generating16668.com
16668.com 时间:2021-03-19 阅读:()
Rac-RelatedGTP-BindingProteininElicitor-InducedReactiveOxygenGenerationbySuspension-CulturedSoybeanCells1JumokPark,Hyun-JungChoi,SuminLee,TaehoonLee,ZhenbiaoYang,andYoungsookLee*DivisionofMolecularLifeScience,PohangUniversityofScienceandTechnology,Pohang,790–784,Korea(J.
P.
,H.
-J.
C.
,S.
L.
,T.
L.
,Y.
L.
);andDepartmentofBotanyandPlantSciences,UniversityofCalifornia,Riverside,California92521–0124(Z.
Y.
)Plantcellsproducereactiveoxygenspecies(ROS)inresponsetomanystimuli.
However,themechanismofROSbiosyn-thesisremainsunclear.
Wehaveexploredthehypothesisthatthesuperoxideburstinplantsmechanisticallyresemblestheoxidativeburstinneutrophils.
FirstwehaveconfirmedthatROSproduction,whichoccursinsuspension-culturedsoybean(Glycinemax)cellsinresponsetohypo-osmoticshock,isinhibitedbydiphenyleneiodonium,aninhibitoroftheflavin-dependentoxidaseofneutrophils.
BecauseaRacfamilyGproteinisanessentialregulatorofthisNADPHoxidase,andbecausemanyplanthomologsofRachavebeencloned,wenextexaminedwhetherRac-likeproteinsmightbeinvolvedintheoxidativeburstinthesoybeancells.
WeidentifiedaRac-like21-kDsoybeanproteinthatcross-reactswithantibodiestohumanRacandgardenpeaRopandalsobinds[-35S]GTP,adiagnostictraitofsmallGproteins.
ThisRac-relatedproteintranslocatedfromthecytosoltomicrosomesduringtheoxidativeburst.
Moreover,soybeancellstransientlytransformedwitheitheradominantnegative(RacN17)oradominantpositive(RacV12)formofRac1showedtheanticipatedalteredresponsestothreedifferentstimuli:hypo-osmoticshock,oligo-GalUA,andharpin.
Inresponsetothesestimuli,cellstransformedwithRacN17producedlessROSandcellstransformedwithRacV12generatedmoreROSthancontrolcells.
TheseresultsstronglysuggestthataRac-relatedproteinparticipatesintheregulationofROSproductioninsoybeancells,possiblyviaactivationofanenzymecomplexsimilartotheNADPHoxidaseofphagocytesinanimalsystems.
Reactiveoxygenspecies(ROS)areproducedinplantcellsinresponsetoabroadrangeofbiologicalandphysicalstimuli,includingelicitors,pathogeninfections,osmoticshock,andwounding(Doke,1983;Apostoletal.
,1989;Atkinsonetal.
,1990;Chan-draandLow,1997;Stennisetal.
,1998).
ThetransientburstofROSproductionknownastheoxidativebursthasadirectantimicrobialeffectandisinvolvedininducingmanyotherdefenseresponses(Keppleretal.
,1989;PengandKuc,1992).
Despiteintensiveinvestigation,themechanismofROSproductioninplantcellshasyettobeclearlydefined.
EnzymesthatmayberesponsibleforROSproductioninplantsincludeperoxidaseandNADPHoxidase,aswellasseveralotheroxidases(AuhandMurphy,1995;Desi-kanetal.
,1996;Dwyeretal.
,1996;Kiefferetal.
,1997;Xingetal.
,1997;Bestwicketal.
,1998;Bolwelletal.
,1998).
ThatNADPHoxidasemayplayaroleinROSproductioninplantcellsisindicatedbytwolinesofevidence.
First,NADPHoxidaseisresponsibleforROSformationinneutrophils,andthebiochemicalcharacteristicsofROSproductionincludingmaxi-mumrate,rapidactivationkinetics,desensitization,andsignaltransductionpathwaysthattriggertheoxidativeburstaresimilarinplantcellsandneutro-phils(Dwyeretal.
,1996).
Second,thereexistplantproteinsthatcross-reactwithantibodiesthatrecog-nizethesubunitsofNADPHoxidasefromanimalcells(Desikanetal.
,1996;Dwyeretal.
,1996;Kiefferetal.
,1997;Xingetal.
,1997).
Together,theseobser-vationssuggestthatNADPHoxidasemaybein-volvedinamechanismofROSproductioncommontothedefensesystemsofbothplantandanimalcells.
Inneutrophils,thelowMrGprotein,Rac,playsanessentialroleasaregulatoroftheNADPHoxi-dase(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
TranslocationofRactotheplasmamembraneisrequiredforassemblyandactivationoftheNADPHoxidasecomplex(Hanetal.
,1998).
RecentreportssuggestthatRacisalsoinvolvedinROSproductioninsomenon-phagocyticcellsinwhichtheenzyme(s)responsibleforROSproductionremainunknown(Sundaresanetal.
,1996;Iranietal.
,1997;Kheradmandetal.
,1998;Yehetal.
,1999).
RacproteinsbelongtotheconservedRHOfamilyofsmallGTPases.
Inanimals,RHOisdividedintoseveralsubfamilies,includingRho,Cdc42,andRac.
AlthoughorthologsoftheseRHOGTPaseshavenotbeenidentifiedinplants,plantspossessauniquesubfamilyofRhoGTPases,termedRop,thatismostcloselyrelatedtotheRac(YangandWatson,1993;Delmeretal.
,1995;Xiaetal.
,1996;Wingeetal.
,1997;Lietal.
,1998).
Rop1anditscloserelativeRop5/Arac11ThisworkwassupportedbygrantsfromtheKoreaScienceandEngineeringFoundation(toY.
L.
)andfromtheNationalSci-enceFoundation(no.
MCD–9724047toZ.
Y.
).
*Correspondingauthor;e-mailylee@postech.
ac.
kr;fax82–54–279–2199.
PlantPhysiology,October2000,Vol.
124,pp.
725–732,www.
plantphysiol.
org2000AmericanSocietyofPlantPhysiologists725https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
playaroleintheregulationofpolarizedgrowthofpollentubesinpeaandArabidopsis(LinandYang,1997;Lietal.
,1998,1999;Kostetal.
,1999).
Inaddi-tion,thereisemergingevidencethatRopplaysim-portantrolesintheregulationofROSproduction.
Usingananti-Rac2polyclonalantibody,Xingetal.
(1997)showedthata21-kDRac2-relatedproteinfromtomatoistranslocatedtotheplasmamembraneinresponsetorace-specificelicitors.
Rac13mayalsobeinvolvedasaregulatorofH2O2productioninsec-ondarycellwalldifferentiationduringcottonfiberdevelopment(Potikhaetal.
,1999).
Inrice,OsRac1isinvolvedinROSproductionandcelldeath(Ka-wasakietal.
,1999).
Theseresults,togetherwiththeindirectevidencedescribedabovefortheinvolve-mentofNADPHoxidaseinROSproductioninplantcells,suggestthataplantRachomologmaybein-volvedintheregulationofaplantNADPHoxidase.
However,itisnotknownwhethertheRac/Rop-likeproteinsofplantsaretruefunctionalequivalentsofRacintheNADPHoxidasecomplexinanimalcells.
Inthisreportweshowthata21-kDGTP-bindingprotein,whichcross-reactswithRop-specificandwithanimalRac1-andRac2-specificantibodies,translo-catesfromthecytosoltothemembraneduringtheoxidativeburstinsuspension-culturedsoybean(Gly-cinemax)cells.
Furthermore,transientexpressionofhumanRac1proteinsinthesoybeancellsmodulatesROSproductioninresponsetoosmoticstressandelicitors.
Theseresultsprovideevidencefortheexis-tenceofasoybeanRop-likeGTPase,whichfunctionsasaregulatoroftheplantNADPHoxidase,possiblyanalogoustoRacintheNADPHoxidasecomplexinanimalcells.
RESULTSHypo-OsmoticShock-InducedSuperoxideAnion(O2)FormationTotestthehypothesisthatNADPHoxidaseisin-volvedinROSgenerationinsoybeancells,weusedastimulusthatreliablyinducesO2production,hypo-osmoticshock,andaflavin-containingoxidasein-hibitor,diphenyleneiodonium(DPI;O'Donnelletal.
,1993;Dwyeretal.
,1996;MurphyandAuh,1996).
ProductionofO2,measuredbymonitoringtheformationofreducedCytc,wasinducedwhensus-pension-culturedsoybeancellsweresubjectedtohypo-osmoticshock(dilutionofthemediumwithwaterin1:1ratio),andtheinductionwasalmostcompletelyinhibitedinthepresenceof30mDPI(Fig.
1A),supportingourhypothesis.
Inaddition,thelowamplitudeoftheresponseandthetransientpeaksuggestedthatsuperoxideproducedbyNADPHox-idasewasrapidlyconvertedtoH2O2byendogenousSOD.
Indeed,weobservedthatinthepresenceofDDC,aninhibitorofSOD,thereducedCytccontin-uedtoaccumulate(Fig.
1B).
DDCalonewithoutos-moticshockdidnotinduceoxidativeburst(datanotshown).
Theseresultsstronglysuggestthathypo-osmoticshockactivatesNADPHoxidaseinsoybeancells.
IdentificationofaRac-RelatedProteininSuspension-CulturedSoybeanCellsTotestwhetheraRac-orRop-likeproteinmightexistinsoybeancells,proteinsextractedfromsuspension-Figure1.
Productionofsuperoxideanion(O2)inresponsetohypo-osmoticshock.
A,InhibitionbyDPIofO2production.
Thecellswerepretreatedwithorwithout30MDPIfor30min,thenstimu-latedbyhypo-osmoticshock.
Controlcellswerenotsubjectedtothestimulus.
DimethylsulfoxidealonewasaddedtothesolventcontrolofDPI,whichwasalsostimulatedbyhypo-osmoticshock.
B,Accu-mulationofO2inthepresenceofasuperoxidedismutase(SOD)inhibitordiethyldithiocarbamate(DDC).
Thecellswerepretreatedwithorwithout1mMDDCfor10min,thenstimulatedbyhypo-osmoticshock.
Controlcellswerenotsubjectedtothestimulus.
Resultsshownarerepresentativesfromthree(A)andtwo(B)similarindependentexperiments.
Parketal.
726PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
culturedsoybeancellsweretestedforGTPbindingandcross-reactionwithantibodiesraisedagainstRac1,Rac2,andRac1(C189S)fromhuman,andRop1Psfromgardenpea.
Rac1(C189S)isanisoprenylation-deficientmutantofRac1,andourpolyclonalantibodyraisedagainsttheentireproteincross-reactedwithbothRac1andRac2proteins(datanotshown).
Figure2showsthatasoybeanproteinwithanapparentmolecularmassof21kD,aboutthesizeofRacandRop,bound[-35S]GTPaswellascross-reactedwithalltheantibodiestested.
Thisre-sultsuggeststhataRac/Rop-likeGTP-bindingpro-teinexistsinsoybeancells.
Thisproteinwasex-pressedatallgrowthstagesoftheculturedcells(datanotshown).
TranslocationoftheEndogenousRac-RelatedProteinduringtheOxidativeBurstInneutrophils,RacactivationofNADPHoxidaseinresponsetostimulationwithchemo-attractantorphorbolesterisaccompaniedbyRactranslocationfromthecytosoltothemembrane(Quinnetal.
,1993;Nisimotoetal.
,1997).
IftheRac-relatedproteininsoybeancellshasaroleanalogoustothatofRacofneutrophils,similarmembranetranslocationofthisproteinmightbeexpectedduringtheoxidativeburst.
ThereforeweexaminedthelocationoftheRac-relatedsoybeanproteinusinganti-Rac1(C189S)anti-body,sincethisantiserumexhibitedthehighestcross-reactivitywiththisproteinamongthefouran-tiseratested.
Examinationofmicrosomalandcytoso-licfractionsofsoybeancellspreparedbeforeandafterhypo-osmoticshockshowedthatmostoftheRac-relatedproteinwaspresentinthecytosolbeforetheshocktreatment,andthataconsiderableportionwasalsofoundinthemicrosomalfractionat5minaftershocktreatment(Fig.
3)whenH2O2productionwasmaximal.
TheRac-relatedproteinwaspresentinthemicrosomeevenaftertheoxidativeburstendedat20minaftertheshock(Fig.
3).
TheremaybeotherfactorsthatinactivateNADPHoxidasebeforeRacpro-teinsreturntothecytosol(Sathyamoorthyetal.
,1997).
AlteredRatesofOxidativeBurstinMutantRac1-ExpressingCellsSincetranslocationoftheendogenousRac-relatedproteinsuggestedthatsoybeancellsmayhaveaROS-generatingmechanismsimilartothatofanimalcells,wethenexaminedwhetherRacofanimalorigincouldmodulateROSgenerationbysoybeancells.
MutanthumanRac1genesweretransientlyex-pressedinsuspension-culturedsoybeancellsandtheoxidativeburstofthecellsinresponsetomechanicalstress(osmoticshock)andelicitors(oligo-GalUA[OGA]andharpin)thatinducedefenseresponseswereanalyzed.
OGAisaplantcellwallcomponentreleasedduringpathogenattackorwounding,andharpinisaproteinaceousbacterialelicitorfromEr-winiaamylovora(ChandraandLow,1997).
Thesethreestimuliinducetheoxidativeburstviadistinctsignaltransductionpathways,althoughtheidentitiesoftheintermediatesinthesepathwaysremainlargelyunknown(LowandMerida,1996).
AsseeninFigure4,osmoticshockandOGAinducedanoxida-tiveburstwithin2to3minincellstransformedwith-glucuronidase(GUS)onlyandasimilarresponsewasstimulatedbyharpinabout5minafterelicita-tion.
TolearnwhethermammalianRacmightalterthisresponse,RacV12,adominantpositiveRac1mu-tantwithconstitutiveactivity(defectiveinitsintrin-sicGTPaseactivity;Diekmannetal.
,1991)wastrans-formedintothesoybeancells.
Forasimilarpurpose,RacN17,adominantnegativeRac1mutantthatislockedintoitsinactivestatebyitspreferentialaffin-ityforGDP(FarnsworthandFeig,1991)wasalsotestedforitsinfluenceontheburst.
BecauseRacN17alsocompetesforRac'sguaninenucleotideexchangefactor(FarnsworthandFeig,1991;Jungetal.
,1994),itmightalsobeexpectedtoreduceactivationofanyendogenousplantRacisoforms.
AsshownintheinsettoFigure4A,expressionofbothmutantRac1genescouldbedemonstratedinthesoybeancellsbyFigure2.
IdentificationofaRac/Rop-relatedproteininsoybeancellsbyimmunoblottingand[-35S]GTP-bindingassay.
Crudeextractsfromsuspension-culturedsoybeancellswereseparatedby15%SDS-PAGE,transferredtonitrocellulosemembrane,andprobedwithantibodiesraisedagainstRac1(C189S)(lane1),Rop(lane2),Rac1(lane3),andRac2(lane4).
Nitrocellulosemembranepreparedinthesamemannerwasalsousedin[-35S]GTP-bindingassay,theresultofwhichwasvisualizedbyautoradiography(lane5).
Representativesfromtwoindependentexperimentswithsimilarresultsareshown.
Figure3.
TranslocationoftheRac-relatedproteinofsoybeancellsfromthecytosoltothemicrosome.
Microsomalandcytosolicfrac-tionswerepreparedfromsuspension-culturedsoybeancellsbeforeand5and20minafterhypo-osmoticshocktreatment.
Theendoge-nousRac-relatedproteinwasdetectedbyimmunoblottingwithan-tibodiesraisedagainstRac1(C189S).
Seventymicrogramsofproteinwasloadedineachlane.
Resultsshownarerepresentativesfromtwosimilarindependentexperiments.
Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000727https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
immunoblottingwithanti-mycantibody.
Moreim-portantly,noneofthesoybeancellstransformedwithRacN17,RacV12togetherwithGUS,orGUSaloneproducedH2O2intheabsenceofexternalstimuliinnormalculturemedium.
However,inresponsetoeachoftheabovethreestimuli,cellstransformedwithRacN17producedlessH2O2,whereascellstransformedwithRacV12producedmoreH2O2thancontrolcellsthatweretransformedwiththeGUSconstructonly(Fig.
4).
ThesedatasuggestthatRac-relatedproteinscanregulatetheoxidativeburstinsoybeancells.
MembraneTranslocationofHumanRacduringtheOxidativeBurstBecauseanimalRacmodifiedtheoxidativeburstrateinsoybeancells,wenextexaminedwhetherhumanRacexpressedinsoybeancellsmightalsotranslocateinamannersimilartotheendogenousRac-likesoybeanprotein.
Microsomalmembraneandcytosolwerepreparedfromtransformedcellsbeforeand5minafterosmoticshock.
RacV12,identifiedbyanti-mycantibody,wasfoundonlyinthecytosolintheabsenceofstimulus,butwasalsolocatedinthemicrosomalfractionfollowingosmoticshock(Fig.
5).
DISCUSSIONOurstudiessuggestthata21-kDGTP-bindingpro-tein,whichcross-reactswithbothanti-Ropandanti-Racantibodies(Fig.
2),isassociatedwithproductionofROSinsuspension-culturedsoybeancells.
Thecross-reactivityofthe21-kDproteinwiththeantibod-iessuggestedthatthisproteinislikelyamemberoftheplant-specificRopsubfamilyofRHOGTPasesbe-causeRopismostcloselyrelatedtoRac,sharing65%aminoacidsequenceidentitywitheachother(Lietal.
,1998).
Duringtheoxidativeburst,thisRop-likeproteinistranslocatedtothemicrosomalmembrane(Fig.
3),asisRac,aregulatorycomponentoftheNADPHoxi-dasecomplexinneutrophilcells(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
Inasimilarman-ner,thesubcellularlocationofa21-kDproteinoftobaccoandtomatocellschangedinresponsetoelic-itortreatment(Kiefferetal.
,1997;Xingetal.
,1997).
However,inthelatterstudies,anti-Rac2,butnotanti-Rac1antibodywasfoundtorecognizethecross-reactingbandat21kD,whereasinourexperimentsbothRac1andRac2antibodiescross-reactedwiththe21-kDsoybeanprotein.
Thereasonforthisdiscrep-ancyisnotclear.
BecausetheseantibodieswereraisedagainsttheC-terminalregionoftheirrespectiveRacproteins,itispossiblethattheC-terminalregionforFigure4.
AlteredratesofH2O2productionbymutantRac1-transformedsoybeancells.
Oxidativeburstassayofsuspension-culturedsoybeancellstransformedwithRacN17(b),RacV12to-getherwithGUS(d),orGUSalone(c)inresponsetohypo-osmoticshock(A),OGA(B),andharpin(C).
a,AcontrolshowingRacV12-transformedcellsintheabsenceofanystimuli.
Controlsusingallothertransformantsshowedsimilarresponse.
TheinsetofAshowswesternhybridizationusinganti-mycantibodytoprobetotalproteinextractedfromsoybeancellstransformedwithmyc-taggedRacV12(lane1),RacN17(lane2),andtheGUSgenewithoutanepitopetag(lane3).
Resultsshownarerepresentativesfromfour(osmoticshock),five(OGA),andtwo(harpin)similarindependentexperiments.
Eachexperimentconsistedofthreereplicateseachforeachsampletype.
Parketal.
728PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
thetomatoandtobaccoRop-likeproteinsisquitedif-ferentfromthatforthesoybeanRop-likeprotein.
RopisencodedbyalargegenefamilyinArabidopsisandprobablyinotherplantspeciesaswell(Lietal.
,1998).
FurtherstudiesareobviouslyneededtodeterminewhichRop-likeproteinisassociatedwiththeoxida-tiveburst.
WehaveshownthattheconstitutivelyactiveanddominantnegativeformsofhumanRacincreasedanddecreased,respectively,therateofROSproduc-tioninducedinsoybeancellsbyvariousstimuli(Fig.
4),similartotheireffectsinneutrophils(Iranietal.
,1997;Kheradmandetal.
,1998).
ThisresultsuggeststhataRop-likeproteinmaypromotetheoxidativeburstinsoybeancells,assuggestedforOsRac1inriceandRac13incottonfibercells(Kawasakietal.
,1999;Potikhaetal.
,1999).
O2generationbysoybeancellsexposedtohypo-osmoticshockanditsinhibitionbyDPIalsosuggestthatNADPHoxidaseislikelyre-sponsibleforatleastsomepartoftheoxidativeburstofsoybeancells(Fig.
1).
Moreover,threedifferentstimuli,whichactthroughdifferentsignalingcas-cades,inducedthesamechangesintheratesofROSproductioninRac-transformedsoybeancells,sug-gestingthattheRacproteinperformsacommonfunctionindiverseoxidativeburstsignalingpath-ways.
Finally,thesoybeanRac-relatedproteinandtheheterologouslyexpressedmammalianRacbothtranslocatedtothemembranefromthecytosoldur-ingROSproduction(Figs.
3and5),asdoesRacintheNADPHoxidasecomplexofneutrophils(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
Takentogether,theseresultsindicateaconservedroleforaRac-likeGproteininregulationofROSinsoybeancellsandtheysuggestthatsoybeancellsemployaRac/Rop-likeproteintomodulateanenzymaticsys-temsimilartotheNADPHoxidaseofneutrophils.
WehavealsoshownthattheconstitutivelyactiveRacmutantdoesnotconstitutivelyactivatetheoxi-dativeburstinsoybeancells;instead,inductionoftheoxidativeburstbythedominantpositiveRacmutantwasstimulus-dependent(Fig.
4).
Stimulus-dependencewasalsofoundinitstranslocationtothemicrosomes(Fig.
5).
Thesedatasuggestthatthestimulus-mediatedactivationofNADPHoxidaseinsoybeancellsinvolvesatleasttwosteps:onethatactivatesRacandanotherthatisindependentofRacactivation.
ThesecondstepismostlikelyrequiredforthetranslocationofRactotheplasmamembrane,assuggestedbystimulus-activatedRactranslocationtomembranes.
Thestimulus-dependentpromotionoftheoxidativeburstbyactivatedhumanRacinsoy-beancellsisdifferentfromtheactionofactivatedRopinriceorcottonfibercellswhereconstitutivelyactiveRopcausesconstitutiveactivationofH2O2production(Kawasakietal.
,1999;Potikhaetal.
,1999).
RopinthesesystemscouldhaveafunctiondistinctfromthatoftheRop-likeproteininsoybean.
Ontheotherhand,thedifferencemaybeduetodifferentfunctionsofplantRopandheterologoushumanRacinplantcells;RopsmayfunctionbothasaregulatorycomponentofNADPHoxidaseandasanupstreamregulatorofthesignalingcascadethateventuallyactivatesNADPHoxidase,whereastheheterologousmammalianRacmaynothavethelatterfunctioninplantcells.
IdentificationofthesoybeanRopproteininvolvedintheregulationofNADPHoxidasewillhelptoaddressthisproblem.
Asafinalpoint,weshouldpointoutthatalthoughwepresentedevidenceforpossiblesimilaritybe-tweentheROSproducingmechanismsofsoybeancellsandneutrophils,ourdatadonotexcludethepossibilitythattheRac/Rop-likeproteinofsoybeanorheterologouslyexpressedRacmodulateoxidativeburstatasitedifferentfromtheNADPHoxidase.
ROShasbeenrecentlyshowntobeproducedinsomeanimalcellswhereexistenceofthecomponentsoftheNADPHoxidaseisquestionable,andthereactivatedRacalsoenhancestherateofROSproduction(Sundaresanetal.
,1996;Iranietal.
,1997;Kherad-mandetal.
,1998;Yehetal.
,1999).
FurtherstudiesarenecessarytounderstandtheexactmechanismofmodulationofROSproductionbyRac/Rop-likepro-teininsoybeancells.
MATERIALSANDMETHODSPlantCellCultureSuspension-culturedsoybean(Glycinemax)cellsweremaintainedinMurashigeandSkoogmedium(MurashigeandSkoog,1962)containing0.
1mgkinetin,3mg2,4-dichlorophenoxyaceticacid,1gcasein,and0.
5gMES[2-(N-morpholino)ethanesulfonicacid]perliter.
Twomil-lilitersofcellswastransferredto25mLoffreshMurashigeandSkoogmediumevery7d.
Thecellsweregrowninanincubatorat23°C,withshakingat80to90rpmand18-hlight/6-hdarkcycles.
RacConstructsTheplasmidsincludingpEXV-RacV12andpEXV-RacN17weregiftsfromDr.
Jae-HongKim(KimandKim,1997;Kimetal.
,1997).
Forparticlebombardment,RacconstructswerepreparedbyinsertingtheRac1codingFigure5.
TranslocationoftheRacV12ofanimaloriginfromthecytosoltothemicrosome.
Microsomalandcytosolicfractionswerepreparedfromsuspension-culturedsoybeancellstransformedwithRacV12beforeand5minafterhypo-osmoticshocktreatment.
RacV12proteinwasdetectedbyimmunoblottingwithanti-mycan-tibody.
Representativesfromtwoindependentexperimentswithsim-ilarresultsareshown.
Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000729https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
regionintoEcoRIsiteofthebinaryvectorpGA748(provid-edbyDr.
GynheungAn)under35Spromoter,andallconstructsweretaggedwithanN-terminal9E10epitope.
DetectionofH2O2ProductionbyFluorescenceQuenchingH2O2productioninsuspension-culturedsoybeancellswasdetectedbymonitoringtheoxidativequenchingofthefluorescentdye,pyranine(8-hydroxypyrene-1,3,6-tri-sulfonicacidtrisodiumsalt:ex405nm,em512nm,Mo-lecularProbes,Eugene,OR)usingaspectrofluorimeter(RF5000,Shimadzu,Kyoto,Japan)aspreviouslydescribed(Dwyeretal.
,1996).
Hypo-osmoticshocktreatmentwasperformedbydilutingthecellsuspensionwithanequalvolumeofdistilledwater.
ElicitationwithOGAandharpin(kindgiftsofDrs.
PhilipS.
LowandStevenBeer,respec-tively;ChandraandLow,1997)wascarriedoutbytheadditionof10gofOGAor60gofharpinto1.
5mLofcellsuspension.
Thecellswerestirredgentlyinaquartzcuvetteduringfluorescencemeasurements.
SuperoxideAnionDeterminationbyCytcReductionAssayTheamountofO2accumulatedinthesoybeancellsuspensionduringtheoxidativeburstwasmeasuredbymonitoringthereductionofCytc,whichdisplaysachangeinabsorbancewhenitacceptsanelectronfromthesuper-oxideanion.
Attimezero,Cytc(typeVI;horseheart,Sigma,St.
Louis)wasaddedto50Lofcellsuspensionatafinalconcentrationof50m,andthecellsweresubjectedtoosmoticshockbytheadditionofanequalvolumeofdistilledwater.
ReducedCytcwasquantifiedbymonitor-ingA550inabio-kineticsreader(EL312e,BIO-TEKInstru-ments,Denkendorf,Germany;Curnutteetal.
,1989).
ToassesswhethertheO2accumulationinvolvesNADPHoxidase,DPI,aninhibitorofflavinoxidase,wasdissolvedindimethylsulfoxideandaddedatafinalconcentrationof30m30minbeforethestartoftheCytcreductionassay.
ToconfirmtheidentityofthereducingagentofCytcassuperoxide,weusedaninhibitorofSOD,DDC(Sigma).
DDCwasaddedto0.
5mLofcellsatafinalconcentrationof1mm,10minbeforethestartoftheassay.
TheCytcreductionassaywasperformedinthesamemannerasdescribedabove,exceptinthiscasethereducedCytcwasquantifiedwithaspectrophotometer(UV-160A,Shimadzu).
PreparationofMicrosomalandCytosolicFractionsCellswerecollectedbyfiltrationandhomogenizedingrindingmedium{100mmKCl,3mmNaCl,1mmATP,3.
5mmMgCl2,5mmSuc,1mmphenylmethylsulfonylfluo-ride,2mmdithiothreitol,and10mmPIPES[piperazine-N,N-bis-(2-ethanesulfonicacid)],pH7.
3}usingamortarandapestle.
Thehomogenatewascentrifugedat8,000gfor15min.
Thesupernatantwascollectedandcentrifugedagainat100,000gfor1h.
Theresultingpelletwasresus-pendedingrindingmediumandusedasthemicrosomalfraction.
Thecytosolicfractionwaspreparedbyconcentrat-ingthesupernatantfromthesecondcentrifugationstepusingCentriconfilters(10-kDcut-offvalue;Millipore,Bed-fold,MA),givingafinalproteinconcentrationof3to4mgmL1.
ProteinconcentrationsweremeasuredaccordingtotheBradfordmethod(Bradford,1976)usingbovineserumalbuminasastandard.
ElectrophoresisandProteinImmunoblottingProteinextracts(100g)weresubjectedto15%SDS-PAGEandthenelectrophoreticallytransferredtoa0.
22-mnitrocellulosemembrane(Schleicher&Schuell,Keene,NH).
Themembranewasblockedfor1hinTTBSbuffer{20mmTris[Tris(hydroxymethyl)aminomethane],130mmNaCl,0.
05%[w/v]NaN3,and0.
05%[v/v]Tween20,pH7.
4}supplementedwith5%(w/v)non-fatmilkpowder.
Primaryantibodieswerethenaddedat1:500dilutioninTTBS.
Anti-Rac1(C189S)antiserumwaspreparedbyimmunizingrab-bitswithpurifiedRac1(C189S)protein(Krecketal.
,1994)accordingtostandardprotocolsaspreviouslydescribed(Hanetal.
,1998).
Anti-Ropantiserumwaspreparedaspreviouslydescribed(Linetal.
,1996).
Anti-myc(Invitrogen,Carlsbad,CA),anti-Rac1,andanti-Rac2antibodies(SantaCruzBiotechnology,SantaCruz,CA)werepurchasedfromcommercialsuppliersasindicated.
Horseradishperoxidase-conjugatedanti-mouseIgGantibody(Amersham,Bucking-hamshire,UK)wasusedfordetectionofanti-mycantibody,andalkalinephosphatase-conjugatedanti-rabbitIgGanti-body(Promega,Madison,WI)wasusedfordetectionofanti-Rac1(C189S),anti-Rac1,anti-Rac2,andanti-Ropanti-bodies.
Secondaryantibodieswerediluted1:2,000inTTBS.
Allprimaryantibodiesexceptanti-mycantibodywerepoly-clonal.
Anti-Rac1(C189S)andanti-Ropantibodieswereraisedagainstwholeproteins,whereasRac1-andRac2-specificantibodieswereraisedagainsttheC-terminal11aminoacidsoftherespectiveproteins.
[-35S]GTP-BindingAssaySoybeanproteinsweretransferredontoanitrocellulosemembraneasdescribedabove.
Themembranewaswashedwith100mLofrenaturationbuffer(50mmTris-HCl,pH7.
5,0.
1%[w/v]bovineserumalbumin,5mmMgCl2,2mmdithiothreitol,and0.
1%[v/v]TritonX-100)for90minandthenincubatedin10mLoffreshrenaturationbuffercon-taining2.
7nm[-35S]GTPwithgentleagitationforafurther90min.
Duringthenext90min,themembranewaswashedsixtimeswithrenaturationbuffer.
Allreactionswereperformedatroomtemperature.
Themembranewasair-driedandradioactivebandswerevisualizedbyautoradiography.
MicroprojectileBombardmentofSuspension-CulturedSoybeanCellswithMutantRacGenesOnemilliliterofsoybeancellsuspensionculturewasharvested7daftersubculture.
CellswerespreadinathinlayeroverafilterpapermoistenedwithasmallamountofParketal.
730PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
growthmedium.
M-10tungstenparticles(Bio-Rad,Her-cules,CA)werecoatedwith30gofplasmidDNA(con-tainingequalamountofRacandGUSconstructs)andusedasthemicroprojectilesinthetransfections.
Aftermicroprojectilebombardment(BiolisticPDS-1000/HeSystem,Bio-Rad)accordingtothemanufacturer'sinstruc-tions,thecellswerescrapedfromthefilterpaperandtransferredinto5mLoffreshgrowthmedium.
Thecellsweregrowninashakingincubatorasdescribedabovefor24hbeforemeasurementoftheoxidativeburst.
Theeffi-ciencyoftransformationwasestimatedbydeterminingthepercentageofcellsexpressingGUS.
GUSactivitywasassayedbystainingthecellswithasubstratesolution(100mmsodiumphosphate,pH7.
0,1mmEDTA,5mmpotas-siumferrocyanide,5mmpotassiumferricyanide,1%[v/v]TritonX-100,and1mgmL15-bromo-4-chloro-3-indoyl--d-GlcUAcyclohexylaminesaltfromRoseScien-tific,Edmonton,AB,Canada;Citovskyetal.
,1994).
Trans-formationefficiencynormallyreached30%to60%.
ExpressionofRacproteinsinthesoybeancellswascon-firmedbywesternanalysisusinganti-mycantibody.
Tooptimizetransformationefficiency,wesometimesperformedtheosmotictreatmentdescribedbyVainetal.
(1993)byplacingthefilteredsoybeancellsontoanosmoticum-containingsolidmediumfor4hbeforeand16hafterbombardment.
Theosmoticumconsistedofanequimolarmixtureofmannitolandsorbitoltogiveafinalconcentrationof0.
25m.
ACKNOWLEDGMENTSWethankDrs.
PhilipS.
LowandSungHoRyuforusefuldiscussionsandcriticalreadingofthemanuscript.
WealsothankDrs.
Jae-HongKim,GynheungAn,PhilipS.
Low,StevenBeer,andJ.
DavidLambethforprovidinguswiththemutantRacgeneconstructs,binaryvectors,OGA,harpin,andanti-Rac1(C189S)antibody,respectively,andWon-YongSongfortechnicalassistance.
ReceivedFebruary18,2000;acceptedJune27,2000.
LITERATURECITEDApostolI,HeinsteinPF,LowPS(1989)Rapidstimulationofanoxidativeburstduringelicitationofculturedplantcells:roleindefenseandsignaltransduction.
PlantPhysiol90:109–116AtkinsonMM,KepplerLD,OrlandiEW,BakerCJ,MischkeCF(1990)InvolvementofplasmamembranecalciuminfluxinbacterialinductionoftheK/Hex-changeandhypersensitiveresponsesintobacco.
PlantPhysiol92:215–221AuhCK,MurphyTM(1995)PlasmamembraneredoxenzymeisinvolvedinthesynthesisofO2andH2O2byPhytophthoraelicitor-stimulatedrosecells.
PlantPhysiol107:1241–1247BestwickCS,BrownIR,MansfieldJW(1998)Localizedchangesinperoxidaseactivityaccompanyhydrogenper-oxidegenerationduringthedevelopmentofanonhosthypersensitivereactioninlettuce.
PlantPhysiol118:1067–1078BokochGM(1994)RegulationofthehumanneutrophilNADPHoxidasebytheRacGTP-bindingproteins.
CurrOpinCellBiol6:212–218BolwellGP,DaviesDR,GerrishC,AuhCK,MurphyTM(1998)ComparativebiochemistryoftheoxidativeburstproducedbyroseandFrenchbeancellsrevealstwodistinctmechanisms.
PlantPhysiol116:1379–1385BradfordMM(1976)Arapidandsensitivemethodforthequantificationofmicrogramquantitiesofproteinutiliz-ingtheprincipleofprotein-dyebinding.
AnalBiochem72:248–254ChandraS,LowPS(1997)MeasurementofCa2fluxesduringelicitationoftheoxidativeburstinaequorin-transformedtobaccocells.
JBiolChem272:28274–28280CitovskyV,WarnickD,ZambryskiP(1994)Nuclearim-portofAgrobacteriumVirD2andVirE2proteinsinmaizeandtobacco.
ProcNatlAcadSciUSA91:3210–3214CurnutteJT,ScottPJ,MayoLA(1989)Cytosoliccompo-nentsoftherespiratoryburstoxidase:resolutionoffourcomponents,twoofwhicharemissingincomplementingtypesofchronicgranulomatousdisease.
ProcNatlAcadSciUSA86:825–829DelmerDP,PearJR,AndrawisA,StalkerDM(1995)GenesencodingsmallGTP-bindingproteinsanalogoustomammalianracarepreferentiallyexpressedindevel-opingcottonfibers.
MolGenGenet248:43–51DesikanR,HancockJT,CoffeyMJ,NeillSJ(1996)Gen-erationofactiveoxygeninelicitedcellsofArabidopsisthalianaismediatedbyaNADPHoxidase-likeenzyme.
FEBSLett382:213–217DiekmannD,BrillS,GarrettMD,TottyN,HsuanJ,Mon-friesC,HallC,LimL,HallA(1991)BcrencodesaGTPaseactivatingproteinforp21rac.
Nature351:400–402DokeN(1983)Involvementofsuperoxideaniongenera-tioninhypersensitiveresponseofpotatotubertissuestoinfectionwithanincompatibleraceofPhytophthorainfes-tans.
PhysiolPlantPathol23:345–347DwyerSC,LegendreL,LowPS,LetoTL(1996)Plantandhumanneutrophiloxidativeburstcomplexescontainim-munologicallyrelatedproteins.
BiochimBiophysActa1289:231–237FarnsworthCA,FeigLA(1991)Dominantinhibitorymu-tationsintheMg2-bindingsiteofRasHpreventitsactivationbyGTP.
MolCellBiol11:4822–4829HanCH,FreemanJLR,LeeT,MotalebiSA,LambethJD(1998)Regulationoftheneutrophilrespiratoryburstox-idase:identificationofanactivationdomaininp67phox.
JBiolChem273:16663–16668IraniK,Goldschmidt-ClermontPJ(1998)Ras,superoxideandsignaltransduction.
BiochemPharmacol55:1339–1346IraniK,XiaY,ZweierJL,SollottSJ,DerCJ,FearonER,SundaresanM,FinkelT,Goldschmidt-ClermontPJ(1997)MitogenicsignalingmediatedbyoxidantsinRas-transformedfibroblasts.
Science275:1649–1652Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000731https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
JungV,WeiW,BallesterR,CamonisJ,MiS,VanAelstL,WiglerM,BroekD(1994)TwotypesofRASmutantsthatdominantlyinterferewithactivatorsofRAS.
MolCellBiol14:3707–3718KawasakiT,HenmiK,OnoE,HatakeyamaS,IwanoM,SatohH,ShimamotoK(1999)ThesmallGTP-bindingproteinRacisaregulatorofcelldeathinplants.
ProcNatlAcadSciUSA96:10922–10926KepplerLD,BakerCJ,AtkinsonMM(1989)Activatedoxygenproductionduringabacteria-inducedhypersen-sitivereactionintobaccosuspensioncells.
Phytopathol-ogy79:974–978KheradmandF,WernerE,TrembleP,SymonsM,WerbZ(1998)RoleofRac1andoxygenradicalsincollagenase-1expressioninducedbycellshapechange.
Science280:898–902KiefferF,Simon-PlasF,MaumeBF,BleinJP(1997)To-baccocellscontainaprotein,immunologicallyrelatedtotheneutrophilsmallGproteinRac2andinvolvedinelicitor-inducedoxidativeburst.
FEBSLett403:149–153KimBC,KimJH(1997)NuclearsignallingbyRacGTPase:essentialroleofphospholipaseA2.
BiochemJ326:333–337KimJH,KwackHJ,ChoiSE,KimBC,KimYS,KangIJ,KumarCC(1997)EssentialroleofRacGTPaseinhydro-genperoxide-inducedactivationofc-fosserumresponseelement.
FEBSLett406:93–96KostB,LemichezE,SpielhoferP,HongY,ToliasK,CarpenterC,ChuaNH(1999)Rachomologuesandcom-partmentalizedphosphatidylinositol4,5-bisphosphateactinacommonpathwaytoregulatepolarpollentubegrowth.
JCellBiol145:317–330KreckML,UhlingerDJ,TyagiSR,IngeKL,LambethJD(1994)ParticipationofthesmallmolecularweightGTP-bindingproteinRac1incell-freeactivationandassemblyoftherespiratoryburstoxidase.
JBiolChem269:4161–4168LiH,LinY,HeathRM,ZhuMX,YangZ(1999)ControlofpollentubetipgrowthbyaRopGTPases-dependentpathwaythatleadstotip-localizedcalciuminflux.
PlantCell11:1731–1741LiH,WuG,WareD,DavisKR,YangZ(1998)ArabidopsisRho-relatedGTPases:differentialgeneexpressioninpol-lenandpolarlocalizationinfissionyeast.
PlantPhysiol118:407–417LinY,WangY,ZhuJ,YangZ(1996)LocalizationofaRhoGTPaseimpliesaroleintipgrowthandmovementofthegenerativecellinpollentubes.
PlantCell8:293–303LinY,YangZ(1997)Inhibitionofpollentubeelongationbymicroinjectedanti-Rop1Psantibodiessuggestacru-cialroleforRho-typeGTPasesinthecontroloftipgrowth.
PlantCell9:1647–1659LowPS,MeridaJR(1996)Theoxidativeburstinplantdefense:functionandsignaltransduction.
PhysiolPlant96:533–542MurashigeT,SkoogF(1962)Arevisedmediumforrapidgrowthandbioassayswithtobaccotissuecultures.
PhysiolPlant15:473–497MurphyTM,AuhCK(1996)Thesuperoxidesynthasesofplasmamembranepreparationsfromculturedrosecells.
PlantPhysiol110:621–629NisimotoY,FreemanJLR,MotalebiSA,HirshbergM,LambethJD(1997)Racbindingtop67phox:structuralbasisforinteractionsoftheRac1effectorregionandinsertregionwithcomponentsoftherespiratoryburstoxidase.
JBiolChem272:18834–18841O'DonnellVB,TewDG,JonesOTG,EnglandPJ(1993)Studiesontheinhibitorymechanismofiodoniumcom-poundswithspecialreferencetoneutrophilNADPHoxidase.
BiochemJ290:41–49PengM,KucJ(1992)Peroxidase-generatedhydrogenper-oxideasasourceofantifungalactivityinvitroandontobaccoleafdisk.
Phytopathology82:696–699PotikhaTS,CollinsCC,JohnsonDI,DelmerDP,LevineA(1999)Theinvolvementofhydrogenperoxideinthedifferentiationofsecondarywallsincottonfibers.
PlantPhysiol119:849–858QuinnMT,EvansT,LoetterleLR,JesaitisAJ,BokochGM(1993)TranslocationofRaccorrelateswithNADPHox-idaseactivation.
JBiolChem268:20983–20987SathyamoorthyM,MendezID,AdamsAG,LetoTL(1997)p40phoxdown-regulatesNADPHoxidaseactivitythroughinteractionswithitsSH3domain.
JBiolChem272:9141–9146StennisMJ,ChandraS,RyanCA,LowPS(1998)Systeminpotentiatestheoxidativeburstinculturedtomatocells.
PlantPhysiol117:1031–1036SundaresanM,YuZX,FerransVJ,SulcinerDJ,GutkindJS,IraniK,Goldschmidt-ClermontPJ,FinkelT(1996)Regulationofreactive-oxygen-speciesgenerationinfi-broblastsbyRac1.
BiochemJ318:379–382VainP,McMullenMD,FinerJJ(1993)Osmotictreatmentenhancesparticlebombardment-mediatedtransientandstabletransformationofmaize.
PlantCellReports12:84–88WingeP,BrembuT,BonesAM(1997)Cloningandchar-acterizationofrac-likecDNAsfromArabidopsisthaliana.
PlantMolBiol35:483–495XiaG,RamachandranS,HongY,ChanYS,SimanisV,ChuaNH(1996)Identificationofplantcytoskeletal,cellcycle-relatedandpolarity-relatedproteinsusingSchizo-saccharomycespombe.
PlantJ10:761–769XingT,HigginsVJ,BlumwaldE(1997)Race-specificelic-itorsofCladosporiumfulvumpromotetranslocationofcytosoliccomponentsofNADPHoxidasetotheplasmamembraneoftomatocells.
PlantCell9:249–259YangZ,WatsonJC(1993)Molecularcloningandcharac-terizationofrho,aras-relatedsmallGTP-bindingproteinfromthegardenpea.
ProcNatlAcadSciUSA90:8732–8736YehLH,ParkYJ,HansaliaRJ,AhmedIS,DeshpandeSS,Goldschmidt-ClermontPJ,IraniK,AlevriadouBR(1999)Shear-inducedtyrosinephosphorylationinendo-thelialcellsrequiresRac1-dependentproductionofROS.
AmJPhysiol276:C838–C847Parketal.
732PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
P.
,H.
-J.
C.
,S.
L.
,T.
L.
,Y.
L.
);andDepartmentofBotanyandPlantSciences,UniversityofCalifornia,Riverside,California92521–0124(Z.
Y.
)Plantcellsproducereactiveoxygenspecies(ROS)inresponsetomanystimuli.
However,themechanismofROSbiosyn-thesisremainsunclear.
Wehaveexploredthehypothesisthatthesuperoxideburstinplantsmechanisticallyresemblestheoxidativeburstinneutrophils.
FirstwehaveconfirmedthatROSproduction,whichoccursinsuspension-culturedsoybean(Glycinemax)cellsinresponsetohypo-osmoticshock,isinhibitedbydiphenyleneiodonium,aninhibitoroftheflavin-dependentoxidaseofneutrophils.
BecauseaRacfamilyGproteinisanessentialregulatorofthisNADPHoxidase,andbecausemanyplanthomologsofRachavebeencloned,wenextexaminedwhetherRac-likeproteinsmightbeinvolvedintheoxidativeburstinthesoybeancells.
WeidentifiedaRac-like21-kDsoybeanproteinthatcross-reactswithantibodiestohumanRacandgardenpeaRopandalsobinds[-35S]GTP,adiagnostictraitofsmallGproteins.
ThisRac-relatedproteintranslocatedfromthecytosoltomicrosomesduringtheoxidativeburst.
Moreover,soybeancellstransientlytransformedwitheitheradominantnegative(RacN17)oradominantpositive(RacV12)formofRac1showedtheanticipatedalteredresponsestothreedifferentstimuli:hypo-osmoticshock,oligo-GalUA,andharpin.
Inresponsetothesestimuli,cellstransformedwithRacN17producedlessROSandcellstransformedwithRacV12generatedmoreROSthancontrolcells.
TheseresultsstronglysuggestthataRac-relatedproteinparticipatesintheregulationofROSproductioninsoybeancells,possiblyviaactivationofanenzymecomplexsimilartotheNADPHoxidaseofphagocytesinanimalsystems.
Reactiveoxygenspecies(ROS)areproducedinplantcellsinresponsetoabroadrangeofbiologicalandphysicalstimuli,includingelicitors,pathogeninfections,osmoticshock,andwounding(Doke,1983;Apostoletal.
,1989;Atkinsonetal.
,1990;Chan-draandLow,1997;Stennisetal.
,1998).
ThetransientburstofROSproductionknownastheoxidativebursthasadirectantimicrobialeffectandisinvolvedininducingmanyotherdefenseresponses(Keppleretal.
,1989;PengandKuc,1992).
Despiteintensiveinvestigation,themechanismofROSproductioninplantcellshasyettobeclearlydefined.
EnzymesthatmayberesponsibleforROSproductioninplantsincludeperoxidaseandNADPHoxidase,aswellasseveralotheroxidases(AuhandMurphy,1995;Desi-kanetal.
,1996;Dwyeretal.
,1996;Kiefferetal.
,1997;Xingetal.
,1997;Bestwicketal.
,1998;Bolwelletal.
,1998).
ThatNADPHoxidasemayplayaroleinROSproductioninplantcellsisindicatedbytwolinesofevidence.
First,NADPHoxidaseisresponsibleforROSformationinneutrophils,andthebiochemicalcharacteristicsofROSproductionincludingmaxi-mumrate,rapidactivationkinetics,desensitization,andsignaltransductionpathwaysthattriggertheoxidativeburstaresimilarinplantcellsandneutro-phils(Dwyeretal.
,1996).
Second,thereexistplantproteinsthatcross-reactwithantibodiesthatrecog-nizethesubunitsofNADPHoxidasefromanimalcells(Desikanetal.
,1996;Dwyeretal.
,1996;Kiefferetal.
,1997;Xingetal.
,1997).
Together,theseobser-vationssuggestthatNADPHoxidasemaybein-volvedinamechanismofROSproductioncommontothedefensesystemsofbothplantandanimalcells.
Inneutrophils,thelowMrGprotein,Rac,playsanessentialroleasaregulatoroftheNADPHoxi-dase(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
TranslocationofRactotheplasmamembraneisrequiredforassemblyandactivationoftheNADPHoxidasecomplex(Hanetal.
,1998).
RecentreportssuggestthatRacisalsoinvolvedinROSproductioninsomenon-phagocyticcellsinwhichtheenzyme(s)responsibleforROSproductionremainunknown(Sundaresanetal.
,1996;Iranietal.
,1997;Kheradmandetal.
,1998;Yehetal.
,1999).
RacproteinsbelongtotheconservedRHOfamilyofsmallGTPases.
Inanimals,RHOisdividedintoseveralsubfamilies,includingRho,Cdc42,andRac.
AlthoughorthologsoftheseRHOGTPaseshavenotbeenidentifiedinplants,plantspossessauniquesubfamilyofRhoGTPases,termedRop,thatismostcloselyrelatedtotheRac(YangandWatson,1993;Delmeretal.
,1995;Xiaetal.
,1996;Wingeetal.
,1997;Lietal.
,1998).
Rop1anditscloserelativeRop5/Arac11ThisworkwassupportedbygrantsfromtheKoreaScienceandEngineeringFoundation(toY.
L.
)andfromtheNationalSci-enceFoundation(no.
MCD–9724047toZ.
Y.
).
*Correspondingauthor;e-mailylee@postech.
ac.
kr;fax82–54–279–2199.
PlantPhysiology,October2000,Vol.
124,pp.
725–732,www.
plantphysiol.
org2000AmericanSocietyofPlantPhysiologists725https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
playaroleintheregulationofpolarizedgrowthofpollentubesinpeaandArabidopsis(LinandYang,1997;Lietal.
,1998,1999;Kostetal.
,1999).
Inaddi-tion,thereisemergingevidencethatRopplaysim-portantrolesintheregulationofROSproduction.
Usingananti-Rac2polyclonalantibody,Xingetal.
(1997)showedthata21-kDRac2-relatedproteinfromtomatoistranslocatedtotheplasmamembraneinresponsetorace-specificelicitors.
Rac13mayalsobeinvolvedasaregulatorofH2O2productioninsec-ondarycellwalldifferentiationduringcottonfiberdevelopment(Potikhaetal.
,1999).
Inrice,OsRac1isinvolvedinROSproductionandcelldeath(Ka-wasakietal.
,1999).
Theseresults,togetherwiththeindirectevidencedescribedabovefortheinvolve-mentofNADPHoxidaseinROSproductioninplantcells,suggestthataplantRachomologmaybein-volvedintheregulationofaplantNADPHoxidase.
However,itisnotknownwhethertheRac/Rop-likeproteinsofplantsaretruefunctionalequivalentsofRacintheNADPHoxidasecomplexinanimalcells.
Inthisreportweshowthata21-kDGTP-bindingprotein,whichcross-reactswithRop-specificandwithanimalRac1-andRac2-specificantibodies,translo-catesfromthecytosoltothemembraneduringtheoxidativeburstinsuspension-culturedsoybean(Gly-cinemax)cells.
Furthermore,transientexpressionofhumanRac1proteinsinthesoybeancellsmodulatesROSproductioninresponsetoosmoticstressandelicitors.
Theseresultsprovideevidencefortheexis-tenceofasoybeanRop-likeGTPase,whichfunctionsasaregulatoroftheplantNADPHoxidase,possiblyanalogoustoRacintheNADPHoxidasecomplexinanimalcells.
RESULTSHypo-OsmoticShock-InducedSuperoxideAnion(O2)FormationTotestthehypothesisthatNADPHoxidaseisin-volvedinROSgenerationinsoybeancells,weusedastimulusthatreliablyinducesO2production,hypo-osmoticshock,andaflavin-containingoxidasein-hibitor,diphenyleneiodonium(DPI;O'Donnelletal.
,1993;Dwyeretal.
,1996;MurphyandAuh,1996).
ProductionofO2,measuredbymonitoringtheformationofreducedCytc,wasinducedwhensus-pension-culturedsoybeancellsweresubjectedtohypo-osmoticshock(dilutionofthemediumwithwaterin1:1ratio),andtheinductionwasalmostcompletelyinhibitedinthepresenceof30mDPI(Fig.
1A),supportingourhypothesis.
Inaddition,thelowamplitudeoftheresponseandthetransientpeaksuggestedthatsuperoxideproducedbyNADPHox-idasewasrapidlyconvertedtoH2O2byendogenousSOD.
Indeed,weobservedthatinthepresenceofDDC,aninhibitorofSOD,thereducedCytccontin-uedtoaccumulate(Fig.
1B).
DDCalonewithoutos-moticshockdidnotinduceoxidativeburst(datanotshown).
Theseresultsstronglysuggestthathypo-osmoticshockactivatesNADPHoxidaseinsoybeancells.
IdentificationofaRac-RelatedProteininSuspension-CulturedSoybeanCellsTotestwhetheraRac-orRop-likeproteinmightexistinsoybeancells,proteinsextractedfromsuspension-Figure1.
Productionofsuperoxideanion(O2)inresponsetohypo-osmoticshock.
A,InhibitionbyDPIofO2production.
Thecellswerepretreatedwithorwithout30MDPIfor30min,thenstimu-latedbyhypo-osmoticshock.
Controlcellswerenotsubjectedtothestimulus.
DimethylsulfoxidealonewasaddedtothesolventcontrolofDPI,whichwasalsostimulatedbyhypo-osmoticshock.
B,Accu-mulationofO2inthepresenceofasuperoxidedismutase(SOD)inhibitordiethyldithiocarbamate(DDC).
Thecellswerepretreatedwithorwithout1mMDDCfor10min,thenstimulatedbyhypo-osmoticshock.
Controlcellswerenotsubjectedtothestimulus.
Resultsshownarerepresentativesfromthree(A)andtwo(B)similarindependentexperiments.
Parketal.
726PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
culturedsoybeancellsweretestedforGTPbindingandcross-reactionwithantibodiesraisedagainstRac1,Rac2,andRac1(C189S)fromhuman,andRop1Psfromgardenpea.
Rac1(C189S)isanisoprenylation-deficientmutantofRac1,andourpolyclonalantibodyraisedagainsttheentireproteincross-reactedwithbothRac1andRac2proteins(datanotshown).
Figure2showsthatasoybeanproteinwithanapparentmolecularmassof21kD,aboutthesizeofRacandRop,bound[-35S]GTPaswellascross-reactedwithalltheantibodiestested.
Thisre-sultsuggeststhataRac/Rop-likeGTP-bindingpro-teinexistsinsoybeancells.
Thisproteinwasex-pressedatallgrowthstagesoftheculturedcells(datanotshown).
TranslocationoftheEndogenousRac-RelatedProteinduringtheOxidativeBurstInneutrophils,RacactivationofNADPHoxidaseinresponsetostimulationwithchemo-attractantorphorbolesterisaccompaniedbyRactranslocationfromthecytosoltothemembrane(Quinnetal.
,1993;Nisimotoetal.
,1997).
IftheRac-relatedproteininsoybeancellshasaroleanalogoustothatofRacofneutrophils,similarmembranetranslocationofthisproteinmightbeexpectedduringtheoxidativeburst.
ThereforeweexaminedthelocationoftheRac-relatedsoybeanproteinusinganti-Rac1(C189S)anti-body,sincethisantiserumexhibitedthehighestcross-reactivitywiththisproteinamongthefouran-tiseratested.
Examinationofmicrosomalandcytoso-licfractionsofsoybeancellspreparedbeforeandafterhypo-osmoticshockshowedthatmostoftheRac-relatedproteinwaspresentinthecytosolbeforetheshocktreatment,andthataconsiderableportionwasalsofoundinthemicrosomalfractionat5minaftershocktreatment(Fig.
3)whenH2O2productionwasmaximal.
TheRac-relatedproteinwaspresentinthemicrosomeevenaftertheoxidativeburstendedat20minaftertheshock(Fig.
3).
TheremaybeotherfactorsthatinactivateNADPHoxidasebeforeRacpro-teinsreturntothecytosol(Sathyamoorthyetal.
,1997).
AlteredRatesofOxidativeBurstinMutantRac1-ExpressingCellsSincetranslocationoftheendogenousRac-relatedproteinsuggestedthatsoybeancellsmayhaveaROS-generatingmechanismsimilartothatofanimalcells,wethenexaminedwhetherRacofanimalorigincouldmodulateROSgenerationbysoybeancells.
MutanthumanRac1genesweretransientlyex-pressedinsuspension-culturedsoybeancellsandtheoxidativeburstofthecellsinresponsetomechanicalstress(osmoticshock)andelicitors(oligo-GalUA[OGA]andharpin)thatinducedefenseresponseswereanalyzed.
OGAisaplantcellwallcomponentreleasedduringpathogenattackorwounding,andharpinisaproteinaceousbacterialelicitorfromEr-winiaamylovora(ChandraandLow,1997).
Thesethreestimuliinducetheoxidativeburstviadistinctsignaltransductionpathways,althoughtheidentitiesoftheintermediatesinthesepathwaysremainlargelyunknown(LowandMerida,1996).
AsseeninFigure4,osmoticshockandOGAinducedanoxida-tiveburstwithin2to3minincellstransformedwith-glucuronidase(GUS)onlyandasimilarresponsewasstimulatedbyharpinabout5minafterelicita-tion.
TolearnwhethermammalianRacmightalterthisresponse,RacV12,adominantpositiveRac1mu-tantwithconstitutiveactivity(defectiveinitsintrin-sicGTPaseactivity;Diekmannetal.
,1991)wastrans-formedintothesoybeancells.
Forasimilarpurpose,RacN17,adominantnegativeRac1mutantthatislockedintoitsinactivestatebyitspreferentialaffin-ityforGDP(FarnsworthandFeig,1991)wasalsotestedforitsinfluenceontheburst.
BecauseRacN17alsocompetesforRac'sguaninenucleotideexchangefactor(FarnsworthandFeig,1991;Jungetal.
,1994),itmightalsobeexpectedtoreduceactivationofanyendogenousplantRacisoforms.
AsshownintheinsettoFigure4A,expressionofbothmutantRac1genescouldbedemonstratedinthesoybeancellsbyFigure2.
IdentificationofaRac/Rop-relatedproteininsoybeancellsbyimmunoblottingand[-35S]GTP-bindingassay.
Crudeextractsfromsuspension-culturedsoybeancellswereseparatedby15%SDS-PAGE,transferredtonitrocellulosemembrane,andprobedwithantibodiesraisedagainstRac1(C189S)(lane1),Rop(lane2),Rac1(lane3),andRac2(lane4).
Nitrocellulosemembranepreparedinthesamemannerwasalsousedin[-35S]GTP-bindingassay,theresultofwhichwasvisualizedbyautoradiography(lane5).
Representativesfromtwoindependentexperimentswithsimilarresultsareshown.
Figure3.
TranslocationoftheRac-relatedproteinofsoybeancellsfromthecytosoltothemicrosome.
Microsomalandcytosolicfrac-tionswerepreparedfromsuspension-culturedsoybeancellsbeforeand5and20minafterhypo-osmoticshocktreatment.
Theendoge-nousRac-relatedproteinwasdetectedbyimmunoblottingwithan-tibodiesraisedagainstRac1(C189S).
Seventymicrogramsofproteinwasloadedineachlane.
Resultsshownarerepresentativesfromtwosimilarindependentexperiments.
Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000727https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
immunoblottingwithanti-mycantibody.
Moreim-portantly,noneofthesoybeancellstransformedwithRacN17,RacV12togetherwithGUS,orGUSaloneproducedH2O2intheabsenceofexternalstimuliinnormalculturemedium.
However,inresponsetoeachoftheabovethreestimuli,cellstransformedwithRacN17producedlessH2O2,whereascellstransformedwithRacV12producedmoreH2O2thancontrolcellsthatweretransformedwiththeGUSconstructonly(Fig.
4).
ThesedatasuggestthatRac-relatedproteinscanregulatetheoxidativeburstinsoybeancells.
MembraneTranslocationofHumanRacduringtheOxidativeBurstBecauseanimalRacmodifiedtheoxidativeburstrateinsoybeancells,wenextexaminedwhetherhumanRacexpressedinsoybeancellsmightalsotranslocateinamannersimilartotheendogenousRac-likesoybeanprotein.
Microsomalmembraneandcytosolwerepreparedfromtransformedcellsbeforeand5minafterosmoticshock.
RacV12,identifiedbyanti-mycantibody,wasfoundonlyinthecytosolintheabsenceofstimulus,butwasalsolocatedinthemicrosomalfractionfollowingosmoticshock(Fig.
5).
DISCUSSIONOurstudiessuggestthata21-kDGTP-bindingpro-tein,whichcross-reactswithbothanti-Ropandanti-Racantibodies(Fig.
2),isassociatedwithproductionofROSinsuspension-culturedsoybeancells.
Thecross-reactivityofthe21-kDproteinwiththeantibod-iessuggestedthatthisproteinislikelyamemberoftheplant-specificRopsubfamilyofRHOGTPasesbe-causeRopismostcloselyrelatedtoRac,sharing65%aminoacidsequenceidentitywitheachother(Lietal.
,1998).
Duringtheoxidativeburst,thisRop-likeproteinistranslocatedtothemicrosomalmembrane(Fig.
3),asisRac,aregulatorycomponentoftheNADPHoxi-dasecomplexinneutrophilcells(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
Inasimilarman-ner,thesubcellularlocationofa21-kDproteinoftobaccoandtomatocellschangedinresponsetoelic-itortreatment(Kiefferetal.
,1997;Xingetal.
,1997).
However,inthelatterstudies,anti-Rac2,butnotanti-Rac1antibodywasfoundtorecognizethecross-reactingbandat21kD,whereasinourexperimentsbothRac1andRac2antibodiescross-reactedwiththe21-kDsoybeanprotein.
Thereasonforthisdiscrep-ancyisnotclear.
BecausetheseantibodieswereraisedagainsttheC-terminalregionoftheirrespectiveRacproteins,itispossiblethattheC-terminalregionforFigure4.
AlteredratesofH2O2productionbymutantRac1-transformedsoybeancells.
Oxidativeburstassayofsuspension-culturedsoybeancellstransformedwithRacN17(b),RacV12to-getherwithGUS(d),orGUSalone(c)inresponsetohypo-osmoticshock(A),OGA(B),andharpin(C).
a,AcontrolshowingRacV12-transformedcellsintheabsenceofanystimuli.
Controlsusingallothertransformantsshowedsimilarresponse.
TheinsetofAshowswesternhybridizationusinganti-mycantibodytoprobetotalproteinextractedfromsoybeancellstransformedwithmyc-taggedRacV12(lane1),RacN17(lane2),andtheGUSgenewithoutanepitopetag(lane3).
Resultsshownarerepresentativesfromfour(osmoticshock),five(OGA),andtwo(harpin)similarindependentexperiments.
Eachexperimentconsistedofthreereplicateseachforeachsampletype.
Parketal.
728PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
thetomatoandtobaccoRop-likeproteinsisquitedif-ferentfromthatforthesoybeanRop-likeprotein.
RopisencodedbyalargegenefamilyinArabidopsisandprobablyinotherplantspeciesaswell(Lietal.
,1998).
FurtherstudiesareobviouslyneededtodeterminewhichRop-likeproteinisassociatedwiththeoxida-tiveburst.
WehaveshownthattheconstitutivelyactiveanddominantnegativeformsofhumanRacincreasedanddecreased,respectively,therateofROSproduc-tioninducedinsoybeancellsbyvariousstimuli(Fig.
4),similartotheireffectsinneutrophils(Iranietal.
,1997;Kheradmandetal.
,1998).
ThisresultsuggeststhataRop-likeproteinmaypromotetheoxidativeburstinsoybeancells,assuggestedforOsRac1inriceandRac13incottonfibercells(Kawasakietal.
,1999;Potikhaetal.
,1999).
O2generationbysoybeancellsexposedtohypo-osmoticshockanditsinhibitionbyDPIalsosuggestthatNADPHoxidaseislikelyre-sponsibleforatleastsomepartoftheoxidativeburstofsoybeancells(Fig.
1).
Moreover,threedifferentstimuli,whichactthroughdifferentsignalingcas-cades,inducedthesamechangesintheratesofROSproductioninRac-transformedsoybeancells,sug-gestingthattheRacproteinperformsacommonfunctionindiverseoxidativeburstsignalingpath-ways.
Finally,thesoybeanRac-relatedproteinandtheheterologouslyexpressedmammalianRacbothtranslocatedtothemembranefromthecytosoldur-ingROSproduction(Figs.
3and5),asdoesRacintheNADPHoxidasecomplexofneutrophils(Bokoch,1994;IraniandGoldschmidt-Clermont,1998).
Takentogether,theseresultsindicateaconservedroleforaRac-likeGproteininregulationofROSinsoybeancellsandtheysuggestthatsoybeancellsemployaRac/Rop-likeproteintomodulateanenzymaticsys-temsimilartotheNADPHoxidaseofneutrophils.
WehavealsoshownthattheconstitutivelyactiveRacmutantdoesnotconstitutivelyactivatetheoxi-dativeburstinsoybeancells;instead,inductionoftheoxidativeburstbythedominantpositiveRacmutantwasstimulus-dependent(Fig.
4).
Stimulus-dependencewasalsofoundinitstranslocationtothemicrosomes(Fig.
5).
Thesedatasuggestthatthestimulus-mediatedactivationofNADPHoxidaseinsoybeancellsinvolvesatleasttwosteps:onethatactivatesRacandanotherthatisindependentofRacactivation.
ThesecondstepismostlikelyrequiredforthetranslocationofRactotheplasmamembrane,assuggestedbystimulus-activatedRactranslocationtomembranes.
Thestimulus-dependentpromotionoftheoxidativeburstbyactivatedhumanRacinsoy-beancellsisdifferentfromtheactionofactivatedRopinriceorcottonfibercellswhereconstitutivelyactiveRopcausesconstitutiveactivationofH2O2production(Kawasakietal.
,1999;Potikhaetal.
,1999).
RopinthesesystemscouldhaveafunctiondistinctfromthatoftheRop-likeproteininsoybean.
Ontheotherhand,thedifferencemaybeduetodifferentfunctionsofplantRopandheterologoushumanRacinplantcells;RopsmayfunctionbothasaregulatorycomponentofNADPHoxidaseandasanupstreamregulatorofthesignalingcascadethateventuallyactivatesNADPHoxidase,whereastheheterologousmammalianRacmaynothavethelatterfunctioninplantcells.
IdentificationofthesoybeanRopproteininvolvedintheregulationofNADPHoxidasewillhelptoaddressthisproblem.
Asafinalpoint,weshouldpointoutthatalthoughwepresentedevidenceforpossiblesimilaritybe-tweentheROSproducingmechanismsofsoybeancellsandneutrophils,ourdatadonotexcludethepossibilitythattheRac/Rop-likeproteinofsoybeanorheterologouslyexpressedRacmodulateoxidativeburstatasitedifferentfromtheNADPHoxidase.
ROShasbeenrecentlyshowntobeproducedinsomeanimalcellswhereexistenceofthecomponentsoftheNADPHoxidaseisquestionable,andthereactivatedRacalsoenhancestherateofROSproduction(Sundaresanetal.
,1996;Iranietal.
,1997;Kherad-mandetal.
,1998;Yehetal.
,1999).
FurtherstudiesarenecessarytounderstandtheexactmechanismofmodulationofROSproductionbyRac/Rop-likepro-teininsoybeancells.
MATERIALSANDMETHODSPlantCellCultureSuspension-culturedsoybean(Glycinemax)cellsweremaintainedinMurashigeandSkoogmedium(MurashigeandSkoog,1962)containing0.
1mgkinetin,3mg2,4-dichlorophenoxyaceticacid,1gcasein,and0.
5gMES[2-(N-morpholino)ethanesulfonicacid]perliter.
Twomil-lilitersofcellswastransferredto25mLoffreshMurashigeandSkoogmediumevery7d.
Thecellsweregrowninanincubatorat23°C,withshakingat80to90rpmand18-hlight/6-hdarkcycles.
RacConstructsTheplasmidsincludingpEXV-RacV12andpEXV-RacN17weregiftsfromDr.
Jae-HongKim(KimandKim,1997;Kimetal.
,1997).
Forparticlebombardment,RacconstructswerepreparedbyinsertingtheRac1codingFigure5.
TranslocationoftheRacV12ofanimaloriginfromthecytosoltothemicrosome.
Microsomalandcytosolicfractionswerepreparedfromsuspension-culturedsoybeancellstransformedwithRacV12beforeand5minafterhypo-osmoticshocktreatment.
RacV12proteinwasdetectedbyimmunoblottingwithanti-mycan-tibody.
Representativesfromtwoindependentexperimentswithsim-ilarresultsareshown.
Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000729https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
regionintoEcoRIsiteofthebinaryvectorpGA748(provid-edbyDr.
GynheungAn)under35Spromoter,andallconstructsweretaggedwithanN-terminal9E10epitope.
DetectionofH2O2ProductionbyFluorescenceQuenchingH2O2productioninsuspension-culturedsoybeancellswasdetectedbymonitoringtheoxidativequenchingofthefluorescentdye,pyranine(8-hydroxypyrene-1,3,6-tri-sulfonicacidtrisodiumsalt:ex405nm,em512nm,Mo-lecularProbes,Eugene,OR)usingaspectrofluorimeter(RF5000,Shimadzu,Kyoto,Japan)aspreviouslydescribed(Dwyeretal.
,1996).
Hypo-osmoticshocktreatmentwasperformedbydilutingthecellsuspensionwithanequalvolumeofdistilledwater.
ElicitationwithOGAandharpin(kindgiftsofDrs.
PhilipS.
LowandStevenBeer,respec-tively;ChandraandLow,1997)wascarriedoutbytheadditionof10gofOGAor60gofharpinto1.
5mLofcellsuspension.
Thecellswerestirredgentlyinaquartzcuvetteduringfluorescencemeasurements.
SuperoxideAnionDeterminationbyCytcReductionAssayTheamountofO2accumulatedinthesoybeancellsuspensionduringtheoxidativeburstwasmeasuredbymonitoringthereductionofCytc,whichdisplaysachangeinabsorbancewhenitacceptsanelectronfromthesuper-oxideanion.
Attimezero,Cytc(typeVI;horseheart,Sigma,St.
Louis)wasaddedto50Lofcellsuspensionatafinalconcentrationof50m,andthecellsweresubjectedtoosmoticshockbytheadditionofanequalvolumeofdistilledwater.
ReducedCytcwasquantifiedbymonitor-ingA550inabio-kineticsreader(EL312e,BIO-TEKInstru-ments,Denkendorf,Germany;Curnutteetal.
,1989).
ToassesswhethertheO2accumulationinvolvesNADPHoxidase,DPI,aninhibitorofflavinoxidase,wasdissolvedindimethylsulfoxideandaddedatafinalconcentrationof30m30minbeforethestartoftheCytcreductionassay.
ToconfirmtheidentityofthereducingagentofCytcassuperoxide,weusedaninhibitorofSOD,DDC(Sigma).
DDCwasaddedto0.
5mLofcellsatafinalconcentrationof1mm,10minbeforethestartoftheassay.
TheCytcreductionassaywasperformedinthesamemannerasdescribedabove,exceptinthiscasethereducedCytcwasquantifiedwithaspectrophotometer(UV-160A,Shimadzu).
PreparationofMicrosomalandCytosolicFractionsCellswerecollectedbyfiltrationandhomogenizedingrindingmedium{100mmKCl,3mmNaCl,1mmATP,3.
5mmMgCl2,5mmSuc,1mmphenylmethylsulfonylfluo-ride,2mmdithiothreitol,and10mmPIPES[piperazine-N,N-bis-(2-ethanesulfonicacid)],pH7.
3}usingamortarandapestle.
Thehomogenatewascentrifugedat8,000gfor15min.
Thesupernatantwascollectedandcentrifugedagainat100,000gfor1h.
Theresultingpelletwasresus-pendedingrindingmediumandusedasthemicrosomalfraction.
Thecytosolicfractionwaspreparedbyconcentrat-ingthesupernatantfromthesecondcentrifugationstepusingCentriconfilters(10-kDcut-offvalue;Millipore,Bed-fold,MA),givingafinalproteinconcentrationof3to4mgmL1.
ProteinconcentrationsweremeasuredaccordingtotheBradfordmethod(Bradford,1976)usingbovineserumalbuminasastandard.
ElectrophoresisandProteinImmunoblottingProteinextracts(100g)weresubjectedto15%SDS-PAGEandthenelectrophoreticallytransferredtoa0.
22-mnitrocellulosemembrane(Schleicher&Schuell,Keene,NH).
Themembranewasblockedfor1hinTTBSbuffer{20mmTris[Tris(hydroxymethyl)aminomethane],130mmNaCl,0.
05%[w/v]NaN3,and0.
05%[v/v]Tween20,pH7.
4}supplementedwith5%(w/v)non-fatmilkpowder.
Primaryantibodieswerethenaddedat1:500dilutioninTTBS.
Anti-Rac1(C189S)antiserumwaspreparedbyimmunizingrab-bitswithpurifiedRac1(C189S)protein(Krecketal.
,1994)accordingtostandardprotocolsaspreviouslydescribed(Hanetal.
,1998).
Anti-Ropantiserumwaspreparedaspreviouslydescribed(Linetal.
,1996).
Anti-myc(Invitrogen,Carlsbad,CA),anti-Rac1,andanti-Rac2antibodies(SantaCruzBiotechnology,SantaCruz,CA)werepurchasedfromcommercialsuppliersasindicated.
Horseradishperoxidase-conjugatedanti-mouseIgGantibody(Amersham,Bucking-hamshire,UK)wasusedfordetectionofanti-mycantibody,andalkalinephosphatase-conjugatedanti-rabbitIgGanti-body(Promega,Madison,WI)wasusedfordetectionofanti-Rac1(C189S),anti-Rac1,anti-Rac2,andanti-Ropanti-bodies.
Secondaryantibodieswerediluted1:2,000inTTBS.
Allprimaryantibodiesexceptanti-mycantibodywerepoly-clonal.
Anti-Rac1(C189S)andanti-Ropantibodieswereraisedagainstwholeproteins,whereasRac1-andRac2-specificantibodieswereraisedagainsttheC-terminal11aminoacidsoftherespectiveproteins.
[-35S]GTP-BindingAssaySoybeanproteinsweretransferredontoanitrocellulosemembraneasdescribedabove.
Themembranewaswashedwith100mLofrenaturationbuffer(50mmTris-HCl,pH7.
5,0.
1%[w/v]bovineserumalbumin,5mmMgCl2,2mmdithiothreitol,and0.
1%[v/v]TritonX-100)for90minandthenincubatedin10mLoffreshrenaturationbuffercon-taining2.
7nm[-35S]GTPwithgentleagitationforafurther90min.
Duringthenext90min,themembranewaswashedsixtimeswithrenaturationbuffer.
Allreactionswereperformedatroomtemperature.
Themembranewasair-driedandradioactivebandswerevisualizedbyautoradiography.
MicroprojectileBombardmentofSuspension-CulturedSoybeanCellswithMutantRacGenesOnemilliliterofsoybeancellsuspensionculturewasharvested7daftersubculture.
CellswerespreadinathinlayeroverafilterpapermoistenedwithasmallamountofParketal.
730PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
growthmedium.
M-10tungstenparticles(Bio-Rad,Her-cules,CA)werecoatedwith30gofplasmidDNA(con-tainingequalamountofRacandGUSconstructs)andusedasthemicroprojectilesinthetransfections.
Aftermicroprojectilebombardment(BiolisticPDS-1000/HeSystem,Bio-Rad)accordingtothemanufacturer'sinstruc-tions,thecellswerescrapedfromthefilterpaperandtransferredinto5mLoffreshgrowthmedium.
Thecellsweregrowninashakingincubatorasdescribedabovefor24hbeforemeasurementoftheoxidativeburst.
Theeffi-ciencyoftransformationwasestimatedbydeterminingthepercentageofcellsexpressingGUS.
GUSactivitywasassayedbystainingthecellswithasubstratesolution(100mmsodiumphosphate,pH7.
0,1mmEDTA,5mmpotas-siumferrocyanide,5mmpotassiumferricyanide,1%[v/v]TritonX-100,and1mgmL15-bromo-4-chloro-3-indoyl--d-GlcUAcyclohexylaminesaltfromRoseScien-tific,Edmonton,AB,Canada;Citovskyetal.
,1994).
Trans-formationefficiencynormallyreached30%to60%.
ExpressionofRacproteinsinthesoybeancellswascon-firmedbywesternanalysisusinganti-mycantibody.
Tooptimizetransformationefficiency,wesometimesperformedtheosmotictreatmentdescribedbyVainetal.
(1993)byplacingthefilteredsoybeancellsontoanosmoticum-containingsolidmediumfor4hbeforeand16hafterbombardment.
Theosmoticumconsistedofanequimolarmixtureofmannitolandsorbitoltogiveafinalconcentrationof0.
25m.
ACKNOWLEDGMENTSWethankDrs.
PhilipS.
LowandSungHoRyuforusefuldiscussionsandcriticalreadingofthemanuscript.
WealsothankDrs.
Jae-HongKim,GynheungAn,PhilipS.
Low,StevenBeer,andJ.
DavidLambethforprovidinguswiththemutantRacgeneconstructs,binaryvectors,OGA,harpin,andanti-Rac1(C189S)antibody,respectively,andWon-YongSongfortechnicalassistance.
ReceivedFebruary18,2000;acceptedJune27,2000.
LITERATURECITEDApostolI,HeinsteinPF,LowPS(1989)Rapidstimulationofanoxidativeburstduringelicitationofculturedplantcells:roleindefenseandsignaltransduction.
PlantPhysiol90:109–116AtkinsonMM,KepplerLD,OrlandiEW,BakerCJ,MischkeCF(1990)InvolvementofplasmamembranecalciuminfluxinbacterialinductionoftheK/Hex-changeandhypersensitiveresponsesintobacco.
PlantPhysiol92:215–221AuhCK,MurphyTM(1995)PlasmamembraneredoxenzymeisinvolvedinthesynthesisofO2andH2O2byPhytophthoraelicitor-stimulatedrosecells.
PlantPhysiol107:1241–1247BestwickCS,BrownIR,MansfieldJW(1998)Localizedchangesinperoxidaseactivityaccompanyhydrogenper-oxidegenerationduringthedevelopmentofanonhosthypersensitivereactioninlettuce.
PlantPhysiol118:1067–1078BokochGM(1994)RegulationofthehumanneutrophilNADPHoxidasebytheRacGTP-bindingproteins.
CurrOpinCellBiol6:212–218BolwellGP,DaviesDR,GerrishC,AuhCK,MurphyTM(1998)ComparativebiochemistryoftheoxidativeburstproducedbyroseandFrenchbeancellsrevealstwodistinctmechanisms.
PlantPhysiol116:1379–1385BradfordMM(1976)Arapidandsensitivemethodforthequantificationofmicrogramquantitiesofproteinutiliz-ingtheprincipleofprotein-dyebinding.
AnalBiochem72:248–254ChandraS,LowPS(1997)MeasurementofCa2fluxesduringelicitationoftheoxidativeburstinaequorin-transformedtobaccocells.
JBiolChem272:28274–28280CitovskyV,WarnickD,ZambryskiP(1994)Nuclearim-portofAgrobacteriumVirD2andVirE2proteinsinmaizeandtobacco.
ProcNatlAcadSciUSA91:3210–3214CurnutteJT,ScottPJ,MayoLA(1989)Cytosoliccompo-nentsoftherespiratoryburstoxidase:resolutionoffourcomponents,twoofwhicharemissingincomplementingtypesofchronicgranulomatousdisease.
ProcNatlAcadSciUSA86:825–829DelmerDP,PearJR,AndrawisA,StalkerDM(1995)GenesencodingsmallGTP-bindingproteinsanalogoustomammalianracarepreferentiallyexpressedindevel-opingcottonfibers.
MolGenGenet248:43–51DesikanR,HancockJT,CoffeyMJ,NeillSJ(1996)Gen-erationofactiveoxygeninelicitedcellsofArabidopsisthalianaismediatedbyaNADPHoxidase-likeenzyme.
FEBSLett382:213–217DiekmannD,BrillS,GarrettMD,TottyN,HsuanJ,Mon-friesC,HallC,LimL,HallA(1991)BcrencodesaGTPaseactivatingproteinforp21rac.
Nature351:400–402DokeN(1983)Involvementofsuperoxideaniongenera-tioninhypersensitiveresponseofpotatotubertissuestoinfectionwithanincompatibleraceofPhytophthorainfes-tans.
PhysiolPlantPathol23:345–347DwyerSC,LegendreL,LowPS,LetoTL(1996)Plantandhumanneutrophiloxidativeburstcomplexescontainim-munologicallyrelatedproteins.
BiochimBiophysActa1289:231–237FarnsworthCA,FeigLA(1991)Dominantinhibitorymu-tationsintheMg2-bindingsiteofRasHpreventitsactivationbyGTP.
MolCellBiol11:4822–4829HanCH,FreemanJLR,LeeT,MotalebiSA,LambethJD(1998)Regulationoftheneutrophilrespiratoryburstox-idase:identificationofanactivationdomaininp67phox.
JBiolChem273:16663–16668IraniK,Goldschmidt-ClermontPJ(1998)Ras,superoxideandsignaltransduction.
BiochemPharmacol55:1339–1346IraniK,XiaY,ZweierJL,SollottSJ,DerCJ,FearonER,SundaresanM,FinkelT,Goldschmidt-ClermontPJ(1997)MitogenicsignalingmediatedbyoxidantsinRas-transformedfibroblasts.
Science275:1649–1652Rac-RelatedProteininReactiveOxygenSpeciesGenerationPlantPhysiol.
Vol.
124,2000731https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
JungV,WeiW,BallesterR,CamonisJ,MiS,VanAelstL,WiglerM,BroekD(1994)TwotypesofRASmutantsthatdominantlyinterferewithactivatorsofRAS.
MolCellBiol14:3707–3718KawasakiT,HenmiK,OnoE,HatakeyamaS,IwanoM,SatohH,ShimamotoK(1999)ThesmallGTP-bindingproteinRacisaregulatorofcelldeathinplants.
ProcNatlAcadSciUSA96:10922–10926KepplerLD,BakerCJ,AtkinsonMM(1989)Activatedoxygenproductionduringabacteria-inducedhypersen-sitivereactionintobaccosuspensioncells.
Phytopathol-ogy79:974–978KheradmandF,WernerE,TrembleP,SymonsM,WerbZ(1998)RoleofRac1andoxygenradicalsincollagenase-1expressioninducedbycellshapechange.
Science280:898–902KiefferF,Simon-PlasF,MaumeBF,BleinJP(1997)To-baccocellscontainaprotein,immunologicallyrelatedtotheneutrophilsmallGproteinRac2andinvolvedinelicitor-inducedoxidativeburst.
FEBSLett403:149–153KimBC,KimJH(1997)NuclearsignallingbyRacGTPase:essentialroleofphospholipaseA2.
BiochemJ326:333–337KimJH,KwackHJ,ChoiSE,KimBC,KimYS,KangIJ,KumarCC(1997)EssentialroleofRacGTPaseinhydro-genperoxide-inducedactivationofc-fosserumresponseelement.
FEBSLett406:93–96KostB,LemichezE,SpielhoferP,HongY,ToliasK,CarpenterC,ChuaNH(1999)Rachomologuesandcom-partmentalizedphosphatidylinositol4,5-bisphosphateactinacommonpathwaytoregulatepolarpollentubegrowth.
JCellBiol145:317–330KreckML,UhlingerDJ,TyagiSR,IngeKL,LambethJD(1994)ParticipationofthesmallmolecularweightGTP-bindingproteinRac1incell-freeactivationandassemblyoftherespiratoryburstoxidase.
JBiolChem269:4161–4168LiH,LinY,HeathRM,ZhuMX,YangZ(1999)ControlofpollentubetipgrowthbyaRopGTPases-dependentpathwaythatleadstotip-localizedcalciuminflux.
PlantCell11:1731–1741LiH,WuG,WareD,DavisKR,YangZ(1998)ArabidopsisRho-relatedGTPases:differentialgeneexpressioninpol-lenandpolarlocalizationinfissionyeast.
PlantPhysiol118:407–417LinY,WangY,ZhuJ,YangZ(1996)LocalizationofaRhoGTPaseimpliesaroleintipgrowthandmovementofthegenerativecellinpollentubes.
PlantCell8:293–303LinY,YangZ(1997)Inhibitionofpollentubeelongationbymicroinjectedanti-Rop1Psantibodiessuggestacru-cialroleforRho-typeGTPasesinthecontroloftipgrowth.
PlantCell9:1647–1659LowPS,MeridaJR(1996)Theoxidativeburstinplantdefense:functionandsignaltransduction.
PhysiolPlant96:533–542MurashigeT,SkoogF(1962)Arevisedmediumforrapidgrowthandbioassayswithtobaccotissuecultures.
PhysiolPlant15:473–497MurphyTM,AuhCK(1996)Thesuperoxidesynthasesofplasmamembranepreparationsfromculturedrosecells.
PlantPhysiol110:621–629NisimotoY,FreemanJLR,MotalebiSA,HirshbergM,LambethJD(1997)Racbindingtop67phox:structuralbasisforinteractionsoftheRac1effectorregionandinsertregionwithcomponentsoftherespiratoryburstoxidase.
JBiolChem272:18834–18841O'DonnellVB,TewDG,JonesOTG,EnglandPJ(1993)Studiesontheinhibitorymechanismofiodoniumcom-poundswithspecialreferencetoneutrophilNADPHoxidase.
BiochemJ290:41–49PengM,KucJ(1992)Peroxidase-generatedhydrogenper-oxideasasourceofantifungalactivityinvitroandontobaccoleafdisk.
Phytopathology82:696–699PotikhaTS,CollinsCC,JohnsonDI,DelmerDP,LevineA(1999)Theinvolvementofhydrogenperoxideinthedifferentiationofsecondarywallsincottonfibers.
PlantPhysiol119:849–858QuinnMT,EvansT,LoetterleLR,JesaitisAJ,BokochGM(1993)TranslocationofRaccorrelateswithNADPHox-idaseactivation.
JBiolChem268:20983–20987SathyamoorthyM,MendezID,AdamsAG,LetoTL(1997)p40phoxdown-regulatesNADPHoxidaseactivitythroughinteractionswithitsSH3domain.
JBiolChem272:9141–9146StennisMJ,ChandraS,RyanCA,LowPS(1998)Systeminpotentiatestheoxidativeburstinculturedtomatocells.
PlantPhysiol117:1031–1036SundaresanM,YuZX,FerransVJ,SulcinerDJ,GutkindJS,IraniK,Goldschmidt-ClermontPJ,FinkelT(1996)Regulationofreactive-oxygen-speciesgenerationinfi-broblastsbyRac1.
BiochemJ318:379–382VainP,McMullenMD,FinerJJ(1993)Osmotictreatmentenhancesparticlebombardment-mediatedtransientandstabletransformationofmaize.
PlantCellReports12:84–88WingeP,BrembuT,BonesAM(1997)Cloningandchar-acterizationofrac-likecDNAsfromArabidopsisthaliana.
PlantMolBiol35:483–495XiaG,RamachandranS,HongY,ChanYS,SimanisV,ChuaNH(1996)Identificationofplantcytoskeletal,cellcycle-relatedandpolarity-relatedproteinsusingSchizo-saccharomycespombe.
PlantJ10:761–769XingT,HigginsVJ,BlumwaldE(1997)Race-specificelic-itorsofCladosporiumfulvumpromotetranslocationofcytosoliccomponentsofNADPHoxidasetotheplasmamembraneoftomatocells.
PlantCell9:249–259YangZ,WatsonJC(1993)Molecularcloningandcharac-terizationofrho,aras-relatedsmallGTP-bindingproteinfromthegardenpea.
ProcNatlAcadSciUSA90:8732–8736YehLH,ParkYJ,HansaliaRJ,AhmedIS,DeshpandeSS,Goldschmidt-ClermontPJ,IraniK,AlevriadouBR(1999)Shear-inducedtyrosinephosphorylationinendo-thelialcellsrequiresRac1-dependentproductionofROS.
AmJPhysiol276:C838–C847Parketal.
732PlantPhysiol.
Vol.
124,2000https://plantphysiol.
orgDownloadedonFebruary9,2021.
-PublishedbyCopyright(c)2020AmericanSocietyofPlantBiologists.
Allrightsreserved.
- generating16668.com相关文档
- 物质16668.com
- synthesized16668.com
- addressing16668.com
- 广发证券16668.com
- excitation16668.com
- sample16668.com
RAKSmart VPS主机半价活动 支持Windows系统 包含香港、日本机房
RAKSmart 商家最近动作还是比较大的,比如他们也在增加云服务器产品,目前已经包含美国圣何塞和洛杉矶机房,以及这个月有新增的中国香港机房,根据大趋势云服务器算是比较技术流的趋势。传统的VPS主机架构方案在技术层面上稍微落后一些,当然也是可以用的。不清楚是商家出于对于传统VPS主机清理库存,还是多渠道的产品化营销,看到RAKSmart VPS主机提供美国、香港和日本机房的半价促销,当然也包括其他...
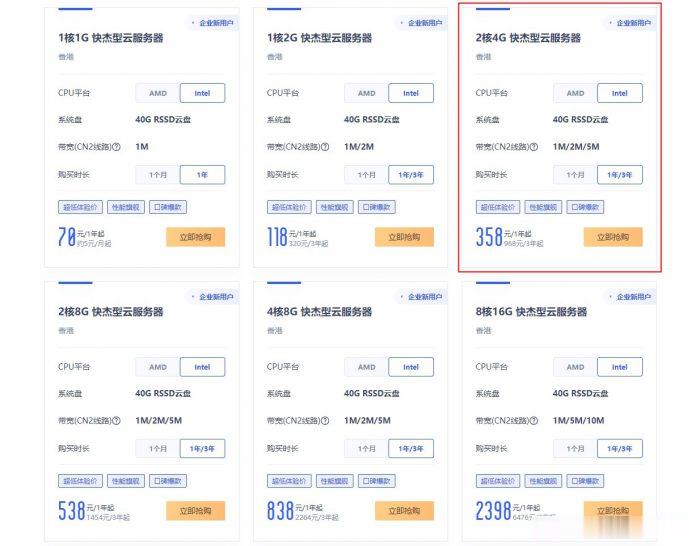
ucloud香港服务器优惠活动:香港2核4G云服务器低至358元/年,968元/3年
ucloud香港服务器优惠降价活动开始了!此前,ucloud官方全球云大促活动的香港云服务器一度上涨至2核4G配置752元/年,2031元/3年。让很多想购买ucloud香港云服务器的新用户望而却步!不过,目前,ucloud官方下调了香港服务器价格,此前2核4G香港云服务器752元/年,现在降至358元/年,968元/3年,价格降了快一半了!UCloud活动路子和阿里云、腾讯云不同,活动一步到位,...
安徽BGP云服务器 1核 1G 5M 29元/月 香港云服务器 1核 1G 19元首月 麻花云
麻花云怎么样?麻花云公司成立于2007年,当前主打产品为安徽移动BGP线路,数据中心连入移动骨干网。提供5M,10M大带宽云主机,香港云服务器产品,数据中心为香港将军澳机房,香港宽频机房 cn2-GIA优质线路、采用HYPER-V,KVM虚拟技术架构一、麻花云官网点击直达麻花云官方网站合肥网联网络科技有限公司优惠码: 专属优惠码:F1B07B 享受85折优惠。最新活动 :双11 云上嗨购 香港云主...
16668.com为你推荐
-
kaixin001.com耍开心网的具体步骤有哪些?公司网络被攻击受到网络人身攻击如何处理?openeuler电脑上显示openser是什么意思?sherylsandberg这个文章什么意思 给个翻译好吗 谢谢了百度商城百度知道一般一天能挣多少钱?嘉兴商标注册个人如何申请商标注册月神谭求男变女类的变身小说www.765.com有没好的学习网站lcoc.top日本Ni-TOP是什么意思?lcoc.toptop weenie 是什么?