60601-1-6:2013Medicalelectricalequipment–Part1-6:Generalrequirementsforbasicsafetyandessentialperformance–Collateralstandard:UsabilityTheSpryHealthLoopSystempassedallelectricalsafetyandEMCtesting.
BiocompatibilityTestingBiocompatibilitytestingwasconductedinaccordancewithISO10993-1:2009/AC:2010,Biologicalevaluationofmedicaldevices–Part1:Evaluationandtestingwithinariskmanagementprocess,andFDA'sguidancedocuments,UseofInternationalStandardISO10993-1,"Biologicalevaluationofmedicaldevices-Part1:Evaluationandtestingwithinariskmanagementprocess"issuedJune16,2016.
ThistestingdemonstratesthatthematerialsintheLoopBandwillnotcauseanadversebiocompatibilityreactionwhenusedasintended.
K181352:LoopSystemTraditionalPremarketNotificationPage8ClinicalStudiesTwoclinicalstudieswereconductedtodemonstratetheperformanceoftheLoopSystem.
Oneclinicalstudyinvestigatedtheaccuracyofrespirationratemonitoringin12healthyadultsubjectswitharangeofskintypesandrespiratoryrates.
TheLoopSystemaccuracywasshowntobeequivalenttoagoldstandardmonitoringprocedure.
Anotherclinicalstudyinvestigatedtheaccuracyofthepulseoximetrymonitoringin12healthyadultsubjectswitharangeofskintypes.
TheLoopSystemaccuracywascomparedtothegoldstandard,arterialbloodgasanalysis.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
G.
ConclusionSpryHealthconcludesthattheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracyinmonitoringrespirationrate,heartrateandpulseoximetrysaturation.
- Servicesrewrite相关文档
- sectionrewrite
- strategiesrewrite
- discrepanciesrewrite
- dependentrewrite
- relationrewrite
- joinedrewrite
快云科技,免云服务器75折优惠服务器快云21元/月
近日快云科技发布了最新的夏季优惠促销活动,主要针对旗下的香港CN2 GIA系列的VPS云服务器产品推送的最新的75折优惠码,国内回程三网CN2 GIA,平均延迟50ms以下,硬件配置方面采用E5 2696v2、E5 2696V4 铂金Platinum等,基于KVM虚拟架构,采用SSD硬盘存储,RAID10阵列保障数据安全,有需要香港免备案CN2服务器的朋友可以关注一下。快云科技怎么样?快云科技好不...
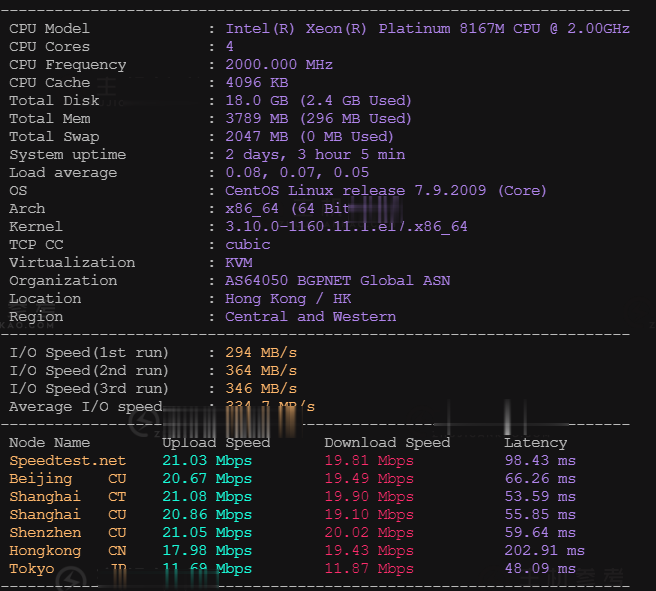
易速互联月付299元,美国独立服务器促销,加州地区,BGP直连线路,10G防御
易速互联怎么样?易速互联是国人老牌主机商家,至今已经成立9年,商家销售虚拟主机、VPS及独立服务器,目前商家针对美国加州萨克拉门托RH数据中心进行促销,线路采用BGP直连线路,自带10G防御,美国加州地区,100M带宽不限流量,月付299元起,有需要美国不限流量独立服务器的朋友可以看看。点击进入:易速互联官方网站美国独立服务器优惠套餐:RH数据中心位于美国加州、配置丰富性价比高、10G DDOS免...
BuyVM($5/月),1Gbps不限流量流媒体VPS主机
BuyVM针对中国客户推出了China Special - STREAM RYZEN VPS主机,带Streaming Optimized IP,帮你解锁多平台流媒体,适用于对于海外流媒体有需求的客户,主机开设在拉斯维加斯机房,AMD Ryzen+NVMe磁盘,支持Linux或者Windows操作系统,IPv4+IPv6,1Gbps不限流量,最低月付5加元起,比美元更低一些,现在汇率1加元=0.7...
rewrite为你推荐
-
海贼王644海贼王第644集在哪看非主流桌面背景图片给我找几张好看的桌面图片??聚酯纤维和棉哪个好纯棉和聚酯纤维的最佳比例燃气热水器和电热水器哪个好燃气热水器好还是电热水器好?压缩软件哪个好压缩软件那个最好,360压缩软件好?还是快压、好压软件好呢?录音软件哪个好录音软件哪个好用又简单录音软件哪个好有什么录音软件好用??手机杀毒软件哪个好手机杀毒软件那个好用手机音乐播放器哪个好手机哪个音乐播放器的音质更好?手机浏览器哪个好用手机哪个浏览器最好用