ideawww.vtigu.com
www.vtigu.com 时间:2021-03-19 阅读:()
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org357TThheerraannoossttiiccss2015;5(4):357-370.
doi:10.
7150/thno.
10657ReviewOfftotheOrganelles-KillingCancerCellswithTargetedGoldNanoparticlesMohamedKodiha1,YiMengWang1,ElizaHutter2,DusicaMaysinger2,UrsulaStochaj11.
DepartmentofPhysiology,McGillUniversity,Montreal,Canada;2.
DepartmentofPharmacology&Therapeutics,McGillUniversity,Montreal,Canada.
Correspondingauthor:UrsulaStochaj,Ph.
D.
DepartmentofPhysiology,McGillUniversity,3655PromenadeSirWilliamOsler,Montreal,QC,Canada,H3G1Y6.
Phone:514-398-2949Fax:514-398-7452E-mail:ursula.
stochaj@mcgill.
ca.
IvyspringInternationalPublisher.
Thisisanopen-accessarticledistributedunderthetermsoftheCreativeCommonsLicense(http://creativecommons.
org/licenses/by-nc-nd/3.
0/).
Reproductionispermittedforpersonal,noncommercialuse,providedthatthearticleisinwhole,unmodified,andproperlycited.
Received:2014.
09.
27;Accepted:2014.
12.
16;Published:2015.
01.
21AbstractGoldnanoparticles(AuNPs)areexcellenttoolsforcancercellimagingandbasicresearch.
However,theyhaveyettoreachtheirfullpotentialintheclinic.
Atpresent,weareonlybeginningtounderstandthemolecularmechanismsthatunderliethebiologicaleffectsofAuNPs,includingthestructuralandfunctionalchangesofcancercells.
Thisknowledgeiscriticalfortwoaspectsofnanomedicine.
First,itwilldefinetheAuNP-inducedeventsatthesubcellularandmolecularlevel,therebypossiblyidentifyingnewtargetsforcancertreatment.
Second,itcouldprovidenewstrategiestoimproveAuNP-dependentcancerdiagnosisandtreatment.
OurreviewsummarizestheimpactofAuNPsonselectedsubcellularorganellesthatarerelevanttocancertherapy.
Wefocusonthenucleus,itssubcompartments,andmitochondria,becausetheyareintimatelylinkedtocancercellsurvival,growth,proliferationanddeath.
Whilenon-targetedAuNPscandamagetumorcells,concentratingAuNPsinparticularsubcellularlocationswilllikelyimprovetumorcellkilling.
Thus,itwillincreasecancercelldamagebyphotothermalablation,mechanicalinjuryorlocalizeddrugdelivery.
Thisconceptispromising,butAuNPshavetoovercomemultiplehurdlestoperformthesetasks.
AuNPsize,morphologyandsurfacemodifi-cationarecriticalparametersfortheirdeliverytoorganelles.
Recentstrategiesexploredallofthesevariables,andsurfacefunctionalizationhasbecomecrucialtoconcentrateAuNPsinsub-cellularcompartments.
Here,wehighlighttheuseofAuNPstodamagecancercellsandtheirorganelles.
WediscusscurrentlimitationsofAuNP-basedcancerresearchandconcludewithfuturedirectionsforAuNP-dependentcancertreatment.
Keywords:Goldnanoparticles,AuNPs,cancercellimagingThisreviewprovidesanupdateonthethera-peuticpotentialofgoldnanoparticles(AuNPs)foroncology.
Tothisend,weintroduceAuNPsasthera-peutictools,summarizethecurrentstrategiesthattargetAuNPstospecificcellcompartmentsanddis-cusshowthistargetingimpactscancercellkilling.
Forsubcellulartargeting,ourfocusisonnucleiandmi-tochondria,sincebothorganellesareintimatelylinkedtocancercellsurvival,growthandprolifera-tionandthereforeprimarytargetsforanti-canceragents[1,2].
Weconcludebyhighlightingunsolvedquestionsandpotentialroadblocksinthefield.
1.
IntroductionNanotechnologyisinthespotlightoftherapeuticinnovation[3],andAuNPsareparticularlypromisingtoolstoimprovecancertreatment[4].
Duetotheiruniqueopticalproperties,non-toxicnature,relativelysimplepreparationandfunctionalization,AuNPsareexcellentcandidatesformanybiologicalapplications,IvyspringInternationalPublisherTheranostics2015,Vol.
5,Issue4http://www.
thno.
org358suchasimaging,drugdeliveryandphotothermaltherapy.
Theseapplicationscommonlytakead-vantageoftheparticles'stronglightscattering,in-tenseabsorption,andelectromagneticfieldenhance-mentthatresultfromlocalizedsurfaceplasmonres-onance[5,6].
AuNPscanbeproducedinlargequantitieswithdefinedshapesandsizes.
Themostcommonap-proachessynthesizeAuNPsinsituthroughchemicalreductionofgoldsaltsandseed-mediatedgrowth[7],whichenlargestheparticlesstepbystep.
ThismethodisidealtocontrolAuNPsizeandshape[8-10]andusedtoproducelargespherical,semi-spherical,rod-like,branchedorotherparticleshapes[7].
AuNPsurfacesareamenabletocovalentandnon-covalentsurfacemodifications;thispropertyiscrucialforcel-lularandsubcellulartargeting.
Asthephysi-co-chemicalcharacterizationofAuNPsandtheirde-tectionhavebeenreviewedbyothers[11-15],itwillnotbediscussedhere.
ThedevelopmentofAuNP-basedstrategiesfortheeradicationofcancercellsisimportant,becauseeffectivetherapiesarefrequentlynotavailableforrapidlyprogressingcancers[16].
Sofar,manyofthestudiesonAuNPssuggestthatcancercellsareespe-ciallyvulnerabletotheseparticles.
Thus,AuNP-basedtreatmentcandestroycancercells,withminimalin-jurytohealthycells[17].
ThetherapeuticvalueofAuNPsisbasedon(i)theirdistinctivephysicalpropertiesand(ii)theirabil-itytointeractwithtumorsanddamagecancercells.
Thus,theenhancedpermeabilityandretention(EPR)characteristicsofmany,butnotall,tumorsfacilitateAuNPinfiltrationintothetumor[18].
Duetothispassivetargeting,AuNPs(~6-200nm)accessthetu-mortissue,wheretheyaccumulateintheextracellularmatrixbeforeenteringthecells[19].
Followingtheirassociationwithtumorcells,AuNPspromoteuniquewaysofkilling(Fig.
1).
Theycandestroycancercellsbyphotothermalablation,asexemplifiedbyAuro-Shell[20,21],throughmechanicaldamage,orasdrugdeliverysystemsforanticanceragents,suchastumornecrosisfactor[21,22]ordoxorubicin[23,24].
WhatarethebenefitsofsubcellularAuNPtargetingWhileAuNPsarerelevantfordifferentclinicalapplications,furtherimprovementsofAuNP-basedstrategiesareexpectedtooptimizethetherapeuticoutcomes.
OnesuchimprovementisbasedontheconceptthatAuNPtargetingtospecificorganellesmaximizestheimpactontumorcells.
Tothisend,AuNPsarebeingdevelopedthataccumulateinsub-cellularcompartmentswheretheydestroyintrinsiccancercellfunctionsthatareessentialfortumorsur-vival.
Onceintheirproperintracellularlocation,AuNPscanenhancecancercelldestructionbydif-ferentmeans.
Thisincludestheconfineddeliveryofanti-canceragents[25],localizedsubcellularmechan-icaldamage,andimprovedefficiencyofphotothermalablationduetohighlocalAuNPconcentrations[26,27].
SuchcontrolledAuNPactionwillnotonlyin-creasecancercellkilling,butalsodiminishtoxicsideeffects,becauseitreducesthenecessaryamountsofAuNPsanddrug-load.
Candidatecompoundsfornanoparticle-dependentsubcellulardeliveryaredox-orubicin[23],platinum-baseddrugs[28]andpaclitaxel[29].
Theseanticanceragentsinterferewithnuclearandmitochondrialfunctions,respectively[30-33]andhavebeenusedtofunctionalizeAuNPs[23,34-37].
Asidefromdrugs,AuNPscanalsodeliveroligonucleotidestoaltergeneexpressionorsplicing([38]andreferencestherein).
Figure1.
ImpactofAuNPsoncancercells.
Size,morphology,functionalgroupsontheAuNPsurfaceandthecelltypedeterminethesubcellulardistributionofAuNPs.
AuNPscancausetumorcelldeathbyphotothermalablation,me-chanicaldamage,andincreaseinthelocalizeddrugconcentration.
Theseeventscanbecombinedtoenhancetheirkillingefficiency.
WhatarethebottlenecksforAuNPtargetingtospecificsubcellularcompartmentsOnceaccumulatedintumortissue,AuNPshavetoovercomemultipleobstaclesbeforetheycancon-centrateinthedesiredcellcompartment:(i)cellsur-facebinding,(ii)cellularuptake,(iii)escapefromly-sosomes/endosomes,and(iv)associationwithapar-ticularsubcellularlocation,suchasnucleiormito-chondria(Fig.
2;[39]).
ThefirstthreestepsaregeneralfeaturesthatregulatetheintracellulardestinationofallAuNPs.
Thesestepshavebeenreviewedexten-sively[13,39-42];wewillonlybrieflysummarizethemhereandthenprovideamoredetaileddiscus-sionofAuNPtargetingtonuclei,mitochondriaandtheER.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org359Figure2.
ObstaclesAuNPshavetoovercomeforsuccessfultargetingtointracellularorganellesorcompartments.
OnceAuNPsareintheextracellularmatrixofthetumor(ECM,barrier1),theyhavetobindtothecancercellsurface.
Cellularuptakerequirestranslocationacrosstheplasmamembrane(barrier2),byendocytosisorothermechanisms.
Insidethecell,AuNPshavetoescapefromendosomesorlysosomes(barrier3)tosubsequentlyassociatewiththedesiredorganelleorcellcompartment(barrier4).
Possiblefinaldestinationsarethenucleus(blue)ormitochondria(yellow).
Bindingtothecancercellsurface,internaliza-tionandescapefromendosomes/lysosomesAuNPuptake,subcellulardistributionandtox-icityaredeterminedbyparticlesize,morphologyandsurfacemodification.
AlthoughAuNPsofdifferentshapes(spherical,shells,rods,diamonds)orsizes(1-100nm)accumulateinvariouscancercells,theiruptakekineticsandtoxicitymayvaryprofoundly(Fig.
1,reviewedby[13]).
Besidesshapeandsize,AuNP-basedbio-nanointeractionsarefurthermodu-latedbytheirfunctionalization[11,43,44].
Ingeneral,positivechargesontheAuNPsurfacestimulatecel-lularuptake,possiblyduetoelectrostaticinteractionswiththecellsurface[45].
PositivechargescanalsoimproveAuNPtransporttothenucleus,becausenu-clearlocalizationsequences(NLSs)ofmanyproteinsareenrichedforbasicaminoacidresidues.
ParticleuptakenotonlydependsonAuNPproperties,butalsoreliesonthecelltype.
Whengrowninculture,cancerandnon-tumorigeniccellsdiffersignificantlyinthisrespect.
Notably,tumorcellsareoftenmorevulnerabletoAuNPs[46,47].
Nevertheless,thereisvariabilityevenamongcancercells;forexample,AuNPuptakediffersinhepatocel-lularcarcinoma(HepG2)andcervixcarcinomacells(HeLa)[48].
Despitesuchcell-typespecificdiffer-ences,nanoparticlebindingtocancercellscanbeen-hancedbyexploitingtumor-relatedchangesinplas-mamembranecomposition(AdditionalFile1:TableS1andassociatedreferences[41-91]).
Tothisend,particlesurfaceshavebeenfunctionalizedwithlig-andsthatimprovedockingatthetumorcellmem-brane.
LigandsthatpromotehighaviditybindingtocancercellsincludeEGF(epidermalgrowthfactor;associateswithEGFR)orpeptidescontainingtheRGDmotif(arginine-glycine-asparticacid;recognizedbysomeintegrinfamilymembers)[32,92-94].
Nucle-olin,transferrinorantibodiesagainstHer2andEGFR[55,95-97]canalsoenhancenanoparticlebindingtocancercells.
Onceboundtothecellsurface,AuNPsenterthecell;inmostcasesthisoccursbyanenergy-dependentprocess,forwhichendocytosisisthemainroute(re-viewedin[13,14]).
Followingcellularuptake,AuNPsinitiallylocatetoendosomesand/orlysosomes,wheretheorganellarmembraneintegritydeterminesAuNPretention(reviewedin[14]).
However,properfunctionalizationofAuNPscanstimulatetheirendo-somal/lysosomalescapeorpromoteuptakebynon-endocytoticpathways([13,14]andreferencestherein).
2.
NucleirepresentprimarytargetsforcancertherapyAsnucleimastermindessentialaspectsoftumorcellbiology,theyhavebecomeimportanttargetsincancertherapyingeneral,andAuNP-dependentin-terventionsinparticular.
Thenucleuscontrolscellgrowth,proliferationandapoptosis,andmanyan-ti-cancerdrugsobliteratethesefunctions.
Atthesametime,nuclearhomeostasisisoftenalteredincancercells,whichdisplaychangesinnuclearsize,shape,envelope,laminaandchromatinorganization,nucle-olarfunctionornucleocytoplasmictrafficking[98-104].
Forexample,theconcentrationofnucleartransportcarriersisfrequentlyincreasedintrans-formedcellsortumorsamples,andthiscorrelateswithaugmentedsignal-mediatednuclearimportandexport[105].
Ascomparedtotheirnormalcounter-parts,transformedcellsoftentransportlargerAuNPs,andAuNPnucleartranslocationismoreefficient[106-108].
Nuclearinteractionsofnon-targetedAuNPsEvenintheabsenceofsubcellulartargetingsig-nals,certainAuNPtypesassociatewiththenucleusanditssubcompartments.
Insomebutnotallcasesthisnuclearlocalizationisaccompaniedbyorganelledamage.
Assuch,AuNPs(3.
7nmaveragediameter)weremodifiedwithboth3-mercaptopropionicacidandpolyethyleneglycol(PEG)andconjugatedtoFITC[58].
AlthoughtheparticlesaccumulatedinHeLacellnucleiaftera24-hourincubationperiod,cellTheranostics2015,Vol.
5,Issue4http://www.
thno.
org360viabilitystillamountedto85%ofthecontrolsamples.
Evenafter72hoursandwithupto10MofAuNPs,cellsurvivalwasmaintainedat70%,suggestinglowcytotoxicityinHeLacells[58].
Ontheotherhand,Au55goldclusters(1.
4nm)enteredthecellnucleuswheretheyinteractedwithDNAandelicitedtoxiceffects[109].
Comparisonofhealthyandtumorcelllinesrevealedmaximumtox-icityforthemetastaticmelanomacelllinesMV3andBLM.
TheinteractionbetweenAu55goldclustersandnuclearDNAislikelytheunderlyingcauseofthistoxicity.
OurrecentstudiesexaminedAuNPsofdifferentsizesandmorphologies.
Inbreastcancercells,nuclearmembranes,nuclearlaminaeandnucleolarfunctionswerecompromisedbysmallsphericalAuNPsorgoldnanoflowers,butnotbylargesphericalAuNPs([46],Fig.
3).
Thedamageinflictedbynon-sphericalgoldnanoflowersisparticularlyinteresting;despitetheirlargesize(40-120nm),theyenteredthenucleusanddestroyednuclearhomeostasisinbreastcancercells,butnotinnormalbreastcells.
Figure3.
(A)Goldnanoflowersandlargegoldnanospheres(red)associatewithnucleiofdifferentbreastcells,i.
e.
MCF7andnon-tumorigenichumanmammaryepithelialcells(HuMEC).
NotethatgoldnanoflowerscanbedetectedinthenuclearinteriorofMCF7cells,wheretheydisruptthenuclearlamina(green).
Scalebarsare10m.
(B)Smallgoldnanospheres(15.
6nmdiameter)andgoldnanoflowers(40-120nm),butnotlargegoldnanospheres(60nm),alterthenuclearorganizationinMCF7cells.
Inparticular,nuclearporecomplexes(NPC,red)andthenuclearlamina(LaminA,green)showseverechanges.
Arrowsmarksomeofthenucleiwithalteredmorphology;scalebaris20m.
(C)SmallgoldnanospheresandgoldnanoflowersinhibitdenovoRNAsynthesis(magenta)inthenucleolus.
Scalebaris3m.
Adaptedfrom[46]withpermission.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org361TargetingAuNPstothenucleusWhichAuNPsizefitsthenucleusTheoptimalAuNPpropertiesfornucleartargetingdependonthedesiredimpactoftheparticle.
IfAuNPsaredestinedforthenuclearinterior,thesizeofthetransportchannelofthenuclearporecomplex(NPC)hastobeconsidered.
Accordingly,sphericalparticlesof9nmorlessindiametermaycrosstheNPCbydiffusion,whereasparticlesupto39nmindiametercanbede-liveredtothenucleusiftheycarryanucleartransportsignal[110].
Thesearenotfixedvalues,asthenucleartransportmachineryisoftenalteredincancercells(seeabove).
Inthecontextofcancertherapy,threedifferentscenarioscanapplytonuclearAuNPs:(i)theyaretransportedintothenuclearinterioror(ii)clogtheNPC,or(iii)releasecytotoxicdrugsinthevicinityofnuclei.
NuclearimportofAuNPs.
Theground-breakingworkbyCarlFeldherrbuiltthefoundationforAuNPtargetingtothenucleus.
HislaboratorycoatedAuNPswithconjugatesthatcontainedserumalbuminandthenuclearlocalizationsequences(NLSs)derivedfromSV40T-antigenornucleoplasmin.
Uponinjec-tionintothecytoplasm,thesefunctionalizedAuNPstranslocatedacrossthenuclearporecomplex(NPC)andaccumulatedinsidethenucleus[111,112].
Feldheim'sgroupwentbeyondthesestudiesanddefinedthedifferentstagesofAuNPnuclearlocaliza-tioninintactculturedcells[39,59].
Thesestudiesshowedthatasidefromtheinitialbinding,cellularuptakeandreleasefromendosomes/lysosomes(seeabove),targetingtothenucleusandpassageacrossthenuclearenvelope(NE)arecriticalstepsthatlimitthedeliverytothenucleus.
WhileAuNPsdestinedforthenucleusshowcell-typespecificdifferencesincel-lularuptake,escapefromendosomes/lysosomesandnucleartargeting[113],theyalsosharecommonfea-tures.
Forexample,uptakeofNLS-modifiedAuNPsistemperature-dependentinculturedcells,consistentwithendocytosis[59,113].
Furthermore,increasingthenumberofNLS-peptidesontheparticlesurfaceenhancesAuNPnuclearlocalizationandtoxicity[59].
Variousstrategieshavebeenemployedtotargetgoldnanoparticlestothenucleus.
Sofar,thereisnoprotocolthathasbeenuniversallysuccessfulfordif-ferenttumorcells.
Therefore,wediscussspecificex-amplesforAuNPsthatwerefunctionalizedwithNLSs,cell-penetratingpeptides,peptidecombina-tions,oligonucleotidesorothermoieties(summarizedinAdditionalFile1:TableS1).
Peptidesequencesaredepictedintheone-lettercode.
ThesuccessofmultiplepeptidemodificationswasdemonstratedforAuNPsmodifiedwithSV40-NLSoranNLSderivedfromadenovirusfiberproteins;theseparticlesdidnotenterthenucleusofHepG2cells.
However,uponfurtheradditionoftheadenovirusreceptor-mediatedendocytosissequence,AuNPslocalizedtonuclei[39].
Notably,whenAuNPscarriedtheNLSandthereceptor-mediatedendocyto-sispeptideasseparateentities,nucleartargetingwasenhancedascomparedtoalongerpeptidethatcom-binesbothsequences.
Ifcombinedintoonepeptide,thetwosequenceelementsmaybelessaccessibletocellularbindingpartners.
Inotherstudies,AuNPsstabilizedwiththenon-naturalaminoacidtiopronin[94]werefunction-alizedwithGRKKRRQRRR,apeptidederivedfromtheHIV-1proteinTat[61].
Aftera1-hourtreatment,Tat-modifiedparticleswerelocatedinthenuclearinteriorofhTERT-BJ1humanfibroblasts.
Bycontrast,AuNPscarryingtioproninaloneaccumulatedincy-toplasmicvacuolesoratthemitochondrialperiphery[61].
Upona24-hourincubationperiodwithupto10MAuNPs(coresize2.
8nm),toxicitywas≤20%forbothtypesofparticles.
Toimprovecellularuptakeandnuclearaccu-mulationof30nmAuNPs,particlesweredecoratedwithpeptidesthatcontainedthesequenceCALNN.
AdditionofmixedpeptidescontainingCALNNandCALNNpluseightarginineresidues(CALNNR8)promotedAuNPaccumulationinnucleiofHeLacells[52].
Afterincubationwith0.
32nMAuNPsfor24hours,celldeathcouldreach95%.
Infurtherexperiments,16or14nmPEGylatedAuNPsweremodifiedwithdifferentCALLN-derivedpeptides.
FunctionalizationwithCALLN-fusionscontainingthecellpenetratingpeptideofTAT(CALLN-AGRKKRRQRRR)orPntn(CALLN-GRQIKIWFQNRRMKWKK)stimulatedcellentry,whereasanNLS(CALLN-GGFSTSLRARKA)wasaddedfornucleartargeting[66,114].
Inthissce-nario,AuNPsmodifiedsimultaneouslywithNLS,TATandPntn-containingpeptidesledtohigherin-tracellulargoldcontentwhencomparedtoAuNPscarryingonepeptidespeciesonly.
Furthermore,sim-ultaneouscoatingwithNLS,TATandPntnpromotedAuNPnuclearlocalization.
Surprisingly,ifAuNPscarryapositivesurfacecharge,nucleartargetingcanevenbeaccomplishedwithpeptidesthathavenosequencesimilaritytoabasicNLS([64],Fig.
4).
Nucleolinisamultifunctionalproteinthatre-sidesinthenucleolusandothercellcompartments,suchastheplasmamembrane.
Forsomecancercellsnucleolinabundanceishighintheplasmamembrane,wheretheproteincanserveasadockingsiteforAuNPs.
TheaptamerAS1411bindsnucleolinandwastestedinphaseIandIIclinicaltrialsforadvancedsolidtumorsandacutemyeloidleukemia[21].
WhenTheranostics2015,Vol.
5,Issue4http://www.
thno.
org362goldnanostarswerefunctionalizedwithAS1411,theyenteredHeLacellsandconcentratedinthevicinityofnuclei[55].
Thisuptakerequiredbothnucleolinonthecellsurfaceandtheaptamer.
AS1411-goldnanostarlocalizationclosetothenucleuscorrelatedwithchangesinnuclearmorphology,suggestingmechan-icaldamagetothenuclearenvelope.
Irradiationlib-eratedtheaptamerfromAuNPs,whichinturnacti-vatedcaspases3and7andincreasedcelldeath.
Col-lectively,thestudysupportsthemodelthattheap-tamerreleaseclosetothenucleusenhanceddamageandtheensuingcelldeath.
Figure4.
Detectionof13nmAuNPsmodifiedwithCIPGNVG-PEG-NH3+in1BR3Gcells(transformedhumanskinfibroblasts).
Cellswereincubatedfor3hourswithfunctionalizedAuNPs,andparticleswerevisualizedbytransmissionelectronmicroscopy.
SomeoftheAuNPswerepresentinthenuclearinterior,asindicatedbytheredarrows.
PanelsAandBdepicttwodifferentnuclei.
Scalebarsare2m.
AdaptedfromOjea-Jiménezetal.
[64]withpermission.
Theexamplesabovesuggestthattargetingtothenucleuscanamplifyorganelle-specificinsults.
ThisideaisvalidatedbyNLS-modifiedAuNPsthatin-creasedDNAdamageandinterferedwithcelldivi-sion,inparticularcytokinesis[56].
Specifically,30nmPEGylatedAuNPsdecoratedwithRGD-andNLS-peptidesaccumulatedinnucleiandtriggeredapoptosisin20%ofthecancercells[56].
Asidefromtargetingtothenuclearinterior,AuNPscanalsobeusedtoobstructtheNPC.
Thus,NLS-modifiedAuNPs(~39or32nminsize)blockednucleartransportinHeLacellsandinducedau-tophagiccelldeath[115].
Interestingly,thiswascelltypespecific,becauseitwasnotobservedinSiHacells,anothercervixcarcinomacellline.
Deliveryofnucleicacids.
OneoftheapplicationsofnuclearAuNPsisthemodulationofgeneexpression;thiscanbeachievedbyknockdownorchangesinsplicing[60,116-119].
Anexampleofthisapproachistheuseof13nmAuNPsthatcarriedoligonucleotidestoadjustthealternativesplicingofmRNAsforpro-survivalfactorsindifferentexperimentalsystems[119].
Assuch,theparticlesweredetectedinnucleiofculturedcellsandledtotumorshrinkageinaxeno-graftmodelbasedonLoVocells(humancoloncarci-noma).
InMCF7breastcancercells,sphericalultrasmall(6and2nm)tiopronin-coatedAuNPsresideinthecytoplasmandenterthenucleusaftera24-hourin-cubationperiod[60].
Whenconjugatedtoafluores-centFITC-tag,only2nmparticlesweredetectedinMCF7nuclei.
FITC-labelingincreasedthesizeof6nmAuNPsto10nm,whichmayhavelimitedthepassagethroughNPCs.
Toinducecelldeath,anoligonucleo-tidewasaddedto2nmtiopronin-AuNPs.
Thisoligo-nucleotidegeneratedtriplexstructureswiththeP2promoterofc-MYC.
Asaresult,expressionoftheproto-oncogenewasdownregulatedandcellviabilitywasreducedto70%.
Whencomparedtothetri-plex-formingoligonucleotidealone,AuNP-attachedoligonucleotidesincreasedc-MYCsilencingandMCF7celldeath[60].
Interestingly,AuNPsfunctionalizedwithdexa-methasonealsodeliveredplasmidDNAtonuclei[76],suggestingthatsteroidhormonescanbeusedforAuNPnucleartargeting.
Thesestudieswereper-formedinculturedcellsandexperimentalanimals;theyarediscussedinsection5.
Deliveryofanti-cancerdrugs.
AuNPsthatlocatetothenuclearperipherycanprovidetransportvehiclesforanti-cancerdrugs.
Onesuchdeliverysystemcar-rieddoxorubicinandwastestedinHeLa(cervixcar-cinoma),A549(lungcarcinoma)andNIH3T3-L1cells(fibroblastswithpre-adiposecharacteristics).
Tothisend,25nmPEGylatedsphericalAuNPswerefunc-tionalizedwithcellpenetratingpeptides,suchasTAT[24].
TAT-AuNPsweretakenupinallcellstested,whereasotherpeptidesdisplayedcelltype-specificdifferences.
Doxorubicin-loadedAuNPswereespe-ciallycytotoxictoHeLaandA549cells,butNIH3T3-L1cellswerelessaffected.
Celldeathwasattributedtothereleaseofdoxorubicinclosetothenucleus,whereAuNPsconcentrated.
3.
TargetingcancercellmitochondriawithAuNPsMitochondriaarethemajorsitesofcellularen-ergyproduction,andtheirdysfunctionisassociatedwithawiderangeofdiseasesandpathophysiologies,includingcancer[120].
Changesinbioenergeticsareahallmarkofmanytumors,butmitochondriaarealsoTheranostics2015,Vol.
5,Issue4http://www.
thno.
org363keyregulatorsofapoptoticcelldeath.
Theseproper-tiesmakemitochondriaprimetargetsforcancertreatment[121,122].
AuNPsoftenimpairmitochon-drialfunctionsandtherebyinducecelldeath.
How-ever,thisdoesnotalwaysinvolvestablephysicalcontactbetweenAuNPsandtheorganelle.
Here,wefocusonexamplesthatreportAuNPassociationwithmitochondria.
TheemphasisisonstudiesthatweredesignedtodeliverAuNPsspecificallytomitochon-dria.
WhichAuNPsizefitsmitochondriaTheconcep-tualdifferencesforAuNPtargetingtonucleiandmi-tochondriaarebasedonthedistinctorganelleborderstothecytoplasm.
Unlikethenucleus,mitochondriadonotcontainlargeporesthatprovideeasyaccessforAuNPs.
BoththeouterandtheinnermitochondrialmembranespresentbarrierstoAuNPsthataredes-tinedforthemitochondrialmatrix.
Inheartcells,3nmparticles,butnot6nmAuNPs,translocatedacrosstheoutermitochondrialmembrane[86].
Intheinnermi-tochondrialmembrane,proteinimportchannelspro-videopeningsof<2nm([123],reviewedin[124]).
ThisrestrictsAuNPentryintothematrixofintactmito-chondria.
Accordingly,mitochondrialtargetingse-quences,whichinteractwiththemitochondrialpro-teinimportapparatus,haveonlylimitedvalueforAuNPdelivery.
However,AuNPtranslocationacrossthemitochondrialmembranesisnotobligatorytodamagetheorganelle,andotherstrategieshavebeensuccessful.
CommoneffectsofAuNPsonmitochon-driaaremorphologicalchanges,lossofmembranepotentialandproductionofreactiveoxygenspecies.
MitochondrialdeliveryofAuNPs.
Analternativetopeptide-derivedAuNPtargetingwasliposomesthatfusewithmitochondrialmembranes[87].
AuNPs(8-12nm)weredeliveredbyencapsulationinMi-to-porter;thisenvelopecontainsfusogeniclipidsandocta-argininesurfacemodifications.
Octa-arginineservedmultiplefunctions;itstimulatedparticleup-takebymicropinocytosis,escapeintothecytosol,andbindingtomitochondria.
Oncetheparticlesboundtomitochondria,Mito-porterlipidsmediatedmembranefusion,andAuNPswerereleasedintomitochondria.
AnotherapproachwasbasedonAuNPfunc-tionalizationwithcetyltrimethylammoniumbromide(CTAB)[88].
Thiscompounddamageslipidbilayersandcanfacilitatethepermeationofmembranes,leadingtothemitochondrialassociationofgoldna-norods.
CTABstimulatedlysosomalescapeinsomecelllines,likelybecauseitcompromisedlysosomalmembraneintegrity.
Amonglungcarcinoma(A549),normalbronchialepithelial(16HBE)andprimaryadultstemcells,thenanoparticlesaffectedA549cellstothegreatestextent[47].
SuchCTAB-goldnanorodsaccumulatedinmitochondria(Fig.
5)andcausedcelldeaththroughacollapseofthemitochondrialmem-branepotentialandthegenerationofoxidativestress.
Figure5.
A549cellswereincubatedfor24hourswithgoldnanorods.
Swellingandroundingwasobservedforafractionofthemitochondria.
Moreover,somecristaewerelostandvacuolesappearedinmitochondria.
Goldnanorodsassociatedasaggregateswithmitochondria(M),asindicatedbythearrows.
Thetransmissionelectronmicrographwasadaptedfrom[47]withpermission.
ThebiologicalpropertiesofmitochondriacanbeexploitedforAuNPtargetingwithtri-phenylphosphonium.
Drivenbythemitochondrialmembranepotential,thislipophiliccationcanin-creasetheconcentrationofvariousagentsinthemi-tochondrialmatrix(reviewedin[125]).
Tri-phenylphosphoniumtargetedgoldnanoclusterstoHeLacellmitochondria[89].
However,undertheconditionstested,thecytotoxicityofthesegoldnanoclusterswaslow,as≥80%ofthecellsremainedviable.
Pre-photoactivationseemedtoenhancethedel-eteriouseffectsofAuNPsoncancercells[90].
Thus,AuNPsweremoretoxic,ifphotoexcitedbeforeaddi-tiontopancreaticcancercells(1.
4E7cells).
Theirheightenedtoxicitycorrelatedwithincreasedmito-chondrialdamage,suchasswellingandlossofmito-chondrialmembranepotential.
Pre-photocativationalsopromotedAuNPlocationtomitochondriaandtheproductionofreactiveoxygenspecies.
Apro-apoptoticpeptidecontainingthesequence(KLAKLAK)2targeted13.
5nmAuNPstoHeLacellmitochondria[33].
Thesepeptide-functionalizedAuNPsassociatedwithmitochondriaandinducedtheirswelling,lossofmembranepotentialandulti-matelycelldeath.
Peptide-modifiedAuNPsweremoretoxicthantheirnon-functionalizedcounterpartsorthepro-apoptoticpeptidealone.
Takentogether,nucleiandmitochondriahavebeensuccessfullytargetedbyAuNPs.
ThegeneralprinciplesthatrouteAuNPstothenucleusormito-chondriahavebeenidentified(Fig.
6),butmechanisticdetailsarefrequentlyunknown.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org364Figure6.
UptakeandsubcellulartargetingofAuNPs.
Non-targeted(left)andtargeted(right)AuNPsbindtotheplasmamembrane;thismayinvolvere-ceptorsonthecellsurface.
Uponinternalization,AuNPsinitiallyconcentrateinendosomesorlysosomes.
Afterescapefromthesemembrane-boundcom-partments,AuNPsassociatewithnucleiormitochondria,wheretheycancauseirreversibledamagethatculminatesincancercelldeath.
CellularinjuryisenhancedifAuNPsaretargetedtonucleiormitochondria(right);thissubcel-lulartargetingincreasescancercellkilling.
4.
TargetingAuNPstotheERUnlikeAuNPdeliverytonucleiormitochondria,strategiesforERtargetingarelesswelldeveloped.
However,giventheimportanceoftheERformanytumortypesandthelinksbetweenmitochondriaandtheER[126],AuNPslocatedintheERcouldplayasignificantroleincancertherapy.
AuNPshavebeendetectedinthismembranesystem;forexample,non-functionalized13nmsphericalAuNPscolocal-izedwiththeERandGolgiapparatusinB16F10melanomacells[127].
InHeLacells,18nmAuNPsassociatedwithcytoplasmicvesiclesthatwerepre-sumablypartoftheER[128].
InKupffercellsoftheliver,13nmPEGylatedAuNPslocatedtolysosomesandthesmoothER[129],andAuNPsderivatizedwithCALNNR8peptidesweretrappedintheERofHeLacells[52].
SimultaneousassociationwithnucleiandtheERhasalsobeenreportedforK562cells(human,chronicmyelogenousleukemia)[130].
Notably,theseparticlesinducedERstress,whichlikelycontributedtotheirtoxicity.
5.
TargetingAuNPstosubcellularorga-nellesinvivoAscomparedtoculturedcellsorspheroids,AuNPtargetingtocellorganellesinvivofacesextrahurdles.
Theseadditionalchallengesincludeaccu-mulationinhealthyorgans,tissue-specificbarriers,clearancebythereticuloendothelialsystemandin-sufficientconcentrationatthetumorsite.
Onceac-cumulatedinthetumor,AuNPswillfollowthesamestepsdepictedinFig.
1.
Assuch,aftercellularuptake,particleshavetoescapelysosomes/endosomesandlocatetotheirfinalsubcellulardestination.
Toovercometheseobstacles,severalvariablescanbechanged,includingsize,surfacemodification,dosageandrouteofentry.
Accumulationatthetumorsitesinvivoisaprerequisiteforsubcellulartargeting,andthistopichasbeendiscussedinpreviousreviews[16,18].
Atpresent,onlyalimitednumberofstudieshaveattemptedtooptimizeAuNPstoreachasub-cellulardestinationinvivo.
Here,wediscussseveralstudiesthatshoworganelletargetingwithAuNPsbothinculturedcellsandinexperimentalanimals.
Targetingnucleiinvitroandinvivo.
Huangetal.
[85]comparedtheperformanceofAuNPsinthreedifferentmodelsystems.
Sphericaltiopronin-coatedAuNPsof2,6or15nmsizewereanalyzedinMCF7cells,growninmonolayersorspheroids.
Theseparti-cleswerealsoexaminedinxenograftsofBalb/cnudemice.
Inallofthesemodelsystems,cellularuptakewassize-dependentandmoreefficientforsmallerAuNPs.
Moreover,particlesizedeterminedthesub-cellularAuNPdistribution(seeAdditionalFile1:Ta-bleS1).
SmallAuNPsof2and6nmlocatedtothenu-cleusandcytoplasm,whereas15nmparticleswererestrictedtothecytoplasm.
Followingintravenousinjection,allAuNPswereclearedfromtheblood;thiswasmostefficientfor15nmparticles.
Bycontrast,tumorsaccumulatedpreferentially2nmAuNPs,but2nmparticlesalsoconcentratedinthekidneyandlung.
AuNPsof15nmsizeassociatedpredominantlywithnon-tumortissue,withpreferencefortheliverandspleen.
Collectively,theseresultssuggestthatthesubcellulardistributionoftiopronin-coatedAuNPsissimilarinMCF7cellmonolayers,spheroidsandxen-ografts.
Chenetal.
[76]examinedAuNPnucleartarget-inginvitroinHep3B(hepatocellularcarcinoma)and293Tcells(humanembryonickidneycellscontainingSV40T-antigen);theexperimentswereextendedtoHep3Bcell-derivedxenografts.
TodeliverDNAeffi-cientlytothecellnucleus,AuNPswerecoatedwithdifferentmoleculesinasandwich-likefashion.
ThesandwichincludedplasmidDNA,polyethylenimine(PEI)anddexamethasone,asyntheticagonistoftheglucocorticoidreceptor.
PEIenhancedendosomalescape,whiledexamethasoneservedseveralpurpos-es.
First,itimproveduptake,whichreached82.
5%inculturedHepB3cells.
Second,dexamethasonestimu-latedAuNPnucleartargetingthroughitsassociationwiththeglucocorticoidreceptor.
Specifically,func-tionalizedAuNPs(carryingdexamethasone,DNAandPEI)formcomplexeswithglucocorticoidreceptorinthecytoplasmandthentranslocatetothenucleus.
Withthisapproach,culturedcellsweretransfectedeffectively,whilecytotoxicitywaslow.
Buildingontheseinvitrostudies,functionalizedAuNPswerealsousedforgenedeliveryinvivoTheranostics2015,Vol.
5,Issue4http://www.
thno.
org365(Balb/cnudemicebearingHepB3-derivedtumors).
DNAencodingtumornecrosisfactor-relatedapopto-sis-inducingligand(TRAIL)wasintroducedintotheanimalsusingAuNPsof~55nmsize.
Tumorspro-ducedthehighestlevelsofTRAILwhentheDNAwasdeliveredbyanAuNPsandwichthatcontainedbothPEIanddexamethasone.
ConcomitantwithefficientDNAdelivery,tumorgrowthwasinhibited.
Thus,functionalizedAuNPsinducedTRAILsynthesisinvivoandtherebyinterferedwithtumorgrowth[76],whileTRAILexpressionwaslowinnon-tumortis-sues.
ThisstudydemonstratedAuNPnucleartarget-inginculturedcells.
Althoughnotshownexperi-mentallyinvivo,resultssuggestthatnucleartargetingalsooccurredinexperimentalanimals.
Thus,AuNPfunctionalizationwithdexamethasonecanbeusefultotargettumorsthatsynthesizeglucocorticoidre-ceptor.
Targetingmitochondriainvitroandinvivo.
ThepreferredrouteofATPproductioninmanyformsofcancerisanaerobicglycolysis;thispathwayreliesontheenzymehexokinasewhichsynthesizesglu-cose-6-phosphate.
Hexokinase2isespeciallyim-portantamongthefourenzymeisoforms,becauseitishighlyabundantinaggressivetumorcellsandpre-dominantlyassociatedwiththeoutermitochondrialmembrane.
Sincehexokinase2interactionwiththeoutermitochondrialmembranestimulatesglycolyticenergyproductionandreducesapoptosis,theenzymehasbecomeatherapeutictargetforcancertherapy.
Hexokinase2isinhibitedby3-bromopyruvate,atoxiccompoundwithoff-targeteffects.
Toimprove3-bromopyruvatespecificityanddiminishtoxicity,AuNPsweredevelopedformitochondrialdelivery[91].
PEGylatedsphericalAuNPs(2.
9–4.
3nm)weremodifiedwithTPP,therebygeneratingT-AuNPswhicharecharacterizedbypositivelychargedandlipophilicmoietiesonthesurface.
ThesepropertiesimprovedT-AuNPuptakeandmitochondrialassoci-ationinPC3cells.
ResultsweresimilarwhenT-AuNPswerefurthermodifiedwith3-bromopyruvate(T-3-BP-AuNP).
After4hours,T-3-BP-AuNPsboundtotheoutermitochondrialmembranewherehexokinase2resides.
TheseAuNPsaccumulatedinthemitochondrialmatrixat12hours.
T-3-BP-AuNPsreducedPC3andDU145(prostatecarcinoma)cellviability,buthadlittleeffectonhu-manmesenchymalstemcells.
Theimpactoncancercellswasattributedtothelossofmitochondrialfunc-tionsandglycolysis.
Overall,AuNPsmodifiedwith3-BPweremoretoxictocancerthannormalcells.
Atthesametime,AuNPstargetedtomitochondria(T-AuNPs)weremoreharmfulthantheirnon-targetedcounterparts(NT-AuNPs).
ThetoxicityofAuNPswasfurtherenhancedbya1minlaserirra-diationat660nm.
Toevaluateinvivoeffects,thebio-distributionandpharmacokineticsofT-AuNPandNT-AuNPwereassessedinmaleSpragueDawleyrats.
ClearancefromtheplasmawasfasterforT-AuNPsthanNT-AuNPs.
Bothtypesofparticlesaccumulatedintheliverandspleen,buttheirsubcel-lularlocalizationwasnotdetermined[91].
6.
Perspectives:LimitationsandfuturedirectionsThepromiseofAuNP-dependentcancertherapyisemphasizedbynumerouspublicationsandclinicaltrials[21].
Atpresent,severalhurdleslimittherapidimprovementofAuNP-basedtreatments.
Forexam-ple,theapproachesforAuNPtargetingtosubcellularlocationsdifferwidely.
SinceAuNPsize,morphology,functionalization,concentrationandthecelltypesanalyzedvarysignificantly,itisdifficulttocompareresultsfromdifferentlaboratories.
Moreover,alt-houghessentialtoimproveAuNP-basedintervention,thebiologicalmechanismsunderlyingAuNP-inducedcellkillingareoftennotdefined.
Nevertheless,somegeneralconclusionscanbedrawnforthediversestrategiesthatleadtoAuNPassociationwithsubcel-lularorganelles(Table1).
OurreviewdiscussesthepotentialbenefitsoftargetingAuNPstospecificcellorganelles,andwepresentthebarriersAuNPshavetoovercometoreachtheirintracellulardestination.
Asidefromthere-strictionsthesebarriersimpose,AuNP-mediatedcancercellkillingcouldbelimitedbyexportviaexo-cytosis(reviewedin[131]).
However,suchparticlelosscanbereducedwithappropriatesurfacefunc-tionalization[132].
Attheorganismallevel,itwillfurtherbeimportanttodesignstrategiesthatcontrolAuNPclearance.
Clearanceismostlyachievedthroughthehepatobiliarysystemandthekidney,anditismodulatedbyAuNPsurfacemodification[133].
Astheimmunesystemcontributestonanoparticleclearance[134],clearancerateslikelydifferamongpatients.
Althoughthesevariationschallengetheuniversalapplicationforcancertherapy,theymayneverthelessoffertheopportunitytoproduceAuNPsforpersonalizedmedicine.
Celltypedependentdifferencesinuptakeandsubsequentintracellulartargetingcomplicatetheop-timizationofAuNPsforcancertherapy.
Ontheotherhand,ifsystematicallyexamined,thesedifferencescouldbeexploredtodevelopAuNPsthatareselectiveforspecifictypesofcancer.
Moreover,AuNPtarget-ingtotumorcellorganellescouldbeenhancedbyexploitingthedifferencesbetweennormalandcancercells.
Forexample,nucleartransportismoreefficientinproliferatingcancercellsascomparedtotheirnon-tumorigeniccounterparts(seeabove).
Thisis-inTheranostics2015,Vol.
5,Issue4http://www.
thno.
org366part-causedbytheoverexpressionofimportin-αandimportin-βfamilymembersandothersolublefactorsornucleoporinsthatsupportsignal-mediatednuclearimport[135-137].
Todate,AuNPnucleartargetingreliespredominantlyonNLSsthatbindimportin-α(SV40T-antigen,nucleoplasmin)orimportin-β1(TAT,[138]).
Inthefuture,nucleardeliverycouldbeimprovedthroughAuNPfunctionalizationthatstim-ulatesNPCbinding.
Somenucleoporinsaremoreabundantincancercells[139],andthereforeprovidepotentialAuNPdockingsitesatthenuclearpore.
Withrespecttomitochondria,functionalizingsmallsphericalorrod-shapedAuNPswithmito-chondrialtargetingsequencesmaybesuitabletodamageorganellarfunctionsorevenclogmitochon-drialproteinimportsites,analogoustowhathasbeenobservedforNPCs.
Whiletargetingtosubcellularorganellesisofparticularinteresttonanomedicine,themajorbottle-neckaftercellularuptakeisAuNPescapefromen-dosomes/lysosomes.
Recentstudiessuggestaddi-tionalstrategiestoenhancethisescape.
Besideslowenergylaserirradiation[140],particlesurfacemodi-ficationsthatincreasethe"protonspongeeffect"couldpromoteendosomeswellingandAuNPrelease[141].
Moreover,AuNPsthatundergopH-dependentaggregationorenhancepH-dependentlipidrupturecouldbeusefulfortheliberationfromendosomes.
ReleasefromendosomesiscriticalforAuNPstoreachnuclei;however,itmaynotbemandatoryformito-chondria.
Forinstance,fusogeniclipidsasdescribedfortheMito-portercouldcircumventtheneedforendosomalescape.
Ontheotherhand,itisconceiva-blethatthedirectdeliveryofmaterialfromendo-somestomitochondria[142]willbeexploitedtobringAuNPstomitochondria.
Sofar,cellculturemodelshavedominatedthecharacterizationofAuNPbio-nanointeractions.
Morerecently,AuNP-inducedcelldamageanddeathhavebeenassessedintumorcellspheroids[85,143],amodelsystemthatmimicsmultipleaspectsoftumortissuesinvivo.
Forexample,AuNPpenetrationacrossmultiplecelllayerswasstudiedinspheroidsderivedfromU87glioblastomacells[143].
TheprogressinspheroidproductionsetsthestagetoexplorethismodelfurtherandexamineAuNPtargetingtonucleiandmitochondria.
Todate,thereareonlyfewstudiesthatexaminethesubcellulardistributionofAuNPsinvivo.
WhileinvitroanalyseslocatedAuNPsinnucleiormitochondria,itisinmostcasesnotclearwhethertheseAuNPsaretargetedtothesametumorcellor-ganellesinexperimentalanimals.
Clearly,thisinfor-mationhastobeprovidedinthefuturetofullyassessthevalueofAuNPsubcellulartargetinginvivo.
Table1.
AdvantagesandlimitationsofcurrentapproachesforAuNPdeliverytosubcellularorganelles.
ApproachSubcellularorganelleAdvantagesLimitationsExploitationofAuNPphysicalprop-ertiesfororganellardeliverywithoutspecifictargetingmoieties:size(e.
g.
smallorlargeparticles,nanoclusters),shape(spheres,rods,flowers,urchins),chargenonspecificsubcellulardistribution,oftenconcentratedinendo-somes/lysosomes,particlesmayescapeendosomes/lysosomesandassociatewithnucleiand/ormito-chondriaeasytoprepare,lowcost,fastclearancemaylimittoxicity,PEGylatedsmallAuNPsoftendamagecancercells,positivechargesfrequentlyenhanceup-takeuptakenotspecifictocancercells,fastclearancemaylimitaccumulationintumor,effectofpositivechargescell-typede-pendent,lowendosomal/lysosomalescape,lowconcentrationinsubcellularorganelles,hightoxicity,thusdamagetonon-tumortissuesTargetingtocellsurface:RGDtrans-ferrin,EGF,antibodiesoraptamersthatbindcellsurfacecomponentsnonspecificsubcellulardistribution,oftenconcentratedinendo-somes/lysosomes,particlesmayescapeendosomes/lysosomesandassociatewithnucleiand/ormito-chondriaenhancedtargetingtocancercells,therebyimproveduptakebytumorcellsendosomalescapeandsubcellulardeliverymayrequireadditionalmodifications;cellsurfacereceptorsignaturesforefficienttumortargetingnotavailableforallcancertypesImprovedcellularuptake:cellpene-trating(CPP)andotherpeptides;e.
g.
CALNN,CALNNR8,TAT,Pntn,lysosomalsortingpeptidesnucleusandothersubcellularcom-partmentsmayenhancenucleartargetingthroughincreaseofcellularuptake;someCPPsalsofunctionasnuclearlocali-zationsignalsomepeptidesinefficientforendoso-mal/lysosomalescapeandnucleartarget-ing;nuclearlocalizationcandependoncelltypeNuclearlocalizationsignals(NLSs):biologicalandsyntheticsignals;linearandcyclicpeptides:SV40-NLS,adenoviralNLS,cyclic[KW]5enrichedinnucleuspositivechargesofNLSenhancecellularuptake;specificandefficientnucleartargeting;frequentlylowtoxicityinvitro;usefulfordrugdeliverytonucleusmayneedadditionalmodificationstoimprovetumortargetinginvivoandtofacilitateendosomal/lysosomalescapeCombinationofpeptideswithdiffer-entfunctions:e.
g.
CPP+NLS,RGD+NLSenrichedinnucleusimprovedtumortargetingandcellularuptake;usefultoexploitcellsurfacereceptorsfunctionalizationwithmultiplepeptides;specificratioofpeptidesmayberequiredOthermolecules:CTAB,tiopronin,cysteamine,thioglucose,dexamethasonecanleadtoenrichmentinnucleusmaystimulatecellularuptake,endoso-mal/lysosomalescapeorboth;thiscanenhancenuclearassociationsomemodificationshighlytoxic(e.
g.
CTAB);exceptfordexamethasone,nucleartarget-ingnotefficient;targetingmaybecelltypespecific(e.
g.
nuclearaccumulationbydexamethasonereliesonglucocorticoidreceptor)Differenttypesoffunctionalization:octa-arginine,CTAB,TPP,chitosan,polyvinylpyrrolidoneenrichedinmitochondriacanstimulatecellularuptakeand/orendosomal/lysosomalescape;thiscanenhancemitochondrialassociationfrequentlytoxic(e.
g.
TPP);mayrequirepermeabilizationofplasmamembrane(e.
g.
polyvinylpyrrolidone)Theranostics2015,Vol.
5,Issue4http://www.
thno.
org367AsthemodelsystemstoevaluateAuNPper-formancearebecomingmorediverse,soareAuNPs.
Assuch,AuNPscanassembleintochains,plasmonicvesicles[144,145]orotherstructuresthatprovidemultiplefunctionsatthesametime,includingdrugdelivery,enhancedphotothermalablationandparti-cletracking.
LargecomplexAuNPsthatareabletodisassembleintosmallerAuNPunits[145,146]couldhelpovercomethedifferentroadblocksthatlimitAuNPsubcellulartargeting(Fig.
2).
SuchcomplexAuNPs,whendisassembled,mayhavetheadditionalbenefitofrapidrenalorhepatobiliaryclearance.
Takentogether,AuNPsofferuniqueopportuni-tiestotranslatetheinsightsofbasicresearchintoclinicalapplications.
GiventhesuccessofAuNPsforphotothermalablation,mechanicalinjuryandtar-geteddrugdelivery,futurestrategiesthatcombinetheseeffectsforAuNP-dependentcancercellkillingareparticularlypromising.
7.
SummaryandhighlightsRecentstudieshavebeguntorevealhowAuNPsimpingeonthestructural/functionalorganizationofcancerandnormalcells.
Thisknowledgeiscriticalfortwoaspectsofnanomedicine.
First,itwillhelpdefinetheAuNP-inducedeventsatthesubcellularlevel.
Thiswillsetthestagefortheidentificationofnewmoleculartargetsforcancertherapy.
Second,itwilldirectthedesignofAuNPswithphysico-chemicalpropertiesthatovercomethecurrentlimitationstheseparticlesfaceinbasicresearch,diagnosisandtherapy.
Thus,optimizationofAuNPsurfacesforcellandor-ganelle-specificdeliveryisanticipatedtoenhancetheefficiencyofcancercellkilling,whileminimizingtheimpactonnon-tumortissues.
Basedontheiressentialroleforcancercellsurvival,nucleiandmitochondriaareprimetargetsforthisapproach.
AbbreviationsAuNP:goldnanoparticleCALNN:cys-ala-leu-asp-aspCTAB:cetyltrimethylammoniumbromideEGF:epidermalgrowthfactorEGFR:EGFreceptorEPR:enhancedpermeabilityandretentionLSP:localizedsurfaceplasmonresonanceMTS:mitochondrialtargetingsequenceNIR:nearinfraredradiationNLS:nuclearlocalizationsequencePEG:polyethyleneglycol,surfacecoatingofAuNPsthatreducesparticleaggregation,non-specificinteractionswithbiomoleculesanduptakebythere-ticuloendothelialsystemPEI:polyethyleniminePntn:Penetratin,peptidesequencederivedfromDrosophilaproteinAntennapediathatpromotesentryintothecellTAT:HIV-1-trans-activatingproteinTPP:triphenylphosphoniumTRAIL:tumornecrosisfactor-relatedapopto-sis-inducingligandSupplementaryMaterialAdditionalFile1:TableS1:ImpactofAuNPsize,morphologyandfunctionalizationoncellularuptake,subcellularlo-calizationandcellsurvival.
http://www.
thno.
org/v05p0357s1.
pdfAuthorContributionsThemanuscriptwaswrittenthroughcontribu-tionsofallauthors.
Allauthorshavegivenapprovaltothefinalversionofthemanuscript.
CompetingInterestsTheauthorshavedeclaredthatnocompetinginterestexists.
References1.
HanahanD,WeinbergRobertA.
Hallmarksofcancer:Thenextgeneration.
Cell.
2011;144:646-74.
2.
UbahOC,WallaceHM.
Cancertherapy:Targetingmitochondriaandothersub-cellularorganelles.
CurrPharmDes.
2014;20:201-22.
3.
WrightJ.
Nanotechnology:Deliveronapromise.
Nature.
2014;509:S58-S9.
4.
GhoshP,HanG,DeM,KimCK,RotelloVM.
Goldnanoparticlesindeliveryapplications.
AdvDrugDelRev.
2008;60:1307-15.
5.
HutterE,MaysingerD.
Goldnanoparticlesandquantumdotsforbioimaging.
MicroscResTech.
2011;74:592-604.
6.
HutterE,MaysingerD.
Gold-nanoparticle-basedbiosensorsfordetectionofenzymeactivity.
TrendsPharmacolSci.
2013;34:497-507.
7.
ZhaoP,LiN,AstrucD.
Stateoftheartingoldnanoparticlesynthesis.
CoordChemRev.
2013;257:638-65.
8.
JanaNR,GearheartL,MurphyCJ.
Wetchemicalsynthesisofhighaspectratiocylindricalgoldnanorods.
JPhysChemB.
2001;105:4065-7.
9.
BrownKR,NatanMJ.
Hydroxylamineseedingofcolloidalaunanoparticlesinsolutionandonsurfaces.
Langmuir.
1998;14:726-8.
10.
NikoobakhtB,El-SayedMA.
Preparationandgrowthmechanismofgoldnanorods(NRs)usingseed-mediatedgrowthmethod.
ChemMater.
2003;15:1957-62.
11.
SapsfordKE,AlgarWR,BertiL,GemmillKB,CaseyBJ,OhE,etal.
Function-alizingnanoparticleswithbiologicalmolecules:Developingchemistriesthatfacilitatenanotechnology.
ChemRev.
2013;113:1904-2074.
12.
HuefnerA,SeptiadiD,WiltsBD,PatelII,KuanW-L,FragniereA,etal.
Goldnanoparticlesexplorecells:Cellularuptakeandtheiruseasintracellularprobes.
Methods.
2014;68:354–63.
13.
DykmanLA,KhlebtsovNG.
Uptakeofengineeredgoldnanoparticlesintomammaliancells.
ChemRev.
2014;114:1258-88.
14.
LévyR,ShaheenU,CesbronY,SéeV.
Goldnanoparticlesdeliveryinmam-malianlivecells:Acriticalreview.
NanoReviews.
2010;1:10.
3402/nano.
v1i0.
4889.
15.
JeremicB,AguerriAR,FilipovicN.
Radiosensitizationbygoldnanoparticles.
ClinTranslatOncol.
2013;15:593-601.
16.
JainS,HirstDG,O'SullivanJM.
Goldnanoparticlesasnovelagentsforcancertherapy.
BrJRadiol.
2012;85:101-13.
17.
AhmadMZ,AkhterS,RahmanZ,AkhterS,AnwarM,MallikN,etal.
Na-nometricgoldincancernanotechnology:Currentstatusandfutureprospect.
JPharmPharmacol.
2013;65:634-51.
18.
DreadenEC,AustinLA,MackeyMA,El-SayedMA.
Sizematters:Goldna-noparticlesintargetedcancerdrugdelivery.
TherDeliv.
2012;3:457-78.
19.
HolbackH,YeoY.
Intratumoraldrugdeliverywithnanoparticulatecarriers.
PharmRes.
2011;28:1819-30.
20.
LeungJ,WuS,ChouK,SignorellR.
Investigationofsub-100nmgoldnano-particlesforlaser-inducedthermotherapyofcancer.
Nanomaterials.
2013;3:86-106.
21.
[Internet]NIH:Clinicaltrials.
2014.
http://clinicaltrials.
gov/Theranostics2015,Vol.
5,Issue4http://www.
thno.
org36822.
LibuttiSK,PaciottiGF,ByrnesAA,AlexanderHR,GannonWE,WalkerM,etal.
PhaseIandpharmacokineticstudiesofCYT-6091,anovelPEGylatedcol-loidalgold-rhTNFnanomedicine.
ClinCancerRes.
2010;16:6139-49.
23.
AlexanderCM,HamnerKL,MayeMM,DabrowiakJC.
MultifunctionalDNA-goldnanoparticlesfortargeteddoxorubicindelivery.
BioconjugChem.
2014.
24.
ParkH,TsutsumiH,MiharaH.
Cell-selectiveintracellulardrugdeliveryusingdoxorubicinandα-helicalpeptidesconjugatedtogoldnanoparticles.
Bio-materials.
2014;35:3480-7.
25.
SakhraniNM,PadhH.
Organelletargeting:Thirdlevelofdrugtargeting.
DrugDesDevelTher.
2013;7:585-99.
26.
YuanH,FalesAM,Vo-DinhT.
Tatpeptide-functionalizedgoldnanostars:Enhancedintracellulardeliveryandefficientnirphotothermaltherapyusingultralowirradiance.
JAmChemSoc.
2012;134:11358-61.
27.
KongT,ZengJ,WangX,YangX,YangJ,McQuarrieS,etal.
Enhancementofradiationcytotoxicityinbreast-cancercellsbylocalizedattachmentofgoldnanoparticles.
Small.
2008;4:1537-43.
28.
BrownSD,NativoP,SmithJ-A,StirlingD,EdwardsPR,VenugopalB,etal.
Goldnanoparticlesfortheimprovedanticancerdrugdeliveryoftheactivecomponentofoxaliplatin.
JAmChemSoc.
2010;132:4678-84.
29.
SeidmanAD,ConlinAK,BachA,MoynahanME,LakeD,ForeroA,etal.
RandomizedphaseIItrialofweeklyvs.
every2weeksvs.
every3weeksna-noparticlealbumin-boundpaclitaxelwithbevacizumabasfirst-linechemo-therapyformetastaticbreastcancer.
ClinBreastCancer.
2013;13:239-46.
e1.
30.
JaggiAS,SinghN.
Mechanismsincancer-chemotherapeuticdrugs-inducedperipheralneuropathy.
Toxicology.
2012;291:1-9.
31.
VyasD,LaputG,VyasAK.
Chemotherapy-enhancedinflammationmayleadtothefailureoftherapyandmetastasis.
OncoTargetsTher.
2014;7:1015-23.
32.
MackeyMA,SairaF,MahmoudMA,El-SayedMA.
Inducingcancercelldeathbytargetingitsnucleus:Solidgoldnanospheresversushollowgoldnanocag-es.
BioconjugChem.
2013;24:897-906.
33.
ChenW-H,ChenJ-X,ChengH,ChenC-S,YangJ,XuX-D,etal.
Anewan-ti-cancerstrategyofdamagingmitochondriabypro-apoptoticpeptidefunc-tionalizedgoldnanoparticles.
ChemCommun(Camb).
2013;49:6403-5.
34.
WangF,WangY-C,DouS,XiongM-H,SunT-M,WangJ.
Doxorubi-cin-tetheredresponsivegoldnanoparticlesfacilitateintracellulardrugdeliv-eryforovercomingmultidrugresistanceincancercells.
ACSNano.
2011;5:3679-92.
35.
GibsonJD,KhanalBP,ZubarevER.
Paclitaxel-functionalizedgoldnanoparti-cles.
JAmChemSoc.
2007;129:11653-61.
36.
KumarA,HuoS,ZhangX,LiuJ,TanA,LiS,etal.
Neuropilin-1-targetedgoldnanoparticlesenhancetherapeuticefficacyofplatinum(IV)drugforprostatecancertreatment.
ACSNano.
2014;8:4205-20.
37.
ChenH,ZhangX,DaiS,MaY,CuiS,AchilefuS,etal.
Multifunctionalgoldnanostarconjugatesfortumorimagingandcombinedphotothermalandchemo-therapy.
Theranostics.
2013;3:633-49.
38.
AlmeidaJPM,FigueroaER,DrezekRA.
Goldnanoparticlemediatedcancerimmunotherapy.
NanomedNanotechnolBiolMed.
2014;10:503-14.
39.
TkachenkoAG,XieH,ColemanD,GlommW,RyanJ,AndersonMF,etal.
Multifunctionalgoldnanoparticle-peptidecomplexesfornucleartargeting.
JAmChemSoc.
2003;125:4700-1.
40.
AkhterS,AhmadMZ,AhmadFJ,StormG,KokRJ.
Goldnanoparticlesintheranosticoncology:Currentstate-of-the-art.
ExpertOpinDrugDeliv.
2012;9:1225-43.
41.
MurphyCJ,GoleAM,StoneJW,SiscoPN,AlkilanyAM,GoldsmithEC,etal.
Goldnanoparticlesinbiology:Beyondtoxicitytocellularimaging.
AccChemRes.
2008;41:1721-30.
42.
DreadenEC,El-SayedMA.
Detectinganddestroyingcancercellsinmorethanonewaywithnoblemetalsanddifferentconfinementpropertiesonthena-noscale.
AccChemRes.
2012;45:1854-65.
43.
VermaA,StellacciF.
Effectofsurfacepropertiesonnanoparticle–cellinterac-tions.
Small.
2010;6:12-21.
44.
LlevotA,AstrucD.
Applicationsofvectorizedgoldnanoparticlestothediagnosisandtherapyofcancer.
ChemSocRev.
2012;41:242-57.
45.
ChoEC,XieJ,WurmPA,XiaY.
Understandingtheroleofsurfacechargesincellularadsorptionversusinternalizationbyselectivelyremovinggoldnano-particlesonthecellsurfacewithaI2/KIetchant.
NanoLett.
2009;9:1080-4.
46.
KodihaM,HutterE,BoridyS,JuhasM,MaysingerD,StochajU.
Goldnano-particlesinducenucleardamageinbreastcancercellswhichisfurtherampli-fiedbyhyperthermia.
CellMolLifeSci.
2014;71:4259-73.
47.
WangL,LiuY,LiW,JiangX,JiY,WuX,etal.
Selectivetargetingofgoldnanorodsatthemitochondriaofcancercells:Implicationsforcancertherapy.
NanoLett.
2010;11:772-80.
48.
TkachenkoAG,XieH,LiuYL,ColemanD,RyanJ,GlommWR,etal.
Cellulartrajectoriesofpeptide-modifiedgoldparticlecomplexes:Comparisonofnu-clearlocalizationsignalsandpeptidetransductiondomains.
BioconjugChem.
2004;15:482-90.
49.
ChithraniBD,GhazaniAA,ChanWCW.
Determiningthesizeandshapedependenceofgoldnanoparticleuptakeintomammaliancells.
NanoLett.
2006;6:662-8.
50.
ChithraniBD,ChanWCW.
Elucidatingthemechanismofcellularuptakeandremovalofprotein-coatedgoldnanoparticlesofdifferentsizesandshapes.
NanoLett.
2007;7:1542-50.
51.
MandalD,MaranA,YaszemskiM,BolanderM,SarkarG.
Cellularuptakeofgoldnanoparticlesdirectlycross-linkedwithcarrierpeptidesbyosteosarcomacells.
JMaterSciMaterMed.
2009;20:347-50.
52.
SunL,LiuD,WangZ.
Functionalgoldnanoparticlepeptidecomplexesascell-targetingagents.
Langmuir.
2008;24:10293-7.
53.
DekiwadiaCD,LawrieAC,FecondoJV.
Peptide-mediatedcellpenetrationandtargeteddeliveryofgoldnanoparticlesintolysosomes.
JPeptSci.
2012;18:527-34.
54.
SongJ,ZhouJ,DuanH.
Self-assembledplasmonicvesiclesofsers-encodedamphiphilicgoldnanoparticlesforcancercelltargetingandtraceableintra-cellulardrugdelivery.
JAmChemSoc.
2012;134:13458-69.
55.
DamDHM,LeeJH,SiscoPN,CoDT,ZhangM,WasielewskiMR,etal.
Directobservationofnanoparticle–cancercellnucleusinteractions.
ACSNano.
2012;6:3318-26.
56.
KangB,MackeyMA,El-SayedMA.
NucleartargetingofgoldnanoparticlesincancercellsinducesDNAdamage,causingcytokinesisarrestandapoptosis.
JAmChemSoc.
2010;132:1517-9.
57.
ShahNB,DongJ,BischofJC.
Cellularuptakeandnanoscalelocalizationofgoldnanoparticlesincancerusinglabel-freeconfocalramanmicroscopy.
MolPharm.
2010;8:176-84.
58.
GuY-J,ChengJ,LinC-C,LamYW,ChengSH,WongW-T.
Nuclearpenetra-tionofsurfacefunctionalizedgoldnanoparticles.
ToxicolApplPharmacol.
2009;237:196-204.
59.
RyanJA,OvertonKW,SpeightME,OldenburgCM,LooL,RobargeW,etal.
Cellularuptakeofgoldnanoparticlespassivatedwithbsa-sv40largetantigenconjugates.
AnalChem.
2007;79:9150-9.
60.
HuoS,JinS,MaX,XueX,YangK,KumarA,etal.
Ultrasmallgoldnanoparti-clesascarriersfornucleus-basedgenetherapyduetosize-dependentnuclearentry.
ACSNano.
2014;8:5852-62.
61.
delaFuenteJM,BerryCC.
Tatpeptideasanefficientmoleculetotranslocategoldnanoparticlesintothecellnucleus.
BioconjugChem.
2005;16:1176-80.
62.
BerryCC,delaFuenteJM,MullinM,ChuSW,CurtisAS.
NuclearlocalizationofHIV-1tatfunctionalizedgoldnanoparticles.
IEEETransNanobioscience.
2007;6:262-9.
63.
ArvizoRR,MirandaOR,ThompsonMA,PabelickCM,BhattacharyaR,Ro-bertsonJD,etal.
Effectofnanoparticlesurfacechargeattheplasmamembraneandbeyond.
NanoLett.
2010;10:2543-8.
64.
Ojea-JimenezI,Garcia-FernandezL,LorenzoJ,PuntesVF.
Facilepreparationofcationicgoldnanoparticle-bioconjugatesforcellpenetrationandnucleartargeting.
ACSNano.
2012;6:7692-702.
65.
ChithraniBD,StewartJ,AllenC,JaffrayDA.
Intracellularuptake,transport,andprocessingofnanostructuresincancercells.
NanomedNanotechnolBiolMed.
2009;5:118-27.
66.
NativoP,PriorIA,BrustM.
Uptakeandintracellularfateofsurface-modifiedgoldnanoparticles.
ACSNano.
2008;2:1639-44.
67.
OyelereAK,ChenPC,HuangX,El-SayedIH,El-SayedMA.
Pep-tide-conjugatedgoldnanorodsfornucleartargeting.
BioconjugChem.
2007;18:1490-7.
68.
YangC,UertzJ,YohanD,ChithraniBD.
Peptidemodifiedgoldnanoparticlesforimprovedcellularuptake,nucleartransport,andintracellularretention.
Nanoscale.
2014;6:12026-33.
69.
ConnorEE,MwamukaJ,GoleA,MurphyCJ,WyattMD.
Goldnanoparticlesaretakenupbyhumancellsbutdonotcauseacutecytotoxicity.
Small.
2005;1:325-7.
70.
DixitV,VandenBosscheJ,ShermanDM,ThompsonDH,AndresRP.
Syn-thesisandgraftingofthiocticacidPEGfolateconjugatesontoaunanoparti-clesforselectivetargetingoffolatereceptor-positivetumorcells.
BioconjugChem.
2006;17:603-9.
71.
HuffTB,HansenMN,ZhaoY,ChengJ-X,WeiA.
Controllingthecellularuptakeofgoldnanorods.
Langmuir.
2007;23:1596-9.
72.
HuffTB,TongL,ZhaoY,HansenMN,ChengJ-X,WeiA.
Hyperthermiceffectsofgoldnanorodsontumorcells.
Nanomedicine.
2007;2:125-32.
73.
BhattacharyaR,PatraCR,EarlA,WangS,KataryaA,LuL,etal.
Attachingfolicacidongoldnanoparticlesusingnoncovalentinteractionviadifferentpolyethyleneglycolbackbonesandtargetingofcancercells.
NanomedNano-technolBiolMed.
2007;3:224-38.
74.
ShiraziAN,TiwariRK,OhD,SullivanB,McCaffreyK,MandalD,etal.
Surfacedecoratedgoldnanoparticlesbylinearandcyclicpeptidesasmolec-ulartransporters.
MolPharm.
2013;10:10.
1021/mp400199e.
75.
ShuklaR,BansalV,ChaudharyM,BasuA,BhondeRR,SastryM.
Biocom-patibilityofgoldnanoparticlesandtheirendocytoticfateinsidethecellularcompartment:Amicroscopicoverview.
Langmuir.
2005;21:10644-54.
76.
ChenZ,ZhangL,HeY,LiY.
Sandwich-typeAu-PEI/DNA/PEI-Dexanano-complexfornucleus-targetedgenedeliveryinvitroandinvivo.
ACSApplMaterInterfaces.
2014;6:14196-206.
77.
HanG,YouC-C,KimB-j,TuringanRS,ForbesNS,MartinCT,etal.
Light-regulatedreleaseofDNAanditsdeliverytonucleibymeansofpho-tolabilegoldnanoparticles.
AngewChemIntEdEngl.
2006;45:3165-9.
78.
El-SayedIH,HuangX,El-SayedMA.
Surfaceplasmonresonancescatteringandabsorptionofanti-egfrantibodyconjugatedgoldnanoparticlesincancerdiagnostics:Applicationsinoralcancer.
NanoLett.
2005;5:829-34.
79.
HuangX,El-SayedIH,QianW,El-SayedMA.
Cancercellimagingandpho-tothermaltherapyinthenear-infraredregionbyusinggoldnanorods.
JAmChemSoc.
2006;128:2115-20.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org36980.
HauckTS,GhazaniAA,ChanWCW.
Assessingtheeffectofsurfacechemistryongoldnanoroduptake,toxicity,andgeneexpressioninmammaliancells.
Small.
2008;4:153-9.
81.
OhE,DelehantyJB,SapsfordKE,SusumuK,GoswamiR,Blanco-CanosaJB,etal.
CellularuptakeandfateofPEGylatedgoldnanoparticlesisdependentonbothcell-penetrationpeptidesandparticlesize.
ACSNano.
2011;5:6434-48.
82.
LiuX,HuangN,LiH,JinQ,JiJ.
Surfaceandsizeeffectsoncellinteractionofgoldnanoparticleswithbothphagocyticandnonphagocyticcells.
Langmuir.
2013;29:9138-48.
83.
CoradeghiniR,GioriaS,GarcíaCP,NativoP,FranchiniF,GillilandD,etal.
Size-dependenttoxicityandcellinteractionmechanismsofgoldnanoparticlesonmousefibroblasts.
ToxicolLett.
2013;217:205-16.
84.
PanY,NeussS,LeifertA,FischlerM,WenF,SimonU,etal.
Size-dependentcytotoxicityofgoldnanoparticles.
Small.
2007;3:1941-9.
85.
HuangK,MaH,LiuJ,HuoS,KumarA,WeiT,etal.
Size-dependentlocaliza-tionandpenetrationofultrasmallgoldnanoparticlesincancercells,multi-cellularspheroids,andtumorsinvivo.
ACSNano.
2012;6:4483-93.
86.
SalnikovV,LukyanenkoYO,FrederickCA,LedererWJ,LukyanenkoV.
Probingtheoutermitochondrialmembraneincardiacmitochondriawithna-noparticles.
BiophysJ.
2007;92:1058-71.
87.
YamadaY,AkitaH,KamiyaH,KogureK,YamamotoT,ShinoharaY,etal.
Mito-porter:Aliposome-basedcarriersystemfordeliveryofmacromoleculesintomitochondriaviamembranefusion.
BiochimBiophysActa.
2008;1778:423-32.
88.
WangL,JiangX,JiY,BaiR,ZhaoY,WuX,etal.
Surfacechemistryofgoldnanorods:Originofcellmembranedamageandcytotoxicity.
Nanoscale.
2013;5:8384-91.
89.
ZhuangQ,JiaH,DuL,LiY,ChenZ,HuangS,etal.
Targetedsur-face-functionalizedgoldnanoclustersformitochondrialimaging.
BiosensorsBioelectron.
2014;55:76-82.
90.
MocanL,IlieI,TabaranFA,DanaB,ZaharieF,ZdrehusC,etal.
Surfaceplasmonresonance-inducedphotoactivationofgoldnanoparticlesasmito-chondria-targetedtherapeuticagentsforpancreaticcancer.
ExpertOpinTherTargets.
2013;17:1383-93.
91.
MarracheS,DharS.
Theenergyblockerinsidethepowerhouse:Mitochondriatargeteddeliveryof3-bromopyruvate.
ChemScience.
2015.
doi:10.
1039/C4SC01963.
92.
ChengY,MeyersJD,AgnesRS,DoaneTL,KenneyME,BroomeA-M,etal.
Addressingbraintumorswithtargetedgoldnanoparticles:AnewgoldstandardforhydrophobicdrugdeliverySmall.
2011;7:2301-6.
93.
ScarìG,PortaF,FascioU,AvvakumovaS,DalSantoV,DeSimoneM,etal.
Goldnanoparticlescappedbyagc-containingpeptidefunctionalizedwithanrgdmotifforintegrintargeting.
BioconjugChem.
2012;23:340-9.
94.
delaFuenteJM,BerryCC,RiehleMO,CurtisASG.
Nanoparticletargetingatcells.
Langmuir.
2006;22:3286-93.
95.
SykesEA,ChenJ,ZhengG,ChanWCW.
Investigatingtheimpactofnano-particlesizeonactiveandpassivetumortargetingefficiency.
ACSNano.
2014;8:5696-706.
96.
EghtedariM,LiopoAV,CoplandJA,OraevskyAA,MotamediM.
Engineer-ingofhetero-functionalgoldnanorodsfortheinvivomoleculartargetingofbreastcancercells.
NanoLett.
2008;9:287-91.
97.
El-SayedIH,HuangX,El-SayedMA.
Selectivelaserphoto-thermaltherapyofepithelialcarcinomausinganti-EGFRantibodyconjugatedgoldnanoparticles.
CancerLett.
2006;239:129-35.
98.
delasHerasJI,BatrakouDG,SchirmerEC.
Cancerbiologyandthenuclearenvelope:Aconvolutedrelationship.
SeminCancerBiol.
2013;23:125-37.
99.
JevtiP,EdensLJ,VukoviLD,LevyDL.
Sizingandshapingthenucleus:Mechanismsandsignificance.
CurrOpinCellBiol.
2014;28:16-27.
100.
MatchettKB,McFarlaneS,HamiltonSE,EltuhamyYS,DavidsonMA,MurrayJT,etal.
RanGTPaseinnuclearenvelopeformationandcancermetastasis.
AdvExpMedBiol.
2014;773:323-51.
101.
MiyamotoY,LovelandKL,YonedaY.
Nuclearimportinalphaanditsphysi-ologicalimportance.
CommunIntegrBiol.
2012;5:220-2.
102.
QuinJE,DevlinJR,CameronD,HannanKM,PearsonRB,HannanRD.
Tar-getingthenucleolusforcancerintervention.
BiochimBiophysActa.
2014;1842:802-16.
103.
RuggeroD.
Revisitingthenucleolus:Frommarkertodynamicintegratorofcancersignaling.
SciSignal.
2012;5:pe38.
104.
MorA,WhiteMA,FontouraBMA.
Nucleartraffickinginhealthanddisease.
CurrOpinCellBiol.
2014;28:28-35.
105.
KuusistoHV,WagstaffKM,AlvisiG,RothDM,JansDA.
Globalenhancementofnuclearlocalization-dependentnucleartransportintransformedcells.
FASEBJ.
2012;26:1181-93.
106.
FeldherrCM,AkinD.
Thepermeabilityofthenuclearenvelopeindividingandnondividingcellcultures.
JCellBiol.
1990;111:1-8.
107.
FeldherrCM,AkinD.
Signal-mediatednucleartransportinproliferatingandgrowth-arrestedBALB/c3T3cells.
JCellBiol.
1991;115:933-9.
108.
FeldherrCM,AkinD.
Regulationofnucleartransportinproliferatingandquiescentcells.
ExpCellRes.
1993;205:179-86.
109.
TsoliM,KuhnH,BrandauW,EscheH,SchmidG.
CellularuptakeandtoxicityofAu55clusters.
Small.
2005;1:841-4.
110.
PantéN,KannM.
Nuclearporecomplexisabletotransportmacromoleculeswithdiametersof39nm.
MolBiolCell.
2002;13:425-34.
111.
FeldherrCM,AkinD,CohenRJ.
Regulationoffunctionalnuclearporesizeinfibroblasts.
JCellSci.
2001;114:4621-7.
112.
FeldherrCM,AkinD.
Thelocationofthetransportgateinthenuclearporecomplex.
JCellSci.
1997;110:3065-70.
113.
NovakJP,NickersonC,FranzenS,FeldheimDL.
Purificationofmolecularlybridgedmetalnanoparticlearraysbycentrifugationandsizeexclusionchro-matography.
AnalChem.
2001;73:5758-61.
114.
Krpeti,SaleemiS,PriorIA,SéeV,QureshiR,BrustM.
NegotiationofintracellularmembranebarriersbyTAT-modifiedgoldnanoparticles.
ACSNano.
2011;5:5195-201.
115.
TsaiTL,HouCC,WangHC,YangZS,YehCS,ShiehDB,etal.
Nucleocyto-plasmictransportblockagebySV40peptide-modifiedgoldnanoparticlesin-ducescellularautophagy.
IntJNanomedicine.
2012;7:5215-34.
116.
HuschkaR,BarhoumiA,LiuQ,RothJA,JiL,HalasNJ.
Genesilencingbygoldnanoshell-mediateddeliveryandlaser-triggeredreleaseofantisenseoligonu-cleotideandsiRNA.
ACSNano.
2012;6:7681-91.
117.
RyouS-M,ParkM,KimJ-M,JeonCO,YunC-H,HanSH,etal.
Inhibitionofxenografttumorgrowthinmicebygoldnanoparticle-assisteddeliveryofshorthairpinRNAsagainstMcl-1L.
JBiotechnol.
2011;156:89-94.
118.
KimJ-H,JangHH,RyouS-M,KimS,BaeJ,LeeK,etal.
Afunctionalizedgoldnanoparticles-assisteduniversalcarrierforantisenseDNA.
ChemCommun(Camb).
2010;46:4151-3.
119.
KimD-W,KimJ-H,ParkM,YeomJ-H,GoH,KimS,etal.
ModulationofbiologicalprocessesinthenucleusbydeliveryofDNAoligonucleotidescon-jugatedwithgoldnanoparticles.
Biomaterials.
2011;32:2593-604.
120.
WallaceDC.
Amitochondrialparadigmofmetabolicanddegenerativedis-eases,aging,andcancer:Adawnforevolutionarymedicine.
AnnuRevGenet.
2005;39:359-407.
121.
PathaniaD,MillardM,NeamatiN.
Opportunitiesindiscoveryanddeliveryofanticancerdrugstargetingmitochondriaandcancercellmetabolism.
AdvDrugDelRev.
2009;61:1250-75.
122.
WenS,ZhuD,HuangP.
Targetingcancercellmitochondriaasatherapeuticapproach.
FutureMedChem.
2012;5:53-67.
123.
RehlingP,ModelK,BrandnerK,KovermannP,SickmannA,MeyerHE,etal.
Proteininsertionintothemitochondrialinnermembranebyatwin-poretranslocase.
Science.
2003;299:1747-51.
124.
SzaboI,ZorattiM.
Mitochondrialchannels:Ionfluxesandmore.
PhysiolRev.
2014;94:519-608.
125.
SmithRA,HartleyRC,MurphyMP.
Mitochondria-targetedsmallmoleculetherapeuticsandprobes.
AntioxidRedoxSignal.
2011;15:3021-38.
126.
ClarkeHannaJ,ChambersJosephE,LinikerE,MarciniakStefanJ.
Endoplas-micreticulumstressinmalignancy.
CancerCell.
2014;25:563-73.
127.
ChangM-Y,ShiauA-L,ChenY-H,ChangC-J,ChenHHW,WuC-L.
Increasedapoptoticpotentialanddose-enhancingeffectofgoldnanoparticlesincom-binationwithsingle-doseclinicalelectronbeamsontumor-bearingmice.
CancerSci.
2008;99:1479-84.
128.
KhanJA,PillaiB,DasTK,SinghY,MaitiS.
MoleculareffectsofuptakeofgoldnanoparticlesinHeLacells.
ChemBioChem.
2007;8:1237-40.
129.
ChoW-S,ChoM,JeongJ,ChoiM,ChoH-Y,HanBS,etal.
Acutetoxicityandpharmacokineticsof13nm-sizedPEG-coatedgoldnanoparticles.
ToxicolApplPharmacol.
2009;236:16-24.
130.
TsaiY-Y,HuangY-H,ChaoY-L,HuK-Y,ChinL-T,ChouS-H,etal.
Identifi-cationofthenanogoldparticle-inducedendoplasmicreticulumstressbyomictechniquesandsystemsbiologyanalysis.
ACSNano.
2011;5:9354-69.
131.
KimCS,LeNDB,XingY,YanB,TongaGY,KimC,etal.
Theroleofsurfacefunctionalityinnanoparticleexocytosis.
AdvHealthcMater.
2014;3:1200-2132.
OhN,ParkJ-H.
Surfacechemistryofgoldnanoparticlesmediatestheirexo-cytosisinmacrophages.
ACSNano.
2014;8:6232-41.
133.
SimpsonCA,SallengKJ,CliffelDE,FeldheimDL.
Invivotoxicity,biodistri-bution,andclearanceofglutathione-coatedgoldnanoparticles.
Nanomedi-cine.
2013;9:257-63.
134.
JonesSW,RobertsRA,RobbinsGR,PerryJL,KaiMP,ChenK,etal.
Nanopar-ticleclearanceisgovernedbyTh1/Th2immunityandstrainbackground.
JClinInvest.
2013;123:3061-73.
135.
vanderWattPJ,StowellCL,LeanerVD.
Thenuclearimportreceptorkpnb1anditspotentialasananticancertherapeutictarget.
CritRevEukaryotGeneExpr.
2013;23:1-10.
136.
ChristiansenA,DyrskjtL.
Thefunctionalroleofthenovelbiomarkerkary-opherinα2(KPNA2)incancer.
CancerLett.
2013;331:18-23.
137.
FanH,LuY,QinH,ZhouY,GuY,ZhouJ,etal.
Highranleveliscorrelatedwithpoorprognosisinpatientswithcolorectalcancer.
IntJClinOncol.
2013;18:856-63.
138.
TruantR,CullenBR.
Thearginine-richdomainspresentinhumanimmuno-deficiencyvirustype1TatandRevfunctionasdirectimportinβ-dependentnuclearlocalizationsignals.
MolCellBiol.
1999;19:1210-7.
139.
OriA,BanterleN,IskarM,Andrés‐PonsA,EscherC,KhanhBuiH,etal.
Celltype‐specificnuclearpores:Acaseinpointforcontext‐dependentstoichiom-etryofmolecularmachines.
MolSystBiol.
2013;9:648140.
KrpeticZ,NativoP,SeeV,PriorIA,BrustM,VolkM.
Inflictingcontrollednonthermaldamagetosubcellularstructuresbylaser-activatedgoldnano-particles.
NanoLett.
2010;10:4549-54.
141.
NeubergP,KichlerA.
Recentdevelopmentsinnucleicaciddeliverywithpolyethylenimines.
AdvGenet.
2014;88:263-88.
142.
SheftelAD,ZhangAS,BrownC,ShirihaiOS,PonkaP.
Directinterorganellartransferofironfromendosometomitochondrion.
Blood.
2007;110:125-32.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org370143.
HuoS,MaH,HuangK,LiuJ,WeiT,JinS,etal.
Superiorpenetrationandretentionbehaviorof50nmgoldnanoparticlesintumors.
CancerRes.
2013;73:319-30.
144.
LinM,GuoC,LiJ,ZhouD,LiuK,ZhangX,etal.
Polypyrrole-coatedchainlikegoldnanoparticlearchitectureswiththe808nmphotothermaltransductionefficiencyupto70%.
ACSApplMaterInterfaces.
2014;6:5860-8.
145.
SongJ,FangZ,WangC,ZhouJ,DuanB,PuL,etal.
Photolabileplasmonicvesiclesassembledfromamphiphilicgoldnanoparticlesforremote-controlledtraceabledrugdelivery.
Nanoscale.
2013;5:5816-24.
146.
KanarasAG,WangZ,BrustM,CosstickR,BatesAD.
EnzymaticdisassemblyofDNA–goldnanostructures.
Small.
2007;3:590-4.
5,Issue4http://www.
thno.
org357TThheerraannoossttiiccss2015;5(4):357-370.
doi:10.
7150/thno.
10657ReviewOfftotheOrganelles-KillingCancerCellswithTargetedGoldNanoparticlesMohamedKodiha1,YiMengWang1,ElizaHutter2,DusicaMaysinger2,UrsulaStochaj11.
DepartmentofPhysiology,McGillUniversity,Montreal,Canada;2.
DepartmentofPharmacology&Therapeutics,McGillUniversity,Montreal,Canada.
Correspondingauthor:UrsulaStochaj,Ph.
D.
DepartmentofPhysiology,McGillUniversity,3655PromenadeSirWilliamOsler,Montreal,QC,Canada,H3G1Y6.
Phone:514-398-2949Fax:514-398-7452E-mail:ursula.
stochaj@mcgill.
ca.
IvyspringInternationalPublisher.
Thisisanopen-accessarticledistributedunderthetermsoftheCreativeCommonsLicense(http://creativecommons.
org/licenses/by-nc-nd/3.
0/).
Reproductionispermittedforpersonal,noncommercialuse,providedthatthearticleisinwhole,unmodified,andproperlycited.
Received:2014.
09.
27;Accepted:2014.
12.
16;Published:2015.
01.
21AbstractGoldnanoparticles(AuNPs)areexcellenttoolsforcancercellimagingandbasicresearch.
However,theyhaveyettoreachtheirfullpotentialintheclinic.
Atpresent,weareonlybeginningtounderstandthemolecularmechanismsthatunderliethebiologicaleffectsofAuNPs,includingthestructuralandfunctionalchangesofcancercells.
Thisknowledgeiscriticalfortwoaspectsofnanomedicine.
First,itwilldefinetheAuNP-inducedeventsatthesubcellularandmolecularlevel,therebypossiblyidentifyingnewtargetsforcancertreatment.
Second,itcouldprovidenewstrategiestoimproveAuNP-dependentcancerdiagnosisandtreatment.
OurreviewsummarizestheimpactofAuNPsonselectedsubcellularorganellesthatarerelevanttocancertherapy.
Wefocusonthenucleus,itssubcompartments,andmitochondria,becausetheyareintimatelylinkedtocancercellsurvival,growth,proliferationanddeath.
Whilenon-targetedAuNPscandamagetumorcells,concentratingAuNPsinparticularsubcellularlocationswilllikelyimprovetumorcellkilling.
Thus,itwillincreasecancercelldamagebyphotothermalablation,mechanicalinjuryorlocalizeddrugdelivery.
Thisconceptispromising,butAuNPshavetoovercomemultiplehurdlestoperformthesetasks.
AuNPsize,morphologyandsurfacemodifi-cationarecriticalparametersfortheirdeliverytoorganelles.
Recentstrategiesexploredallofthesevariables,andsurfacefunctionalizationhasbecomecrucialtoconcentrateAuNPsinsub-cellularcompartments.
Here,wehighlighttheuseofAuNPstodamagecancercellsandtheirorganelles.
WediscusscurrentlimitationsofAuNP-basedcancerresearchandconcludewithfuturedirectionsforAuNP-dependentcancertreatment.
Keywords:Goldnanoparticles,AuNPs,cancercellimagingThisreviewprovidesanupdateonthethera-peuticpotentialofgoldnanoparticles(AuNPs)foroncology.
Tothisend,weintroduceAuNPsasthera-peutictools,summarizethecurrentstrategiesthattargetAuNPstospecificcellcompartmentsanddis-cusshowthistargetingimpactscancercellkilling.
Forsubcellulartargeting,ourfocusisonnucleiandmi-tochondria,sincebothorganellesareintimatelylinkedtocancercellsurvival,growthandprolifera-tionandthereforeprimarytargetsforanti-canceragents[1,2].
Weconcludebyhighlightingunsolvedquestionsandpotentialroadblocksinthefield.
1.
IntroductionNanotechnologyisinthespotlightoftherapeuticinnovation[3],andAuNPsareparticularlypromisingtoolstoimprovecancertreatment[4].
Duetotheiruniqueopticalproperties,non-toxicnature,relativelysimplepreparationandfunctionalization,AuNPsareexcellentcandidatesformanybiologicalapplications,IvyspringInternationalPublisherTheranostics2015,Vol.
5,Issue4http://www.
thno.
org358suchasimaging,drugdeliveryandphotothermaltherapy.
Theseapplicationscommonlytakead-vantageoftheparticles'stronglightscattering,in-tenseabsorption,andelectromagneticfieldenhance-mentthatresultfromlocalizedsurfaceplasmonres-onance[5,6].
AuNPscanbeproducedinlargequantitieswithdefinedshapesandsizes.
Themostcommonap-proachessynthesizeAuNPsinsituthroughchemicalreductionofgoldsaltsandseed-mediatedgrowth[7],whichenlargestheparticlesstepbystep.
ThismethodisidealtocontrolAuNPsizeandshape[8-10]andusedtoproducelargespherical,semi-spherical,rod-like,branchedorotherparticleshapes[7].
AuNPsurfacesareamenabletocovalentandnon-covalentsurfacemodifications;thispropertyiscrucialforcel-lularandsubcellulartargeting.
Asthephysi-co-chemicalcharacterizationofAuNPsandtheirde-tectionhavebeenreviewedbyothers[11-15],itwillnotbediscussedhere.
ThedevelopmentofAuNP-basedstrategiesfortheeradicationofcancercellsisimportant,becauseeffectivetherapiesarefrequentlynotavailableforrapidlyprogressingcancers[16].
Sofar,manyofthestudiesonAuNPssuggestthatcancercellsareespe-ciallyvulnerabletotheseparticles.
Thus,AuNP-basedtreatmentcandestroycancercells,withminimalin-jurytohealthycells[17].
ThetherapeuticvalueofAuNPsisbasedon(i)theirdistinctivephysicalpropertiesand(ii)theirabil-itytointeractwithtumorsanddamagecancercells.
Thus,theenhancedpermeabilityandretention(EPR)characteristicsofmany,butnotall,tumorsfacilitateAuNPinfiltrationintothetumor[18].
Duetothispassivetargeting,AuNPs(~6-200nm)accessthetu-mortissue,wheretheyaccumulateintheextracellularmatrixbeforeenteringthecells[19].
Followingtheirassociationwithtumorcells,AuNPspromoteuniquewaysofkilling(Fig.
1).
Theycandestroycancercellsbyphotothermalablation,asexemplifiedbyAuro-Shell[20,21],throughmechanicaldamage,orasdrugdeliverysystemsforanticanceragents,suchastumornecrosisfactor[21,22]ordoxorubicin[23,24].
WhatarethebenefitsofsubcellularAuNPtargetingWhileAuNPsarerelevantfordifferentclinicalapplications,furtherimprovementsofAuNP-basedstrategiesareexpectedtooptimizethetherapeuticoutcomes.
OnesuchimprovementisbasedontheconceptthatAuNPtargetingtospecificorganellesmaximizestheimpactontumorcells.
Tothisend,AuNPsarebeingdevelopedthataccumulateinsub-cellularcompartmentswheretheydestroyintrinsiccancercellfunctionsthatareessentialfortumorsur-vival.
Onceintheirproperintracellularlocation,AuNPscanenhancecancercelldestructionbydif-ferentmeans.
Thisincludestheconfineddeliveryofanti-canceragents[25],localizedsubcellularmechan-icaldamage,andimprovedefficiencyofphotothermalablationduetohighlocalAuNPconcentrations[26,27].
SuchcontrolledAuNPactionwillnotonlyin-creasecancercellkilling,butalsodiminishtoxicsideeffects,becauseitreducesthenecessaryamountsofAuNPsanddrug-load.
Candidatecompoundsfornanoparticle-dependentsubcellulardeliveryaredox-orubicin[23],platinum-baseddrugs[28]andpaclitaxel[29].
Theseanticanceragentsinterferewithnuclearandmitochondrialfunctions,respectively[30-33]andhavebeenusedtofunctionalizeAuNPs[23,34-37].
Asidefromdrugs,AuNPscanalsodeliveroligonucleotidestoaltergeneexpressionorsplicing([38]andreferencestherein).
Figure1.
ImpactofAuNPsoncancercells.
Size,morphology,functionalgroupsontheAuNPsurfaceandthecelltypedeterminethesubcellulardistributionofAuNPs.
AuNPscancausetumorcelldeathbyphotothermalablation,me-chanicaldamage,andincreaseinthelocalizeddrugconcentration.
Theseeventscanbecombinedtoenhancetheirkillingefficiency.
WhatarethebottlenecksforAuNPtargetingtospecificsubcellularcompartmentsOnceaccumulatedintumortissue,AuNPshavetoovercomemultipleobstaclesbeforetheycancon-centrateinthedesiredcellcompartment:(i)cellsur-facebinding,(ii)cellularuptake,(iii)escapefromly-sosomes/endosomes,and(iv)associationwithapar-ticularsubcellularlocation,suchasnucleiormito-chondria(Fig.
2;[39]).
ThefirstthreestepsaregeneralfeaturesthatregulatetheintracellulardestinationofallAuNPs.
Thesestepshavebeenreviewedexten-sively[13,39-42];wewillonlybrieflysummarizethemhereandthenprovideamoredetaileddiscus-sionofAuNPtargetingtonuclei,mitochondriaandtheER.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org359Figure2.
ObstaclesAuNPshavetoovercomeforsuccessfultargetingtointracellularorganellesorcompartments.
OnceAuNPsareintheextracellularmatrixofthetumor(ECM,barrier1),theyhavetobindtothecancercellsurface.
Cellularuptakerequirestranslocationacrosstheplasmamembrane(barrier2),byendocytosisorothermechanisms.
Insidethecell,AuNPshavetoescapefromendosomesorlysosomes(barrier3)tosubsequentlyassociatewiththedesiredorganelleorcellcompartment(barrier4).
Possiblefinaldestinationsarethenucleus(blue)ormitochondria(yellow).
Bindingtothecancercellsurface,internaliza-tionandescapefromendosomes/lysosomesAuNPuptake,subcellulardistributionandtox-icityaredeterminedbyparticlesize,morphologyandsurfacemodification.
AlthoughAuNPsofdifferentshapes(spherical,shells,rods,diamonds)orsizes(1-100nm)accumulateinvariouscancercells,theiruptakekineticsandtoxicitymayvaryprofoundly(Fig.
1,reviewedby[13]).
Besidesshapeandsize,AuNP-basedbio-nanointeractionsarefurthermodu-latedbytheirfunctionalization[11,43,44].
Ingeneral,positivechargesontheAuNPsurfacestimulatecel-lularuptake,possiblyduetoelectrostaticinteractionswiththecellsurface[45].
PositivechargescanalsoimproveAuNPtransporttothenucleus,becausenu-clearlocalizationsequences(NLSs)ofmanyproteinsareenrichedforbasicaminoacidresidues.
ParticleuptakenotonlydependsonAuNPproperties,butalsoreliesonthecelltype.
Whengrowninculture,cancerandnon-tumorigeniccellsdiffersignificantlyinthisrespect.
Notably,tumorcellsareoftenmorevulnerabletoAuNPs[46,47].
Nevertheless,thereisvariabilityevenamongcancercells;forexample,AuNPuptakediffersinhepatocel-lularcarcinoma(HepG2)andcervixcarcinomacells(HeLa)[48].
Despitesuchcell-typespecificdiffer-ences,nanoparticlebindingtocancercellscanbeen-hancedbyexploitingtumor-relatedchangesinplas-mamembranecomposition(AdditionalFile1:TableS1andassociatedreferences[41-91]).
Tothisend,particlesurfaceshavebeenfunctionalizedwithlig-andsthatimprovedockingatthetumorcellmem-brane.
LigandsthatpromotehighaviditybindingtocancercellsincludeEGF(epidermalgrowthfactor;associateswithEGFR)orpeptidescontainingtheRGDmotif(arginine-glycine-asparticacid;recognizedbysomeintegrinfamilymembers)[32,92-94].
Nucle-olin,transferrinorantibodiesagainstHer2andEGFR[55,95-97]canalsoenhancenanoparticlebindingtocancercells.
Onceboundtothecellsurface,AuNPsenterthecell;inmostcasesthisoccursbyanenergy-dependentprocess,forwhichendocytosisisthemainroute(re-viewedin[13,14]).
Followingcellularuptake,AuNPsinitiallylocatetoendosomesand/orlysosomes,wheretheorganellarmembraneintegritydeterminesAuNPretention(reviewedin[14]).
However,properfunctionalizationofAuNPscanstimulatetheirendo-somal/lysosomalescapeorpromoteuptakebynon-endocytoticpathways([13,14]andreferencestherein).
2.
NucleirepresentprimarytargetsforcancertherapyAsnucleimastermindessentialaspectsoftumorcellbiology,theyhavebecomeimportanttargetsincancertherapyingeneral,andAuNP-dependentin-terventionsinparticular.
Thenucleuscontrolscellgrowth,proliferationandapoptosis,andmanyan-ti-cancerdrugsobliteratethesefunctions.
Atthesametime,nuclearhomeostasisisoftenalteredincancercells,whichdisplaychangesinnuclearsize,shape,envelope,laminaandchromatinorganization,nucle-olarfunctionornucleocytoplasmictrafficking[98-104].
Forexample,theconcentrationofnucleartransportcarriersisfrequentlyincreasedintrans-formedcellsortumorsamples,andthiscorrelateswithaugmentedsignal-mediatednuclearimportandexport[105].
Ascomparedtotheirnormalcounter-parts,transformedcellsoftentransportlargerAuNPs,andAuNPnucleartranslocationismoreefficient[106-108].
Nuclearinteractionsofnon-targetedAuNPsEvenintheabsenceofsubcellulartargetingsig-nals,certainAuNPtypesassociatewiththenucleusanditssubcompartments.
Insomebutnotallcasesthisnuclearlocalizationisaccompaniedbyorganelledamage.
Assuch,AuNPs(3.
7nmaveragediameter)weremodifiedwithboth3-mercaptopropionicacidandpolyethyleneglycol(PEG)andconjugatedtoFITC[58].
AlthoughtheparticlesaccumulatedinHeLacellnucleiaftera24-hourincubationperiod,cellTheranostics2015,Vol.
5,Issue4http://www.
thno.
org360viabilitystillamountedto85%ofthecontrolsamples.
Evenafter72hoursandwithupto10MofAuNPs,cellsurvivalwasmaintainedat70%,suggestinglowcytotoxicityinHeLacells[58].
Ontheotherhand,Au55goldclusters(1.
4nm)enteredthecellnucleuswheretheyinteractedwithDNAandelicitedtoxiceffects[109].
Comparisonofhealthyandtumorcelllinesrevealedmaximumtox-icityforthemetastaticmelanomacelllinesMV3andBLM.
TheinteractionbetweenAu55goldclustersandnuclearDNAislikelytheunderlyingcauseofthistoxicity.
OurrecentstudiesexaminedAuNPsofdifferentsizesandmorphologies.
Inbreastcancercells,nuclearmembranes,nuclearlaminaeandnucleolarfunctionswerecompromisedbysmallsphericalAuNPsorgoldnanoflowers,butnotbylargesphericalAuNPs([46],Fig.
3).
Thedamageinflictedbynon-sphericalgoldnanoflowersisparticularlyinteresting;despitetheirlargesize(40-120nm),theyenteredthenucleusanddestroyednuclearhomeostasisinbreastcancercells,butnotinnormalbreastcells.
Figure3.
(A)Goldnanoflowersandlargegoldnanospheres(red)associatewithnucleiofdifferentbreastcells,i.
e.
MCF7andnon-tumorigenichumanmammaryepithelialcells(HuMEC).
NotethatgoldnanoflowerscanbedetectedinthenuclearinteriorofMCF7cells,wheretheydisruptthenuclearlamina(green).
Scalebarsare10m.
(B)Smallgoldnanospheres(15.
6nmdiameter)andgoldnanoflowers(40-120nm),butnotlargegoldnanospheres(60nm),alterthenuclearorganizationinMCF7cells.
Inparticular,nuclearporecomplexes(NPC,red)andthenuclearlamina(LaminA,green)showseverechanges.
Arrowsmarksomeofthenucleiwithalteredmorphology;scalebaris20m.
(C)SmallgoldnanospheresandgoldnanoflowersinhibitdenovoRNAsynthesis(magenta)inthenucleolus.
Scalebaris3m.
Adaptedfrom[46]withpermission.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org361TargetingAuNPstothenucleusWhichAuNPsizefitsthenucleusTheoptimalAuNPpropertiesfornucleartargetingdependonthedesiredimpactoftheparticle.
IfAuNPsaredestinedforthenuclearinterior,thesizeofthetransportchannelofthenuclearporecomplex(NPC)hastobeconsidered.
Accordingly,sphericalparticlesof9nmorlessindiametermaycrosstheNPCbydiffusion,whereasparticlesupto39nmindiametercanbede-liveredtothenucleusiftheycarryanucleartransportsignal[110].
Thesearenotfixedvalues,asthenucleartransportmachineryisoftenalteredincancercells(seeabove).
Inthecontextofcancertherapy,threedifferentscenarioscanapplytonuclearAuNPs:(i)theyaretransportedintothenuclearinterioror(ii)clogtheNPC,or(iii)releasecytotoxicdrugsinthevicinityofnuclei.
NuclearimportofAuNPs.
Theground-breakingworkbyCarlFeldherrbuiltthefoundationforAuNPtargetingtothenucleus.
HislaboratorycoatedAuNPswithconjugatesthatcontainedserumalbuminandthenuclearlocalizationsequences(NLSs)derivedfromSV40T-antigenornucleoplasmin.
Uponinjec-tionintothecytoplasm,thesefunctionalizedAuNPstranslocatedacrossthenuclearporecomplex(NPC)andaccumulatedinsidethenucleus[111,112].
Feldheim'sgroupwentbeyondthesestudiesanddefinedthedifferentstagesofAuNPnuclearlocaliza-tioninintactculturedcells[39,59].
Thesestudiesshowedthatasidefromtheinitialbinding,cellularuptakeandreleasefromendosomes/lysosomes(seeabove),targetingtothenucleusandpassageacrossthenuclearenvelope(NE)arecriticalstepsthatlimitthedeliverytothenucleus.
WhileAuNPsdestinedforthenucleusshowcell-typespecificdifferencesincel-lularuptake,escapefromendosomes/lysosomesandnucleartargeting[113],theyalsosharecommonfea-tures.
Forexample,uptakeofNLS-modifiedAuNPsistemperature-dependentinculturedcells,consistentwithendocytosis[59,113].
Furthermore,increasingthenumberofNLS-peptidesontheparticlesurfaceenhancesAuNPnuclearlocalizationandtoxicity[59].
Variousstrategieshavebeenemployedtotargetgoldnanoparticlestothenucleus.
Sofar,thereisnoprotocolthathasbeenuniversallysuccessfulfordif-ferenttumorcells.
Therefore,wediscussspecificex-amplesforAuNPsthatwerefunctionalizedwithNLSs,cell-penetratingpeptides,peptidecombina-tions,oligonucleotidesorothermoieties(summarizedinAdditionalFile1:TableS1).
Peptidesequencesaredepictedintheone-lettercode.
ThesuccessofmultiplepeptidemodificationswasdemonstratedforAuNPsmodifiedwithSV40-NLSoranNLSderivedfromadenovirusfiberproteins;theseparticlesdidnotenterthenucleusofHepG2cells.
However,uponfurtheradditionoftheadenovirusreceptor-mediatedendocytosissequence,AuNPslocalizedtonuclei[39].
Notably,whenAuNPscarriedtheNLSandthereceptor-mediatedendocyto-sispeptideasseparateentities,nucleartargetingwasenhancedascomparedtoalongerpeptidethatcom-binesbothsequences.
Ifcombinedintoonepeptide,thetwosequenceelementsmaybelessaccessibletocellularbindingpartners.
Inotherstudies,AuNPsstabilizedwiththenon-naturalaminoacidtiopronin[94]werefunction-alizedwithGRKKRRQRRR,apeptidederivedfromtheHIV-1proteinTat[61].
Aftera1-hourtreatment,Tat-modifiedparticleswerelocatedinthenuclearinteriorofhTERT-BJ1humanfibroblasts.
Bycontrast,AuNPscarryingtioproninaloneaccumulatedincy-toplasmicvacuolesoratthemitochondrialperiphery[61].
Upona24-hourincubationperiodwithupto10MAuNPs(coresize2.
8nm),toxicitywas≤20%forbothtypesofparticles.
Toimprovecellularuptakeandnuclearaccu-mulationof30nmAuNPs,particlesweredecoratedwithpeptidesthatcontainedthesequenceCALNN.
AdditionofmixedpeptidescontainingCALNNandCALNNpluseightarginineresidues(CALNNR8)promotedAuNPaccumulationinnucleiofHeLacells[52].
Afterincubationwith0.
32nMAuNPsfor24hours,celldeathcouldreach95%.
Infurtherexperiments,16or14nmPEGylatedAuNPsweremodifiedwithdifferentCALLN-derivedpeptides.
FunctionalizationwithCALLN-fusionscontainingthecellpenetratingpeptideofTAT(CALLN-AGRKKRRQRRR)orPntn(CALLN-GRQIKIWFQNRRMKWKK)stimulatedcellentry,whereasanNLS(CALLN-GGFSTSLRARKA)wasaddedfornucleartargeting[66,114].
Inthissce-nario,AuNPsmodifiedsimultaneouslywithNLS,TATandPntn-containingpeptidesledtohigherin-tracellulargoldcontentwhencomparedtoAuNPscarryingonepeptidespeciesonly.
Furthermore,sim-ultaneouscoatingwithNLS,TATandPntnpromotedAuNPnuclearlocalization.
Surprisingly,ifAuNPscarryapositivesurfacecharge,nucleartargetingcanevenbeaccomplishedwithpeptidesthathavenosequencesimilaritytoabasicNLS([64],Fig.
4).
Nucleolinisamultifunctionalproteinthatre-sidesinthenucleolusandothercellcompartments,suchastheplasmamembrane.
Forsomecancercellsnucleolinabundanceishighintheplasmamembrane,wheretheproteincanserveasadockingsiteforAuNPs.
TheaptamerAS1411bindsnucleolinandwastestedinphaseIandIIclinicaltrialsforadvancedsolidtumorsandacutemyeloidleukemia[21].
WhenTheranostics2015,Vol.
5,Issue4http://www.
thno.
org362goldnanostarswerefunctionalizedwithAS1411,theyenteredHeLacellsandconcentratedinthevicinityofnuclei[55].
Thisuptakerequiredbothnucleolinonthecellsurfaceandtheaptamer.
AS1411-goldnanostarlocalizationclosetothenucleuscorrelatedwithchangesinnuclearmorphology,suggestingmechan-icaldamagetothenuclearenvelope.
Irradiationlib-eratedtheaptamerfromAuNPs,whichinturnacti-vatedcaspases3and7andincreasedcelldeath.
Col-lectively,thestudysupportsthemodelthattheap-tamerreleaseclosetothenucleusenhanceddamageandtheensuingcelldeath.
Figure4.
Detectionof13nmAuNPsmodifiedwithCIPGNVG-PEG-NH3+in1BR3Gcells(transformedhumanskinfibroblasts).
Cellswereincubatedfor3hourswithfunctionalizedAuNPs,andparticleswerevisualizedbytransmissionelectronmicroscopy.
SomeoftheAuNPswerepresentinthenuclearinterior,asindicatedbytheredarrows.
PanelsAandBdepicttwodifferentnuclei.
Scalebarsare2m.
AdaptedfromOjea-Jiménezetal.
[64]withpermission.
Theexamplesabovesuggestthattargetingtothenucleuscanamplifyorganelle-specificinsults.
ThisideaisvalidatedbyNLS-modifiedAuNPsthatin-creasedDNAdamageandinterferedwithcelldivi-sion,inparticularcytokinesis[56].
Specifically,30nmPEGylatedAuNPsdecoratedwithRGD-andNLS-peptidesaccumulatedinnucleiandtriggeredapoptosisin20%ofthecancercells[56].
Asidefromtargetingtothenuclearinterior,AuNPscanalsobeusedtoobstructtheNPC.
Thus,NLS-modifiedAuNPs(~39or32nminsize)blockednucleartransportinHeLacellsandinducedau-tophagiccelldeath[115].
Interestingly,thiswascelltypespecific,becauseitwasnotobservedinSiHacells,anothercervixcarcinomacellline.
Deliveryofnucleicacids.
OneoftheapplicationsofnuclearAuNPsisthemodulationofgeneexpression;thiscanbeachievedbyknockdownorchangesinsplicing[60,116-119].
Anexampleofthisapproachistheuseof13nmAuNPsthatcarriedoligonucleotidestoadjustthealternativesplicingofmRNAsforpro-survivalfactorsindifferentexperimentalsystems[119].
Assuch,theparticlesweredetectedinnucleiofculturedcellsandledtotumorshrinkageinaxeno-graftmodelbasedonLoVocells(humancoloncarci-noma).
InMCF7breastcancercells,sphericalultrasmall(6and2nm)tiopronin-coatedAuNPsresideinthecytoplasmandenterthenucleusaftera24-hourin-cubationperiod[60].
Whenconjugatedtoafluores-centFITC-tag,only2nmparticlesweredetectedinMCF7nuclei.
FITC-labelingincreasedthesizeof6nmAuNPsto10nm,whichmayhavelimitedthepassagethroughNPCs.
Toinducecelldeath,anoligonucleo-tidewasaddedto2nmtiopronin-AuNPs.
Thisoligo-nucleotidegeneratedtriplexstructureswiththeP2promoterofc-MYC.
Asaresult,expressionoftheproto-oncogenewasdownregulatedandcellviabilitywasreducedto70%.
Whencomparedtothetri-plex-formingoligonucleotidealone,AuNP-attachedoligonucleotidesincreasedc-MYCsilencingandMCF7celldeath[60].
Interestingly,AuNPsfunctionalizedwithdexa-methasonealsodeliveredplasmidDNAtonuclei[76],suggestingthatsteroidhormonescanbeusedforAuNPnucleartargeting.
Thesestudieswereper-formedinculturedcellsandexperimentalanimals;theyarediscussedinsection5.
Deliveryofanti-cancerdrugs.
AuNPsthatlocatetothenuclearperipherycanprovidetransportvehiclesforanti-cancerdrugs.
Onesuchdeliverysystemcar-rieddoxorubicinandwastestedinHeLa(cervixcar-cinoma),A549(lungcarcinoma)andNIH3T3-L1cells(fibroblastswithpre-adiposecharacteristics).
Tothisend,25nmPEGylatedsphericalAuNPswerefunc-tionalizedwithcellpenetratingpeptides,suchasTAT[24].
TAT-AuNPsweretakenupinallcellstested,whereasotherpeptidesdisplayedcelltype-specificdifferences.
Doxorubicin-loadedAuNPswereespe-ciallycytotoxictoHeLaandA549cells,butNIH3T3-L1cellswerelessaffected.
Celldeathwasattributedtothereleaseofdoxorubicinclosetothenucleus,whereAuNPsconcentrated.
3.
TargetingcancercellmitochondriawithAuNPsMitochondriaarethemajorsitesofcellularen-ergyproduction,andtheirdysfunctionisassociatedwithawiderangeofdiseasesandpathophysiologies,includingcancer[120].
Changesinbioenergeticsareahallmarkofmanytumors,butmitochondriaarealsoTheranostics2015,Vol.
5,Issue4http://www.
thno.
org363keyregulatorsofapoptoticcelldeath.
Theseproper-tiesmakemitochondriaprimetargetsforcancertreatment[121,122].
AuNPsoftenimpairmitochon-drialfunctionsandtherebyinducecelldeath.
How-ever,thisdoesnotalwaysinvolvestablephysicalcontactbetweenAuNPsandtheorganelle.
Here,wefocusonexamplesthatreportAuNPassociationwithmitochondria.
TheemphasisisonstudiesthatweredesignedtodeliverAuNPsspecificallytomitochon-dria.
WhichAuNPsizefitsmitochondriaTheconcep-tualdifferencesforAuNPtargetingtonucleiandmi-tochondriaarebasedonthedistinctorganelleborderstothecytoplasm.
Unlikethenucleus,mitochondriadonotcontainlargeporesthatprovideeasyaccessforAuNPs.
BoththeouterandtheinnermitochondrialmembranespresentbarrierstoAuNPsthataredes-tinedforthemitochondrialmatrix.
Inheartcells,3nmparticles,butnot6nmAuNPs,translocatedacrosstheoutermitochondrialmembrane[86].
Intheinnermi-tochondrialmembrane,proteinimportchannelspro-videopeningsof<2nm([123],reviewedin[124]).
ThisrestrictsAuNPentryintothematrixofintactmito-chondria.
Accordingly,mitochondrialtargetingse-quences,whichinteractwiththemitochondrialpro-teinimportapparatus,haveonlylimitedvalueforAuNPdelivery.
However,AuNPtranslocationacrossthemitochondrialmembranesisnotobligatorytodamagetheorganelle,andotherstrategieshavebeensuccessful.
CommoneffectsofAuNPsonmitochon-driaaremorphologicalchanges,lossofmembranepotentialandproductionofreactiveoxygenspecies.
MitochondrialdeliveryofAuNPs.
Analternativetopeptide-derivedAuNPtargetingwasliposomesthatfusewithmitochondrialmembranes[87].
AuNPs(8-12nm)weredeliveredbyencapsulationinMi-to-porter;thisenvelopecontainsfusogeniclipidsandocta-argininesurfacemodifications.
Octa-arginineservedmultiplefunctions;itstimulatedparticleup-takebymicropinocytosis,escapeintothecytosol,andbindingtomitochondria.
Oncetheparticlesboundtomitochondria,Mito-porterlipidsmediatedmembranefusion,andAuNPswerereleasedintomitochondria.
AnotherapproachwasbasedonAuNPfunc-tionalizationwithcetyltrimethylammoniumbromide(CTAB)[88].
Thiscompounddamageslipidbilayersandcanfacilitatethepermeationofmembranes,leadingtothemitochondrialassociationofgoldna-norods.
CTABstimulatedlysosomalescapeinsomecelllines,likelybecauseitcompromisedlysosomalmembraneintegrity.
Amonglungcarcinoma(A549),normalbronchialepithelial(16HBE)andprimaryadultstemcells,thenanoparticlesaffectedA549cellstothegreatestextent[47].
SuchCTAB-goldnanorodsaccumulatedinmitochondria(Fig.
5)andcausedcelldeaththroughacollapseofthemitochondrialmem-branepotentialandthegenerationofoxidativestress.
Figure5.
A549cellswereincubatedfor24hourswithgoldnanorods.
Swellingandroundingwasobservedforafractionofthemitochondria.
Moreover,somecristaewerelostandvacuolesappearedinmitochondria.
Goldnanorodsassociatedasaggregateswithmitochondria(M),asindicatedbythearrows.
Thetransmissionelectronmicrographwasadaptedfrom[47]withpermission.
ThebiologicalpropertiesofmitochondriacanbeexploitedforAuNPtargetingwithtri-phenylphosphonium.
Drivenbythemitochondrialmembranepotential,thislipophiliccationcanin-creasetheconcentrationofvariousagentsinthemi-tochondrialmatrix(reviewedin[125]).
Tri-phenylphosphoniumtargetedgoldnanoclusterstoHeLacellmitochondria[89].
However,undertheconditionstested,thecytotoxicityofthesegoldnanoclusterswaslow,as≥80%ofthecellsremainedviable.
Pre-photoactivationseemedtoenhancethedel-eteriouseffectsofAuNPsoncancercells[90].
Thus,AuNPsweremoretoxic,ifphotoexcitedbeforeaddi-tiontopancreaticcancercells(1.
4E7cells).
Theirheightenedtoxicitycorrelatedwithincreasedmito-chondrialdamage,suchasswellingandlossofmito-chondrialmembranepotential.
Pre-photocativationalsopromotedAuNPlocationtomitochondriaandtheproductionofreactiveoxygenspecies.
Apro-apoptoticpeptidecontainingthesequence(KLAKLAK)2targeted13.
5nmAuNPstoHeLacellmitochondria[33].
Thesepeptide-functionalizedAuNPsassociatedwithmitochondriaandinducedtheirswelling,lossofmembranepotentialandulti-matelycelldeath.
Peptide-modifiedAuNPsweremoretoxicthantheirnon-functionalizedcounterpartsorthepro-apoptoticpeptidealone.
Takentogether,nucleiandmitochondriahavebeensuccessfullytargetedbyAuNPs.
ThegeneralprinciplesthatrouteAuNPstothenucleusormito-chondriahavebeenidentified(Fig.
6),butmechanisticdetailsarefrequentlyunknown.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org364Figure6.
UptakeandsubcellulartargetingofAuNPs.
Non-targeted(left)andtargeted(right)AuNPsbindtotheplasmamembrane;thismayinvolvere-ceptorsonthecellsurface.
Uponinternalization,AuNPsinitiallyconcentrateinendosomesorlysosomes.
Afterescapefromthesemembrane-boundcom-partments,AuNPsassociatewithnucleiormitochondria,wheretheycancauseirreversibledamagethatculminatesincancercelldeath.
CellularinjuryisenhancedifAuNPsaretargetedtonucleiormitochondria(right);thissubcel-lulartargetingincreasescancercellkilling.
4.
TargetingAuNPstotheERUnlikeAuNPdeliverytonucleiormitochondria,strategiesforERtargetingarelesswelldeveloped.
However,giventheimportanceoftheERformanytumortypesandthelinksbetweenmitochondriaandtheER[126],AuNPslocatedintheERcouldplayasignificantroleincancertherapy.
AuNPshavebeendetectedinthismembranesystem;forexample,non-functionalized13nmsphericalAuNPscolocal-izedwiththeERandGolgiapparatusinB16F10melanomacells[127].
InHeLacells,18nmAuNPsassociatedwithcytoplasmicvesiclesthatwerepre-sumablypartoftheER[128].
InKupffercellsoftheliver,13nmPEGylatedAuNPslocatedtolysosomesandthesmoothER[129],andAuNPsderivatizedwithCALNNR8peptidesweretrappedintheERofHeLacells[52].
SimultaneousassociationwithnucleiandtheERhasalsobeenreportedforK562cells(human,chronicmyelogenousleukemia)[130].
Notably,theseparticlesinducedERstress,whichlikelycontributedtotheirtoxicity.
5.
TargetingAuNPstosubcellularorga-nellesinvivoAscomparedtoculturedcellsorspheroids,AuNPtargetingtocellorganellesinvivofacesextrahurdles.
Theseadditionalchallengesincludeaccu-mulationinhealthyorgans,tissue-specificbarriers,clearancebythereticuloendothelialsystemandin-sufficientconcentrationatthetumorsite.
Onceac-cumulatedinthetumor,AuNPswillfollowthesamestepsdepictedinFig.
1.
Assuch,aftercellularuptake,particleshavetoescapelysosomes/endosomesandlocatetotheirfinalsubcellulardestination.
Toovercometheseobstacles,severalvariablescanbechanged,includingsize,surfacemodification,dosageandrouteofentry.
Accumulationatthetumorsitesinvivoisaprerequisiteforsubcellulartargeting,andthistopichasbeendiscussedinpreviousreviews[16,18].
Atpresent,onlyalimitednumberofstudieshaveattemptedtooptimizeAuNPstoreachasub-cellulardestinationinvivo.
Here,wediscussseveralstudiesthatshoworganelletargetingwithAuNPsbothinculturedcellsandinexperimentalanimals.
Targetingnucleiinvitroandinvivo.
Huangetal.
[85]comparedtheperformanceofAuNPsinthreedifferentmodelsystems.
Sphericaltiopronin-coatedAuNPsof2,6or15nmsizewereanalyzedinMCF7cells,growninmonolayersorspheroids.
Theseparti-cleswerealsoexaminedinxenograftsofBalb/cnudemice.
Inallofthesemodelsystems,cellularuptakewassize-dependentandmoreefficientforsmallerAuNPs.
Moreover,particlesizedeterminedthesub-cellularAuNPdistribution(seeAdditionalFile1:Ta-bleS1).
SmallAuNPsof2and6nmlocatedtothenu-cleusandcytoplasm,whereas15nmparticleswererestrictedtothecytoplasm.
Followingintravenousinjection,allAuNPswereclearedfromtheblood;thiswasmostefficientfor15nmparticles.
Bycontrast,tumorsaccumulatedpreferentially2nmAuNPs,but2nmparticlesalsoconcentratedinthekidneyandlung.
AuNPsof15nmsizeassociatedpredominantlywithnon-tumortissue,withpreferencefortheliverandspleen.
Collectively,theseresultssuggestthatthesubcellulardistributionoftiopronin-coatedAuNPsissimilarinMCF7cellmonolayers,spheroidsandxen-ografts.
Chenetal.
[76]examinedAuNPnucleartarget-inginvitroinHep3B(hepatocellularcarcinoma)and293Tcells(humanembryonickidneycellscontainingSV40T-antigen);theexperimentswereextendedtoHep3Bcell-derivedxenografts.
TodeliverDNAeffi-cientlytothecellnucleus,AuNPswerecoatedwithdifferentmoleculesinasandwich-likefashion.
ThesandwichincludedplasmidDNA,polyethylenimine(PEI)anddexamethasone,asyntheticagonistoftheglucocorticoidreceptor.
PEIenhancedendosomalescape,whiledexamethasoneservedseveralpurpos-es.
First,itimproveduptake,whichreached82.
5%inculturedHepB3cells.
Second,dexamethasonestimu-latedAuNPnucleartargetingthroughitsassociationwiththeglucocorticoidreceptor.
Specifically,func-tionalizedAuNPs(carryingdexamethasone,DNAandPEI)formcomplexeswithglucocorticoidreceptorinthecytoplasmandthentranslocatetothenucleus.
Withthisapproach,culturedcellsweretransfectedeffectively,whilecytotoxicitywaslow.
Buildingontheseinvitrostudies,functionalizedAuNPswerealsousedforgenedeliveryinvivoTheranostics2015,Vol.
5,Issue4http://www.
thno.
org365(Balb/cnudemicebearingHepB3-derivedtumors).
DNAencodingtumornecrosisfactor-relatedapopto-sis-inducingligand(TRAIL)wasintroducedintotheanimalsusingAuNPsof~55nmsize.
Tumorspro-ducedthehighestlevelsofTRAILwhentheDNAwasdeliveredbyanAuNPsandwichthatcontainedbothPEIanddexamethasone.
ConcomitantwithefficientDNAdelivery,tumorgrowthwasinhibited.
Thus,functionalizedAuNPsinducedTRAILsynthesisinvivoandtherebyinterferedwithtumorgrowth[76],whileTRAILexpressionwaslowinnon-tumortis-sues.
ThisstudydemonstratedAuNPnucleartarget-inginculturedcells.
Althoughnotshownexperi-mentallyinvivo,resultssuggestthatnucleartargetingalsooccurredinexperimentalanimals.
Thus,AuNPfunctionalizationwithdexamethasonecanbeusefultotargettumorsthatsynthesizeglucocorticoidre-ceptor.
Targetingmitochondriainvitroandinvivo.
ThepreferredrouteofATPproductioninmanyformsofcancerisanaerobicglycolysis;thispathwayreliesontheenzymehexokinasewhichsynthesizesglu-cose-6-phosphate.
Hexokinase2isespeciallyim-portantamongthefourenzymeisoforms,becauseitishighlyabundantinaggressivetumorcellsandpre-dominantlyassociatedwiththeoutermitochondrialmembrane.
Sincehexokinase2interactionwiththeoutermitochondrialmembranestimulatesglycolyticenergyproductionandreducesapoptosis,theenzymehasbecomeatherapeutictargetforcancertherapy.
Hexokinase2isinhibitedby3-bromopyruvate,atoxiccompoundwithoff-targeteffects.
Toimprove3-bromopyruvatespecificityanddiminishtoxicity,AuNPsweredevelopedformitochondrialdelivery[91].
PEGylatedsphericalAuNPs(2.
9–4.
3nm)weremodifiedwithTPP,therebygeneratingT-AuNPswhicharecharacterizedbypositivelychargedandlipophilicmoietiesonthesurface.
ThesepropertiesimprovedT-AuNPuptakeandmitochondrialassoci-ationinPC3cells.
ResultsweresimilarwhenT-AuNPswerefurthermodifiedwith3-bromopyruvate(T-3-BP-AuNP).
After4hours,T-3-BP-AuNPsboundtotheoutermitochondrialmembranewherehexokinase2resides.
TheseAuNPsaccumulatedinthemitochondrialmatrixat12hours.
T-3-BP-AuNPsreducedPC3andDU145(prostatecarcinoma)cellviability,buthadlittleeffectonhu-manmesenchymalstemcells.
Theimpactoncancercellswasattributedtothelossofmitochondrialfunc-tionsandglycolysis.
Overall,AuNPsmodifiedwith3-BPweremoretoxictocancerthannormalcells.
Atthesametime,AuNPstargetedtomitochondria(T-AuNPs)weremoreharmfulthantheirnon-targetedcounterparts(NT-AuNPs).
ThetoxicityofAuNPswasfurtherenhancedbya1minlaserirra-diationat660nm.
Toevaluateinvivoeffects,thebio-distributionandpharmacokineticsofT-AuNPandNT-AuNPwereassessedinmaleSpragueDawleyrats.
ClearancefromtheplasmawasfasterforT-AuNPsthanNT-AuNPs.
Bothtypesofparticlesaccumulatedintheliverandspleen,buttheirsubcel-lularlocalizationwasnotdetermined[91].
6.
Perspectives:LimitationsandfuturedirectionsThepromiseofAuNP-dependentcancertherapyisemphasizedbynumerouspublicationsandclinicaltrials[21].
Atpresent,severalhurdleslimittherapidimprovementofAuNP-basedtreatments.
Forexam-ple,theapproachesforAuNPtargetingtosubcellularlocationsdifferwidely.
SinceAuNPsize,morphology,functionalization,concentrationandthecelltypesanalyzedvarysignificantly,itisdifficulttocompareresultsfromdifferentlaboratories.
Moreover,alt-houghessentialtoimproveAuNP-basedintervention,thebiologicalmechanismsunderlyingAuNP-inducedcellkillingareoftennotdefined.
Nevertheless,somegeneralconclusionscanbedrawnforthediversestrategiesthatleadtoAuNPassociationwithsubcel-lularorganelles(Table1).
OurreviewdiscussesthepotentialbenefitsoftargetingAuNPstospecificcellorganelles,andwepresentthebarriersAuNPshavetoovercometoreachtheirintracellulardestination.
Asidefromthere-strictionsthesebarriersimpose,AuNP-mediatedcancercellkillingcouldbelimitedbyexportviaexo-cytosis(reviewedin[131]).
However,suchparticlelosscanbereducedwithappropriatesurfacefunc-tionalization[132].
Attheorganismallevel,itwillfurtherbeimportanttodesignstrategiesthatcontrolAuNPclearance.
Clearanceismostlyachievedthroughthehepatobiliarysystemandthekidney,anditismodulatedbyAuNPsurfacemodification[133].
Astheimmunesystemcontributestonanoparticleclearance[134],clearancerateslikelydifferamongpatients.
Althoughthesevariationschallengetheuniversalapplicationforcancertherapy,theymayneverthelessoffertheopportunitytoproduceAuNPsforpersonalizedmedicine.
Celltypedependentdifferencesinuptakeandsubsequentintracellulartargetingcomplicatetheop-timizationofAuNPsforcancertherapy.
Ontheotherhand,ifsystematicallyexamined,thesedifferencescouldbeexploredtodevelopAuNPsthatareselectiveforspecifictypesofcancer.
Moreover,AuNPtarget-ingtotumorcellorganellescouldbeenhancedbyexploitingthedifferencesbetweennormalandcancercells.
Forexample,nucleartransportismoreefficientinproliferatingcancercellsascomparedtotheirnon-tumorigeniccounterparts(seeabove).
Thisis-inTheranostics2015,Vol.
5,Issue4http://www.
thno.
org366part-causedbytheoverexpressionofimportin-αandimportin-βfamilymembersandothersolublefactorsornucleoporinsthatsupportsignal-mediatednuclearimport[135-137].
Todate,AuNPnucleartargetingreliespredominantlyonNLSsthatbindimportin-α(SV40T-antigen,nucleoplasmin)orimportin-β1(TAT,[138]).
Inthefuture,nucleardeliverycouldbeimprovedthroughAuNPfunctionalizationthatstim-ulatesNPCbinding.
Somenucleoporinsaremoreabundantincancercells[139],andthereforeprovidepotentialAuNPdockingsitesatthenuclearpore.
Withrespecttomitochondria,functionalizingsmallsphericalorrod-shapedAuNPswithmito-chondrialtargetingsequencesmaybesuitabletodamageorganellarfunctionsorevenclogmitochon-drialproteinimportsites,analogoustowhathasbeenobservedforNPCs.
Whiletargetingtosubcellularorganellesisofparticularinteresttonanomedicine,themajorbottle-neckaftercellularuptakeisAuNPescapefromen-dosomes/lysosomes.
Recentstudiessuggestaddi-tionalstrategiestoenhancethisescape.
Besideslowenergylaserirradiation[140],particlesurfacemodi-ficationsthatincreasethe"protonspongeeffect"couldpromoteendosomeswellingandAuNPrelease[141].
Moreover,AuNPsthatundergopH-dependentaggregationorenhancepH-dependentlipidrupturecouldbeusefulfortheliberationfromendosomes.
ReleasefromendosomesiscriticalforAuNPstoreachnuclei;however,itmaynotbemandatoryformito-chondria.
Forinstance,fusogeniclipidsasdescribedfortheMito-portercouldcircumventtheneedforendosomalescape.
Ontheotherhand,itisconceiva-blethatthedirectdeliveryofmaterialfromendo-somestomitochondria[142]willbeexploitedtobringAuNPstomitochondria.
Sofar,cellculturemodelshavedominatedthecharacterizationofAuNPbio-nanointeractions.
Morerecently,AuNP-inducedcelldamageanddeathhavebeenassessedintumorcellspheroids[85,143],amodelsystemthatmimicsmultipleaspectsoftumortissuesinvivo.
Forexample,AuNPpenetrationacrossmultiplecelllayerswasstudiedinspheroidsderivedfromU87glioblastomacells[143].
TheprogressinspheroidproductionsetsthestagetoexplorethismodelfurtherandexamineAuNPtargetingtonucleiandmitochondria.
Todate,thereareonlyfewstudiesthatexaminethesubcellulardistributionofAuNPsinvivo.
WhileinvitroanalyseslocatedAuNPsinnucleiormitochondria,itisinmostcasesnotclearwhethertheseAuNPsaretargetedtothesametumorcellor-ganellesinexperimentalanimals.
Clearly,thisinfor-mationhastobeprovidedinthefuturetofullyassessthevalueofAuNPsubcellulartargetinginvivo.
Table1.
AdvantagesandlimitationsofcurrentapproachesforAuNPdeliverytosubcellularorganelles.
ApproachSubcellularorganelleAdvantagesLimitationsExploitationofAuNPphysicalprop-ertiesfororganellardeliverywithoutspecifictargetingmoieties:size(e.
g.
smallorlargeparticles,nanoclusters),shape(spheres,rods,flowers,urchins),chargenonspecificsubcellulardistribution,oftenconcentratedinendo-somes/lysosomes,particlesmayescapeendosomes/lysosomesandassociatewithnucleiand/ormito-chondriaeasytoprepare,lowcost,fastclearancemaylimittoxicity,PEGylatedsmallAuNPsoftendamagecancercells,positivechargesfrequentlyenhanceup-takeuptakenotspecifictocancercells,fastclearancemaylimitaccumulationintumor,effectofpositivechargescell-typede-pendent,lowendosomal/lysosomalescape,lowconcentrationinsubcellularorganelles,hightoxicity,thusdamagetonon-tumortissuesTargetingtocellsurface:RGDtrans-ferrin,EGF,antibodiesoraptamersthatbindcellsurfacecomponentsnonspecificsubcellulardistribution,oftenconcentratedinendo-somes/lysosomes,particlesmayescapeendosomes/lysosomesandassociatewithnucleiand/ormito-chondriaenhancedtargetingtocancercells,therebyimproveduptakebytumorcellsendosomalescapeandsubcellulardeliverymayrequireadditionalmodifications;cellsurfacereceptorsignaturesforefficienttumortargetingnotavailableforallcancertypesImprovedcellularuptake:cellpene-trating(CPP)andotherpeptides;e.
g.
CALNN,CALNNR8,TAT,Pntn,lysosomalsortingpeptidesnucleusandothersubcellularcom-partmentsmayenhancenucleartargetingthroughincreaseofcellularuptake;someCPPsalsofunctionasnuclearlocali-zationsignalsomepeptidesinefficientforendoso-mal/lysosomalescapeandnucleartarget-ing;nuclearlocalizationcandependoncelltypeNuclearlocalizationsignals(NLSs):biologicalandsyntheticsignals;linearandcyclicpeptides:SV40-NLS,adenoviralNLS,cyclic[KW]5enrichedinnucleuspositivechargesofNLSenhancecellularuptake;specificandefficientnucleartargeting;frequentlylowtoxicityinvitro;usefulfordrugdeliverytonucleusmayneedadditionalmodificationstoimprovetumortargetinginvivoandtofacilitateendosomal/lysosomalescapeCombinationofpeptideswithdiffer-entfunctions:e.
g.
CPP+NLS,RGD+NLSenrichedinnucleusimprovedtumortargetingandcellularuptake;usefultoexploitcellsurfacereceptorsfunctionalizationwithmultiplepeptides;specificratioofpeptidesmayberequiredOthermolecules:CTAB,tiopronin,cysteamine,thioglucose,dexamethasonecanleadtoenrichmentinnucleusmaystimulatecellularuptake,endoso-mal/lysosomalescapeorboth;thiscanenhancenuclearassociationsomemodificationshighlytoxic(e.
g.
CTAB);exceptfordexamethasone,nucleartarget-ingnotefficient;targetingmaybecelltypespecific(e.
g.
nuclearaccumulationbydexamethasonereliesonglucocorticoidreceptor)Differenttypesoffunctionalization:octa-arginine,CTAB,TPP,chitosan,polyvinylpyrrolidoneenrichedinmitochondriacanstimulatecellularuptakeand/orendosomal/lysosomalescape;thiscanenhancemitochondrialassociationfrequentlytoxic(e.
g.
TPP);mayrequirepermeabilizationofplasmamembrane(e.
g.
polyvinylpyrrolidone)Theranostics2015,Vol.
5,Issue4http://www.
thno.
org367AsthemodelsystemstoevaluateAuNPper-formancearebecomingmorediverse,soareAuNPs.
Assuch,AuNPscanassembleintochains,plasmonicvesicles[144,145]orotherstructuresthatprovidemultiplefunctionsatthesametime,includingdrugdelivery,enhancedphotothermalablationandparti-cletracking.
LargecomplexAuNPsthatareabletodisassembleintosmallerAuNPunits[145,146]couldhelpovercomethedifferentroadblocksthatlimitAuNPsubcellulartargeting(Fig.
2).
SuchcomplexAuNPs,whendisassembled,mayhavetheadditionalbenefitofrapidrenalorhepatobiliaryclearance.
Takentogether,AuNPsofferuniqueopportuni-tiestotranslatetheinsightsofbasicresearchintoclinicalapplications.
GiventhesuccessofAuNPsforphotothermalablation,mechanicalinjuryandtar-geteddrugdelivery,futurestrategiesthatcombinetheseeffectsforAuNP-dependentcancercellkillingareparticularlypromising.
7.
SummaryandhighlightsRecentstudieshavebeguntorevealhowAuNPsimpingeonthestructural/functionalorganizationofcancerandnormalcells.
Thisknowledgeiscriticalfortwoaspectsofnanomedicine.
First,itwillhelpdefinetheAuNP-inducedeventsatthesubcellularlevel.
Thiswillsetthestagefortheidentificationofnewmoleculartargetsforcancertherapy.
Second,itwilldirectthedesignofAuNPswithphysico-chemicalpropertiesthatovercomethecurrentlimitationstheseparticlesfaceinbasicresearch,diagnosisandtherapy.
Thus,optimizationofAuNPsurfacesforcellandor-ganelle-specificdeliveryisanticipatedtoenhancetheefficiencyofcancercellkilling,whileminimizingtheimpactonnon-tumortissues.
Basedontheiressentialroleforcancercellsurvival,nucleiandmitochondriaareprimetargetsforthisapproach.
AbbreviationsAuNP:goldnanoparticleCALNN:cys-ala-leu-asp-aspCTAB:cetyltrimethylammoniumbromideEGF:epidermalgrowthfactorEGFR:EGFreceptorEPR:enhancedpermeabilityandretentionLSP:localizedsurfaceplasmonresonanceMTS:mitochondrialtargetingsequenceNIR:nearinfraredradiationNLS:nuclearlocalizationsequencePEG:polyethyleneglycol,surfacecoatingofAuNPsthatreducesparticleaggregation,non-specificinteractionswithbiomoleculesanduptakebythere-ticuloendothelialsystemPEI:polyethyleniminePntn:Penetratin,peptidesequencederivedfromDrosophilaproteinAntennapediathatpromotesentryintothecellTAT:HIV-1-trans-activatingproteinTPP:triphenylphosphoniumTRAIL:tumornecrosisfactor-relatedapopto-sis-inducingligandSupplementaryMaterialAdditionalFile1:TableS1:ImpactofAuNPsize,morphologyandfunctionalizationoncellularuptake,subcellularlo-calizationandcellsurvival.
http://www.
thno.
org/v05p0357s1.
pdfAuthorContributionsThemanuscriptwaswrittenthroughcontribu-tionsofallauthors.
Allauthorshavegivenapprovaltothefinalversionofthemanuscript.
CompetingInterestsTheauthorshavedeclaredthatnocompetinginterestexists.
References1.
HanahanD,WeinbergRobertA.
Hallmarksofcancer:Thenextgeneration.
Cell.
2011;144:646-74.
2.
UbahOC,WallaceHM.
Cancertherapy:Targetingmitochondriaandothersub-cellularorganelles.
CurrPharmDes.
2014;20:201-22.
3.
WrightJ.
Nanotechnology:Deliveronapromise.
Nature.
2014;509:S58-S9.
4.
GhoshP,HanG,DeM,KimCK,RotelloVM.
Goldnanoparticlesindeliveryapplications.
AdvDrugDelRev.
2008;60:1307-15.
5.
HutterE,MaysingerD.
Goldnanoparticlesandquantumdotsforbioimaging.
MicroscResTech.
2011;74:592-604.
6.
HutterE,MaysingerD.
Gold-nanoparticle-basedbiosensorsfordetectionofenzymeactivity.
TrendsPharmacolSci.
2013;34:497-507.
7.
ZhaoP,LiN,AstrucD.
Stateoftheartingoldnanoparticlesynthesis.
CoordChemRev.
2013;257:638-65.
8.
JanaNR,GearheartL,MurphyCJ.
Wetchemicalsynthesisofhighaspectratiocylindricalgoldnanorods.
JPhysChemB.
2001;105:4065-7.
9.
BrownKR,NatanMJ.
Hydroxylamineseedingofcolloidalaunanoparticlesinsolutionandonsurfaces.
Langmuir.
1998;14:726-8.
10.
NikoobakhtB,El-SayedMA.
Preparationandgrowthmechanismofgoldnanorods(NRs)usingseed-mediatedgrowthmethod.
ChemMater.
2003;15:1957-62.
11.
SapsfordKE,AlgarWR,BertiL,GemmillKB,CaseyBJ,OhE,etal.
Function-alizingnanoparticleswithbiologicalmolecules:Developingchemistriesthatfacilitatenanotechnology.
ChemRev.
2013;113:1904-2074.
12.
HuefnerA,SeptiadiD,WiltsBD,PatelII,KuanW-L,FragniereA,etal.
Goldnanoparticlesexplorecells:Cellularuptakeandtheiruseasintracellularprobes.
Methods.
2014;68:354–63.
13.
DykmanLA,KhlebtsovNG.
Uptakeofengineeredgoldnanoparticlesintomammaliancells.
ChemRev.
2014;114:1258-88.
14.
LévyR,ShaheenU,CesbronY,SéeV.
Goldnanoparticlesdeliveryinmam-malianlivecells:Acriticalreview.
NanoReviews.
2010;1:10.
3402/nano.
v1i0.
4889.
15.
JeremicB,AguerriAR,FilipovicN.
Radiosensitizationbygoldnanoparticles.
ClinTranslatOncol.
2013;15:593-601.
16.
JainS,HirstDG,O'SullivanJM.
Goldnanoparticlesasnovelagentsforcancertherapy.
BrJRadiol.
2012;85:101-13.
17.
AhmadMZ,AkhterS,RahmanZ,AkhterS,AnwarM,MallikN,etal.
Na-nometricgoldincancernanotechnology:Currentstatusandfutureprospect.
JPharmPharmacol.
2013;65:634-51.
18.
DreadenEC,AustinLA,MackeyMA,El-SayedMA.
Sizematters:Goldna-noparticlesintargetedcancerdrugdelivery.
TherDeliv.
2012;3:457-78.
19.
HolbackH,YeoY.
Intratumoraldrugdeliverywithnanoparticulatecarriers.
PharmRes.
2011;28:1819-30.
20.
LeungJ,WuS,ChouK,SignorellR.
Investigationofsub-100nmgoldnano-particlesforlaser-inducedthermotherapyofcancer.
Nanomaterials.
2013;3:86-106.
21.
[Internet]NIH:Clinicaltrials.
2014.
http://clinicaltrials.
gov/Theranostics2015,Vol.
5,Issue4http://www.
thno.
org36822.
LibuttiSK,PaciottiGF,ByrnesAA,AlexanderHR,GannonWE,WalkerM,etal.
PhaseIandpharmacokineticstudiesofCYT-6091,anovelPEGylatedcol-loidalgold-rhTNFnanomedicine.
ClinCancerRes.
2010;16:6139-49.
23.
AlexanderCM,HamnerKL,MayeMM,DabrowiakJC.
MultifunctionalDNA-goldnanoparticlesfortargeteddoxorubicindelivery.
BioconjugChem.
2014.
24.
ParkH,TsutsumiH,MiharaH.
Cell-selectiveintracellulardrugdeliveryusingdoxorubicinandα-helicalpeptidesconjugatedtogoldnanoparticles.
Bio-materials.
2014;35:3480-7.
25.
SakhraniNM,PadhH.
Organelletargeting:Thirdlevelofdrugtargeting.
DrugDesDevelTher.
2013;7:585-99.
26.
YuanH,FalesAM,Vo-DinhT.
Tatpeptide-functionalizedgoldnanostars:Enhancedintracellulardeliveryandefficientnirphotothermaltherapyusingultralowirradiance.
JAmChemSoc.
2012;134:11358-61.
27.
KongT,ZengJ,WangX,YangX,YangJ,McQuarrieS,etal.
Enhancementofradiationcytotoxicityinbreast-cancercellsbylocalizedattachmentofgoldnanoparticles.
Small.
2008;4:1537-43.
28.
BrownSD,NativoP,SmithJ-A,StirlingD,EdwardsPR,VenugopalB,etal.
Goldnanoparticlesfortheimprovedanticancerdrugdeliveryoftheactivecomponentofoxaliplatin.
JAmChemSoc.
2010;132:4678-84.
29.
SeidmanAD,ConlinAK,BachA,MoynahanME,LakeD,ForeroA,etal.
RandomizedphaseIItrialofweeklyvs.
every2weeksvs.
every3weeksna-noparticlealbumin-boundpaclitaxelwithbevacizumabasfirst-linechemo-therapyformetastaticbreastcancer.
ClinBreastCancer.
2013;13:239-46.
e1.
30.
JaggiAS,SinghN.
Mechanismsincancer-chemotherapeuticdrugs-inducedperipheralneuropathy.
Toxicology.
2012;291:1-9.
31.
VyasD,LaputG,VyasAK.
Chemotherapy-enhancedinflammationmayleadtothefailureoftherapyandmetastasis.
OncoTargetsTher.
2014;7:1015-23.
32.
MackeyMA,SairaF,MahmoudMA,El-SayedMA.
Inducingcancercelldeathbytargetingitsnucleus:Solidgoldnanospheresversushollowgoldnanocag-es.
BioconjugChem.
2013;24:897-906.
33.
ChenW-H,ChenJ-X,ChengH,ChenC-S,YangJ,XuX-D,etal.
Anewan-ti-cancerstrategyofdamagingmitochondriabypro-apoptoticpeptidefunc-tionalizedgoldnanoparticles.
ChemCommun(Camb).
2013;49:6403-5.
34.
WangF,WangY-C,DouS,XiongM-H,SunT-M,WangJ.
Doxorubi-cin-tetheredresponsivegoldnanoparticlesfacilitateintracellulardrugdeliv-eryforovercomingmultidrugresistanceincancercells.
ACSNano.
2011;5:3679-92.
35.
GibsonJD,KhanalBP,ZubarevER.
Paclitaxel-functionalizedgoldnanoparti-cles.
JAmChemSoc.
2007;129:11653-61.
36.
KumarA,HuoS,ZhangX,LiuJ,TanA,LiS,etal.
Neuropilin-1-targetedgoldnanoparticlesenhancetherapeuticefficacyofplatinum(IV)drugforprostatecancertreatment.
ACSNano.
2014;8:4205-20.
37.
ChenH,ZhangX,DaiS,MaY,CuiS,AchilefuS,etal.
Multifunctionalgoldnanostarconjugatesfortumorimagingandcombinedphotothermalandchemo-therapy.
Theranostics.
2013;3:633-49.
38.
AlmeidaJPM,FigueroaER,DrezekRA.
Goldnanoparticlemediatedcancerimmunotherapy.
NanomedNanotechnolBiolMed.
2014;10:503-14.
39.
TkachenkoAG,XieH,ColemanD,GlommW,RyanJ,AndersonMF,etal.
Multifunctionalgoldnanoparticle-peptidecomplexesfornucleartargeting.
JAmChemSoc.
2003;125:4700-1.
40.
AkhterS,AhmadMZ,AhmadFJ,StormG,KokRJ.
Goldnanoparticlesintheranosticoncology:Currentstate-of-the-art.
ExpertOpinDrugDeliv.
2012;9:1225-43.
41.
MurphyCJ,GoleAM,StoneJW,SiscoPN,AlkilanyAM,GoldsmithEC,etal.
Goldnanoparticlesinbiology:Beyondtoxicitytocellularimaging.
AccChemRes.
2008;41:1721-30.
42.
DreadenEC,El-SayedMA.
Detectinganddestroyingcancercellsinmorethanonewaywithnoblemetalsanddifferentconfinementpropertiesonthena-noscale.
AccChemRes.
2012;45:1854-65.
43.
VermaA,StellacciF.
Effectofsurfacepropertiesonnanoparticle–cellinterac-tions.
Small.
2010;6:12-21.
44.
LlevotA,AstrucD.
Applicationsofvectorizedgoldnanoparticlestothediagnosisandtherapyofcancer.
ChemSocRev.
2012;41:242-57.
45.
ChoEC,XieJ,WurmPA,XiaY.
Understandingtheroleofsurfacechargesincellularadsorptionversusinternalizationbyselectivelyremovinggoldnano-particlesonthecellsurfacewithaI2/KIetchant.
NanoLett.
2009;9:1080-4.
46.
KodihaM,HutterE,BoridyS,JuhasM,MaysingerD,StochajU.
Goldnano-particlesinducenucleardamageinbreastcancercellswhichisfurtherampli-fiedbyhyperthermia.
CellMolLifeSci.
2014;71:4259-73.
47.
WangL,LiuY,LiW,JiangX,JiY,WuX,etal.
Selectivetargetingofgoldnanorodsatthemitochondriaofcancercells:Implicationsforcancertherapy.
NanoLett.
2010;11:772-80.
48.
TkachenkoAG,XieH,LiuYL,ColemanD,RyanJ,GlommWR,etal.
Cellulartrajectoriesofpeptide-modifiedgoldparticlecomplexes:Comparisonofnu-clearlocalizationsignalsandpeptidetransductiondomains.
BioconjugChem.
2004;15:482-90.
49.
ChithraniBD,GhazaniAA,ChanWCW.
Determiningthesizeandshapedependenceofgoldnanoparticleuptakeintomammaliancells.
NanoLett.
2006;6:662-8.
50.
ChithraniBD,ChanWCW.
Elucidatingthemechanismofcellularuptakeandremovalofprotein-coatedgoldnanoparticlesofdifferentsizesandshapes.
NanoLett.
2007;7:1542-50.
51.
MandalD,MaranA,YaszemskiM,BolanderM,SarkarG.
Cellularuptakeofgoldnanoparticlesdirectlycross-linkedwithcarrierpeptidesbyosteosarcomacells.
JMaterSciMaterMed.
2009;20:347-50.
52.
SunL,LiuD,WangZ.
Functionalgoldnanoparticlepeptidecomplexesascell-targetingagents.
Langmuir.
2008;24:10293-7.
53.
DekiwadiaCD,LawrieAC,FecondoJV.
Peptide-mediatedcellpenetrationandtargeteddeliveryofgoldnanoparticlesintolysosomes.
JPeptSci.
2012;18:527-34.
54.
SongJ,ZhouJ,DuanH.
Self-assembledplasmonicvesiclesofsers-encodedamphiphilicgoldnanoparticlesforcancercelltargetingandtraceableintra-cellulardrugdelivery.
JAmChemSoc.
2012;134:13458-69.
55.
DamDHM,LeeJH,SiscoPN,CoDT,ZhangM,WasielewskiMR,etal.
Directobservationofnanoparticle–cancercellnucleusinteractions.
ACSNano.
2012;6:3318-26.
56.
KangB,MackeyMA,El-SayedMA.
NucleartargetingofgoldnanoparticlesincancercellsinducesDNAdamage,causingcytokinesisarrestandapoptosis.
JAmChemSoc.
2010;132:1517-9.
57.
ShahNB,DongJ,BischofJC.
Cellularuptakeandnanoscalelocalizationofgoldnanoparticlesincancerusinglabel-freeconfocalramanmicroscopy.
MolPharm.
2010;8:176-84.
58.
GuY-J,ChengJ,LinC-C,LamYW,ChengSH,WongW-T.
Nuclearpenetra-tionofsurfacefunctionalizedgoldnanoparticles.
ToxicolApplPharmacol.
2009;237:196-204.
59.
RyanJA,OvertonKW,SpeightME,OldenburgCM,LooL,RobargeW,etal.
Cellularuptakeofgoldnanoparticlespassivatedwithbsa-sv40largetantigenconjugates.
AnalChem.
2007;79:9150-9.
60.
HuoS,JinS,MaX,XueX,YangK,KumarA,etal.
Ultrasmallgoldnanoparti-clesascarriersfornucleus-basedgenetherapyduetosize-dependentnuclearentry.
ACSNano.
2014;8:5852-62.
61.
delaFuenteJM,BerryCC.
Tatpeptideasanefficientmoleculetotranslocategoldnanoparticlesintothecellnucleus.
BioconjugChem.
2005;16:1176-80.
62.
BerryCC,delaFuenteJM,MullinM,ChuSW,CurtisAS.
NuclearlocalizationofHIV-1tatfunctionalizedgoldnanoparticles.
IEEETransNanobioscience.
2007;6:262-9.
63.
ArvizoRR,MirandaOR,ThompsonMA,PabelickCM,BhattacharyaR,Ro-bertsonJD,etal.
Effectofnanoparticlesurfacechargeattheplasmamembraneandbeyond.
NanoLett.
2010;10:2543-8.
64.
Ojea-JimenezI,Garcia-FernandezL,LorenzoJ,PuntesVF.
Facilepreparationofcationicgoldnanoparticle-bioconjugatesforcellpenetrationandnucleartargeting.
ACSNano.
2012;6:7692-702.
65.
ChithraniBD,StewartJ,AllenC,JaffrayDA.
Intracellularuptake,transport,andprocessingofnanostructuresincancercells.
NanomedNanotechnolBiolMed.
2009;5:118-27.
66.
NativoP,PriorIA,BrustM.
Uptakeandintracellularfateofsurface-modifiedgoldnanoparticles.
ACSNano.
2008;2:1639-44.
67.
OyelereAK,ChenPC,HuangX,El-SayedIH,El-SayedMA.
Pep-tide-conjugatedgoldnanorodsfornucleartargeting.
BioconjugChem.
2007;18:1490-7.
68.
YangC,UertzJ,YohanD,ChithraniBD.
Peptidemodifiedgoldnanoparticlesforimprovedcellularuptake,nucleartransport,andintracellularretention.
Nanoscale.
2014;6:12026-33.
69.
ConnorEE,MwamukaJ,GoleA,MurphyCJ,WyattMD.
Goldnanoparticlesaretakenupbyhumancellsbutdonotcauseacutecytotoxicity.
Small.
2005;1:325-7.
70.
DixitV,VandenBosscheJ,ShermanDM,ThompsonDH,AndresRP.
Syn-thesisandgraftingofthiocticacidPEGfolateconjugatesontoaunanoparti-clesforselectivetargetingoffolatereceptor-positivetumorcells.
BioconjugChem.
2006;17:603-9.
71.
HuffTB,HansenMN,ZhaoY,ChengJ-X,WeiA.
Controllingthecellularuptakeofgoldnanorods.
Langmuir.
2007;23:1596-9.
72.
HuffTB,TongL,ZhaoY,HansenMN,ChengJ-X,WeiA.
Hyperthermiceffectsofgoldnanorodsontumorcells.
Nanomedicine.
2007;2:125-32.
73.
BhattacharyaR,PatraCR,EarlA,WangS,KataryaA,LuL,etal.
Attachingfolicacidongoldnanoparticlesusingnoncovalentinteractionviadifferentpolyethyleneglycolbackbonesandtargetingofcancercells.
NanomedNano-technolBiolMed.
2007;3:224-38.
74.
ShiraziAN,TiwariRK,OhD,SullivanB,McCaffreyK,MandalD,etal.
Surfacedecoratedgoldnanoparticlesbylinearandcyclicpeptidesasmolec-ulartransporters.
MolPharm.
2013;10:10.
1021/mp400199e.
75.
ShuklaR,BansalV,ChaudharyM,BasuA,BhondeRR,SastryM.
Biocom-patibilityofgoldnanoparticlesandtheirendocytoticfateinsidethecellularcompartment:Amicroscopicoverview.
Langmuir.
2005;21:10644-54.
76.
ChenZ,ZhangL,HeY,LiY.
Sandwich-typeAu-PEI/DNA/PEI-Dexanano-complexfornucleus-targetedgenedeliveryinvitroandinvivo.
ACSApplMaterInterfaces.
2014;6:14196-206.
77.
HanG,YouC-C,KimB-j,TuringanRS,ForbesNS,MartinCT,etal.
Light-regulatedreleaseofDNAanditsdeliverytonucleibymeansofpho-tolabilegoldnanoparticles.
AngewChemIntEdEngl.
2006;45:3165-9.
78.
El-SayedIH,HuangX,El-SayedMA.
Surfaceplasmonresonancescatteringandabsorptionofanti-egfrantibodyconjugatedgoldnanoparticlesincancerdiagnostics:Applicationsinoralcancer.
NanoLett.
2005;5:829-34.
79.
HuangX,El-SayedIH,QianW,El-SayedMA.
Cancercellimagingandpho-tothermaltherapyinthenear-infraredregionbyusinggoldnanorods.
JAmChemSoc.
2006;128:2115-20.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org36980.
HauckTS,GhazaniAA,ChanWCW.
Assessingtheeffectofsurfacechemistryongoldnanoroduptake,toxicity,andgeneexpressioninmammaliancells.
Small.
2008;4:153-9.
81.
OhE,DelehantyJB,SapsfordKE,SusumuK,GoswamiR,Blanco-CanosaJB,etal.
CellularuptakeandfateofPEGylatedgoldnanoparticlesisdependentonbothcell-penetrationpeptidesandparticlesize.
ACSNano.
2011;5:6434-48.
82.
LiuX,HuangN,LiH,JinQ,JiJ.
Surfaceandsizeeffectsoncellinteractionofgoldnanoparticleswithbothphagocyticandnonphagocyticcells.
Langmuir.
2013;29:9138-48.
83.
CoradeghiniR,GioriaS,GarcíaCP,NativoP,FranchiniF,GillilandD,etal.
Size-dependenttoxicityandcellinteractionmechanismsofgoldnanoparticlesonmousefibroblasts.
ToxicolLett.
2013;217:205-16.
84.
PanY,NeussS,LeifertA,FischlerM,WenF,SimonU,etal.
Size-dependentcytotoxicityofgoldnanoparticles.
Small.
2007;3:1941-9.
85.
HuangK,MaH,LiuJ,HuoS,KumarA,WeiT,etal.
Size-dependentlocaliza-tionandpenetrationofultrasmallgoldnanoparticlesincancercells,multi-cellularspheroids,andtumorsinvivo.
ACSNano.
2012;6:4483-93.
86.
SalnikovV,LukyanenkoYO,FrederickCA,LedererWJ,LukyanenkoV.
Probingtheoutermitochondrialmembraneincardiacmitochondriawithna-noparticles.
BiophysJ.
2007;92:1058-71.
87.
YamadaY,AkitaH,KamiyaH,KogureK,YamamotoT,ShinoharaY,etal.
Mito-porter:Aliposome-basedcarriersystemfordeliveryofmacromoleculesintomitochondriaviamembranefusion.
BiochimBiophysActa.
2008;1778:423-32.
88.
WangL,JiangX,JiY,BaiR,ZhaoY,WuX,etal.
Surfacechemistryofgoldnanorods:Originofcellmembranedamageandcytotoxicity.
Nanoscale.
2013;5:8384-91.
89.
ZhuangQ,JiaH,DuL,LiY,ChenZ,HuangS,etal.
Targetedsur-face-functionalizedgoldnanoclustersformitochondrialimaging.
BiosensorsBioelectron.
2014;55:76-82.
90.
MocanL,IlieI,TabaranFA,DanaB,ZaharieF,ZdrehusC,etal.
Surfaceplasmonresonance-inducedphotoactivationofgoldnanoparticlesasmito-chondria-targetedtherapeuticagentsforpancreaticcancer.
ExpertOpinTherTargets.
2013;17:1383-93.
91.
MarracheS,DharS.
Theenergyblockerinsidethepowerhouse:Mitochondriatargeteddeliveryof3-bromopyruvate.
ChemScience.
2015.
doi:10.
1039/C4SC01963.
92.
ChengY,MeyersJD,AgnesRS,DoaneTL,KenneyME,BroomeA-M,etal.
Addressingbraintumorswithtargetedgoldnanoparticles:AnewgoldstandardforhydrophobicdrugdeliverySmall.
2011;7:2301-6.
93.
ScarìG,PortaF,FascioU,AvvakumovaS,DalSantoV,DeSimoneM,etal.
Goldnanoparticlescappedbyagc-containingpeptidefunctionalizedwithanrgdmotifforintegrintargeting.
BioconjugChem.
2012;23:340-9.
94.
delaFuenteJM,BerryCC,RiehleMO,CurtisASG.
Nanoparticletargetingatcells.
Langmuir.
2006;22:3286-93.
95.
SykesEA,ChenJ,ZhengG,ChanWCW.
Investigatingtheimpactofnano-particlesizeonactiveandpassivetumortargetingefficiency.
ACSNano.
2014;8:5696-706.
96.
EghtedariM,LiopoAV,CoplandJA,OraevskyAA,MotamediM.
Engineer-ingofhetero-functionalgoldnanorodsfortheinvivomoleculartargetingofbreastcancercells.
NanoLett.
2008;9:287-91.
97.
El-SayedIH,HuangX,El-SayedMA.
Selectivelaserphoto-thermaltherapyofepithelialcarcinomausinganti-EGFRantibodyconjugatedgoldnanoparticles.
CancerLett.
2006;239:129-35.
98.
delasHerasJI,BatrakouDG,SchirmerEC.
Cancerbiologyandthenuclearenvelope:Aconvolutedrelationship.
SeminCancerBiol.
2013;23:125-37.
99.
JevtiP,EdensLJ,VukoviLD,LevyDL.
Sizingandshapingthenucleus:Mechanismsandsignificance.
CurrOpinCellBiol.
2014;28:16-27.
100.
MatchettKB,McFarlaneS,HamiltonSE,EltuhamyYS,DavidsonMA,MurrayJT,etal.
RanGTPaseinnuclearenvelopeformationandcancermetastasis.
AdvExpMedBiol.
2014;773:323-51.
101.
MiyamotoY,LovelandKL,YonedaY.
Nuclearimportinalphaanditsphysi-ologicalimportance.
CommunIntegrBiol.
2012;5:220-2.
102.
QuinJE,DevlinJR,CameronD,HannanKM,PearsonRB,HannanRD.
Tar-getingthenucleolusforcancerintervention.
BiochimBiophysActa.
2014;1842:802-16.
103.
RuggeroD.
Revisitingthenucleolus:Frommarkertodynamicintegratorofcancersignaling.
SciSignal.
2012;5:pe38.
104.
MorA,WhiteMA,FontouraBMA.
Nucleartraffickinginhealthanddisease.
CurrOpinCellBiol.
2014;28:28-35.
105.
KuusistoHV,WagstaffKM,AlvisiG,RothDM,JansDA.
Globalenhancementofnuclearlocalization-dependentnucleartransportintransformedcells.
FASEBJ.
2012;26:1181-93.
106.
FeldherrCM,AkinD.
Thepermeabilityofthenuclearenvelopeindividingandnondividingcellcultures.
JCellBiol.
1990;111:1-8.
107.
FeldherrCM,AkinD.
Signal-mediatednucleartransportinproliferatingandgrowth-arrestedBALB/c3T3cells.
JCellBiol.
1991;115:933-9.
108.
FeldherrCM,AkinD.
Regulationofnucleartransportinproliferatingandquiescentcells.
ExpCellRes.
1993;205:179-86.
109.
TsoliM,KuhnH,BrandauW,EscheH,SchmidG.
CellularuptakeandtoxicityofAu55clusters.
Small.
2005;1:841-4.
110.
PantéN,KannM.
Nuclearporecomplexisabletotransportmacromoleculeswithdiametersof39nm.
MolBiolCell.
2002;13:425-34.
111.
FeldherrCM,AkinD,CohenRJ.
Regulationoffunctionalnuclearporesizeinfibroblasts.
JCellSci.
2001;114:4621-7.
112.
FeldherrCM,AkinD.
Thelocationofthetransportgateinthenuclearporecomplex.
JCellSci.
1997;110:3065-70.
113.
NovakJP,NickersonC,FranzenS,FeldheimDL.
Purificationofmolecularlybridgedmetalnanoparticlearraysbycentrifugationandsizeexclusionchro-matography.
AnalChem.
2001;73:5758-61.
114.
Krpeti,SaleemiS,PriorIA,SéeV,QureshiR,BrustM.
NegotiationofintracellularmembranebarriersbyTAT-modifiedgoldnanoparticles.
ACSNano.
2011;5:5195-201.
115.
TsaiTL,HouCC,WangHC,YangZS,YehCS,ShiehDB,etal.
Nucleocyto-plasmictransportblockagebySV40peptide-modifiedgoldnanoparticlesin-ducescellularautophagy.
IntJNanomedicine.
2012;7:5215-34.
116.
HuschkaR,BarhoumiA,LiuQ,RothJA,JiL,HalasNJ.
Genesilencingbygoldnanoshell-mediateddeliveryandlaser-triggeredreleaseofantisenseoligonu-cleotideandsiRNA.
ACSNano.
2012;6:7681-91.
117.
RyouS-M,ParkM,KimJ-M,JeonCO,YunC-H,HanSH,etal.
Inhibitionofxenografttumorgrowthinmicebygoldnanoparticle-assisteddeliveryofshorthairpinRNAsagainstMcl-1L.
JBiotechnol.
2011;156:89-94.
118.
KimJ-H,JangHH,RyouS-M,KimS,BaeJ,LeeK,etal.
Afunctionalizedgoldnanoparticles-assisteduniversalcarrierforantisenseDNA.
ChemCommun(Camb).
2010;46:4151-3.
119.
KimD-W,KimJ-H,ParkM,YeomJ-H,GoH,KimS,etal.
ModulationofbiologicalprocessesinthenucleusbydeliveryofDNAoligonucleotidescon-jugatedwithgoldnanoparticles.
Biomaterials.
2011;32:2593-604.
120.
WallaceDC.
Amitochondrialparadigmofmetabolicanddegenerativedis-eases,aging,andcancer:Adawnforevolutionarymedicine.
AnnuRevGenet.
2005;39:359-407.
121.
PathaniaD,MillardM,NeamatiN.
Opportunitiesindiscoveryanddeliveryofanticancerdrugstargetingmitochondriaandcancercellmetabolism.
AdvDrugDelRev.
2009;61:1250-75.
122.
WenS,ZhuD,HuangP.
Targetingcancercellmitochondriaasatherapeuticapproach.
FutureMedChem.
2012;5:53-67.
123.
RehlingP,ModelK,BrandnerK,KovermannP,SickmannA,MeyerHE,etal.
Proteininsertionintothemitochondrialinnermembranebyatwin-poretranslocase.
Science.
2003;299:1747-51.
124.
SzaboI,ZorattiM.
Mitochondrialchannels:Ionfluxesandmore.
PhysiolRev.
2014;94:519-608.
125.
SmithRA,HartleyRC,MurphyMP.
Mitochondria-targetedsmallmoleculetherapeuticsandprobes.
AntioxidRedoxSignal.
2011;15:3021-38.
126.
ClarkeHannaJ,ChambersJosephE,LinikerE,MarciniakStefanJ.
Endoplas-micreticulumstressinmalignancy.
CancerCell.
2014;25:563-73.
127.
ChangM-Y,ShiauA-L,ChenY-H,ChangC-J,ChenHHW,WuC-L.
Increasedapoptoticpotentialanddose-enhancingeffectofgoldnanoparticlesincom-binationwithsingle-doseclinicalelectronbeamsontumor-bearingmice.
CancerSci.
2008;99:1479-84.
128.
KhanJA,PillaiB,DasTK,SinghY,MaitiS.
MoleculareffectsofuptakeofgoldnanoparticlesinHeLacells.
ChemBioChem.
2007;8:1237-40.
129.
ChoW-S,ChoM,JeongJ,ChoiM,ChoH-Y,HanBS,etal.
Acutetoxicityandpharmacokineticsof13nm-sizedPEG-coatedgoldnanoparticles.
ToxicolApplPharmacol.
2009;236:16-24.
130.
TsaiY-Y,HuangY-H,ChaoY-L,HuK-Y,ChinL-T,ChouS-H,etal.
Identifi-cationofthenanogoldparticle-inducedendoplasmicreticulumstressbyomictechniquesandsystemsbiologyanalysis.
ACSNano.
2011;5:9354-69.
131.
KimCS,LeNDB,XingY,YanB,TongaGY,KimC,etal.
Theroleofsurfacefunctionalityinnanoparticleexocytosis.
AdvHealthcMater.
2014;3:1200-2132.
OhN,ParkJ-H.
Surfacechemistryofgoldnanoparticlesmediatestheirexo-cytosisinmacrophages.
ACSNano.
2014;8:6232-41.
133.
SimpsonCA,SallengKJ,CliffelDE,FeldheimDL.
Invivotoxicity,biodistri-bution,andclearanceofglutathione-coatedgoldnanoparticles.
Nanomedi-cine.
2013;9:257-63.
134.
JonesSW,RobertsRA,RobbinsGR,PerryJL,KaiMP,ChenK,etal.
Nanopar-ticleclearanceisgovernedbyTh1/Th2immunityandstrainbackground.
JClinInvest.
2013;123:3061-73.
135.
vanderWattPJ,StowellCL,LeanerVD.
Thenuclearimportreceptorkpnb1anditspotentialasananticancertherapeutictarget.
CritRevEukaryotGeneExpr.
2013;23:1-10.
136.
ChristiansenA,DyrskjtL.
Thefunctionalroleofthenovelbiomarkerkary-opherinα2(KPNA2)incancer.
CancerLett.
2013;331:18-23.
137.
FanH,LuY,QinH,ZhouY,GuY,ZhouJ,etal.
Highranleveliscorrelatedwithpoorprognosisinpatientswithcolorectalcancer.
IntJClinOncol.
2013;18:856-63.
138.
TruantR,CullenBR.
Thearginine-richdomainspresentinhumanimmuno-deficiencyvirustype1TatandRevfunctionasdirectimportinβ-dependentnuclearlocalizationsignals.
MolCellBiol.
1999;19:1210-7.
139.
OriA,BanterleN,IskarM,Andrés‐PonsA,EscherC,KhanhBuiH,etal.
Celltype‐specificnuclearpores:Acaseinpointforcontext‐dependentstoichiom-etryofmolecularmachines.
MolSystBiol.
2013;9:648140.
KrpeticZ,NativoP,SeeV,PriorIA,BrustM,VolkM.
Inflictingcontrollednonthermaldamagetosubcellularstructuresbylaser-activatedgoldnano-particles.
NanoLett.
2010;10:4549-54.
141.
NeubergP,KichlerA.
Recentdevelopmentsinnucleicaciddeliverywithpolyethylenimines.
AdvGenet.
2014;88:263-88.
142.
SheftelAD,ZhangAS,BrownC,ShirihaiOS,PonkaP.
Directinterorganellartransferofironfromendosometomitochondrion.
Blood.
2007;110:125-32.
Theranostics2015,Vol.
5,Issue4http://www.
thno.
org370143.
HuoS,MaH,HuangK,LiuJ,WeiT,JinS,etal.
Superiorpenetrationandretentionbehaviorof50nmgoldnanoparticlesintumors.
CancerRes.
2013;73:319-30.
144.
LinM,GuoC,LiJ,ZhouD,LiuK,ZhangX,etal.
Polypyrrole-coatedchainlikegoldnanoparticlearchitectureswiththe808nmphotothermaltransductionefficiencyupto70%.
ACSApplMaterInterfaces.
2014;6:5860-8.
145.
SongJ,FangZ,WangC,ZhouJ,DuanB,PuL,etal.
Photolabileplasmonicvesiclesassembledfromamphiphilicgoldnanoparticlesforremote-controlledtraceabledrugdelivery.
Nanoscale.
2013;5:5816-24.
146.
KanarasAG,WangZ,BrustM,CosstickR,BatesAD.
EnzymaticdisassemblyofDNA–goldnanostructures.
Small.
2007;3:590-4.
- ideawww.vtigu.com相关文档
- slovenskegawww.vtigu.com
- 节点www.vtigu.com
- Cassakwww.vtigu.com
- 71620.635@compuserve.com
- behaviourswww.vtigu.com
- TRMswww.vtigu.com
BuyVM新设立的迈阿密机房速度怎么样?简单的测评速度性能
BuyVM商家算是一家比较老牌的海外主机商,公司设立在加拿大,曾经是低价便宜VPS主机的代表,目前为止有提供纽约、拉斯维加斯、卢森堡机房,以及新增加的美国迈阿密机房。如果我们有需要选择BuyVM商家的机器需要注意的是注册信息的时候一定要规范,否则很容易出现欺诈订单,甚至你开通后都有可能被禁止账户,也是这个原因,曾经被很多人吐槽的。这里我们简单的对于BuyVM商家新增加的迈阿密机房进行简单的测评。如...
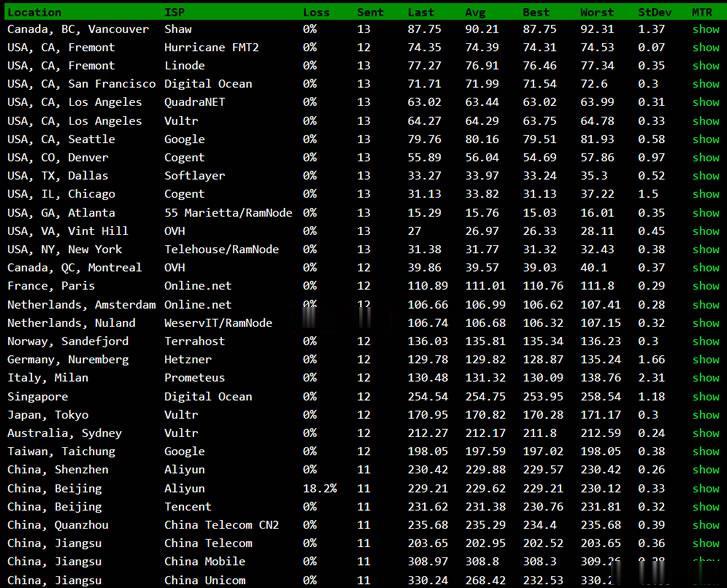
特网云-新上线香港五区补货资源充足限时抢 虚拟主机6折,低至38元!
官方网站:点击访问特网云官网活动方案:===========================香港云限时购==============================支持Linux和Windows操作系统,配置都是可以自选的,非常的灵活,宽带充足新老客户活动期间新购活动款产品都可以享受续费折扣(只限在活动期间购买活动款产品才可享受续费折扣 优惠码:AADE01),购买折扣与续费折扣不叠加,都是在原价...

HostNamaste$24 /年,美国独立日VPS优惠/1核1G/30GB/1Gbps不限流量/可选达拉斯和纽约机房/免费Windows系统/
HostNamaste是一家成立于2016年3月的印度IDC商家,目前有美国洛杉矶、达拉斯、杰克逊维尔、法国鲁贝、俄罗斯莫斯科、印度孟买、加拿大魁北克机房。其中洛杉矶是Quadranet也就是我们常说的QN机房(也有CC机房,可发工单让客服改机房);达拉斯是ColoCrossing也就是我们常说的CC机房;杰克逊维尔和法国鲁贝是OVH的高防机房。采用主流的OpenVZ和KVM架构,支持ipv6,免...

www.vtigu.com为你推荐
-
存储备份存储备份软件哪个好?求推荐sherylsandberg这个文章什么意思 给个翻译好吗 谢谢了netlife熊猫烧香图片硬盘工作原理硬盘跟光盘的工作原理?xyq.163.cbg.com梦幻西游藏宝阁同ip域名两个网站同一个IP怎么绑定两个域名百度关键词工具百度有关键字分析工具吗?Google AdWords有的haole018.com为什么www.haole008.com在我这里打不开啊,是不是haole008换新的地址了?www.5ff.comhttp://www.940777.com/网站,是不是真的网投六合www.zhiboba.com看NBA直播的网站哪个知道