suspiggycase
piggycase 时间:2021-03-23 阅读:()
InhibitionHasLittleEffectonResponseLatenciesintheInferiorColliculusZOLTANM.
FUZESSERY,1JEFFREYJ.
WENSTRUP,2JIMC.
HALL,3ANDSCOTTLEROY21DepartmentofZoologyandPhysiology,UniversityofWyoming,Laramie,WY82071,USA2DepartmentofNeurobiologyandPharmacology,NortheasternOhioUniversitiesCollegeofMedicine,Rootstown,OH44272,USA3DepartmentofBiochemistryandCellularandMolecularBiology,UniversityofTennessee,Knoxville,TN37996,USAReceived:27November2001;Accepted:3June2002;Onlinepublication:19August2002ABSTRACTTheinferiorcolliculiofallmammalsarecharacter-izedbyawiderangeofrst-spikeresponselatenciesthatcangreatlyexceedtheminimumtimerequiredforthetransmissionofinputthroughthelowerbrainstem.
Themechanismsthataccountforlongresponselatenciesofupto50msareunclear,butonehypothesisisthatanearlyinhibitionplaysaroleinshapinglatency.
Totestthishypothesis,responsela-tenciesweremeasuredintheinferiorcolliculiofthepallidandmustachedbatsbeforeandduringtheblockadeofGABAaandglycinereceptors.
Theeffectofblockinginhibitiononresponselatencywascom-paredunderstimulusconditionsthatproducedtheshortestlatencyinthepredrugcondition.
Multibarrel''piggyback''electrodeswereusedtoiontophoreti-callyapplybicucullineandstrychninesequentiallywhilerecordingfromsingleneurons.
Predruglaten-ciesrangedfrom9to26msinthepallidbatandfrom4to17msinthemustachedbat.
Despitelargein-creasesinresponsemagnitudeandresponsedurationfollowingdisinhibition,theblockadeofinhibitoryreceptorshadmodesteffectsonresponselatency.
Inthepallidbat,blockingGABAreceptorsproducedlatencychangesthatrangedfrom)3.
8to+0.
2ms,whileblockingglycinereceptorsproducedchangesfrom)0.
1to+1.
7ms.
Similarly,inthemustachedbat,blockingGABAreceptorscausedchangesrangingfrom)10.
3to+1.
4ms;blockingglycinereceptorsinthemustachedbatcausedchangesfrom)3.
6to+1.
0ms.
Thelargechangeof)10.
3mswasanexception.
Inbothspecies,themajorityofneuronsshowedchangesof40kHz)arelocatedmoremediallyanddonotextendtothelateralwalloftheIC(Fig.
2).
NeuronsinthelateralIChaveresponselatenciesasshortas5ms,theshortestrecordedintheIC(Fig.
2).
Basedontheaboveestimateofthearrivaltimesofafferentinput,theseresponselatenciesprobablyrepresenttheshortestpossibleresponsetimesinthepallidbatICC.
Incontrast,fewneuronstunedtothesamelow-frequencyrangeinthemedialIChadre-sponselatenciesof50kHz(Fig.
4).
Onlyonehadalowerbestfrequencyof25.
4kHz.
Allneuronswerecombi-nation-sensitive,asdescribedinPortforsandWenst-rup(1999).
Theirpredrugresponselatenciesrangedfrom4.
0to17.
2ms(Fig.
4),arangewhichissimilartothosereportedinpreviousstudiesofneuronstunedtothehigherharmonicsoftheecholocationpulse(HattoriandSuga1997;PortforsandWenstrup2001),butwhichdoesnotincludethemuchlongerlatencies(upto45ms)reportedinthehypertrophied60kHzregionofthemustachedbatinferiorcollicu-lus(ParkandPollak1993).
Inall38neuronstestedinthepallidbat,andin21ofthe52neuronstestedinthemustachedbat,re-sponselatenciesbeforeandduringdisinhibitionweremeasuredovertheirdynamicintensityrangebecauseintensitylevelcanhavealargeeffectonlatency(e.
g.
,IrvineandGago1990;Klugetal.
2000),withlatencytypicallydecreasingwithincreasingintensitylevel(Figs.
5and6,rightcolumns).
ThechangeinlatencyascribedtodisinhibitionwasmeasuredattheintensitylevelthatproducedtheshortestlatencyinFIG.
3.
Left:ThreeelectrodepenetrationsthroughthepallidbatIC,varyinginlateromediallocation.
Right:Thelatenciesandbestfrequenciesofneuronsrecordedinthethreepenetrationsshowingthatlatencyvariedasafunctionofbestfrequencyandlocation.
FIG.
2.
Left:TransversesectionofthepallidbatICshowingisofrequencycontoursandfunctionallydenedmedialandlateralregions(verticaldashedline).
Right:ThedistributionofresponselatenciesinthepallidbatICasafunctionofbestfrequencyandlocationinthemedial(lledcircles)andlateral(opensquares)regionsoftheIC.
NeuronsinthelateralICaretunedto0.
5mswereobservedonlywhenglycinereceptorswereblocked.
Similarchangesinlatencywereobservedacrossbestfrequencies(Fig.
7C)andalsoacrosstherangeofpredruglatencies(Fig.
7A).
TheaveragelatencychangewhenblockingGABAareceptorswas)0.
7ms(range=)3.
8to+0.
2ms,SD=1.
0ms).
Theaveragechangewhenblockingglycinereceptorswas)0.
1ms(range=)1.
3to+1.
7ms,SD=0.
8ms).
Inthemustachedbat,changesinresponselatencyrangedfromadecreaseof10.
3mstoanincreaseof1.
4ms,but,asinthepallidbat,themajorityshowedchangesof0.
65).
RepresentativechangesintemporalresponsepropertiesareshowninFigure8.
Inthepallidbat(Fig.
8,leftcolumn),theneuroninFigure8A,Bshowedanaverage0.
8msdecreaseinlatencyinre-sponsetobicuculline.
Inthepredrugcondition,theneuronredonespike.
Duringbicucullineapplica-tion,itredtwospikesinthisintialburst,therstspikeoccurring0.
5–12msearlierthaninthepredrugcondition,followedbyathirdspikeofirregulartim-FIG.
4.
ThedistributionofnormalresponselatenciesofneuronsintheICsofthepallidbat(left)andmustachedbat(right)priortoblockadeoftheirinhibitoryinputs.
Forthemustachedbat,neuronsinwhichresponselatenciesweretestedoverthedynamicintensityrangeoftheneuronareindicatedbyopencircles.
Thosetestedat10dBabovethresholdareindicatedbylledcircles.
FUZESSERYETAL.
:InhibitionandResponseLatency65ing.
Bicucullineappearedtoreleasethisneuronfrominhibitionthatoccurredbothbeforeandafterthenormalexcitation.
DisinhibitionbybicucullinehadsimilarbutgreatereffectsontheneuroninFigure8C,D.
Itsometimesredarstspikeapproximately2msearlierthaninthepredrugcondition,butbecausethisearlyresponsewasinconsistent,itsaveragela-tencywaslongerandtherewasonlya0.
8msdiffer-enceinmeanlatenciesunderthetwoconditions.
Thetimingofthesecondpeakofrst-spiketimingduringbicucullineapplicationissimilartothatoftherstpeakinthepredrugcondition.
ThisresultsuggeststhatbicucullineblockedanearlyGABAergicinhi-bitionthatretardedtheresponselatencyofthisneuron.
Asinthepreviousneuron(Fig.
8A,B),bic-ucullinealsoremovedalateinhibitionandgreatlyincreasedtheresponseduration.
TheneuroninFigure8E–Gwassequentiallyex-posedtobicucullineandstrychnine.
Bicucullinein-creasedthedurationandmagnitudeofitsresponseanddecreasedresponselatencyanaverageof1.
0ms(Fig.
8G).
Incontrast,strychnineincreaseditslatencybyanaverageof1.
7ms(Fig.
8F).
AswastypicallytheFIG.
5.
FourneuronsfromthepallidbatICshowingtheeffectofblockingGABAergicinputwithbicuculline(Bic)orglycinergicinputwithstrychnine(Strych)relativetothepredrug(Pre)condition.
Leftcolumnshowstheeffectofdisinhibitiononintensity–ratefunctions.
Rightcolumnshowstheeffectofresponselatencyasafunctionofintensitylevel.
Thearrowsintherightcolumnindicatetheintensitylevelatwhichchangesinresponselatencyweremeasured.
Thesamesymbolsareusedinthepairsofguresintheleftandrightcolumnstoindicatedrugandpredrugconditions.
66FUZESSERYETAL.
:InhibitionandResponseLatencycase,bicucullineincreasedresponsemagnitudemorethanstrychnine.
Similarchangesinpoststimulustimehistogramswereseeninthemustachedbat(Fig.
8,rightcol-umn).
Figure8H,Ishowsthelargestlatencychangeobservedafterapplicationofanantagonistinthisstudy,10.
3ms.
Incontroltests,theunitrespondedonaveragewithonespiketothebestfrequencystimulus.
Therstspikeoccurredaslittleas11msafterstim-ulusonset,but,becauseofthehighvariability(SD=6.
0ms),themedianlatencywas17.
5ms.
Withbicuculline,theresponserateincreasedbyafactorof5.
Theearliestlatenciesdecreasedto7msandthedistributionoflatencieswasmuchtighter(SD=1.
1ms,medianlatency=7.
2ms).
Thus,bicucullinere-vealedtwolargeeffectsofGABAainhibitiononla-tency:adecreaseintherst-spiketimingandanincreaseintheconsistencyoftherstspike.
Suchlargecombinedeffectswerenotseeninotherunits.
Figure8J,Kshowsaverysmalleffectofbicucullineonlatencydespiteamajoreffectonresponsemagni-tude.
Theunit(alsoshowninFig.
6G,H)respondedweaklyinthecontrolcondition,butthelatencyofresponsevariedlittle(SD=0.
8ms,medianlaten-FIG.
6.
FourneuronsfromthemustachedbatIC.
ThesameconventionasinFig.
4wasusedtoshowtheeffectsofbicucullineand/orstrychnineonintensity–ratefunctionsandintensity–latencyfunctions.
FUZESSERYETAL.
:InhibitionandResponseLatency67cy=8.
4ms).
Withbicuculline,theresponseratein-creasedbyafactorof6butneitherthelatencynoritsvariabilitychangedmuch(SD=0.
6ms,medianlaten-cy=8.
7ms).
TheunitinFigure8L–Nshowedonlysmalleffectsofbothbicucullineandstrychnineonresponsela-tency(seealsoFig.
6C,D).
Theunit'scontrolre-sponsewasweak,partlyaresultofinhibitionoccurringathigherstimuluslevels.
Medianrstspikelatencywas10.
7ms,withlittlevariation(SD=1.
2ms).
Withstrychnine,theresponselevelroughlydoubled,butrst-spikelatencyremainednearlythesamewithlittlevariation(SD=1.
3ms,medianla-tency=10.
6ms).
Withbicuculline,theresponserateincreasedfurther,butthemedianrst-spikelatencydecreasedtoonly9.
8ms(SD=1.
5ms).
DISCUSSIONThisstudyfocusedontheroleofGABAandglycin-ergicinputinregulatingtherst-spikeresponsela-tenciesofICneurons.
Inbothspeciestested,inhibitionhadpronouncedeffectsonthedurationandmagnitudeofresponses,aswellasontheshapesofintensity–ratefunctions,butonlymodesteffectsonresponselatency.
Themajorityofneuronsshowedlatencyshiftsof5ms)inresponselatencywereob-servedintheguineapig(LeBeauetal.
1996),theywerespecicallyassociatedwithneuronswithsus-tainedresponses.
Neuronselicitingphasicburstsatonsettypicallyshowedlesschange.
ParkandPollak(1993)alsonotedthelargestlatencychangesinneuronswithsustaineddischargesandsuggestedthatitwasbecauseGABAergicdisinhibitionallowstheirrstspikestooccurinatightertemporalregister,therebydecreasingtheiraverageresponselatencies.
Thiswouldsuggestthatinhibitiondecreasestheoverallresponsivenessofthesetonicrespondersbutdoesnotnecessarilydeterminerst-spikelatency.
Incontrasttothesemodesteffects,ParkandPollak(1993)reportedthat17%ofneuronsinthehyper-trophied60kHzrepresentationofmustachedbatICshoweddecreasesrangingfrom5to25ms.
NeuronswiththelargestdecreasesinlatencywereOFFre-sponders(neuronsthatrespondedonlyattheendofastimulus)thatwereconvertedtoONresponderswhendisinhibited.
Suchneuronscanbediscountedfromthepresentdiscussionsincetheirresponsela-tenciesaredependentonstimulusduration,andla-tencychangesobservedfollowingdisinhibitionwillFIG.
8.
Representativeexamplesofthepoststimulustimehistogramsofneuronsfromthepallidbat(leftcolumn)andmustachedbat(rightcolumn)beforeandduringdisinhibition.
Theverticaldashedlinesshowmeanrst-spikelatenciesinthepallidbatandmedianrst-spikelatenciesinthemustachedbatfordifferentconditions(seetext).
Allneuronsweretestedwithstimulusparametersthatevokedthegreatestresponsesandshortestresponselatencies.
Forthepallidbat(leftcolumn)allweretestedwithbestfrequencytonesat15–20dBaboveresponsethreshold.
Sincetwooftheseneurons(A,BandE–G)weredurationselective,theyweretestedattheirbestdurationsof5and1ms,respectively.
TheneuroninC,Dwastestedwitha10msdurationtone.
FUZESSERYETAL.
:InhibitionandResponseLatency69alsodependuponstimulusduration.
However,thiscannotaccountforallofthelargedecreasesobservedinthemustachedbat.
AtrendnotedbyParkandPollak(1993)isthatneuronsinthedorsalpartofthe60kHzrepresentationintheIChadthelongestla-tencies(seealsoHattoriandSuga1997),andthispopulationshowedthegreatesteffectofdisinhibitiononresponselatency.
Presentresultsfromneuronsinthemustachedbat's60kHzrepresentationshowin-dicationsofasimilartrend(Fig.
6B);thelongestla-tencyneuronstunedto60kHzweretheonlyonestoshowlatencydecreasesof3msormorefollowingremovalofinhibition.
Ifmore60kHzneuronswithlongerresponselatencieshadbeenincluded,ourresultsmightbemoresimilartothoseofParkandPollak.
Therewasnoevidenceofthistrendamongneuronstunedinthe72–95kHzrangeinmustachedbats,orinthepallidbat(Fig.
6A).
Basedontheirdata,ParkandPollak(1993)pro-posedthatGABAergicinhibitionshapeslatencymapsthroughoutthetonotopicallyorganizedIC.
Theyfurthermoreproposedthattheorganizationofla-tencyintheICcouldprovidedelaylinesnecessaryfortheconstructionofcoincidencedetectorsselectiveforpulse-echodelay(seealsoSaitohandSuga1995;HattoriandSuga1997).
Althoughtherangeofla-tenciesinthe60kHzrepresentationofthemus-tachedbatICisnotunusual,norisitsorganizationoflatency,thedependenceoflatencyoninhibitionisremarkable.
Thisledtospeculationthatthedepen-denceoflatencyoninhibitionisaspecializedfeatureusedbythemustachedbatfortheconstructionofcoincidencedetectorstoanalyzesonarechoes(LeBeauetal.
1996).
However,resultsofseveralrecentstudiesshowthat(1)coincidencedetectorsarecre-atedintheIC,notinthemedialgeniculatebodyastheseproposalsrequire(MittmannandWenstrup1995;WenstrupandLeroy2001);(2)latencymapsintheIChavenoapparentrelationshipwiththedelaytuningofcoincidencedetectorsintheIC(PortforsandWenstrup1999,2001;Wenstrupetal.
1999);and(3)amongthedelay-tunedneuronsrecordedfromthemustachedbat'sICinthepresentstudy(withBFsof72–89kHz),neitherGABAergicnorglycinergicinhibitionappearstocontributesignicantlytothelatencyofresponsetoBFsignals.
Moregenerally,thepresentresultsinbats,asinothermammals,showthatGABAergicandglycinergicinhibitionsdonotcontributesignicantlytoresponselatencyinthegreatmajorityofthepopulationofICneurons.
MechanismsshapingresponselatencyAshorteningofresponselatencyfollowingtheblockadeofGABAaorglycinereceptors,regardlessofthemagnitudeoftheeffect,indicatesthatashort-latencyinhibitoryinput,eitherprecedingorcoin-cidingwiththearrivalofexcitatoryinput,isabletocounterdepolarizationanddelayspikegeneration(ParkandPollak1993;Halpeaetal.
1994;SaitohandSuga1995;CassedayandCovey1995).
PrecedingIPSPsintheIChavebeenobservedinwhole-cellpatch-clamp(Coveyetal.
1996)andintracellular(NelsonandErulkar1963;Kuwadaetal.
1997)re-cordingsaswellasintracellularbrainslicerecordings(Smith1992;Wagner1996;Lietal.
1999).
TheseearlyIPSPswereobservedevenwhenaneuronwaspresentedwithitsmostexcitatorystimulus(Cassedayetal.
1994;Coveyetal.
1996).
Inadditiontoinu-encingtheonsetofaresponse,inhibitionalsoshapesitsdurationandoffset,sinceblockinginhibitionof-tenconvertsneuronswithphasicresponsestotonicresponderswhoseresponsedurationslastwellbeyondthestimulusduration(e.
g.
,Fig.
8).
Thislattereffectofdisinhibitionistypicallymoreprofoundthantheeffectonrst-spikelatency.
Inadditiontodecreasingresponselatency,block-inginhibitoryreceptorscanalsoincreaselatencybyupto3ms.
Inthepresentstudyofthepallidbat,thisoccurredonlywhenglycinergicinputwasblocked.
Inthemustachedbat,thisoccurredfollowingblockadeofeitherglycinergicorGABAergicinput.
Inbothspecies,neuronswereobservedthatshowedde-creasedlatenciesinresponsetobicucullineandin-creasedlatenciesinresponsetostrychnine.
Otherstudies(ParkandPollak1993;Luetal.
1997)haveobservedthiswhenblockingGABAergicinput.
Whyresponselatenciesshouldincreaseduringdisinhibi-tionisnotclear.
Onepossibilityisthatinhibitoryin-putsthemselvesaresubjecttoinhibition,andareleasefromthisinhibitionwouldallowtheinhibi-toryinputtoexertagreaterinhibitoryeffectontherecordedneuron.
If,forexample,alocalGABAergicinhibitoryneuronclosetothesiteofdrugapplicationreceivedinhibitionfromaglycinergicinput,thentheapplicationofstrychninewouldreleasetheinhibitoryneuronfrominhibition,allowingittoexertagreaterinhibitoryeffectontherecordedneuronandin-creaseitsresponselatency.
Asecondpossiblemechanismisthatareboundfrominhibitionmaycontributetoexcitationinsomeneurons.
Thus,eliminationofanearlyinhibitioncouldslowdepolarizationandincreaselatency.
In-creasedexcitationfollowinghyperpolarizationhasbeenreportedinvitrointhecochlearnucleus(Manis1990)andinthedorsalnucleusofthelaterallem-niscus(WuandKelly1995),aswellasintheIC(Smith1992;Perruzietal.
2000).
Invivo,basedonwhole-cellpatch-damprecordings,Cassedayetal.
(1994)haveproposedthatareboundfromearlyin-hibition,coincidingwitharrivalofalateexcitatoryinput,mayunderlieaselectivityforsoundduration70FUZESSERYETAL.
:InhibitionandResponseLatencyobservedintheICofthebigbrownbat.
Additionalevidencethatinhibitionmaycontributetoexcitationintheinferiorcolliculuscomesfromarecentstudyofcombination-sensitiveneuronsinthemustachedbat(WenstrupandLeroy2001).
Thefacilitatedresponseproducedbyappropriatecombinationsoftonesiseliminatedbyblockinginhibitoryglycinergicinput.
Thisparadoxicalresultcouldbetheresultofblock-ingapostinhibitoryreboundthatcontributestoex-citation.
Inhibitioncanindeedinuencetheresponsela-tencyofICneurons,but,withtheexceptionoflong-latencyneuronsinthemustachedbat(ParkandPo-llak1993),therathersmalleffectsobservedinotherspeciesafterblockinginhibitioncannotaccountforthewiderangeoflatenciespresentinthemidbrain.
Othermechanismsrequiregreaterattention.
Wavepropogationtimesalongthecochlearbasilarmem-branecancontributetoashort,frequency-dependentdelayofaround1ms,withhigher-frequencyinputarrivingrst(RhodeandSmith1986).
TheinuenceofthiscochleardelayonICresponselatenciesismostlikelyinsignicant,particularlyinthelateralregionofthepallidbatIC,whereneuronswithlowbestfre-quencieshavetheshortestresponselatencies.
Whiletherangeoflatenciesatlowfrequencieshasbeenfoundtobebroader,minimumlatencieshavebeenfoundtobelargelyindependentoffrequencytuning(e.
g.
,LangnerandSchriener1988;Halpeaetal.
1994).
Otherfactorsthatcaninuenceresponselatencyareaxonlength,conductionvelocity,thenumberofsynapsesalonganascendingpathway,andsynapticintegrationtimes.
ThecontributionofeachofthesefactorsinICresponselatenciesislikelytovaryacrossneurons.
Forexample,studiesthatusedelectricalstimulationoftheauditorynerve(Snyderetal.
1995)anddorsalcochlearnucleus(SempleandAitkin1980)inplaceofnormalacousticstimulationreportawiderangeofresponselatenciesintheIC,leadingtotheconclusionthatitisunlikelythataxonlengthsorthenumberofintercalatedsynapsescouldaccountfor10–20msdifferencesinresponselatenciesintheIC(Snyderetal.
1995).
Theincreaseinresponsela-tenciesalongtheaxisoftheIC(e.
g.
,LangnerandSchriener1988;HattoriandSuga1997)isdifculttoaccountforthroughadditionaltraveltime.
ThissamegradientofresponselatencieswasobservedinthepallidbatIC,wheremorethan10msincreasesinlatencyoccuroveraseparationofonly1.
5mm.
Instead,muchofthedelayinresponseonsetmayresideinmechanismsthatareintrinsictotheIC.
IntracellularbrainslicerecordingsoftheICC(Wag-ner1996)reportlongresponselatencies(to12ms),evenwhenneuronsareexcitedbyelectricalstimula-tionofthelaterallemniscusimmediatelyventraltotheIC.
InthecorticesofthecatIC,regionsthatre-ceiveprimarilyintracollicularandneocorticalinputs,excitatorypostsynapticpotentials(EPSP)latenciesofupto11mswerereportedfollowingstimulationofthecommissureoftheIC(Smith1992).
Theselonglatenciesfollowingintracollicularelectricalstimula-tionsuggestthatmultisynapticcircuitsand/orlongsynapticintegrationtimeswithintheICcanpoten-tiallyimposeadelayinresponsethatcanbelongerthanthetimerequiredforthetransmissionofin-formationfromthecochleatotheIC.
Indeed,Halpeaetal.
(1994)emphasizedthat,inthebigbrownbat,theminimumresponselatenciesfromthelevelsofthecochlearnucleustothelaterallemniscalnucleitotheICdonotincreaseappreciably.
Rather,itistherangeofresponselatenciesthatincreasesdramati-callyasoneascendstheauditorybrainstem.
OnemechanismthatmaycontributetothewidelatencyrangeisadifferenceinthetypesofexcitatoryandinhibitoryreceptortypesacrosstheICandthemannerinwhichtheyinteracttocontrolthetimingofsynapticintegration.
AMPAandNMDAglutamatereceptors,forexample,arereportedtoactivatefastandslowpostsynapticexcitatorypotentials,respec-tively(Yangetal.
1999;KellyandKidd2000;ZhangandKelly2001).
Similarly,GABAaandGABAbre-ceptorsappeartoberesponsibleforinhibitorypo-tentialswithdifferenttimecourses(CrunelliandLeresche1991),andarereportedtobedifferentiallydistributedacrosstheIC(Vateretal.
1992;Fubaraetal.
1996).
SincethepresentstudyexaminedtherolesofonlyfastinhibitoryeventsmediatedbyGABAaandglycinereceptors,thepossiblerolesofotherreceptortypesmediatingslower,longer-lastingexcitatoryandinhibitoryeventsinshapingresponselatencywerenotrevealed.
Inconclusion,thisreportdoesnotquestiontheimportanceofinhibitioninregulatingthetimingofresponsesinthecentralauditorysystem.
Thereisgrowingevidencethatthetimingofinhibitoryandexcitatoryinputsplaysanimportantroleinshapingresponseselectivityfortemporalfeatures,suchasduration,andforcomplex,behaviorallyrelevantsounds,particularlythosewithcomponentsthataredelayedintime.
Moreover,neuronswithOFFre-sponsesduetoinhibitionimposedduringthecourseofasoundwillexhibitlongdelaysthatvarywithsoundduration.
Rather,thepresentstudyexaminedICneuronsthatrespondedatsignalonset,someofwhichhadlongrst-spikelatencies(10–25ms)thatfarexceededtheminimumtimerequiredforinputtotheIC.
Weaskedwhethertheselatenciescouldbefurtherdecreasedbytheblockadeofinhibitoryin-putswhencarehadbeentakentoensurethatthesoundspresentedevokedresponsesattheshortestpossiblelatenciespriortodisinhibitionbyinhibitoryFUZESSERYETAL.
:InhibitionandResponseLatency71transmitterblockers.
Despitelargeeffectsonre-sponsemagnitudeandduration,theblockadeofGABAaandglycinereceptorstypicallyproducedsmallchangesinresponselatency(<1ms).
Thisnding,coupledwiththosefrommostspecies,sug-geststhatfastinhibitorypathwaysmaynotplayadominantroleincreatingthewiderangeofresponselatenciesobservedinthemammalianIC.
ACKNOWLEDGMENTSWethankCarolGrosefortechnicalassistanceandTerriZumstegforeditingthemanuscript.
WearegratefultotheWildlifeSectionoftheMinistryofAgriculture,Land,andMarineResourcesofTrinidadandTobagoforpermissiontoexportmustachedbats.
ResearchwassupportedbyfundsfromtheNationalInstitutesofHealth(RO1DC00054toZMFandRO1DC00937toJJW)andtheNationalScienceFoundation(IBN-9828599toZMF).
REFERENCESBROWNP.
Vocalcommunicationinthepallidbat,Antrozouspallidus.
Z.
Tierpsychol.
41:34–54,1976.
CARNEYLH,YINTCT.
Responsesoflow-frequencycellsintheinfe-riorcolliculustointerauraltimedifferencesinclicks:excitatoryandinhibitorycomponents.
J.
Neurophysiol.
62:144–161,1989.
CASSEDAYJH,EHRLICHD,COVEYE.
Neuraltuningforsounddura-tion:roleofinhibitorymechanismsintheinferiorcolliculus.
Science264:847–850,1994.
CASSEDAYJH,COVEYE.
Mechanismsfortheanalysisofauditorytemporalpatternsinthebrainstemofecholocatingbats.
In:COVEYE,HAWKINGSHL,PORTRF(eds)NeuralRepresentationsofTemporalPatternsPlenum,NewYork,1995,pp25–52CASSEDAYJH,COVEYE.
Aneuroethologicaltheoryoftheoperationoftheinferiorcolliculus.
BrainBehav.
Evol.
47:311–336,1996.
COVEYE,KAUERJA,CASSEDAYJH.
Whole-cellpatch-clamprecordingrevealssubthresholdsound-evokedpostsynapticcurrentsintheinferiorcolliculusofawakebats.
J.
Neurosci.
16:3009–3018,1996.
CRUNELLIV,LERESCHEN.
AroleforGABAbreceptorsinexcitationandinhibitionofthalamocorticalcells.
TrendsNeurosci.
14:16–21,1991.
DEARSP,SIMMONSJA,FRITZJ.
Apossibleneuronalbasisfortherepresentationofacousticscenesinauditorycortexofthebigbrownbat.
Nature364:620–623,1993.
DEARSP,SUGAN.
Delay-tunedneuronsinthemidbrainofthebigbrownbat.
J.
Neurophysiol.
73:1084–1100,1995.
EHRETG,ROMANDR.
Developmentoftoneresponsethresholds,latenciesandtuninginthemouseinferiorcolliculus.
Dev.
BrainRes.
67:317–326,1992.
FENGAS,SIMMONSJA,KICKSA.
Echodetectionandtarget-rangingneuronsintheauditorysystemofthebat,Eptesicusfuscus.
Sci-ence202:645–648,1978.
FERRAGAMOMJ,HARESIGNT,SIMMONSJA.
Frequencytuning,laten-ciesandresponsestofrequency-modulatedsweepsintheinfe-riorcolliculusoftheecholocatingbat,Eptesicusfuscus.
J.
Comp.
Physiol.
182:65–79,1998.
FITZPATRICKDC,KUWADAS,BATRAR,TRAHIOTISC.
Neuralresponsestosimplesimulatedechoesintheauditorybrainstemoftheunanesthetizedrabbit.
J.
Neurophysiol.
74:2469–2486,1995.
FUBARABM,CASSEDAYJH,COVEYE,SCHARTZ-BLOOMRD.
DistributionofGABAA,GABABandglycinereceptorsinthecentralauditorysystemofthebigbrownbat,Eptesicusfuscus.
J.
Comp.
Neurol.
369:83–92,1996.
FUZESSERYZM,BUTTENHOFFP,ANDREWSB,KENNEDYJM.
Passivesoundlocalizationofpreybythepallidbat(Antrozousp.
palli-dus).
J.
Comp.
Physiol.
171:767–777,1993.
FUZESSERYZM.
Acutesensitivitytointerauraltimedifferencesintheinferiorcolliculusofabatthatreliesofpassivesoundlocaliza-tion.
Hear.
Res.
109:46–62,1997.
FUZESSERYZM,HALLJC.
Sounddurationselectivityinthepallidbatinferiorcolliculus.
Hear.
Res.
137:137–154,1999.
HALPEAS,COVEYE,CASSEDAYJH.
Frequencytuningandre-sponselatenciesatthreelevelsinthebrainstemoftheecholo-catingbat,Eptesicusfuscus.
J.
Comp.
Physiol.
174:671–682,1994.
HARRISONRV,PALMERAR.
Neuroneresponselatencyintheinferiorcolliculusinrelationtotheauditorybrainstemresponses(ABR)intheguineapig.
Scand.
Audiol.
13:275–281,1984.
HATTORIT,SUGAN.
Theinferiorcolliculusofthemustachedbathasfrequency-vs-latencycoordinates.
J.
Comp.
Physiol.
180:271–284,1997.
HAVEYDL,CASPARYDM.
Asimpletechniqueforconstructing''piggy-back''multibarrelmicroelectrodes.
Electroencephalogr.
Clin.
Neurophysiol.
48:249–251,1980.
HEILP,NEUBAUERH.
Temporalintegrationofsoundpressurede-terminesthresholdsofauditory-nervebers.
J.
Neurosci.
27:7404–7415,2001.
HENSONOW,POLLAKGD,KOBLERJB,HENSONMM,GOLDMANLJ.
Cochlearmicrophonicpotentialselicitedbybiosonarinyingbats,Pteronotusp.
parnelli.
Hear.
Res.
7:127–147,1982.
IRVINEDR,GAGOG.
Binauralinteractionsinhigh-frequencyneu-ronsininferiorcolliculusofthecat:effectsofvariationsinsoundpressurelevelonsensitivitytointerauralintensitydif-ferences.
J.
Neurophysiol.
63:570–591,1990.
JENPH-S,SCHLEGELPA.
Auditoryphysiologicalpropertiesoftheneuronsintheinferiorcolliculusofthebigbrownbat,Eptesicusfuscus.
J.
Comp.
Physiol.
147:351–363,1982.
JOHNSONPABR.
GABAergicandglycinergicinhibitioninthecen-tralnucleusoftheinferiorcolliculusofthebigbrownbat.
PhDthesis,DukeUniversity,Durham,NC,1993.
KELLYJB,KIDDSA.
NMDAandAMPAreceptorsinthedorsalnu-cleusofthelaterallemniscusshapebinauralresponsesinratinferiorcolliculus.
J.
Neurophysiol.
83:1403–1414,2000.
KITZESLM,GIBSONMM,ROSEJE,HINDJE.
Initialdischargelatencyandthresholdconsiderationsforsomeneuronsinthecochlearnucleuscomplexofthecat.
J.
Neurophysiol.
41:1165–1182,1978.
KLUGA,KHANA,BURGERRM,BAUEREE,HURLEYLM,YANGL,GROTHEB,HALVORSENMB,PARKTJ.
Latencyasafunctionofintensityinauditoryneurons:inuencesofcentralprocessing.
Hear.
Res.
148:107–123,2000.
KUWADAS,BATRAR,YINTCT,OLIVERDL,HABERLYLB,STANFORDTR.
Intracellularrecordingsinresponsetomonauralandbinauralstimulationofneuronsintheinferiorcolliculusofthecat.
J.
Neurosci.
17:7565–7581,1997.
LANGNERG,SCHRIENERC,MERZENICHMM.
Covarianceoflatencyandtemporalresolutionintheinferiorcolliculusofthecat.
Hear.
Res.
31:197–202,1987.
LANGNERG,SCHRIENERC.
Periodicitycodingintheinferiorcolli-culusofthecat.
I.
Neuronalmechanisms.
J.
Neurophysiol.
60:1799–1822,1988.
LEBEAUFEN,REESA,MALMIERCAMS.
ContributionofGABA-andglycine-mediatedinhibitiontothemonauraltemporalresponsepropertiesofneuronsintheinferiorcolliculus.
J.
Neurophysiol.
75:902–919,1996.
72FUZESSERYETAL.
:InhibitionandResponseLatencyLIY,EVANSMS,FAINGOLDCL.
Synapticresponsepatternsofneu-ronsinthecortexofratinferiorcolliculus.
Hear.
Res.
137:15–28,1999.
LUY,JENPH-S,ZHENGQ-Y.
GABAergicdisinhibitionchangestherecoverycycleofbatinferiorcolliculusneurons.
J.
Comp.
Physiol.
181:331–341,1997.
MANISPB.
Membranepropertiesanddischargecharacteristicsofguinea-pigdorsalcochlearnucleusneuronsstudiesinvitro.
J.
Neurosci.
10:2338–2351,1990.
MITTMANNDH,WENSTRUPJJ.
Combination-sensitiveneuronsintheinferiorcolliculus.
Hear.
Res.
90:185–191,1995.
NELSONPG,ERULKARSD.
Synapticmechanismsofexcitationandinhibitioninthecentralauditorypathway.
J.
Neurophysiol.
26:908–923,1963.
PARKTJ,POLLAKGD.
GABAshapesatopographicorganizationofresponselatencyinthemustachebat'sinferiorcolliculus.
J.
Neurosci.
13:5172–5187,1993.
PERUZZID,SIVARAMAKRISHNANS,OLIVERDL.
Identicationofcelltypesinbrainslicesoftheinferiorcolliculus.
Neuroscience101:403–416,2000.
POLLAKGD.
Timeistradedforintensityinthebat'sauditorysystem.
Hear.
Res.
36:107–124,1988.
PORTFORSCV,WENSTRUPJJ.
Delay-tunedneuronsintheinferiorcolliculusofthemustachedbat:implicationsforanalysesoftargetdistance.
J.
Neurophysiol.
82:1326–1338,1999.
PORTFORSCV,WENSTRUPJJ.
Topographicaldistributionofdelay-tunedresponsesinthemustachedbatinferiorcolliculus.
Hear.
Res.
151:95–105,2001.
REESA,MOLLERAR.
ResponsesofneuronsintheinferiorcolliculusoftherattoAMandFMtones.
Hear.
Res.
10:301–330,1983.
RHODEWS,SMITHPH.
Physiologicalstudiesonneuronsintheventralcochlearnucleusofthecat.
J.
Neurophysiol.
56:287–307,1986.
SAITOHI,SUGAN.
Longdelaylinesforrangingarecreatedbyin-hibitionintheinferiorcolliculusofthemustachedbat.
J.
Neurophysiol.
74:1–11,1995.
SCHULLERG,BEUTERK,RUBSAMENR.
DynamicpropertiesofthecompensationsystemforDopplershiftsinthebat,Rhinolophusferrumequinum.
J.
Comp.
Physiol.
97:113–125,1975.
SEMPLEMN,AITKINLM.
Physiologyofpathwayfromdorsalcochlearnucleustoinferiorcolliculusrevealedbyelectricalandauditorystimulation.
Exp.
BrainRes.
41:19–28,1980.
SMITHPH.
Anatomyandphysiologyofmultipolarcellsintheratinferiorcollicularcortexusingtheinvitrobrainslicetechnique.
J.
Neurosci.
12:3700–3715,1992.
SNYDERR,LEAKEP,REBSCHERS,BEITELR.
Temporalresolutionofneuronsincatinferiorcolliculustointracochlearelectricalstimulation:Effectsofneonataldeafeningandchronicstimu-lation.
J.
Neurophysiol.
73:449–467,1995.
SULLIVANWE.
PossibleneuronalmechanismsoftargetdistancecodingintheauditorysystemoftheecholocatingbatMyotislucifugus.
J.
Neurophysiol.
48:1573–1626,1982.
SYKAJ,POPELARJ,KVASNAKE,ASTLJ.
Responsepropertiesofneuronsinthecentralnucleusandexternalanddorsalcorticesoftheinferiorcolliculusoftheguineapig.
Exp.
BrainRes.
133:254–266,2000.
VATERM,KOSSLM,HORNAKE.
GAD-andGABA-immunoreactivityintheascendingauditorypathwayofthehorseshoeandmus-tachebats.
J.
Comp.
Neurol.
385:183–206,1992.
WAGNERT.
Lemniscalinputtoidentiedneuronsofthecentralnucleusofmouseinferiorcolliculus:anintracellularbrainslicestudy.
Eur.
J.
Neurosci.
8:1231–1239,1996.
WENSTRUPJJ,LEROYSA.
Spectralintegrationintheinferiorcolli-culus:roleofglycinergicinhibitioninresponsefacilitation.
J.
Neurosci.
21:RC124(1–6),2001.
WENSTRUPJJ,MITTMANNDH,GROSECD.
Inputstocombination-sensitiveneuronsoftheinferiorcolliculus.
J.
Comp.
Neurol.
409:509–528,1999.
WUSH,KELLYJB.
Invitrobrainslicestudiesoftherat'sdorsalnucleusofthelaterallemniscus.
I.
Membraneandsynapticre-sponseproperties.
J.
Neurophysiol.
73:780–793,1995.
YANJ,SUGAN.
Themidbraincreatesandthethalamussharpensecho-delaytuningforthecorticalrepresentationoftarget-dis-tanceinformationinthemustachedbat.
Hear.
Res.
93:101–110,1996.
YANGL,EVANSMS,FAINGOLDCL.
Synapticresponsepatternsofneuronsinthecortexofratinferiorcolliculus.
Hear.
Res.
137:15–28,1999.
YINTCT,HIRSCHJA,CHANJCK.
Responsesinthecatssuperiorcolliculustoacousticstimuli.
II.
Amodelofinterauralintensitysensitivity.
J.
Neurophysiol.
53:746–758,1985.
ZHANGHM,KELLYJB.
AMPAandNMDAreceptorsregulatere-sponsesofneuronsintherat'sinferiorcolliculus.
J.
Neuro-physiol.
86:871–880,2001.
FUZESSERYETAL.
:InhibitionandResponseLatency73
FUZESSERY,1JEFFREYJ.
WENSTRUP,2JIMC.
HALL,3ANDSCOTTLEROY21DepartmentofZoologyandPhysiology,UniversityofWyoming,Laramie,WY82071,USA2DepartmentofNeurobiologyandPharmacology,NortheasternOhioUniversitiesCollegeofMedicine,Rootstown,OH44272,USA3DepartmentofBiochemistryandCellularandMolecularBiology,UniversityofTennessee,Knoxville,TN37996,USAReceived:27November2001;Accepted:3June2002;Onlinepublication:19August2002ABSTRACTTheinferiorcolliculiofallmammalsarecharacter-izedbyawiderangeofrst-spikeresponselatenciesthatcangreatlyexceedtheminimumtimerequiredforthetransmissionofinputthroughthelowerbrainstem.
Themechanismsthataccountforlongresponselatenciesofupto50msareunclear,butonehypothesisisthatanearlyinhibitionplaysaroleinshapinglatency.
Totestthishypothesis,responsela-tenciesweremeasuredintheinferiorcolliculiofthepallidandmustachedbatsbeforeandduringtheblockadeofGABAaandglycinereceptors.
Theeffectofblockinginhibitiononresponselatencywascom-paredunderstimulusconditionsthatproducedtheshortestlatencyinthepredrugcondition.
Multibarrel''piggyback''electrodeswereusedtoiontophoreti-callyapplybicucullineandstrychninesequentiallywhilerecordingfromsingleneurons.
Predruglaten-ciesrangedfrom9to26msinthepallidbatandfrom4to17msinthemustachedbat.
Despitelargein-creasesinresponsemagnitudeandresponsedurationfollowingdisinhibition,theblockadeofinhibitoryreceptorshadmodesteffectsonresponselatency.
Inthepallidbat,blockingGABAreceptorsproducedlatencychangesthatrangedfrom)3.
8to+0.
2ms,whileblockingglycinereceptorsproducedchangesfrom)0.
1to+1.
7ms.
Similarly,inthemustachedbat,blockingGABAreceptorscausedchangesrangingfrom)10.
3to+1.
4ms;blockingglycinereceptorsinthemustachedbatcausedchangesfrom)3.
6to+1.
0ms.
Thelargechangeof)10.
3mswasanexception.
Inbothspecies,themajorityofneuronsshowedchangesof40kHz)arelocatedmoremediallyanddonotextendtothelateralwalloftheIC(Fig.
2).
NeuronsinthelateralIChaveresponselatenciesasshortas5ms,theshortestrecordedintheIC(Fig.
2).
Basedontheaboveestimateofthearrivaltimesofafferentinput,theseresponselatenciesprobablyrepresenttheshortestpossibleresponsetimesinthepallidbatICC.
Incontrast,fewneuronstunedtothesamelow-frequencyrangeinthemedialIChadre-sponselatenciesof50kHz(Fig.
4).
Onlyonehadalowerbestfrequencyof25.
4kHz.
Allneuronswerecombi-nation-sensitive,asdescribedinPortforsandWenst-rup(1999).
Theirpredrugresponselatenciesrangedfrom4.
0to17.
2ms(Fig.
4),arangewhichissimilartothosereportedinpreviousstudiesofneuronstunedtothehigherharmonicsoftheecholocationpulse(HattoriandSuga1997;PortforsandWenstrup2001),butwhichdoesnotincludethemuchlongerlatencies(upto45ms)reportedinthehypertrophied60kHzregionofthemustachedbatinferiorcollicu-lus(ParkandPollak1993).
Inall38neuronstestedinthepallidbat,andin21ofthe52neuronstestedinthemustachedbat,re-sponselatenciesbeforeandduringdisinhibitionweremeasuredovertheirdynamicintensityrangebecauseintensitylevelcanhavealargeeffectonlatency(e.
g.
,IrvineandGago1990;Klugetal.
2000),withlatencytypicallydecreasingwithincreasingintensitylevel(Figs.
5and6,rightcolumns).
ThechangeinlatencyascribedtodisinhibitionwasmeasuredattheintensitylevelthatproducedtheshortestlatencyinFIG.
3.
Left:ThreeelectrodepenetrationsthroughthepallidbatIC,varyinginlateromediallocation.
Right:Thelatenciesandbestfrequenciesofneuronsrecordedinthethreepenetrationsshowingthatlatencyvariedasafunctionofbestfrequencyandlocation.
FIG.
2.
Left:TransversesectionofthepallidbatICshowingisofrequencycontoursandfunctionallydenedmedialandlateralregions(verticaldashedline).
Right:ThedistributionofresponselatenciesinthepallidbatICasafunctionofbestfrequencyandlocationinthemedial(lledcircles)andlateral(opensquares)regionsoftheIC.
NeuronsinthelateralICaretunedto0.
5mswereobservedonlywhenglycinereceptorswereblocked.
Similarchangesinlatencywereobservedacrossbestfrequencies(Fig.
7C)andalsoacrosstherangeofpredruglatencies(Fig.
7A).
TheaveragelatencychangewhenblockingGABAareceptorswas)0.
7ms(range=)3.
8to+0.
2ms,SD=1.
0ms).
Theaveragechangewhenblockingglycinereceptorswas)0.
1ms(range=)1.
3to+1.
7ms,SD=0.
8ms).
Inthemustachedbat,changesinresponselatencyrangedfromadecreaseof10.
3mstoanincreaseof1.
4ms,but,asinthepallidbat,themajorityshowedchangesof0.
65).
RepresentativechangesintemporalresponsepropertiesareshowninFigure8.
Inthepallidbat(Fig.
8,leftcolumn),theneuroninFigure8A,Bshowedanaverage0.
8msdecreaseinlatencyinre-sponsetobicuculline.
Inthepredrugcondition,theneuronredonespike.
Duringbicucullineapplica-tion,itredtwospikesinthisintialburst,therstspikeoccurring0.
5–12msearlierthaninthepredrugcondition,followedbyathirdspikeofirregulartim-FIG.
4.
ThedistributionofnormalresponselatenciesofneuronsintheICsofthepallidbat(left)andmustachedbat(right)priortoblockadeoftheirinhibitoryinputs.
Forthemustachedbat,neuronsinwhichresponselatenciesweretestedoverthedynamicintensityrangeoftheneuronareindicatedbyopencircles.
Thosetestedat10dBabovethresholdareindicatedbylledcircles.
FUZESSERYETAL.
:InhibitionandResponseLatency65ing.
Bicucullineappearedtoreleasethisneuronfrominhibitionthatoccurredbothbeforeandafterthenormalexcitation.
DisinhibitionbybicucullinehadsimilarbutgreatereffectsontheneuroninFigure8C,D.
Itsometimesredarstspikeapproximately2msearlierthaninthepredrugcondition,butbecausethisearlyresponsewasinconsistent,itsaveragela-tencywaslongerandtherewasonlya0.
8msdiffer-enceinmeanlatenciesunderthetwoconditions.
Thetimingofthesecondpeakofrst-spiketimingduringbicucullineapplicationissimilartothatoftherstpeakinthepredrugcondition.
ThisresultsuggeststhatbicucullineblockedanearlyGABAergicinhi-bitionthatretardedtheresponselatencyofthisneuron.
Asinthepreviousneuron(Fig.
8A,B),bic-ucullinealsoremovedalateinhibitionandgreatlyincreasedtheresponseduration.
TheneuroninFigure8E–Gwassequentiallyex-posedtobicucullineandstrychnine.
Bicucullinein-creasedthedurationandmagnitudeofitsresponseanddecreasedresponselatencyanaverageof1.
0ms(Fig.
8G).
Incontrast,strychnineincreaseditslatencybyanaverageof1.
7ms(Fig.
8F).
AswastypicallytheFIG.
5.
FourneuronsfromthepallidbatICshowingtheeffectofblockingGABAergicinputwithbicuculline(Bic)orglycinergicinputwithstrychnine(Strych)relativetothepredrug(Pre)condition.
Leftcolumnshowstheeffectofdisinhibitiononintensity–ratefunctions.
Rightcolumnshowstheeffectofresponselatencyasafunctionofintensitylevel.
Thearrowsintherightcolumnindicatetheintensitylevelatwhichchangesinresponselatencyweremeasured.
Thesamesymbolsareusedinthepairsofguresintheleftandrightcolumnstoindicatedrugandpredrugconditions.
66FUZESSERYETAL.
:InhibitionandResponseLatencycase,bicucullineincreasedresponsemagnitudemorethanstrychnine.
Similarchangesinpoststimulustimehistogramswereseeninthemustachedbat(Fig.
8,rightcol-umn).
Figure8H,Ishowsthelargestlatencychangeobservedafterapplicationofanantagonistinthisstudy,10.
3ms.
Incontroltests,theunitrespondedonaveragewithonespiketothebestfrequencystimulus.
Therstspikeoccurredaslittleas11msafterstim-ulusonset,but,becauseofthehighvariability(SD=6.
0ms),themedianlatencywas17.
5ms.
Withbicuculline,theresponserateincreasedbyafactorof5.
Theearliestlatenciesdecreasedto7msandthedistributionoflatencieswasmuchtighter(SD=1.
1ms,medianlatency=7.
2ms).
Thus,bicucullinere-vealedtwolargeeffectsofGABAainhibitiononla-tency:adecreaseintherst-spiketimingandanincreaseintheconsistencyoftherstspike.
Suchlargecombinedeffectswerenotseeninotherunits.
Figure8J,Kshowsaverysmalleffectofbicucullineonlatencydespiteamajoreffectonresponsemagni-tude.
Theunit(alsoshowninFig.
6G,H)respondedweaklyinthecontrolcondition,butthelatencyofresponsevariedlittle(SD=0.
8ms,medianlaten-FIG.
6.
FourneuronsfromthemustachedbatIC.
ThesameconventionasinFig.
4wasusedtoshowtheeffectsofbicucullineand/orstrychnineonintensity–ratefunctionsandintensity–latencyfunctions.
FUZESSERYETAL.
:InhibitionandResponseLatency67cy=8.
4ms).
Withbicuculline,theresponseratein-creasedbyafactorof6butneitherthelatencynoritsvariabilitychangedmuch(SD=0.
6ms,medianlaten-cy=8.
7ms).
TheunitinFigure8L–Nshowedonlysmalleffectsofbothbicucullineandstrychnineonresponsela-tency(seealsoFig.
6C,D).
Theunit'scontrolre-sponsewasweak,partlyaresultofinhibitionoccurringathigherstimuluslevels.
Medianrstspikelatencywas10.
7ms,withlittlevariation(SD=1.
2ms).
Withstrychnine,theresponselevelroughlydoubled,butrst-spikelatencyremainednearlythesamewithlittlevariation(SD=1.
3ms,medianla-tency=10.
6ms).
Withbicuculline,theresponserateincreasedfurther,butthemedianrst-spikelatencydecreasedtoonly9.
8ms(SD=1.
5ms).
DISCUSSIONThisstudyfocusedontheroleofGABAandglycin-ergicinputinregulatingtherst-spikeresponsela-tenciesofICneurons.
Inbothspeciestested,inhibitionhadpronouncedeffectsonthedurationandmagnitudeofresponses,aswellasontheshapesofintensity–ratefunctions,butonlymodesteffectsonresponselatency.
Themajorityofneuronsshowedlatencyshiftsof5ms)inresponselatencywereob-servedintheguineapig(LeBeauetal.
1996),theywerespecicallyassociatedwithneuronswithsus-tainedresponses.
Neuronselicitingphasicburstsatonsettypicallyshowedlesschange.
ParkandPollak(1993)alsonotedthelargestlatencychangesinneuronswithsustaineddischargesandsuggestedthatitwasbecauseGABAergicdisinhibitionallowstheirrstspikestooccurinatightertemporalregister,therebydecreasingtheiraverageresponselatencies.
Thiswouldsuggestthatinhibitiondecreasestheoverallresponsivenessofthesetonicrespondersbutdoesnotnecessarilydeterminerst-spikelatency.
Incontrasttothesemodesteffects,ParkandPollak(1993)reportedthat17%ofneuronsinthehyper-trophied60kHzrepresentationofmustachedbatICshoweddecreasesrangingfrom5to25ms.
NeuronswiththelargestdecreasesinlatencywereOFFre-sponders(neuronsthatrespondedonlyattheendofastimulus)thatwereconvertedtoONresponderswhendisinhibited.
Suchneuronscanbediscountedfromthepresentdiscussionsincetheirresponsela-tenciesaredependentonstimulusduration,andla-tencychangesobservedfollowingdisinhibitionwillFIG.
8.
Representativeexamplesofthepoststimulustimehistogramsofneuronsfromthepallidbat(leftcolumn)andmustachedbat(rightcolumn)beforeandduringdisinhibition.
Theverticaldashedlinesshowmeanrst-spikelatenciesinthepallidbatandmedianrst-spikelatenciesinthemustachedbatfordifferentconditions(seetext).
Allneuronsweretestedwithstimulusparametersthatevokedthegreatestresponsesandshortestresponselatencies.
Forthepallidbat(leftcolumn)allweretestedwithbestfrequencytonesat15–20dBaboveresponsethreshold.
Sincetwooftheseneurons(A,BandE–G)weredurationselective,theyweretestedattheirbestdurationsof5and1ms,respectively.
TheneuroninC,Dwastestedwitha10msdurationtone.
FUZESSERYETAL.
:InhibitionandResponseLatency69alsodependuponstimulusduration.
However,thiscannotaccountforallofthelargedecreasesobservedinthemustachedbat.
AtrendnotedbyParkandPollak(1993)isthatneuronsinthedorsalpartofthe60kHzrepresentationintheIChadthelongestla-tencies(seealsoHattoriandSuga1997),andthispopulationshowedthegreatesteffectofdisinhibitiononresponselatency.
Presentresultsfromneuronsinthemustachedbat's60kHzrepresentationshowin-dicationsofasimilartrend(Fig.
6B);thelongestla-tencyneuronstunedto60kHzweretheonlyonestoshowlatencydecreasesof3msormorefollowingremovalofinhibition.
Ifmore60kHzneuronswithlongerresponselatencieshadbeenincluded,ourresultsmightbemoresimilartothoseofParkandPollak.
Therewasnoevidenceofthistrendamongneuronstunedinthe72–95kHzrangeinmustachedbats,orinthepallidbat(Fig.
6A).
Basedontheirdata,ParkandPollak(1993)pro-posedthatGABAergicinhibitionshapeslatencymapsthroughoutthetonotopicallyorganizedIC.
Theyfurthermoreproposedthattheorganizationofla-tencyintheICcouldprovidedelaylinesnecessaryfortheconstructionofcoincidencedetectorsselectiveforpulse-echodelay(seealsoSaitohandSuga1995;HattoriandSuga1997).
Althoughtherangeofla-tenciesinthe60kHzrepresentationofthemus-tachedbatICisnotunusual,norisitsorganizationoflatency,thedependenceoflatencyoninhibitionisremarkable.
Thisledtospeculationthatthedepen-denceoflatencyoninhibitionisaspecializedfeatureusedbythemustachedbatfortheconstructionofcoincidencedetectorstoanalyzesonarechoes(LeBeauetal.
1996).
However,resultsofseveralrecentstudiesshowthat(1)coincidencedetectorsarecre-atedintheIC,notinthemedialgeniculatebodyastheseproposalsrequire(MittmannandWenstrup1995;WenstrupandLeroy2001);(2)latencymapsintheIChavenoapparentrelationshipwiththedelaytuningofcoincidencedetectorsintheIC(PortforsandWenstrup1999,2001;Wenstrupetal.
1999);and(3)amongthedelay-tunedneuronsrecordedfromthemustachedbat'sICinthepresentstudy(withBFsof72–89kHz),neitherGABAergicnorglycinergicinhibitionappearstocontributesignicantlytothelatencyofresponsetoBFsignals.
Moregenerally,thepresentresultsinbats,asinothermammals,showthatGABAergicandglycinergicinhibitionsdonotcontributesignicantlytoresponselatencyinthegreatmajorityofthepopulationofICneurons.
MechanismsshapingresponselatencyAshorteningofresponselatencyfollowingtheblockadeofGABAaorglycinereceptors,regardlessofthemagnitudeoftheeffect,indicatesthatashort-latencyinhibitoryinput,eitherprecedingorcoin-cidingwiththearrivalofexcitatoryinput,isabletocounterdepolarizationanddelayspikegeneration(ParkandPollak1993;Halpeaetal.
1994;SaitohandSuga1995;CassedayandCovey1995).
PrecedingIPSPsintheIChavebeenobservedinwhole-cellpatch-clamp(Coveyetal.
1996)andintracellular(NelsonandErulkar1963;Kuwadaetal.
1997)re-cordingsaswellasintracellularbrainslicerecordings(Smith1992;Wagner1996;Lietal.
1999).
TheseearlyIPSPswereobservedevenwhenaneuronwaspresentedwithitsmostexcitatorystimulus(Cassedayetal.
1994;Coveyetal.
1996).
Inadditiontoinu-encingtheonsetofaresponse,inhibitionalsoshapesitsdurationandoffset,sinceblockinginhibitionof-tenconvertsneuronswithphasicresponsestotonicresponderswhoseresponsedurationslastwellbeyondthestimulusduration(e.
g.
,Fig.
8).
Thislattereffectofdisinhibitionistypicallymoreprofoundthantheeffectonrst-spikelatency.
Inadditiontodecreasingresponselatency,block-inginhibitoryreceptorscanalsoincreaselatencybyupto3ms.
Inthepresentstudyofthepallidbat,thisoccurredonlywhenglycinergicinputwasblocked.
Inthemustachedbat,thisoccurredfollowingblockadeofeitherglycinergicorGABAergicinput.
Inbothspecies,neuronswereobservedthatshowedde-creasedlatenciesinresponsetobicucullineandin-creasedlatenciesinresponsetostrychnine.
Otherstudies(ParkandPollak1993;Luetal.
1997)haveobservedthiswhenblockingGABAergicinput.
Whyresponselatenciesshouldincreaseduringdisinhibi-tionisnotclear.
Onepossibilityisthatinhibitoryin-putsthemselvesaresubjecttoinhibition,andareleasefromthisinhibitionwouldallowtheinhibi-toryinputtoexertagreaterinhibitoryeffectontherecordedneuron.
If,forexample,alocalGABAergicinhibitoryneuronclosetothesiteofdrugapplicationreceivedinhibitionfromaglycinergicinput,thentheapplicationofstrychninewouldreleasetheinhibitoryneuronfrominhibition,allowingittoexertagreaterinhibitoryeffectontherecordedneuronandin-creaseitsresponselatency.
Asecondpossiblemechanismisthatareboundfrominhibitionmaycontributetoexcitationinsomeneurons.
Thus,eliminationofanearlyinhibitioncouldslowdepolarizationandincreaselatency.
In-creasedexcitationfollowinghyperpolarizationhasbeenreportedinvitrointhecochlearnucleus(Manis1990)andinthedorsalnucleusofthelaterallem-niscus(WuandKelly1995),aswellasintheIC(Smith1992;Perruzietal.
2000).
Invivo,basedonwhole-cellpatch-damprecordings,Cassedayetal.
(1994)haveproposedthatareboundfromearlyin-hibition,coincidingwitharrivalofalateexcitatoryinput,mayunderlieaselectivityforsoundduration70FUZESSERYETAL.
:InhibitionandResponseLatencyobservedintheICofthebigbrownbat.
Additionalevidencethatinhibitionmaycontributetoexcitationintheinferiorcolliculuscomesfromarecentstudyofcombination-sensitiveneuronsinthemustachedbat(WenstrupandLeroy2001).
Thefacilitatedresponseproducedbyappropriatecombinationsoftonesiseliminatedbyblockinginhibitoryglycinergicinput.
Thisparadoxicalresultcouldbetheresultofblock-ingapostinhibitoryreboundthatcontributestoex-citation.
Inhibitioncanindeedinuencetheresponsela-tencyofICneurons,but,withtheexceptionoflong-latencyneuronsinthemustachedbat(ParkandPo-llak1993),therathersmalleffectsobservedinotherspeciesafterblockinginhibitioncannotaccountforthewiderangeoflatenciespresentinthemidbrain.
Othermechanismsrequiregreaterattention.
Wavepropogationtimesalongthecochlearbasilarmem-branecancontributetoashort,frequency-dependentdelayofaround1ms,withhigher-frequencyinputarrivingrst(RhodeandSmith1986).
TheinuenceofthiscochleardelayonICresponselatenciesismostlikelyinsignicant,particularlyinthelateralregionofthepallidbatIC,whereneuronswithlowbestfre-quencieshavetheshortestresponselatencies.
Whiletherangeoflatenciesatlowfrequencieshasbeenfoundtobebroader,minimumlatencieshavebeenfoundtobelargelyindependentoffrequencytuning(e.
g.
,LangnerandSchriener1988;Halpeaetal.
1994).
Otherfactorsthatcaninuenceresponselatencyareaxonlength,conductionvelocity,thenumberofsynapsesalonganascendingpathway,andsynapticintegrationtimes.
ThecontributionofeachofthesefactorsinICresponselatenciesislikelytovaryacrossneurons.
Forexample,studiesthatusedelectricalstimulationoftheauditorynerve(Snyderetal.
1995)anddorsalcochlearnucleus(SempleandAitkin1980)inplaceofnormalacousticstimulationreportawiderangeofresponselatenciesintheIC,leadingtotheconclusionthatitisunlikelythataxonlengthsorthenumberofintercalatedsynapsescouldaccountfor10–20msdifferencesinresponselatenciesintheIC(Snyderetal.
1995).
Theincreaseinresponsela-tenciesalongtheaxisoftheIC(e.
g.
,LangnerandSchriener1988;HattoriandSuga1997)isdifculttoaccountforthroughadditionaltraveltime.
ThissamegradientofresponselatencieswasobservedinthepallidbatIC,wheremorethan10msincreasesinlatencyoccuroveraseparationofonly1.
5mm.
Instead,muchofthedelayinresponseonsetmayresideinmechanismsthatareintrinsictotheIC.
IntracellularbrainslicerecordingsoftheICC(Wag-ner1996)reportlongresponselatencies(to12ms),evenwhenneuronsareexcitedbyelectricalstimula-tionofthelaterallemniscusimmediatelyventraltotheIC.
InthecorticesofthecatIC,regionsthatre-ceiveprimarilyintracollicularandneocorticalinputs,excitatorypostsynapticpotentials(EPSP)latenciesofupto11mswerereportedfollowingstimulationofthecommissureoftheIC(Smith1992).
Theselonglatenciesfollowingintracollicularelectricalstimula-tionsuggestthatmultisynapticcircuitsand/orlongsynapticintegrationtimeswithintheICcanpoten-tiallyimposeadelayinresponsethatcanbelongerthanthetimerequiredforthetransmissionofin-formationfromthecochleatotheIC.
Indeed,Halpeaetal.
(1994)emphasizedthat,inthebigbrownbat,theminimumresponselatenciesfromthelevelsofthecochlearnucleustothelaterallemniscalnucleitotheICdonotincreaseappreciably.
Rather,itistherangeofresponselatenciesthatincreasesdramati-callyasoneascendstheauditorybrainstem.
OnemechanismthatmaycontributetothewidelatencyrangeisadifferenceinthetypesofexcitatoryandinhibitoryreceptortypesacrosstheICandthemannerinwhichtheyinteracttocontrolthetimingofsynapticintegration.
AMPAandNMDAglutamatereceptors,forexample,arereportedtoactivatefastandslowpostsynapticexcitatorypotentials,respec-tively(Yangetal.
1999;KellyandKidd2000;ZhangandKelly2001).
Similarly,GABAaandGABAbre-ceptorsappeartoberesponsibleforinhibitorypo-tentialswithdifferenttimecourses(CrunelliandLeresche1991),andarereportedtobedifferentiallydistributedacrosstheIC(Vateretal.
1992;Fubaraetal.
1996).
SincethepresentstudyexaminedtherolesofonlyfastinhibitoryeventsmediatedbyGABAaandglycinereceptors,thepossiblerolesofotherreceptortypesmediatingslower,longer-lastingexcitatoryandinhibitoryeventsinshapingresponselatencywerenotrevealed.
Inconclusion,thisreportdoesnotquestiontheimportanceofinhibitioninregulatingthetimingofresponsesinthecentralauditorysystem.
Thereisgrowingevidencethatthetimingofinhibitoryandexcitatoryinputsplaysanimportantroleinshapingresponseselectivityfortemporalfeatures,suchasduration,andforcomplex,behaviorallyrelevantsounds,particularlythosewithcomponentsthataredelayedintime.
Moreover,neuronswithOFFre-sponsesduetoinhibitionimposedduringthecourseofasoundwillexhibitlongdelaysthatvarywithsoundduration.
Rather,thepresentstudyexaminedICneuronsthatrespondedatsignalonset,someofwhichhadlongrst-spikelatencies(10–25ms)thatfarexceededtheminimumtimerequiredforinputtotheIC.
Weaskedwhethertheselatenciescouldbefurtherdecreasedbytheblockadeofinhibitoryin-putswhencarehadbeentakentoensurethatthesoundspresentedevokedresponsesattheshortestpossiblelatenciespriortodisinhibitionbyinhibitoryFUZESSERYETAL.
:InhibitionandResponseLatency71transmitterblockers.
Despitelargeeffectsonre-sponsemagnitudeandduration,theblockadeofGABAaandglycinereceptorstypicallyproducedsmallchangesinresponselatency(<1ms).
Thisnding,coupledwiththosefrommostspecies,sug-geststhatfastinhibitorypathwaysmaynotplayadominantroleincreatingthewiderangeofresponselatenciesobservedinthemammalianIC.
ACKNOWLEDGMENTSWethankCarolGrosefortechnicalassistanceandTerriZumstegforeditingthemanuscript.
WearegratefultotheWildlifeSectionoftheMinistryofAgriculture,Land,andMarineResourcesofTrinidadandTobagoforpermissiontoexportmustachedbats.
ResearchwassupportedbyfundsfromtheNationalInstitutesofHealth(RO1DC00054toZMFandRO1DC00937toJJW)andtheNationalScienceFoundation(IBN-9828599toZMF).
REFERENCESBROWNP.
Vocalcommunicationinthepallidbat,Antrozouspallidus.
Z.
Tierpsychol.
41:34–54,1976.
CARNEYLH,YINTCT.
Responsesoflow-frequencycellsintheinfe-riorcolliculustointerauraltimedifferencesinclicks:excitatoryandinhibitorycomponents.
J.
Neurophysiol.
62:144–161,1989.
CASSEDAYJH,EHRLICHD,COVEYE.
Neuraltuningforsounddura-tion:roleofinhibitorymechanismsintheinferiorcolliculus.
Science264:847–850,1994.
CASSEDAYJH,COVEYE.
Mechanismsfortheanalysisofauditorytemporalpatternsinthebrainstemofecholocatingbats.
In:COVEYE,HAWKINGSHL,PORTRF(eds)NeuralRepresentationsofTemporalPatternsPlenum,NewYork,1995,pp25–52CASSEDAYJH,COVEYE.
Aneuroethologicaltheoryoftheoperationoftheinferiorcolliculus.
BrainBehav.
Evol.
47:311–336,1996.
COVEYE,KAUERJA,CASSEDAYJH.
Whole-cellpatch-clamprecordingrevealssubthresholdsound-evokedpostsynapticcurrentsintheinferiorcolliculusofawakebats.
J.
Neurosci.
16:3009–3018,1996.
CRUNELLIV,LERESCHEN.
AroleforGABAbreceptorsinexcitationandinhibitionofthalamocorticalcells.
TrendsNeurosci.
14:16–21,1991.
DEARSP,SIMMONSJA,FRITZJ.
Apossibleneuronalbasisfortherepresentationofacousticscenesinauditorycortexofthebigbrownbat.
Nature364:620–623,1993.
DEARSP,SUGAN.
Delay-tunedneuronsinthemidbrainofthebigbrownbat.
J.
Neurophysiol.
73:1084–1100,1995.
EHRETG,ROMANDR.
Developmentoftoneresponsethresholds,latenciesandtuninginthemouseinferiorcolliculus.
Dev.
BrainRes.
67:317–326,1992.
FENGAS,SIMMONSJA,KICKSA.
Echodetectionandtarget-rangingneuronsintheauditorysystemofthebat,Eptesicusfuscus.
Sci-ence202:645–648,1978.
FERRAGAMOMJ,HARESIGNT,SIMMONSJA.
Frequencytuning,laten-ciesandresponsestofrequency-modulatedsweepsintheinfe-riorcolliculusoftheecholocatingbat,Eptesicusfuscus.
J.
Comp.
Physiol.
182:65–79,1998.
FITZPATRICKDC,KUWADAS,BATRAR,TRAHIOTISC.
Neuralresponsestosimplesimulatedechoesintheauditorybrainstemoftheunanesthetizedrabbit.
J.
Neurophysiol.
74:2469–2486,1995.
FUBARABM,CASSEDAYJH,COVEYE,SCHARTZ-BLOOMRD.
DistributionofGABAA,GABABandglycinereceptorsinthecentralauditorysystemofthebigbrownbat,Eptesicusfuscus.
J.
Comp.
Neurol.
369:83–92,1996.
FUZESSERYZM,BUTTENHOFFP,ANDREWSB,KENNEDYJM.
Passivesoundlocalizationofpreybythepallidbat(Antrozousp.
palli-dus).
J.
Comp.
Physiol.
171:767–777,1993.
FUZESSERYZM.
Acutesensitivitytointerauraltimedifferencesintheinferiorcolliculusofabatthatreliesofpassivesoundlocaliza-tion.
Hear.
Res.
109:46–62,1997.
FUZESSERYZM,HALLJC.
Sounddurationselectivityinthepallidbatinferiorcolliculus.
Hear.
Res.
137:137–154,1999.
HALPEAS,COVEYE,CASSEDAYJH.
Frequencytuningandre-sponselatenciesatthreelevelsinthebrainstemoftheecholo-catingbat,Eptesicusfuscus.
J.
Comp.
Physiol.
174:671–682,1994.
HARRISONRV,PALMERAR.
Neuroneresponselatencyintheinferiorcolliculusinrelationtotheauditorybrainstemresponses(ABR)intheguineapig.
Scand.
Audiol.
13:275–281,1984.
HATTORIT,SUGAN.
Theinferiorcolliculusofthemustachedbathasfrequency-vs-latencycoordinates.
J.
Comp.
Physiol.
180:271–284,1997.
HAVEYDL,CASPARYDM.
Asimpletechniqueforconstructing''piggy-back''multibarrelmicroelectrodes.
Electroencephalogr.
Clin.
Neurophysiol.
48:249–251,1980.
HEILP,NEUBAUERH.
Temporalintegrationofsoundpressurede-terminesthresholdsofauditory-nervebers.
J.
Neurosci.
27:7404–7415,2001.
HENSONOW,POLLAKGD,KOBLERJB,HENSONMM,GOLDMANLJ.
Cochlearmicrophonicpotentialselicitedbybiosonarinyingbats,Pteronotusp.
parnelli.
Hear.
Res.
7:127–147,1982.
IRVINEDR,GAGOG.
Binauralinteractionsinhigh-frequencyneu-ronsininferiorcolliculusofthecat:effectsofvariationsinsoundpressurelevelonsensitivitytointerauralintensitydif-ferences.
J.
Neurophysiol.
63:570–591,1990.
JENPH-S,SCHLEGELPA.
Auditoryphysiologicalpropertiesoftheneuronsintheinferiorcolliculusofthebigbrownbat,Eptesicusfuscus.
J.
Comp.
Physiol.
147:351–363,1982.
JOHNSONPABR.
GABAergicandglycinergicinhibitioninthecen-tralnucleusoftheinferiorcolliculusofthebigbrownbat.
PhDthesis,DukeUniversity,Durham,NC,1993.
KELLYJB,KIDDSA.
NMDAandAMPAreceptorsinthedorsalnu-cleusofthelaterallemniscusshapebinauralresponsesinratinferiorcolliculus.
J.
Neurophysiol.
83:1403–1414,2000.
KITZESLM,GIBSONMM,ROSEJE,HINDJE.
Initialdischargelatencyandthresholdconsiderationsforsomeneuronsinthecochlearnucleuscomplexofthecat.
J.
Neurophysiol.
41:1165–1182,1978.
KLUGA,KHANA,BURGERRM,BAUEREE,HURLEYLM,YANGL,GROTHEB,HALVORSENMB,PARKTJ.
Latencyasafunctionofintensityinauditoryneurons:inuencesofcentralprocessing.
Hear.
Res.
148:107–123,2000.
KUWADAS,BATRAR,YINTCT,OLIVERDL,HABERLYLB,STANFORDTR.
Intracellularrecordingsinresponsetomonauralandbinauralstimulationofneuronsintheinferiorcolliculusofthecat.
J.
Neurosci.
17:7565–7581,1997.
LANGNERG,SCHRIENERC,MERZENICHMM.
Covarianceoflatencyandtemporalresolutionintheinferiorcolliculusofthecat.
Hear.
Res.
31:197–202,1987.
LANGNERG,SCHRIENERC.
Periodicitycodingintheinferiorcolli-culusofthecat.
I.
Neuronalmechanisms.
J.
Neurophysiol.
60:1799–1822,1988.
LEBEAUFEN,REESA,MALMIERCAMS.
ContributionofGABA-andglycine-mediatedinhibitiontothemonauraltemporalresponsepropertiesofneuronsintheinferiorcolliculus.
J.
Neurophysiol.
75:902–919,1996.
72FUZESSERYETAL.
:InhibitionandResponseLatencyLIY,EVANSMS,FAINGOLDCL.
Synapticresponsepatternsofneu-ronsinthecortexofratinferiorcolliculus.
Hear.
Res.
137:15–28,1999.
LUY,JENPH-S,ZHENGQ-Y.
GABAergicdisinhibitionchangestherecoverycycleofbatinferiorcolliculusneurons.
J.
Comp.
Physiol.
181:331–341,1997.
MANISPB.
Membranepropertiesanddischargecharacteristicsofguinea-pigdorsalcochlearnucleusneuronsstudiesinvitro.
J.
Neurosci.
10:2338–2351,1990.
MITTMANNDH,WENSTRUPJJ.
Combination-sensitiveneuronsintheinferiorcolliculus.
Hear.
Res.
90:185–191,1995.
NELSONPG,ERULKARSD.
Synapticmechanismsofexcitationandinhibitioninthecentralauditorypathway.
J.
Neurophysiol.
26:908–923,1963.
PARKTJ,POLLAKGD.
GABAshapesatopographicorganizationofresponselatencyinthemustachebat'sinferiorcolliculus.
J.
Neurosci.
13:5172–5187,1993.
PERUZZID,SIVARAMAKRISHNANS,OLIVERDL.
Identicationofcelltypesinbrainslicesoftheinferiorcolliculus.
Neuroscience101:403–416,2000.
POLLAKGD.
Timeistradedforintensityinthebat'sauditorysystem.
Hear.
Res.
36:107–124,1988.
PORTFORSCV,WENSTRUPJJ.
Delay-tunedneuronsintheinferiorcolliculusofthemustachedbat:implicationsforanalysesoftargetdistance.
J.
Neurophysiol.
82:1326–1338,1999.
PORTFORSCV,WENSTRUPJJ.
Topographicaldistributionofdelay-tunedresponsesinthemustachedbatinferiorcolliculus.
Hear.
Res.
151:95–105,2001.
REESA,MOLLERAR.
ResponsesofneuronsintheinferiorcolliculusoftherattoAMandFMtones.
Hear.
Res.
10:301–330,1983.
RHODEWS,SMITHPH.
Physiologicalstudiesonneuronsintheventralcochlearnucleusofthecat.
J.
Neurophysiol.
56:287–307,1986.
SAITOHI,SUGAN.
Longdelaylinesforrangingarecreatedbyin-hibitionintheinferiorcolliculusofthemustachedbat.
J.
Neurophysiol.
74:1–11,1995.
SCHULLERG,BEUTERK,RUBSAMENR.
DynamicpropertiesofthecompensationsystemforDopplershiftsinthebat,Rhinolophusferrumequinum.
J.
Comp.
Physiol.
97:113–125,1975.
SEMPLEMN,AITKINLM.
Physiologyofpathwayfromdorsalcochlearnucleustoinferiorcolliculusrevealedbyelectricalandauditorystimulation.
Exp.
BrainRes.
41:19–28,1980.
SMITHPH.
Anatomyandphysiologyofmultipolarcellsintheratinferiorcollicularcortexusingtheinvitrobrainslicetechnique.
J.
Neurosci.
12:3700–3715,1992.
SNYDERR,LEAKEP,REBSCHERS,BEITELR.
Temporalresolutionofneuronsincatinferiorcolliculustointracochlearelectricalstimulation:Effectsofneonataldeafeningandchronicstimu-lation.
J.
Neurophysiol.
73:449–467,1995.
SULLIVANWE.
PossibleneuronalmechanismsoftargetdistancecodingintheauditorysystemoftheecholocatingbatMyotislucifugus.
J.
Neurophysiol.
48:1573–1626,1982.
SYKAJ,POPELARJ,KVASNAKE,ASTLJ.
Responsepropertiesofneuronsinthecentralnucleusandexternalanddorsalcorticesoftheinferiorcolliculusoftheguineapig.
Exp.
BrainRes.
133:254–266,2000.
VATERM,KOSSLM,HORNAKE.
GAD-andGABA-immunoreactivityintheascendingauditorypathwayofthehorseshoeandmus-tachebats.
J.
Comp.
Neurol.
385:183–206,1992.
WAGNERT.
Lemniscalinputtoidentiedneuronsofthecentralnucleusofmouseinferiorcolliculus:anintracellularbrainslicestudy.
Eur.
J.
Neurosci.
8:1231–1239,1996.
WENSTRUPJJ,LEROYSA.
Spectralintegrationintheinferiorcolli-culus:roleofglycinergicinhibitioninresponsefacilitation.
J.
Neurosci.
21:RC124(1–6),2001.
WENSTRUPJJ,MITTMANNDH,GROSECD.
Inputstocombination-sensitiveneuronsoftheinferiorcolliculus.
J.
Comp.
Neurol.
409:509–528,1999.
WUSH,KELLYJB.
Invitrobrainslicestudiesoftherat'sdorsalnucleusofthelaterallemniscus.
I.
Membraneandsynapticre-sponseproperties.
J.
Neurophysiol.
73:780–793,1995.
YANJ,SUGAN.
Themidbraincreatesandthethalamussharpensecho-delaytuningforthecorticalrepresentationoftarget-dis-tanceinformationinthemustachedbat.
Hear.
Res.
93:101–110,1996.
YANGL,EVANSMS,FAINGOLDCL.
Synapticresponsepatternsofneuronsinthecortexofratinferiorcolliculus.
Hear.
Res.
137:15–28,1999.
YINTCT,HIRSCHJA,CHANJCK.
Responsesinthecatssuperiorcolliculustoacousticstimuli.
II.
Amodelofinterauralintensitysensitivity.
J.
Neurophysiol.
53:746–758,1985.
ZHANGHM,KELLYJB.
AMPAandNMDAreceptorsregulatere-sponsesofneuronsintherat'sinferiorcolliculus.
J.
Neuro-physiol.
86:871–880,2001.
FUZESSERYETAL.
:InhibitionandResponseLatency73
- suspiggycase相关文档
- 研究piggycase
- distinctpiggycase
- systemspiggycase
- Limpiggycase
- servicepiggycase
- sensitivepiggycase
华纳云新人下单立减40元/香港云服务器月付60元起,香港双向CN2(GIA)
华纳云(HNCloud Limited)是一家专业的全球数据中心基础服务提供商,总部在香港,隶属于香港联合通讯国际有限公司,拥有香港政府颁发的商业登记证明,保证用户的安全性和合规性。 华纳云是APNIC 和 ARIN 会员单位。主要提供数据中心基础服务、互联网业务解决方案, 以及香港服务器租用、香港服务器托管、香港云服务器、美国云服务器,云计算、云安全技术研发等产品和服务。其中云服务器基于成熟的 ...

Megalayer促销:美国圣何塞CN2线路VPS月付48元起/香港VPS月付59元起/香港E3独服月付499元起
Megalayer是新晋崛起的国外服务器商,成立于2019年,一直都处于稳定发展的状态,机房目前有美国机房,香港机房,菲律宾机房。其中圣何塞包括CN2或者国际线路,Megalayer商家提供了一些VPS特价套餐,譬如15M带宽CN2线路主机最低每月48元起,基于KVM架构,支持windows或者Linux操作系统。。Megalayer技术团队行业经验丰富,分别来自于蓝汛、IBM等知名企业。Mega...

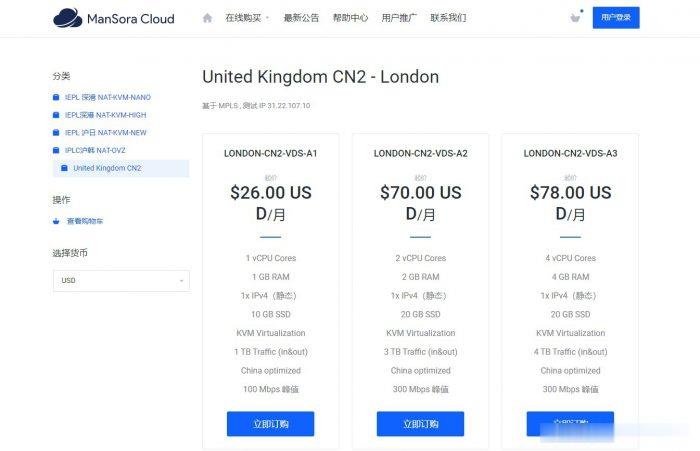
ManSora:英国CN2 VPS,1核/1GB内存/10GB SSD/1TB流量/100Mbps/KVM,$18.2/月
mansora怎么样?mansora是一家国人商家,主要提供沪韩IEPL、沪日IEPL、深港IEPL等专线VPS。现在新推出了英国CN2 KVM VPS,线路为AS4809 AS9929,可解锁 Netflix,并有永久8折优惠。英国CN2 VPS,$18.2/月/1GB内存/10GB SSD空间/1TB流量/100Mbps端口/KVM,有需要的可以关注一下。点击进入:mansora官方网站地址m...
piggycase为你推荐
-
www.hao360.cn主页设置为http://hao.360.cn/,但打开360浏览器先显示www.yes125.com后转换为www.2345.com,搜索注册表和关键字关键词编故事百花百游百花百游的五滴自游进程www.522av.com现在怎样在手机上看AVwww.bbb551.comHUNTA551第一个第二个妹子是谁呀??广告法请问违反了广告法,罚款的标准是什么www.36ybyb.com有什么网址有很多动漫可以看的啊?我知道的有www.hnnn.net.很多好看的!但是...都看了!我想看些别人哦!还有优酷网也不错...www.175qq.com最炫的qq分组梦遗姐我姐姐很漂亮,她24了,我才15,晚上我和他睡在一起,我经常挨遗精,咋办?彪言彪语()言() 语