basedm.2828dy.com
m.2828dy.com 时间:2021-03-19 阅读:()
AdvancedMetalsandAlloysAdvancedMagneticCoolingHigh-PressureIntermetallicHydridesLightweightMetalMatrixNanocompositesSelf-PropagatingReactionsByMechanicalAlloyingTMVol.
2,No.
4AdvancingTechnology—imagineaworldwithoutAldrichChemicalCo.
,Inc.
Sigma-AldrichCorporation6000N.
TeutoniaAve.
Milwaukee,WI53209,USAToPlaceOrdersTelephone800-325-3010(USA)FAX800-325-5052(USA)Customer&TechnicalServicesCustomerInquiries800-325-3010TechnicalService800-231-8327SAFC800-244-1173CustomSynthesis800-244-1173Flavors&Fragrances800-227-4563International414-438-385024-HourEmergency414-438-3850WebSitesigma-aldrich.
comEmailaldrich@sial.
comSubscriptionsTorequestyourFREEsubscriptiontoMaterialMatters,pleasecontactusby:Phone:800-325-3010(USA)Mail:Attn:MarketingCommunicationsAldrichChemicalCo.
,Inc.
Sigma-AldrichCorporationP.
O.
Box355Milwaukee,WI53201-9358Email:sams-usa@sial.
comInternationalcustomers,pleasecontactyourlocalSigma-Aldrichoffice.
Forworldwidecontactinformation,pleaseseebackcover.
MaterialMattersisalsoavailableinPDFformatontheInternetatsigma-aldrich.
com/matsci.
AldrichbrandproductsaresoldthroughSigma-Aldrich,Inc.
Sigma-Aldrich,Inc.
warrantsthatitsproductsconformtotheinformationcontainedinthisandotherSigma-Aldrichpublications.
Purchasermustdeterminethesuitabilityoftheproductforitsparticularuse.
Seereversesideofinvoiceorpackingslipforadditionaltermsandconditionsofsale.
Allpricesaresubjecttochangewithoutnotice.
MaterialMatters(ISSN1933–9631)isapublicationofAldrichChemicalCo.
,Inc.
AldrichisamemberoftheSigma-AldrichGroup.
2007Sigma-AldrichCo.
TMVol.
2No.
4IntroductionAboutOurCoverMetalsandalloyscouldbeconsideredthebackboneofhumancivilization.
Thecastingofsuchmetalsascopperandtintomakebronze,thefirstfunctionalmetalalloy,pavedourwaytothemoderntechnologicalsociety.
Mostofthemetalscrystallizeintocloselypackedcubic(forexample,Al,Cu,Fe)orhexagonalcrystallattices(shownonthecover,forexample,Gd,Ti,Zr,rareearthmetals),whicharelargelyresponsiblefortheirremarkableproperties.
Thecovershowsmetalcasting,thebeginningofalongtechnologicalprocessthatconcludesinsuchmarvelsasairplanes.
IntroductionNoothermaterialshavecontributedmoretothedevelopmentofmankindoverthemillenniathanmetalsandalloys.
Throughoutcenturies,studiesofmetalsbelongedtooneoftheoldestbranchesofAppliedMaterialsScience–Metallurgy.
Thischangedinthelate19thandearly20thcenturies,whenapplicationsofmetallicmaterialsspreadintootherareasofscienceandtechnologyincludingelectronics,energy,aeronauticsandspacetravel,tonameafew.
Currently,it'simpossibletoimagineaworldinwhichwecouldsuccessfullyfunctionwithoutmetalsandalloys.
Traditionally,metalsareportrayedasshinysolids,mostofwhicharegoodconductorsofheatandelectricity.
Theyareductileandmostwillmeltathightemperatures.
Metalandalloyshapescaneasilybechangedbymechanicalprocessing,atechniquethatcanbeusedforthepreparation,modificationandchemicalconversionofmetalalloysandcomposites.
Presently,thereare87knownmetals,61ofwhichareavailablethroughSigma-Aldrichinvariousformsandmodifications;thesearehighlightedinredintheperiodictablechartbelow.
Seepages14–15foranexpandedchart.
HHeLiBeBCNOFNeNaMgAlSiPSClArKCaScTiVCrMnFeCoNiCuZnGaGeAsSeBrKrRbSrYZrNbMoTcRuRhPdAgCdInSnSbTeIXeCsBaLaHfTaWReOsIrPtAuHgTlPbBiPoAtRnFrRaAc104105106CePrNdPmSmEuGdTbDyHoErTmYbLuThPaUNpPuAmCmBkCfEsFmMdNoLrPuremetalsatSigma-Aldrichvisitsigma-aldrich.
com/metalsfordetailsThisissueofMaterialMattersfocusesonmetalsandalloysforadvancedapplicationsincludingmagneticrefrigeration;high-pressure,high-capacity,hydrogenabsorbingsystems;nanocomposites;andmechanicallyinducedconversionofmetallicsolids.
LeadingexpertsfromtheAmesLaboratoryoftheU.
S.
DepartmentofEnergy,MoscowStateUniversity,UniversityofWisconsin–Milwaukee,andUniversityofMarylanddiscussrecentexperimentalresultsandshareideasandtechniquesassociatedwithmetals,alloys,andtheirapplications.
Inside,you'llalsofindnewlyintroducedproducts,whichincludebutarenotlimitedtomagneticrefrigerationalloys,hydrideformingintermetallics,magneticmaterialsandhigh-purityrareearthmetalsandfoils.
Inthe"YourMaterialsMatter"feature,wearepleasedtointroduceAlignedMulti-WalledCarbonNanotubes(MWCNTs)—anewSigma-AldrichproductsuggestedbyDr.
KarlGross,CEOofHy-EnergyLLC.
OurgoalatSigma-AldrichMaterialsScienceistoprovideinnovativematerialsthataccelerateyourresearch.
Forproductdetailsandtechnicalinformation,pleasevisitsigma-aldrich.
com/matsci.
Haveacomment,questionorsuggestionaboutMaterialMattersPleasecontactusatmatsci@sial.
com.
ViktorBalema,Ph.
D.
MaterialsScienceSigma-AldrichCorporationForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
"YourMaterialsMatter.
"AlignedMulti-WalledCarbonNanotubes(MWCNTs)JoePorwoll,PresidentAldrichChemicalCo.
,Inc.
Dr.
KarlGross,ofHy-EnergyLLC,kindlysuggestedthathigh-qualitycarbonnanotubearraysareneededforresearchanddevelopmentofhigh-sensitivitygassensors.
Wearepleasedtoofferhighlyalignedarraysofmulti-wallcarbonnanotubes(MWCNTs)asnewproductsinourcatalog.
Thesearrays,availableonsilicone(AldrichProductNo.
687804)orcopper(AldrichProductNo.
687812)substrates,canprovideauniqueplatformforawiderangeofmaterialsresearchanddevelopment,includinggasadsorptionsensorandsensorsubstrates,1catalysts,electronemissionsources,2andbatteryandcapacitordevelopment.
3Carbonnanotubearray,multi-walled,verticallyaligned,onsiliconwafersubstrate687804-1EACarbonnanotubearray,multi-walled,verticallyaligned,oncoppersubstrate687812-1EAReferences:(1)Collins,P.
G.
,Bradley,K.
Ishigami,M.
Zettl,A.
,Science,2000,287,1801.
(2)Bonard,J.
M.
,Stockli,T.
,Maier,F.
,DeHerr,W.
A.
,Chatelain,A.
,Ugarte,D.
,Salvetat,J.
P.
,Forro,L.
,PhysicalReviewLetters,1998,81,1441.
(3)Frackowiak,E.
,Gautier,S.
,Gaucher,H.
,Bonnamy,S.
,Beguin,F.
,Carbon,1999,37,61IntroductionFeatures:99.
9%asMWCNTCNTdiametersare100nm±10nmCNTlengthsare30m±3mArraydensity~2x109MWCNT/cm2Arraydimensions1sq.
cmGrownbyplasma-enhancedCVD(PECVD)GrownwiththenickelcatalysttipintactPackagedinacleanroom;stored,andshippedinsemiconductorgradepackaging.
DoyouhaveacompoundthatyouwishSigma-AldrichcouldlisttohelpmaterialsresearchIfitisneededtoaccelerateyourresearch,itmatters—pleasesendyoursuggestiontomatsci@sial.
comandwewillbehappytogiveitcarefulconsideration.
CNTarray(blacksquare)inthesemiconductorgradepackage.
InsetshowsSEMimageoftheverticallyalignedMWCNTs.
AdvancedMetalsandAlloysFeaturedinThisIssueMaterialsCategoryContentPageMaterialsforMagneticCoolingMaterialsexhibitinggiantmagnetocaloriceffect7UltraHigh-PurityMetalsforPreparationofMagneticRefrigerationMaterialsSigma-Aldrichproductssuitableforthepreparationofnovelsystemswithgiantmagnetocaloriceffect7MagneticAlloysandIntermetallicsMetalalloysusedaspermanentmagnets8HydrogenAbsorbingAlloysMetallicmaterialscapableofabsorbinglargequantitiesofhydrogenatvarioustemperaturesandpressures13High-PurityIron,Nickel,andZirconiumMaterialsusedforthepreparationofintermetallichydrogenabsorbingsystems13PureMetalsfromSigma-AldrichPeriodicTablechartdisplayingmetalproductoffering14–15CarbonNanotubesSingle-andMulti-walledcarbonnanotubes19High-PurityAluminumVariousformsofaluminummetalavailableinhighpurity20BinaryMetalAlloysMetalalloysconsistingoftwodifferentmetals24BinaryMetalCompoundsMetalcarbides,silicides,andphosphides25High-PurityMetalsManufacturedbyAAPLHighpuritymagnesium,calcium,strontium,andbariummanufacturedatAAPL—aSigma-AldrichMaterialsChemistryCenterofExcellence26High-PurityRareEarthMetalFoilsMetalfoilsforcoatingsandPVDprocesses27TOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
AdvancedMaterialsforMagneticCoolingProf.
V.
K.
PecharskyandProf.
K.
A.
Gschneidner,Jr.
AmesLaboratory,IowaStateUniversityIntroductionToday,near-room-temperaturerefrigerationisalmostentirelybasedonavapor-compressionrefrigerationcycle.
Overtheyears,allpartsofacommercialrefrigerator,suchasthecompressor,heatexchangers,refrigerant,andpackaging,havebeenimprovedconsiderablyduetotheextensiveresearchanddevelopmenteffortscarriedoutbyacademiaandindustry.
However,theachievedandanticipatedimprovementsinconventionalrefrigerationtechnologyareincrementalsincethistechnologyisalreadynearitsfundamentallimitofenergyefficiency.
Furthermore,chlorofluorocarbons,hydrofluorocarbons,andotherchemicalsusedasrefrigerantseventuallyescapeintotheenvironmentpromotingozonelayerdepletionandglobalwarming.
Ingeneral,liquidchemical-basedrefrigerationisamajorfactorcontributingtodeleterious,cumulativechangesintheglobalclimate.
Refrigerationisbasedontheuseofaworkingbodythatchangesitstemperatureinresponsetocertainthermodynamictriggerstocoolanobject.
Thesevariationsmustbeachievedquickly,repeatedly,reversibly,andwithminimumenergylosses.
Sinceamagneticfieldeffectivelycouplestomagneticmomentsofindividualatomsinasolid,magneticfieldisoneofthecommonthermodynamicvariablesthatcanalterthetemperatureofamagneticsolid.
Heatingandcoolingofsoftferromagneticmaterialsinresponsetoincreasinganddecreasingmagneticfields,respectively,hasbeenknownsincethelatterpartofthe19thcenturywhenWarburgreportedsmallbutmeasurablereversibletemperaturechangesinpureironinresponsetomagneticfieldchanges.
1Today,thisphenomenonisrecognizedastheMagnetocaloricEffect(MCE)andmaterialsexhibitinglarge,reversibletemperaturechangesinresponsetochangingmagneticfieldsareusuallyreferredtoasmagnetocaloricmaterials.
Theefficiencygainwhenreplacingamechanicalprocess(compressionandexpansionofavapor)withanelectronicprocess(magnetizationanddemagnetizationofasolid)toobtainareversiblechangeoftemperatureissubstantial,thusmakingmagneticrefrigerationoneofthefewviable,energy-efficientsolid-statecoolingtechnologies.
MagnetocaloricEffectThemagnetocaloriceffectoccurswhenamagneticsublatticeiscoupledwithanexternalmagneticfield,affectingthemagneticpartofthetotalentropyofasolid.
Similartotheisothermalcompressionofagas,duringwhichthepositionaldisorderandthecorrespondingcomponentofthetotalentropyofthegaseoussystemaresuppressed,exposingaparamagnetnearabsolutezerotemperatureoraferromagnetnearitsCurietemperature,TC,toachangeinmagneticfield(B)fromzerotoanynon-zerovalue,oringeneral,fromanyinitialvalueBitoafinalhighervalueBf(DB=Bf–Bi>0)greatlyreducesdisorderofaspinsystem.
Thus,themagneticpart(SM)ofthetotalentropy(S)issubstantiallylowered.
Inareversibleprocess,whichresemblestheexpansionofthegasatconstanttemperature,isothermaldemagnetization(DB1atm);p=pressure(atm);T=temperature(K);V=systemvolume(cm3);R=universalgasconstant(82.
06cm3atm/moleK).
ThermodynamicparametersofthedesorptionreactionweredeterminedusingtheVan'tHoffequationandfugacityvalues,correspondingtoexperimentalpressurevalues:RTln(fp)=DH–TDS(2)where,fp=fugacity;H=enthalpychange;S=entropychange.
Finally,thefugacityvalueswerecalculatedusingEquation3andrealmolarvolumesobtainedfromEquation1:RTln(fp)=RTlnp–*(Vid–Vreal)dpp0(3),where,Vreal=realabsorbed/desorbedhydrogenmolarvolumeVid=idealabsorbed/desorbedhydrogenmolarvolumeItisalsoworthnotingthatoursystemallowsforconductingexperimentalstudiesofhydrogenabsorptionbyintermetallicsaswellasinvestigatingthebehaviorofvariousmaterialsathighhydrogenpressures.
YNi5–H2SystemInteractionofhydrogenwithAB5-typeintermetallicsathighpressureshasbeenreported.
6ForLaCo5,La0.
5Ce0.
5Co5,andLaNi5itwasshownthatathighhydrogenpressurestheyformintermetallichydridesoftheapproximatecompositionRT5H9(R=rareearthmetal;T=transitionmetal),whichagreeswiththeoreticalpredictions.
6Forourstudies,wechoseaYNi5alloybecauseofitsuniqueproperties.
YNi5doesnoteasilyabsorbhydrogen7–9atlowpressures.
However,asshownbyTakeshita10,applying1550atmtothematerialsallowsthesynthesisofYNi5H3.
5hydride.
Pressure-composition-temperature(PCT)isothermsobtainedledtoaconclusionthatthepressureappliedwasnotsufficienttoobtainafullyhydrogenatedsample.
Theresultsofotherauthors11differedconsiderablyfromthatofTakeshita.
OurstudiesshowedthatanactiveinteractionofYNi5andhydrogenstartsatpressuresover500atmandtheequilibriumhydrogenabsorptionpressureat293Kis674atmwhilecorrespondingequilibriumdesorptionpressureis170atm.
Hydridecompositionat1887atmcorrespondstoYNi5H5(1.
3mass.
%H2).
Absorption-desorptionPCT-isothermsatthetemperaturesrangingfrom–20to80°CareshowninFigure2.
Ourdatadiffersfromthosereportedinreference10,wheretherearetwodesorptionplateausat300and1000atm(293K)andthehydridecompositioncorrespondstoYNi5H3.
5.
Ourdataalsodiffersfromtheresultsreportedinreference11,wherethedissociationpressureisonly12atmandthehydridecompositionisYNi5H4.
4.
101001000100000123456H/YNi5353Kdes313Kdes293Kdes273Kdes253KdesP(H2)(atm)353Kabs293KabsFigure2.
Absorption-desorptionisothermsforYNi5–H2system.
Theinconsistencyinthenumbersreportedbydifferentgroupsmaybeexplainedbyverylowhydrogenabsorptionanddesorptionratesobserved.
Inourcase,thetimetoreachequilibriumintheplateauregionwasbetween2and4hours;seeFigure3whichshowsthereadingsofthepressuretransducerinseveralconsecutivehydrogendesorptionsteps.
YNi5-H2020406080100120t,h22Cdes15129630PH2,atm18Figure3.
Dependenceofpressurechange(P)ontime(t)toequilibrium.
Anumberofauthors7–9comparedhydrogenabsorptionpropertiesofYNi5tootherAB5alloysandconcludedthatpeculiaritiesinitsinteractionwithhydrogencanbeexplainedneitherbythelow-temperatureheatcapacitynortheelectronicstructure,norbythesurfaceoxidationofYNi5.
Inouropinion,themostpossibleexplanationwasgiveninreference8,whereitwasshownthatamongallbinaryAB5-typeintermetalliccompoundsYNi5hasthelowestcompressibility.
Thus,thelowvolumeoftheYNi5unitcellcouldinfluencehydrogenabsorptionproperties.
UsingalnP(H2)vs.
1/Tplot,wefoundthevaluesofhydrogendesorptionenthalpyandentropyofaYNi5hydridetobe21.
86kJ/molH2and115.
8J/K·molH2respectively.
IntermetallicHydridesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
11AB2–H2SystemsAsbasismaterialsforthesestudies,weselectedLavesphasesZrFe2andTiFe2withhighhydrogendesorptionpressures,whichdonotabsorbhydrogenatrelativelylowpressures.
Ithasbeenreportedthatusingtheultra-highpressureof10,000atm,itispossibletosynthesiseaZrFe2hydride.
12–14Ourstudiesshowedthatnoticeablehydrogenabsorptioninthefirstabsorption-desorptioncyclestartsatapproximately800atmwithoutanypreliminaryactivation.
Duringsubsequentcycling,absorptionstartsatlowerpressures.
Absorptionequilibriumpressureinthefirstrunhasbeenfoundtobe1120atmwhileinsecondandfurthercyclesitdecreasedto690atm(Figure4).
Thehydrogencontentinthehydrogenatedmaterialatroomtemperatureand1800atmis3.
5H/formulaunit.
Atthelowtemperatureof218Kandhydrogenpressureof1900atm,thematerial'scompositionisZrFe2H3.
7.
TheisothermsshowninFigure4revealanobvioushysteresis—atroomtemperaturetheabsorptionequilibriumpressureisabout690atm,whilethedesorptiononeisonly325atm.
1010010001000000.
511.
522.
533.
54H/ZrFe2PH2(atm)313Kdes293K1abs293K1des293K2abs293K2des273Kdes253KdesFigure4.
Absorption-desorptionisothermsforZrFe2–H2system.
Partialsubstitutionofzirconiumforscandiumreducesthehydrogendesorptionpressureofthehydride.
SimilartoZrFe2,Zr0.
5Sc0.
5Fe2andZr0.
8Sc0.
2Fe2crystallizeasC15Lavesphases.
HydrogenabsorptioninZr0.
5Sc0.
5Fe2startsat~100atmwithoutanypreliminaryactivation.
Thereisnosignificanthysteresisinthissystem,i.
e.
theabsorptionanddesorptionpressuresareveryclose.
Hydrogencontentinthematerialat295Kand1560atmreaches3.
6H/formulaunit(Figure5).
11010010001000000.
511.
522.
533.
54313Kdes293Kdes273Kdes253KdesH/Zr0.
5Sc0.
5Fe2PH2(atm)Figure5.
Absorption-desorptionisothermsforZr0.
8Sc0.
2Fe2–H2system.
Theshapeofthehydrogenabsorption-desorptionisothermssuggeststheformationoftwohydridephasesintheZr0.
5Sc0.
5Fe2–H2system.
Atroomtemperature,thecompositionofthefirstphase(β1)isclosetoadihydrideandthatofthesecondone(β2)correspondstoatrihydride(Table1).
Hydrogendesorptionenthalpiesandentropieshavebeencalculatedforbothphases.
Remarkably,thebehaviorofZr0.
5Sc0.
5Fe2resemblesthatoftheScFe1.
8–H2system,wherethestablemonohydrideandthemuchlessstableScFe1.
8H2.
4arealsoformed.
15,16Inourcase,however,substitutionofhalfofthescandiumforzirconiumleadstoasignificantincreaseofstabilityofthelowerhydridewithanenthalpyofformationlowerthanthatofthetrihydride(Table1).
Table1.
ThermodynamicparametersforZr1–xScxFe2–H2systems.
IMC*H/IMCPdes,atmT,Kfdes**,atmH,kJ/moleH2/S,J/K·moleH2ZrFe22.
086170325.
1468.
8253.
1273295.
731390.
5188396.
761921.
3(3)/121(1)Zr0.
5Sc0.
5Fe21.
32.
512.
518.
738.
860.
52246.
4105177254.
1272.
6295.
1310.
9254.
1272.
6295.
1310.
912.
518.
739.
762.
52247.
4111.
5195.
519(2)/95(6)25.
4(4)/125(1)Zr0.
8Sc0.
2Fe21.
849190290253.
4292.
1313.
15021234321(1)/117(5)*Intermetalliccompound**FugacityIntermetallicHydridesTOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
12ComparinghydridesScFe1.
8H1.
8,Zr0.
5Sc0.
5Fe2H2.
5(secondplateau)andZr0.
8Sc0.
2Fe2H1.
8showsthattheincreaseinzirconiumcontentinthealloysleadstothedecreaseinitshydrogendesorptionenthalpy.
However,entropychangegoesthroughamaximumatZr0.
5Sc0.
5Fe2H2.
5(Table1).
ForZr0.
8Sc0.
2Fe2–H2,hydrogenabsorptionalsostartsat100atmwithoutactivation.
Hydrogencompositionatroomtemperature(293K)and1650atmisZr0.
8Sc0.
2Fe2H3.
7(Figure6).
Furthercoolingto219Kwithsimultaneousincreaseinhydrogenpressureto1730atmresultsinmaximumhydridecompositioncorrespondingtoZr0.
8Sc0.
2Fe2H3.
8.
1010010001000000.
511.
522.
533.
54313Kdes293Kabs293Kdes253KdesH/Zr0.
8Sc0.
2Fe2P(H2)(atm)Figure6.
DesorptionisothermsforZr0.
5Sc0.
5Fe2–H2system.
PartialsubstitutionoftitaniumforscandiumallowedustosynthesizethefirstpseudobinaryintermetallichydrideinTi0.
5Sc0.
5Fe2–H2system.
Remarkably,whileTi0.
8Sc0.
2Fe2doesnotabsorbhydrogenevenatpressuresupto2500atm,at223K,thereactionbetweenTi0.
5Sc0.
5Fe2andhydrogenstartsatroomtemperaturealreadyat100atmwithoutanypreliminaryactivation(Figure7).
ThehydrogencontentofthehydridecorrespondstoTi0.
5Sc0.
5Fe2H3.
1(2.
0mass%).
1010010001000000.
511.
522.
533.
54333Kdes295Kdes273KdesP(H2)(atm)H/Ti0.
5Sc0.
5Fe2Figure7.
DesorptionisothermsforTi0.
5Sc0.
5Fe2–H2systemTheroomtemperatureequilibriumabsorptionanddesorptionpressuresforthismaterialare195and175atmaccordingly.
Coolingthehydrogenatedmaterialtobelow223Kshowedthatnonewhydridephasetransitionoccurs.
Hydrogencontentat217Kat2700atmis3.
4H/f.
u.
(2.
16mass.
%)andcalculatedthermodynamicparameterswereareΔH(kJ/moleH2)=17.
7(2)andΔS(J/K·moleH2)=103.
8(7)ConclusionsInteractionofhydrogenwithmulti-componentintermetalliccompoundsofAB5-andAB2-typewerestudiedinthecurrentwork.
Severalnewintermetallichydrideswithpotentialapplicationsinhigh-capacityhydrogenstoragehavebeenidentifiedandfullycharacterizedusingagas-volumetricanalyticaltechnique.
AcknowledgementsThisworkwassupportedinpartbyGeneralMotorsCorp.
References:(1)LibowitzG.
G.
;HayesH.
F.
;GibbT.
R.
P.
J.
Phys.
Chem.
1958,62,76.
(2)ZijlstraH.
;WestendorpF.
F.
SolidStateComm.
1969,7,857.
(3)vanVuchtJ.
H.
N.
;KuijpersF.
A.
;BruningH.
C.
A.
M.
PhilipsResearchReports1970,25,133.
(4)PeblerA.
;Gulbransen.
E.
A.
Trans.
Met.
Soc.
AIME1967,239,1593.
(5)HemmesH.
;DriessenA.
;GriessenR.
J.
Phys.
C1986,19,3571.
(6)LaknerJ.
F.
;UribeF.
;SteweardS.
A.
J.
Less-CommonMet.
1980,72,87.
(7)TakeshitaT.
;GschneidnerK.
A.
Jr.
;ThomeD.
K.
;McMastersO.
D.
PhysicalReviewB1980.
21,5636.
(8)TakeshitaT.
;WallaceW.
E.
;CraigR.
S.
Inorg.
Chem.
1974,13,2282.
(9)SaryninV.
K.
;BurnashevaV.
V.
;SemenenkoK.
N.
IzvestiyaANSSSR,Metally,1977,4,69.
(10)TakeshitaT.
;GschneidnerK.
A.
Jr.
;LaknerJ.
F.
J.
Less-CommonMet.
1981,78,43.
(11)AndersonJ.
L.
;WallaceT.
C.
;BowmanA.
L.
;RadosewichC.
L.
;CourtneyM.
L.
LosAlamosRep.
LA-5320-MS,InformalReport,1973.
(12)FilipekS.
M.
;JacobI.
;Paul-BoncourV.
;Percheron-GueganA.
;MarchukI.
;MogilyanskiD.
;PielaszekJ.
PolishJournalofChemistry2001,75,1921.
(13)FilipekS.
M.
;Paul-BoncourV.
;Percheron-GueganA.
;JacobI.
;MarchukI.
;DorogovaM.
;HirataT.
;KaszkurZ.
J.
Phys.
:Condens.
Matter.
2002,14,11261.
(14)Paul-BoncourV.
;Bouree-VigneronF.
;FilipekS.
M.
;MarchukI.
;JacobI.
;Percheron-GueganA.
J.
AlloysCompds.
2003,356–357,69.
(15)SemenenkoK.
N.
;SirotinaR.
A.
;SavchenkovaA.
P.
;BurnashevaV.
V.
;LototskiiM.
V.
;FokinaE.
E.
;TroitskayaS.
L.
;FokinV.
N.
J.
Less-CommonMet.
1985,106,349.
(16)SavchenkovaA.
P.
;SirotinaR.
A.
;BurnashevaV.
V.
;KudryavtsevaN.
S.
Sov.
Neorgan.
Materialy1984,20,1507.
IntermetallicHydridesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
13HydrogenAbsorbingAlloysNameChem.
CompositionHydrogenStorageCapacitywt.
%EquilibriumPressurePlateauProd.
No.
Zirconium-IronAlloyZeFe21.
65–1.
75(25°C,1800bar)~690bar(23°C)absorption~325bar(23°C)desorption693812-1GZirconium-IronAlloyZr0.
8Sc0.
2Fe21.
65–1.
75(22°C,1560bar)~190bar(20°C)693804-1GYttriumNickelAlloyYNi51.
25–1.
3(25°C,1890bar)~674bar(20°C)693928-5GLanthanumNickelAlloyLaNi51.
5–1.
6(25°C)~2bar(25°C)685933-10GLanthanumNickelAlloyLaNi4.
5Co0.
51.
4–1.
5(25°C)50636797-250MG636797-1GSingle-wall,short1–2NA.
5–2>90652512-250MGDouble-wall51.
3–2.
00.
550–80637351-250MG637351-1GMulti-wall,ArkemaCVD10–152–6.
1–10>90677248-5GMulti-wall110–170–5–9>90659258-2G659258-10GMulti-wall,powderedcylindercores–2–151–1010–40406074-100MG406074-500MG406074-1G406074-5GMulti-wall,asproduced6–20–1–5>7.
5412988-100MG412988-500MG412988-2G412988-10GGraphite,nanofibers80–2000.
5–10.
5–20>95636398-2G636398-10G636398-50GFormoreinformationabouttheseandotherrelatedmaterials,pleasevisitsigma-aldrich.
com/nano.
LightweightMetalMatrixNanocompositesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
21Self-PropagatingReactionsInducedbyMechanicalAlloyingProf.
LaszloTakacsUniversityofMaryland,BaltimoreCountyIntroductionMechanicalalloyingisa"bruteforce"methodofaffectingalloyingandchemicalreactions.
Themixtureofreactantpowdersandseveralballsareplacedinthemillingjarofahigh-energyballmill,forexample,ashakermilloraplanetarymill(Figure1).
Thecollisionsandfrictionbetweentheballs,andbetweentheballsandthewallofthecontainer,resultindeformation,fragmentation,mixing,andcold-welding.
Thereactivityincreasesduetodefectformationandincreasedinterfacearea,andeventuallyalloyingand/orchemicalreactionstakeplace.
Neitheradditionalheatnorsolventareneeded.
Theproductisapowderthatcanbeconsolidatedusingtheusualmethodsofpowdermetallurgy.
Mechanicalalloyingisaveryflexibletechniqueandhasbeenusedtoprepareabroadvarietyofmaterials,includingdispersion-strengthenedalloys,amorphousalloys,andnanocomposites.
1High-energyballmillingisalsocalledmechanochemicalprocessingwhenused,ofteninconjunctionwithothersteps,forinorganicsynthesis,theprocessingofminerals,andtheactivationofbuildingmaterials.
2(a)(b)Figure1.
Crosssectionviewsofthemillingvialofashakermill(a)andaplanetarymill(b).
Mechanically-inducedSelf-propagatingReactions(MSR)arepossibleinhighlyexothermicpowdermixtures.
3Initially,millingresultsinactivation,similartoanyothermechanicalalloyingprocess.
Butatacriticaltime,calledtheignitiontime,thereactionratebeginstoincrease.
Asaresult,thetemperaturerises,furtherincreasingthereactionrateandeventuallyleadingtoaself-sustainingprocess.
Mostofthereactantsareconsumedwithinseconds.
Atthisstage,thereactionissimilartothermallyignitedself-propagatinghigh-temperaturesynthesis(SHS).
4Theabrupttemperatureincreaseisdetectableontheoutersurfaceofthemillingcontaineranditspresencedistinguishessuchmechanicallyinducedself-propagatingreactions(MSR)fromgradualprocesses(Figure2).
MSRcanhappeninabroadvarietyofsystems,suchasinFe2O3–Al,Ni–Al,Ti–C,Zn–S,andMo–Simixtures.
3Theignitiontimeisanimportantattributeoftheprocess;itcanvaryfromafewsecondstoseveralhoursdependingonthereactionandthemillingconditions.
3230282624220500100015002000250030003500Time(s)Temperature(°C)Figure2.
Temperatureoftheoutsidesurfaceofthevialduringballmillingofa5Ni+2PmixtureinaSPEX8000MixerMill.
Ignitionisindicatedbytherapidtemperatureriseat1220sec.
Thegradualtemperatureincreasebeforeignitioniscausedbydissipatedmechanicalenergy.
TheinvestigationofMSRcontributedconsiderablytoourunderstandingofmechanochemicalprocessesingeneral.
Thevariationoftheignitiontimewithprocessconditionsandmaterialpropertiestellsusaboutthemechanismoftheactivationprocess,whiledetailedstudiesofpartiallyactivatedpowdersprovidesinformationaboutthenatureofthecriticalstate.
MSRhasalsobeenconsideredasapracticalmeansfortheproductionofusefulmaterials,particularlyrefractorycompounds.
3RequirementsForSelf-SustainingReactionsMSR(aswellasSHS)requiresufficientself-heatingtopropagatethereaction.
Ameasureofself-heatingistheadiabatictemperature,definedasthefinaltemperature,ifallthereactionheatisusedtoheattheproducts.
Aruleofthumbisthatself-sustainingreactionsarepossible,iftheadiabatictemperatureisatleast1800K.
Sincethemainissueisself-heatingatthebeginningofthereaction,thequantity–ΔH298/C298(whereH298andC298arereactionenthalpyandspecificheatat298K),writtensimplyasΔH/C,isoftenusedasasimplersubstituteforadiabatictemperature;ΔH/C>2000KistheconditionforMSR.
Thissimpleconditionappliessurprisinglywelltothemostfrequentlystudiedclassesofreactions,namelycombinationreactionsbetweenatransitionmetalandametalloidelement(e.
g.
Ti-B,Nb-C,Mo-Si,Ni-P)andthermite-typereactionsbetweenanoxideandamorereactivemetal(e.
g.
Fe3O4-Al,CuO-Fe,ZnO-Ti).
MuchlowervaluesofΔH/CaresufficientforMSRwithchalcogenidesandchlorides.
Table1containsdataforafewtypicalreactions.
MechanicalAlloyingTOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
22Table1.
Typicalreactionsshowingmechanicallyinducedself-propagationandthecorrespondingreactionheats(ΔH)andratioofΔHintheheatcapacityoftheproducts(C).
Reaction–ΔH/formula(kJ/mol)ΔH/C(K)3CuO+2Al3Cu+Al2O3119078104CuO+3Fe4Cu+Fe3O446118503Fe3O4+4Al9Fe+4Al2O3337662225Ni+2PNi5P24362867Sn+2SSnS21542189Ti+2BTiB23167111Hf+CHfC2266011Mo+2SiMoSi21322055Asmoreexothermicreactionsbecomeincreasinglyeasytoself-sustain,reactionswithhigheradiabatictemperaturesareexpectedtorequireshorteractivationtimesbeforeignition.
Sucharelationshipindeedexists,butonlyiftheothermaterialparametersandthemillingconditionsareverysimilar.
Sofar,thebestcorrelationwasobservedforthereductionofCuO(AldrichProd.
No.
203130,450804,450812),NiO(AldrichProd.
No.
203882,637130,481793),Fe3O4(AldrichProd.
No.
310069,518158,637106),Cu2O(AldrichProd.
No.
208825,566284),andZnO(AldrichProd.
No.
204951,255750,544906),withthesamemetal(Ti,ZrorHf).
3Theseareductile-brittlesystems5wheremillingresultsinafinedispersionoftheoxideparticlesinthemetalmatrix.
Thedevelopmentofthemicrostructuredependsprimarilyontheductilecomponentanditiskeptthesameforeachseries.
Thechangescausedbymechanicalmillingduringtheactivationperiod—decreaseofgrainsize,mixing,andformationoflatticedefects—dependmainlyonthemechanicalpropertiesofthereactants.
Althoughitisdifficulttoquantifythisrelationship,theincreasingwidthoftheX-raydiffractionlinesindicatesthatthecrystallitesizedecreasesandtheaccumulatedlatticestrainofthemetalcomponentincreasesasthepowderapproachesignition.
6,7Whilereducingthegrainsizeandtherebyincreasingtheinterfaceareaiscertainlyakeycomponentoftheactivationprocess,agglomerationisalsonecessarytoensureefficientmatterandheattransfer.
AninterestingcaseisthereductionofMoS2(AldrichProd.
No.
234842)withaluminumpowder(AldrichProd.
No.
202584,653608).
Thisreactionisgradual,althoughΔH/C=2093KiswellovertheacceptedthresholdforanMSRprocess.
However,MoS2preventsagglomeration,reducingtheareaoftheactiveinterface.
8Themechanicalintensityofthemillingactiondependsonthenumberandenergyofthecollisionsbetweenthemillingballsandbetweentheballsandthecontainerwall.
Thechargeischaracterizedbytheball-to-powdermassratio,aparameterapproximatelyproportionaltotherateofspecificenergyinput.
Fortypicalmillingconditions,ignitiontakesplacewhenthepowderhasreceivedacriticalamountofmechanicalenergyandtheignitiontimeisinverselyproportionaltotheball-to-powdermassratio.
Iftoomuchpowderisused,theenergyofeachindividualimpactisdistributedinaverylargevolumeandthestressesarenotlargeenoughtocauseactivation.
Iftheamountofpowderistoolittle,theheatlosstothemillingtoolsandtotheatmospherequenchesanyincipientself-sustainingreaction.
UnderstandingMechanicallyInducedSelf-PropagatingReactionsAmechanicalalloyingexperimentmaylookquitesimple,buttheunderlyingprocessisverycomplexconsistingofthecomponentsonverydifferentlengthandtimescales.
Thus,thecompletemodelingofamechanochemicaleventrequiresanadequatedescriptionofthemacroscopicprocesses,suchastheoperationofthemill,thecollisionsbetweenthemillingballs,andthetransportofthepowderwithinthemillingcontainer.
Onthemicroscopicscale,theeffectofanindividualcollisiononthepowdercaughtbetweentheimpactingsurfacesmustbeunderstoodandtheformationoflatticedefectsandtheelementaryinterfacereactionsmustbedescribed.
SignificantadvancesweremadetowardageneraltheoryofgradualmechanicalalloyingbyProf.
Courtneyandhisstudents.
9ThekeymomentofanMSRisignition.
Onceweunderstandwhatmakesthestateofthematerialcriticalatignition,weshouldbeabletousethisunderstandingofMSRforlearningabouttheinitialphaseofothermechanicalalloyingprocesses.
Unfortunately,manydetailsaresystemspecificandidentifyingthegeneralfeaturesisconsequentlydifficult.
Whetherignitiontakesplaceornotmaybeverysensitivetocompositionandmillingconditions.
Forexample,Figure3showstheX-raydiffractionpatternsoftwox(Zn+S)+(1–x)(Sn+2S)mixtures.
10ThisisanunusualsystemasthecombinationreactionsZn+S→ZnSSn+2S→SnS2arebothself-sustaining,buttheprocessisgradualinmixturesofthetwofor0.
1990%purity357391-250G357391-1KGPowder,<100nm,surfacearea70–90m2/g594911-100G594911-250GTantalum(Ta)TaCPowder,5μm280801-10GTitanium(Ti)TiCPowder,–325mesh307807-100G307807-500GPowder,<4μm594849-25G594849-100GPowder,130nmparticlesize(spherical)636967-25G636967-100G636967-250GTungsten(W)WCPowder,10μm241881-100GZirconium(Zr)ZrCPowder,5μm336351-50G336351-250GPhosphidesCalcium(Ca)Ca3P299%purity400971-100G400971-500GGallium(Ga)GaP99.
99%purity521574-2GNickel(Ni)Ni2PPowder,–100mesh,98%purity372641-10GIndium(In)InPPieces,3–20mesh,99.
998%purity366870-1GIron(P2Fe)P2FePowder,–40mesh,99.
5%purity691593-5GIron(P3Fe)P3FePowder,–40mesh,99.
5%purity691658-5GSilicidesCalcium(Ca)CaSi2Technicalgrade21240-250G-F21240-1KG-FChromium(Cr)CrSi2Powder,–230mesh,99%purity372692-25GMagnesium(Mg)Mg2SiPowder,–20mesh,99%purity343196-25GNiobium(Nb)NbSi2Powder,–325mesh399493-10GTungsten(W)WSi2Powder,–325mesh99%purity399442-10GVanadium(V)VSi2Powder,–325mesh399450-10GZirconium(Zr)ZrSi2Powder,325mesh99%purity399426-50GFormoreinformationabouttheseandotherrelatedmaterials,pleasevisitsigma-aldrich.
com/matsci.
MechanicalAlloyingTheprimaryfocusofourmanufacturingfacilityinUrbana,Illinois,USAisonthepurificationofinorganicsandmetalsforavarietyofhightechnologyapplications.
Ourcapabilitiesintheareaofmetalspurificationutilizeseveralroutestoproducesomeofthepurestalkaliearthandrareearthmetalsintheworld.
Thesetechniquescanalsobeusedinthemanufactureofalloysandothermaterialsforyourresearchorcommercialrequirements.
Ourproprietarytechniquesallowustomanufacturebarium,calcium,strontium,andmagnesiumwithtracemetalpuritiesupto4N(99.
99%).
Wealsomanufacturerareearthmetalsinpuritiesexceeding4Nthroughanumberofdifferenthightemperatureroutes.
OneareaofcustomizationhasfocusedonthemanufactureofprepackagedMolecularBeamEpitaxy(MBE)cruciblesforuseinthinfilmmanufacturingofadvancedmaterials.
Wehavesuccessfullymanufacturedhighpuritybarium,calcium,andstrontium(aswellasotherhighpuritymetals)andmeltedthematerialintoanMBEcrucible.
ThisallowsforincreasedloadinginthecrucibleaswellassignificantlyreducedoutgassingofthematerialsintheMBEvacuumchamber.
Pleasecontactustodayforyourspecificrequirements:matsci@sial.
com.
High-PurityMetalsManufacturedbyAAPL—ASigma-AldrichMaterialsChemistryCenterofExcellenceMetalCommentsPurity,%Prod.
NoMagnesium(Mg)dendriticpieces,purifiedbydistillation99.
998(metals)474754-5G474754-25G99.
99(metals)465992-5G465992-25GCalcium(Ca)dendriticpieces,purifiedbydistillation99.
99(metals)441872-5G441872-25G99.
9(metals)596566-5G596566-25GStrontium(Sr)dendriticpieces,purifiedbydistillation99.
99(metals)441899-5G441899-25G99.
9(metals)460346-5G460346-25GBarium(Ba)dendriticpieces,purifiedbydistillation99.
99(metals)474711-5G474711-25G99.
9(metals)441880-5G441880-25GUltraPureMetalsforHighTechnologyApplicationssigma-aldrich.
comHigh-PurityRareEarthMetalFoilsMetalDimensionsPurity*Prod.
No.
Lanthanum(La)25mmX25mmX1mm,~3.
9gTotalREM:99.
5%La/TotalREM:99.
9%694908Cerium(Ce)25mmX25mmX1mm,~4.
2gTotalREM:99.
5%Ce/TotalREM:99.
9%693766Neodymium(Nd)25mmX25mmX1mm,~4.
4TotalREM:99.
5%Nd/TotalREM:99.
9%693758Samarium(Sm)25mmX25mmX1mm,~4.
9gTotalREM:99.
9%Sm/TotalREM:99.
95%693731GadoliniumGd)25mmX25mmX1mm,~4.
8gTotalREM:99.
5%Gd/TotalREM:99.
95%693723Terbium(Tb)25mmX25mmX1mm,~4.
9gTotalREM:99.
5%Tb/TotalREM:99.
9%693715Dysprosium(Dy)25mmX25mmX1mm,~5.
6gTotalREM:99.
5%Dy/TotalREM:99.
9%693707HolmiumFoil(Ho)25mmX25mmX1mm,~5.
5gTotalREM:99.
5%Dy/TotalREM:99.
9%693693Erbium(Er)25mmX25mmX1mm,~5.
6gTotalREM:99.
5%Dy/TotalREM:99.
9%693685Thulium(Tm)25mmX25mmX1mm,~5.
8gTotalREM:99.
5%Dy/TotalREM:99.
95%693677Ytterbium(Yb)25mmX25mmX1mm,~4.
4gTotalREM:99.
5%Dy/TotalREM:99.
95%693669Lutetium(Lu)25mmX25mmX1mm,~6.
2gTotalREM:99.
5%Dy/TotalREM:99.
9%693650Yttrium(Y)25mmX25mmX1mm,~2.
8gTotalREM:99.
5%Dy/TotalREM:99.
9%693642*REM:RareearthmetalsRareearthmetalfoilsareusedinthermalandelectronbeam(e-beam)evaporationprocessesforcoatingsandthinfilmsviaphysicalvapordeposition(PVD).
Thelow-temperaturee-beamtechniqueisparticularlysuitedforapplicationssuchasfuelcellsandsolarpanels.
Therareearthmetalfoilscanalsobeusedinthepreparationofalloysandcompositesthatcontainhighlyvolatilecomponents(Ca,Mg,etc.
).
Formoreinformation,pleasevisitsigma-aldrich.
com/metals.
JZX03394-5034030107ArgentinaSIGMA-ALDRICHDEARGENTINAS.
A.
FreeTel:08108887446Tel:(+54)1145561472Fax:(+54)1145521698AustraliaSIGMA-ALDRICHPTYLTD.
FreeTel:1800800097FreeFax:1800800096Tel:(+61)298410555Fax:(+61)298410500AustriaSIGMA-ALDRICHHANDELSGmbHTel:(+43)16058110Fax:(+43)16058120BelgiumSIGMA-ALDRICHNV/SA.
FreeTel:080014747FreeFax:080014745Tel:(+32)38991301Fax:(+32)38991311BrazilSIGMA-ALDRICHBRASILLTDA.
FreeTel:08007017425Tel:(+55)1137323100Fax:(+55)1155229895CanadaSIGMA-ALDRICHCANADALTD.
FreeTel:18005651400FreeFax:18002653858Tel:(+1)9058299500Fax:(+1)9058299292ChinaSIGMA-ALDRICH(SHANGHAI)TRADINGCO.
LTD.
FreeTel:8008193336Tel:(+86)2161415566Fax:(+86)2161415567CzechRepublicSIGMA-ALDRICHspol.
sr.
o.
Tel:(+420)246003200Fax:(+420)246003291DenmarkSIGMA-ALDRICHDENMARKA/STel:(+45)43565910Fax:(+45)43565905FinlandSIGMA-ALDRICHFINLANDOYTel:(+358)93509250Fax:(+358)935092555FranceSIGMA-ALDRICHCHIMIES.
à.
r.
l.
FreeTel:0800211408FreeFax:0800031052Tel:(+33)474822800Fax:(+33)474956808GermanySIGMA-ALDRICHCHEMIEGmbHFreeTel:08005155000FreeFax:08006490000Tel:(+49)8965130Fax:(+49)8965131160GreeceSIGMA-ALDRICH(O.
M.
)LTD.
Tel:(+30)2109948010Fax:(+30)2109943831HungarySIGMA-ALDRICHKftIngyeneszldtelefon:0680355355Ingyeneszldfax:0680344344Tel:(+36)12359055Fax:(+36)12359050IndiaSIGMA-ALDRICHCHEMICALSPRIVATELIMITEDTelephoneBangalore:(+91)8066219600NewDelhi:(+91)1141654255Mumbai:(+91)2225702364Hyderabad:(+91)4040155488FaxBangalore:(+91)8066219650NewDelhi:(+91)1141654266Mumbai:(+91)2225797589Hyderabad:(+91)4040155466IrelandSIGMA-ALDRICHIRELANDLTD.
FreeTel:1800200888FreeFax:1800600222Tel:(+353)14041900Fax:(+353)14041910IsraelSIGMA-ALDRICHISRAELLTD.
FreeTel:1800702222Tel:(+972)89484100Fax:(+972)89484200ItalySIGMA-ALDRICHS.
r.
l.
NumeroVerde:800827018Tel:(+39)0233417310Fax:(+39)0238010737JapanSIGMA-ALDRICHJAPANK.
K.
Tel:(+81)357967300Fax:(+81)357967315KoreaSIGMA-ALDRICHKOREAFreeTel:(+82)800237111FreeFax:(+82)800238111Tel:(+82)313299000Fax:(+82)313299090MalaysiaSIGMA-ALDRICH(M)SDN.
BHDTel:(+60)356353321Fax:(+60)356354116MexicoSIGMA-ALDRICHQUMICA,S.
A.
deC.
V.
FreeTel:018000075300FreeFax:018007129920Tel:527222761600Fax:527222761601TheNetherlandsSIGMA-ALDRICHCHEMIEBVFreeTel:08000229088FreeFax:08000229089Tel:(+31)786205411Fax:(+31)786205421NewZealandSIGMA-ALDRICHNEWZEALANDLTD.
FreeTel:0800936666FreeFax:0800937777Tel:(+61)298410555Fax:(+61)298410500NorwaySIGMA-ALDRICHNORWAYASTel:(+47)23176060Fax:(+47)23176050PolandSIGMA-ALDRICHSp.
zo.
o.
Tel:(+48)618290100Fax:(+48)618290120PortugalSIGMA-ALDRICHQUMICA,S.
A.
FreeTel:800202180FreeFax:800202178Tel:(+351)219242555Fax:(+351)219242610RussiaSIGMA-ALDRICHRUS,LLCTel:+7(495)6216037+7(495)6215828Fax:+7(495)6215923SingaporeSIGMA-ALDRICHPTE.
LTD.
Tel:(+65)67791200Fax:(+65)67791822SouthAfricaSIGMA-ALDRICHSOUTHAFRICA(PTY)LTD.
FreeTel:0800110075FreeFax:0800110079Tel:(+27)119791188Fax:(+27)119791119SpainSIGMA-ALDRICHQUMICA,S.
A.
FreeTel:900101376FreeFax:900102028Tel:(+34)916619977Fax:(+34)916619642SwedenSIGMA-ALDRICHSWEDENABTel:(+46)87424200Fax:(+46)87424243SwitzerlandSIGMA-ALDRICHCHEMIEGmbHFreeTel:0800800080FreeFax:0800800081Tel:(+41)817552828Fax:(+41)817552815UnitedKingdomSIGMA-ALDRICHCOMPANYLTD.
FreeTel:0800717181FreeFax:0800378785Tel:(+44)1747833000Fax:(+44)1747833313SAFC(UK)FreeTel:01202712305UnitedStatesSIGMA-ALDRICHP.
O.
Box14508St.
Louis,Missouri63178Toll-Free:8003253010Toll-FreeFax:8003255052CallCollect:(+1)3147715750Tel:(+1)3147715765Fax:(+1)3147715757Internetsigma-aldrich.
comWorldHeadquarters3050SpruceSt.
,St.
Louis,MO63103(314)771-5765sigma-aldrich.
comOrder/CustomerService(800)325-3010Fax(800)325-5052TechnicalService(800)325-5832sigma-aldrich.
com/techserviceDevelopment/BulkManufacturingInquiries(800)244-11732007Sigma-AldrichCo.
Allrightsreserved.
SIGMA,,SAFC,,SIGMA-ALDRICH,ALDRICH,,FLUKA,,andSUPELCO,aretrademarksbelongingtoSigma-AldrichCo.
anditsaffiliateSigma-AldrichBiotechnology,L.
P.
SigmabrandproductsaresoldthroughSigma-Aldrich,Inc.
Sigma-Aldrich,Inc.
warrantsthatitsproductsconformtotheinformationcontainedinthisandotherSigma-Aldrichpublications.
Purchasermustdeterminethesuitabilityoftheproduct(s)fortheirparticularuse.
Additionaltermsandconditionsmayapply.
Pleaseseereversesideoftheinvoiceorpackingslip.
PrintedonRecycledPaper—AWorldwideOperationwithaGlobalCommitmentSigma-Aldrich3050SpruceStreetSt.
Louis,MO63103USAsigma-aldrich.
comAcceleratingCustomers'SuccessthroughLeadershipinLifeScience,HighTechnologyandService
2,No.
4AdvancingTechnology—imagineaworldwithoutAldrichChemicalCo.
,Inc.
Sigma-AldrichCorporation6000N.
TeutoniaAve.
Milwaukee,WI53209,USAToPlaceOrdersTelephone800-325-3010(USA)FAX800-325-5052(USA)Customer&TechnicalServicesCustomerInquiries800-325-3010TechnicalService800-231-8327SAFC800-244-1173CustomSynthesis800-244-1173Flavors&Fragrances800-227-4563International414-438-385024-HourEmergency414-438-3850WebSitesigma-aldrich.
comEmailaldrich@sial.
comSubscriptionsTorequestyourFREEsubscriptiontoMaterialMatters,pleasecontactusby:Phone:800-325-3010(USA)Mail:Attn:MarketingCommunicationsAldrichChemicalCo.
,Inc.
Sigma-AldrichCorporationP.
O.
Box355Milwaukee,WI53201-9358Email:sams-usa@sial.
comInternationalcustomers,pleasecontactyourlocalSigma-Aldrichoffice.
Forworldwidecontactinformation,pleaseseebackcover.
MaterialMattersisalsoavailableinPDFformatontheInternetatsigma-aldrich.
com/matsci.
AldrichbrandproductsaresoldthroughSigma-Aldrich,Inc.
Sigma-Aldrich,Inc.
warrantsthatitsproductsconformtotheinformationcontainedinthisandotherSigma-Aldrichpublications.
Purchasermustdeterminethesuitabilityoftheproductforitsparticularuse.
Seereversesideofinvoiceorpackingslipforadditionaltermsandconditionsofsale.
Allpricesaresubjecttochangewithoutnotice.
MaterialMatters(ISSN1933–9631)isapublicationofAldrichChemicalCo.
,Inc.
AldrichisamemberoftheSigma-AldrichGroup.
2007Sigma-AldrichCo.
TMVol.
2No.
4IntroductionAboutOurCoverMetalsandalloyscouldbeconsideredthebackboneofhumancivilization.
Thecastingofsuchmetalsascopperandtintomakebronze,thefirstfunctionalmetalalloy,pavedourwaytothemoderntechnologicalsociety.
Mostofthemetalscrystallizeintocloselypackedcubic(forexample,Al,Cu,Fe)orhexagonalcrystallattices(shownonthecover,forexample,Gd,Ti,Zr,rareearthmetals),whicharelargelyresponsiblefortheirremarkableproperties.
Thecovershowsmetalcasting,thebeginningofalongtechnologicalprocessthatconcludesinsuchmarvelsasairplanes.
IntroductionNoothermaterialshavecontributedmoretothedevelopmentofmankindoverthemillenniathanmetalsandalloys.
Throughoutcenturies,studiesofmetalsbelongedtooneoftheoldestbranchesofAppliedMaterialsScience–Metallurgy.
Thischangedinthelate19thandearly20thcenturies,whenapplicationsofmetallicmaterialsspreadintootherareasofscienceandtechnologyincludingelectronics,energy,aeronauticsandspacetravel,tonameafew.
Currently,it'simpossibletoimagineaworldinwhichwecouldsuccessfullyfunctionwithoutmetalsandalloys.
Traditionally,metalsareportrayedasshinysolids,mostofwhicharegoodconductorsofheatandelectricity.
Theyareductileandmostwillmeltathightemperatures.
Metalandalloyshapescaneasilybechangedbymechanicalprocessing,atechniquethatcanbeusedforthepreparation,modificationandchemicalconversionofmetalalloysandcomposites.
Presently,thereare87knownmetals,61ofwhichareavailablethroughSigma-Aldrichinvariousformsandmodifications;thesearehighlightedinredintheperiodictablechartbelow.
Seepages14–15foranexpandedchart.
HHeLiBeBCNOFNeNaMgAlSiPSClArKCaScTiVCrMnFeCoNiCuZnGaGeAsSeBrKrRbSrYZrNbMoTcRuRhPdAgCdInSnSbTeIXeCsBaLaHfTaWReOsIrPtAuHgTlPbBiPoAtRnFrRaAc104105106CePrNdPmSmEuGdTbDyHoErTmYbLuThPaUNpPuAmCmBkCfEsFmMdNoLrPuremetalsatSigma-Aldrichvisitsigma-aldrich.
com/metalsfordetailsThisissueofMaterialMattersfocusesonmetalsandalloysforadvancedapplicationsincludingmagneticrefrigeration;high-pressure,high-capacity,hydrogenabsorbingsystems;nanocomposites;andmechanicallyinducedconversionofmetallicsolids.
LeadingexpertsfromtheAmesLaboratoryoftheU.
S.
DepartmentofEnergy,MoscowStateUniversity,UniversityofWisconsin–Milwaukee,andUniversityofMarylanddiscussrecentexperimentalresultsandshareideasandtechniquesassociatedwithmetals,alloys,andtheirapplications.
Inside,you'llalsofindnewlyintroducedproducts,whichincludebutarenotlimitedtomagneticrefrigerationalloys,hydrideformingintermetallics,magneticmaterialsandhigh-purityrareearthmetalsandfoils.
Inthe"YourMaterialsMatter"feature,wearepleasedtointroduceAlignedMulti-WalledCarbonNanotubes(MWCNTs)—anewSigma-AldrichproductsuggestedbyDr.
KarlGross,CEOofHy-EnergyLLC.
OurgoalatSigma-AldrichMaterialsScienceistoprovideinnovativematerialsthataccelerateyourresearch.
Forproductdetailsandtechnicalinformation,pleasevisitsigma-aldrich.
com/matsci.
Haveacomment,questionorsuggestionaboutMaterialMattersPleasecontactusatmatsci@sial.
com.
ViktorBalema,Ph.
D.
MaterialsScienceSigma-AldrichCorporationForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
"YourMaterialsMatter.
"AlignedMulti-WalledCarbonNanotubes(MWCNTs)JoePorwoll,PresidentAldrichChemicalCo.
,Inc.
Dr.
KarlGross,ofHy-EnergyLLC,kindlysuggestedthathigh-qualitycarbonnanotubearraysareneededforresearchanddevelopmentofhigh-sensitivitygassensors.
Wearepleasedtoofferhighlyalignedarraysofmulti-wallcarbonnanotubes(MWCNTs)asnewproductsinourcatalog.
Thesearrays,availableonsilicone(AldrichProductNo.
687804)orcopper(AldrichProductNo.
687812)substrates,canprovideauniqueplatformforawiderangeofmaterialsresearchanddevelopment,includinggasadsorptionsensorandsensorsubstrates,1catalysts,electronemissionsources,2andbatteryandcapacitordevelopment.
3Carbonnanotubearray,multi-walled,verticallyaligned,onsiliconwafersubstrate687804-1EACarbonnanotubearray,multi-walled,verticallyaligned,oncoppersubstrate687812-1EAReferences:(1)Collins,P.
G.
,Bradley,K.
Ishigami,M.
Zettl,A.
,Science,2000,287,1801.
(2)Bonard,J.
M.
,Stockli,T.
,Maier,F.
,DeHerr,W.
A.
,Chatelain,A.
,Ugarte,D.
,Salvetat,J.
P.
,Forro,L.
,PhysicalReviewLetters,1998,81,1441.
(3)Frackowiak,E.
,Gautier,S.
,Gaucher,H.
,Bonnamy,S.
,Beguin,F.
,Carbon,1999,37,61IntroductionFeatures:99.
9%asMWCNTCNTdiametersare100nm±10nmCNTlengthsare30m±3mArraydensity~2x109MWCNT/cm2Arraydimensions1sq.
cmGrownbyplasma-enhancedCVD(PECVD)GrownwiththenickelcatalysttipintactPackagedinacleanroom;stored,andshippedinsemiconductorgradepackaging.
DoyouhaveacompoundthatyouwishSigma-AldrichcouldlisttohelpmaterialsresearchIfitisneededtoaccelerateyourresearch,itmatters—pleasesendyoursuggestiontomatsci@sial.
comandwewillbehappytogiveitcarefulconsideration.
CNTarray(blacksquare)inthesemiconductorgradepackage.
InsetshowsSEMimageoftheverticallyalignedMWCNTs.
AdvancedMetalsandAlloysFeaturedinThisIssueMaterialsCategoryContentPageMaterialsforMagneticCoolingMaterialsexhibitinggiantmagnetocaloriceffect7UltraHigh-PurityMetalsforPreparationofMagneticRefrigerationMaterialsSigma-Aldrichproductssuitableforthepreparationofnovelsystemswithgiantmagnetocaloriceffect7MagneticAlloysandIntermetallicsMetalalloysusedaspermanentmagnets8HydrogenAbsorbingAlloysMetallicmaterialscapableofabsorbinglargequantitiesofhydrogenatvarioustemperaturesandpressures13High-PurityIron,Nickel,andZirconiumMaterialsusedforthepreparationofintermetallichydrogenabsorbingsystems13PureMetalsfromSigma-AldrichPeriodicTablechartdisplayingmetalproductoffering14–15CarbonNanotubesSingle-andMulti-walledcarbonnanotubes19High-PurityAluminumVariousformsofaluminummetalavailableinhighpurity20BinaryMetalAlloysMetalalloysconsistingoftwodifferentmetals24BinaryMetalCompoundsMetalcarbides,silicides,andphosphides25High-PurityMetalsManufacturedbyAAPLHighpuritymagnesium,calcium,strontium,andbariummanufacturedatAAPL—aSigma-AldrichMaterialsChemistryCenterofExcellence26High-PurityRareEarthMetalFoilsMetalfoilsforcoatingsandPVDprocesses27TOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
AdvancedMaterialsforMagneticCoolingProf.
V.
K.
PecharskyandProf.
K.
A.
Gschneidner,Jr.
AmesLaboratory,IowaStateUniversityIntroductionToday,near-room-temperaturerefrigerationisalmostentirelybasedonavapor-compressionrefrigerationcycle.
Overtheyears,allpartsofacommercialrefrigerator,suchasthecompressor,heatexchangers,refrigerant,andpackaging,havebeenimprovedconsiderablyduetotheextensiveresearchanddevelopmenteffortscarriedoutbyacademiaandindustry.
However,theachievedandanticipatedimprovementsinconventionalrefrigerationtechnologyareincrementalsincethistechnologyisalreadynearitsfundamentallimitofenergyefficiency.
Furthermore,chlorofluorocarbons,hydrofluorocarbons,andotherchemicalsusedasrefrigerantseventuallyescapeintotheenvironmentpromotingozonelayerdepletionandglobalwarming.
Ingeneral,liquidchemical-basedrefrigerationisamajorfactorcontributingtodeleterious,cumulativechangesintheglobalclimate.
Refrigerationisbasedontheuseofaworkingbodythatchangesitstemperatureinresponsetocertainthermodynamictriggerstocoolanobject.
Thesevariationsmustbeachievedquickly,repeatedly,reversibly,andwithminimumenergylosses.
Sinceamagneticfieldeffectivelycouplestomagneticmomentsofindividualatomsinasolid,magneticfieldisoneofthecommonthermodynamicvariablesthatcanalterthetemperatureofamagneticsolid.
Heatingandcoolingofsoftferromagneticmaterialsinresponsetoincreasinganddecreasingmagneticfields,respectively,hasbeenknownsincethelatterpartofthe19thcenturywhenWarburgreportedsmallbutmeasurablereversibletemperaturechangesinpureironinresponsetomagneticfieldchanges.
1Today,thisphenomenonisrecognizedastheMagnetocaloricEffect(MCE)andmaterialsexhibitinglarge,reversibletemperaturechangesinresponsetochangingmagneticfieldsareusuallyreferredtoasmagnetocaloricmaterials.
Theefficiencygainwhenreplacingamechanicalprocess(compressionandexpansionofavapor)withanelectronicprocess(magnetizationanddemagnetizationofasolid)toobtainareversiblechangeoftemperatureissubstantial,thusmakingmagneticrefrigerationoneofthefewviable,energy-efficientsolid-statecoolingtechnologies.
MagnetocaloricEffectThemagnetocaloriceffectoccurswhenamagneticsublatticeiscoupledwithanexternalmagneticfield,affectingthemagneticpartofthetotalentropyofasolid.
Similartotheisothermalcompressionofagas,duringwhichthepositionaldisorderandthecorrespondingcomponentofthetotalentropyofthegaseoussystemaresuppressed,exposingaparamagnetnearabsolutezerotemperatureoraferromagnetnearitsCurietemperature,TC,toachangeinmagneticfield(B)fromzerotoanynon-zerovalue,oringeneral,fromanyinitialvalueBitoafinalhighervalueBf(DB=Bf–Bi>0)greatlyreducesdisorderofaspinsystem.
Thus,themagneticpart(SM)ofthetotalentropy(S)issubstantiallylowered.
Inareversibleprocess,whichresemblestheexpansionofthegasatconstanttemperature,isothermaldemagnetization(DB1atm);p=pressure(atm);T=temperature(K);V=systemvolume(cm3);R=universalgasconstant(82.
06cm3atm/moleK).
ThermodynamicparametersofthedesorptionreactionweredeterminedusingtheVan'tHoffequationandfugacityvalues,correspondingtoexperimentalpressurevalues:RTln(fp)=DH–TDS(2)where,fp=fugacity;H=enthalpychange;S=entropychange.
Finally,thefugacityvalueswerecalculatedusingEquation3andrealmolarvolumesobtainedfromEquation1:RTln(fp)=RTlnp–*(Vid–Vreal)dpp0(3),where,Vreal=realabsorbed/desorbedhydrogenmolarvolumeVid=idealabsorbed/desorbedhydrogenmolarvolumeItisalsoworthnotingthatoursystemallowsforconductingexperimentalstudiesofhydrogenabsorptionbyintermetallicsaswellasinvestigatingthebehaviorofvariousmaterialsathighhydrogenpressures.
YNi5–H2SystemInteractionofhydrogenwithAB5-typeintermetallicsathighpressureshasbeenreported.
6ForLaCo5,La0.
5Ce0.
5Co5,andLaNi5itwasshownthatathighhydrogenpressurestheyformintermetallichydridesoftheapproximatecompositionRT5H9(R=rareearthmetal;T=transitionmetal),whichagreeswiththeoreticalpredictions.
6Forourstudies,wechoseaYNi5alloybecauseofitsuniqueproperties.
YNi5doesnoteasilyabsorbhydrogen7–9atlowpressures.
However,asshownbyTakeshita10,applying1550atmtothematerialsallowsthesynthesisofYNi5H3.
5hydride.
Pressure-composition-temperature(PCT)isothermsobtainedledtoaconclusionthatthepressureappliedwasnotsufficienttoobtainafullyhydrogenatedsample.
Theresultsofotherauthors11differedconsiderablyfromthatofTakeshita.
OurstudiesshowedthatanactiveinteractionofYNi5andhydrogenstartsatpressuresover500atmandtheequilibriumhydrogenabsorptionpressureat293Kis674atmwhilecorrespondingequilibriumdesorptionpressureis170atm.
Hydridecompositionat1887atmcorrespondstoYNi5H5(1.
3mass.
%H2).
Absorption-desorptionPCT-isothermsatthetemperaturesrangingfrom–20to80°CareshowninFigure2.
Ourdatadiffersfromthosereportedinreference10,wheretherearetwodesorptionplateausat300and1000atm(293K)andthehydridecompositioncorrespondstoYNi5H3.
5.
Ourdataalsodiffersfromtheresultsreportedinreference11,wherethedissociationpressureisonly12atmandthehydridecompositionisYNi5H4.
4.
101001000100000123456H/YNi5353Kdes313Kdes293Kdes273Kdes253KdesP(H2)(atm)353Kabs293KabsFigure2.
Absorption-desorptionisothermsforYNi5–H2system.
Theinconsistencyinthenumbersreportedbydifferentgroupsmaybeexplainedbyverylowhydrogenabsorptionanddesorptionratesobserved.
Inourcase,thetimetoreachequilibriumintheplateauregionwasbetween2and4hours;seeFigure3whichshowsthereadingsofthepressuretransducerinseveralconsecutivehydrogendesorptionsteps.
YNi5-H2020406080100120t,h22Cdes15129630PH2,atm18Figure3.
Dependenceofpressurechange(P)ontime(t)toequilibrium.
Anumberofauthors7–9comparedhydrogenabsorptionpropertiesofYNi5tootherAB5alloysandconcludedthatpeculiaritiesinitsinteractionwithhydrogencanbeexplainedneitherbythelow-temperatureheatcapacitynortheelectronicstructure,norbythesurfaceoxidationofYNi5.
Inouropinion,themostpossibleexplanationwasgiveninreference8,whereitwasshownthatamongallbinaryAB5-typeintermetalliccompoundsYNi5hasthelowestcompressibility.
Thus,thelowvolumeoftheYNi5unitcellcouldinfluencehydrogenabsorptionproperties.
UsingalnP(H2)vs.
1/Tplot,wefoundthevaluesofhydrogendesorptionenthalpyandentropyofaYNi5hydridetobe21.
86kJ/molH2and115.
8J/K·molH2respectively.
IntermetallicHydridesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
11AB2–H2SystemsAsbasismaterialsforthesestudies,weselectedLavesphasesZrFe2andTiFe2withhighhydrogendesorptionpressures,whichdonotabsorbhydrogenatrelativelylowpressures.
Ithasbeenreportedthatusingtheultra-highpressureof10,000atm,itispossibletosynthesiseaZrFe2hydride.
12–14Ourstudiesshowedthatnoticeablehydrogenabsorptioninthefirstabsorption-desorptioncyclestartsatapproximately800atmwithoutanypreliminaryactivation.
Duringsubsequentcycling,absorptionstartsatlowerpressures.
Absorptionequilibriumpressureinthefirstrunhasbeenfoundtobe1120atmwhileinsecondandfurthercyclesitdecreasedto690atm(Figure4).
Thehydrogencontentinthehydrogenatedmaterialatroomtemperatureand1800atmis3.
5H/formulaunit.
Atthelowtemperatureof218Kandhydrogenpressureof1900atm,thematerial'scompositionisZrFe2H3.
7.
TheisothermsshowninFigure4revealanobvioushysteresis—atroomtemperaturetheabsorptionequilibriumpressureisabout690atm,whilethedesorptiononeisonly325atm.
1010010001000000.
511.
522.
533.
54H/ZrFe2PH2(atm)313Kdes293K1abs293K1des293K2abs293K2des273Kdes253KdesFigure4.
Absorption-desorptionisothermsforZrFe2–H2system.
Partialsubstitutionofzirconiumforscandiumreducesthehydrogendesorptionpressureofthehydride.
SimilartoZrFe2,Zr0.
5Sc0.
5Fe2andZr0.
8Sc0.
2Fe2crystallizeasC15Lavesphases.
HydrogenabsorptioninZr0.
5Sc0.
5Fe2startsat~100atmwithoutanypreliminaryactivation.
Thereisnosignificanthysteresisinthissystem,i.
e.
theabsorptionanddesorptionpressuresareveryclose.
Hydrogencontentinthematerialat295Kand1560atmreaches3.
6H/formulaunit(Figure5).
11010010001000000.
511.
522.
533.
54313Kdes293Kdes273Kdes253KdesH/Zr0.
5Sc0.
5Fe2PH2(atm)Figure5.
Absorption-desorptionisothermsforZr0.
8Sc0.
2Fe2–H2system.
Theshapeofthehydrogenabsorption-desorptionisothermssuggeststheformationoftwohydridephasesintheZr0.
5Sc0.
5Fe2–H2system.
Atroomtemperature,thecompositionofthefirstphase(β1)isclosetoadihydrideandthatofthesecondone(β2)correspondstoatrihydride(Table1).
Hydrogendesorptionenthalpiesandentropieshavebeencalculatedforbothphases.
Remarkably,thebehaviorofZr0.
5Sc0.
5Fe2resemblesthatoftheScFe1.
8–H2system,wherethestablemonohydrideandthemuchlessstableScFe1.
8H2.
4arealsoformed.
15,16Inourcase,however,substitutionofhalfofthescandiumforzirconiumleadstoasignificantincreaseofstabilityofthelowerhydridewithanenthalpyofformationlowerthanthatofthetrihydride(Table1).
Table1.
ThermodynamicparametersforZr1–xScxFe2–H2systems.
IMC*H/IMCPdes,atmT,Kfdes**,atmH,kJ/moleH2/S,J/K·moleH2ZrFe22.
086170325.
1468.
8253.
1273295.
731390.
5188396.
761921.
3(3)/121(1)Zr0.
5Sc0.
5Fe21.
32.
512.
518.
738.
860.
52246.
4105177254.
1272.
6295.
1310.
9254.
1272.
6295.
1310.
912.
518.
739.
762.
52247.
4111.
5195.
519(2)/95(6)25.
4(4)/125(1)Zr0.
8Sc0.
2Fe21.
849190290253.
4292.
1313.
15021234321(1)/117(5)*Intermetalliccompound**FugacityIntermetallicHydridesTOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
12ComparinghydridesScFe1.
8H1.
8,Zr0.
5Sc0.
5Fe2H2.
5(secondplateau)andZr0.
8Sc0.
2Fe2H1.
8showsthattheincreaseinzirconiumcontentinthealloysleadstothedecreaseinitshydrogendesorptionenthalpy.
However,entropychangegoesthroughamaximumatZr0.
5Sc0.
5Fe2H2.
5(Table1).
ForZr0.
8Sc0.
2Fe2–H2,hydrogenabsorptionalsostartsat100atmwithoutactivation.
Hydrogencompositionatroomtemperature(293K)and1650atmisZr0.
8Sc0.
2Fe2H3.
7(Figure6).
Furthercoolingto219Kwithsimultaneousincreaseinhydrogenpressureto1730atmresultsinmaximumhydridecompositioncorrespondingtoZr0.
8Sc0.
2Fe2H3.
8.
1010010001000000.
511.
522.
533.
54313Kdes293Kabs293Kdes253KdesH/Zr0.
8Sc0.
2Fe2P(H2)(atm)Figure6.
DesorptionisothermsforZr0.
5Sc0.
5Fe2–H2system.
PartialsubstitutionoftitaniumforscandiumallowedustosynthesizethefirstpseudobinaryintermetallichydrideinTi0.
5Sc0.
5Fe2–H2system.
Remarkably,whileTi0.
8Sc0.
2Fe2doesnotabsorbhydrogenevenatpressuresupto2500atm,at223K,thereactionbetweenTi0.
5Sc0.
5Fe2andhydrogenstartsatroomtemperaturealreadyat100atmwithoutanypreliminaryactivation(Figure7).
ThehydrogencontentofthehydridecorrespondstoTi0.
5Sc0.
5Fe2H3.
1(2.
0mass%).
1010010001000000.
511.
522.
533.
54333Kdes295Kdes273KdesP(H2)(atm)H/Ti0.
5Sc0.
5Fe2Figure7.
DesorptionisothermsforTi0.
5Sc0.
5Fe2–H2systemTheroomtemperatureequilibriumabsorptionanddesorptionpressuresforthismaterialare195and175atmaccordingly.
Coolingthehydrogenatedmaterialtobelow223Kshowedthatnonewhydridephasetransitionoccurs.
Hydrogencontentat217Kat2700atmis3.
4H/f.
u.
(2.
16mass.
%)andcalculatedthermodynamicparameterswereareΔH(kJ/moleH2)=17.
7(2)andΔS(J/K·moleH2)=103.
8(7)ConclusionsInteractionofhydrogenwithmulti-componentintermetalliccompoundsofAB5-andAB2-typewerestudiedinthecurrentwork.
Severalnewintermetallichydrideswithpotentialapplicationsinhigh-capacityhydrogenstoragehavebeenidentifiedandfullycharacterizedusingagas-volumetricanalyticaltechnique.
AcknowledgementsThisworkwassupportedinpartbyGeneralMotorsCorp.
References:(1)LibowitzG.
G.
;HayesH.
F.
;GibbT.
R.
P.
J.
Phys.
Chem.
1958,62,76.
(2)ZijlstraH.
;WestendorpF.
F.
SolidStateComm.
1969,7,857.
(3)vanVuchtJ.
H.
N.
;KuijpersF.
A.
;BruningH.
C.
A.
M.
PhilipsResearchReports1970,25,133.
(4)PeblerA.
;Gulbransen.
E.
A.
Trans.
Met.
Soc.
AIME1967,239,1593.
(5)HemmesH.
;DriessenA.
;GriessenR.
J.
Phys.
C1986,19,3571.
(6)LaknerJ.
F.
;UribeF.
;SteweardS.
A.
J.
Less-CommonMet.
1980,72,87.
(7)TakeshitaT.
;GschneidnerK.
A.
Jr.
;ThomeD.
K.
;McMastersO.
D.
PhysicalReviewB1980.
21,5636.
(8)TakeshitaT.
;WallaceW.
E.
;CraigR.
S.
Inorg.
Chem.
1974,13,2282.
(9)SaryninV.
K.
;BurnashevaV.
V.
;SemenenkoK.
N.
IzvestiyaANSSSR,Metally,1977,4,69.
(10)TakeshitaT.
;GschneidnerK.
A.
Jr.
;LaknerJ.
F.
J.
Less-CommonMet.
1981,78,43.
(11)AndersonJ.
L.
;WallaceT.
C.
;BowmanA.
L.
;RadosewichC.
L.
;CourtneyM.
L.
LosAlamosRep.
LA-5320-MS,InformalReport,1973.
(12)FilipekS.
M.
;JacobI.
;Paul-BoncourV.
;Percheron-GueganA.
;MarchukI.
;MogilyanskiD.
;PielaszekJ.
PolishJournalofChemistry2001,75,1921.
(13)FilipekS.
M.
;Paul-BoncourV.
;Percheron-GueganA.
;JacobI.
;MarchukI.
;DorogovaM.
;HirataT.
;KaszkurZ.
J.
Phys.
:Condens.
Matter.
2002,14,11261.
(14)Paul-BoncourV.
;Bouree-VigneronF.
;FilipekS.
M.
;MarchukI.
;JacobI.
;Percheron-GueganA.
J.
AlloysCompds.
2003,356–357,69.
(15)SemenenkoK.
N.
;SirotinaR.
A.
;SavchenkovaA.
P.
;BurnashevaV.
V.
;LototskiiM.
V.
;FokinaE.
E.
;TroitskayaS.
L.
;FokinV.
N.
J.
Less-CommonMet.
1985,106,349.
(16)SavchenkovaA.
P.
;SirotinaR.
A.
;BurnashevaV.
V.
;KudryavtsevaN.
S.
Sov.
Neorgan.
Materialy1984,20,1507.
IntermetallicHydridesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
13HydrogenAbsorbingAlloysNameChem.
CompositionHydrogenStorageCapacitywt.
%EquilibriumPressurePlateauProd.
No.
Zirconium-IronAlloyZeFe21.
65–1.
75(25°C,1800bar)~690bar(23°C)absorption~325bar(23°C)desorption693812-1GZirconium-IronAlloyZr0.
8Sc0.
2Fe21.
65–1.
75(22°C,1560bar)~190bar(20°C)693804-1GYttriumNickelAlloyYNi51.
25–1.
3(25°C,1890bar)~674bar(20°C)693928-5GLanthanumNickelAlloyLaNi51.
5–1.
6(25°C)~2bar(25°C)685933-10GLanthanumNickelAlloyLaNi4.
5Co0.
51.
4–1.
5(25°C)50636797-250MG636797-1GSingle-wall,short1–2NA.
5–2>90652512-250MGDouble-wall51.
3–2.
00.
550–80637351-250MG637351-1GMulti-wall,ArkemaCVD10–152–6.
1–10>90677248-5GMulti-wall110–170–5–9>90659258-2G659258-10GMulti-wall,powderedcylindercores–2–151–1010–40406074-100MG406074-500MG406074-1G406074-5GMulti-wall,asproduced6–20–1–5>7.
5412988-100MG412988-500MG412988-2G412988-10GGraphite,nanofibers80–2000.
5–10.
5–20>95636398-2G636398-10G636398-50GFormoreinformationabouttheseandotherrelatedmaterials,pleasevisitsigma-aldrich.
com/nano.
LightweightMetalMatrixNanocompositesForquestions,productdata,ornewproductsuggestions,pleasecontacttheMaterialsScienceteamatmatsci@sial.
com.
21Self-PropagatingReactionsInducedbyMechanicalAlloyingProf.
LaszloTakacsUniversityofMaryland,BaltimoreCountyIntroductionMechanicalalloyingisa"bruteforce"methodofaffectingalloyingandchemicalreactions.
Themixtureofreactantpowdersandseveralballsareplacedinthemillingjarofahigh-energyballmill,forexample,ashakermilloraplanetarymill(Figure1).
Thecollisionsandfrictionbetweentheballs,andbetweentheballsandthewallofthecontainer,resultindeformation,fragmentation,mixing,andcold-welding.
Thereactivityincreasesduetodefectformationandincreasedinterfacearea,andeventuallyalloyingand/orchemicalreactionstakeplace.
Neitheradditionalheatnorsolventareneeded.
Theproductisapowderthatcanbeconsolidatedusingtheusualmethodsofpowdermetallurgy.
Mechanicalalloyingisaveryflexibletechniqueandhasbeenusedtoprepareabroadvarietyofmaterials,includingdispersion-strengthenedalloys,amorphousalloys,andnanocomposites.
1High-energyballmillingisalsocalledmechanochemicalprocessingwhenused,ofteninconjunctionwithothersteps,forinorganicsynthesis,theprocessingofminerals,andtheactivationofbuildingmaterials.
2(a)(b)Figure1.
Crosssectionviewsofthemillingvialofashakermill(a)andaplanetarymill(b).
Mechanically-inducedSelf-propagatingReactions(MSR)arepossibleinhighlyexothermicpowdermixtures.
3Initially,millingresultsinactivation,similartoanyothermechanicalalloyingprocess.
Butatacriticaltime,calledtheignitiontime,thereactionratebeginstoincrease.
Asaresult,thetemperaturerises,furtherincreasingthereactionrateandeventuallyleadingtoaself-sustainingprocess.
Mostofthereactantsareconsumedwithinseconds.
Atthisstage,thereactionissimilartothermallyignitedself-propagatinghigh-temperaturesynthesis(SHS).
4Theabrupttemperatureincreaseisdetectableontheoutersurfaceofthemillingcontaineranditspresencedistinguishessuchmechanicallyinducedself-propagatingreactions(MSR)fromgradualprocesses(Figure2).
MSRcanhappeninabroadvarietyofsystems,suchasinFe2O3–Al,Ni–Al,Ti–C,Zn–S,andMo–Simixtures.
3Theignitiontimeisanimportantattributeoftheprocess;itcanvaryfromafewsecondstoseveralhoursdependingonthereactionandthemillingconditions.
3230282624220500100015002000250030003500Time(s)Temperature(°C)Figure2.
Temperatureoftheoutsidesurfaceofthevialduringballmillingofa5Ni+2PmixtureinaSPEX8000MixerMill.
Ignitionisindicatedbytherapidtemperatureriseat1220sec.
Thegradualtemperatureincreasebeforeignitioniscausedbydissipatedmechanicalenergy.
TheinvestigationofMSRcontributedconsiderablytoourunderstandingofmechanochemicalprocessesingeneral.
Thevariationoftheignitiontimewithprocessconditionsandmaterialpropertiestellsusaboutthemechanismoftheactivationprocess,whiledetailedstudiesofpartiallyactivatedpowdersprovidesinformationaboutthenatureofthecriticalstate.
MSRhasalsobeenconsideredasapracticalmeansfortheproductionofusefulmaterials,particularlyrefractorycompounds.
3RequirementsForSelf-SustainingReactionsMSR(aswellasSHS)requiresufficientself-heatingtopropagatethereaction.
Ameasureofself-heatingistheadiabatictemperature,definedasthefinaltemperature,ifallthereactionheatisusedtoheattheproducts.
Aruleofthumbisthatself-sustainingreactionsarepossible,iftheadiabatictemperatureisatleast1800K.
Sincethemainissueisself-heatingatthebeginningofthereaction,thequantity–ΔH298/C298(whereH298andC298arereactionenthalpyandspecificheatat298K),writtensimplyasΔH/C,isoftenusedasasimplersubstituteforadiabatictemperature;ΔH/C>2000KistheconditionforMSR.
Thissimpleconditionappliessurprisinglywelltothemostfrequentlystudiedclassesofreactions,namelycombinationreactionsbetweenatransitionmetalandametalloidelement(e.
g.
Ti-B,Nb-C,Mo-Si,Ni-P)andthermite-typereactionsbetweenanoxideandamorereactivemetal(e.
g.
Fe3O4-Al,CuO-Fe,ZnO-Ti).
MuchlowervaluesofΔH/CaresufficientforMSRwithchalcogenidesandchlorides.
Table1containsdataforafewtypicalreactions.
MechanicalAlloyingTOORDER:ContactyourlocalSigma-Aldrichoffice(seebackcover),orvisitsigma-aldrich.
com/matsci.
22Table1.
Typicalreactionsshowingmechanicallyinducedself-propagationandthecorrespondingreactionheats(ΔH)andratioofΔHintheheatcapacityoftheproducts(C).
Reaction–ΔH/formula(kJ/mol)ΔH/C(K)3CuO+2Al3Cu+Al2O3119078104CuO+3Fe4Cu+Fe3O446118503Fe3O4+4Al9Fe+4Al2O3337662225Ni+2PNi5P24362867Sn+2SSnS21542189Ti+2BTiB23167111Hf+CHfC2266011Mo+2SiMoSi21322055Asmoreexothermicreactionsbecomeincreasinglyeasytoself-sustain,reactionswithhigheradiabatictemperaturesareexpectedtorequireshorteractivationtimesbeforeignition.
Sucharelationshipindeedexists,butonlyiftheothermaterialparametersandthemillingconditionsareverysimilar.
Sofar,thebestcorrelationwasobservedforthereductionofCuO(AldrichProd.
No.
203130,450804,450812),NiO(AldrichProd.
No.
203882,637130,481793),Fe3O4(AldrichProd.
No.
310069,518158,637106),Cu2O(AldrichProd.
No.
208825,566284),andZnO(AldrichProd.
No.
204951,255750,544906),withthesamemetal(Ti,ZrorHf).
3Theseareductile-brittlesystems5wheremillingresultsinafinedispersionoftheoxideparticlesinthemetalmatrix.
Thedevelopmentofthemicrostructuredependsprimarilyontheductilecomponentanditiskeptthesameforeachseries.
Thechangescausedbymechanicalmillingduringtheactivationperiod—decreaseofgrainsize,mixing,andformationoflatticedefects—dependmainlyonthemechanicalpropertiesofthereactants.
Althoughitisdifficulttoquantifythisrelationship,theincreasingwidthoftheX-raydiffractionlinesindicatesthatthecrystallitesizedecreasesandtheaccumulatedlatticestrainofthemetalcomponentincreasesasthepowderapproachesignition.
6,7Whilereducingthegrainsizeandtherebyincreasingtheinterfaceareaiscertainlyakeycomponentoftheactivationprocess,agglomerationisalsonecessarytoensureefficientmatterandheattransfer.
AninterestingcaseisthereductionofMoS2(AldrichProd.
No.
234842)withaluminumpowder(AldrichProd.
No.
202584,653608).
Thisreactionisgradual,althoughΔH/C=2093KiswellovertheacceptedthresholdforanMSRprocess.
However,MoS2preventsagglomeration,reducingtheareaoftheactiveinterface.
8Themechanicalintensityofthemillingactiondependsonthenumberandenergyofthecollisionsbetweenthemillingballsandbetweentheballsandthecontainerwall.
Thechargeischaracterizedbytheball-to-powdermassratio,aparameterapproximatelyproportionaltotherateofspecificenergyinput.
Fortypicalmillingconditions,ignitiontakesplacewhenthepowderhasreceivedacriticalamountofmechanicalenergyandtheignitiontimeisinverselyproportionaltotheball-to-powdermassratio.
Iftoomuchpowderisused,theenergyofeachindividualimpactisdistributedinaverylargevolumeandthestressesarenotlargeenoughtocauseactivation.
Iftheamountofpowderistoolittle,theheatlosstothemillingtoolsandtotheatmospherequenchesanyincipientself-sustainingreaction.
UnderstandingMechanicallyInducedSelf-PropagatingReactionsAmechanicalalloyingexperimentmaylookquitesimple,buttheunderlyingprocessisverycomplexconsistingofthecomponentsonverydifferentlengthandtimescales.
Thus,thecompletemodelingofamechanochemicaleventrequiresanadequatedescriptionofthemacroscopicprocesses,suchastheoperationofthemill,thecollisionsbetweenthemillingballs,andthetransportofthepowderwithinthemillingcontainer.
Onthemicroscopicscale,theeffectofanindividualcollisiononthepowdercaughtbetweentheimpactingsurfacesmustbeunderstoodandtheformationoflatticedefectsandtheelementaryinterfacereactionsmustbedescribed.
SignificantadvancesweremadetowardageneraltheoryofgradualmechanicalalloyingbyProf.
Courtneyandhisstudents.
9ThekeymomentofanMSRisignition.
Onceweunderstandwhatmakesthestateofthematerialcriticalatignition,weshouldbeabletousethisunderstandingofMSRforlearningabouttheinitialphaseofothermechanicalalloyingprocesses.
Unfortunately,manydetailsaresystemspecificandidentifyingthegeneralfeaturesisconsequentlydifficult.
Whetherignitiontakesplaceornotmaybeverysensitivetocompositionandmillingconditions.
Forexample,Figure3showstheX-raydiffractionpatternsoftwox(Zn+S)+(1–x)(Sn+2S)mixtures.
10ThisisanunusualsystemasthecombinationreactionsZn+S→ZnSSn+2S→SnS2arebothself-sustaining,buttheprocessisgradualinmixturesofthetwofor0.
1990%purity357391-250G357391-1KGPowder,<100nm,surfacearea70–90m2/g594911-100G594911-250GTantalum(Ta)TaCPowder,5μm280801-10GTitanium(Ti)TiCPowder,–325mesh307807-100G307807-500GPowder,<4μm594849-25G594849-100GPowder,130nmparticlesize(spherical)636967-25G636967-100G636967-250GTungsten(W)WCPowder,10μm241881-100GZirconium(Zr)ZrCPowder,5μm336351-50G336351-250GPhosphidesCalcium(Ca)Ca3P299%purity400971-100G400971-500GGallium(Ga)GaP99.
99%purity521574-2GNickel(Ni)Ni2PPowder,–100mesh,98%purity372641-10GIndium(In)InPPieces,3–20mesh,99.
998%purity366870-1GIron(P2Fe)P2FePowder,–40mesh,99.
5%purity691593-5GIron(P3Fe)P3FePowder,–40mesh,99.
5%purity691658-5GSilicidesCalcium(Ca)CaSi2Technicalgrade21240-250G-F21240-1KG-FChromium(Cr)CrSi2Powder,–230mesh,99%purity372692-25GMagnesium(Mg)Mg2SiPowder,–20mesh,99%purity343196-25GNiobium(Nb)NbSi2Powder,–325mesh399493-10GTungsten(W)WSi2Powder,–325mesh99%purity399442-10GVanadium(V)VSi2Powder,–325mesh399450-10GZirconium(Zr)ZrSi2Powder,325mesh99%purity399426-50GFormoreinformationabouttheseandotherrelatedmaterials,pleasevisitsigma-aldrich.
com/matsci.
MechanicalAlloyingTheprimaryfocusofourmanufacturingfacilityinUrbana,Illinois,USAisonthepurificationofinorganicsandmetalsforavarietyofhightechnologyapplications.
Ourcapabilitiesintheareaofmetalspurificationutilizeseveralroutestoproducesomeofthepurestalkaliearthandrareearthmetalsintheworld.
Thesetechniquescanalsobeusedinthemanufactureofalloysandothermaterialsforyourresearchorcommercialrequirements.
Ourproprietarytechniquesallowustomanufacturebarium,calcium,strontium,andmagnesiumwithtracemetalpuritiesupto4N(99.
99%).
Wealsomanufacturerareearthmetalsinpuritiesexceeding4Nthroughanumberofdifferenthightemperatureroutes.
OneareaofcustomizationhasfocusedonthemanufactureofprepackagedMolecularBeamEpitaxy(MBE)cruciblesforuseinthinfilmmanufacturingofadvancedmaterials.
Wehavesuccessfullymanufacturedhighpuritybarium,calcium,andstrontium(aswellasotherhighpuritymetals)andmeltedthematerialintoanMBEcrucible.
ThisallowsforincreasedloadinginthecrucibleaswellassignificantlyreducedoutgassingofthematerialsintheMBEvacuumchamber.
Pleasecontactustodayforyourspecificrequirements:matsci@sial.
com.
High-PurityMetalsManufacturedbyAAPL—ASigma-AldrichMaterialsChemistryCenterofExcellenceMetalCommentsPurity,%Prod.
NoMagnesium(Mg)dendriticpieces,purifiedbydistillation99.
998(metals)474754-5G474754-25G99.
99(metals)465992-5G465992-25GCalcium(Ca)dendriticpieces,purifiedbydistillation99.
99(metals)441872-5G441872-25G99.
9(metals)596566-5G596566-25GStrontium(Sr)dendriticpieces,purifiedbydistillation99.
99(metals)441899-5G441899-25G99.
9(metals)460346-5G460346-25GBarium(Ba)dendriticpieces,purifiedbydistillation99.
99(metals)474711-5G474711-25G99.
9(metals)441880-5G441880-25GUltraPureMetalsforHighTechnologyApplicationssigma-aldrich.
comHigh-PurityRareEarthMetalFoilsMetalDimensionsPurity*Prod.
No.
Lanthanum(La)25mmX25mmX1mm,~3.
9gTotalREM:99.
5%La/TotalREM:99.
9%694908Cerium(Ce)25mmX25mmX1mm,~4.
2gTotalREM:99.
5%Ce/TotalREM:99.
9%693766Neodymium(Nd)25mmX25mmX1mm,~4.
4TotalREM:99.
5%Nd/TotalREM:99.
9%693758Samarium(Sm)25mmX25mmX1mm,~4.
9gTotalREM:99.
9%Sm/TotalREM:99.
95%693731GadoliniumGd)25mmX25mmX1mm,~4.
8gTotalREM:99.
5%Gd/TotalREM:99.
95%693723Terbium(Tb)25mmX25mmX1mm,~4.
9gTotalREM:99.
5%Tb/TotalREM:99.
9%693715Dysprosium(Dy)25mmX25mmX1mm,~5.
6gTotalREM:99.
5%Dy/TotalREM:99.
9%693707HolmiumFoil(Ho)25mmX25mmX1mm,~5.
5gTotalREM:99.
5%Dy/TotalREM:99.
9%693693Erbium(Er)25mmX25mmX1mm,~5.
6gTotalREM:99.
5%Dy/TotalREM:99.
9%693685Thulium(Tm)25mmX25mmX1mm,~5.
8gTotalREM:99.
5%Dy/TotalREM:99.
95%693677Ytterbium(Yb)25mmX25mmX1mm,~4.
4gTotalREM:99.
5%Dy/TotalREM:99.
95%693669Lutetium(Lu)25mmX25mmX1mm,~6.
2gTotalREM:99.
5%Dy/TotalREM:99.
9%693650Yttrium(Y)25mmX25mmX1mm,~2.
8gTotalREM:99.
5%Dy/TotalREM:99.
9%693642*REM:RareearthmetalsRareearthmetalfoilsareusedinthermalandelectronbeam(e-beam)evaporationprocessesforcoatingsandthinfilmsviaphysicalvapordeposition(PVD).
Thelow-temperaturee-beamtechniqueisparticularlysuitedforapplicationssuchasfuelcellsandsolarpanels.
Therareearthmetalfoilscanalsobeusedinthepreparationofalloysandcompositesthatcontainhighlyvolatilecomponents(Ca,Mg,etc.
).
Formoreinformation,pleasevisitsigma-aldrich.
com/metals.
JZX03394-5034030107ArgentinaSIGMA-ALDRICHDEARGENTINAS.
A.
FreeTel:08108887446Tel:(+54)1145561472Fax:(+54)1145521698AustraliaSIGMA-ALDRICHPTYLTD.
FreeTel:1800800097FreeFax:1800800096Tel:(+61)298410555Fax:(+61)298410500AustriaSIGMA-ALDRICHHANDELSGmbHTel:(+43)16058110Fax:(+43)16058120BelgiumSIGMA-ALDRICHNV/SA.
FreeTel:080014747FreeFax:080014745Tel:(+32)38991301Fax:(+32)38991311BrazilSIGMA-ALDRICHBRASILLTDA.
FreeTel:08007017425Tel:(+55)1137323100Fax:(+55)1155229895CanadaSIGMA-ALDRICHCANADALTD.
FreeTel:18005651400FreeFax:18002653858Tel:(+1)9058299500Fax:(+1)9058299292ChinaSIGMA-ALDRICH(SHANGHAI)TRADINGCO.
LTD.
FreeTel:8008193336Tel:(+86)2161415566Fax:(+86)2161415567CzechRepublicSIGMA-ALDRICHspol.
sr.
o.
Tel:(+420)246003200Fax:(+420)246003291DenmarkSIGMA-ALDRICHDENMARKA/STel:(+45)43565910Fax:(+45)43565905FinlandSIGMA-ALDRICHFINLANDOYTel:(+358)93509250Fax:(+358)935092555FranceSIGMA-ALDRICHCHIMIES.
à.
r.
l.
FreeTel:0800211408FreeFax:0800031052Tel:(+33)474822800Fax:(+33)474956808GermanySIGMA-ALDRICHCHEMIEGmbHFreeTel:08005155000FreeFax:08006490000Tel:(+49)8965130Fax:(+49)8965131160GreeceSIGMA-ALDRICH(O.
M.
)LTD.
Tel:(+30)2109948010Fax:(+30)2109943831HungarySIGMA-ALDRICHKftIngyeneszldtelefon:0680355355Ingyeneszldfax:0680344344Tel:(+36)12359055Fax:(+36)12359050IndiaSIGMA-ALDRICHCHEMICALSPRIVATELIMITEDTelephoneBangalore:(+91)8066219600NewDelhi:(+91)1141654255Mumbai:(+91)2225702364Hyderabad:(+91)4040155488FaxBangalore:(+91)8066219650NewDelhi:(+91)1141654266Mumbai:(+91)2225797589Hyderabad:(+91)4040155466IrelandSIGMA-ALDRICHIRELANDLTD.
FreeTel:1800200888FreeFax:1800600222Tel:(+353)14041900Fax:(+353)14041910IsraelSIGMA-ALDRICHISRAELLTD.
FreeTel:1800702222Tel:(+972)89484100Fax:(+972)89484200ItalySIGMA-ALDRICHS.
r.
l.
NumeroVerde:800827018Tel:(+39)0233417310Fax:(+39)0238010737JapanSIGMA-ALDRICHJAPANK.
K.
Tel:(+81)357967300Fax:(+81)357967315KoreaSIGMA-ALDRICHKOREAFreeTel:(+82)800237111FreeFax:(+82)800238111Tel:(+82)313299000Fax:(+82)313299090MalaysiaSIGMA-ALDRICH(M)SDN.
BHDTel:(+60)356353321Fax:(+60)356354116MexicoSIGMA-ALDRICHQUMICA,S.
A.
deC.
V.
FreeTel:018000075300FreeFax:018007129920Tel:527222761600Fax:527222761601TheNetherlandsSIGMA-ALDRICHCHEMIEBVFreeTel:08000229088FreeFax:08000229089Tel:(+31)786205411Fax:(+31)786205421NewZealandSIGMA-ALDRICHNEWZEALANDLTD.
FreeTel:0800936666FreeFax:0800937777Tel:(+61)298410555Fax:(+61)298410500NorwaySIGMA-ALDRICHNORWAYASTel:(+47)23176060Fax:(+47)23176050PolandSIGMA-ALDRICHSp.
zo.
o.
Tel:(+48)618290100Fax:(+48)618290120PortugalSIGMA-ALDRICHQUMICA,S.
A.
FreeTel:800202180FreeFax:800202178Tel:(+351)219242555Fax:(+351)219242610RussiaSIGMA-ALDRICHRUS,LLCTel:+7(495)6216037+7(495)6215828Fax:+7(495)6215923SingaporeSIGMA-ALDRICHPTE.
LTD.
Tel:(+65)67791200Fax:(+65)67791822SouthAfricaSIGMA-ALDRICHSOUTHAFRICA(PTY)LTD.
FreeTel:0800110075FreeFax:0800110079Tel:(+27)119791188Fax:(+27)119791119SpainSIGMA-ALDRICHQUMICA,S.
A.
FreeTel:900101376FreeFax:900102028Tel:(+34)916619977Fax:(+34)916619642SwedenSIGMA-ALDRICHSWEDENABTel:(+46)87424200Fax:(+46)87424243SwitzerlandSIGMA-ALDRICHCHEMIEGmbHFreeTel:0800800080FreeFax:0800800081Tel:(+41)817552828Fax:(+41)817552815UnitedKingdomSIGMA-ALDRICHCOMPANYLTD.
FreeTel:0800717181FreeFax:0800378785Tel:(+44)1747833000Fax:(+44)1747833313SAFC(UK)FreeTel:01202712305UnitedStatesSIGMA-ALDRICHP.
O.
Box14508St.
Louis,Missouri63178Toll-Free:8003253010Toll-FreeFax:8003255052CallCollect:(+1)3147715750Tel:(+1)3147715765Fax:(+1)3147715757Internetsigma-aldrich.
comWorldHeadquarters3050SpruceSt.
,St.
Louis,MO63103(314)771-5765sigma-aldrich.
comOrder/CustomerService(800)325-3010Fax(800)325-5052TechnicalService(800)325-5832sigma-aldrich.
com/techserviceDevelopment/BulkManufacturingInquiries(800)244-11732007Sigma-AldrichCo.
Allrightsreserved.
SIGMA,,SAFC,,SIGMA-ALDRICH,ALDRICH,,FLUKA,,andSUPELCO,aretrademarksbelongingtoSigma-AldrichCo.
anditsaffiliateSigma-AldrichBiotechnology,L.
P.
SigmabrandproductsaresoldthroughSigma-Aldrich,Inc.
Sigma-Aldrich,Inc.
warrantsthatitsproductsconformtotheinformationcontainedinthisandotherSigma-Aldrichpublications.
Purchasermustdeterminethesuitabilityoftheproduct(s)fortheirparticularuse.
Additionaltermsandconditionsmayapply.
Pleaseseereversesideoftheinvoiceorpackingslip.
PrintedonRecycledPaper—AWorldwideOperationwithaGlobalCommitmentSigma-Aldrich3050SpruceStreetSt.
Louis,MO63103USAsigma-aldrich.
comAcceleratingCustomers'SuccessthroughLeadershipinLifeScience,HighTechnologyandService
- basedm.2828dy.com相关文档
- TANKm.2828dy.com
- 0.70m.2828dy.com
- Studem.2828dy.com
- plikm.2828dy.com
- PSL2m.2828dy.com
- Pinkeyedm.2828dy.com
UCloud优刻得,新增1核1G内存AMD快杰云机型,服务器2元/首月,47元/年
UCloud优刻得近日针对全球大促活动进行了一次改版,这次改版更加优惠了,要比之前的优惠价格还要低一些,并且新增了1核心1G内存的快杰云服务器,2元/首年,47元/年,这个价格应该是目前市面上最低最便宜的云服务器产品了,有需要国内外便宜VPS云服务器的朋友可以关注一下。UCloud好不好,UCloud服务器怎么样?UCloud服务器值不值得购买UCloud是优刻得科技股份有限公司旗下拥有的云计算服...
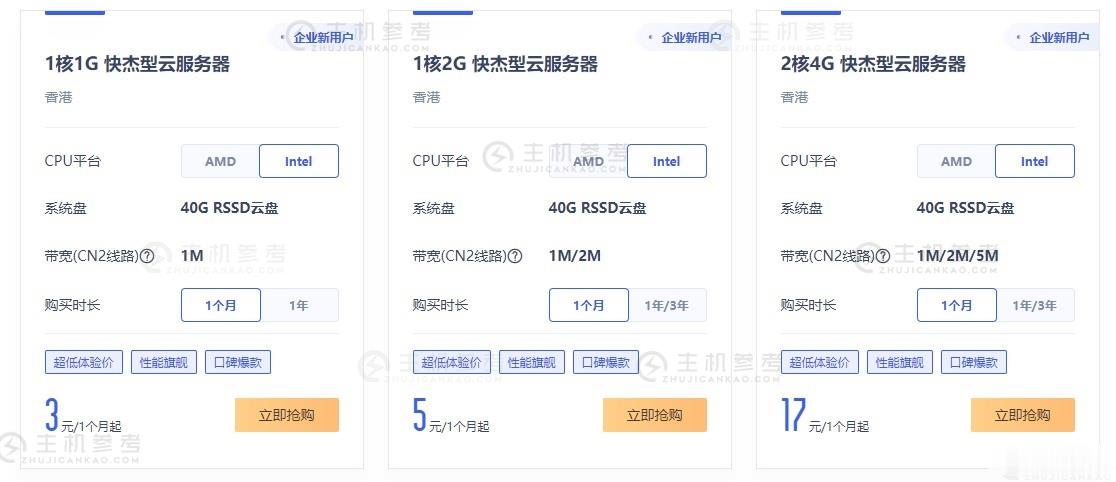
享有云:美国BGP云服务器低至20元/月起,首月打折;香港2核2G2M仅50元/月起
享有云怎么样?享有云是一家新的国内云服务器商家,目前提供国内、香港及海外地区的云服务器,拥有多线路如:BGP线路、CN2线路、高防等云服务器,并且提供稳定、安全、弹性、高性能的云端计算服务,实时满足您的多样性业务需求。目前,美国bgp云服务器,5M带宽,低至20元/月起,270元/年起,首月打折;香港2核2G2M仅50元/月起,450元/年起!点击进入:享有云官方网站地址享有云优惠活动:一、美国B...

Vinahost - 越南VPS主机商月6美元 季付以上赠送时长最多半年
Vinahost,这个主机商还是第一次介绍到,翻看商家的介绍信息,是一家成立于2008年的老牌越南主机商,业务涵盖网站设计、域名、SSL证书、电子邮箱、虚拟主机、越南VPS、云计算、越南服务器出租以及设备托管等,机房主要在越南胡志明市的Viettle和VNPT数据中心,其中VNPT数据中心对于国内是三网直连,速度优。类似很多海外主机商一样,希望拓展自己的业务,必须要降价优惠或者增加机房迎合需求用户...

m.2828dy.com为你推荐
-
急救知识纳入考试应急救护知识应该由哪个部门培训johncusack约翰·库萨克好看的的恐怖片全集微信回应封杀钉钉微信大封杀什么时候结束地图应用手机地图软件那么多,都不知道用哪个好了?www.jjwxc.net在哪个网站看小说?丑福晋历史上真正的八福晋是什么样子的?同ip网站一个域名能对应多个IP吗百度关键词工具百度有关键字分析工具吗?Google AdWords有的网站检测如何进行网站全面诊断ip在线查询我要用eclipse做个ip在线查询功能,用QQwry数据库,可是我不知道怎么把这个数据库放到我的程序里面去,高手帮忙指点下,小弟在这谢谢了