graphics360web
GuidanceforIndustrySafetyConsiderationsforContainerLabelsandCartonLabelingDesigntoMinimizeMedicationErrorsDRAFTGUIDANCEThisguidancedocumentisbeingdistributedforcommentpurposesonly.
Commentsandsuggestionsregardingthisdraftdocumentshouldbesubmittedwithin60daysofpublicationintheFederalRegisterofthenoticeannouncingtheavailabilityofthedraftguidance.
Submitelectroniccommentstohttp://www.
regulations.
gov/.
SubmitwrittencommentstotheDivisionofDocketsManagement(HFA-305),FoodandDrugAdministration,5630FishersLane,rm.
1061,Rockville,MD20852.
AllcommentsshouldbeidentifiedwiththedocketnumberlistedinthenoticeofavailabilitythatpublishesintheFederalRegister.
Forquestionsregardingthisdraftdocumentcontact(CDER),OfficeofSurveillanceandEpidemiology,DivisionofMedicationErrorPreventionandAnalysis,CarolHolquistat301-796-0171.
U.
S.
DepartmentofHealthandHumanServicesFoodandDrugAdministrationCenterforDrugEvaluationandResearch(CDER)April2013DrugSafetyGuidanceforIndustrySafetyConsiderationsforContainerLabelsandCartonLabelingDesigntoMinimizeMedicationErrorsAdditionalcopiesareavailablefrom:OfficeofCommunicationsDivisionofDrugInformation,WO51,Room2201CenterforDrugEvaluationandResearchFoodandDrugAdministration10903NewHampshireAve.
,SilverSpring,MD20993Phone:301-796-3400;Fax:301-847-8714druginfo@fda.
hhs.
govhttp://www.
fda.
gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.
htmU.
S.
DepartmentofHealthandHumanServicesFoodandDrugAdministrationCenterforDrugEvaluationandResearchApril2013DrugSafetyTableofContentsI.
INTRODUCTION.
1II.
BACKGROUND2III.
GENERALCONSIDERATIONS3A.
PoorDesignofProductContainerLabelsandCartonLabelingCanObscureCriticalSafetyInformation.
3B.
RiskAssessmentDuringtheDesignStageCanReducetheRiskofMedicationErrors.
4C.
CriticalProductInformationShouldAppearonthePrincipalDisplayPanel.
4D.
LabelsShouldbeLegible,Readable,andEasytoUnderstand41.
ContainerLabelSize.
52.
TextSizeandStyle53.
ContrastofTextandBackgroundColor.
64.
InformationCrowdingandVisualClutter.
65.
DangerousAbbreviations,Acronyms,andSymbols.
7E.
AvoidLook-alikeContainerLabelsandCartonLabeling71.
CorporateTradeDress.
72.
UseofColor.
8IV.
SPECIALCONSIDERATIONSANDRECOMMENDATIONS9A.
Proprietary,Established,andProperNames.
9B.
ProductStrength.
101.
StrengthDifferentiation102.
StrengthDesignation103.
SmallVolumeParenteralProducts104.
ExpressionofStrengthforDryPowderProducts115.
SaltNomenclature.
116.
Prodrugs127.
MetricMeasurements.
128.
LocationofNetQuantityStatements129.
LeadingandTerminalZeros,Decimals,andCommas12C.
Route(s)ofAdministration.
13D.
WarningsforCriticalInformation.
13E.
ExpirationDates.
13F.
BarCodes.
13G.
NationalDrugCodeNumbers14iH.
ControlledSubstanceSchedule15V.
OTHERSPECIALCONTAINERLABELANDCARTONLABELINGCONSIDERATIONS.
15A.
UnitDoseBlisterPackPresentations.
151.
BlisterCellLabel.
152.
ProductStrength.
153.
BlisterCellLabelMaterialandReadability.
164.
BlisterPackLabelDesign16B.
LabelingofFerrulesandCapOverseals17C.
ColorClosureSystemforConcentratedPotassiumChloride.
17D.
LabelsforLargeVolumeParenterals.
17E.
TransferableorPeel-OffLabelsforInjectableMedications.
19F.
Double-sidedLabelsandLabeling19G.
PharmacyBulkPackages.
19H.
CommunicationofImportantProductChanges19I.
DosingDevices.
19J.
ProductSamples.
20K.
PackageType.
20L.
QuickResponseCode20GLOSSARY.
23iiContainsNonbindingRecommendationsDraft–NotforImplementation1GuidanceforIndustry123SafetyConsiderationsforContainerLabelsandCartonLabeling4DesigntoMinimizeMedicationErrors567Thisdraftguidance,whenfinalized,willrepresenttheFoodandDrugAdministration's(FDA's)current8thinkingonthistopic.
Itdoesnotcreateorconferanyrightsfororonanypersonanddoesnotoperateto9bindFDAorthepublic.
Youcanuseanalternativeapproachiftheapproachsatisfiestherequirementsof10theapplicablestatutesandregulations.
Ifyouwanttodiscussanalternativeapproach,contacttheFDA11staffresponsibleforimplementingthisguidance.
IfyoucannotidentifytheappropriateFDAstaff,call12theappropriatenumberlistedonthetitlepageofthisguidance.
13141516I.
INTRODUCTION1718Thepurposeofthisguidanceistohelpprescriptiondrugandbiologicproductmanufacturers19minimizemedicationerrors2associatedwiththeirproducts.
Thisguidancefocusesonsafety20aspectsofthecontainerlabel3andcartonlabelingdesign,andprovidesasetofprinciplesand21recommendationsforensuringthatcriticalelementsofaproduct'scontainerlabelsandcarton22labelingaredesignedtopromotesafedispensing,administration,anduseoftheproduct.
2324ThisguidanceappliestoprescriptiondrugandCDER-regulatedbiologicalproducts,including25thefollowing:2627Prescriptiondrugproductsmarketedunderanapprovednewdrugapplication(NDA)or28abbreviatednewdrugapplication(ANDA);29PrescriptiondrugsmarketedwithoutanapprovedNDAorANDA;and30Biologicalproductsmarketedunderanapprovedbiologicslicensingapplication(BLA).
3132Inthisguidance,allsuchproductsarejointlyreferredtoasproducts,andpersonsresponsiblefor33designingproductcontainerlabelsandcartonlabelingarereferredtoassponsors.
Referencesto34enduser(s)include,butarenotlimitedto,thepatient,patient'scaregiver,theprescribing35physician,nurse,pharmacist,pharmacytechnician,andotherindividualswhoareinvolvedin36routineprocurement,stocking,storage,andadministrationofmedications(e.
g.
,medication37technicians).
381ThisguidancewaspreparedbytheDivisionofMedicationErrorPreventionandAnalysisintheCenterforDrugEvaluationandResearch(CDER)attheFoodandDrugAdministration.
2Amedicationerrorisanypreventableeventthatmaycauseorleadtoinappropriatemedicationuseorpatientharmwhilethemedicationisinthecontrolofthehealthcareprofessional,patient,orconsumer(NationalCoordinatingCouncilforMedicationErrorReportingandPrevention,http://www.
nccmerp.
org/aboutMedErrors.
html).
3TermsthatappearinboldtypeuponfirstusearedefinedintheGlossarysectionofthisguidance.
1ContainsNonbindingRecommendationsDraft–NotforImplementation39Thisguidancedoesnotapplytoover-the-counter(OTC)drugproducts.
4041ThisisthesecondinaseriesofthreeguidancedocumentsthattheFoodandDrug42Administration(FDA)isissuingtohelpminimizemedicationerrors.
Thefirstguidancefocuses43onminimizingrisksassociatedwiththedesignofthedrugproductanditscontainerclosure44system.
4Thethirdplannedguidancewillfocusonbestpracticesforthedevelopmentandtesting45ofproposedproprietarynamestominimizerisksassociatedwithdrugproductnomenclature,46suchasproprietarynamesthatlookorsoundlikethenameofanotherproduct(e.
g.
,look-alikeor47sound-alikenames).
4849FDA'sguidancedocuments,includingthisguidance,donotestablishlegallyenforceable50responsibilities.
Instead,guidancedocumentsdescribetheFDA'scurrentthinkingonatopicand51shouldbeviewedonlyasrecommendations,unlessspecificregulatoryorstatutoryrequirements52arecited.
TheuseofthewordshouldinFDA'sguidancemeansthatsomethingissuggestedor53recommended,butnotrequired.
5455II.
BACKGROUND5657Medicationerrorsareasignificantpublichealthconcernthataccountforanestimated7,00058deathsannuallyintheUnitedStates.
5InJuly2006,theInstituteofMedicine(IOM)publisheda59reporttitledPreventingMedicationErrors.
Thereportcitedlabelingandpackagingissuesas60thecauseof33percentofallmedicationerrorsand30percentoffatalitiesfrommedication61errors.
6TheIOMemphasizedthat"[p]roductnaming,labeling,andpackagingshouldbe62designedfortheenduser—theproviderintheclinicalenvironmentand/ortheconsumer.
"763Morespecifically,thereporturgedFDAtoaddresssafetyissuesrelatedtoproductlabelingand64nomenclatureusingtheprinciplesofcognitiveandhumanfactorsengineering.
86566OnSeptember27,2007,thereauthorizationandexpansionofthePrescriptionDrugUserFeeAct67(PDUFAIV)wassignedintolawaspartoftheFoodandDrugAdministrationAmendmentsAct68of2007(FDAAA)(PublicLaw110-85).
AspartofthePDUFAIVreauthorization,FDA69committedtocertainperformancegoals,includingmeasurestoreducemedicationerrorsrelated70tolook-alikeandsound-alikeproprietarynames,unclearlabelabbreviations,acronyms,dose4SeetheFDAdraftguidanceforindustrySafetyConsiderationsforProductDesigntoMinimizeMedicationErrors(December2012).
Whenfinal,thisguidancewillrepresentFDA'scurrentthinkingonthistopic.
Forthemostrecentversionofaguidance,checktheFDADrugsguidanceWebpageathttp://www.
fda.
gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.
htm.
5PhillipsDP,ChristenfeldN,andGlynnLM.
IncreaseinUSMedication-ErrorDeathsbetween1983and1993.
TheLancet.
351:643-644,1998.
6AspdenP,WolcottJA,BootmanJL,CronenwettLR,eds.
PreventingMedicationErrors.
InstituteofMedicine,TheNationalAcademiesPress:WashingtonDC.
2006.
Chapter6:p.
275.
7IOM,PreventingMedicationErrors.
Chapter6,Recommendation4,p.
280.
8IOM,PreventingMedicationErrors.
Chapter6,ActionstoImproveDrugNaming,Labeling,andPackaging,p.
281-282.
2ContainsNonbindingRecommendationsDraft–NotforImplementation71designations,anderror-pronelabelingandpackagingdesigns.
9InJune2010,FDAheldapublic72workshopandopenedapublicdockettoreceivecommentsonthesetopics.
10Thisguidanceand73thecompanionguidancesdescribedinsectionIofthisguidancepresentFDA's74recommendationsandconclusionsafterreviewingthispublicinput.
7576III.
GENERALCONSIDERATIONS7778Theformatandcontentofprescriptiondrugandbiologicalproductlabelsandlabelingmust79complywithFDAregulationsin21CFRpart201fordrugsand21CFRpart610SubpartG-80LabelingStandardsforbiologics,andshouldconformwithalllabelingrequirementsrequiredby81theUnitedStatesPharmacopeia(USP).
Althoughthisguidancereferstosomeaspectsofthose82requirementsastheyrelatetothepreventionofmedicationerrors,productsponsorsshouldrefer83totheregulationsandUSPforthefullrequirements.
84A.
PoorDesignofProductContainerLabelsandCartonLabelingCanObscure85CriticalSafetyInformation8687Productcontainerlabelsandcartonlabelingshouldcommunicateinformationthatiscriticalto88thesafeuseofamedicationfromtheinitialprescription,toprocurement,preparationand89dispensingoftheproducttothetimeitisgiventothepatient.
Poorlabeldesigncancontributeto90medicationerrorsbymakingitdifficultforhealthcareprofessionals,caregivers,and/orpatients91toreadilylocateandunderstandcriticalsafetyinformation.
Examplesfromreportsof92medicationerrorsinclude:9394Keyinformation,suchastheproductname,strength,anddosageform,ismissing;is95expressedinaconfusingmanner;orisnotprominentlylocatedanddisplayed.
9697Keyinformationdoesnotappearinthesamefieldofvision(i.
e.
,theinformationisnot98readablewithouthavingtoturnorrotatethecontainer).
99100Containerlabelsandcartonlabelinglooksimilaracrossmultiplestrengthsofthesame101productoracrossmultipleproductswithinacompany'sproductline.
102103Containerlabelsandcartonlabelinglooksimilaramongmultipleproductsfromdifferent104manufacturers.
105106Containerlabelsandcartonlabelingarevisuallyclutteredbyextraneoustextor107distractingimagesandgraphics.
108109Error-proneabbreviationsorsymbolsareused.
9SeelettersfromtheSecretaryofHealthandHumanServicestotheChairmanoftheCommitteeonHealth,Education,Labor,andPensionsoftheSenateandtheChairmanoftheCommitteeonEnergyandCommerceoftheHouseofRepresentatives,assetforthintheCongressionalRecordhttp://www.
fda.
gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm119243.
htm.
10SeeApril12,2010,WorkshopNoticeandRequestforComments(75FR18514),DocketNo.
FDA-2010-N-0168.
3ContainsNonbindingRecommendationsDraft–NotforImplementation110111Textisdifficulttoreadbecauseoffontsizeorstyle,insufficientcolorcontrast,orother112designelements.
113114Overlappingtextisprintedonbothsidesofaclear,transparent,ortranslucentcontainer115labelsuchasthosethatmightbefoundonsyringes,ampules,vials,intravenousbagsor116low-densitypolyethylene(LDPE)vials.
117118B.
RiskAssessmentDuringtheDesignStageCanReducetheRiskof119MedicationErrors120121Itisimportanttoconsidertheendusersandtheirenvironmentofuseduringthedevelopmentand122designofadrugproduct'slabel,labeling,andpackaging.
Sponsorsshouldassessandminimizethe123riskofmedicationerrorsresultingfromthedesignofproductcontainerlabelsandcartonlabeling124beforesubmittingproposedlabelsandlabelingforFDAreviewandapproval.
Medicationerror125riskassessmentshouldtakeintoaccountalloftheprospectiveendusersandtheenvironmentsin126whichtheproductwillbeprescribed,dispensed,andused.
FDArecommendsapplyingthe127principlesdescribedinthisguidanceandtestingtheoveralldesignusingwell-establishedrisk128assessmentmethodsatthepre-INDstageorduringtheearlystagesoflabel,labeling,and129packagingdevelopment,andwhenchangesoradditionstoanalreadymarketeddrugproductoccur130throughouttheproduct'slifecycle.
Formoreinformationandrecommendationsonanalytical131methodsforriskassessments,wereferyoutotheFDAdraftguidanceforindustrySafety132ConsiderationsforProductDesigntoMinimizeMedicationErrors(seefootnote4).
133134C.
CriticalProductInformationShouldAppearonthePrincipalDisplayPanel135136Theprincipaldisplaypanel(PDP)isthepanelofalabelthatismostlikelytobedisplayed,137presented,shown,orexaminedbytheenduser.
WerecommendthatthePDPincludethe138followingcriticalinformation:139140Proprietaryname141Establishednameorpropername142Productstrength143Route(s)ofadministration144Warnings(ifany)orcautionarystatements(ifany)145146TheinformationlistedaboveshouldbethemostprominentinformationonthePDP.
Other147informationonthePDPsuchastheRx-onlystatement,netquantitystatement,manufacturer148name,andlogoshouldnotcompeteinsizeandprominencewiththeimportantinformationlisted149above.
Informationsuchastheproductstrengthequivalencystatement,"eachtabletcontains"150statement,andmanufacturernameandlogoisbestplacedonthesideorbackpaneltomaximize151theprominenceoftheimportantinformationlistedabove.
152153D.
LabelsShouldbeLegible,Readable,andEasytoUnderstand1544ContainsNonbindingRecommendationsDraft–NotforImplementation155FDArecommendsthatthetextonthecontainerlabelandcartonlabelingshouldbe(1)generally156orientedinthesamedirection;(2)placedinthesamefieldofvision(i.
e.
,readablewithout157havingtoturnorrotatethecontainer);and(3)surroundedbyadequatewhitespacetoimprove158readabilityandavoidcrowding.
ForFDAregulationsrelatedtothereadabilityofproduct159labels,see21CFR201.
15.
Importantfactorstoconsiderincludethefollowing:1601611.
ContainerLabelSize162163Thesizeofthecontainerlabelgreatlyinfluencestheoverallcontainerlabeldesign.
FDA164recognizesthatincertaincircumstancesthecontainerclosuresystemmightactuallybe165inseparablefromthecontainerlabel(e.
g.
,LDPEvial,glassampule,syringe,blisterfoilbacking,166andintravenousbag).
Inothercases,thecontainerlabelmightbeapaper,foil,orclearlabelthat167isaffixedtothecontainerclosuresystemorblister.
Ifthecontainerlabelistoosmall,important168informationmaynotalwaysfitontheprincipaldisplaypanelofthecontainerlabel.
169170Ideally,manufacturersshouldexploreapproachestocreatelargercontainerlabelsorunique171packagingtoaccommodateallcriticalinformationontheimmediateproductcontainerlabel.
172FDAregulationsprovideanexemptionfromsomedruglabelingrequirementswhenthe173containeristoosmallorotherwiseunabletoaccommodatealabelwithenoughspacetoinclude174allrequiredinformation,providedthatallrequiredinformationispresentonthecartonlabeling175orintheprescribinginformation(21CFR201.
10(i)).
Insuchcases,thecontainerlabelmust176includeatminimumtheproduct'sproprietaryandestablishedname(ifany);productstrength;lot177178number;andthenameofthemanufacturer,packer,ordistributor.
USPrequiresthelabelofanofficialdrugproducttobearanexpirationdate.
11Therefore,wealsostronglyrecommend179includingtheproduct'sexpirationdate.
Forbiologicalproducts,ataminimum,thenameofthe180product,lotnumber,manufacturername,andtherecommendedindividualdoseformultiple-181dosecontainersmustbeincluded(21CFR610.
60(c)).
Suchexemptionsarenotavailable,182however,ifthelackofspaceiscausedbyfailuretouseallavailablespaceonthecontainer,or183theuseoflabelspaceisfornon-requiredinformationorotherdesign-relatedelements(see21184CFR201.
15(a)(3)through(a)(5)and201.
15(b)).
1851862.
TextSizeandStyle187188Sponsorsshouldchooseafontthatiseasytoread,notlightweightorcondensed.
Anumberof189190publishedreferencesrecommendalargerfontsizesuchas12-pointsansserif(e.
g.
,Arial)toimprovereadability.
12,13FDArecommendstheuseofatleasta12-pointfontwheneverlabelsize191permits.
11USPGeneralNotices:10.
Preservation,Packaging,Storage,AndLabeling;10.
40.
100;ExpirationDateandBeyond-UseDate.
USPhasannouncedplanstorelocatethisinformationtoGeneralChapterLabelinginthenearfuture.
12Designforpatientsafety:Aguidetothegraphicdesignofmedicationpackaging,NationalPatientSafetyAgency,SecondEdition,2007;Designforpatientsafety:Aguidetolabelingandpackagingofinjectablemedicines,NationalPatientSafetyAgency,Edition1,2008.
13RecommendationstotheSafeMedicationUseExpertCommitteebytheHealthLiteracyandPrescriptionContainerLabelingAdvisoryPanel,MayandNovember2009,UnitedStatesPharmacopia(USP),postedApril2010.
5ContainsNonbindingRecommendationsDraft–NotforImplementation1923.
ContrastofTextandBackgroundColor193194Thecolorcontrastbetweenthetextandthecontainerlabelbackgroundcolorshouldbechosento195affordadequatelegibilityofthetext.
Sponsorsshouldavoidcolorcombinationsthatdonot196affordmaximumlegibilityoftext(e.
g.
,paleyellowtextonwhitecontainerlabelbackground).
197198Textthatisraisedorrecessed(i.
e.
,embossedordebossed)onclear,transparent,ortranslucent199containers(e.
g.
,LDPEvials)isgenerallyillegible.
Forthesetypesofcontainerlabels,we200recommendindividuallyoverwrappingtheproductsothatalegiblelabelisappliedtothe201overwrap,andtheproductshouldberetainedintheoverwrapuntilitisadministered.
2022034.
InformationCrowdingandVisualClutter204205Whenlabelsarecrowded,textsizeandprominencearegenerallydecreased,andimportant206informationmaybedifficulttoreadand/oreasilyoverlooked.
Linesorblocksoftextshouldbe207separatedbysufficientwhitespacetoavoidcrowdingorclutter.
Werecommendplacingless208importantinformationonasideorbackpanelofthecontainerlabelandcartonlabeling,rather209thanonthePDP,orplacingit,asappropriate,intheprescribinginformation.
210211Apartfromrequiredinformationaboutaproduct'smanufacturer,distributororpacker(see§212201.
1),informationaboutbusinesspartnershipsshouldnotappearonthelabelorlabeling.
213214Theuseoflogos,bars,stripes,watermarkgraphics,lines,andsymbolsisdiscouragedon215containerlabelsand/orcartonlabelingbecausetheycandistractthereaderfromimportant216informationandaddtolabelclutter.
Whensuchitemsareincluded,thegraphicdesignshould217notcompetewith,interrupt,ordistortimportantinformation.
218219Werecommendnotsuperimposingtextoverimagesorlogosorplacingalogoimmediately220beforeoraftertheproprietaryname,becausethelogocanoftenlooklikeanadditionalletterin221theproprietaryname.
Inaddition,thereshouldbenointerveningwritten,printed,orgraphic222matterbetweentheproprietaryname,establishedname,andproductstrength(see§201.
10(a)).
223224Imagesoftabletsand/orcapsulescanhelppharmacistsorotherhealthcareprovidersconfirm225theyaredispensingthecorrectmedicationwhencomparingtheproducttobedispensedagainst226theproductcontainedinthecommercialcontainerclosuresystem.
Ifanimageisusedonthe227PDP,theimageshouldappearatthebottomofthelabelandshouldnotcompeteinsizeor228prominencewiththeproprietaryand/ornonproprietarynameandstrengthinformation.
Images229shouldrepresenttheactualtabletorcapsuleandreflectthetruesize,color,andimprint.
230Schematicorcomputer-generatedimagesshouldnotbeused.
2316ContainsNonbindingRecommendationsDraft–NotforImplementation2322335.
DangerousAbbreviations,Acronyms,andSymbols234235Certainabbreviations,acronyms,andsymbolsaredangerousandshouldnotbeusedbecause236theyarefrequentlymisinterpretedandcanleadtomistakesthatresultinpatientharm.
For237example,theabbreviationgformicrogramshouldnotbeusedbecauseithasbeenmistakenas238mg,meaningmilligram.
Theabbreviationmcgisanappropriateabbreviationformicrogram.
239TheabbreviationIUforinternationalunitalsoshouldnotbeusedbecauseithasbeenconfused240fortheintravenousrouteofadministration.
Mistakescanalsoresultfromtheuseof241242abbreviations,symbols,anddosedesignationswhosemeaningisnon-standardizedand/orunfamiliartothehealthcareprofessionalorothertargetreader.
14Forthesereasons,sponsors243shouldavoidusingerror-proneabbreviationsorsymbolsforproductnames,doses,andstrength244designationsoncontainerlabelsandcartonlabeling.
WereferyoutoTheJointCommission's245"DoNotUse"list,aswellastheInstituteforSafeMedicationPractices(ISMP)ListofError-246247ProneAbbreviations,Symbols,andDoseDesignationsforalistofcommonlyconfusedabbreviations,symbols,anddosedesignations.
15,16,17248249E.
AvoidLook-alikeContainerLabelsandCartonLabeling250251Look-alikecontainerlabelsandcartonlabelinghavefrequentlycontributedtoproductselection252errorsleadingtothedispensingandadministrationofthewrongdrug,wrongstrengthand/or253wrongdose.
Sponsorsshouldcreateacontainerlabelandcartonlabelingdesignthatis254sufficientlydistinctfromthatoftheirotherproductsandtheproductsofothermanufacturersso255thattheenduserisabletocorrectlyidentify,select,dispense,andadministertheappropriate256medication,strength,anddose.
257258Thepotentialforproductconfusionisespeciallyproblematicwhenproductswithsimilarlooking259containerlabelsorcartonlabelingarecustomarilystoredside-by-sideornearoneanother.
To260reducetheriskoferror,FDArecommendsthefollowing:2612621.
CorporateTradeDress263264Sponsorsshouldavoidorminimizetheuseofcorporatetradedressthatcouldmakeitdifficult265forenduserstodistinguishbetweendifferentmedicationsordifferentstrengthsofthesame266medication.
26714FDA/InstituteforSafeMedicationPracticesCampaigntoEliminateUseofError-ProneAbbreviations,http://www.
ismp.
org/tools/abbreviations/.
15TheJointCommission2001;http://www.
jointcommission.
org/assets/1/18/Official_Do_Not_Use_List_6_111.
PDF.
16TheInstituteforSafeMedicationPractices'ListofError-ProneAbbreviations,Symbols,andDoseDesignations,2010,http://www.
ismp.
org/tools/errorproneabbreviations.
pdf.
17USPGeneralChapters:WrittenPrescriptionDrugInformation-Guidelines.
7ContainsNonbindingRecommendationsDraft–NotforImplementation2682692.
UseofColor270271Acommonfeatureoflook-alikeproductsistheuseofthesameorsimilarcolorsinthecontainer272labelsandcartonlabelingofmultipleproductsacrossacompany'sentireproductlineorwithina273lineofrelatedproducts.
Werecommendthatsponsorsusecolorprudentlytobringattentionto274theproductname,strength,andimportantwarning(s).
Sponsorsalsoshouldbearinmindthat275(1)individualscanperceivecolorsdifferentlyandsomeindividualsmaybecolorblind,(2)276identificationofproductsbycolormightreplacereadingthelabel,(3)therearealimitednumber277ofdiscerniblecolorsavailable,and(4)colorscanlookdifferentundercertainlightingconditions.
278Accordingly,colorshouldnotbetheonlyelementusedasameanstodistinguishdifferent279280containerlabelandcartonlabelingofmultipleproductsacrossacompany'sentireproductlineorwithinalineofrelatedproducts.
18281282Inresponsetomedicationerrorsassociatedwiththeuseofcoloronpharmaceuticalproduct283284containerlabels,cartonlabeling,andpackaging,FDAheldapublichearingonMarch7,2005,todiscusstheprosandconsofeachofthefollowingapplicationsofcolor.
19Basedonthese285discussions,FDArecommendsthefollowing:286287a.
ColorDifferentiation288289Colordifferentiationisaneffectivetoolthatcan(1)differentiateproductswithina290manufacturer'sproductline;(2)differentiatestrengthswithinamanufacturer'sproductline;and291(3)highlightcertainaspectsofthelabel,suchasimportantwarningstatements.
Whenapplying292color,sponsorsshouldensurethatthetexthighlightedbythecolorhasadequatecolorcontrast293againstthebackgroundcolor.
Colordifferentiationismosteffectivewhenthecolorusedhasno294associationwithaparticularfeatureandthereisnopatternintheapplicationofthecolorscheme.
295296b.
ColorCoding297298Colorcodingisatechniquethatusescolortodesignateaspecificmeaning.
FDAgenerally299recommendsavoidingcolorcodinginmostinstances.
Colorcodingisreservedforspecial300circumstancesandonlyafterhumanfactorstestingandfeedbackontheprototypefromallend301usersisreceivedandevaluatedbyFDApriortouse.
302303Theuseofcolorcodingondruglabelshasbeenlimitedandnotwithoutrisk.
Colorcoding304schemesdevelopedtodecreaseerrormayactuallyincreaseerrorwhenthecolorisrelieduponas305ashortcuttoproperidentification(i.
e.
,notreadingthelabel).
Errorscanalsooccurwhenthe306colorcodeisnotmeaningfultoendusersoutsidethelimitedenvironmentwherethecolorcoding307hasanestablisheduse(e.
g.
,BroselowTapeintheemergencyroom,anduser-applied,color-308codedlabelsintheoperatingroom).
18MedicationErrors,SecondEdition,2007,p.
119.
19SeetheFederalRegisternotice"UseofColoronPharmaceuticalProductLabels,Labeling,andPackaging;Publichearing,"February3,2005(70FR5687).
8ContainsNonbindingRecommendationsDraft–NotforImplementation309310Certainapplicationsofcolorcodingareappropriate.
Examplesincludethecolorcodingof311certaindrugproductstrengthssuchaswarfarin,levothyroxine,andconjugatedestrogen-312containingproducts.
Thecolorsofthestrengthsareuniversallycolorcodedacrossall313manufacturers.
Anotherexampleiscolorcodingthecapsusedforophthalmicproductsto314distinguishatherapeuticclass(e.
g.
,beta-blockershaveayellowcap).
Althoughcolorcodingthe315capsisusefultoophthalmologistsandsomepatientsinidentifyingthetherapeuticclassof316medication,itisgenerallynothelpfultoendusersoutsideofophthalmology.
Infact,thecolor317codinghasmadeitdifficultfortheseuserstodifferentiatebetweendrugswithinthesame318therapeuticclasswhenthecolorcodewasusedonthecontainerlabelandcartonlabeling.
319Becausetheseproductsaretypicallystoredneareachother,thesimilarappearanceofthe320containerlabelsandcartonlabelinghasledtodispensingandadministeringthewrongstrength,321wrongdose,andwrongproduct.
Forthesereasons,thecolorcodingofophthalmicproductsis322limitedtothecapcolor.
323324IV.
SPECIALCONSIDERATIONSANDRECOMMENDATIONS325326A.
Proprietary,Established,andProperNames327328Sponsorsshouldmaximizethereadabilityofproprietary,established,andpropernamesonthe329containerlabelandcartonlabeling.
Werecommendcapitalizingonlythefirstletterinthe330proprietarynamebecausewordswritteninall-capitallettersarelesslegiblethanwordswrittenin331mixedcaseletters.
Moreover,theestablishedorpropernameandproprietarynameshouldbe332displayedinamannerconsistentwiththeFDAregulations,takingintoaccountallpertinent333factors,includingtypography,layout,contrast,andotherprintingfeatures(fordrugssee21CFR334201.
10(g)(2));forbiologicproductssee21CFR610.
62).
335336Theestablishednamefordrugproductsshouldincludethefinisheddosageform.
Ifspacedoes337notpermitthefinisheddosageformtoappearonthesamelineastheactiveingredient,we338recommendplacingthefinisheddosageformonthenextlinebelowtheactiveingredient.
339340MydrugMydrugMydrug341(drugozideinjection)or(drugozide)injectionor(drugozide)342Injection343344Forbiologicalproducts,thepropernameforbiologicalproductsshouldnotincludethefinished345dosageform.
Thefinisheddosageformcanappearonthelinebelowthepropername.
346347Mydrugdrugozide348(drugozide)Mydrug349InjectionInjection350351Mixedcaseortallmanletteringonapprovedcontainerlabelsandcartonlabelingcan352sometimesbeusedtohelpdistinguishsimilarlooking,establishednamepairsthathavebeen353confusedpostmarket.
Dissimilarlettersineachoftheestablishednamesareplacedinuppercase9ContainsNonbindingRecommendationsDraft–NotforImplementation354letterstobringattentiontothepointofdissimilaritybetweenthenamesofconcern(e.
g.
,355drugMYversusdrug).
WerecommendthatapplicantsconsultFDA'smedicationerror356preventionstaffbeforeusingthistechniqueandsupplydataconcerningthepostmarketconfusion357concern,adescriptionofhowtheletterstringwasselected,anddatademonstratingthatthe358proposedpresentationwilladequatelydistinguishbetweenthepotentiallyconfusingproduct359names.
Alistofapprovednamepairsthatusemixed-casetypographycanbefoundontheFDA360Webpageatwww.
fda.
gov/Drugs/DrugSafety/MedicationErrors/ucm164587.
htm.
361362B.
ProductStrength363364Aproduct'sstrengthorconcentrationiscriticallyimportantinformationfortheenduser.
Ifthe365productstrengthisnotclearlydisplayedonthecontainerlabel,orisexpressedinunitsof366measurethatareincongruentwiththoseusedinthedosinginstructions,thewrongstrengthcan367beselectedorthewrongdoseadministered(i.
e.
,over-orunder-dosing).
Werecommendthe368followingmeasurestoavoidorminimizecommonlyreporteddosingerrors:3693701.
StrengthDifferentiation371372Productselectionerrorsleadingtounder-orover-dosingcanoccurwhendifferentstrengthsof373thesameproductorsimilarstrengthsofdifferentproductsarestoredordisplayedinclose374proximity.
Sponsorsshouldensurethattheproductstrengthstandsoutonthecontainerlabeland375cartonlabeling.
Appropriatetechniquesforthispurposeincludetheuseofboxing,aprominent376typefaceortypeweight,andcolordifferentiation,amongothers.
3773782.
StrengthDesignation379380Productstrengthdesignationsshoulduseaconsistentunitofmeasureacrossallelementsofthe381labeling(e.
g.
,container,carton,andprescribinginformation).
Theproductstrengthshould382matchtheunitsofmeasuredescribedintheDOSAGEANDADMINISTRATIONsectionofthe383prescribinginformationtoavoiderror.
Forexample,userconfusionanddosingerrorscanoccur384ifproductstrengthisexpressedonthelabelinpercentage,butthedirectionsfordosageand385administrationofthedrugareexpressedinmilligrams.
Sponsorsalsoshouldusethesameunits386ofmeasurewhenlabelingmultipleproductscontainingthesameactiveingredient(e.
g.
,usemg387formilligramtoexpressthestrengthforallnitroglycerinproductsratherthanusingbothmgand388mcg).
3893903.
SmallVolumeParenteralProducts391392Forsmallvolumeparenteralproducts,theproductstrengthshouldbeexpressedastotalquantity393394pertotalvolumefollowedbytheconcentrationpermilliliter(mL),asdescribedintheUSP,GeneralChapterInjection.
20Anumberofoverdoseshaveoccurredwithsmall-volume20USPGeneralChapter:Injections;LabelsandLabeling;strengthandtotalvolumeforsingle-andmultiple-doseinjectabledrugproducts.
USPhasannouncedplanstorelocatethisinformationtoGeneralChapterLabelinginthenearfuture.
10ContainsNonbindingRecommendationsDraft–NotforImplementation395parenteralsbecauseofhealthcarepractitionerandpatientfailuretodeterminethetotalamountof396druginthecontainer.
Inmostcases,theusernoticedtheconcentration(e.
g.
,10mg/mL)but397failedtoseethenetquantity(e.
g.
,10mL),whichoftenappearsinadifferentlocationonthe398containerlabel.
Thisconfusionhasledtoadministrationoftheentirecontentsofthecontainer,399whenonlyaportionofthetotalvolumewasneeded.
400401Toavoidsuchconfusion,thestrengthpertotalvolumeshouldbetheprimaryandprominent402expressionontheprincipaldisplaypanelofthelabel,followedincloseproximitybystrengthper403milliliterenclosedbyparentheses.
Forexample:404405500mg/10mL406(50mg/mL)407408409Iftheproductcontainsavolumeoflessthan1mL,theproductshouldneverbelabeledwitha410concentrationofmg/mL,sincethismayleadenduserstomistakenlythinkthecontainerhas411moredruginitthanitactuallydoes,whichcanleadtounder-dosing.
Forcontainersholdinga412volumeoflessthan1mL,thestrengthperfractionofamillilitershouldbetheonlyexpression413ofstrength.
Forexample:41441512.
5mg/0.
625mL416or41712.
5mgper0.
625mL4184194.
ExpressionofStrengthforDryPowderProducts420421Drypowderproductsshouldexpressthestrengthintermsofthetotalamountofdrugpervialas422follows:423424XXmg/vialorXXmgpervial425426Instructionsforreconstitutingtheproductandtheresultantconcentrationshouldbeincludedon427thevial,ifspacepermits.
Theseinstructionswillinformpersonsresponsibleforpreparingthe428productwhattypeandvolumeofdiluentshouldbeusedforreconstitution,andtheamountof429drugcontainedineachmilliliteroncereconstituted.
Ifspacepermits,informationonthe430expirationdateandpost-reconstitutionstorageshouldalsobeincluded.
4314325.
SaltNomenclature43311ContainsNonbindingRecommendationsDraft–NotforImplementation434Whenaproductcontainsanactiveingredientthatisasalt,theUSPSaltPolicyshouldbeapplied435whennamingandlabelingdrugproducts.
214364376.
Prodrugs438439Forprodrugs,theproductstrengthshouldbeexpressedintermsoftheestablishedorproper440nameoftheprodrug—forexample,Valtrex(valacyclovirhydrochloridetablets)isaprodrugfor441acyclovir.
Afteroraladministration,valacyclovirhydrochlorideisrapidlyabsorbedfromthe442gastrointestinaltractandnearlycompletelyconvertedtoacyclovir.
Therefore,thestrengthof443thisproductisbasedonvalacyclovirratherthanacyclovir.
4444457.
MetricMeasurements446447ThedoseorexpressionofstrengthshouldappearinmetricunitsofmeasuresuchasmL,mg,and448mcg,ratherthanapothecaryorhouseholdmeasurements(e.
g.
,tspforteaspoon,TBSPfor449tablespoon,drams,andgrains)orratios(e.
g.
,1:1000).
450451Fatalerrorshaveoccurredwhenhealthcareprovidersorpatientsmiscalculatedmedicationdoses452whenconvertingfromoneunitofmeasuretoanother(forexample,theusualdoseisexpressed453intermsofamilligramunitofmeasure,buttheproductstrengthisexpressedasaratio,requiring454conversionoftheratiotoamilligramdose).
4554568.
LocationofNetQuantityStatements457458Productselectionordosingerrorscanoccurifthenetquantitystatementismistakenforthe459productstrength,leadingtounder-orover-dosing.
Thiserrorgenerallyoccurswhentheproduct460strengthoverlapswiththeproductnetquantity(e.
g.
,100tabletsversus100mg)orwhenthenet461quantityispresentedmoreprominentlyonthelabelthanistheproductstrength.
Thenet462quantitystatementshouldappearonthePDPbutshouldbeseparatefromandlessprominent463thanthestatementofstrength(e.
g.
,nothighlighted,boxed,orbolded).
4644659.
LeadingandTerminalZeros,Decimals,andCommas466467Numberscontainingdecimalpointsinthedeclarationofstrengthcanleadtotenfolddosing468errorswhenthedecimalpointgoesunseen(e.
g.
,4.
0mgisseenas40mg,or.
4mgisreadas4469mg).
Tominimizesucherrors,thequantityofactiveingredientinthestatementofstrength470shouldbepresentedinwholenumbers,andnotwithadecimalpointthatisfollowedbya471terminalzero(e.
g.
,4mg,not4.
0mg).
Conversely,decimalnumberssmallerthanoneshould472alwaysbeprecededbyazero(e.
g.
,0.
4mg,not.
4mg).
Thisservestoenhancethevisibilityof473thedecimalpoint.
4741221USPGeneralChaptersNomenclature;MonographNamingPolicyforSaltDrugSubstancesinDrugProductsandCompoundedPreparations.
ContainsNonbindingRecommendationsDraft–NotforImplementation475Commasshouldbeusedfornumbers1,000andabovetoimprovethelegibilityoflarger476numerals.
477478C.
Route(s)ofAdministration479Therouteofadministrationshouldbedescribedwithoutabbreviation.
Werecommendusing480positivestatementssuchas"forintravenoususe,""givebysubcutaneousinjection,"or"topical481useonly.
"Negativestatementssuchas"NOTforintrathecaluse"shouldnotbeusedbecauseit482iseasytooverlooktheword"not,"evenwhenitisemphasizedbybolding,underlining,orother483means.
Usingaffirmativestatementswillhelptoensurethatendusersunderstandtheintended484routeofadministration,eveniftheydonotreadeveryword.
22,23,24485486D.
WarningsforCriticalInformation487488Whenwarningstatementsareaddedtothecontainerlabelorcartonlabeling,theyshouldbe489writtenaffirmatively.
Non-affirmativewarningstatementshavebeenconfused.
Forexample,490thewarning"Notforintrathecaluse"hasbeenconfusedas"Forintrathecaluse.
"Affirmative491statementssuchas"ForIntravenousUseOnly,""Fatalifgivenbyanyotherroute,"or"Must492DiluteBeforeUse"aremoreeasilyunderstood.
493E.
ExpirationDates494495Currently,manufacturersusevariouswaystoexpresstheexpirationdateonaproductlabel.
496Someexpresstheexpirationdatewiththemonthandday,whileothersusethemonthandyear.
497Mostuseabbreviationstoexpressthesedates(e.
g.
,MA12).
Theuseofabbreviationsfor498expirationdateshasledtoconfusion,misinterpretation,andsometimesdelaysintreatment499becausetheabbreviationwasinterpretedincorrectly.
Forexample:"MA"couldmeanMarchor500May,whereasthenumber12couldrepresenttheday,month,oryear.
Accordingly,FDA501recommendstheexpirationdatebeexpressedinastandardformat,usingthree-lettertextforthe502month,two-digitnumeralsfortheday(ifincluded),andfour-digitnumeralsfortheyear,as503shownbelow:504505MMMYYYY(e.
g.
,JAN2013)506or507MMMDDYYYY(e.
g.
,JAN012013)508F.
BarCodes5091322EastmanKodakCompany.
ErgonomicDesignforPeopleatWork,SecondEdition.
ChengalurSN,etal.
,eds.
Hoboken,NJ:JohnWiley&Sons,2004.
23HandbookofWarnings.
WogalterMS,ed.
Mahwah,NJ:LawrenceErlbaumAssociates,2006.
24ProctorRW,VuKL.
HandbookofHumanFactorsinWebDesign.
UnitedKingdom:Routledge,2005.
ContainsNonbindingRecommendationsDraft–NotforImplementation510Abarcodeshouldbeplacedontheimmediatecontainerlabelandcartonlabelingofmostdrug511products.
Thebarcodeshouldbesurroundedbyenoughwhitespacetoallowscannerstoread512thebarcodeproperly(see21CFR201.
25(c)(2)).
Printdensityshouldbeconsistenttoallowfor513anaccuratescan.
Thebarcodeshouldbeplacedinaconspicuouslocation(e.
g.
,notonthe514bottomofacarton)whereitwillnotbedifficulttoreadbecauseofdistortedtext.
Additionally,515thebarcodeshouldbeplacedinanareawhereitwillnotbedamagedbecauseitappearsatthe516pointoflabelseparation(e.
g.
,perforation).
517G.
NationalDrugCodeNumbers518519Eachlisteddrugproduct25,26isassignedaunique10-digit,3-segmentnumberknownasthe520521nationaldrugcode(NDC).
TheNDCnumberidentifiesthelabeler,product,andcommercialpackagesize.
27,28WhenselectingtheproductcodeforNDCnumbersofdrugproductswith522multiplestrengths,FDArecommendsthefollowing:523524Avoidassigningproductcodesthatarenumericallysimilaroridentical.
Thesimilarityofthe525productcodenumbershasledtoselectinganddispensingofthewrongstrengthandwrongdrug.
526Themiddledigitsaretraditionallyusedbyhealthcareproviderstocheckthecorrectproduct,527strength,andformulation.
Therefore,assignmentofsequentialnumbersforthemiddledigitsis528notaneffectivedifferentiatingfeature(e.
g.
,6666,6667,and6668),norisusingtheidentical529productcodeforinjectableproductscontainingthesameconcentrationofdrugbutdifferenttotal530volumes.
Forexample,injectableproductsmightcontainthesameproductconcentrationbut531containadifferenttotalamountofdruginthecontainerbecauseofdifferencesinthefillvolume532(e.
g.
,20mg/2mL(10mg/mL),40mg/4mL(10mg/mL)).
Whenthesameproductcodenumber533isusedforallofthedifferentcontainers,healthcarepractitionershavehaddifficulty534distinguishingthedifferenceintotaldrugcontent.
Eachoftheseinjectableproductsshould535thereforehaveadifferentproductcodeassigned.
Anotherexampleiswheninjectableproducts536containthesameconcentrationandsametotaldrugcontentbutdeliversdifferentvolumesor537doses.
Forexample,adrugmightbemarketedintwoprefilledpendevices,eachcontaininga10538mg/mLsolution,andtotalvolumeof3mL.
However,onedevicemightdeliverdosagesof5mg539(0.
5mL)andtheothermightdeliverdosagesof10mg(1mL).
Eachoftheseprefilledpens540shouldbeassignedadifferentproductcodetohelpavoidconfusionbetweenthetwoproducts.
54125Undersection510oftheFederalFood,DrugandCosmeticAct(FD&CAct)(21U.
S.
C.
360),asamended,andpart207ofFDA'sregulations,withsomelimitedexceptions,firmsthatmanufacture,prepare,propagate,compound,orprocessdrugsintheUnitedStatesordrugsthatareofferedforimportintotheUnitedStatesmustberegisteredwiththeFDA(see21U.
S.
C.
360(b),(c),(d),and(i)).
Everypersonwhoregistersmust,atthetimeofinitialregistration,listalldrugsmanufactured,prepared,propagated,compounded,orprocessedforcommercialdistribution(21U.
S.
C.
360(j)(1))(seealso21CFR207.
20).
DruglistinginformationmustbeupdatedinJuneandDecembereachyear.
Theseupdatesmustincludedrugsnotpreviouslylisted(ifany),andcertainchangestoinformationforpreviouslylisteddrugs(21U.
S.
C.
360(j)(2);21CFR207.
21(b)and207.
30).
26InformationregardingFDA'sDrugRegistrationandListingisavailableonFDA'sWebsiteathttp://www.
fda.
gov/Drugs/GuidanceComplianceRegulatoryInformation/DrugRegistrationandListing/default.
htm.
- graphics360web相关文档
- settings360web
- Journal360web
- issues360web
- 英特尔360web
- C:\Users\cdodge\Desktop\New
- 开源奇虎360Web平台部基础架构团队访谈:开源线上数据库中间件Atlas
PacificRack 下架旧款方案 续费涨价 谨慎自动续费
前几天看到网友反馈到PacificRack商家关于处理问题的工单速度慢,于是也有后台提交个工单问问,没有得到答复导致工单自动停止,不清楚商家最近在调整什么。而且看到有网友反馈到,PacificRack 商家的之前年付低价套餐全部下架,而且如果到期续费的话账单中的产品价格会涨价不少。所以,如果我们有需要续费产品的话,谨慎选择。1、特价产品下架我们看到他们的所有原来发布的特价方案均已下架。如果我们已有...
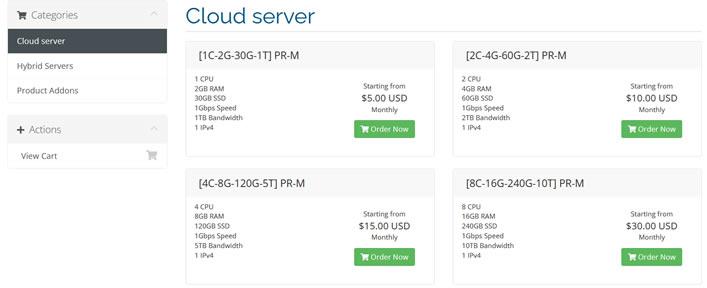
蓝竹云挂机宝25元/年,美国西雅图 1核1G 100M 20元
蓝竹云怎么样 蓝竹云好不好蓝竹云是新商家这次给我们带来的 挂机宝25元/年 美国西雅图云服务器 下面是套餐和评测,废话不说直接开干~~蓝竹云官网链接点击打开官网江西上饶挂机宝宿主机配置 2*E5 2696V2 384G 8*1500G SAS RAID10阵列支持Windows sever 2008,Windows sever 2012,Centos 7.6,Debian 10.3,Ubuntu1...
无视CC攻击CDN ,DDOS打不死高防CDN,免备案CDN,月付58元起
快快CDN主营业务为海外服务器无须备案,高防CDN,防劫持CDN,香港服务器,美国服务器,加速CDN,是一家综合性的主机服务商。美国高防服务器,1800DDOS防御,单机1800G DDOS防御,大陆直链 cn2线路,线路友好。快快CDN全球安全防护平台是一款集 DDOS 清洗、CC 指纹识别、WAF 防护为一体的外加全球加速的超强安全加速网络,为您的各类型业务保驾护航加速前进!价格都非常给力,需...

-
英文域名中文域名和英文域名的区别? 越详细越好域名主机电脑域名是什么香港虚拟空间最稳定香港虚拟主机空间在哪里?jsp虚拟空间JSP虚拟目录及虚拟路径的配置方法免费网站空间申请申请免费空间的网站北京虚拟主机租用北京云主机租用哪家资质正规,价格便宜,服务好?要真云主机不要那种vps的假云主机,机房要在北京的!虚拟主机mysql虚拟主机的数据库有哪些长沙虚拟主机长沙IDC,求长沙本地虚拟主机,大伙推荐推荐域名停靠“域名停靠”怎么挣钱啊?花生壳域名花生壳添加新域名