generationxxx
xxx365 时间:2021-03-01 阅读:()
ReviewGliaandpain:IschronicpainagliopathyRu-RongJia,,TemuginBertaa,MaikenNedergaardbaDepartmentofAnesthesiologyandNeurobiology,DukeUniversityMedicalCenter,Durham,NC,USAbDivisionofGlialDiseaseandTherapeutics,CenterforTranslationalNeuromedicine,UniversityofRochester,Rochester,NY,USAarticleinfoArticlehistory:Received27July2012Receivedinrevisedform23May2013Accepted12June2013AvailableonlinexxxxKeywords:AstrocytesATPreceptorsChemokinesCytokinesHumanMicrogliaRodentsSatelliteglialcellsSpinalcordabstractActivationofglialcellsandneuro–glialinteractionsareemergingaskeymechanismsunderlyingchronicpain.
Accumulatingevidencehasimplicated3typesofglialcellsinthedevelopmentandmain-tenanceofchronicpain:microgliaandastrocytesofthecentralnervoussystem(CNS),andsatelliteglialcellsofthedorsalrootandtrigeminalganglia.
Painfulsyndromesareassociatedwithdifferentglialactivationstates:(1)glialreaction(ie,upregulationofglialmarkerssuchasIBA1andglialbrillaryacidicprotein(GFAP)and/ormorphologicalchanges,includinghypertrophy,proliferation,andmodi-cationsofglialnetworks);(2)phosphorylationofmitogen-activatedproteinkinasesignalingpathways;(3)upregulationofadenosinetriphosphateandchemokinereceptorsandhemichannelsanddownreg-ulationofglutamatetransporters;and(4)synthesisandreleaseofglialmediators(eg,cytokines,che-mokines,growthfactors,andproteases)totheextracellularspace.
Althoughwidelydetectedinchronicpainresultingfromnervetrauma,inammation,cancer,andchemotherapyinrodents,andmorerecently,humanimmunodeciencyvirus-associatedneuropathyinhumanbeings,glialreaction(acti-vationstate1)isnotthoughttomediatepainsensitivitydirectly.
Instead,activationstates2to4havebeendemonstratedtoenhancepainsensitivityviaanumberofsynergisticneuro–glialinteractions.
Glialmediatorshavebeenshowntopowerfullymodulateexcitatoryandinhibitorysynaptictransmis-sionatpresynaptic,postsynaptic,andextrasynapticsites.
Glialactivationalsooccursinacutepaincon-ditions,andacuteopioidtreatmentactivatesperipheralgliatomaskopioidanalgesia.
Thus,chronicpaincouldbearesultof''gliopathy,''thatis,dysregulationofglialfunctionsinthecentralandperiph-eralnervoussystem.
Inthisreview,weprovideanupdateonrecentadvancesanddiscussremainingquestions.
2013InternationalAssociationfortheStudyofPain.
PublishedbyElsevierB.
V.
Allrightsreserved.
1.
IntroductionItisnowwellestablishedthatchronicpain,suchasinamma-torypain,neuropathicpain,andcancerpain,isanexpressionofneuralplasticity,bothintheperipheralnervoussystem(PNS)asperipheralsensitization[11,78]andinthecentralnervoussystem(CNS)ascentralsensitization[111,139].
Themostwidelystudiedneuronalmechanismsarehyperexcitabilityandsensitizationofprimarysensoryneurons(peripheralsensitization)andenhance-mentofexcitatorysynaptictransmissioninspinalcord,brainstem,andcorticalneurons(centralsensitization),causedbytranscrip-tional,translational,andpost-translationalregulation.
Otherneu-ronalmechanismsincludedisinhibition(reducedinhibitorysynaptictransmission),descendingpathwayfacilitation(eg,fromthebrainstemtothespinalcord),andlong-termpotentiation(LTP)inthecortexandspinalcord.
Theseneuronalmechanismshavebeenstronglyimplicatedinthedevelopmentandmainte-nanceofpersistentpaininrodents[11,142,195,205,317].
CentralsensitizationandLTParealsoinvolvedinhumanpainconditions[134,285].
Inparalleltotheprogressintheseneuronalmecha-nismsistheincreasedrecognitionoftheimportanceofnon-neuro-nalcells,especiallyglialcells,intheinitiationandmaintenanceofchronicpain.
Ofnote,overthelast10years,theeldofpainre-searchhaswitnessedadramaticincreaseinthenumberofpubli-cationsstudyinggliaandpain.
Numerousreviewshavebeenpublishedinhigh-impactjournalstoaddressthistopic[24,52,68,80,160,164,200,209,247,273].
Hereweprovideacom-prehensiveandupdatedreviewofgliaandpainbyintegratingre-centadvancesinboththepainandglialresearchelds.
GlialcellsintheCNSconsistof3majorgroups:astrocytes,microglia,andoligodendrocytes[69].
GlialcellsinthePNScon-sistofsatelliteglialcells(SGCs)inthedorsalrootganglia(DRGs)andtrigeminalganglia(TGs)andSchwanncellsintheperipheralnerves.
Thisreviewwillcover3typesofglia—microglia,0304-3959/$36.
002013InternationalAssociationfortheStudyofPain.
PublishedbyElsevierB.
V.
Allrightsreserved.
http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022Correspondingauthor.
Address:DepartmentofAnesthesiology,DukeUniversityMedicalCenter,595LaSalleStreet,GSRB-I,Room1027A,P.
O.
Box3094,NC27710,USA.
Tel.
:+1(919)6849387;fax:+1(919)6842411.
E-mailaddress:ru-rong.
ji@duke.
edu(R.
-R.
Ji).
PAINxxx(2013)xxx–xxxwww.
elsevier.
com/locate/painPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022astrocytes,andSGCs—astheirrolesinpainregulationarewelldocumented.
1.
1.
MicrogliaMicrogliaaremacrophage-likecellsintheCNSthatoriginatefrombonemarrow-derivedmonocytesthatmigrateduringperina-taldevelopment.
TheyareheterogeneouslydistributedthroughouttheCNS.
Undernormalconditions,microgliaarenotasquiescentasmanyinvestigatorsoriginallythought,asithasbeenshownthatmicrogliaactivelysensetheirenvironmentwiththeirramiedpro-cesses[93,175,199].
Notably,microgliadynamicallyinteractwithsynapsestomodulatetheirstructuresandfunctionsinhealthybrain[246].
Duringdevelopment,microglialprocessescanengulfsynapses,andsynapticpruningbymicroglia,whichinvolvestheactivationofthecomplementsystem,isnecessaryfornormalbraindevelopment.
[186,221].
Microgliaarefurtheractivatedaftervariousinsultssuchasnerveinjury,bydisplayingmorphologicalchanges,suchasachangefromramiedtoamoeboidshape[57]andupregulationofmicroglialmarkers(CCR3/CD11b,majorhistocompatibilitycom-plexII[MHCII],andionizedcalcium-bindingadaptormolecule-1[IBA1])[93,227](Table1).
Afterperipheralnerveinjury,microgliainthespinalcordundergorapidproliferation[14,23,55,151],andthisproliferationisalreadyveryprominent2daysaftersparednerveinjury[227].
Numerousstudieshavedemonstratedacriticalroleofmicrogliainthedevelopmentofneuropathicpain[43,113,197,252],aswellasacuteinammatorypain[229,311].
Minocycline,anonselectiveinhibitorofmicroglia,hasbeenshowntoreduceneuropathicpain,inammatorypain,andpostoperativepain[13,86,100,197],butitsroleinreducingtheestablishedlate-phaseneuropathicpainislim-ited[197].
Importantly,recentprogresshasidentiedalargenum-berofmoleculesthatareinducedinmicrogliaafterpainfulinjuries,especiallynervetrauma(Tables1–4).
1.
2.
AstrocytesAstrocytesarethemostabundantcellsintheCNSandwerehis-toricallyregardedassupportcells.
Workoverthepastdecadeindi-catesthatastrocytesplaymultipleactiverolesinacuteandchronicneuronaldiseasessuchasseizure,stroke,andischemia[133].
Un-likemicrogliaandoligodendrocytes,astrocytesformphysicallycouplednetworksmediatedbygapjunctions,which,amongotherfunctions,facilitateintercellulartransmissionofCa2+signalingandexchangeofcytosoliccontents,anddisplayoscillationsinionper-meabilitythroughastrocyticnetworks.
Gapjunctioncommunica-tionismediatedbyhomo-andheteromericassociationsofhemichannels,suchasconnexin-43(Cx43),thepredominantconn-exinexpressedinastrocytes[27].
Althoughastrocytesaretypicallyimmunelabeledbyglialbrillaryacidicprotein(GFAP),GFAPimmunoreactivitylabelsonlymajorbranchesandprocessesofastrocytes.
TheactualterritoryoccupiedbyanastrocyteismuchlargerthanthatrevealedbyGFAPimmunostaining.
Ofnote,eachastrocyteformsanon-overlappingterritoryordomain[106,133],whichcollectivelyresemblealatticeframework,appearingcrystal-lineinnature.
Althoughtheimplicationsofthisorganizationarenotfullyunderstood,itbecomeslostwhenastrocytestransitiontoreactivestates[181].
Inaddition,astrocyteshaveextensivecon-tactswithbothsynapsesandcerebralbloodvessels,andcontroltheincreaseinbloodowevokedbysynapticactivity.
Theastro-cyte-mediatedbloodowincreaseisfundamentaltotheblood-oxygen-level-dependent(BOLD)signaldetectedbyfunctionalmagneticresonanceimaging(fMRI)[106].
Itisestimatedthatasingleastrocytecanenwrap140,000syn-apsesand4to6neuronalsomata,andcancontact300to600neu-ronaldendritesinrodents.
[22,69,180].
Aclosecontactwithneuronsandsynapsesmakesitpossibleforastrocytesnotonlytosupportandnourishneuronsbutalsotoregulatetheexternalchemicalenvironmentduringsynaptictransmission.
Thegrowingappreciationforactiverolesofastrocyteshasledtotheproposalofa''tripartitesynapse''theory,basedonthefactsthat(1)gliarespondtoneuronalactivitywithanelevationoftheirinternalCa2+concentrationandtriggerthereleaseofchemicaltransmittersfromgliathemselves,and(2)glialtransmitterscausefeedbackregulationofneuronalactivityandsynapticstrength.
Table1Distinctreactionofmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions,asexaminedbyupregulationoftheglialmarkersIBA1,CD11b,andglialbrillaryacidicprotein(GFAP).
PainconditionsMicrogliaAstrocytesSGCsNerveinjury%%%Spinalcordinjury%%Pawincision%%InammationM/%%%Jointarthritis%%%BonecancerM/%%%SkincancerM%ChemotherapyM/%%%Diabetes%%HIVneuropathyM%Chronicopioid%%AcuteopioidMM%Detailed,withrelatedreferences,inSection2.
1.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
Table2Phosphorylationofmitogen-activatedproteinkinases(MAPKs;ERK,p38,JNK,ERK5)inmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions.
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryP-ERK%%%P-p38%%P-JNK%P-ERK5%SCIP-ERK%P-p38%PawincisionP-p38%InammationP-ERK%%P-p38%P-JNK%BonecancerPERK%%P-p38%P-JNK%SkincancerP-JNK%DiabetesP-ERK%P-p38%ChronicopioidP-ERK%P-p38%Detailed,withrelatedreferences,inSection2.
2.
SCI=spinalcordinjury.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
2R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022Accordingtothistheory,astrocyticprocessesareactivecomponentsofsynapses,inadditiontopre-andpost-synapticcomponents[7].
Althoughactivecontributiontosynapticactivityremainsapossibility,severalrecentstudieshavechallengedthetheoryofthetripartitesynapse,bydemonstratingthatalterationsinastrocyticCa2+donotmodulatesynaptictransmission[4,172,193].
Inreviewingtheseconclusions,however,itisimportanttonotethatmostoftheclassicalstudiesofthetripartitesynapsearebasedonelectrophysiologicalanalysisofacuteslicespreparedfromrodentpups.
Sincetheexpressionofmembraneproteinsaswellasneuralcircuitsundergosignicantchangesduringdevelopment[16,61],itispossiblethattheconceptofreceptor-mediatedCa2+signalingasakeyfeaturedeningastrocyticparticipationinhigherneuralfunctionwillbeexpandedtoincludeotherintracellularsignalingpathways.
Ofnote,gluta-mate-dependentneuroglialCa2+signalingdiffersbetweentheyoungandadultrodentbrain[223].
Thus,alternativepathwaysforastrocyticmodulationofsynaptictransmissionexist:1oftheessentialhousekeepingdutiesofastrocytesistomaintainpotassiumhemostasis.
Recently,ithasbeenshownthatreceptor-mediatedincreasesinastrocyticCa2+canmodulateneuralnetworkactivitybyactiveuptakeofextracellularK+[263].
BecausetheextracellularconcentrationofK+isanimportantdeterminantoftherestingmembranepotentialandtherebyofneuronalactivity,activeuptakeofK+representsasimpleyetpowerfultoolforrapidmodulationofneuralnetworks.
Studiesusingastroglialtoxins(eg,urocitrateanda-aminoadi-pate),astroglialaconitaseinhibitor(sodiumuoroacetate),orinhibitorsoftheastroglialenzymeglutaminesynthetase(eg,methioninesulfoximine)inadultanimalssuggestthatastrocytesareimportantbothfortheinductionandmaintenanceofinamma-toryandforneuropathicpain[30,31,69,83,110,161,184,200,272].
Proliferationofspinalcordastrocyteshasbeendemonstratedinmodelsofneuropathicpain,suchasrhizotomy[151]andspinalnerveligation[248].
Conversely,inhibitingastrocyteproliferationinthespinalcordwasshowntoreduceneuropathicpain[248].
1.
3.
SatelliteglialcellsSatelliteglialcells(SGCs)areprominentglialcellsinthePNS.
Theyarefoundnotonlyinsensoryglia(DRGsandTGs)butalsoinsympatheticandparasympatheticganglia.
LikeSchwanncells,SGCsarederivedfromneuralcrestcells.
SGCsarecharacterizedbythincellularsheathsthatsurroundtheindividualneurons.
Theyexhibitmanysimilaritiestoastrocytes:(1)bothexpresstheglialmarkersGFAP,S100,andglutaminesynthetase;and(2)bothformgapjunctions[89].
ThenumberofSGCsinDRGsandTGsismuchTable3Regulationofreceptors,channels,transporters,enzymes,andtranscriptionalfactorsinmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions.
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryP2X4%MP2X7%P2Y6%P2Y12%%TLR2MTLR3MTLR4%C1q,3,4,5%MCX3CR1%MCCR2%IFN-cR%Cx43%%Kir4.
1%&TRPM2MMGLT-1&GLAST&COX-1%COX-2%NF-kB%%NOX-2%STAT3%%c-Jun%CB2%SCICx43%InammationTLR3MTLR4%Cx43%TRPM2%GRK2&ALX&JointarthritisCX3CR1%BonecancerCX3CR1%HIVTLR2%TLR9&ChronicopioidP2X4%P2X7%TLR2%TLR4MDetailed,withrelatedreferences,inSection2.
3.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
Table4Regulationoftheglialmediatorscytokines,chemokines,growthfactors,andproteasesinmicroglia,astrocytes,andsatelliteglialcells(SGCs).
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryTNF-a%IL-1b%%%IL-6%IL-18%CCL2%BDNF%bFGF%%MMP-2%%tPA%%CatS%TSP4%SCIIL-1b%InammationTNF-a%IL-1b%%IL-6%BonecancerTNF-a%IL-1b%IL-6%ChronicopioidTNF-a%IL-1b%IL-6%AcutemorphineIL-1b%Detailed,withrelatedreferences,inSection2.
4.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx3Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022lowerthanthatofastroctyesinthespinalcord.
Unlikeastrocytes,eachSGCcontactsonly1neuron.
Strikingly,thegapofextracellu-larspacebetweentheSGCsheathandtheassociatedneuronalplasmamembranemeasuresonly20nm,allowingforcloseinter-actionsandeffectivesignalingbetweenneuronsandSGCs[89].
EmergingevidencesuggeststhatSGCsareactivatedafterpainfulinjuriesandplayanactiveroleinthedevelopmentofpersistentpain[29,54,91,107,150].
SGCsalsoexhibitenhancedcouplinginpersistentinammatoryandneuropathicpain[54,295].
2.
DifferentactivationstatesofgliaafterpainfulstimuliandinjuriesAfterpainfulstimuliandinjuries,gliaexhibitvariablealtera-tionsinfunctionsandmorphologies,includingthefollowing:(1)ionicchanges(eg,intracellularCa2+risesinastrocytes);(2)post-translationalregulation(eg,phosphorylationofmitogen-activatedproteinkinases[MAPK]);(3)translationalandtranscriptionalmodulation(eg,modulationofsurfacemolecules,glialmarkers,pro-andanti-inammatorymediators);(4)morphologicalchanges(eg,hypertrophy);and(5)proliferation.
Thesechangesareassoci-atedwithdifferentactivationstatesofglia(Fig.
1).
Belowwedis-cussactivationstatesthatarefrequentlymeasuredinthepainresearcheld.
2.
1.
Glialreaction:Changesinglialmarkersand/ormorphologyMoststudiesdeneglialactivationasupregulationoftheglialmarkerssuchasCCR3/CD11b,IBA1,andGFAP,whichareoften,butnotalways,associatedwithmorphologicalchanges(eg,hyper-trophyorprocessretraction/extension).
Thus,werefertothisglialactivationstateasglialreaction.
Observationsthatnerveinjuryinducesmicroglialresponsesdatebacktothe1970s[3].
Microglialreaction(microgliosis)inthespinalcordhasbeenintensivelyinvestigatedafterperipheralnerveinjury.
Nervetraumainducesveryrobustmicroglialreaction,suchashypertrophyandupregulationofthemicroglialmarkersCD11b,IBA1,andCD68inthespinalcordandbrainstem[118,252,300](Fig.
2).
IBA1isprobablythemostwidelyusedmar-kerformicroglialreactioninthepaineld,partlybecausetheIBA1antibodyfromWakoChemicalsworksbetterthanotherantibodiesofmicroglialmarkers.
Asexpected,microglialreactionisalsoveryrobustafterspinalcordinjury[86,102].
Furthermore,chronicopi-oidexposure,streptozotocin-induceddiabeticneuropathy,andsurgicalincisionresultinmicroglialreaction[49,192,275,310].
However,microglialreactionislessevidentafterbonecancer[98]andchemotherapy-inducedneuropathy[297,307],dependingonthedosesofchemotherapydrugsandseverityofnervedamageaftertumorgrowth(asshownbyATF-3expressioninDRGneu-rons)[23,303].
Intra-articularbutnotintraplantarinjectionofcompleteFreund'sadjuvant(CFA)inducesmicroglialreaction[222],becauseofdeeptissue(joint)injuryandpossibleaxonalinjury(Table1).
Interestingly,inyoungrats(P10),nerveFig.
1.
Differentactivationstatesofglia.
Gliaexhibitdifferentactivationstatesafterpainfulinjuries.
(1)Glialreactionreferstoupregulationofglialmarkersandmorphologicalchangesofglia(gliosis);(2)upregulationofglialreceptorssuchasadenosinetriphosphate(ATP)receptors,chemokinereceptors,andToll-likerecep-tors,whichwillleadtothethirdactivationstate:(3)activationofintracellularsignalingpathways,suchasmitogen-activatedproteinkinase(MAPK)pathways.
PhosphorylationofMAPKswillleadtothenextactivationstate:(4)upregulationofglialmediators,suchascytokines,chemokines,andgrowthfactors.
Uponrelease,theseglialmediatorscaninteractwithneuronstoelicitpainviacentralandperipheralsensitization.
Unlikeglialreaction(state1),theotheractivationstates(states2–4)havebeenshowntoinducepain.
Fig.
2.
Activationofmicrogliainthespinalcorddorsalhorn3daysaftersparednerveinjury(SNI)inrats.
(A)IB4staininginthespinalcorddorsalhornipsilateralandcontralateraltotheinjuryside.
NotealossofIB4staininginthedorsalhornregioninnervatedbytheinjurednervebranches.
(BandC)CD11b(OX-42)andphosphorylatedp38(p-p38)immunostaininginthedorsalhornipsilateralandcontralateraltotheinjuryside.
NoteoverlappingexpressionpatternsofOX-42andp-p38intheinjuryside.
(D)Doublestainingofp-p38(red)andOX-42(green)intheipsilateraldorsalhorn.
Lowerpanelpresentshigh-magnicationimagesof2microglialcells(indicatedbyarrowandarrowhead)fromtheupperpanel.
Notethatp-p38iscompletelyco-localizedwithOX-42.
Scale,100lm.
ImagesaremodiedfromWenetal.
[276],withpermission.
4R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022injury-evokedspinalmicroglialreactionisnotsoevident,inparal-lelwiththeabsenceofnerveinjury-inducedneuropathicpainintheseyounganimals[170,256].
Furthermore,priorneonatalinjurycan''prime''thespinalmicroglialresponsetoadultinjury,result-inginenhancedmicroglialreactivity[13].
Microgliacanalsobeprimedbypreviousinsultinadults,leadingtoenhancedpainintensityanddurationofthesecondinsult[87].
Comparedtomicroglialreaction,astrocytereactioninthespinalcordismoregeneralandevidentafterpainfulinjuries[69].
Robustastrocytereactionisinducednotonlybynervetraumaandspinalcordinjury[75,76,173,316],butalsobychronicopioidexposure[214],intraplantarorintra-articularCFAinjection[70,83,198,222],bone[98]andskincancer[67],chemotherapy,andhumanimmunodeciencyvirus(HIV)-inducedneuropathy[297].
Inaddition,itappearsthatastrocyticreactionismorepersis-tentthanmicroglialreaction.
IthasbeenshownthatGFAPandCD11bupregulationpeaksat150and14daysafternerveinjury,respectively,althoughCD11bupregulationremainsafter150days[298].
GFAPupregulationisalsoprominent9monthsafterspinalcordinjury[85,173].
Althoughmoststudieshavefocusedonglialreactioninthespinalcordandbrainstem,astrocytereactionhasalsobeenfoundintheforebrain,suchastheanteriorcingulatecor-tex,whichcontributestoaffectivepain[28].
OnecaveatisthatimmunohistochemistryofsomeGFAPantibodiesmaydetectcon-formationalorsolubilitychangesorpost-translationalmodica-tionsoftheproteinbutnotactualchangesinproteinexpression,becauseofdifferentxationconditions[15,56].
Thus,itisidealtovalidatetheresultsofGFAPimmunohistochemistrywithdiffer-entantibodiesanddifferentmethodssuchasWesternblotandquantitativepolymerasechainreaction(PCR).
LessisknownaboutSGCreaction(GFAPupregulation)afterpainfulinjuries.
SGCreactionisinducednotonlybynerveinjury[150,295]butalsobyinammation[235,236]inDRGsandTGs.
NerveinjuryfurtherresultsinSGCproliferation[107].
SGCsreac-tionafternerveinjuryandDRGcompressionisveryrapid,becom-ingevidentwithin4hours.
Thisreactionpeaksat1weekbutdeclinesafter3weeks.
ThistimecourseofSGCreactionsuggestsapossibleroleofSCGsintheinductionandearlymaintenanceofneuropathicpain[150,295].
AdministrationofglialtoxintoDRGshasbeenshowntoreduceneuropathicpain[150].
Also,thereisin-creasedcouplingbetweenSGCsafternerveinjury[185,295]andinammation[54].
Interestingly,evenacuteopioidtreatmentafterasubcutaneousinjectionresultsinmarkedSGCreactioninDRGsat2hourswhenmorphineanalgesiadeclines[18](Table1).
2.
2.
PhosphorylationofMAPKsandSrcingliaTheMAPKfamilyincludes3majormembers:extracellularsig-nal-regulatedkinase1and2(ERK1andERK2,respectively),p38,andc-JunN-terminalkinases(JNK)).
ERK5isanewfamilymemberandwasshowntobeactivatedinspinalmicrogliaafternervein-jury[177].
MAPKpathwaysplayanimportantroleinintracellularsignalinginneuronsandglia,andbotharerequiredforthegenesisofpersistentpain[109,178].
Interestingly,differentMAPKsexhibitdistinctactivation(phosphorylation)patternsinglialcellsafterpainfulinjuries[109](Table2).
Numerousstudieshaveshownincreasedphosphorylation(acti-vation)ofp38(P-p38)inspinalcordmicrogliaafternerveinjury[118,136,251](Fig.
2),spinalcordinjury[46,86],formalin-inducedacuteinammatorypain[229],surgery-evokedpostoperativepain[192,275],andchronicopioidexposure[47].
Nerveinjuryalsoacti-vatesmicroglialp38inthetrigeminalnucleus[194].
Ithasbeenshownthatthebisoformofp38(p38b)isexpressedinmicroglia[228].
Inaddition,P-p38isinducedinneuronsandSGCsofDRGsfollowingnerveinjury[118,179]andalsoinSGCsofTGsafterinammationinthetemporomandibularjoint[65].
P-JNKisinducedinspinalastrocytesafternerveinjury[316],CFA-inducedpersistentinammatorypain[70],bonecancer[267],andmelanoma-inducedskincancer[67].
Consistently,nerveinjuryalsoactivatestheupstreamactivatorofJNK,thetransform-inggrowthfactor-activatedkinase-1(TAK1),andthedownstreameffectorofJNK,c-Juninspinalastrocytes[125,316].
AmongseveralJNKisoforms(JNK1,2,3),JNK1wasshowntobeexpressedinspinalastrocytes[70].
P-ERKinductioningliaafterinjuryishighlydynamic:inductioninspinalmicrogliacorrespondstotheearly-phase(rstweek),andgraduallytransitionstoastrocytesinthelatephaseafternervein-juryandbonecancer[268,314].
CFAalsoinducesP-ERKinspinalastrocytesinthelatephase[279].
Furthermore,nerveinjuryevokesP-ERKinSGCsofDRGs[314],andtemporomandibularjointinammationelicitsP-ERKinSGCsofTGs[65].
MAPKsareactivatedbyproinammatorymediators[109]andinactivatedbyphosphatases,suchasMAPKphosphatase(MKP1,2,3).
Forexample,P-p38expressioninspinalmicrogliaafternerveinjurycanbesuppressedbyMKP3[171].
ActivationofCB2inmicrogliawasshowntoupregulateMKP1andMKP3,leadingtoareductionofP-ERKinmicroglia[202].
InammationinducesrapidupregulationofMKP1,MKP2,andMKP3inSGCsofTGs[65],whichmayregulatetheresolutionofinammatorypain.
MountingevidenceindicatesthatactivationofMAPKsinspinalcordglialcellsisessentialforthedevelopmentofpersistentpain[109].
Thus,intrathecalinjection(s)ofselectiveinhibitorsofMEK(ERKkinase),p38,andJNK,aswellasantisenseknockdownofERK5,attenuatedinammatory,neuropathic,andcancerpaininratsandmice[109].
Systemicinjectionofp38inhibitoralsore-ducedspinalnerveligation-inducedmechanicalallodyniainmice[113].
UpregulationofspinalMKP-3viagenetherapyattenuatesneuropathicpainbysuppressingP-p38[171].
TheimportanceofMAPKpathwaysforneuropathicpainhasalsobeendemonstratedinhumanbeings.
InHIVpatientswithneuropathicpain,P-ERK,P-p38,andP-JNKlevelsinthedorsalhornsaresignicantlyincreased,comparedtothoseinHIVpa-tientswithoutneuropathicpain[211].
Inadouble-blind,pla-cebo-controlledclinicaltrial,oraldeliveryofaselectivep38inhibitor,dilmapimod(SB-681323)attenuatedneuropathicpaininpatientswithnervetrauma,radiculopathy,orcarpaltunnelsyn-drome[6].
NerveinjuryalsoinducesphosphorylationofSrcfamilykinases(Src,Lyn,Fyn)inspinalmicroglia[126,250].
IntrathecalinfusionofaSrcinhibitor(PP2)reducednerveligation-elicitedneuropathicpain.
Ofinterest,PP2suppressedtheactivationofERKbutnotp38inspinalmicroglia[126].
2.
3.
Regulationofreceptors,channels,andtransportersingliaAsshowninTable3,multiplereceptors,channels,andtrans-portersareexpressedinglialcellsandareregulatedindifferentpainconditions.
Althoughthesemoleculesarenotsecreted,theyplayactiverolesinglialintracellularsignalingbyactivatingtheMAPKpathwaysandinducingthesynthesis,release,anduptakeofthesecretedmolecules(Table4).
ATPmodulatesglialactivationviaactivatingP2X(ionchannels)andP2Yreceptors(GPCR-coupled),andtheseATPreceptorsgatemicroglialsignalingforneuropathicpain[244,253].
PeripheralnerveinjuryupregulatesP2X4,P2X7,P2Y6,andP2Y12inspinalmicroglia;and,furthermore,neuropathicpainisreducedafterphar-macologicalinhibition,antisenseknockdown,orgeneticdeletionofP2X4,P2X7,P2Y6,orP2Y12[135–137,215,243,244,252,253].
MicelackingtheP2x4genedisplaydiminishedinammatorypainandbluntedneuropathicpain[249].
P2Y12isalsoinducedinSGCsofTGafternerveinjury,andinjectionofaP2Y12RantagonistintoTGreducestrigeminalneuropathicpain[124].
Moreover,chronicR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx5Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022opioidtreatmentupregulatesP2X4andP2X7inspinalmicroglia,andopioidtoleranceispreventedafterspinalknockdownofP2X4orP2X7[99,108,310].
However,arecentstudydemonstratedthatopioid-inducedhyperalgesiabutnottoleranceismediatedbyopioidreceptor-dependentexpressionofP2X4inmicroglia[60].
Toll-likereceptors(TLRs)areknowntoregulateinnateimmu-nityandhavebeenstronglyimplicatedinglialactivation[152,174].
Lipopolysaccride(LPS),anagonistofTLR4,ishighlypo-tentinactivatingmicroglia.
ItalsoactivatesTLR4inastrocytes[152].
Ofnote,spinalmicroglialreactionandneuropathicpainafternerveinjuryarereducedinTlr2knockoutmice[130]andTlr4mutantmice[238].
Arthriticpaininthelatephaseisalsore-ducedinTlr4knockoutmice[33].
Strikingly,malebutnotfemalemicewithTlr4mutationexhibitreducedneuropathicpain[168],suggestingsexdifferencesinTLR4andmicroglialsignaling.
Chronicmorphinewasshowntoinduceglialresponsesviaactiva-tionofTLR4[271].
Ofnote,opioid-inactiveisomerswereshowntoinducespinalproinammatoryresponsesviaactivationofTLR4[104].
PharmacologicalblockadeofTLR4signalinginvivoattenu-ateddevelopmentofanalgesictolerance,hyperalgesia,andopioidwithdrawalbehaviorsinrats[105].
Incontrast,Ferrinietal.
showedthatchronicmorphine-inducedhyperalgesiaisintactinTlr4mutantmice[60].
Microarrayanalysisrevealsthatthecomplementcomponents(eg,C1q,C3,C4,C5)areamongthemostregulatedtranscriptsinthespinalcordfollowingnerveinjury.
Inparticular,thesecompli-mentcomponentsareupregulatedinspinalmicroglia.
InductionofC5aRinspinalmicrogliahasbeenimplicatedinneuropathicpainsensitization[82].
Increasingevidencesuggeststhatchemokinereceptorscontrib-utetothepathogenesisofchronicpainviamodulatingglialactiva-tionandneuralplasticity[1,35,68,280].
CX3CL1(fractalkine)andCCL2(MCP-1)are2ofthemostwell-studiedchemokinesforpainmodulation.
Althoughachemokinenormallyactivatesmultiplereceptors,CX3CR1appearstobetheonlyknownreceptorforCX3CL1andisexclusivelyexpressedinmicroglia.
Thus,Cx3cr1-GFPmicehavebeenusedforstudyingthelocalizationandactiva-tionofmicroglia[175].
NerveinjuryandjointinammationinducearobustupregulationofCX3CR1inspinalmicroglia,andspinalblockadeofCX3CR1withaneutralizingantibodyinhibitedinam-matoryandneuropathicpain[165,222,257,315].
Consistently,micelackingCx3cr1exhibitedreducedinammatoryandneuro-pathicpain[219].
ComparedtoselectivemicroglialexpressionofCX3CR1,CCR2,amajorreceptorforCCL2,isexpressedinbothneu-ronsandmicroglia[2,72,81,84,121,305].
Nerveinjury-inducedspinalmicroglialreactionisabolishedinCcr2knockoutmice[300],whereasintrathecalCCL2causesmicrogliosisinthespinalcord[239,300].
NeuropathicpainisimpairedinCcr2knockoutmiceorafterspinalinjectionofCCR2antagonist[2,300,305].
Acti-vationofCCR2byCCL2alsorapidlymodulatesDRGneuronalsen-sitivityandspinalcordsynapticplasticity[72,81,315].
LikeLPS,interferon-c(IFN-c)isastrongactivatorofmicroglia,bymeansofinducingmicroglialreaction,P2X4upregulation,andLynphosphorylation[250].
NerveinjuryupregulatesINF-crecep-torsinspinalmicroglia,andnerveinjury-inducedmicroglialreac-tionandmechanicalallodyniaareabrogatedinIfncreceptorknockoutmice[250].
AstrocytesandSGCsarecharacterizedbyforminggapjunction-couplednetworks,leadingtothetransmissionofCa2+signalingthroughnetworks[7,54].
Connexinsarethemajorstructuralcom-ponentsofgapjunctions,andCx30andCx43areknowntobeex-pressedbyastrocytes[27].
Cx43isupregulatedinastrocytesafternervelesion,spinalcordinjury,andinammation[27,62,77,83,145].
Inhibitionofgapjunctionfunctionbycarbenox-olone(CBX),anonselectivegapjunctioninhibitor,reducesinam-matoryandneuropathicpain[140,218].
Inadditiontomodulatinggapjunctioncommunication,recentstudiesalsoproposedapara-crinesignalingofCx43toreleasekeyastrocyticmediatorssuchasATPandglutamate[123,148,240,262].
UnopposedCx43hemichan-nelsareidealformodulatingATPreleasepathways,asthebiophys-icalpropertiesofthesehemichannelsenablethemtoconducthighlevelsofATPefux[17,42,190].
Ofnote,SCI-inducedATPreleaseinthespinalcordisdiminishedafterCx43blockade[45].
IndoubleknockoutmicelackingCx30/Cx43,thedevelopmentofneuropathicpain(heathyperalgesiaandmechanicalallodynia)isprevented,andspinalastroglialreactionisreduced[27].
NerveinjurywasshowntoupregulateCx43inSGCsofTGs.
Ofinterest,reducingCx43expressioninSGCsviaRNAireducedneuropathicpaininnerve-injuredratsbutinducedpain-likebehaviorsinnormalrats,suggestingdifferentrolesofSGCs-Cx43inpainmodulationinnon-injuredvsinjuredanimals[183].
Notably,thegapjunctionblockerCBXalsoinhibitspannexin-1(PNX1),whichisexpressedinastro-cytesandmodulatesATPrelease[74].
TheroleofPNX1inpaincontrolneedsfurtherinvestigation.
Thefollowingionchannelshavealsobeenimplicatedforglialsignalinginpain.
TheK+channelsubunitKir4.
1isexpressedinSGCs,andsilencingthisK+subunitwithRANileadstopainhyper-sensitivity[261].
Thewaterchannelaquaporin-4(AQP4)isinducedinspinalcordastrocytesafterspinalcordinjury[173],andmicelackingAqp4displaydecreasedpainsensitivity(hypoalgesia)[9].
TRPM2isexpressedinmicrogliaandcontributestospinalcordmicroglialactivation.
Inammatoryandneuropathicpainareim-pairedinTrpm2knockoutmice[95].
TheglutamatetransporterssuchasGLT-1andGLASTareex-pressedinastrocytes(Table3)andregulatetheclearanceofgluta-matefromsynapticcleftsandextracellularspace,leadingtoalteredglutamatergictransmissionandneuronalplasticity[203,204].
Nerveinjuryandchronicmorphineelicitasustaineddown-regulation,afteraninitialupregulation,ofglutamatetrans-porter-1(GLT1)andglutamateandasparticacidtransporter(GLAST)inthespinalcord[158,224,288].
Inhibitionofglutamatetransportersresultsinanelevationinspinalextracellulargluta-mateandspontaneouspain[147,278].
Consistently,GLT-1genedeliverytothespinalcordattenuatesinammatoryandneuro-pathicpain[157],supportingaroleofastroglialglutamatetrans-portersintheresolutionofchronicpain.
Severalenzymesarealsoactivelyinvolvedinglialsignalinginpain.
Cyclooxygenase-1and-2(COX-1andCOX-2,respectively)areinducedinmicrogliaaftersurgicalincisionandnerveinjurytofacili-tatepostoperativeandneuropathicpain[306,312,313].
NADPHoxi-dase2(Nox2)expressionisinducedindorsalhornmicrogliaafterL5spinalnervetransection,andNox2-decientmiceshowedde-creasesinoxidativestress,microglialreaction,andproinammatorycytokineexpressioninthespinalcord,aswellasneuropathicpain[131].
Ofinterest,G-protein-coupledreceptorkinase(GRK2)inmicrogliawasimplicatedinthetransitionfromacutetochronicinammatorypain.
Spinalmicroglia/macrophageGRK2expressionisreducedafterinammation,leadingtotheactivationofmicrogliaandpersistentpainviap38andinterleukin-1b(IL-1b)signaling[283].
Furthermore,nerveinjuryupregulatesthetranscriptionalfac-torsinspinalcordglia,includingc-Juninastrocytes[316],signaltransducersandactivatorsoftranscription3(STAT3)inmicroglia[53]andastrocytes[248],andnuclearfactor-jB(NF-jB)inmicroglia[227]andastrocytes[167],toenhanceandmaintainneuropathicpain.
Finally,painfulinjuriesalsoinduceupregulationofanti-inam-matoryreceptorsingliafortheresolutionofacutepain.
Inamma-tionincreaseslipoxinreceptorALXexpressioninspinalcordastrocytes,andlipoxinA4reducesinammatorypainviainhibitingJNKphosphorylationinastrocytes[231].
LipoxinA4alsoattenu-atesmorphinetoleranceviamodulatingglialactivationandcyto-kineexpression[117].
Nerveinjuryincreasedcannabinnoid6R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022receptorCB2expressioninspinalmicroglia[299],andCB2agonistssuppressedmicroglialreactionandneuropathicpain[282].
OfinterestCb2knockoutmicedisplayedincreasedmicroglialandastrocyticreactivityinthespinalcordandenhancedneuropathicpain,whereastransgenicmiceoverexpressingCb2showedattenu-atedglialreactivityandneuropathicpain[196].
2.
4.
Regulationofcytokines,chemokines,growthfactors,andproteasesingliaAkeyissueregardingglialcontrolofpainistounderstandhowglialmediatorsareproducedandreleased.
AsshowninTable4,gliaproducebothlargemolecules(cytokines,chemokines,growthfactors,andproteases)andsmallmolecules(glutamate,ATP,D-serine,andprostaglandinE2(PGE2).
Theseglialmediatorscanmodulateneuronalandsynapticactivityandpainsensitivity.
Proinammatorycytokinessuchastumornecrosisfactor–a(TNF-a),IL-1b,andIL-6areamongthemostwell-studiedglialmediators.
Theyareupregulatedinspinalcordgliaafternervein-jury,inammation,bonecancer,andchronicopioidexposure,andtheycontributetothedevelopmentofandmaintenanceofinam-matory,neuropathic,andcanerpainandmorphinetolerance[52,213,230,273].
TNF-aisprimarilyproducedbymicrogliaandplaysanessentialroleinthegenerationofcentralsensitizationandpersistentpain[92,289,301,308],inadditiontoitswell-docu-mentedroleinmodulatingperipheralsensitization[119,207,216].
IL-1bisinducedinastrocytesafterbonecancer,inammation,andnerveinjury[83,274,279,303].
IL-1bcanalsobeproducedbymicrogliaandneuronsinthespinalcord[36,51,92].
InhibitionofspinalandbrainIL-1bsignalingreducesinammatory,neuro-pathic,andcancerpain[83,163,232,274,303]andenhancesmor-phineanalgesia[120,270].
IL-18ishighlyrelatedtoIL-1b,andbothrequirecaspase-1andinammasomesforactivecleavage[146].
NerveinjuryinducesIL-18expressioninspinalmicroglia[34,167].
Furthermore,painfulinjuriesinducecytokineexpressioninperipheralglia.
Forexample,nerveinjuryandCFAinammationincreaseIL-1bexpressioninSGCsofDRGsandTGs[127,234].
Ofnote,acutemorphineupregulatesIL-1bonlyinperipheralglia(SGCs)inDRGsbutnotincentralglia(microgliaandastrocytes)inthespinalcord[18].
Chemokinesareexpressedinglialcells,particularlyinastro-cytesintheCNS[68],aswellasinneurons[84].
Inprimarycul-turesofastrocytes,TNF-ainducedrapidexpressionofCCL2,CXCL10,andCXCL1[72].
SpinalinjectionofTNF-a-activatedastrocytesresultsinpersistentmechanicalallodyniaviareleasingCCL2[71].
SpinalnerveligationalsoinducesCCL2inspinalastro-cytes,andintrathecaladministrationofanMCP-1neutralizingantibodyreducesneuropathicpain[72].
CCL2expressionisfur-therincreasedinastrocytesofthemedullarydorsalhornandcon-tributestotrigeminalneuropathicpain[305].
Consistently,micewithCCL2overexpressioninastrocytesdisplaypainhypersensi-tivity[162].
Growthfactorsareknowntobeinducedinspinalgliabynerveinjury.
Inparticular,nerveligationupregulatesbrain-derivedneu-rotrophicfactor(BDNF)inspinalmicroglia,viaactivationofP2X4andp38[244,254].
SpinalinjectionofATP-activatedmicrogliaissufcienttoinducemechanicalallodyniaviareleasingBDNF,and,conversely,neuropathicpainissuppressedbyspinalblockadeoftheBDNFreceptorTrkB[43].
Furthermore,treatmentofmicroglialcultureswithmorphineincreasesBDNFrelease,whichdoesnotre-quirel-opioidreceptorandTLR[60].
BDNFisalsoinducedinDRGneuronsafternerveinjuryandcanbereleasedfromprimaryaffer-entsinthespinalcord[66,143].
UnlikeBDNF,basicbroblastgrowthfactor(bFGForFGF-2)isinducedinreactiveastrocytesofthespinalcordinthelatephase(3weeks)ofnerveinjury[110].
IntrathecalinfusionofbFGFproducespersistentactivationofspinalastrocytes(upregulationofP-JNKandGFAP)andsustainedmechanicalallodynia[110].
Bycontrast,intrathecaladministrationofabFGF-neutralizingantibodyattenuatesestablishedneuro-pathicpain[156].
Therefore,bFGFmaintainschronicpainviaacti-vationofastrocytes.
Proteasesarealsoupregulatedinspinalgliaafternerveinjury.
Notably,spinalnerveligationinducesmatrixmetalloprotease-2(MMP-2)inspinalcordastrocytesandDRGSGCsinthelatephaseofneuropathicpaintomaintainneuropathicpain,viaactivationofIL-1bandERK[127].
NerveinjuryfurtherinducescathepsinSinspinalmicroglia[37]andtissuetypeplasminogenactivator(tPA)inspinalastrocytes[138]toenhanceneuropathicpain.
Arecentstudyshowedthatnerveinjuryincreasestheexpressionofthrombospondin-4(TSP4),anextracellularmatrixglycoprotein,inspinalcordastrocytes.
Thisincreaseisnotonlycorrelatedbutalsorequiredforthedevelopmentneuropathicpain[132].
TSP4releasefromastrocytescanpromotesynaptogenesis.
Ofgreatinterest,thea2d-1calciumchannelsubunit,apossibletargetofgabapentin,wasshowntobeaneuronalreceptorofTSP4.
Thus,gabapentinmayinhibitneuropathicpainviamodulatingsynaptogenesis[58].
AstrocytesalsoproducesmallmoleculemediatorssuchasD-serine,ATP,andglutamatetoenhancepainstates[69].
Interestingly,inhibi-tionofglycinergictransmission,whichisknowntooccurinchronicpain,resultsinD-serinereleasefromastrocytestogeneratetactileallodynia[166].
D-serineisknownasanagonistofglycinesiteofN-methyl-D-aspartate(NMDA)receptors[176].
Inadditiontothepro-inammatoryandpronociceptivemedia-tors,glialcellsmayalsoproduceanti-inammatoryandantinoci-ceptivemediators,suchasIL-4,IL-10,andTGF-b[92]fortherecoveryandresolutionofpain[41,92,94,114,164].
Enhancementofendogenousproductionofinterleukin-10viagenetherapyhasbeenshowntoproducelong-termreliefinneuropathicpain[212].
Ofinterest,apossibleoff-targeteffectofhighdosesofsiR-NAsistoinduceIFN-ainspinalastrocytesforelicitingantinocicep-tiveeffects[237].
3.
Neuronal–glialandglial–glialinteractionsinpersistentpainBecausepainisconveyedonlybyneurotransmissionintheneu-ralcircuits,gliamustinteractwithneuronstomodulatepainsen-sitivity.
Herewefocusonneuronal–glial(neuronal–glial)(Section3.
1)andglial–glial(Section3.
2)interactionsintheCNSunderpersistentpainconditions(Fig.
3).
Wealsodiscussneuro–glialinteractionsinthePNSafterpainfulinjuriesandacutemor-phinetreatment(Section3.
3)(Fig.
4).
3.
1.
Neuronal–glialinteractions:SignalsfromneuronstogliaItisgenerallybelievedthatinjury-inducedspontaneousdis-chargefromprimaryafferentsdrivesneuropathicpain[149,155,286].
Severallinesofevidencesuggestthatnervein-jury-releasedsignalingmoleculesfromprimaryafferentcentralterminalstriggermicroglialactivation(Fig.
3).
Abrief,low-frequencyelectricalstimulationoftheperipheralC-berswasshowntoinducespinalmicroglialreactionwithoutcausingnotice-ablenerveinjury[97].
Sustainednerveblockadeviabupivacainemicrospherespreventednerveinjury-inducedmicroglialresponses(CD11bexpressionandP-p38induction)[276,287].
However,inhi-bitionofC-beractivityaloneinthesciaticnervewithresininfera-toxinmaynotbesufcienttopreventsparednerveinjury-inducedmicroglialactivation[226],suggestingpossiblecontributionoflargeA-bers.
Consistently,deletionofvesicularglutamatetrans-porter-2(vGluT2)inNav1.
8-expressingnociceptorsdidnotpre-ventnerveinjury-inducedspinalmicroglialreaction,suggestingthatglutamatereleasefromnociceptorsmaynotbesufcienttoR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx7Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022drivemicroglialreaction[208].
Althoughspontaneousactivityisimportantfortheinitiationofmicroglialactivation,itisnotsocrit-icalforthemaintenanceofmicroglialactivation[276].
Chemokines,suchasCCL2,CCL21,andCX3CL1,areidealformediatingneuronal–microglialinteractions,giventhedistinctexpressionoftheirligandsandreceptors.
NerveinjuryinducesCCL2andCCL21expressioninDRGneurons[19,281,298].
Stimula-tionofthedorsalrootresultsinactivity-dependentCCL2releaseinthespinalcord[239,255].
ChemokinesmayactivatemicrogliaviaP2X4signaling:CCL2inducesthesurfacetrafckingofP2X4[242],andCCL21increasestheexpressionofP2X4[19].
ActivationofP2X4resultedinBDNFexpressionandreleasefrommicrogliaviap38activation[245].
Proteaseshavealsobeenimplicatedinmicroglialactivation.
NerveinjuryinducesrapidandtransientupregulationofMMP-9inDRGneurons,whichisessentialfortheearly-phasedevelopmentofneuropathicpain[115].
Activity-dependentre-leaseofMMP-9fromprimarysensoryneuronswasimplicatedinmicroglialactivation,inpartthroughIL-1bcleavage[127].
Cathep-sinSisalsoinvolvedinmicroglial–neuronal–microglialsignaling.
Nerveinjury-evokedreleaseofcathepsinSfrommicrogliaresultsinfurtheractivationofmicroglia,throughthecleavageandreleaseofCX3CL1fromprimarysensoryneurons[35,37].
Thegrowthfactorneuregulin-1(NRG1)playsanactiveroleinmicroglialactivation.
AlthoughNRG1isexpressedinDRGneurons,itsreceptor,erbB2,isexpressedinmicroglia.
NRG1wasshowntoFig.
3.
Schematicofneuronal–glialandglial–glialinteractionsinthespinalcordinpersistentpain.
Spontaneousdischargeafterapainfulinjury(eg,nerveinjury)resultsinthereleaseofATP,chemokines(CCL2,CCL21,CX3CL1),MMP-9,NRG1,andCRGPfromprimaryafferentcentralterminals,leadingtoactivationofmicrogliainthedorsalhorn.
SpinalmicrogliaexpressthereceptorsforATP(P2X4,P2X7,P2Y6,P2Y12),andchemokines(CX3CR1,CCR2),andNRG1(ErB2).
Activationofthesereceptorsinducesphosphorylationofp38andERK(earlyphase)inmicroglia,leadingtotheproductionandreleaseoftheproinammatorycytokines(TNF-a,IL-1b,IL-18)andthegrowthfactorBDNF,andtheconsequentsensitizationofdorsalhornneurons.
Astrocytescanbeactivatedbymicroglialmediators(TNF-aandIL-18),aswellasastrocyticmediators(matrixmetalloprotein-2(MMP-2)andbFGF).
SubsequentphosphorylationofJNKandP-ERKinastrocytesresultsintheproductionandreleaseofchemokines(eg,CCL2)andcytokines(eg,interleukin-1b[IL-1b]).
Astrocytesalsoproduceadenosinetriphosphate(ATP)andglutamateaftertheactivationofthehemichannels(Cx43andPNX1).
Afternerveinjury,downregulationofastrocyticGLT1resultsindecreaseinastrocyticuptakeofglutamate.
Releaseofastrocyticmediators(CCL2,interleukin-1b[IL-1b],glutamate)canelicitNMDAR-mediatedcentralsensitization.
Releaseofadenosinetriphosphate(ATP)andCCL2fromastrocytescanfurthermaintainmicroglialactivation.
8R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022stimulatemicroglialproliferation,chemotaxis,andIL-1breleaseviaerbB2[26].
BlockadeoftheerbB2receptororsequestrationofendogenousNRG1reducesnerveinjury-inducedmicroglialproliferation,p38activation,andneuropathicpain[26].
NRG1alsoinducesmicroglialproliferationviaphosphorylationofERKandAKT[23].
Inaddition,releaseoftheneuropeptideCGRPfrompri-marysensoryneuronsisnotonlyinvolvedinneurotransmissionbutalsocontributestomicroglialactivationafterchronicmor-phineexposure[269].
p38MAPKservesasakeysignalingmoleculeinmicrogliabyintegratingvariousinputtomicroglia[112].
Microgliap38isacti-vatedbyATP[79],TNF-a[230],andIL-1b[225].
Afternerveinjury,p38isphosphorylatedfollowingtheactivationofmultiplerecep-tors,suchasATPreceptors(P2X4andP2Y12)[136,245]andchemo-kinereceptors(CCR2andCX3CR1)[2,315].
Microglialp38isalsoactivatedbyCGRPinchronicmorphine-inducedtolerance[269].
Notably,minocyclineinhibitsmicroglialactivationbyinhibitingspinalmicroglialp38activationafterinammationandchronicmorphinetreatment[48,100].
Uponactivation,p38inducesthesynthesisandreleaseofmicroglialmediatorsTNF-a,IL-1b,andBDNF[277].
Althoughp38iscriticalforthesynthesisandreleaseofinammatorymediators,ithasalimitedroleinmorphologicalchanges(microgliosis)andproliferationofmicroglia,whichcouldbemediatedbyanotherMAPKfamilymember,ERK[25].
Neuronalsignalsarealsoimportantfortheactivationofastro-cytes.
Forexample,neuronalactivityappearstodriveastrocyteactivationafternerveinjury[287]andinammation[264].
Basicbroblastgrowthfactor(bFGForFGF-2)isinducedinprimarysen-soryneuronsafternerveinjuryandhasanactiveroleinneuron–astrocytesignaling.
Asawell-knownactivatorofastrocytes,bFGFelicitsmitosis,growth,differentiation,andgliosisofastrocytes[110].
NerveinjurynotonlyinducesbFGFinDRGneurons[116]butalsoproducesadelayedbFGFupregulationinastrocytesformaintainingneuropathicpain[110].
3.
2.
Glial–glialinteractionsAstrocyticreactionisoftenprecededbymicroglialreaction,andmicroglialactivationisknowntodriveastrocyteactivation[197].
TNF-a,akeysignalmoleculeproducedbymicroglia,causesrapidJNKactivationinastrocytes[72].
Ofinterest,nerveinjuryelicitsIL-18andIL-18Rexpressioninspinalmicrogliaandastrocytes,respectively,andIL-18releasedfrommicrogliawasshowntoactivateIL-18RinastrocytestoupregulateNF-jBandfacilitateneuropathicpain[167].
Ontheotherhand,astrocytescanalsoreleasesignalingmole-culestoactivatemicroglia.
Afterspinalcordinjury,Cx43isupreg-ulatedandgainsanewfunctionofparacrinesignaling,leadingtothereleaseofATPandglutamate[123,148,240,262].
IncreasesinFig.
4.
Glialmediatorsmodulateexcitatoryandinhibitorysynaptictransmissioninthespinalcord.
(A)Modulationofexcitatorysynaptictransmissionatpresynaptic,postsynaptic,andextrasynapticsitesbyglialmediators.
Presynaptically,tumornecrosisfactor-a(TNF-a),interleukin-1b(IL-1b),CCL2,interferon-c(IFN-c),andTSP4increaseglutamatereleasetoenhanceEPSCfrequency.
Postsynaptically,IL-1bTNF-a,andCCL2increaseAMPARactivity.
Extrasynaptically,TNF-a,IL-1b,CCL2,andD-serineincreaseNMDAR-NR2BactivityandenhanceNMDA-inducedcurrents.
Astrocyte-releasedglutamatecanfurtherinduceNR2B-mediatedinwardcurrentsinsurroundingneurons.
(B)Modulationofinhibitorysynaptictransmissionatpresynaptic,postsynaptic,andextrasynapticsites.
Presynaptically,IL-1bandIL-6decreaseGABAandglycinereleasetodecreaseIPSCfrequency.
Postsynaptically,IL-1bdecreasesGABA/GlyRactivityandIPSCamplitude.
ProstaglandinE2(PGE2)inhibitsevokedglycinecurrent.
Extrasynaptically,IL-1b,CCL2,andIFN-csuppressGABA-and/orglycine-inducedcurrents.
TNF-ainhibitsactionpotentialsininhibitoryneurons.
InlaminaIneurons,BDNFproducesdisinhibitionbyalteringchloridereversepotential.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx9Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022extracellularATPhavebeendocumentedinawiderangeofperiph-eralandcentralnervoussysteminjuries,suchassciaticnerveentrapment[159],traumaticbraininjury[50,64],andspinalcordinjury[191,265].
ATPiscriticalfornerveinjury-evokedmicroglialactivationviaactivationofP2X4,P2X7,P2Y6,andP2Y12receptors[244,253].
Ofnote,CCL2isinducednotonlyinprimarysensoryneuronsbutalsoinastrocytes[72,281].
AlthoughDRG-CCL2inducesmicroglialactivation,astrocytic-CCL2maymaintainmicroglialactivation.
IFN-c,astrongmicroglialactivatorandneu-ropathicpaininducer[250],isalsoproducedbyastrocytes[196].
Finally,microgliaandastrocytescouldbeself-activatedviaautocrineorparacrinesignals.
Forexample,bFGFisupregulatedinspinalastrocytesafternerveinjurytomaintainastrocyteactiva-tion[110].
Nerveinjury-inducedastrocyticMMP-2upregulationinthelatephasecanmaintainastrocyticactivationandneuropathicpainthroughIL-1bcleavage(activation)andphosphorylationofERKinastrocytes[127].
3.
3.
Neuronal–glialinteractionsindorsalrootandtrigeminalgangliainthePNSSGCsinDRGsandTGsaretightlyassociatedwithsensoryneu-ronsviagapjunction;andgapjunctioncommunicationbetweenSGCsandSGCandneuronsisgreatlyenhancedinpersistentpainconditions[54,90,91].
Nerveinjury-inducedSGCactivationre-quiresneuronalactivityandlocalinammation[144,287].
Puriner-gicsignalingiscriticallyinvolvedinneuronal–glialcommunicationinDRGs[29,304].
Forexample,activity-dependentATPreleasefromneuronalsomaactivatesP2X7inSGCs[304],leadingtoTNF-areleasefromSGCs,whichcaninturnactonsurroundingneuronstoincreasetheirexcitability[119,216](Fig.
4).
ATPcanalsobereleasedfromSGCstoactivateP2X3receptor,whichisex-pressedinprimarysensoryneuronsandplaysanimportantroleinperipheralsensitization[40,217].
Ofnote,MMP-9mediatesneuron–SGCinteractioninDRGsafteracutemorphinetreatment,whichcanmaskmorphineanalgesia[153](Fig.
5).
Systemicmorphineadministrationwasshowntoeli-citrapidMMP-9upregulationinDRGneuronsintherecoveryphaseofmorphineanalgesia(2hours),whichrequiresactivationofl-opioidreceptors[153].
Notably,morphineanalgesiaisen-hancedandprolongedinMmp9knockoutmice[153].
Acutemor-phinealsoupregulatesGFAPandIL-1binSGCsofDRGs,andbothrequireMMP-9[18].
MMP-9releasefromneuronsresultsinIL-1bcleavageandrelease,whichinturnactivatesIL-1breceptorsinsensoryneuronstoelicitactionpotentials[20].
IL-1bisknowntoincreasetheexcitabilityofsensoryneuronsviaenhancingso-diumcurrentsandsuppressingpotassiumcurrents[20,233,236].
Ofinterest,IL-1bhasalsobeenshowntomaskmorphine-inducedanalgesia[103,120].
Thus,targetingperipheralneuronal–glialinteractions,inadditiontopreviouslyrecognizedcentralneuro-nal–glialinteractions,canalsoenhanceopioidanalgesia.
4.
GlialmediatorsmodulateexcitatoryandinhibitorysynaptictransmissionAkeyissueregardingglialcontrolofpainishowglialmedia-torsregulatesynaptictransmission.
Strikingly,glialmediatorscanmodulatespinalcordsynaptictransmissionatverylowconcen-trations.
Althoughneurotransmitters(eg,glutamate,GABA,gly-cine,andsubstanceP)normallyregulateneuronalandsynapticactivityatmicromolarconcentrations,glialmediators(cytokines,chemokines,andgrowthfactors)canchangesynapticactivityatnanomolarconcentrationsinvitro[43,72,128].
Inparticular,glialmediatorscanmodulatebothexcitatoryandinhibitorysynaptictransmission(Fig.
5).
Althoughmoststudiesusedyoung(3-to5-week-old)andadultanimals(ratsandmice)forrecordingspinalneuronalactivities[43,72,128,189,296],somestudiesusedneonatalanimals[73,81,259].
Itiswellknownthatthegeneexpressionprolesofprimarysensoryandspinalcordneurons,glialresponses,aswellasspinalcordpaincircuitsundergodra-maticchangesintherst2weeksafterbirth[16,61,172].
Thus,cautionmustbetakentointerpretthedatafromneonatalanimals.
4.
1.
ModulationofexcitatorysynaptictransmissionGlialmediatorscanmodulateexcitatorysynaptictransmissionviapre-,post-,andextrasynapticmechanisms(Fig.
5).
Theeffectsofproinammatorycytokinesandchemokinesonexcitatorypost-synapticcurrents(EPSCs)havebeenexaminedinlaminaIIneuronsusingexvivospinalcordslicepreparations[293].
AlthoughtheEPSCfrequencychangemayresultfrompresynapticmechanisms(duetoglutamatereleasefrompresynapticterminals),theEPSCamplitudeincreaseiscausedbyenhancedsignalingofglutamatereceptors(AMPAsubtype)inpost-synapticsites.
IncubationofspinalcordsliceswithTNF-a,IL-1b,andCCL2veryrapidly(withinminutes)increasedspontaneousEPSC(sEPSC)fre-quency[72,128,296].
ChronicexposureofcultureddorsalhornneuronstoIFN-calsoincreasedsEPSCfrequency[260](Fig.
5A),supportingapossiblepresynapticmodulation.
TNF-aincreasessEPSCfrequencyviaactivationofTRPV1inpresynapticterminals,asthissEPSCincreaseisabolishedinTrpv1knockoutmice.
Sin-gle-cellPCRanalysisindicatesthatTNF-a-respondinglaminaIIinterneuronsareexclusivelyexcitatoryones,becausetheyallex-pressvesicularglutamatetransporter-2(vGluT2).
TheselaminaIIneuronsalsoreceiveinputfromTRPV1-expressingC-bersandmakesynapsestolamina-Iprojectionneurons[241],formingaFig.
5.
Schematicrepresentationofneuronal–glialinteractionsindorsalrootandtrigeminalgangliaoftheperipheralnervoussystem(PNS).
Spontaneousneuronaldischargeafterpainfulinjuryresultsinadenosinetriphosphate(ATP)releaseinneuronalsomata,leadingtotheactivationofP2X7andsubsequentreleaseoftumornecrosisfactor-a(TNF-a)insatelliteglialcells(SGCs).
Persistentnociceptiveactivityoractivationofopioidreceptorsbymorphinealsoresultsinmatrixmetalloproteinase-9(MMP-9)releasefromprimarysensoryneurons,causingthecleavage(activation)andreleaseofinterleukin-1b(IL-1b)inSGCs.
TNF-aandIL-1bbindrespectiveTNFRandIL-1Ronsensoryneuronstoelicithyperexcitability.
SGCscanalsoreleaseATPviahemichannels(Cx43andPNX1)orgapjunctioncommu-nicationtoactivateP2X3inneuronsfortriggeringperipheralsensitization.
Inaddition,SGCsexpressKir4.
1tomaintainhomeostasisofextracellularK+levelsofsensoryneurons,andinjury-induceddownregulationofKir4.
1inSGCswilldisruptthisK+homeostasisandgenerateneuronalhyperexcitability.
10R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022spinalcircuittomediateTNF-a-inducedpain.
ArecentstudyalsodemonstratedthatTSP4,producedbyastrocytes,increasedsEPSCfrequency[132].
GlialmediatorssuchasIL-1bandCCL2alsoincreasetheampli-tudesofsEPSCs,viaAMPA-mediatedpostsynapticmechanisms(Fig.
5A).
TNF-aisknowntoinducethetrafckingandsurfaceexpressionofAMPAreceptorsinhippocampalneurons[12,220].
Afterspinalcordinjury,TNF-ainducesrapidtrafckingofGluR2-lackingAMPARstotheplasmamembraneinspinalcordmotorneurons[59].
Ofnote,inammationinducesaTNF-a–dependentsurfacetrafckingofGluR1-AMPARsinthedorsalhorn[32].
AlthoughTNFR1isthepredominantreceptormediatingtheeffectsofTNF-a,bothTNFR1andTNFR2arerequiredfortheinductionofcentralsensitization[301,309].
Pro-inammatorycytokinesandchemokinesfurtherinducecentralsensitizationviaextrasynapticmechanisms(Fig.
5A).
NMDAcurrentsinlaminaIIneurons,inducedbybathapplicationofNMDAtospinalcordslices,areenhancedbyIL-1b,TNF-a,orCCL2[72,128].
TNF-aincreasesNMDAreceptor(NMDAR)activitythroughphosphorylationofERKindorsalhornneurons[292].
IL-1binducesphosphorylationoftheNR1subunitinspinalcordneurons[302].
AstrocyticD-serineenhancesNMDAcurrentsviabindingtheglycinesiteofNMDAreceptors[201].
Interestingly,astrocyticglutamatereleasecanbedetectedasslowinwardcur-rents,viapatch-clamprecordingsinnearbyneurons.
SlowinwardcurrentsaremediatedbyextrasynapticNR2Breceptorsandin-ducedinspinaldorsalhornneuronsafterinammation[10].
4.
2.
ModulationofinhibitorysynaptictransmissionReductionorlossofinhibitorysynaptictransmission(disinhibi-tion)inthespinalcordpaincircuithasbeenstronglyimplicatedinthegenesisofcentralsensitizationandchronicpain[8,44,169,294].
Disinhibitionafterperipheralnerveinjuryinvolvesatrans-synapticreductionintheexpressionofthepotassium-chlo-rideco-transporterKCC2andsubsequentdisruptionofanionhomeostasis(chloridehomeostasis)inspinallaminaIneurons.
Insomecases,theshiftinthetransmembraneaniongradientcanconvertnormallyinhibitoryanionicsynapticcurrentstobeexcit-atory[44].
GlialmediatorssuchasBDNF,cytokines,chemokines,andPGE2canalsomodulateinhibitorysynaptictransmissionviapre-,post-,andextrasynapticmechanisms(Fig.
5B).
Presynaptically,IL-1bandIL-6wereshowntoinhibitthefrequencyofspontaneouspostsyn-apticcurrents(sIPSCs)inspinallaminaIIneurons[128].
Postsyn-aptically,IL-1breducesthesIPSCamplitude[128].
PGE2inhibitsglycinergicneurotransmissioninthedorsalhornviapost-synapticGlyR3andthecAMP/PKApathway[5,96].
Atextrasynapticsites,GABAandglycinecurrents,inducedbybathapplicationofGABAandglycine,canbesuppressedbyIL-1bandIL-6[128].
BDNFactsonspinallaminaIneuronstoreverseGABAinhibitionbyalteringchloridereversepotential[43].
Fur-thermore,ATPormorphine-stimulatedmicrogliaresultinadepo-larizingshiftintheanionreversalpotentialbyreleasingBDNF[43,60].
Likenerveinjury,administrationofATP-stimulatedmicrogliaorpharmacologicaldisruptionofchloridetransportinvivoalterthephenotypeofspinallaminaIoutputneurons,lead-ingtoneuropathicpainphenotypes[129].
TNF-awasalsoshowntosuppressactionpotentialsinGAD67+inhibitoryneuronsinspinalcordslices[296].
Moreover,CCL2andIFN-cinhibitGABA-inducedresponsesinspinalcordneurons[81,259].
Itremainstobeinvestigatedhowanti-inammatorycytokines(eg,IL-4,IL-10,TGF-b)regulatesynapticplasticity.
ItappearsthatIL-10cansuppressTNF-a-inducedsynapticplasticity(unpublishedobservations).
Inparticular,theanti-inammatorylipidmediatorssuchasresolvinE1(RvE1)andneuroprotectin(NPD1)blockedTNF-a-inducedsynapticplasticity(sEPSCfrequencyincrease)[292].
NPD1andRvD2furtherreversedinammation-inducedsynapticplasticityandtetanicstimulation-inducedspinallong-termpotentiation(LTP)[189].
Finally,theproinammatorycytokinesTNF-a,IL-1b,andIL-6alsoelicitlong-termneuronalplasticityinthepaincircuitbyinducingthephosphorylationofthetranscriptionfactorcAMPre-sponseelement-bindingprotein(CREB),leadingtothetranscrip-tionofCREB-mediatedpronociceptivegenes(eg,cyclooxygenase-2[COX-2],neurokinin-1[NK-1])inspinalcordneurons[111,128,206].
Ofnote,TNF-aissufcienttoinducespinalLTPafternerveinjury[154],andtetanicstimulation-inducedspinalLTPisabolishedinTNFR1orTNFR2knockoutmice[188].
4.
3.
ConcludingremarksInthepastdecadegreatprogresshasbeenmadetodemon-stratecriticalrolesofglialcells,suchasmicroglia,astrocytes,andSGCsinthegenesisofpersistentpain.
Asevidenceemerges,thelistofglial-derivedsignalingmoleculesandmediatorscontin-uestogrow(Tables1–4).
Gliacancommunicatewithneuronsby''listening''and''talking''toneurons.
Itisincreasinglyappreciatedthatchronicpaincanmanifestnotonlybyneuralplasticitybutalsobydysfunctionofglialcells.
Underthenormalphysiologicalconditions,astrocytesandSGCsprovidetrophicsupporttoneuronsandmaintainthehomeostasisofK+,glutamate,andH2OinCNSandPNS[258].
AstrocytesandSGCscouldalso''insulate''theneuralcircuitofpainbyformingastructuralbarrierandkeepthecircuitsilentbyreleasinginhibitorymediators[172].
Nervein-jury-inducedchronicpainisassociatednotonlywithneuropathybutalsowith''gliopathy.
''AstrocyteslosetheirabilitytomaintainthehomeostasisofK+andglutamate,leadingtoneuronalheperex-citability,asaresultofhigherextracellularlevelsofglutamateandK+.
Dysfunctionofastrocyticwaterchannel(AQP4)willalsoresultinedemaintheCNSandPNS[258].
Asaresultofgliopathy,gliacannolongerinsulatethepaincircuit;insteadtheyserveasanampli-erofpain,byproducingproinammatoryandpronociceptivemediators.
PainfulinjuriesevokerapidreactionofSGCsinthePNS,fol-lowedbymicroglialandastrocyticreactionintheCNS.
Moststud-iesongliaandpainfocusonmicrogliaandastrocytesinthespinalcord.
Uponactivation,presumablyinitiatedbyneuronalsignals,gliasynthesizeandreleaseproinammatoryandpronociceptivemediators(eg,proinammatorycytokinesandchemokinesandgrowthfactors)toenhancepainstates,viaactivationofkeysignal-ingpathways,suchastheMAPkinasepathways.
Activationofhemichannels(eg,Cx43andPNX1)andP2X7resultsinthereleaseofATPandglutamatefromastrocytes.
Importantly,glialmediators(eg,TNF-a,IL-1b,IL-6,CCL2,BDNF)canpowerfullymodulateexcit-atoryandinhibitorysynaptictransmissionatcomparablylowerconcentrations.
Glialmediators(ATP,CCL2,IFN-c,bFGF,MMP-2)alsoresultinfurtheractivationofglialcellsviaparacrineorauto-crineregulation.
Lastbutnottheleast,gliamayalsoproduceanti-inammatoryandantinociceptivemediatorsfortheresolutionofacutepain.
Furtherinquiryisneededtodeterminewhetherfailureintheproductionoftheseresolutionmediatorsleadstothetransi-tionfromacutepaintochronicpain.
5.
Remainingquestionsandfuturedirections5.
1.
IsglialactivationassociatedwithpainDespitethegrowingimportanceofglialcellsinpainregulation,''glialactivation''isnotwelldened.
Moststudiesintheelduseglialreaction(upregulationoftheglialmarkersIBA1,CD11b,andR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx11Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022GFAPtodenetheactivationofmicroglia(IBA1/CD11b),astrocytes(GFAP),andSGCs(GFAP)(Table1).
Althoughtheupregulationofthesemarkersisassociatedwithpainbehaviors,especiallyintheinductionphase,thereareseveralcaveatsrelatedtothesemarkers.
First,dissociationbetweenmicroglialmarkerexpressionandpainbehaviorshasbeenreportedbydifferentgroups[21,38,307].
Com-paredtomicroglialmarkers(IBA1andCD11b),theastrocyticmar-kerGFAPisbettercorrelatedwithpainbehaviors,especiallyafterinammation,bonecancer,chemotherapy,andHIVneuropathy[98,211,297,307].
Second,weshouldnotexcludemicroglialactiva-tionifthereisnochangeinIBA1expression.
Glialactivationcanalsomanifestasquickresponses,suchasCa2+changesandphos-phorylationofsignalingmolecules(eg,MAPKs)thatcouldoccurwithinminutesafterastimulationorinsult.
Indeed,sensorywhis-kerstimulationwasshowntoevokerapidincreases,withinseveralseconds,inastrocyticcytosolicCa2+inthebarrelcortexofadultmice[266].
Third,evenundertheactivationstateswithupregula-tionofglialmarkersandhypertrophy,microgliacouldstillhavedifferentfunctionalstatesbyexhibitingeitherpro-inammatory(neurotoxic,M1)andanti-inammatory(neuroprotective,M2)phenotypes[93,284].
Finally,andimportantly,glialreactivityandmorphologicalchangesdonotdirectlymodulatepain.
Neuronalactivityandpainsensitivityarecontrolledbytheglialmediators(cytokines,chemokines,ATP,BDNF,glutamate).
Thus,theregula-tionofglialsignalingmoleculesandglialmediatorsafterpainfulinjuries(Tables2–4)couldbebetterassociatedwithpainstatesthanglialreactivity.
5.
2.
CanwetargetgliaforpaintherapyHowcanwedesigndrugstotargetglialactivityforpaincon-trolDowereallyneedglia-selectivedrugsIndeed,itisextremelydifculttodesigndrugsthattargetonlyglialcellswithoutaffect-ingneurons.
Furthermore,eliminationofglialcellswithglia-selec-tivetoxinsmaycausedetrimentaleffects,giventhesupportiveandprotectiverolesofglia.
Instead,therearealternativestrategies:(1)totargettheMAPKsignalingpathways(ERK,p38,JNK),hemichan-nels(eg,Cx43andPNX1),orP2X7tosuppressthereleaseofglialmediators;(2)totargettheupstreamactivatorsofglia,suchasP2X4,P2Y6/12,MMP-9/2,andcathepsinS;and(3)totargetthedownstreammediatorsreleasedbyglia,suchasTNF-a,IL-1b,IL-6,orBDNF.
Weshouldlearnlessonsfromrecentfailuresin2clinicaltrials:1trialwithaglialmodulator,propentofylline,whichshowednoefcacyinreducingneuropathicpaininpatientswithpost-her-peticneuralgia[141];anothertrialwithaCCR2antagonistAZD2423,whichshowednosignicanteffects,comparedtopla-cebo,inpost-traumaticneuralgiapatients[122].
Thefailuresmayresultfrommultiplereasons,includinglackoftranslationfromrodentstohumanbeings,differentwaysofpainmeasurementinrodentsandhumanbeings(evokedpainvsspontaneouspain),anddifferentpainconditionstestedinrodentsandhumanbeings(nervetrauma-inducedpainhypersensitivityinseveralweeksvspost-herpetic/traumaticneuralgiaaftermanyyears).
Ofnote,pro-pentofyllineisawell-knowninhibitorofphosphodiesterase,andthereforecouldaltercAMPlevelsinglialandnon-glialcells[63].
Propentofyllineisalsoanadenosineuptakeinhibitor[63].
Com-paredtothecompletelackofeffectofpropentofylline,AZD2423(150mg)showedsometrendstowardreductioninparoxysmalpainandparesthesia/dysesthesia,indicatingthataCCR2antago-nistmayhavesomepossibleeffectsforsomesensorycomponentsofpain[122].
Notably,thevariabilitybetweenandwithinindivid-ualswasveryhigh,inpartbecauseofthenatureofamulticentertrial.
ItisalsoaconcernthatinhibitionofglialresponsesintheCNScannotbevalidatedinthistrial,becauseofthelackofeffectiveimagingtechniquefordetectingglialresponses(seeSection6.
3).
Theoretically,itshouldbemoreeffectiveforadrugtotargetbothneuronsandgliaforpainrelief.
Forexample,p38isactivatedbothinspinalcordmicrogliaandDRGneurons,andsystemicp38inhibitorhasbeenshowntoalleviateneuropathicpaininaclinicaltrial[6].
Recentstudieshavedemonstratedthattheanti-inamma-toryandpro-resolutionlipidmediatorssuchasresolvins(RvD1,RvD2,RvE1),protectins/neuroprotectins(PD1/NPD1),andlipoxins(LXA4)couldpotentlyreduceinammatoryandpostoperativepain,atverylowdoses[101,114,231].
Peri-surgicalapplicationofPD1/NPD1effectivelyprotectsnervetrauma-inducedneuropathicpainandspinalcordglialactivationinmice[291].
RvE1andPD1furtherinhibitglialactivationincultures[290,291].
Thereceptorsofthesemediators,suchasChemR23(RvE1)andALX(RvD1andLXA4)arewidelyexpressedinneurons,glia,andimmunecells[39,114,210,231].
Thus,theselipidmediatorsnotonlyinhibitglialactivationandinammationbutalsoinhibitTRPchannels(eg,TRPA1/V1)andreversesynapticplasticityinneurons[114,188,189].
Giventhepotencyandsafety,theseendogenousli-pidmediators,ortheiranalogs,orsmall-moleculeagonistsoftheirFig.
6.
Glialbrillaryacidicprotein(GFAP)immunostainingofmouse,rhesusmonkey,andhumanastrocytesincortex.
Notestrikingdifferencesinthesizesofmouse,monkey,andhumanastrocytes.
Alsonotedifferencesinthenumberandlengthsofbranchesofastrocytesfrommouse,monkey,andhumanbeing.
Sizesofastrocytesincreasewithincreasingcomplexityofbrainfunction.
Scale,50lm.
ImagesarereproducedfromKimelbergandNedergaard[133],withpermission.
12R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022receptors,couldbedevelopedforpreventingandtreatingchronicpain,viatargetingbothneuronalandnon-neuronal(immuneandglial)mechanisms.
5.
3.
HowmuchdoweknowabouthumangliaLittleisknownabouttheroleofhumangliainpaincontrol.
In-deed,astrocytesfrommice,monkeys,andhumanbeingsarequitedifferentintheirsizes[180,182](Fig.
6).
ThehumanbrainappearstocontainsubtypesofGFAP-positiveastrocytesthatarenotrepre-sentedinrodents.
Inhumancortex,astrocytesaremorethan2-foldlargerindiameterandextend10-foldmoreGFAP-positiveprimaryprocessesthantheirrodentcounterparts(Fig.
6).
Thedomainofasinglehumanastrocytehasbeenestimatedtocontactupto2mil-lionsynapses[133,180].
Remarkably,humanglialprogenitorcells(GPCs),afterbeingimplantedintoneonatalimmunodecientmice,aregapjunction-coupledtohostastroglia,propagateCa2+signals3-foldfasterthantheirhosts,andexhibitenhancedLTPandlearn-ingcapability[88].
Hence,humanastrocytescouldplayamoresophisticatedroleinchronicpainthanrodentastrocytes.
Impor-tantly,astrocytereaction,butnotmicroglialreaction,isassociatedwithchronicpaininHIV-infectedpatients[211].
ActivationoftheMAPKpathwaysisalsocorrelatedwithneuropathicpaininthesepatients[211].
Futureresearchshouldfocusonthefollowing:studyingtheresponsesofhumangliainculturesandhumangliatransplantationinmice;investigatingthechangesinhumangliainpainfuldiseaseconditionsinpostmortemtissues;andimagingreal-timeglialactivationinpatientswithchronicpain.
ConictofintereststatementTheauthorsdeclarenoconictofinterestinregardtothiswork.
AcknowledgmentsThisworkwassupportedbyNationalInstitutesofHealthGrantsNS67686andDE17794(toR.
R.
J.
)andDE22743(toR.
R.
J.
andM.
N.
).
References[1]AbbadieC,BhangooS,DeKoninckY,MalcangioM,Melik-ParsadaniantzS,WhiteFA.
Chemokinesandpainmechanisms.
BrainResRev2009;60:125–34.
[2]AbbadieC,LindiaJA,CumiskeyAM,PetersonLB,MudgettJS,BayneEK,DeMartinoJA,MacIntyreDE,ForrestMJ.
ImpairedneuropathicpainresponsesinmicelackingthechemokinereceptorCCR2.
ProcNatlAcadSciUSA2003;100:7947–52.
[3]AdrianJrEK,WilliamsMG,GeorgeFC.
Finestructureofreactivecellsininjurednervoustissuelabeledwith3H-thymidineinjectedbeforeinjury.
JCompNeurol1978;180:815–39.
[4]AgulhonC,FiaccoTA,McCarthyKD.
Hippocampalshort-andlong-termplasticityarenotmodulatedbyastrocyteCa2+signaling.
Science2010;327:1250–4.
[5]AhmadiS,LipprossS,NeuhuberWL,ZeilhoferHU.
PGE(2)selectivelyblocksinhibitoryglycinergicneurotransmissionontoratsupercialdorsalhornneurons.
NatNeurosci2002;5:34–40.
[6]AnandP,ShenoyR,PalmerJE,BainesAJ,LaiRY,RobertsonJ,BirdN,OstenfeldT,ChizhBA.
Clinicaltrialofthep38MAPkinaseinhibitordilmapimodinneuropathicpainfollowingnerveinjury.
EurJPain2011;15:1040–8.
[7]AraqueA,ParpuraV,SanzgiriRP,HaydonPG.
Tripartitesynapses:glia,theunacknowledgedpartner.
TrendsNeurosci1999;22:208–15.
[8]BabaH,JiRR,KohnoT,MooreKA,AtakaT,WakaiA,OkamotoM,WoolfCJ.
RemovalofGABAergicinhibitionfacilitatespolysynapticAber-mediatedexcitatorytransmissiontothesupercialspinaldorsalhorn.
MolCellNeurosci2003;24:818–30.
[9]BaoF,ChenM,ZhangY,ZhaoZ.
Hypoalgesiainmicelackingaquaporin-4waterchannels.
BrainResBull2010;83:298–303.
[10]BardoniR,GhirriA,ZontaM,BetelliC,VitaleG,RuggieriV,SandriniM,CarmignotoG.
Glutamate-mediatedastrocyte-to-neuronsignallingintheratdorsalhorn.
JPhysiol2010;588:831–46.
[11]BasbaumAI,BautistaDM,ScherrerG,JuliusD.
Cellularandmolecularmechanismsofpain.
Cell2009;139:267–84.
[12]BeattieEC,StellwagenD,MorishitaW,BresnahanJC,HaBK,VonZastrowM,BeattieMS,MalenkaRC.
ControlofsynapticstrengthbyglialTNFalpha.
Science2002;295:2282–5.
[13]BeggsS,CurrieG,SalterMW,FitzgeraldM,WalkerSM.
Primingofadultpainresponsesbyneonatalpainexperience:maintenancebycentralneuroimmuneactivity.
Brain2012;135:404–17.
[14]BeggsS,SalterMW.
Stereologicalandsomatotopicanalysisofthespinalmicroglialresponsetoperipheralnerveinjury.
BrainBehavImmun2007;21:624–33.
[15]BellJrPB,RundquistI,SvenssonI,CollinsVP.
FormaldehydesensitivityofaGFAPepitope,removedbyextractionofthecytoskeletonwithhighsalt.
JHistochemCytochem1987;35:1375–80.
[16]BennSC,CostiganM,TateS,FitzgeraldM,WoolfCJ.
DevelopmentalexpressionoftheTTX-resistantvoltage-gatedsodiumchannelsNav1.
8(SNS)andNav1.
9(SNS2)inprimarysensoryneurons.
JNeurosci2001;21:6077–85.
[17]BennettMV,ContrerasJE,BukauskasFF,SaezJC.
Newrolesforastrocytes:gapjunctionhemichannelshavesomethingtocommunicate.
TrendsNeurosci2003;26:610–7.
[18]BertaT,LiuT,LiuYC,XuZZ,JiRR.
Acutemorphineactivatessatelliteglialcellsandup-regulatesIL-1betaindorsalrootgangliainmiceviamatrixmetalloprotease-9.
MolPain2012;8:18.
[19]BiberK,TsudaM,Tozaki-SaitohH,TsukamotoK,ToyomitsuE,MasudaT,BoddekeH,InoueK.
NeuronalCCL21up-regulatesmicrogliaP2X4expressionandinitiatesneuropathicpaindevelopment.
EMBOJ2011;30:1864–73.
[20]BinshtokAM,WangH,ZimmermannK,AmayaF,VardehD,ShiL,BrennerGJ,JiRR,BeanBP,WoolfCJ,SamadTA.
Nociceptorsareinterleukin-1betasensors.
JNeurosci2008;28:14062–73.
[21]BlackbeardJ,WallaceVC,O'DeaKP,HasnieF,SegerdahlA,PhebyT,FieldMJ,TakataM,RiceAS.
Thecorrelationbetweenpain-relatedbehaviourandspinalmicrogliosisinfourdistinctmodelsofperipheralneuropathy.
EurJPain2012;16(10):1357–67.
[22]BushongEA,MartoneME,JonesYZ,EllismanMH.
ProtoplasmicastrocytesinCA1stratumradiatumoccupyseparateanatomicaldomains.
JNeurosci2002;22:183–92.
[23]CalvoM,BennettDL.
Themechanismsofmicrogliosisandpainfollowingperipheralnerveinjury.
ExpNeurol2012;234:271–82.
[24]CalvoM,DawesJM,BennettDL.
Theroleoftheimmunesysteminthegenerationofneuropathicpain.
LancetNeurol2012;11:629–42.
[25]CalvoM,ZhuN,GristJ,MaZ,LoebJA,BennettDL.
Followingnerveinjuryneuregulin-1drivesmicroglialproliferationandneuropathicpainviatheMEK/ERKpathway.
Glia2011;59:554–68.
[26]CalvoM,ZhuN,TsantoulasC,MaZ,GristJ,LoebJA,BennettDL.
Neuregulin-ErbBsignalingpromotesmicroglialproliferationandchemotaxiscontributingtomicrogliosisandpainafterperipheralnerveinjury.
JNeurosci2010;30:5437–50.
[27]ChenMJ,KressB,HanX,MollK,PengW,JiRR,NedergaardM.
AstrocyticCx43hemichannelsandgapjunctionsplayacrucialroleindevelopmentofchronicneuropathicpainfollowingspinalcordinjury.
Glia2012;60:1660–70.
[28]ChenFL,DongYL,ZhangZJ,CaoDL,XuJ,HuiJ,ZhuL,GaoYJ.
Activationofastrocytesintheanteriorcingulatecortexcontributestotheaffectivecomponentofpaininaninammatorypainmodel.
BrainResBull2012;87:60–6.
[29]ChenY,ZhangX,WangC,LiG,GuY,HuangLY.
ActivationofP2X7receptorsinglialsatellitecellsreducespainthroughdownregulationofP2X3receptorsinnociceptiveneurons.
ProcNatlAcadSciUSA2008;105:16773–8.
[30]ChiangCY,SessleBJ,DostrovskyJO.
Roleofastrocytesinpain.
NeurochemRes2012;37(11):2419–31.
[31]ChiangCY,WangJ,XieYF,ZhangS,HuJW,DostrovskyJO,SessleBJ.
Astroglialglutamate–glutamineshuttleisinvolvedincentralsensitizationofnociceptiveneuronsinratmedullarydorsalhorn.
JNeurosci2007;27:9068–76.
[32]ChoiJI,SvenssonCI,KoehrnFJ,BhuskuteA,SorkinLS.
PeripheralinammationinducestumornecrosisfactordependentAMPAreceptortrafckingandAktphosphorylationinspinalcordinadditiontopainbehavior.
PAIN2010;149:243–53.
[33]ChristiansonCA,DumlaoDS,StokesJA,DennisEA,SvenssonCI,CorrM,YakshTL.
SpinalTLR4mediatesthetransitiontoapersistentmechanicalhypersensitivityaftertheresolutionofinammationinserum-transferredarthritis.
PAIN2011;152:2881–91.
[34]ChuYX,ZhangYQ,ZhaoZQ.
Involvementofmicrogliaandinterleukin-18intheinductionoflong-termpotentiationofspinalnociceptiveresponsesinducedbytetanicsciaticstimulation.
NeurosciBull2012;28:49–60.
[35]ClarkAK,StanilandAA,MalcangioM.
Fractalkine/CX3CR1signallinginchronicpainandinammation.
CurrPharmBiotechnol2011;12:1707–14.
[36]ClarkAK,StanilandAA,MarchandF,KaanTK,McMahonSB,MalcangioM.
P2X7-dependentreleaseofinterleukin-1betaandnociceptioninthespinalcordfollowinglipopolysaccharide.
JNeurosci2010;30:573–82.
[37]ClarkAK,YipPK,GristJ,GentryC,StanilandAA,MarchandF,DehvariM,WotherspoonG,WinterJ,UllahJ,BevanS,MalcangioM.
InhibitionofspinalmicroglialcathepsinSforthereversalofneuropathicpain.
ProcNatlAcadSciUSA2007;104:10655–60.
[38]ColburnRW,DeLeoJA,RickmanAJ,YeagerMP,KwonP,HickeyWF.
Dissociationofmicroglialactivationandneuropathicpainbehaviorsfollowingperipheralnerveinjuryintherat.
JNeuroimmunol1997;79:163–75.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx13Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[39]ConnorKM,SanGiovanniJP,LofqvistC,AdermanCM,ChenJ,HiguchiA,HongS,PravdaEA,MajchrzakS,CarperD,HellstromA,KangJX,ChewEY,SalemJrN,SerhanCN,SmithLE.
Increaseddietaryintakeofomega-3-polyunsaturatedfattyacidsreducespathologicalretinalangiogenesis.
NatMed2007;13:868–73.
[40]CookSP,VulchanovaL,HargreavesKM,EldeR,McCleskeyEW.
DistinctATPreceptorsonpain-sensingandstretch-sensingneurons.
Nature1997;387:505–8.
[41]CorreaF,Hernangomez-HerreroM,MestreL,LoriaF,DocagneF,GuazaC.
TheendocannabinoidanandamidedownregulatesIL-23andIL-12subunitsinaviralmodelofmultiplesclerosis:evidenceforacross-talkbetweenIL-12p70/IL-23axisandIL-10inmicroglialcells.
BrainBehavImmun2011;25:736–49.
[42]CotrinaML,LinJH,Alves-RodriguesA,LiuS,LiJ,Azmi-GhadimiH,KangJ,NausCC,NedergaardM.
ConnexinsregulatecalciumsignalingbycontrollingATPrelease.
ProcNatlAcadSciUSA1998;95:15735–40.
[43]CoullJA,BeggsS,BoudreauD,BoivinD,TsudaM,InoueK,GravelC,SalterMW,DeKoninckY.
BDNFfrommicrogliacausestheshiftinneuronalaniongradientunderlyingneuropathicpain.
Nature2005;438:1017–21.
[44]CoullJA,BoudreauD,BachandK,PrescottSA,NaultF,SikA,DeKoninckP,DeKoninckY.
Trans-synapticshiftinaniongradientinspinallaminaIneuronsasamechanismofneuropathicpain.
Nature2003;424:938–42.
[45]CroninM,AndersonPN,CookJE,GreenCR,BeckerDL.
Blockingconnexin43expressionreducesinammationandimprovesfunctionalrecoveryafterspinalcordinjury.
MolCellNeurosci2008;39:152–60.
[46]CrownED,GwakYS,YeZ,JohnsonKM,HulseboschCE.
Activationofp38MAPkinaseisinvolvedincentralneuropathicpainfollowingspinalcordinjury.
ExpNeurol2008;213:257–67.
[47]CuiY,ChenY,ZhiJL,GuoRX,FengJQ,ChenPX.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliamediatesmorphineantinociceptivetolerance.
BrainRes2006;1069:235–43.
[48]CuiY,LiaoXX,LiuW,GuoRX,WuZZ,ZhaoCM,ChenPX,FengJQ.
Anovelroleofminocycline:attenuatingmorphineantinociceptivetolerancebyinhibitionofp38MAPKintheactivatedspinalmicroglia.
BrainBehavImmun2008;22:114–23.
[49]DaulhacL,MalletC,CourteixC,EtienneM,DurouxE,PrivatAM,EschalierA,FialipJ.
Diabetes-inducedmechanicalhyperalgesiainvolvesspinalmitogen-activatedproteinkinaseactivationinneuronsandmicrogliaviaN-methyl-D-aspartate-dependentmechanisms.
MolPharmacol2006;70:1246–54.
[50]DavalosD,GrutzendlerJ,YangG,KimJV,ZuoY,JungS,LittmanDR,DustinML,GanWB.
ATPmediatesrapidmicroglialresponsetolocalbraininjuryinvivo.
NatNeurosci2005;8:752–8.
[51]DeLeoJA,ColburnRW,RickmanAJ.
Cytokineandgrowthfactorimmunohistochemicalspinalprolesintwoanimalmodelsofmononeuropathy.
BrainRes1997;759:50–7.
[52]DeLeoJA,YezierskiRP.
Theroleofneuroinammationandneuroimmuneactivationinpersistentpain.
PAIN2001;90:1–6.
[53]DominguezE,RivatC,PommierB,MauborgneA,PohlM.
JAK/STAT3pathwayisactivatedinspinalcordmicrogliaafterperipheralnerveinjuryandcontributestoneuropathicpaindevelopmentinrat.
JNeurochem2008;107:50–60.
[54]DublinP,HananiM.
Satelliteglialcellsinsensoryganglia:theirpossiblecontributiontoinammatorypain.
BrainBehavImmun2007;21:592–8.
[55]EcheverryS,ShiXQ,ZhangJ.
Characterizationofcellproliferationinratspinalcordfollowingperipheralnerveinjuryandtherelationshipwithneuropathicpain.
PAIN2008;135:37–47.
[56]EngLF,GhirnikarRS,LeeYL.
Glialbrillaryacidicprotein:GFAP—thirty-oneyears(1969–2000).
NeurochemRes2000;25:1439–51.
[57]ErikssonNP,PerssonJK,SvenssonM,ArvidssonJ,MolanderC,AldskogiusH.
Aquantitativeanalysisofthemicroglialcellreactionincentralprimarysensoryprojectionterritoriesfollowingperipheralnerveinjuryintheadultrat.
ExpBrainRes1993;96:19–27.
[58]ErogluC,AllenNJ,SusmanMW,O'RourkeNA,ParkCY,OzkanE,ChakrabortyC,MulinyaweSB,AnnisDS,HubermanAD,GreenEM,LawlerJ,DolmetschR,GarciaKC,SmithSJ,LuoZD,RosenthalA,MosherDF,BarresBA.
Gabapentinreceptoralpha2delta-1isaneuronalthrombospondinreceptorresponsibleforexcitatoryCNSsynaptogenesis.
Cell2009;139:380–92.
[59]FergusonAR,ChristensenRN,GenselJC,MillerBA,SunF,BeattieEC,BresnahanJC,BeattieMS.
CelldeathafterspinalcordinjuryisexacerbatedbyrapidTNFalpha-inducedtrafckingofGluR2-lackingAMPARstotheplasmamembrane.
JNeurosci2008;28:11391–400.
[60]FerriniF,TrangT,MattioliTA,LaffrayS,Del'guidiceT,LorenzoLE,CastonguayA,DoyonN,ZhangW,GodinAG,MohrD,BeggsS,VandalK,BeaulieuJM,CahillCM,SalterMW,DeKY.
Morphinehyperalgesiagatedthroughmicroglia-mediateddisruptionofneuronalCl()homeostasis.
NatNeurosci2013;16(2):183–92.
[61]FitzgeraldM.
Thedevelopmentofnociceptivecircuits.
NatRevNeurosci2005;6:507–20.
[62]FonsecaCG,GreenCR,NicholsonLF.
Upregulationinastrocyticconnexin43gapjunctionlevelsmayexacerbategeneralizedseizuresinmesialtemporallobeepilepsy.
BrainRes2002;929:105–16.
[63]FramptonM,HarveyRJ,KirchnerV.
Propentofyllinefordementia.
CochraneDatabaseSystRev2003:CD002853.
[64]FrankeH,GrummichB,HartigW,GroscheJ,RegenthalR,EdwardsRH,IllesP,KrugelU.
Changesinpurinergicsignalingaftercerebralinjury—involvementofglutamatergicmechanismsIntJDevNeurosci2006;24:123–32.
[65]FreemanSE,PatilVV,DurhamPL.
Nitricoxide-protonstimulationoftrigeminalganglionneuronsincreasesmitogen-activatedproteinkinaseandphosphataseexpressioninneuronsandsatelliteglialcells.
Neuroscience2008;157:542–55.
[66]FukuokaT,KondoE,DaiY,HashimotoN,NoguchiK.
Brain-derivedneurotrophicfactorincreasesintheuninjureddorsalrootganglionneuronsinselectivespinalnerveligationmodel.
JNeurosci2001;21:4891–900.
[67]GaoYJ,ChengJK,ZengQ,XuZZ,DecosterdI,XuX,JiRR.
SelectiveinhibitionofJNKwithapeptideinhibitorattenuatespainhypersensitivityandtumorgrowthinamouseskincancerpainmodel.
ExpNeurol2009;219:146–55.
[68]GaoYJ,JiRR.
Chemokines,neuronal–glialinteractions,andcentralprocessingofneuropathicpain.
PharmacolTher2010;126:56–68.
[69]GaoYJ,JiRR.
Targetingastrocytesignalingforchronicpain.
Neurotherapeutics2010;7:482–93.
[70]GaoYJ,XuZZ,LiuYC,WenYR,DecosterdI,JiRR.
Thec-JunN-terminalkinase1(JNK1)inspinalastrocytesisrequiredforthemaintenanceofbilateralmechanicalallodyniaunderapersistentinammatorypaincondition.
PAIN2010;148:309–19.
[71]GaoYJ,ZhangL,JiRR.
SpinalinjectionofTNF-alpha-activatedastrocytesproducespersistentpainsymptommechanicalallodyniabyreleasingmonocytechemoattractantprotein-1.
Glia2010;58:1871–80.
[72]GaoYJ,ZhangL,SamadOA,SuterMR,YasuhikoK,XuZZ,ParkJY,LindAL,MaQ,JiRR.
JNK-inducedMCP-1productioninspinalcordastrocytescontributestocentralsensitizationandneuropathicpain.
JNeurosci2009;29:4096–108.
[73]GarrawaySM,PetruskaJC,MendellLM.
BDNFsensitizestheresponseoflaminaIIneuronstohighthresholdprimaryafferentinputs.
EurJNeurosci2003;18:2467–76.
[74]GarreJM,RetamalMA,CassinaP,BarbeitoL,BukauskasFF,SaezJC,BennettMV,AbudaraV.
FGF-1inducesATPreleasefromspinalastrocytesincultureandopenspannexinandconnexinhemichannels.
ProcNatlAcadSciUSA2010;107:22659–64.
[75]GarrisonCJ,DoughertyPM,CarltonSM.
GFAPexpressioninlumbarspinalcordofnaiveandneuropathicratstreatedwithMK-801.
ExpNeurol1994;129:237–43.
[76]GarrisonCJ,DoughertyPM,KajanderKC,CarltonSM.
Stainingofglialbrillaryacidicprotein(GFAP)inlumbarspinalcordincreasesfollowingasciaticnerveconstrictioninjury.
BrainRes1991;565:1–7.
[77]GiaumeC,McCarthyKD.
Controlofgap-junctionalcommunicationinastrocyticnetworks.
TrendsNeurosci1996;19:319–25.
[78]GoldMS,GebhartGF.
Nociceptorsensitizationinpainpathogenesis.
NatMed2010;16:1248–57.
[79]GongQJ,LiYY,XinWJ,ZangY,RenWJ,WeiXH,LiYY,ZhangT,LiuXG.
ATPinduceslong-termpotentiationofC-ber-evokedeldpotentialsinspinaldorsalhorn:therolesofP2X4receptorsandp38MAPKinmicroglia.
Glia2009;57:583–91.
[80]GosselinRD,SuterMR,JiRR,DecosterdI.
Glialcellsandchronicpain.
Neuroscientist2010;16:519–31.
[81]GosselinRD,VarelaC,BanisadrG,MechighelP,RosteneW,KitabgiP,Melik-ParsadaniantzS.
ConstitutiveexpressionofCCR2chemokinereceptorandinhibitionbyMCP-1/CCL2ofGABA-inducedcurrentsinspinalcordneurones.
JNeurochem2005;95:1023–34.
[82]GrifnRS,CostiganM,BrennerGJ,MaCH,ScholzJ,MossA,AllchorneAJ,StahlGL,WoolfCJ.
ComplementinductioninspinalcordmicrogliaresultsinanaphylatoxinC5a-mediatedpainhypersensitivity.
JNeurosci2007;27:8699–708.
[83]GuoW,WangH,WatanabeM,ShimizuK,ZouS,LaGraizeSC,WeiF,DubnerR,RenK.
Glial-cytokine-neuronalinteractionsunderlyingthemechanismsofpersistentpain.
JNeurosci2007;27:6006–18.
[84]GuoW,WangH,ZouS,DubnerR,RenK.
Chemokinesignalinginvolvingchemokine(C–Cmotif)ligand2playsaroleindescendingpainfacilitation.
NeurosciBull2012;28:193–207.
[85]GwakYS,KangJ,UnabiaGC,HulseboschCE.
Spatialandtemporalactivationofspinalglialcells:roleofgliopathyincentralneuropathicpainfollowingspinalcordinjuryinrats.
ExpNeurol2012;234:362–72.
[86]HainsBC,WaxmanSG.
Activatedmicrogliacontributetothemaintenanceofchronicpainafterspinalcordinjury.
JNeurosci2006;26:4308–17.
[87]HainsLE,LoramLC,WeiselerJL,FrankMG,BlossEB,SholarP,TaylorFR,HarrisonJA,MartinTJ,EisenachJC,MaierSF,WatkinsLR.
Painintensityanddurationcanbeenhancedbypriorchallenge:initialevidencesuggestiveofaroleofmicroglialpriming.
JPain2010;11:1004–14.
[88]HanX,ChenM,WangF,WindremM,WangS,ShanzS,XuQ,OberheimNA,BekarL,BetstadtS,SilvaAJ,TakanoT,GoldmanSA,NedergaardM.
Forebrainengraftmentbyhumanglialprogenitorcellsenhancessynapticplasticityandlearninginadultmice.
CellStemCell2013;12:342–53.
[89]HananiM.
Satelliteglialcellsinsensoryganglia:fromformtofunction.
BrainResBrainResRev2005;48:457–76.
[90]HananiM.
Intercellularcommunicationinsensorygangliabypurinergicreceptorsandgapjunctions:Implicationsforchronicpain.
BrainRes2012;1487:183–91.
[91]HananiM,HuangTY,CherkasPS,LeddaM,PanneseE.
Glialcellplasticityinsensorygangliainducedbynervedamage.
Neuroscience2002;114:279–83.
[92]HanischUK.
Microgliaasasourceandtargetofcytokines.
Glia2002;40:140–55.
[93]HanischUK,KettenmannH.
Microglia:activesensorandversatileeffectorcellsinthenormalandpathologicbrain.
NatNeurosci2007;10:1387–94.
14R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[94]HaoS,MataM,GloriosoJC,FinkDJ.
HSV-mediatedexpressionofinterleukin-4indorsalrootganglionneuronsreducesneuropathicpain.
MolPain2006;2:6.
[95]HaraguchiK,KawamotoA,IsamiK,MaedaS,KusanoA,AsakuraK,ShirakawaH,MoriY,NakagawaT,KanekoS.
TRPM2contributestoinammatoryandneuropathicpainthroughtheaggravationofpronociceptiveinammatoryresponsesinmice.
JNeurosci2012;32:3931–41.
[96]HarveyRJ,DepnerUB,WassleH,AhmadiS,HeindlC,ReinoldH,SmartTG,HarveyK,SchutzB,Abo-SalemOM,ZimmerA,PoisbeauP,WelzlH,WolferDP,BetzH,ZeilhoferHU,MullerU.
GlyRalpha3:anessentialtargetforspinalPGE2-mediatedinammatorypainsensitization.
Science2004;304:884–7.
[97]HathwayGJ,Vega-AvelairaD,MossA,IngramR,FitzgeraldM.
Brief,lowfrequencystimulationofratperipheralC-bresevokesprolongedmicroglial-inducedcentralsensitizationinadultsbutnotinneonates.
PAIN2009;144:110–8.
[98]HonoreP,RogersSD,SchweiMJ,Salak-JohnsonJL,LugerNM,SabinoMC,ClohisyDR,MantyhPW.
Murinemodelsofinammatory,neuropathicandcancerpaineachgeneratesauniquesetofneurochemicalchangesinthespinalcordandsensoryneurons.
Neuroscience2000;98:585–98.
[99]HorvathRJ,DeLeoJA.
MorphineenhancesmicroglialmigrationthroughmodulationofP2X4receptorsignaling.
JNeurosci2009;29:998–1005.
[100]HuaXY,SvenssonCI,MatsuiT,FitzsimmonsB,YakshTL,WebbM.
Intrathecalminocyclineattenuatesperipheralinammation-inducedhyperalgesiabyinhibitingp38MAPKinspinalmicroglia.
EurJNeurosci2005;22:2431–40.
[101]HuangL,WangCF,SerhanCN,StrichartzG.
EnduringpreventionandtransientreductionofpostoperativepainbyintrathecalresolvinD1.
PAIN2011;152:557–65.
[102]HulseboschCE,HainsBC,CrownED,CarltonSM.
Mechanismsofchroniccentralneuropathicpainafterspinalcordinjury.
BrainResRev2009;60:202–13.
[103]HutchinsonMR,CoatsBD,LewisSS,ZhangY,SprungerDB,RezvaniN,BakerEM,JekichBM,WieselerJL,SomogyiAA,MartinD,PooleS,JuddCM,MaierSF,WatkinsLR.
Proinammatorycytokinesopposeopioid-inducedacuteandchronicanalgesia.
BrainBehavImmun2008;22:1178–89.
[104]HutchinsonMR,LewisSS,CoatsBD,RezvaniN,ZhangY,WieselerJL,SomogyiAA,YinH,MaierSF,RiceKC,WatkinsLR.
Possibleinvolvementoftoll-likereceptor4/myeloiddifferentiationfactor-2activityofopioidinactiveisomerscausesspinalproinammationandrelatedbehavioralconsequences.
Neuroscience2010;167:880–93.
[105]HutchinsonMR,ZhangY,ShridharM,EvansJH,BuchananMM,ZhaoTX,SlivkaPF,CoatsBD,RezvaniN,WieselerJ,HughesTS,LandgrafKE,ChanS,FongS,PhippsS,FalkeJJ,LeinwandLA,MaierSF,YinH,RiceKC,WatkinsLR.
Evidencethatopioidsmayhavetoll-likereceptor4andMD-2effects.
BrainBehavImmun2010;24:83–95.
[106]IadecolaC,NedergaardM.
Glialregulationofthecerebralmicrovasculature.
NatNeurosci2007;10:1369–76.
[107]JasminL,VitJP,BhargavaA,OharaPT.
CansatelliteglialcellsbetherapeutictargetsforpaincontrolNeuronGliaBiol2010;6:63–71.
[108]JiRR.
Targetingmicroglialpurinergicsignalingtoimprovemorphineanalgesia.
PAIN2010;150:377–8.
[109]JiRR,GereauRW,MalcangioM,StrichartzGR.
MAPkinaseandpain.
BrainResRev2009;60:135–48.
[110]JiRR,KawasakiY,ZhuangZY,WenYR,DecosterdI.
Possibleroleofspinalastrocytesinmaintainingchronicpainsensitization:reviewofcurrentevidencewithfocusonbFGF/JNKpathway.
NeuronGliaBiol2006;2:259–69.
[111]JiRR,KohnoT,MooreKA,WoolfCJ.
CentralsensitizationandLTP:dopainandmemorysharesimilarmechanismsTrendsNeurosci2003;26:696–705.
[112]JiRR,StrichartzG.
Cellsignalingandthegenesisofneuropathicpain.
ScienceSignaling(ScienceSig),partofScience2004:reE14.
[113]JiRR,SuterMR.
P38MAPK,microglialsignaling,andneuropathicpain.
MolPain2007;3:33.
[114]JiRR,XuZZ,StrichartzG,SerhanCN.
Emergingrolesofresolvinsintheresolutionofinammationandpain.
TrendsNeurosci2011;34(11):599–609.
[115]JiRR,XuZZ,WangX,LoEH.
Matrixmetalloproteaseregulationofneuropathicpain.
TrendsPharmacolSci2009;30:336–40.
[116]JiRR,ZhangQ,ZhangX,PiehlF,ReillyT,PetterssonRF,HokfeltT.
ProminentexpressionofbFGFindorsalrootgangliaafteraxotomy.
EurJNeurosci1995;7:2458–68.
[117]JinH,LiYH,XuJS,GuoGQ,ChenDL,BoY.
LipoxinA4analogattenuatesmorphineantinociceptivetolerance,withdrawal-inducedhyperalgesia,andglialreactionandcytokineexpressioninthespinalcordofrat.
Neuroscience2012;208:1–10.
[118]JinSX,ZhuangZY,WoolfCJ,JiRR.
P38mitogen-activatedproteinkinaseisactivatedafteraspinalnerveligationinspinalcordmicrogliaanddorsalrootganglionneuronsandcontributestothegenerationofneuropathicpain.
JNeurosci2003;23:4017–22.
[119]JinX,GereauRW.
Acutep38-mediatedmodulationoftetrodotoxin-resistantsodiumchannelsinmousesensoryneuronsbytumornecrosisfactor-alpha.
JNeurosci2006;26:246–55.
[120]JohnstonIN,MilliganED,Wieseler-FrankJ,FrankMG,ZapataV,CampisiJ,LangerS,MartinD,GreenP,FleshnerM,LeinwandL,MaierSF,WatkinsLR.
Aroleforproinammatorycytokinesandfractalkineinanalgesia,tolerance,andsubsequentpainfacilitationinducedbychronicintrathecalmorphine.
JNeurosci2004;24:7353–65.
[121]JungH,BhangooS,BanisadrG,FreitagC,RenD,WhiteFA,MillerRJ.
VisualizationofchemokinereceptoractivationintransgenicmicerevealsperipheralactivationofCCR2receptorsinstatesofneuropathicpain.
JNeurosci2009;29:8051–62.
[122]KalliomakiJ,AttalN,JonzonB,BachFW,HuizarK,RatcliffeS,ErikssonB,JaneckiM,DanilovA,BouhassiraD.
Arandomized,double-blind,placebo-controlledtrialofachemokinereceptor2(CCR2)antagonistinposttraumaticneuralgia.
PAIN2013;154:761–7.
[123]KangJ,KangN,LovattD,TorresA,ZhaoZ,LinJ,NedergaardM.
Connexin43hemichannelsarepermeabletoATP.
JNeurosci2008;28:4702–11.
[124]KatagiriA,ShinodaM,HondaK,ToyofukuA,SessleBJ,IwataK.
SatelliteglialcellP2Y12receptorinthetrigeminalganglionisinvolvedinlingualneuropathicpainmechanismsinrats.
MolPain2012;8:23.
[125]KatsuraH,ObataK,MiyoshiK,KondoT,YamanakaH,KobayashiK,DaiY,FukuokaT,SakagamiM,NoguchiK.
Transforminggrowthfactor-activatedkinase1inducedinspinalastrocytescontributestomechanicalhypersensitivityafternerveinjury.
Glia2008;56:723–33.
[126]KatsuraH,ObataK,MizushimaT,SakuraiJ,KobayashiK,YamanakaH,DaiY,FukuokaT,SakagamiM,NoguchiK.
ActivationofSrc-familykinasesinspinalmicrogliacontributestomechanicalhypersensitivityafternerveinjury.
JNeurosci2006;26:8680–90.
[127]KawasakiY,XuZZ,WangX,ParkJY,ZhuangZY,TanPH,GaoYJ,RoyK,CorfasG,LoEH,JiRR.
Distinctrolesofmatrixmetalloproteasesintheearly-andlate-phasedevelopmentofneuropathicpain.
NatMed2008;14:331–6.
[128]KawasakiY,ZhangL,ChengJK,JiRR.
Cytokinemechanismsofcentralsensitization:distinctandoverlappingroleofinterleukin-1beta,interleukin-6,andtumornecrosisfactor-alphainregulatingsynapticandneuronalactivityinthesupercialspinalcord.
JNeurosci2008;28:5189–94.
[129]KellerAF,BeggsS,SalterMW,DeKY.
TransformationoftheoutputofspinallaminaIneuronsafternerveinjuryandmicrogliastimulationunderlyingneuropathicpain.
MolPain2007;3:27.
[130]KimD,KimMA,ChoIH,KimMS,LeeS,JoEK,ChoiSY,ParkK,KimJS,AkiraS,NaHS,OhSB,LeeSJ.
Acriticalroleoftoll-likereceptor2innerveinjury-inducedspinalcordglialcellactivationandpainhypersensitivity.
JBiolChem2007;282:14975–83.
[131]KimD,YouB,JoEK,HanSK,SimonMI,LeeSJ.
NADPHoxidase2-derivedreactiveoxygenspeciesinspinalcordmicrogliacontributetoperipheralnerveinjury-inducedneuropathicpain.
ProcNatlAcadSciUSA2010;107:14851–6.
[132]KimDS,LiKW,BoroujerdiA,PeterYY,ZhouCY,DengP,ParkJ,ZhangX,LeeJ,CorpeM,SharpK,StewardO,ErogluC,BarresB,ZauckeF,XuZC,LuoZD.
Thrombospondin-4contributestospinalsensitizationandneuropathicpainstates.
JNeurosci2012;32:8977–87.
[133]KimelbergHK,NedergaardM.
Functionsofastrocytesandtheirpotentialastherapeutictargets.
Neurotherapeutics2010;7:338–53.
[134]KleinT,MagerlW,HopfHC,SandkuhlerJ,TreedeRD.
Perceptualcorrelatesofnociceptivelong-termpotentiationandlong-termdepressioninhumans.
JNeurosci2004;24:964–71.
[135]KobayashiK,TakahashiE,MiyagawaY,YamanakaH,NoguchiK.
InductionoftheP2X7receptorinspinalmicrogliainaneuropathicpainmodel.
NeurosciLett2011;504:57–61.
[136]KobayashiK,YamanakaH,FukuokaT,DaiY,ObataK,NoguchiK.
P2Y12receptorupregulationinactivatedmicrogliaisagatewayofp38signalingandneuropathicpain.
JNeurosci2008;28:2892–902.
[137]KobayashiK,YamanakaH,YanamotoF,OkuboM,NoguchiK.
MultipleP2Ysubtypesinspinalmicrogliaareinvolvedinneuropathicpainafterperipheralnerveinjury.
Glia2012;60(10):1529–39.
[138]KozaiT,YamanakaH,DaiY,ObataK,KobayashiK,MashimoT,NoguchiK.
Tissuetypeplasminogenactivatorinducedinratdorsalhornastrocytescontributestomechanicalhypersensitivityfollowingdorsalrootinjury.
Glia2007;55:595–603.
[139]KunerR.
Centralmechanismsofpathologicalpain.
NatMed2010;16:1258–66.
[140]LanL,YuanH,DuanL,CaoR,GaoB,ShenJ,XiongY,ChenLW,RaoZR.
Blockingtheglialfunctionsuppressessubcutaneousformalin-inducednociceptivebehaviorintherat.
NeurosciRes2007;57:112–9.
[141]LandryRP,JacobsVL,Romero-SandovalEA,DeLeoJA.
Propentofylline,aCNSglialmodulatordoesnotdecreasepaininpost-herpeticneuralgiapatients:invitroevidencefordifferentialresponsesinhumanandrodentmicrogliaandmacrophages.
ExpNeurol2012;234:340–50.
[142]LatremoliereA,WoolfCJ.
Centralsensitization:ageneratorofpainhypersensitivitybycentralneuralplasticity.
JPain2009;10:895–926.
[143]LeverIJ,BradburyEJ,CunninghamJR,AdelsonDW,JonesMG,McMahonSB,MarvizonJC,MalcangioM.
Brain-derivedneurotrophicfactorisreleasedinthedorsalhornbydistinctivepatternsofafferentberstimulation.
JNeurosci2001;21:4469–77.
[144]LiJY,XieW,StrongJA,GuoQL,ZhangJM.
Mechanicalhypersensitivity,sympatheticsprouting,andglialactivationareattenuatedbylocalinjectionofcorticosteroidnearthelumbarganglioninaratmodelofneuropathicpain.
RegAnesthPainMed2011;36:56–62.
[145]LiWE,NagyJI.
Activationofbresinratsciaticnervealtersphosphorylationstateofconnexin-43atastrocyticgapjunctionsinspinalcord:evidenceforjunctionregulationbyneuronal–glialinteractions.
Neuroscience2000;97:113–23.
[146]LiWW,GuoTZ,LiangD,ShiX,WeiT,KingeryWS,ClarkJD.
TheNALP1inammasomecontrolscytokineproductionandnociceptioninaratfracturemodelofcomplexregionalpainsyndrome.
PAIN2009;147:277–86.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx15Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[147]LiawWJ,StephensJrRL,BinnsBC,ChuY,SepkutyJP,JohnsRA,RothsteinJD,TaoYX.
Spinalglutamateuptakeiscriticalformaintainingnormalsensorytransmissioninratspinalcord.
PAIN2005;115:60–70.
[148]LinJH,LouN,KangN,TakanoT,HuF,HanX,XuQ,LovattD,TorresA,WilleckeK,YangJ,KangJ,NedergaardM.
Acentralroleofconnexin43inhypoxicpreconditioning.
JNeurosci2008;28:681–95.
[149]LiuCN,WallPD,BenDorE,MichaelisM,AmirR,DevorM.
TactileallodyniaintheabsenceofC-beractivation:alteredringpropertiesofDRGneuronsfollowingspinalnerveinjury.
PAIN2000;85:503–21.
[150]LiuFY,SunYN,WangFT,LiQ,SuL,ZhaoZF,MengXL,ZhaoH,WuX,SunQ,XingGG,WanY.
Activationofsatelliteglialcellsinlumbardorsalrootgangliacontributestoneuropathicpainafterspinalnerveligation.
BrainRes2012;1427:65–77.
[151]LiuL,RudinM,KozlovaEN.
Glialcellproliferationinthespinalcordafterdorsalrhizotomyorsciaticnervetransectionintheadultrat.
ExpBrainRes2000;131:64–73.
[152]LiuT,GaoYJ,JiRR.
EmergingroleofToll-likereceptorsinthecontrolofpainanditch.
NeurosciBull2012;28:131–44.
[153]LiuYC,BertaT,LiuT,TanPH,JiRR.
Acutemorphineinducesmatrixmetalloproteinase-9up-regulationinprimarysensoryneuronstomaskopioid-inducedanalgesiainmice.
MolPain2012;8:19.
[154]LiuYL,ZhouLJ,HuNW,XuJT,WuCY,ZhangT,LiYY,LiuXG.
Tumornecrosisfactor-alphainduceslong-termpotentiationofC-berevokedeldpotentialsinspinaldorsalhorninratswithnerveinjury:theroleofNF-kappaB,JNKandp38MAPK.
Neuropharmacology2007;52:708–15.
[155]MaC,ShuY,ZhengZ,ChenY,YaoH,GreenquistKW,WhiteFA,LaMotteRH.
Similarelectrophysiologicalchangesinaxotomizedandneighboringintactdorsalrootganglionneurons.
JNeurophysiol2003;89:1588–602.
[156]MadiaiF,GoettlVM,HussainSR,ClairmontAR,StephensJrRL,HackshawKV.
Anti-broblastgrowthfactor-2antibodiesattenuatemechanicalallodyniainaratmodelofneuropathicpain.
JMolNeurosci2005;27:315–24.
[157]MaedaS,KawamotoA,YataniY,ShirakawaH,NakagawaT,KanekoS.
GenetransferofGLT-1,aglialglutamatetransporter,intothespinalcordbyrecombinantadenovirusattenuatesinammatoryandneuropathicpaininrats.
MolPain2008;4:65.
[158]MaoJ,SungB,JiRR,LimG.
Chronicmorphineinducesdownregulationofspinalglutamatetransporters:implicationsinmorphinetoleranceandabnormalpainsensitivity.
JNeurosci2002;22:8312–23.
[159]MatsukaY,OnoT,IwaseH,MitrirattanakulS,OmotoKS,ChoT,LamYY,SnyderB,SpigelmanI.
AlteredATPreleaseandmetabolismindorsalrootgangliaofneuropathicrats.
MolPain2008;4:66.
[160]McMahonSB,MalcangioM.
Currentchallengesinglia-painbiology.
Neuron2009;64:46–54.
[161]MellerST,DykstraC,GrzybyckiD,MurphyS,GebhartGF.
Thepossibleroleofgliainnociceptiveprocessingandhyperalgesiainthespinalcordoftherat.
Neuropharmacology1994;33:1471–8.
[162]MenetskiJ,MistryS,LuM,MudgettJS,RansohoffRM,DeMartinoJA,MacIntyreDE,AbbadieC.
Miceoverexpressingchemokineligand2(CCL2)inastrocytesdisplayenhancednociceptiveresponses.
Neuroscience2007;149:706–14.
[163]MilliganED,TwiningC,ChacurM,BiedenkappJ,O'ConnorK,PooleS,TraceyK,MartinD,MaierSF,WatkinsLR.
Spinalgliaandproinammatorycytokinesmediatemirror-imageneuropathicpaininrats.
JNeurosci2003;23:1026–40.
[164]MilliganED,WatkinsLR.
Pathologicalandprotectiverolesofgliainchronicpain.
NatRevNeurosci2009;10:23–36.
[165]MilliganED,ZapataV,ChacurM,SchoenigerD,BiedenkappJ,O'ConnorKA,VergeGM,ChapmanG,GreenP,FosterAC,NaeveGS,MaierSF,WatkinsLR.
Evidencethatexogenousandendogenousfractalkinecaninducespinalnociceptivefacilitationinrats.
EurJNeurosci2004;20:2294–302.
[166]MiraucourtLS,PeirsC,DallelR,VoisinDL.
Glycineinhibitorydysfunctionturnstouchintopainthroughastrocyte-derivedD-serine.
PAIN2011;152:1340–8.
[167]MiyoshiK,ObataK,KondoT,OkamuraH,NoguchiK.
Interleukin-18-mediatedmicroglia/astrocyteinteractioninthespinalcordenhancesneuropathicpainprocessingafternerveinjury.
JNeurosci2008;28:12775–87.
[168]MogilJS.
Sexdifferencesinpainandpaininhibition:multipleexplanationsofacontroversialphenomenon.
NatRevNeurosci2012;13:859–66.
[169]MooreKA,KohnoT,KarchewskiLA,ScholzJ,BabaH,WoolfCJ.
PartialperipheralnerveinjurypromotesaselectivelossofGABAergicinhibitioninthesupercialdorsalhornofthespinalcord.
JNeurosci2002;22:6724–31.
[170]MossA,BeggsS,Vega-AvelairaD,CostiganM,HathwayGJ,SalterMW,FitzgeraldM.
Spinalmicrogliaandneuropathicpaininyoungrats.
PAIN2007;128:215–24.
[171]NdongC,LandryRP,DeLeoJA,Romero-SandovalEA.
Mitogenactivatedproteinkinasephosphatase-1preventsthedevelopmentoftactilesensitivityinarodentmodelofneuropathicpain.
MolPain2012;8:34.
[172]NedergaardM,VerkhratskyA.
Artifactversusreality—howastrocytescontributetosynapticevents.
Glia2012;60:1013–23.
[173]NesicO,LeeJ,JohnsonKM,YeZ,XuGY,UnabiaGC,WoodTG,McAdooDJ,WestlundKN,HulseboschCE,ReginoPerez-PoloJ.
Transcriptionalprolingofspinalcordinjury-inducedcentralneuropathicpain.
JNeurochem2005;95:998–1014.
[174]NicotraL,LoramLC,WatkinsLR,HutchinsonMR.
Toll-likereceptorsinchronicpain.
ExpNeurol2012;234(2):316–29.
[175]NimmerjahnA,KirchhoffF,HelmchenF.
Restingmicroglialcellsarehighlydynamicsurveillantsofbrainparenchymainvivo.
Science2005;308:1314–8.
[176]NongY,HuangYQ,JuW,KaliaLV,AhmadianG,WangYT,SalterMW.
GlycinebindingprimesNMDAreceptorinternalization.
Nature2003;422:302–7.
[177]ObataK,KatsuraH,MizushimaT,SakuraiJ,KobayashiK,YamanakaH,DaiY,FukuokaT,NoguchiK.
Rolesofextracellularsignal-regulatedproteinkinases5inspinalmicrogliaandprimarysensoryneuronsforneuropathicpain.
JNeurochem2007;102:1569–84.
[178]ObataK,NoguchiK.
MAPKactivationinnociceptiveneuronsandpainhypersensitivity.
LifeSci2004;74:2643–53.
[179]ObataK,YamanakaH,KobayashiK,DaiY,MizushimaT,KatsuraH,FukuokaT,TokunagaA,NoguchiK.
Roleofmitogen-activatedproteinkinaseactivationininjuredandintactprimaryafferentneuronsformechanicalandheathypersensitivityafterspinalnerveligation.
JNeurosci2004;24:10211–22.
[180]OberheimNA,TakanoT,HanX,HeW,LinJH,WangF,XuQ,WyattJD,PilcherW,OjemannJG,RansomBR,GoldmanSA,NedergaardM.
Uniquelyhominidfeaturesofadulthumanastrocytes.
JNeurosci2009;29:3276–87.
[181]OberheimNA,TianGF,HanX,PengW,TakanoT,RansomB,NedergaardM.
Lossofastrocyticdomainorganizationintheepilepticbrain.
JNeurosci2008;28:3264–76.
[182]OberheimNA,WangX,GoldmanS,NedergaardM.
Astrocyticcomplexitydistinguishesthehumanbrain.
TrendsNeurosci2006;29:547–53.
[183]OharaPT,VitJP,BhargavaA,JasminL.
Evidenceforaroleofconnexin43intrigeminalpainusingRNAinterferenceinvivo.
JNeurophysiol2008;100:3064–73.
[184]Okada-OgawaA,SuzukiI,SessleBJ,ChiangCY,SalterMW,DostrovskyJO,TsuboiY,KondoM,KitagawaJ,KobayashiA,NomaN,ImamuraY,IwataK.
Astrogliainmedullarydorsalhorn(trigeminalspinalsubnucleuscaudalis)areinvolvedintrigeminalneuropathicpainmechanisms.
JNeurosci2009;29:11161–71.
[185]PanneseE,LeddaM,CherkasPS,HuangTY,HananiM.
Satellitecellreactionstoaxoninjuryofsensoryganglionneurons:increaseinnumberofgapjunctionsandformationofbridgesconnectingpreviouslyseparateperineuronalsheaths.
AnatEmbryol(Berl)2003;206:337–47.
[186]PaolicelliRC,BolascoG,PaganiF,MaggiL,ScianniM,PanzanelliP,GiustettoM,FerreiraTA,GuiducciE,DumasL,RagozzinoD,GrossCT.
Synapticpruningbymicrogliaisnecessaryfornormalbraindevelopment.
Science2011;333:1456–8.
[188]ParkCK,LuN,XuZZ,LiuT,SerhanCN,JiRR.
ResolvingTRPV1-andTNF-mediatedspinalcordsynapticplasticityandinammatorypainwithneuroprotectinD1.
JNeurosci2011;31:15072–85.
[189]ParkCK,XuZZ,LiuT,LuN,SerhanCN,JiRR.
Resolvind2isapotentendogenousinhibitorfortransientreceptorpotentialsubtypev1/a1,inammatorypain,andspinalcordsynapticplasticityinmice:distinctrolesofresolvind1,d2,ande1.
JNeurosci2011;31:18433–8.
[190]ParpuraV,ScemesE,SprayDC.
Mechanismsofglutamatereleasefromastrocytes:gapjunction''hemichannels'',purinergicreceptorsandexocytoticrelease.
NeurochemInt2004;45:259–64.
[191]PengW,CotrinaML,HanX,YuH,BekarL,BlumL,TakanoT,TianGF,GoldmanSA,NedergaardM.
SystemicadministrationofanantagonistoftheATP-sensitivereceptorP2X7improvesrecoveryafterspinalcordinjury.
ProcNatlAcadSciUSA2009;106:12489–93.
[192]PetersCM,EisenachJC.
Contributionofthechemokine(C–Cmotif)ligand2(CCL2)tomechanicalhypersensitivityaftersurgicalincisioninrats.
Anesthesiology2010;112:1250–8.
[193]PetraviczJ,FiaccoTA,McCarthyKD.
LossofIP3receptor-dependentCa2+increasesinhippocampalastrocytesdoesnotaffectbaselineCA1pyramidalneuronsynapticactivity.
JNeurosci2008;28:4967–73.
[194]PiaoZG,ChoIH,ParkCK,HongJP,ChoiSY,LeeSJ,LeeS,ParkK,KimJS,OhSB.
Activationofgliaandmicroglialp38MAPKinmedullarydorsalhorncontributestotactilehypersensitivityfollowingtrigeminalsensorynerveinjury.
PAIN2006;121:219–31.
[195]PorrecaF,OssipovMH,GebhartGF.
Chronicpainandmedullarydescendingfacilitation.
TrendsNeurosci2002;25:319–25.
[196]RaczI,NadalX,AlferinkJ,BanosJE,RehneltJ,MartinM,PintadoB,Gutierrez-AdanA,SanguinoE,BelloraN,ManzanaresJ,ZimmerA,MaldonadoR.
Interferon-gammaisacriticalmodulatorofCB(2)cannabinoidreceptorsignalingduringneuropathicpain.
JNeurosci2008;28:12136–45.
[197]RaghavendraV,TangaF,DeLeoJA.
Inhibitionofmicroglialactivationattenuatesthedevelopmentbutnotexistinghypersensitivityinaratmodelofneuropathy.
JPharmacolExpTher2003;306:624–30.
[198]RaghavendraV,TangaFY,DeLeoJA.
CompleteFreundsadjuvant-inducedperipheralinammationevokesglialactivationandproinammatorycytokineexpressionintheCNS.
EurJNeurosci2004;20:467–73.
[199]RaivichG.
Likecopsonthebeat:theactiveroleofrestingmicroglia.
TrendsNeurosci2005;28:571–3.
[200]RenK,DubnerR.
Interactionsbetweentheimmuneandnervoussystemsinpain.
NatMed2010;16:1267–76.
[201]RenWH,GuoJD,CaoH,WangH,WangPF,ShaH,JiRR,ZhaoZQ,ZhangYQ.
IsendogenousD-serineintherostralanteriorcingulatecortexnecessaryforpain-relatednegativeaffectJNeurochem2006;96:1636–47.
[202]Romero-SandovalEA,HorvathR,LandryRP,DeLeoJA.
Cannabinoidreceptortype2activationinducesamicroglialanti-inammatoryphenotypeandreducesmigrationviaMKPinductionandERKdephosphorylation.
MolPain2009;5:25.
16R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[203]RothsteinJD,Dykes-HobergM,PardoCA,BristolLA,JinL,KunclRW,KanaiY,HedigerMA,WangY,SchielkeJP,WeltyDF.
Knockoutofglutamatetransportersrevealsamajorroleforastroglialtransportinexcitotoxicityandclearanceofglutamate.
Neuron1996;16:675–86.
[204]RothsteinJD,MartinL,LeveyAI,Dykes-HobergM,JinL,WuD,NashN,KunclRW.
Localizationofneuronalandglialglutamatetransporters.
Neuron1994;13:713–25.
[205]RuscheweyhR,Wilder-SmithO,DrdlaR,LiuXG,SandkuhlerJ.
Long-termpotentiationinspinalnociceptivepathwaysasanoveltargetforpaintherapy.
MolPain2011;7:20.
[206]SamadTA,MooreKA,SapirsteinA,BilletS,AllchorneA,PooleS,BonventreJV,WoolfCJ.
Interleukin-1beta-mediatedinductionofCox-2intheCNScontributestoinammatorypainhypersensitivity.
Nature2001;410:471–5.
[207]SchafersM,LeeDH,BrorsD,YakshTL,SorkinLS.
Increasedsensitivityofinjuredandadjacentuninjuredratprimarysensoryneuronstoexogenoustumornecrosisfactor-alphaafterspinalnerveligation.
JNeurosci2003;23:3028–38.
[208]ScherrerG,LowSA,WangX,ZhangJ,YamanakaH,UrbanR,SolorzanoC,HarperB,HnaskoTS,EdwardsRH,BasbaumAI.
VGLUT2expressioninprimaryafferentneuronsisessentialfornormalacutepainandinjury-inducedheathypersensitivity.
ProcNatlAcadSciUSA2010;107:22296–301.
[209]ScholzJ,WoolfCJ.
Theneuropathicpaintriad:neurons,immunecellsandglia.
NatNeurosci2007;10:1361–8.
[210]SerhanCN,ChiangN,VanDykeTE.
Resolvinginammation:dualanti-inammatoryandpro-resolutionlipidmediators.
NatRevImmunol2008;8:349–61.
[211]ShiYQ.
Chronic-pain-associatedastrocyticreactioninthespinalcordofHIV-infectedpatients.
JNeurosci2012;32(32):10833–40.
[212]SloaneEM,SoderquistRG,MaierSF,MahoneyMJ,WatkinsLR,MilliganED.
Long-termcontrolofneuropathicpaininanon-viralgenetherapyparadigm.
GeneTher2009;16:470–5.
[213]SommerC,SchafersM,MarziniakM,ToykaKV.
Etanerceptreduceshyperalgesiainexperimentalpainfulneuropathy.
JPeripherNervSyst2001;6:67–72.
[214]SongP,ZhaoZQ.
Theinvolvementofglialcellsinthedevelopmentofmorphinetolerance.
NeurosciRes2001;39:281–6.
[215]SorgeRE,TrangT,DorfmanR,SmithSB,BeggsS,RitchieJ,AustinJS,ZaykinDV,VanderMH,CostiganM,HerbertTA,Yarkoni-AbitbulM,TichauerD,LivnehJ,GershonE,ZhengM,TanK,JohnSL,SladeGD,JordanJ,WoolfCJ,PeltzG,MaixnerW,DiatchenkoL,SeltzerZ,SalterMW,MogilJS.
GeneticallydeterminedP2X7receptorporeformationregulatesvariabilityinchronicpainsensitivity.
NatMed2012;18:595–9.
[216]SorkinLS,XiaoWH,WagnerR,MyersRR.
Tumournecrosisfactor-alphainducesectopicactivityinnociceptiveprimaryafferentbres.
Neuroscience1997;81:255–62.
[217]SouslovaV,CesareP,DingY,AkopianAN,StanfaL,SuzukiR,CarpenterK,DickensonA,BoyceS,HillR,Nebenuis-OosthuizenD,SmithAJ,KiddEJ,WoodJN.
Warm-codingdecitsandaberrantinammatorypaininmicelackingP2X3receptors.
Nature2000;407:1015–7.
[218]SpataroLE,SloaneEM,MilliganED,Wieseler-FrankJ,SchoenigerD,JekichBM,BarrientosRM,MaierSF,WatkinsLR.
Spinalgapjunctions:potentialinvolvementinpainfacilitation.
JPain2004;5:392–405.
[219]StanilandAA,ClarkAK,WodarskiR,SassoO,MaioneF,D'AcquistoF,MalcangioM.
Reducedinammatoryandneuropathicpainanddecreasedspinalmicroglialresponseinfractalkinereceptor(CX3CR1)knockoutmice.
JNeurochem2010;114:1143–57.
[220]StellwagenD,BeattieEC,SeoJY,MalenkaRC.
DifferentialregulationofAMPAreceptorandGABAreceptortrafckingbytumornecrosisfactor-alpha.
JNeurosci2005;25:3219–28.
[221]StephanAH,BarresBA,StevensB.
Thecomplementsystem:anunexpectedroleinsynapticpruningduringdevelopmentanddisease.
AnnuRevNeurosci2012;35:369–89.
[222]SunS,CaoH,HanM,LiTT,PanHL,ZhaoZQ,ZhangYQ.
Newevidencefortheinvolvementofspinalfractalkinereceptorinpainfacilitationandspinalglialactivationinratmodelofmonoarthritis.
PAIN2007;129:64–75.
[223]SunW,McConnellE,PareJF,XuQ,ChenM,PengW,LovattD,HanX,SmithY,NedergaardM.
Glutamate-dependentneuroglialcalciumsignalingdiffersbetweenyoungandadultbrain.
Science2013;339:197–200.
[224]SungB,LimG,MaoJ.
Alteredexpressionanduptakeactivityofspinalglutamatetransportersafternerveinjurycontributetothepathogenesisofneuropathicpaininrats.
JNeurosci2003;23:2899–910.
[225]SungCS,WenZH,ChangWK,ChanKH,HoST,TsaiSK,ChangYC,WongCS.
Inhibitionofp38mitogen-activatedproteinkinaseattenuatesinterleukin-1beta-inducedthermalhyperalgesiaandinduciblenitricoxidesynthaseexpressioninthespinalcord.
JNeurochem2005;94:742–52.
[226]SuterMR,BertaT,GaoYJ,DecosterdI,JiRR.
LargeA-beractivityisrequiredformicroglialproliferationandp38MAPKactivationinthespinalcord:differenteffectsofresiniferatoxinandbupivacaineonspinalmicroglialchangesaftersparednerveinjury.
MolPain2009;5:53.
[227]SuterMR,WenYR,DecosterdI,JiRR.
DoglialcellscontrolpainNeuronGliaBiol2007;3:255–68.
[228]SvenssonCI,FitzsimmonsB,AziziS,PowellHC,HuaXY,YakshTL.
Spinalp38betaisoformmediatestissueinjury-inducedhyperalgesiaandspinalsensitization.
JNeurochem2005;92:1508–20.
[229]SvenssonCI,MarsalaM,WesterlundA,CalcuttNA,CampanaWM,FreshwaterJD,CatalanoR,FengY,ProtterAA,ScottB,YakshTL.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliaisacriticallinkininammation-inducedspinalpainprocessing.
JNeurochem2003;86:1534–44.
[230]SvenssonCI,SchafersM,JonesTL,PowellH,SorkinLS.
SpinalblockadeofTNFblocksspinalnerveligation-inducedincreasesinspinalP-p38.
NeurosciLett2005;379:209–13.
[231]SvenssonCI,ZattoniM,SerhanCN.
Lipoxinsandaspirin-triggeredlipoxininhibitinammatorypainprocessing.
JExpMed2007;204:245–52.
[232]SweitzerS,MartinD,DeLeoJA.
Intrathecalinterleukin-1receptorantagonistincombinationwithsolubletumornecrosisfactorreceptorexhibitsananti-allodynicactioninaratmodelofneuropathicpain.
Neuroscience2001;103:529–39.
[233]TakedaM,KitagawaJ,TakahashiM,MatsumotoS.
Activationofinterleukin-1betareceptorsuppressesthevoltage-gatedpotassiumcurrentsinthesmall-diametertrigeminalganglionneuronsfollowingperipheralinammation.
PAIN2008;139:594–602.
[234]TakedaM,TakahashiM,MatsumotoS.
Contributionofactivatedinterleukinreceptorsintrigeminalganglionneuronstohyperalgesiaviasatelliteglialinterleukin-1betaparacrinemechanism.
BrainBehavImmun2008;22:1016–23.
[235]TakedaM,TakahashiM,MatsumotoS.
Contributionoftheactivationofsatellitegliainsensorygangliatopathologicalpain.
NeurosciBiobehavRev2009;33:784–92.
[236]TakedaM,TanimotoT,KadoiJ,NasuM,TakahashiM,KitagawaJ,MatsumotoS.
Enhancedexcitabilityofnociceptivetrigeminalganglionneuronsbysatelliteglialcytokinefollowingperipheralinammation.
PAIN2007;129:155–66.
[237]TanPH,GaoYJ,BertaT,XuZZ,JiRR.
Shortsmall-interferingRNAsproduceinterferon-alpha-mediatedanalgesia.
BrJAnaesth2012;108:662–9.
[238]TangaFY,Nutile-McMenemyN,DeLeoJA.
TheCNSroleofToll-likereceptor4ininnateneuroimmunityandpainfulneuropathy.
ProcNatlAcadSciUSA2005.
[239]ThackerMA,ClarkAK,BishopT,GristJ,YipPK,MoonLD,ThompsonSW,MarchandF,McMahonSB.
CCL2isakeymediatorofmicrogliaactivationinneuropathicpainstates.
EurJPain2009;13:263–72.
[240]TheriaultE,FrankensteinUN,HertzbergEL,NagyJI.
Connexin43andastrocyticgapjunctionsintheratspinalcordafteracutecompressioninjury.
JCompNeurol1997;382:199–214.
[241]ToddAJ.
Neuronalcircuitryforpainprocessinginthedorsalhorn.
NatRevNeurosci2010;11:823–36.
[242]ToyomitsuE,TsudaM,YamashitaT,Tozaki-SaitohH,TanakaY,InoueK.
CCL2promotesP2X4receptortrafckingtothecellsurfaceofmicroglia.
PurinergicSignal2012;8:301–10.
[243]Tozaki-SaitohH,TsudaM,MiyataH,UedaK,KohsakaS,InoueK.
P2Y12receptorsinspinalmicrogliaarerequiredforneuropathicpainafterperipheralnerveinjury.
JNeurosci2008;28:4949–56.
[244]TrangT,BeggsS,SalterMW.
ATPreceptorsgatemicrogliasignalinginneuropathicpain.
ExpNeurol2012;234:354–61.
[245]TrangT,BeggsS,WanX,SalterMW.
P2X4-receptor-mediatedsynthesisandreleaseofbrain-derivedneurotrophicfactorinmicrogliaisdependentoncalciumandp38-mitogen-activatedproteinkinaseactivation.
JNeurosci2009;29:3518–28.
[246]TremblayME,StevensB,SierraA,WakeH,BessisA,NimmerjahnA.
Theroleofmicrogliainthehealthybrain.
JNeurosci2011;31:16064–9.
[247]TsudaM,InoueK,SalterMW.
Neuropathicpainandspinalmicroglia:abigproblemfrommoleculesin''small''glia.
TrendsNeurosci2005;28:101–7.
[248]TsudaM,KohroY,YanoT,TsujikawaT,KitanoJ,Tozaki-SaitohH,KoyanagiS,OhdoS,JiRR,SalterMW,InoueK.
JAK-STAT3pathwayregulatesspinalastrocyteproliferationandneuropathicpainmaintenanceinrats.
Brain2011;134(4):1127–39.
[249]TsudaM,KuboyamaK,InoueT,NagataK,Tozaki-SaitohH,InoueK.
BehavioralphenotypesofmicelackingpurinergicP2X4receptorsinacuteandchronicpainassays.
MolPain2009;5:28.
[250]TsudaM,MasudaT,KitanoJ,ShimoyamaH,Tozaki-SaitohH,InoueK.
IFN-gammareceptorsignalingmediatesspinalmicrogliaactivationdrivingneuropathicpain.
ProcNatlAcadSciUSA2009;106:8032–7.
[251]TsudaM,MizokoshiA,Shigemoto-MogamiY,KoizumiS,InoueK.
Activationofp38mitogen-activatedproteinkinaseinspinalhyperactivemicrogliacontributestopainhypersensitivityfollowingperipheralnerveinjury.
Glia2004;45:89–95.
[252]TsudaM,Shigemoto-MogamiY,KoizumiS,MizokoshiA,KohsakaS,SalterMW,InoueK.
P2X4receptorsinducedinspinalmicrogliagatetactileallodyniaafternerveinjury.
Nature2003;424:778–83.
[253]TsudaM,Tozaki-SaitohH,InoueK.
Painandpurinergicsignaling.
BrainResRev2010;63:222–32.
[254]UlmannL,HatcherJP,HughesJP,ChaumontS,GreenPJ,ConquetF,BuellGN,ReeveAJ,ChessellIP,RassendrenF.
Up-regulationofP2X4receptorsinspinalmicrogliaafterperipheralnerveinjurymediatesBDNFreleaseandneuropathicpain.
JNeurosci2008;28:11263–8.
[255]VanSJ,Reaux-LeGA,PommierB,MauborgneA,DansereauMA,KitabgiP,SarretP,PohlM,MelikPS.
CCL2releasedfromneuronalsynapticvesiclesinthespinalcordisamajormediatoroflocalinammationandpainafterperipheralnerveinjury.
JNeurosci2011;31:5865–75.
[256]Vega-AvelairaD,McKelveyR,HathwayG,FitzgeraldM.
Theemergenceofadolescentonsetpainhypersensitivityfollowingneonatalnerveinjury.
MolPain2012;8:30.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx17Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[257]VergeGM,MilliganED,MaierSF,WatkinsLR,NaeveGS,FosterAC.
Fractalkine(CX3CL1)andfractalkinereceptor(CX3CR1)distributioninspinalcordanddorsalrootgangliaunderbasalandneuropathicpainconditions.
EurJNeurosci2004;20:1150–60.
[258]VerkhratskyA,SofroniewMV,MessingA,deLanerolleNC,RempeD,RodriguezJJ,NedergaardM.
Neurologicaldiseasesasprimarygliopathies:areassessmentofneurocentrism.
ASNNeuro2012;4.
[259]VikmanKS,DugganAW,SiddallPJ.
Interferon-gammainduceddisruptionofGABAergicinhibitioninthespinaldorsalhorninvivo.
PAIN2007;133:18–28.
[260]VikmanKS,HillRH,BackstromE,RobertsonB,KristenssonK.
Interferon-gammainducescharacteristicsofcentralsensitizationinspinaldorsalhornneuronsinvitro.
PAIN2003;106:241–51.
[261]VitJP,OharaPT,BhargavaA,KelleyK,JasminL.
SilencingtheKir4.
1potassiumchannelsubunitinsatelliteglialcellsoftherattrigeminalganglionresultsinpain-likebehaviorintheabsenceofnerveinjury.
JNeurosci2008;28:4161–71.
[262]WallraffA,KohlingR,HeinemannU,TheisM,WilleckeK,SteinhauserC.
Theimpactofastrocyticgapjunctionalcouplingonpotassiumbufferinginthehippocampus.
JNeurosci2006;26:5438–47.
[263]WangF,SmithNA,XuQ,FujitaT,BabaA,MatsudaT,TakanoT,BekarL,NedergaardM.
AstrocytesmodulateneuralnetworkactivitybyCa(2)(+)-dependentuptakeofextracellularK(+).
SciSignal2012;5:ra26.
[264]WangH,GuoW,YangK,WeiF,DubnerR,RenK.
Contributionofprimaryafferentinputtotrigeminalastroglialhyperactivity,cytokineinductionandNMDAreceptorphosphorylation.
OpenPainJ2010:144–52.
[265]WangX,ArcuinoG,TakanoT,LinJ,PengWG,WanP,LiP,XuQ,LiuQS,GoldmanSA,NedergaardM.
P2X7receptorinhibitionimprovesrecoveryafterspinalcordinjury.
NatMed2004;10:821–7.
[266]WangX,LouN,XuQ,TianGF,PengWG,HanX,KangJ,TakanoT,NedergaardM.
AstrocyticCa2+signalingevokedbysensorystimulationinvivo.
NatNeurosci2006;9:816–23.
[267]WangXW,HuS,Mao-YingQL,LiQ,YangCJ,ZhangH,MiWL,WuGC,WangYQ.
Activationofc-junN-terminalkinaseinspinalcordcontributestobreastcancerinducedbonepaininrats.
MolBrain2012;5:21.
[268]WangXW,LiTT,ZhaoJ,Mao-YingQL,ZhangH,HuS,LiQ,MiWL,WuGC,ZhangYQ,WangYQ.
Extracellularsignal-regulatedkinaseactivationinspinalastrocytesandmicrogliacontributestocancer-inducedbonepaininrats.
Neuroscience2012;217:172–81.
[269]WangZ,MaW,ChabotJG,QuirionR.
Calcitoningene-relatedpeptideasaregulatorofneuronalCaMKII-CREB,microglialp38-NFkappaBandastroglialERK-Stat1/3cascadesmediatingthedevelopmentoftolerancetomorphine-inducedanalgesia.
PAIN2010;151:194–205.
[270]WatkinsLR,HutchinsonMR,JohnstonIN,MaierSF.
Glia:novelcounter-regulatorsofopioidanalgesia.
TrendsNeurosci2005;28:661–9.
[271]WatkinsLR,HutchinsonMR,RiceKC,MaierSF.
The''toll''ofopioid-inducedglialactivation:improvingtheclinicalefcacyofopioidsbytargetingglia.
TrendsPharmacolSci2009;30:581–91.
[272]WatkinsLR,MartinD,UlrichP,TraceyKJ,MaierSF.
Evidencefortheinvolvementofspinalcordgliainsubcutaneousformalininducedhyperalgesiaintherat.
PAIN1997;71:225–35.
[273]WatkinsLR,MilliganED,MaierSF.
Glialactivation:adrivingforceforpathologicalpain.
TrendsNeurosci2001;24:450–5.
[274]WeiF,GuoW,ZouS,RenK,DubnerR.
Supraspinalglial–neuronalinteractionscontributetodescendingpainfacilitation.
JNeurosci2008;28:10482–95.
[275]WenYR,SuterMR,JiRR,YehGC,WuYS,WangKC,KohnoT,SunWZ,WangCC.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliacontributestoincision-inducedmechanicalallodynia.
Anesthesiology2009;110:155–65.
[276]WenYR,SuterMR,KawasakiY,HuangJ,PertinM,KohnoT,BerdeCB,DecosterdI,JiRR.
Nerveconductionblockadeinthesciaticnervepreventsbutdoesnotreversetheactivationofp38mitogen-activatedproteinkinaseinspinalmicrogliaintheratsparednerveinjurymodel.
Anesthesiology2007;107:312–21.
[277]WenYR,TanPH,ChengJK,LiuYC,JiRR.
Microglia:apromisingtargetfortreatingneuropathicandpostoperativepain,andmorphinetolerance.
JFormosMedAssoc2011;110:487–94.
[278]WengHR,ChenJH,CataJP.
Inhibitionofglutamateuptakeinthespinalcordinduceshyperalgesiaandincreasedresponsesofspinaldorsalhornneuronstoperipheralafferentstimulation.
Neuroscience2006;138:1351–60.
[279]WeyerbacherAR,XuQ,TamasdanC,ShinSJ,InturrisiCE.
N-methyl-D-aspartatereceptor(NMDAR)independentmaintenanceofinammatorypain.
PAIN2010;148:237–46.
[280]WhiteFA,JungH,MillerRJ.
Chemokinesandthepathophysiologyofneuropathicpain.
ProcNatlAcadSciUSA2007;104:20151–8.
[281]WhiteFA,SunJ,WatersSM,MaC,RenD,RipschM,SteikJ,CortrightDN,LaMotteRH,MillerRJ.
Excitatorymonocytechemoattractantprotein-1signalingisup-regulatedinsensoryneuronsafterchroniccompressionofthedorsalrootganglion.
ProcNatlAcadSciUSA2005;102:14092–7.
[282]WilkersonJL,GentryKR,DenglerEC,WallaceJA,KerwinAA,ArmijoLM,KuhnMN,ThakurGA,MakriyannisA,MilliganED.
IntrathecalcannabilactoneCB(2)Ragonist,AM1710,controlspathologicalpainandrestoresbasalcytokinelevels.
PAIN2012;153:1091–106.
[283]WillemenHL,EijkelkampN,WangH,DantzerR,DornGW,KelleyKW,HeijnenCJ,KavelaarsA.
Microglial/macrophageGRK2determinesdurationofperipheralIL-1beta-inducedhyperalgesia:contributionofspinalcordCX3CR1,p38andIL-1signaling.
PAIN2010;150:550–60.
[284]WillemenHL,HuoXJ,Mao-YingQL,ZijlstraJ,HeijnenCJ,KavelaarsA.
MicroRNA-124asanoveltreatmentforpersistenthyperalgesia.
JNeuroinamm2012;9:143.
[285]WoolfCJ.
Centralsensitization:implicationsforthediagnosisandtreatmentofpain.
PAIN2011;152:S2–S15.
[286]WuG,RingkampM,HartkeTV,MurinsonBB,CampbellJN,GrifnJW,MeyerRA.
EarlyonsetofspontaneousactivityinuninjuredC-bernociceptorsafterinjurytoneighboringnervebers.
JNeurosci2001;21:RC140.
[287]XieW,StrongJA,ZhangJM.
Earlyblockadeofinjuredprimarysensoryafferentsreducesglialcellactivationintworatneuropathicpainmodels.
Neuroscience2009;160:847–57.
[288]XinWJ,WengHR,DoughertyPM.
PlasticityinexpressionoftheglutamatetransportersGLT-1andGLASTinspinaldorsalhornglialcellsfollowingpartialsciaticnerveligation.
MolPain2009;5:15.
[289]XuJT,XinWJ,ZangY,WuCY,LiuXG.
Theroleoftumornecrosisfactor-alphaintheneuropathicpaininducedbylumbar5ventralroottransectioninrat.
PAIN2006;123:306–21.
[290]XuZZ,BertaT,JiRR.
ResolvinE1inhibitsneuropathicpainandspinalcordmicroglialactivationfollowingperipheralnerveinjury.
JNeuroimmunPharmacol2013;8:37–41.
[291]XuZZ,LiuXJ,BertaT,ParkC,LuN,SerhanCN,JiRR.
Neuroprotectin/protectin-1protectsneuropathicpaininmicefollowingnervetrauma.
AnnNeurol2013.
http://dx.
doi.
org/10.
1002/ana.
23928.
[Epubaheadofprint].
[292]XuZZ,ZhangL,LiuT,ParkJY,BertaT,YangR,SerhanCN,JiRR.
ResolvinsRvE1andRvD1attenuateinammatorypainviacentralandperipheralactions.
NatMed2010;16:1p.
[293]YoshimuraM,JessellTM.
Primaryafferent-evokedsynapticresponsesandslowpotentialgenerationinratsubstantiagelatinosaneuronsinvitro.
JNeurophysiol1989;62:96–108.
[294]ZeilhoferHU,BenkeD,YevenesGE.
Chronicpainstates:pharmacologicalstrategiestorestorediminishedinhibitoryspinalpaincontrol.
AnnuRevPharmacolToxicol2012;52:111–33.
[295]ZhangH,MeiX,ZhangP,MaC,WhiteFA,DonnellyDF,LaMotteRH.
Alteredfunctionalpropertiesofsatelliteglialcellsincompressedspinalganglia.
Glia2009;57:1588–99.
[296]ZhangH,NeiH,DoughertyPM.
Ap38mitogen-activatedproteinkinase-dependentmechanismofdisinhibitioninspinalsynaptictransmissioninducedbytumornecrosisfactor-alpha.
JNeurosci2010;30:12844–55.
[297]ZhangH,YoonSY,ZhangH,DoughertyPM.
Evidencethatspinalastrocytesbutnotmicrogliacontributetothepathogenesisofpaclitaxel-inducedpainfulneuropathy.
JPain2012;13:293–303.
[298]ZhangJ,DeKoninckY.
Spatialandtemporalrelationshipbetweenmonocytechemoattractantprotein-1expressionandspinalglialactivationfollowingperipheralnerveinjury.
JNeurochem2006;97:772–83.
[299]ZhangJ,HoffertC,VuHK,GroblewskiT,AhmadS,O'DonnellD.
InductionofCB2receptorexpressionintheratspinalcordofneuropathicbutnotinammatorychronicpainmodels.
EurJNeurosci2003;17:2750–4.
[300]ZhangJ,ShiXQ,EcheverryS,MogilJS,DeKoninckY,RivestS.
ExpressionofCCR2inbothresidentandbonemarrow-derivedmicrogliaplaysacriticalroleinneuropathicpain.
JNeurosci2007;27:12396–406.
[301]ZhangL,BertaT,XuZZ,LiuT,ParkJY,JiRR.
TNF-alphacontributestospinalcordsynapticplasticityandinammatorypain:distinctroleofTNFreceptorsubtypes1and2.
PAIN2011;152:419–27.
[302]ZhangRX,LiA,LiuB,WangL,RenK,ZhangH,BermanBM,LaoL.
IL-1raalleviatesinammatoryhyperalgesiathroughpreventingphosphorylationofNMDAreceptorNR-1subunitinrats.
PAIN2008;135:232–9.
[303]ZhangRX,LiuB,WangL,RenK,QiaoJT,BermanBM,LaoL.
Spinalglialactivationinanewratmodelofbonecancerpainproducedbyprostatecancercellinoculationofthetibia.
PAIN2005;118:125–36.
[304]ZhangX,ChenY,WangC,HuangLY.
NeuronalsomaticATPreleasetriggersneuron-satelliteglialcellcommunicationindorsalrootganglia.
ProcNatlAcadSciUSA2007;104:9864–9.
[305]ZhangZJ,DongYL,LuY,CaoS,ZhaoZQ,GaoYJ.
ChemokineCCL2anditsreceptorCCR2inthemedullarydorsalhornareinvolvedintrigeminalneuropathicpain.
JNeuroinamm2012;9:136.
[306]ZhaoP,WaxmanSG,HainsBC.
Extracellularsignal-regulatedkinase-regulatedmicroglia-neuronsignalingbyprostaglandinE2contributestopainafterspinalcordinjury.
JNeurosci2007;27:2357–68.
[307]ZhengFY,XiaoWH,BennettGJ.
Theresponseofspinalmicrogliatochemotherapy-evokedpainfulperipheralneuropathiesisdistinctfromthatevokedbytraumaticnerveinjuries.
Neuroscience2011;176:447–54.
[308]ZhengW,OuyangH,ZhengX,LiuS,MataM,FinkDJ,HaoS.
GlialTNFalphainthespinalcordregulatesneuropathicpaininducedbyHIVgp120applicationinrats.
MolPain2011;7:40.
[309]ZhongY,ZhouLJ,RenWJ,XinWJ,LiYY,ZhangT,LiuXG.
ThedirectionofsynapticplasticitymediatedbyC-bersinspinaldorsalhornisdecidedbySrc-familykinasesinmicroglia:theroleoftumornecrosisfactor-alpha.
BrainBehavImmun2010;24:874–80.
[310]ZhouD,ChenML,ZhangYQ,ZhaoZQ.
InvolvementofspinalmicroglialP2X7receptoringenerationoftolerancetomorphineanalgesiainrats.
JNeurosci2010;30:8042–7.
[311]ZhouZ,PengX,HaoS,FinkDJ,MataM.
HSV-mediatedtransferofinterleukin-10reducesinammatorypainthroughmodulationofmembranetumornecrosisfactoralphainspinalcordmicroglia.
GeneTher2008;15:183–90.
18R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[312]ZhuX,ConklinD,EisenachJC.
Cyclooxygenase-1inthespinalcordplaysanimportantroleinpostoperativepain.
PAIN2003;104:15–23.
[313]ZhuX,EisenachJC.
Cyclooxygenase-1inthespinalcordisalteredafterperipheralnerveinjury.
Anesthesiology2003;99:1175–9.
[314]ZhuangZY,GernerP,WoolfCJ,JiRR.
ERKissequentiallyactivatedinneurons,microglia,andastrocytesbyspinalnerveligationandcontributestomechanicalallodyniainthisneuropathicpainmodel.
PAIN2005;114:149–59.
[315]ZhuangZY,KawasakiY,TanPH,WenYR,HuangJ,JiRR.
RoleoftheCX3CR1/p38MAPKpathwayinspinalmicrogliaforthedevelopmentofneuropathicpainfollowingnerveinjury-inducedcleavageoffractalkine.
BrainBehavImmun2007;21:642–51.
[316]ZhuangZY,WenYR,ZhangDR,BorselloT,BonnyC,StrichartzGR,DecosterdI,JiRR.
Apeptidec-JunN-terminalkinase(JNK)inhibitorblocksmechanicalallodyniaafterspinalnerveligation:respectiverolesofJNKactivationinprimarysensoryneuronsandspinalastrocytesforneuropathicpaindevelopmentandmaintenance.
JNeurosci2006;26:3551–60.
[317]ZhuoM.
Corticalexcitationandchronicpain.
TrendsNeurosci2008;31:199–207.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx19Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022
Accumulatingevidencehasimplicated3typesofglialcellsinthedevelopmentandmain-tenanceofchronicpain:microgliaandastrocytesofthecentralnervoussystem(CNS),andsatelliteglialcellsofthedorsalrootandtrigeminalganglia.
Painfulsyndromesareassociatedwithdifferentglialactivationstates:(1)glialreaction(ie,upregulationofglialmarkerssuchasIBA1andglialbrillaryacidicprotein(GFAP)and/ormorphologicalchanges,includinghypertrophy,proliferation,andmodi-cationsofglialnetworks);(2)phosphorylationofmitogen-activatedproteinkinasesignalingpathways;(3)upregulationofadenosinetriphosphateandchemokinereceptorsandhemichannelsanddownreg-ulationofglutamatetransporters;and(4)synthesisandreleaseofglialmediators(eg,cytokines,che-mokines,growthfactors,andproteases)totheextracellularspace.
Althoughwidelydetectedinchronicpainresultingfromnervetrauma,inammation,cancer,andchemotherapyinrodents,andmorerecently,humanimmunodeciencyvirus-associatedneuropathyinhumanbeings,glialreaction(acti-vationstate1)isnotthoughttomediatepainsensitivitydirectly.
Instead,activationstates2to4havebeendemonstratedtoenhancepainsensitivityviaanumberofsynergisticneuro–glialinteractions.
Glialmediatorshavebeenshowntopowerfullymodulateexcitatoryandinhibitorysynaptictransmis-sionatpresynaptic,postsynaptic,andextrasynapticsites.
Glialactivationalsooccursinacutepaincon-ditions,andacuteopioidtreatmentactivatesperipheralgliatomaskopioidanalgesia.
Thus,chronicpaincouldbearesultof''gliopathy,''thatis,dysregulationofglialfunctionsinthecentralandperiph-eralnervoussystem.
Inthisreview,weprovideanupdateonrecentadvancesanddiscussremainingquestions.
2013InternationalAssociationfortheStudyofPain.
PublishedbyElsevierB.
V.
Allrightsreserved.
1.
IntroductionItisnowwellestablishedthatchronicpain,suchasinamma-torypain,neuropathicpain,andcancerpain,isanexpressionofneuralplasticity,bothintheperipheralnervoussystem(PNS)asperipheralsensitization[11,78]andinthecentralnervoussystem(CNS)ascentralsensitization[111,139].
Themostwidelystudiedneuronalmechanismsarehyperexcitabilityandsensitizationofprimarysensoryneurons(peripheralsensitization)andenhance-mentofexcitatorysynaptictransmissioninspinalcord,brainstem,andcorticalneurons(centralsensitization),causedbytranscrip-tional,translational,andpost-translationalregulation.
Otherneu-ronalmechanismsincludedisinhibition(reducedinhibitorysynaptictransmission),descendingpathwayfacilitation(eg,fromthebrainstemtothespinalcord),andlong-termpotentiation(LTP)inthecortexandspinalcord.
Theseneuronalmechanismshavebeenstronglyimplicatedinthedevelopmentandmainte-nanceofpersistentpaininrodents[11,142,195,205,317].
CentralsensitizationandLTParealsoinvolvedinhumanpainconditions[134,285].
Inparalleltotheprogressintheseneuronalmecha-nismsistheincreasedrecognitionoftheimportanceofnon-neuro-nalcells,especiallyglialcells,intheinitiationandmaintenanceofchronicpain.
Ofnote,overthelast10years,theeldofpainre-searchhaswitnessedadramaticincreaseinthenumberofpubli-cationsstudyinggliaandpain.
Numerousreviewshavebeenpublishedinhigh-impactjournalstoaddressthistopic[24,52,68,80,160,164,200,209,247,273].
Hereweprovideacom-prehensiveandupdatedreviewofgliaandpainbyintegratingre-centadvancesinboththepainandglialresearchelds.
GlialcellsintheCNSconsistof3majorgroups:astrocytes,microglia,andoligodendrocytes[69].
GlialcellsinthePNScon-sistofsatelliteglialcells(SGCs)inthedorsalrootganglia(DRGs)andtrigeminalganglia(TGs)andSchwanncellsintheperipheralnerves.
Thisreviewwillcover3typesofglia—microglia,0304-3959/$36.
002013InternationalAssociationfortheStudyofPain.
PublishedbyElsevierB.
V.
Allrightsreserved.
http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022Correspondingauthor.
Address:DepartmentofAnesthesiology,DukeUniversityMedicalCenter,595LaSalleStreet,GSRB-I,Room1027A,P.
O.
Box3094,NC27710,USA.
Tel.
:+1(919)6849387;fax:+1(919)6842411.
E-mailaddress:ru-rong.
ji@duke.
edu(R.
-R.
Ji).
PAINxxx(2013)xxx–xxxwww.
elsevier.
com/locate/painPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022astrocytes,andSGCs—astheirrolesinpainregulationarewelldocumented.
1.
1.
MicrogliaMicrogliaaremacrophage-likecellsintheCNSthatoriginatefrombonemarrow-derivedmonocytesthatmigrateduringperina-taldevelopment.
TheyareheterogeneouslydistributedthroughouttheCNS.
Undernormalconditions,microgliaarenotasquiescentasmanyinvestigatorsoriginallythought,asithasbeenshownthatmicrogliaactivelysensetheirenvironmentwiththeirramiedpro-cesses[93,175,199].
Notably,microgliadynamicallyinteractwithsynapsestomodulatetheirstructuresandfunctionsinhealthybrain[246].
Duringdevelopment,microglialprocessescanengulfsynapses,andsynapticpruningbymicroglia,whichinvolvestheactivationofthecomplementsystem,isnecessaryfornormalbraindevelopment.
[186,221].
Microgliaarefurtheractivatedaftervariousinsultssuchasnerveinjury,bydisplayingmorphologicalchanges,suchasachangefromramiedtoamoeboidshape[57]andupregulationofmicroglialmarkers(CCR3/CD11b,majorhistocompatibilitycom-plexII[MHCII],andionizedcalcium-bindingadaptormolecule-1[IBA1])[93,227](Table1).
Afterperipheralnerveinjury,microgliainthespinalcordundergorapidproliferation[14,23,55,151],andthisproliferationisalreadyveryprominent2daysaftersparednerveinjury[227].
Numerousstudieshavedemonstratedacriticalroleofmicrogliainthedevelopmentofneuropathicpain[43,113,197,252],aswellasacuteinammatorypain[229,311].
Minocycline,anonselectiveinhibitorofmicroglia,hasbeenshowntoreduceneuropathicpain,inammatorypain,andpostoperativepain[13,86,100,197],butitsroleinreducingtheestablishedlate-phaseneuropathicpainislim-ited[197].
Importantly,recentprogresshasidentiedalargenum-berofmoleculesthatareinducedinmicrogliaafterpainfulinjuries,especiallynervetrauma(Tables1–4).
1.
2.
AstrocytesAstrocytesarethemostabundantcellsintheCNSandwerehis-toricallyregardedassupportcells.
Workoverthepastdecadeindi-catesthatastrocytesplaymultipleactiverolesinacuteandchronicneuronaldiseasessuchasseizure,stroke,andischemia[133].
Un-likemicrogliaandoligodendrocytes,astrocytesformphysicallycouplednetworksmediatedbygapjunctions,which,amongotherfunctions,facilitateintercellulartransmissionofCa2+signalingandexchangeofcytosoliccontents,anddisplayoscillationsinionper-meabilitythroughastrocyticnetworks.
Gapjunctioncommunica-tionismediatedbyhomo-andheteromericassociationsofhemichannels,suchasconnexin-43(Cx43),thepredominantconn-exinexpressedinastrocytes[27].
Althoughastrocytesaretypicallyimmunelabeledbyglialbrillaryacidicprotein(GFAP),GFAPimmunoreactivitylabelsonlymajorbranchesandprocessesofastrocytes.
TheactualterritoryoccupiedbyanastrocyteismuchlargerthanthatrevealedbyGFAPimmunostaining.
Ofnote,eachastrocyteformsanon-overlappingterritoryordomain[106,133],whichcollectivelyresemblealatticeframework,appearingcrystal-lineinnature.
Althoughtheimplicationsofthisorganizationarenotfullyunderstood,itbecomeslostwhenastrocytestransitiontoreactivestates[181].
Inaddition,astrocyteshaveextensivecon-tactswithbothsynapsesandcerebralbloodvessels,andcontroltheincreaseinbloodowevokedbysynapticactivity.
Theastro-cyte-mediatedbloodowincreaseisfundamentaltotheblood-oxygen-level-dependent(BOLD)signaldetectedbyfunctionalmagneticresonanceimaging(fMRI)[106].
Itisestimatedthatasingleastrocytecanenwrap140,000syn-apsesand4to6neuronalsomata,andcancontact300to600neu-ronaldendritesinrodents.
[22,69,180].
Aclosecontactwithneuronsandsynapsesmakesitpossibleforastrocytesnotonlytosupportandnourishneuronsbutalsotoregulatetheexternalchemicalenvironmentduringsynaptictransmission.
Thegrowingappreciationforactiverolesofastrocyteshasledtotheproposalofa''tripartitesynapse''theory,basedonthefactsthat(1)gliarespondtoneuronalactivitywithanelevationoftheirinternalCa2+concentrationandtriggerthereleaseofchemicaltransmittersfromgliathemselves,and(2)glialtransmitterscausefeedbackregulationofneuronalactivityandsynapticstrength.
Table1Distinctreactionofmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions,asexaminedbyupregulationoftheglialmarkersIBA1,CD11b,andglialbrillaryacidicprotein(GFAP).
PainconditionsMicrogliaAstrocytesSGCsNerveinjury%%%Spinalcordinjury%%Pawincision%%InammationM/%%%Jointarthritis%%%BonecancerM/%%%SkincancerM%ChemotherapyM/%%%Diabetes%%HIVneuropathyM%Chronicopioid%%AcuteopioidMM%Detailed,withrelatedreferences,inSection2.
1.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
Table2Phosphorylationofmitogen-activatedproteinkinases(MAPKs;ERK,p38,JNK,ERK5)inmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions.
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryP-ERK%%%P-p38%%P-JNK%P-ERK5%SCIP-ERK%P-p38%PawincisionP-p38%InammationP-ERK%%P-p38%P-JNK%BonecancerPERK%%P-p38%P-JNK%SkincancerP-JNK%DiabetesP-ERK%P-p38%ChronicopioidP-ERK%P-p38%Detailed,withrelatedreferences,inSection2.
2.
SCI=spinalcordinjury.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
2R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022Accordingtothistheory,astrocyticprocessesareactivecomponentsofsynapses,inadditiontopre-andpost-synapticcomponents[7].
Althoughactivecontributiontosynapticactivityremainsapossibility,severalrecentstudieshavechallengedthetheoryofthetripartitesynapse,bydemonstratingthatalterationsinastrocyticCa2+donotmodulatesynaptictransmission[4,172,193].
Inreviewingtheseconclusions,however,itisimportanttonotethatmostoftheclassicalstudiesofthetripartitesynapsearebasedonelectrophysiologicalanalysisofacuteslicespreparedfromrodentpups.
Sincetheexpressionofmembraneproteinsaswellasneuralcircuitsundergosignicantchangesduringdevelopment[16,61],itispossiblethattheconceptofreceptor-mediatedCa2+signalingasakeyfeaturedeningastrocyticparticipationinhigherneuralfunctionwillbeexpandedtoincludeotherintracellularsignalingpathways.
Ofnote,gluta-mate-dependentneuroglialCa2+signalingdiffersbetweentheyoungandadultrodentbrain[223].
Thus,alternativepathwaysforastrocyticmodulationofsynaptictransmissionexist:1oftheessentialhousekeepingdutiesofastrocytesistomaintainpotassiumhemostasis.
Recently,ithasbeenshownthatreceptor-mediatedincreasesinastrocyticCa2+canmodulateneuralnetworkactivitybyactiveuptakeofextracellularK+[263].
BecausetheextracellularconcentrationofK+isanimportantdeterminantoftherestingmembranepotentialandtherebyofneuronalactivity,activeuptakeofK+representsasimpleyetpowerfultoolforrapidmodulationofneuralnetworks.
Studiesusingastroglialtoxins(eg,urocitrateanda-aminoadi-pate),astroglialaconitaseinhibitor(sodiumuoroacetate),orinhibitorsoftheastroglialenzymeglutaminesynthetase(eg,methioninesulfoximine)inadultanimalssuggestthatastrocytesareimportantbothfortheinductionandmaintenanceofinamma-toryandforneuropathicpain[30,31,69,83,110,161,184,200,272].
Proliferationofspinalcordastrocyteshasbeendemonstratedinmodelsofneuropathicpain,suchasrhizotomy[151]andspinalnerveligation[248].
Conversely,inhibitingastrocyteproliferationinthespinalcordwasshowntoreduceneuropathicpain[248].
1.
3.
SatelliteglialcellsSatelliteglialcells(SGCs)areprominentglialcellsinthePNS.
Theyarefoundnotonlyinsensoryglia(DRGsandTGs)butalsoinsympatheticandparasympatheticganglia.
LikeSchwanncells,SGCsarederivedfromneuralcrestcells.
SGCsarecharacterizedbythincellularsheathsthatsurroundtheindividualneurons.
Theyexhibitmanysimilaritiestoastrocytes:(1)bothexpresstheglialmarkersGFAP,S100,andglutaminesynthetase;and(2)bothformgapjunctions[89].
ThenumberofSGCsinDRGsandTGsismuchTable3Regulationofreceptors,channels,transporters,enzymes,andtranscriptionalfactorsinmicroglia,astrocytes,andsatelliteglialcells(SGCs)indifferentpainconditions.
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryP2X4%MP2X7%P2Y6%P2Y12%%TLR2MTLR3MTLR4%C1q,3,4,5%MCX3CR1%MCCR2%IFN-cR%Cx43%%Kir4.
1%&TRPM2MMGLT-1&GLAST&COX-1%COX-2%NF-kB%%NOX-2%STAT3%%c-Jun%CB2%SCICx43%InammationTLR3MTLR4%Cx43%TRPM2%GRK2&ALX&JointarthritisCX3CR1%BonecancerCX3CR1%HIVTLR2%TLR9&ChronicopioidP2X4%P2X7%TLR2%TLR4MDetailed,withrelatedreferences,inSection2.
3.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
Table4Regulationoftheglialmediatorscytokines,chemokines,growthfactors,andproteasesinmicroglia,astrocytes,andsatelliteglialcells(SGCs).
PainconditionsMicrogliaAstrocytesSGCsNerveinjuryTNF-a%IL-1b%%%IL-6%IL-18%CCL2%BDNF%bFGF%%MMP-2%%tPA%%CatS%TSP4%SCIIL-1b%InammationTNF-a%IL-1b%%IL-6%BonecancerTNF-a%IL-1b%IL-6%ChronicopioidTNF-a%IL-1b%IL-6%AcutemorphineIL-1b%Detailed,withrelatedreferences,inSection2.
4.
Symbols:Right-upwarddiagonalarrow(%)denotesupregulation;right&lefthori-zontalarrow(M)denotesnoregulation;right-downwarddiagonalarrow(&)denotesdownregulation.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx3Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022lowerthanthatofastroctyesinthespinalcord.
Unlikeastrocytes,eachSGCcontactsonly1neuron.
Strikingly,thegapofextracellu-larspacebetweentheSGCsheathandtheassociatedneuronalplasmamembranemeasuresonly20nm,allowingforcloseinter-actionsandeffectivesignalingbetweenneuronsandSGCs[89].
EmergingevidencesuggeststhatSGCsareactivatedafterpainfulinjuriesandplayanactiveroleinthedevelopmentofpersistentpain[29,54,91,107,150].
SGCsalsoexhibitenhancedcouplinginpersistentinammatoryandneuropathicpain[54,295].
2.
DifferentactivationstatesofgliaafterpainfulstimuliandinjuriesAfterpainfulstimuliandinjuries,gliaexhibitvariablealtera-tionsinfunctionsandmorphologies,includingthefollowing:(1)ionicchanges(eg,intracellularCa2+risesinastrocytes);(2)post-translationalregulation(eg,phosphorylationofmitogen-activatedproteinkinases[MAPK]);(3)translationalandtranscriptionalmodulation(eg,modulationofsurfacemolecules,glialmarkers,pro-andanti-inammatorymediators);(4)morphologicalchanges(eg,hypertrophy);and(5)proliferation.
Thesechangesareassoci-atedwithdifferentactivationstatesofglia(Fig.
1).
Belowwedis-cussactivationstatesthatarefrequentlymeasuredinthepainresearcheld.
2.
1.
Glialreaction:Changesinglialmarkersand/ormorphologyMoststudiesdeneglialactivationasupregulationoftheglialmarkerssuchasCCR3/CD11b,IBA1,andGFAP,whichareoften,butnotalways,associatedwithmorphologicalchanges(eg,hyper-trophyorprocessretraction/extension).
Thus,werefertothisglialactivationstateasglialreaction.
Observationsthatnerveinjuryinducesmicroglialresponsesdatebacktothe1970s[3].
Microglialreaction(microgliosis)inthespinalcordhasbeenintensivelyinvestigatedafterperipheralnerveinjury.
Nervetraumainducesveryrobustmicroglialreaction,suchashypertrophyandupregulationofthemicroglialmarkersCD11b,IBA1,andCD68inthespinalcordandbrainstem[118,252,300](Fig.
2).
IBA1isprobablythemostwidelyusedmar-kerformicroglialreactioninthepaineld,partlybecausetheIBA1antibodyfromWakoChemicalsworksbetterthanotherantibodiesofmicroglialmarkers.
Asexpected,microglialreactionisalsoveryrobustafterspinalcordinjury[86,102].
Furthermore,chronicopi-oidexposure,streptozotocin-induceddiabeticneuropathy,andsurgicalincisionresultinmicroglialreaction[49,192,275,310].
However,microglialreactionislessevidentafterbonecancer[98]andchemotherapy-inducedneuropathy[297,307],dependingonthedosesofchemotherapydrugsandseverityofnervedamageaftertumorgrowth(asshownbyATF-3expressioninDRGneu-rons)[23,303].
Intra-articularbutnotintraplantarinjectionofcompleteFreund'sadjuvant(CFA)inducesmicroglialreaction[222],becauseofdeeptissue(joint)injuryandpossibleaxonalinjury(Table1).
Interestingly,inyoungrats(P10),nerveFig.
1.
Differentactivationstatesofglia.
Gliaexhibitdifferentactivationstatesafterpainfulinjuries.
(1)Glialreactionreferstoupregulationofglialmarkersandmorphologicalchangesofglia(gliosis);(2)upregulationofglialreceptorssuchasadenosinetriphosphate(ATP)receptors,chemokinereceptors,andToll-likerecep-tors,whichwillleadtothethirdactivationstate:(3)activationofintracellularsignalingpathways,suchasmitogen-activatedproteinkinase(MAPK)pathways.
PhosphorylationofMAPKswillleadtothenextactivationstate:(4)upregulationofglialmediators,suchascytokines,chemokines,andgrowthfactors.
Uponrelease,theseglialmediatorscaninteractwithneuronstoelicitpainviacentralandperipheralsensitization.
Unlikeglialreaction(state1),theotheractivationstates(states2–4)havebeenshowntoinducepain.
Fig.
2.
Activationofmicrogliainthespinalcorddorsalhorn3daysaftersparednerveinjury(SNI)inrats.
(A)IB4staininginthespinalcorddorsalhornipsilateralandcontralateraltotheinjuryside.
NotealossofIB4staininginthedorsalhornregioninnervatedbytheinjurednervebranches.
(BandC)CD11b(OX-42)andphosphorylatedp38(p-p38)immunostaininginthedorsalhornipsilateralandcontralateraltotheinjuryside.
NoteoverlappingexpressionpatternsofOX-42andp-p38intheinjuryside.
(D)Doublestainingofp-p38(red)andOX-42(green)intheipsilateraldorsalhorn.
Lowerpanelpresentshigh-magnicationimagesof2microglialcells(indicatedbyarrowandarrowhead)fromtheupperpanel.
Notethatp-p38iscompletelyco-localizedwithOX-42.
Scale,100lm.
ImagesaremodiedfromWenetal.
[276],withpermission.
4R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022injury-evokedspinalmicroglialreactionisnotsoevident,inparal-lelwiththeabsenceofnerveinjury-inducedneuropathicpainintheseyounganimals[170,256].
Furthermore,priorneonatalinjurycan''prime''thespinalmicroglialresponsetoadultinjury,result-inginenhancedmicroglialreactivity[13].
Microgliacanalsobeprimedbypreviousinsultinadults,leadingtoenhancedpainintensityanddurationofthesecondinsult[87].
Comparedtomicroglialreaction,astrocytereactioninthespinalcordismoregeneralandevidentafterpainfulinjuries[69].
Robustastrocytereactionisinducednotonlybynervetraumaandspinalcordinjury[75,76,173,316],butalsobychronicopioidexposure[214],intraplantarorintra-articularCFAinjection[70,83,198,222],bone[98]andskincancer[67],chemotherapy,andhumanimmunodeciencyvirus(HIV)-inducedneuropathy[297].
Inaddition,itappearsthatastrocyticreactionismorepersis-tentthanmicroglialreaction.
IthasbeenshownthatGFAPandCD11bupregulationpeaksat150and14daysafternerveinjury,respectively,althoughCD11bupregulationremainsafter150days[298].
GFAPupregulationisalsoprominent9monthsafterspinalcordinjury[85,173].
Althoughmoststudieshavefocusedonglialreactioninthespinalcordandbrainstem,astrocytereactionhasalsobeenfoundintheforebrain,suchastheanteriorcingulatecor-tex,whichcontributestoaffectivepain[28].
OnecaveatisthatimmunohistochemistryofsomeGFAPantibodiesmaydetectcon-formationalorsolubilitychangesorpost-translationalmodica-tionsoftheproteinbutnotactualchangesinproteinexpression,becauseofdifferentxationconditions[15,56].
Thus,itisidealtovalidatetheresultsofGFAPimmunohistochemistrywithdiffer-entantibodiesanddifferentmethodssuchasWesternblotandquantitativepolymerasechainreaction(PCR).
LessisknownaboutSGCreaction(GFAPupregulation)afterpainfulinjuries.
SGCreactionisinducednotonlybynerveinjury[150,295]butalsobyinammation[235,236]inDRGsandTGs.
NerveinjuryfurtherresultsinSGCproliferation[107].
SGCsreac-tionafternerveinjuryandDRGcompressionisveryrapid,becom-ingevidentwithin4hours.
Thisreactionpeaksat1weekbutdeclinesafter3weeks.
ThistimecourseofSGCreactionsuggestsapossibleroleofSCGsintheinductionandearlymaintenanceofneuropathicpain[150,295].
AdministrationofglialtoxintoDRGshasbeenshowntoreduceneuropathicpain[150].
Also,thereisin-creasedcouplingbetweenSGCsafternerveinjury[185,295]andinammation[54].
Interestingly,evenacuteopioidtreatmentafterasubcutaneousinjectionresultsinmarkedSGCreactioninDRGsat2hourswhenmorphineanalgesiadeclines[18](Table1).
2.
2.
PhosphorylationofMAPKsandSrcingliaTheMAPKfamilyincludes3majormembers:extracellularsig-nal-regulatedkinase1and2(ERK1andERK2,respectively),p38,andc-JunN-terminalkinases(JNK)).
ERK5isanewfamilymemberandwasshowntobeactivatedinspinalmicrogliaafternervein-jury[177].
MAPKpathwaysplayanimportantroleinintracellularsignalinginneuronsandglia,andbotharerequiredforthegenesisofpersistentpain[109,178].
Interestingly,differentMAPKsexhibitdistinctactivation(phosphorylation)patternsinglialcellsafterpainfulinjuries[109](Table2).
Numerousstudieshaveshownincreasedphosphorylation(acti-vation)ofp38(P-p38)inspinalcordmicrogliaafternerveinjury[118,136,251](Fig.
2),spinalcordinjury[46,86],formalin-inducedacuteinammatorypain[229],surgery-evokedpostoperativepain[192,275],andchronicopioidexposure[47].
Nerveinjuryalsoacti-vatesmicroglialp38inthetrigeminalnucleus[194].
Ithasbeenshownthatthebisoformofp38(p38b)isexpressedinmicroglia[228].
Inaddition,P-p38isinducedinneuronsandSGCsofDRGsfollowingnerveinjury[118,179]andalsoinSGCsofTGsafterinammationinthetemporomandibularjoint[65].
P-JNKisinducedinspinalastrocytesafternerveinjury[316],CFA-inducedpersistentinammatorypain[70],bonecancer[267],andmelanoma-inducedskincancer[67].
Consistently,nerveinjuryalsoactivatestheupstreamactivatorofJNK,thetransform-inggrowthfactor-activatedkinase-1(TAK1),andthedownstreameffectorofJNK,c-Juninspinalastrocytes[125,316].
AmongseveralJNKisoforms(JNK1,2,3),JNK1wasshowntobeexpressedinspinalastrocytes[70].
P-ERKinductioningliaafterinjuryishighlydynamic:inductioninspinalmicrogliacorrespondstotheearly-phase(rstweek),andgraduallytransitionstoastrocytesinthelatephaseafternervein-juryandbonecancer[268,314].
CFAalsoinducesP-ERKinspinalastrocytesinthelatephase[279].
Furthermore,nerveinjuryevokesP-ERKinSGCsofDRGs[314],andtemporomandibularjointinammationelicitsP-ERKinSGCsofTGs[65].
MAPKsareactivatedbyproinammatorymediators[109]andinactivatedbyphosphatases,suchasMAPKphosphatase(MKP1,2,3).
Forexample,P-p38expressioninspinalmicrogliaafternerveinjurycanbesuppressedbyMKP3[171].
ActivationofCB2inmicrogliawasshowntoupregulateMKP1andMKP3,leadingtoareductionofP-ERKinmicroglia[202].
InammationinducesrapidupregulationofMKP1,MKP2,andMKP3inSGCsofTGs[65],whichmayregulatetheresolutionofinammatorypain.
MountingevidenceindicatesthatactivationofMAPKsinspinalcordglialcellsisessentialforthedevelopmentofpersistentpain[109].
Thus,intrathecalinjection(s)ofselectiveinhibitorsofMEK(ERKkinase),p38,andJNK,aswellasantisenseknockdownofERK5,attenuatedinammatory,neuropathic,andcancerpaininratsandmice[109].
Systemicinjectionofp38inhibitoralsore-ducedspinalnerveligation-inducedmechanicalallodyniainmice[113].
UpregulationofspinalMKP-3viagenetherapyattenuatesneuropathicpainbysuppressingP-p38[171].
TheimportanceofMAPKpathwaysforneuropathicpainhasalsobeendemonstratedinhumanbeings.
InHIVpatientswithneuropathicpain,P-ERK,P-p38,andP-JNKlevelsinthedorsalhornsaresignicantlyincreased,comparedtothoseinHIVpa-tientswithoutneuropathicpain[211].
Inadouble-blind,pla-cebo-controlledclinicaltrial,oraldeliveryofaselectivep38inhibitor,dilmapimod(SB-681323)attenuatedneuropathicpaininpatientswithnervetrauma,radiculopathy,orcarpaltunnelsyn-drome[6].
NerveinjuryalsoinducesphosphorylationofSrcfamilykinases(Src,Lyn,Fyn)inspinalmicroglia[126,250].
IntrathecalinfusionofaSrcinhibitor(PP2)reducednerveligation-elicitedneuropathicpain.
Ofinterest,PP2suppressedtheactivationofERKbutnotp38inspinalmicroglia[126].
2.
3.
Regulationofreceptors,channels,andtransportersingliaAsshowninTable3,multiplereceptors,channels,andtrans-portersareexpressedinglialcellsandareregulatedindifferentpainconditions.
Althoughthesemoleculesarenotsecreted,theyplayactiverolesinglialintracellularsignalingbyactivatingtheMAPKpathwaysandinducingthesynthesis,release,anduptakeofthesecretedmolecules(Table4).
ATPmodulatesglialactivationviaactivatingP2X(ionchannels)andP2Yreceptors(GPCR-coupled),andtheseATPreceptorsgatemicroglialsignalingforneuropathicpain[244,253].
PeripheralnerveinjuryupregulatesP2X4,P2X7,P2Y6,andP2Y12inspinalmicroglia;and,furthermore,neuropathicpainisreducedafterphar-macologicalinhibition,antisenseknockdown,orgeneticdeletionofP2X4,P2X7,P2Y6,orP2Y12[135–137,215,243,244,252,253].
MicelackingtheP2x4genedisplaydiminishedinammatorypainandbluntedneuropathicpain[249].
P2Y12isalsoinducedinSGCsofTGafternerveinjury,andinjectionofaP2Y12RantagonistintoTGreducestrigeminalneuropathicpain[124].
Moreover,chronicR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx5Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022opioidtreatmentupregulatesP2X4andP2X7inspinalmicroglia,andopioidtoleranceispreventedafterspinalknockdownofP2X4orP2X7[99,108,310].
However,arecentstudydemonstratedthatopioid-inducedhyperalgesiabutnottoleranceismediatedbyopioidreceptor-dependentexpressionofP2X4inmicroglia[60].
Toll-likereceptors(TLRs)areknowntoregulateinnateimmu-nityandhavebeenstronglyimplicatedinglialactivation[152,174].
Lipopolysaccride(LPS),anagonistofTLR4,ishighlypo-tentinactivatingmicroglia.
ItalsoactivatesTLR4inastrocytes[152].
Ofnote,spinalmicroglialreactionandneuropathicpainafternerveinjuryarereducedinTlr2knockoutmice[130]andTlr4mutantmice[238].
Arthriticpaininthelatephaseisalsore-ducedinTlr4knockoutmice[33].
Strikingly,malebutnotfemalemicewithTlr4mutationexhibitreducedneuropathicpain[168],suggestingsexdifferencesinTLR4andmicroglialsignaling.
Chronicmorphinewasshowntoinduceglialresponsesviaactiva-tionofTLR4[271].
Ofnote,opioid-inactiveisomerswereshowntoinducespinalproinammatoryresponsesviaactivationofTLR4[104].
PharmacologicalblockadeofTLR4signalinginvivoattenu-ateddevelopmentofanalgesictolerance,hyperalgesia,andopioidwithdrawalbehaviorsinrats[105].
Incontrast,Ferrinietal.
showedthatchronicmorphine-inducedhyperalgesiaisintactinTlr4mutantmice[60].
Microarrayanalysisrevealsthatthecomplementcomponents(eg,C1q,C3,C4,C5)areamongthemostregulatedtranscriptsinthespinalcordfollowingnerveinjury.
Inparticular,thesecompli-mentcomponentsareupregulatedinspinalmicroglia.
InductionofC5aRinspinalmicrogliahasbeenimplicatedinneuropathicpainsensitization[82].
Increasingevidencesuggeststhatchemokinereceptorscontrib-utetothepathogenesisofchronicpainviamodulatingglialactiva-tionandneuralplasticity[1,35,68,280].
CX3CL1(fractalkine)andCCL2(MCP-1)are2ofthemostwell-studiedchemokinesforpainmodulation.
Althoughachemokinenormallyactivatesmultiplereceptors,CX3CR1appearstobetheonlyknownreceptorforCX3CL1andisexclusivelyexpressedinmicroglia.
Thus,Cx3cr1-GFPmicehavebeenusedforstudyingthelocalizationandactiva-tionofmicroglia[175].
NerveinjuryandjointinammationinducearobustupregulationofCX3CR1inspinalmicroglia,andspinalblockadeofCX3CR1withaneutralizingantibodyinhibitedinam-matoryandneuropathicpain[165,222,257,315].
Consistently,micelackingCx3cr1exhibitedreducedinammatoryandneuro-pathicpain[219].
ComparedtoselectivemicroglialexpressionofCX3CR1,CCR2,amajorreceptorforCCL2,isexpressedinbothneu-ronsandmicroglia[2,72,81,84,121,305].
Nerveinjury-inducedspinalmicroglialreactionisabolishedinCcr2knockoutmice[300],whereasintrathecalCCL2causesmicrogliosisinthespinalcord[239,300].
NeuropathicpainisimpairedinCcr2knockoutmiceorafterspinalinjectionofCCR2antagonist[2,300,305].
Acti-vationofCCR2byCCL2alsorapidlymodulatesDRGneuronalsen-sitivityandspinalcordsynapticplasticity[72,81,315].
LikeLPS,interferon-c(IFN-c)isastrongactivatorofmicroglia,bymeansofinducingmicroglialreaction,P2X4upregulation,andLynphosphorylation[250].
NerveinjuryupregulatesINF-crecep-torsinspinalmicroglia,andnerveinjury-inducedmicroglialreac-tionandmechanicalallodyniaareabrogatedinIfncreceptorknockoutmice[250].
AstrocytesandSGCsarecharacterizedbyforminggapjunction-couplednetworks,leadingtothetransmissionofCa2+signalingthroughnetworks[7,54].
Connexinsarethemajorstructuralcom-ponentsofgapjunctions,andCx30andCx43areknowntobeex-pressedbyastrocytes[27].
Cx43isupregulatedinastrocytesafternervelesion,spinalcordinjury,andinammation[27,62,77,83,145].
Inhibitionofgapjunctionfunctionbycarbenox-olone(CBX),anonselectivegapjunctioninhibitor,reducesinam-matoryandneuropathicpain[140,218].
Inadditiontomodulatinggapjunctioncommunication,recentstudiesalsoproposedapara-crinesignalingofCx43toreleasekeyastrocyticmediatorssuchasATPandglutamate[123,148,240,262].
UnopposedCx43hemichan-nelsareidealformodulatingATPreleasepathways,asthebiophys-icalpropertiesofthesehemichannelsenablethemtoconducthighlevelsofATPefux[17,42,190].
Ofnote,SCI-inducedATPreleaseinthespinalcordisdiminishedafterCx43blockade[45].
IndoubleknockoutmicelackingCx30/Cx43,thedevelopmentofneuropathicpain(heathyperalgesiaandmechanicalallodynia)isprevented,andspinalastroglialreactionisreduced[27].
NerveinjurywasshowntoupregulateCx43inSGCsofTGs.
Ofinterest,reducingCx43expressioninSGCsviaRNAireducedneuropathicpaininnerve-injuredratsbutinducedpain-likebehaviorsinnormalrats,suggestingdifferentrolesofSGCs-Cx43inpainmodulationinnon-injuredvsinjuredanimals[183].
Notably,thegapjunctionblockerCBXalsoinhibitspannexin-1(PNX1),whichisexpressedinastro-cytesandmodulatesATPrelease[74].
TheroleofPNX1inpaincontrolneedsfurtherinvestigation.
Thefollowingionchannelshavealsobeenimplicatedforglialsignalinginpain.
TheK+channelsubunitKir4.
1isexpressedinSGCs,andsilencingthisK+subunitwithRANileadstopainhyper-sensitivity[261].
Thewaterchannelaquaporin-4(AQP4)isinducedinspinalcordastrocytesafterspinalcordinjury[173],andmicelackingAqp4displaydecreasedpainsensitivity(hypoalgesia)[9].
TRPM2isexpressedinmicrogliaandcontributestospinalcordmicroglialactivation.
Inammatoryandneuropathicpainareim-pairedinTrpm2knockoutmice[95].
TheglutamatetransporterssuchasGLT-1andGLASTareex-pressedinastrocytes(Table3)andregulatetheclearanceofgluta-matefromsynapticcleftsandextracellularspace,leadingtoalteredglutamatergictransmissionandneuronalplasticity[203,204].
Nerveinjuryandchronicmorphineelicitasustaineddown-regulation,afteraninitialupregulation,ofglutamatetrans-porter-1(GLT1)andglutamateandasparticacidtransporter(GLAST)inthespinalcord[158,224,288].
Inhibitionofglutamatetransportersresultsinanelevationinspinalextracellulargluta-mateandspontaneouspain[147,278].
Consistently,GLT-1genedeliverytothespinalcordattenuatesinammatoryandneuro-pathicpain[157],supportingaroleofastroglialglutamatetrans-portersintheresolutionofchronicpain.
Severalenzymesarealsoactivelyinvolvedinglialsignalinginpain.
Cyclooxygenase-1and-2(COX-1andCOX-2,respectively)areinducedinmicrogliaaftersurgicalincisionandnerveinjurytofacili-tatepostoperativeandneuropathicpain[306,312,313].
NADPHoxi-dase2(Nox2)expressionisinducedindorsalhornmicrogliaafterL5spinalnervetransection,andNox2-decientmiceshowedde-creasesinoxidativestress,microglialreaction,andproinammatorycytokineexpressioninthespinalcord,aswellasneuropathicpain[131].
Ofinterest,G-protein-coupledreceptorkinase(GRK2)inmicrogliawasimplicatedinthetransitionfromacutetochronicinammatorypain.
Spinalmicroglia/macrophageGRK2expressionisreducedafterinammation,leadingtotheactivationofmicrogliaandpersistentpainviap38andinterleukin-1b(IL-1b)signaling[283].
Furthermore,nerveinjuryupregulatesthetranscriptionalfac-torsinspinalcordglia,includingc-Juninastrocytes[316],signaltransducersandactivatorsoftranscription3(STAT3)inmicroglia[53]andastrocytes[248],andnuclearfactor-jB(NF-jB)inmicroglia[227]andastrocytes[167],toenhanceandmaintainneuropathicpain.
Finally,painfulinjuriesalsoinduceupregulationofanti-inam-matoryreceptorsingliafortheresolutionofacutepain.
Inamma-tionincreaseslipoxinreceptorALXexpressioninspinalcordastrocytes,andlipoxinA4reducesinammatorypainviainhibitingJNKphosphorylationinastrocytes[231].
LipoxinA4alsoattenu-atesmorphinetoleranceviamodulatingglialactivationandcyto-kineexpression[117].
Nerveinjuryincreasedcannabinnoid6R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022receptorCB2expressioninspinalmicroglia[299],andCB2agonistssuppressedmicroglialreactionandneuropathicpain[282].
OfinterestCb2knockoutmicedisplayedincreasedmicroglialandastrocyticreactivityinthespinalcordandenhancedneuropathicpain,whereastransgenicmiceoverexpressingCb2showedattenu-atedglialreactivityandneuropathicpain[196].
2.
4.
Regulationofcytokines,chemokines,growthfactors,andproteasesingliaAkeyissueregardingglialcontrolofpainistounderstandhowglialmediatorsareproducedandreleased.
AsshowninTable4,gliaproducebothlargemolecules(cytokines,chemokines,growthfactors,andproteases)andsmallmolecules(glutamate,ATP,D-serine,andprostaglandinE2(PGE2).
Theseglialmediatorscanmodulateneuronalandsynapticactivityandpainsensitivity.
Proinammatorycytokinessuchastumornecrosisfactor–a(TNF-a),IL-1b,andIL-6areamongthemostwell-studiedglialmediators.
Theyareupregulatedinspinalcordgliaafternervein-jury,inammation,bonecancer,andchronicopioidexposure,andtheycontributetothedevelopmentofandmaintenanceofinam-matory,neuropathic,andcanerpainandmorphinetolerance[52,213,230,273].
TNF-aisprimarilyproducedbymicrogliaandplaysanessentialroleinthegenerationofcentralsensitizationandpersistentpain[92,289,301,308],inadditiontoitswell-docu-mentedroleinmodulatingperipheralsensitization[119,207,216].
IL-1bisinducedinastrocytesafterbonecancer,inammation,andnerveinjury[83,274,279,303].
IL-1bcanalsobeproducedbymicrogliaandneuronsinthespinalcord[36,51,92].
InhibitionofspinalandbrainIL-1bsignalingreducesinammatory,neuro-pathic,andcancerpain[83,163,232,274,303]andenhancesmor-phineanalgesia[120,270].
IL-18ishighlyrelatedtoIL-1b,andbothrequirecaspase-1andinammasomesforactivecleavage[146].
NerveinjuryinducesIL-18expressioninspinalmicroglia[34,167].
Furthermore,painfulinjuriesinducecytokineexpressioninperipheralglia.
Forexample,nerveinjuryandCFAinammationincreaseIL-1bexpressioninSGCsofDRGsandTGs[127,234].
Ofnote,acutemorphineupregulatesIL-1bonlyinperipheralglia(SGCs)inDRGsbutnotincentralglia(microgliaandastrocytes)inthespinalcord[18].
Chemokinesareexpressedinglialcells,particularlyinastro-cytesintheCNS[68],aswellasinneurons[84].
Inprimarycul-turesofastrocytes,TNF-ainducedrapidexpressionofCCL2,CXCL10,andCXCL1[72].
SpinalinjectionofTNF-a-activatedastrocytesresultsinpersistentmechanicalallodyniaviareleasingCCL2[71].
SpinalnerveligationalsoinducesCCL2inspinalastro-cytes,andintrathecaladministrationofanMCP-1neutralizingantibodyreducesneuropathicpain[72].
CCL2expressionisfur-therincreasedinastrocytesofthemedullarydorsalhornandcon-tributestotrigeminalneuropathicpain[305].
Consistently,micewithCCL2overexpressioninastrocytesdisplaypainhypersensi-tivity[162].
Growthfactorsareknowntobeinducedinspinalgliabynerveinjury.
Inparticular,nerveligationupregulatesbrain-derivedneu-rotrophicfactor(BDNF)inspinalmicroglia,viaactivationofP2X4andp38[244,254].
SpinalinjectionofATP-activatedmicrogliaissufcienttoinducemechanicalallodyniaviareleasingBDNF,and,conversely,neuropathicpainissuppressedbyspinalblockadeoftheBDNFreceptorTrkB[43].
Furthermore,treatmentofmicroglialcultureswithmorphineincreasesBDNFrelease,whichdoesnotre-quirel-opioidreceptorandTLR[60].
BDNFisalsoinducedinDRGneuronsafternerveinjuryandcanbereleasedfromprimaryaffer-entsinthespinalcord[66,143].
UnlikeBDNF,basicbroblastgrowthfactor(bFGForFGF-2)isinducedinreactiveastrocytesofthespinalcordinthelatephase(3weeks)ofnerveinjury[110].
IntrathecalinfusionofbFGFproducespersistentactivationofspinalastrocytes(upregulationofP-JNKandGFAP)andsustainedmechanicalallodynia[110].
Bycontrast,intrathecaladministrationofabFGF-neutralizingantibodyattenuatesestablishedneuro-pathicpain[156].
Therefore,bFGFmaintainschronicpainviaacti-vationofastrocytes.
Proteasesarealsoupregulatedinspinalgliaafternerveinjury.
Notably,spinalnerveligationinducesmatrixmetalloprotease-2(MMP-2)inspinalcordastrocytesandDRGSGCsinthelatephaseofneuropathicpaintomaintainneuropathicpain,viaactivationofIL-1bandERK[127].
NerveinjuryfurtherinducescathepsinSinspinalmicroglia[37]andtissuetypeplasminogenactivator(tPA)inspinalastrocytes[138]toenhanceneuropathicpain.
Arecentstudyshowedthatnerveinjuryincreasestheexpressionofthrombospondin-4(TSP4),anextracellularmatrixglycoprotein,inspinalcordastrocytes.
Thisincreaseisnotonlycorrelatedbutalsorequiredforthedevelopmentneuropathicpain[132].
TSP4releasefromastrocytescanpromotesynaptogenesis.
Ofgreatinterest,thea2d-1calciumchannelsubunit,apossibletargetofgabapentin,wasshowntobeaneuronalreceptorofTSP4.
Thus,gabapentinmayinhibitneuropathicpainviamodulatingsynaptogenesis[58].
AstrocytesalsoproducesmallmoleculemediatorssuchasD-serine,ATP,andglutamatetoenhancepainstates[69].
Interestingly,inhibi-tionofglycinergictransmission,whichisknowntooccurinchronicpain,resultsinD-serinereleasefromastrocytestogeneratetactileallodynia[166].
D-serineisknownasanagonistofglycinesiteofN-methyl-D-aspartate(NMDA)receptors[176].
Inadditiontothepro-inammatoryandpronociceptivemedia-tors,glialcellsmayalsoproduceanti-inammatoryandantinoci-ceptivemediators,suchasIL-4,IL-10,andTGF-b[92]fortherecoveryandresolutionofpain[41,92,94,114,164].
Enhancementofendogenousproductionofinterleukin-10viagenetherapyhasbeenshowntoproducelong-termreliefinneuropathicpain[212].
Ofinterest,apossibleoff-targeteffectofhighdosesofsiR-NAsistoinduceIFN-ainspinalastrocytesforelicitingantinocicep-tiveeffects[237].
3.
Neuronal–glialandglial–glialinteractionsinpersistentpainBecausepainisconveyedonlybyneurotransmissionintheneu-ralcircuits,gliamustinteractwithneuronstomodulatepainsen-sitivity.
Herewefocusonneuronal–glial(neuronal–glial)(Section3.
1)andglial–glial(Section3.
2)interactionsintheCNSunderpersistentpainconditions(Fig.
3).
Wealsodiscussneuro–glialinteractionsinthePNSafterpainfulinjuriesandacutemor-phinetreatment(Section3.
3)(Fig.
4).
3.
1.
Neuronal–glialinteractions:SignalsfromneuronstogliaItisgenerallybelievedthatinjury-inducedspontaneousdis-chargefromprimaryafferentsdrivesneuropathicpain[149,155,286].
Severallinesofevidencesuggestthatnervein-jury-releasedsignalingmoleculesfromprimaryafferentcentralterminalstriggermicroglialactivation(Fig.
3).
Abrief,low-frequencyelectricalstimulationoftheperipheralC-berswasshowntoinducespinalmicroglialreactionwithoutcausingnotice-ablenerveinjury[97].
Sustainednerveblockadeviabupivacainemicrospherespreventednerveinjury-inducedmicroglialresponses(CD11bexpressionandP-p38induction)[276,287].
However,inhi-bitionofC-beractivityaloneinthesciaticnervewithresininfera-toxinmaynotbesufcienttopreventsparednerveinjury-inducedmicroglialactivation[226],suggestingpossiblecontributionoflargeA-bers.
Consistently,deletionofvesicularglutamatetrans-porter-2(vGluT2)inNav1.
8-expressingnociceptorsdidnotpre-ventnerveinjury-inducedspinalmicroglialreaction,suggestingthatglutamatereleasefromnociceptorsmaynotbesufcienttoR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx7Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022drivemicroglialreaction[208].
Althoughspontaneousactivityisimportantfortheinitiationofmicroglialactivation,itisnotsocrit-icalforthemaintenanceofmicroglialactivation[276].
Chemokines,suchasCCL2,CCL21,andCX3CL1,areidealformediatingneuronal–microglialinteractions,giventhedistinctexpressionoftheirligandsandreceptors.
NerveinjuryinducesCCL2andCCL21expressioninDRGneurons[19,281,298].
Stimula-tionofthedorsalrootresultsinactivity-dependentCCL2releaseinthespinalcord[239,255].
ChemokinesmayactivatemicrogliaviaP2X4signaling:CCL2inducesthesurfacetrafckingofP2X4[242],andCCL21increasestheexpressionofP2X4[19].
ActivationofP2X4resultedinBDNFexpressionandreleasefrommicrogliaviap38activation[245].
Proteaseshavealsobeenimplicatedinmicroglialactivation.
NerveinjuryinducesrapidandtransientupregulationofMMP-9inDRGneurons,whichisessentialfortheearly-phasedevelopmentofneuropathicpain[115].
Activity-dependentre-leaseofMMP-9fromprimarysensoryneuronswasimplicatedinmicroglialactivation,inpartthroughIL-1bcleavage[127].
Cathep-sinSisalsoinvolvedinmicroglial–neuronal–microglialsignaling.
Nerveinjury-evokedreleaseofcathepsinSfrommicrogliaresultsinfurtheractivationofmicroglia,throughthecleavageandreleaseofCX3CL1fromprimarysensoryneurons[35,37].
Thegrowthfactorneuregulin-1(NRG1)playsanactiveroleinmicroglialactivation.
AlthoughNRG1isexpressedinDRGneurons,itsreceptor,erbB2,isexpressedinmicroglia.
NRG1wasshowntoFig.
3.
Schematicofneuronal–glialandglial–glialinteractionsinthespinalcordinpersistentpain.
Spontaneousdischargeafterapainfulinjury(eg,nerveinjury)resultsinthereleaseofATP,chemokines(CCL2,CCL21,CX3CL1),MMP-9,NRG1,andCRGPfromprimaryafferentcentralterminals,leadingtoactivationofmicrogliainthedorsalhorn.
SpinalmicrogliaexpressthereceptorsforATP(P2X4,P2X7,P2Y6,P2Y12),andchemokines(CX3CR1,CCR2),andNRG1(ErB2).
Activationofthesereceptorsinducesphosphorylationofp38andERK(earlyphase)inmicroglia,leadingtotheproductionandreleaseoftheproinammatorycytokines(TNF-a,IL-1b,IL-18)andthegrowthfactorBDNF,andtheconsequentsensitizationofdorsalhornneurons.
Astrocytescanbeactivatedbymicroglialmediators(TNF-aandIL-18),aswellasastrocyticmediators(matrixmetalloprotein-2(MMP-2)andbFGF).
SubsequentphosphorylationofJNKandP-ERKinastrocytesresultsintheproductionandreleaseofchemokines(eg,CCL2)andcytokines(eg,interleukin-1b[IL-1b]).
Astrocytesalsoproduceadenosinetriphosphate(ATP)andglutamateaftertheactivationofthehemichannels(Cx43andPNX1).
Afternerveinjury,downregulationofastrocyticGLT1resultsindecreaseinastrocyticuptakeofglutamate.
Releaseofastrocyticmediators(CCL2,interleukin-1b[IL-1b],glutamate)canelicitNMDAR-mediatedcentralsensitization.
Releaseofadenosinetriphosphate(ATP)andCCL2fromastrocytescanfurthermaintainmicroglialactivation.
8R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022stimulatemicroglialproliferation,chemotaxis,andIL-1breleaseviaerbB2[26].
BlockadeoftheerbB2receptororsequestrationofendogenousNRG1reducesnerveinjury-inducedmicroglialproliferation,p38activation,andneuropathicpain[26].
NRG1alsoinducesmicroglialproliferationviaphosphorylationofERKandAKT[23].
Inaddition,releaseoftheneuropeptideCGRPfrompri-marysensoryneuronsisnotonlyinvolvedinneurotransmissionbutalsocontributestomicroglialactivationafterchronicmor-phineexposure[269].
p38MAPKservesasakeysignalingmoleculeinmicrogliabyintegratingvariousinputtomicroglia[112].
Microgliap38isacti-vatedbyATP[79],TNF-a[230],andIL-1b[225].
Afternerveinjury,p38isphosphorylatedfollowingtheactivationofmultiplerecep-tors,suchasATPreceptors(P2X4andP2Y12)[136,245]andchemo-kinereceptors(CCR2andCX3CR1)[2,315].
Microglialp38isalsoactivatedbyCGRPinchronicmorphine-inducedtolerance[269].
Notably,minocyclineinhibitsmicroglialactivationbyinhibitingspinalmicroglialp38activationafterinammationandchronicmorphinetreatment[48,100].
Uponactivation,p38inducesthesynthesisandreleaseofmicroglialmediatorsTNF-a,IL-1b,andBDNF[277].
Althoughp38iscriticalforthesynthesisandreleaseofinammatorymediators,ithasalimitedroleinmorphologicalchanges(microgliosis)andproliferationofmicroglia,whichcouldbemediatedbyanotherMAPKfamilymember,ERK[25].
Neuronalsignalsarealsoimportantfortheactivationofastro-cytes.
Forexample,neuronalactivityappearstodriveastrocyteactivationafternerveinjury[287]andinammation[264].
Basicbroblastgrowthfactor(bFGForFGF-2)isinducedinprimarysen-soryneuronsafternerveinjuryandhasanactiveroleinneuron–astrocytesignaling.
Asawell-knownactivatorofastrocytes,bFGFelicitsmitosis,growth,differentiation,andgliosisofastrocytes[110].
NerveinjurynotonlyinducesbFGFinDRGneurons[116]butalsoproducesadelayedbFGFupregulationinastrocytesformaintainingneuropathicpain[110].
3.
2.
Glial–glialinteractionsAstrocyticreactionisoftenprecededbymicroglialreaction,andmicroglialactivationisknowntodriveastrocyteactivation[197].
TNF-a,akeysignalmoleculeproducedbymicroglia,causesrapidJNKactivationinastrocytes[72].
Ofinterest,nerveinjuryelicitsIL-18andIL-18Rexpressioninspinalmicrogliaandastrocytes,respectively,andIL-18releasedfrommicrogliawasshowntoactivateIL-18RinastrocytestoupregulateNF-jBandfacilitateneuropathicpain[167].
Ontheotherhand,astrocytescanalsoreleasesignalingmole-culestoactivatemicroglia.
Afterspinalcordinjury,Cx43isupreg-ulatedandgainsanewfunctionofparacrinesignaling,leadingtothereleaseofATPandglutamate[123,148,240,262].
IncreasesinFig.
4.
Glialmediatorsmodulateexcitatoryandinhibitorysynaptictransmissioninthespinalcord.
(A)Modulationofexcitatorysynaptictransmissionatpresynaptic,postsynaptic,andextrasynapticsitesbyglialmediators.
Presynaptically,tumornecrosisfactor-a(TNF-a),interleukin-1b(IL-1b),CCL2,interferon-c(IFN-c),andTSP4increaseglutamatereleasetoenhanceEPSCfrequency.
Postsynaptically,IL-1bTNF-a,andCCL2increaseAMPARactivity.
Extrasynaptically,TNF-a,IL-1b,CCL2,andD-serineincreaseNMDAR-NR2BactivityandenhanceNMDA-inducedcurrents.
Astrocyte-releasedglutamatecanfurtherinduceNR2B-mediatedinwardcurrentsinsurroundingneurons.
(B)Modulationofinhibitorysynaptictransmissionatpresynaptic,postsynaptic,andextrasynapticsites.
Presynaptically,IL-1bandIL-6decreaseGABAandglycinereleasetodecreaseIPSCfrequency.
Postsynaptically,IL-1bdecreasesGABA/GlyRactivityandIPSCamplitude.
ProstaglandinE2(PGE2)inhibitsevokedglycinecurrent.
Extrasynaptically,IL-1b,CCL2,andIFN-csuppressGABA-and/orglycine-inducedcurrents.
TNF-ainhibitsactionpotentialsininhibitoryneurons.
InlaminaIneurons,BDNFproducesdisinhibitionbyalteringchloridereversepotential.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx9Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022extracellularATPhavebeendocumentedinawiderangeofperiph-eralandcentralnervoussysteminjuries,suchassciaticnerveentrapment[159],traumaticbraininjury[50,64],andspinalcordinjury[191,265].
ATPiscriticalfornerveinjury-evokedmicroglialactivationviaactivationofP2X4,P2X7,P2Y6,andP2Y12receptors[244,253].
Ofnote,CCL2isinducednotonlyinprimarysensoryneuronsbutalsoinastrocytes[72,281].
AlthoughDRG-CCL2inducesmicroglialactivation,astrocytic-CCL2maymaintainmicroglialactivation.
IFN-c,astrongmicroglialactivatorandneu-ropathicpaininducer[250],isalsoproducedbyastrocytes[196].
Finally,microgliaandastrocytescouldbeself-activatedviaautocrineorparacrinesignals.
Forexample,bFGFisupregulatedinspinalastrocytesafternerveinjurytomaintainastrocyteactiva-tion[110].
Nerveinjury-inducedastrocyticMMP-2upregulationinthelatephasecanmaintainastrocyticactivationandneuropathicpainthroughIL-1bcleavage(activation)andphosphorylationofERKinastrocytes[127].
3.
3.
Neuronal–glialinteractionsindorsalrootandtrigeminalgangliainthePNSSGCsinDRGsandTGsaretightlyassociatedwithsensoryneu-ronsviagapjunction;andgapjunctioncommunicationbetweenSGCsandSGCandneuronsisgreatlyenhancedinpersistentpainconditions[54,90,91].
Nerveinjury-inducedSGCactivationre-quiresneuronalactivityandlocalinammation[144,287].
Puriner-gicsignalingiscriticallyinvolvedinneuronal–glialcommunicationinDRGs[29,304].
Forexample,activity-dependentATPreleasefromneuronalsomaactivatesP2X7inSGCs[304],leadingtoTNF-areleasefromSGCs,whichcaninturnactonsurroundingneuronstoincreasetheirexcitability[119,216](Fig.
4).
ATPcanalsobereleasedfromSGCstoactivateP2X3receptor,whichisex-pressedinprimarysensoryneuronsandplaysanimportantroleinperipheralsensitization[40,217].
Ofnote,MMP-9mediatesneuron–SGCinteractioninDRGsafteracutemorphinetreatment,whichcanmaskmorphineanalgesia[153](Fig.
5).
Systemicmorphineadministrationwasshowntoeli-citrapidMMP-9upregulationinDRGneuronsintherecoveryphaseofmorphineanalgesia(2hours),whichrequiresactivationofl-opioidreceptors[153].
Notably,morphineanalgesiaisen-hancedandprolongedinMmp9knockoutmice[153].
Acutemor-phinealsoupregulatesGFAPandIL-1binSGCsofDRGs,andbothrequireMMP-9[18].
MMP-9releasefromneuronsresultsinIL-1bcleavageandrelease,whichinturnactivatesIL-1breceptorsinsensoryneuronstoelicitactionpotentials[20].
IL-1bisknowntoincreasetheexcitabilityofsensoryneuronsviaenhancingso-diumcurrentsandsuppressingpotassiumcurrents[20,233,236].
Ofinterest,IL-1bhasalsobeenshowntomaskmorphine-inducedanalgesia[103,120].
Thus,targetingperipheralneuronal–glialinteractions,inadditiontopreviouslyrecognizedcentralneuro-nal–glialinteractions,canalsoenhanceopioidanalgesia.
4.
GlialmediatorsmodulateexcitatoryandinhibitorysynaptictransmissionAkeyissueregardingglialcontrolofpainishowglialmedia-torsregulatesynaptictransmission.
Strikingly,glialmediatorscanmodulatespinalcordsynaptictransmissionatverylowconcen-trations.
Althoughneurotransmitters(eg,glutamate,GABA,gly-cine,andsubstanceP)normallyregulateneuronalandsynapticactivityatmicromolarconcentrations,glialmediators(cytokines,chemokines,andgrowthfactors)canchangesynapticactivityatnanomolarconcentrationsinvitro[43,72,128].
Inparticular,glialmediatorscanmodulatebothexcitatoryandinhibitorysynaptictransmission(Fig.
5).
Althoughmoststudiesusedyoung(3-to5-week-old)andadultanimals(ratsandmice)forrecordingspinalneuronalactivities[43,72,128,189,296],somestudiesusedneonatalanimals[73,81,259].
Itiswellknownthatthegeneexpressionprolesofprimarysensoryandspinalcordneurons,glialresponses,aswellasspinalcordpaincircuitsundergodra-maticchangesintherst2weeksafterbirth[16,61,172].
Thus,cautionmustbetakentointerpretthedatafromneonatalanimals.
4.
1.
ModulationofexcitatorysynaptictransmissionGlialmediatorscanmodulateexcitatorysynaptictransmissionviapre-,post-,andextrasynapticmechanisms(Fig.
5).
Theeffectsofproinammatorycytokinesandchemokinesonexcitatorypost-synapticcurrents(EPSCs)havebeenexaminedinlaminaIIneuronsusingexvivospinalcordslicepreparations[293].
AlthoughtheEPSCfrequencychangemayresultfrompresynapticmechanisms(duetoglutamatereleasefrompresynapticterminals),theEPSCamplitudeincreaseiscausedbyenhancedsignalingofglutamatereceptors(AMPAsubtype)inpost-synapticsites.
IncubationofspinalcordsliceswithTNF-a,IL-1b,andCCL2veryrapidly(withinminutes)increasedspontaneousEPSC(sEPSC)fre-quency[72,128,296].
ChronicexposureofcultureddorsalhornneuronstoIFN-calsoincreasedsEPSCfrequency[260](Fig.
5A),supportingapossiblepresynapticmodulation.
TNF-aincreasessEPSCfrequencyviaactivationofTRPV1inpresynapticterminals,asthissEPSCincreaseisabolishedinTrpv1knockoutmice.
Sin-gle-cellPCRanalysisindicatesthatTNF-a-respondinglaminaIIinterneuronsareexclusivelyexcitatoryones,becausetheyallex-pressvesicularglutamatetransporter-2(vGluT2).
TheselaminaIIneuronsalsoreceiveinputfromTRPV1-expressingC-bersandmakesynapsestolamina-Iprojectionneurons[241],formingaFig.
5.
Schematicrepresentationofneuronal–glialinteractionsindorsalrootandtrigeminalgangliaoftheperipheralnervoussystem(PNS).
Spontaneousneuronaldischargeafterpainfulinjuryresultsinadenosinetriphosphate(ATP)releaseinneuronalsomata,leadingtotheactivationofP2X7andsubsequentreleaseoftumornecrosisfactor-a(TNF-a)insatelliteglialcells(SGCs).
Persistentnociceptiveactivityoractivationofopioidreceptorsbymorphinealsoresultsinmatrixmetalloproteinase-9(MMP-9)releasefromprimarysensoryneurons,causingthecleavage(activation)andreleaseofinterleukin-1b(IL-1b)inSGCs.
TNF-aandIL-1bbindrespectiveTNFRandIL-1Ronsensoryneuronstoelicithyperexcitability.
SGCscanalsoreleaseATPviahemichannels(Cx43andPNX1)orgapjunctioncommu-nicationtoactivateP2X3inneuronsfortriggeringperipheralsensitization.
Inaddition,SGCsexpressKir4.
1tomaintainhomeostasisofextracellularK+levelsofsensoryneurons,andinjury-induceddownregulationofKir4.
1inSGCswilldisruptthisK+homeostasisandgenerateneuronalhyperexcitability.
10R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022spinalcircuittomediateTNF-a-inducedpain.
ArecentstudyalsodemonstratedthatTSP4,producedbyastrocytes,increasedsEPSCfrequency[132].
GlialmediatorssuchasIL-1bandCCL2alsoincreasetheampli-tudesofsEPSCs,viaAMPA-mediatedpostsynapticmechanisms(Fig.
5A).
TNF-aisknowntoinducethetrafckingandsurfaceexpressionofAMPAreceptorsinhippocampalneurons[12,220].
Afterspinalcordinjury,TNF-ainducesrapidtrafckingofGluR2-lackingAMPARstotheplasmamembraneinspinalcordmotorneurons[59].
Ofnote,inammationinducesaTNF-a–dependentsurfacetrafckingofGluR1-AMPARsinthedorsalhorn[32].
AlthoughTNFR1isthepredominantreceptormediatingtheeffectsofTNF-a,bothTNFR1andTNFR2arerequiredfortheinductionofcentralsensitization[301,309].
Pro-inammatorycytokinesandchemokinesfurtherinducecentralsensitizationviaextrasynapticmechanisms(Fig.
5A).
NMDAcurrentsinlaminaIIneurons,inducedbybathapplicationofNMDAtospinalcordslices,areenhancedbyIL-1b,TNF-a,orCCL2[72,128].
TNF-aincreasesNMDAreceptor(NMDAR)activitythroughphosphorylationofERKindorsalhornneurons[292].
IL-1binducesphosphorylationoftheNR1subunitinspinalcordneurons[302].
AstrocyticD-serineenhancesNMDAcurrentsviabindingtheglycinesiteofNMDAreceptors[201].
Interestingly,astrocyticglutamatereleasecanbedetectedasslowinwardcur-rents,viapatch-clamprecordingsinnearbyneurons.
SlowinwardcurrentsaremediatedbyextrasynapticNR2Breceptorsandin-ducedinspinaldorsalhornneuronsafterinammation[10].
4.
2.
ModulationofinhibitorysynaptictransmissionReductionorlossofinhibitorysynaptictransmission(disinhibi-tion)inthespinalcordpaincircuithasbeenstronglyimplicatedinthegenesisofcentralsensitizationandchronicpain[8,44,169,294].
Disinhibitionafterperipheralnerveinjuryinvolvesatrans-synapticreductionintheexpressionofthepotassium-chlo-rideco-transporterKCC2andsubsequentdisruptionofanionhomeostasis(chloridehomeostasis)inspinallaminaIneurons.
Insomecases,theshiftinthetransmembraneaniongradientcanconvertnormallyinhibitoryanionicsynapticcurrentstobeexcit-atory[44].
GlialmediatorssuchasBDNF,cytokines,chemokines,andPGE2canalsomodulateinhibitorysynaptictransmissionviapre-,post-,andextrasynapticmechanisms(Fig.
5B).
Presynaptically,IL-1bandIL-6wereshowntoinhibitthefrequencyofspontaneouspostsyn-apticcurrents(sIPSCs)inspinallaminaIIneurons[128].
Postsyn-aptically,IL-1breducesthesIPSCamplitude[128].
PGE2inhibitsglycinergicneurotransmissioninthedorsalhornviapost-synapticGlyR3andthecAMP/PKApathway[5,96].
Atextrasynapticsites,GABAandglycinecurrents,inducedbybathapplicationofGABAandglycine,canbesuppressedbyIL-1bandIL-6[128].
BDNFactsonspinallaminaIneuronstoreverseGABAinhibitionbyalteringchloridereversepotential[43].
Fur-thermore,ATPormorphine-stimulatedmicrogliaresultinadepo-larizingshiftintheanionreversalpotentialbyreleasingBDNF[43,60].
Likenerveinjury,administrationofATP-stimulatedmicrogliaorpharmacologicaldisruptionofchloridetransportinvivoalterthephenotypeofspinallaminaIoutputneurons,lead-ingtoneuropathicpainphenotypes[129].
TNF-awasalsoshowntosuppressactionpotentialsinGAD67+inhibitoryneuronsinspinalcordslices[296].
Moreover,CCL2andIFN-cinhibitGABA-inducedresponsesinspinalcordneurons[81,259].
Itremainstobeinvestigatedhowanti-inammatorycytokines(eg,IL-4,IL-10,TGF-b)regulatesynapticplasticity.
ItappearsthatIL-10cansuppressTNF-a-inducedsynapticplasticity(unpublishedobservations).
Inparticular,theanti-inammatorylipidmediatorssuchasresolvinE1(RvE1)andneuroprotectin(NPD1)blockedTNF-a-inducedsynapticplasticity(sEPSCfrequencyincrease)[292].
NPD1andRvD2furtherreversedinammation-inducedsynapticplasticityandtetanicstimulation-inducedspinallong-termpotentiation(LTP)[189].
Finally,theproinammatorycytokinesTNF-a,IL-1b,andIL-6alsoelicitlong-termneuronalplasticityinthepaincircuitbyinducingthephosphorylationofthetranscriptionfactorcAMPre-sponseelement-bindingprotein(CREB),leadingtothetranscrip-tionofCREB-mediatedpronociceptivegenes(eg,cyclooxygenase-2[COX-2],neurokinin-1[NK-1])inspinalcordneurons[111,128,206].
Ofnote,TNF-aissufcienttoinducespinalLTPafternerveinjury[154],andtetanicstimulation-inducedspinalLTPisabolishedinTNFR1orTNFR2knockoutmice[188].
4.
3.
ConcludingremarksInthepastdecadegreatprogresshasbeenmadetodemon-stratecriticalrolesofglialcells,suchasmicroglia,astrocytes,andSGCsinthegenesisofpersistentpain.
Asevidenceemerges,thelistofglial-derivedsignalingmoleculesandmediatorscontin-uestogrow(Tables1–4).
Gliacancommunicatewithneuronsby''listening''and''talking''toneurons.
Itisincreasinglyappreciatedthatchronicpaincanmanifestnotonlybyneuralplasticitybutalsobydysfunctionofglialcells.
Underthenormalphysiologicalconditions,astrocytesandSGCsprovidetrophicsupporttoneuronsandmaintainthehomeostasisofK+,glutamate,andH2OinCNSandPNS[258].
AstrocytesandSGCscouldalso''insulate''theneuralcircuitofpainbyformingastructuralbarrierandkeepthecircuitsilentbyreleasinginhibitorymediators[172].
Nervein-jury-inducedchronicpainisassociatednotonlywithneuropathybutalsowith''gliopathy.
''AstrocyteslosetheirabilitytomaintainthehomeostasisofK+andglutamate,leadingtoneuronalheperex-citability,asaresultofhigherextracellularlevelsofglutamateandK+.
Dysfunctionofastrocyticwaterchannel(AQP4)willalsoresultinedemaintheCNSandPNS[258].
Asaresultofgliopathy,gliacannolongerinsulatethepaincircuit;insteadtheyserveasanampli-erofpain,byproducingproinammatoryandpronociceptivemediators.
PainfulinjuriesevokerapidreactionofSGCsinthePNS,fol-lowedbymicroglialandastrocyticreactionintheCNS.
Moststud-iesongliaandpainfocusonmicrogliaandastrocytesinthespinalcord.
Uponactivation,presumablyinitiatedbyneuronalsignals,gliasynthesizeandreleaseproinammatoryandpronociceptivemediators(eg,proinammatorycytokinesandchemokinesandgrowthfactors)toenhancepainstates,viaactivationofkeysignal-ingpathways,suchastheMAPkinasepathways.
Activationofhemichannels(eg,Cx43andPNX1)andP2X7resultsinthereleaseofATPandglutamatefromastrocytes.
Importantly,glialmediators(eg,TNF-a,IL-1b,IL-6,CCL2,BDNF)canpowerfullymodulateexcit-atoryandinhibitorysynaptictransmissionatcomparablylowerconcentrations.
Glialmediators(ATP,CCL2,IFN-c,bFGF,MMP-2)alsoresultinfurtheractivationofglialcellsviaparacrineorauto-crineregulation.
Lastbutnottheleast,gliamayalsoproduceanti-inammatoryandantinociceptivemediatorsfortheresolutionofacutepain.
Furtherinquiryisneededtodeterminewhetherfailureintheproductionoftheseresolutionmediatorsleadstothetransi-tionfromacutepaintochronicpain.
5.
Remainingquestionsandfuturedirections5.
1.
IsglialactivationassociatedwithpainDespitethegrowingimportanceofglialcellsinpainregulation,''glialactivation''isnotwelldened.
Moststudiesintheelduseglialreaction(upregulationoftheglialmarkersIBA1,CD11b,andR.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx11Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022GFAPtodenetheactivationofmicroglia(IBA1/CD11b),astrocytes(GFAP),andSGCs(GFAP)(Table1).
Althoughtheupregulationofthesemarkersisassociatedwithpainbehaviors,especiallyintheinductionphase,thereareseveralcaveatsrelatedtothesemarkers.
First,dissociationbetweenmicroglialmarkerexpressionandpainbehaviorshasbeenreportedbydifferentgroups[21,38,307].
Com-paredtomicroglialmarkers(IBA1andCD11b),theastrocyticmar-kerGFAPisbettercorrelatedwithpainbehaviors,especiallyafterinammation,bonecancer,chemotherapy,andHIVneuropathy[98,211,297,307].
Second,weshouldnotexcludemicroglialactiva-tionifthereisnochangeinIBA1expression.
Glialactivationcanalsomanifestasquickresponses,suchasCa2+changesandphos-phorylationofsignalingmolecules(eg,MAPKs)thatcouldoccurwithinminutesafterastimulationorinsult.
Indeed,sensorywhis-kerstimulationwasshowntoevokerapidincreases,withinseveralseconds,inastrocyticcytosolicCa2+inthebarrelcortexofadultmice[266].
Third,evenundertheactivationstateswithupregula-tionofglialmarkersandhypertrophy,microgliacouldstillhavedifferentfunctionalstatesbyexhibitingeitherpro-inammatory(neurotoxic,M1)andanti-inammatory(neuroprotective,M2)phenotypes[93,284].
Finally,andimportantly,glialreactivityandmorphologicalchangesdonotdirectlymodulatepain.
Neuronalactivityandpainsensitivityarecontrolledbytheglialmediators(cytokines,chemokines,ATP,BDNF,glutamate).
Thus,theregula-tionofglialsignalingmoleculesandglialmediatorsafterpainfulinjuries(Tables2–4)couldbebetterassociatedwithpainstatesthanglialreactivity.
5.
2.
CanwetargetgliaforpaintherapyHowcanwedesigndrugstotargetglialactivityforpaincon-trolDowereallyneedglia-selectivedrugsIndeed,itisextremelydifculttodesigndrugsthattargetonlyglialcellswithoutaffect-ingneurons.
Furthermore,eliminationofglialcellswithglia-selec-tivetoxinsmaycausedetrimentaleffects,giventhesupportiveandprotectiverolesofglia.
Instead,therearealternativestrategies:(1)totargettheMAPKsignalingpathways(ERK,p38,JNK),hemichan-nels(eg,Cx43andPNX1),orP2X7tosuppressthereleaseofglialmediators;(2)totargettheupstreamactivatorsofglia,suchasP2X4,P2Y6/12,MMP-9/2,andcathepsinS;and(3)totargetthedownstreammediatorsreleasedbyglia,suchasTNF-a,IL-1b,IL-6,orBDNF.
Weshouldlearnlessonsfromrecentfailuresin2clinicaltrials:1trialwithaglialmodulator,propentofylline,whichshowednoefcacyinreducingneuropathicpaininpatientswithpost-her-peticneuralgia[141];anothertrialwithaCCR2antagonistAZD2423,whichshowednosignicanteffects,comparedtopla-cebo,inpost-traumaticneuralgiapatients[122].
Thefailuresmayresultfrommultiplereasons,includinglackoftranslationfromrodentstohumanbeings,differentwaysofpainmeasurementinrodentsandhumanbeings(evokedpainvsspontaneouspain),anddifferentpainconditionstestedinrodentsandhumanbeings(nervetrauma-inducedpainhypersensitivityinseveralweeksvspost-herpetic/traumaticneuralgiaaftermanyyears).
Ofnote,pro-pentofyllineisawell-knowninhibitorofphosphodiesterase,andthereforecouldaltercAMPlevelsinglialandnon-glialcells[63].
Propentofyllineisalsoanadenosineuptakeinhibitor[63].
Com-paredtothecompletelackofeffectofpropentofylline,AZD2423(150mg)showedsometrendstowardreductioninparoxysmalpainandparesthesia/dysesthesia,indicatingthataCCR2antago-nistmayhavesomepossibleeffectsforsomesensorycomponentsofpain[122].
Notably,thevariabilitybetweenandwithinindivid-ualswasveryhigh,inpartbecauseofthenatureofamulticentertrial.
ItisalsoaconcernthatinhibitionofglialresponsesintheCNScannotbevalidatedinthistrial,becauseofthelackofeffectiveimagingtechniquefordetectingglialresponses(seeSection6.
3).
Theoretically,itshouldbemoreeffectiveforadrugtotargetbothneuronsandgliaforpainrelief.
Forexample,p38isactivatedbothinspinalcordmicrogliaandDRGneurons,andsystemicp38inhibitorhasbeenshowntoalleviateneuropathicpaininaclinicaltrial[6].
Recentstudieshavedemonstratedthattheanti-inamma-toryandpro-resolutionlipidmediatorssuchasresolvins(RvD1,RvD2,RvE1),protectins/neuroprotectins(PD1/NPD1),andlipoxins(LXA4)couldpotentlyreduceinammatoryandpostoperativepain,atverylowdoses[101,114,231].
Peri-surgicalapplicationofPD1/NPD1effectivelyprotectsnervetrauma-inducedneuropathicpainandspinalcordglialactivationinmice[291].
RvE1andPD1furtherinhibitglialactivationincultures[290,291].
Thereceptorsofthesemediators,suchasChemR23(RvE1)andALX(RvD1andLXA4)arewidelyexpressedinneurons,glia,andimmunecells[39,114,210,231].
Thus,theselipidmediatorsnotonlyinhibitglialactivationandinammationbutalsoinhibitTRPchannels(eg,TRPA1/V1)andreversesynapticplasticityinneurons[114,188,189].
Giventhepotencyandsafety,theseendogenousli-pidmediators,ortheiranalogs,orsmall-moleculeagonistsoftheirFig.
6.
Glialbrillaryacidicprotein(GFAP)immunostainingofmouse,rhesusmonkey,andhumanastrocytesincortex.
Notestrikingdifferencesinthesizesofmouse,monkey,andhumanastrocytes.
Alsonotedifferencesinthenumberandlengthsofbranchesofastrocytesfrommouse,monkey,andhumanbeing.
Sizesofastrocytesincreasewithincreasingcomplexityofbrainfunction.
Scale,50lm.
ImagesarereproducedfromKimelbergandNedergaard[133],withpermission.
12R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022receptors,couldbedevelopedforpreventingandtreatingchronicpain,viatargetingbothneuronalandnon-neuronal(immuneandglial)mechanisms.
5.
3.
HowmuchdoweknowabouthumangliaLittleisknownabouttheroleofhumangliainpaincontrol.
In-deed,astrocytesfrommice,monkeys,andhumanbeingsarequitedifferentintheirsizes[180,182](Fig.
6).
ThehumanbrainappearstocontainsubtypesofGFAP-positiveastrocytesthatarenotrepre-sentedinrodents.
Inhumancortex,astrocytesaremorethan2-foldlargerindiameterandextend10-foldmoreGFAP-positiveprimaryprocessesthantheirrodentcounterparts(Fig.
6).
Thedomainofasinglehumanastrocytehasbeenestimatedtocontactupto2mil-lionsynapses[133,180].
Remarkably,humanglialprogenitorcells(GPCs),afterbeingimplantedintoneonatalimmunodecientmice,aregapjunction-coupledtohostastroglia,propagateCa2+signals3-foldfasterthantheirhosts,andexhibitenhancedLTPandlearn-ingcapability[88].
Hence,humanastrocytescouldplayamoresophisticatedroleinchronicpainthanrodentastrocytes.
Impor-tantly,astrocytereaction,butnotmicroglialreaction,isassociatedwithchronicpaininHIV-infectedpatients[211].
ActivationoftheMAPKpathwaysisalsocorrelatedwithneuropathicpaininthesepatients[211].
Futureresearchshouldfocusonthefollowing:studyingtheresponsesofhumangliainculturesandhumangliatransplantationinmice;investigatingthechangesinhumangliainpainfuldiseaseconditionsinpostmortemtissues;andimagingreal-timeglialactivationinpatientswithchronicpain.
ConictofintereststatementTheauthorsdeclarenoconictofinterestinregardtothiswork.
AcknowledgmentsThisworkwassupportedbyNationalInstitutesofHealthGrantsNS67686andDE17794(toR.
R.
J.
)andDE22743(toR.
R.
J.
andM.
N.
).
References[1]AbbadieC,BhangooS,DeKoninckY,MalcangioM,Melik-ParsadaniantzS,WhiteFA.
Chemokinesandpainmechanisms.
BrainResRev2009;60:125–34.
[2]AbbadieC,LindiaJA,CumiskeyAM,PetersonLB,MudgettJS,BayneEK,DeMartinoJA,MacIntyreDE,ForrestMJ.
ImpairedneuropathicpainresponsesinmicelackingthechemokinereceptorCCR2.
ProcNatlAcadSciUSA2003;100:7947–52.
[3]AdrianJrEK,WilliamsMG,GeorgeFC.
Finestructureofreactivecellsininjurednervoustissuelabeledwith3H-thymidineinjectedbeforeinjury.
JCompNeurol1978;180:815–39.
[4]AgulhonC,FiaccoTA,McCarthyKD.
Hippocampalshort-andlong-termplasticityarenotmodulatedbyastrocyteCa2+signaling.
Science2010;327:1250–4.
[5]AhmadiS,LipprossS,NeuhuberWL,ZeilhoferHU.
PGE(2)selectivelyblocksinhibitoryglycinergicneurotransmissionontoratsupercialdorsalhornneurons.
NatNeurosci2002;5:34–40.
[6]AnandP,ShenoyR,PalmerJE,BainesAJ,LaiRY,RobertsonJ,BirdN,OstenfeldT,ChizhBA.
Clinicaltrialofthep38MAPkinaseinhibitordilmapimodinneuropathicpainfollowingnerveinjury.
EurJPain2011;15:1040–8.
[7]AraqueA,ParpuraV,SanzgiriRP,HaydonPG.
Tripartitesynapses:glia,theunacknowledgedpartner.
TrendsNeurosci1999;22:208–15.
[8]BabaH,JiRR,KohnoT,MooreKA,AtakaT,WakaiA,OkamotoM,WoolfCJ.
RemovalofGABAergicinhibitionfacilitatespolysynapticAber-mediatedexcitatorytransmissiontothesupercialspinaldorsalhorn.
MolCellNeurosci2003;24:818–30.
[9]BaoF,ChenM,ZhangY,ZhaoZ.
Hypoalgesiainmicelackingaquaporin-4waterchannels.
BrainResBull2010;83:298–303.
[10]BardoniR,GhirriA,ZontaM,BetelliC,VitaleG,RuggieriV,SandriniM,CarmignotoG.
Glutamate-mediatedastrocyte-to-neuronsignallingintheratdorsalhorn.
JPhysiol2010;588:831–46.
[11]BasbaumAI,BautistaDM,ScherrerG,JuliusD.
Cellularandmolecularmechanismsofpain.
Cell2009;139:267–84.
[12]BeattieEC,StellwagenD,MorishitaW,BresnahanJC,HaBK,VonZastrowM,BeattieMS,MalenkaRC.
ControlofsynapticstrengthbyglialTNFalpha.
Science2002;295:2282–5.
[13]BeggsS,CurrieG,SalterMW,FitzgeraldM,WalkerSM.
Primingofadultpainresponsesbyneonatalpainexperience:maintenancebycentralneuroimmuneactivity.
Brain2012;135:404–17.
[14]BeggsS,SalterMW.
Stereologicalandsomatotopicanalysisofthespinalmicroglialresponsetoperipheralnerveinjury.
BrainBehavImmun2007;21:624–33.
[15]BellJrPB,RundquistI,SvenssonI,CollinsVP.
FormaldehydesensitivityofaGFAPepitope,removedbyextractionofthecytoskeletonwithhighsalt.
JHistochemCytochem1987;35:1375–80.
[16]BennSC,CostiganM,TateS,FitzgeraldM,WoolfCJ.
DevelopmentalexpressionoftheTTX-resistantvoltage-gatedsodiumchannelsNav1.
8(SNS)andNav1.
9(SNS2)inprimarysensoryneurons.
JNeurosci2001;21:6077–85.
[17]BennettMV,ContrerasJE,BukauskasFF,SaezJC.
Newrolesforastrocytes:gapjunctionhemichannelshavesomethingtocommunicate.
TrendsNeurosci2003;26:610–7.
[18]BertaT,LiuT,LiuYC,XuZZ,JiRR.
Acutemorphineactivatessatelliteglialcellsandup-regulatesIL-1betaindorsalrootgangliainmiceviamatrixmetalloprotease-9.
MolPain2012;8:18.
[19]BiberK,TsudaM,Tozaki-SaitohH,TsukamotoK,ToyomitsuE,MasudaT,BoddekeH,InoueK.
NeuronalCCL21up-regulatesmicrogliaP2X4expressionandinitiatesneuropathicpaindevelopment.
EMBOJ2011;30:1864–73.
[20]BinshtokAM,WangH,ZimmermannK,AmayaF,VardehD,ShiL,BrennerGJ,JiRR,BeanBP,WoolfCJ,SamadTA.
Nociceptorsareinterleukin-1betasensors.
JNeurosci2008;28:14062–73.
[21]BlackbeardJ,WallaceVC,O'DeaKP,HasnieF,SegerdahlA,PhebyT,FieldMJ,TakataM,RiceAS.
Thecorrelationbetweenpain-relatedbehaviourandspinalmicrogliosisinfourdistinctmodelsofperipheralneuropathy.
EurJPain2012;16(10):1357–67.
[22]BushongEA,MartoneME,JonesYZ,EllismanMH.
ProtoplasmicastrocytesinCA1stratumradiatumoccupyseparateanatomicaldomains.
JNeurosci2002;22:183–92.
[23]CalvoM,BennettDL.
Themechanismsofmicrogliosisandpainfollowingperipheralnerveinjury.
ExpNeurol2012;234:271–82.
[24]CalvoM,DawesJM,BennettDL.
Theroleoftheimmunesysteminthegenerationofneuropathicpain.
LancetNeurol2012;11:629–42.
[25]CalvoM,ZhuN,GristJ,MaZ,LoebJA,BennettDL.
Followingnerveinjuryneuregulin-1drivesmicroglialproliferationandneuropathicpainviatheMEK/ERKpathway.
Glia2011;59:554–68.
[26]CalvoM,ZhuN,TsantoulasC,MaZ,GristJ,LoebJA,BennettDL.
Neuregulin-ErbBsignalingpromotesmicroglialproliferationandchemotaxiscontributingtomicrogliosisandpainafterperipheralnerveinjury.
JNeurosci2010;30:5437–50.
[27]ChenMJ,KressB,HanX,MollK,PengW,JiRR,NedergaardM.
AstrocyticCx43hemichannelsandgapjunctionsplayacrucialroleindevelopmentofchronicneuropathicpainfollowingspinalcordinjury.
Glia2012;60:1660–70.
[28]ChenFL,DongYL,ZhangZJ,CaoDL,XuJ,HuiJ,ZhuL,GaoYJ.
Activationofastrocytesintheanteriorcingulatecortexcontributestotheaffectivecomponentofpaininaninammatorypainmodel.
BrainResBull2012;87:60–6.
[29]ChenY,ZhangX,WangC,LiG,GuY,HuangLY.
ActivationofP2X7receptorsinglialsatellitecellsreducespainthroughdownregulationofP2X3receptorsinnociceptiveneurons.
ProcNatlAcadSciUSA2008;105:16773–8.
[30]ChiangCY,SessleBJ,DostrovskyJO.
Roleofastrocytesinpain.
NeurochemRes2012;37(11):2419–31.
[31]ChiangCY,WangJ,XieYF,ZhangS,HuJW,DostrovskyJO,SessleBJ.
Astroglialglutamate–glutamineshuttleisinvolvedincentralsensitizationofnociceptiveneuronsinratmedullarydorsalhorn.
JNeurosci2007;27:9068–76.
[32]ChoiJI,SvenssonCI,KoehrnFJ,BhuskuteA,SorkinLS.
PeripheralinammationinducestumornecrosisfactordependentAMPAreceptortrafckingandAktphosphorylationinspinalcordinadditiontopainbehavior.
PAIN2010;149:243–53.
[33]ChristiansonCA,DumlaoDS,StokesJA,DennisEA,SvenssonCI,CorrM,YakshTL.
SpinalTLR4mediatesthetransitiontoapersistentmechanicalhypersensitivityaftertheresolutionofinammationinserum-transferredarthritis.
PAIN2011;152:2881–91.
[34]ChuYX,ZhangYQ,ZhaoZQ.
Involvementofmicrogliaandinterleukin-18intheinductionoflong-termpotentiationofspinalnociceptiveresponsesinducedbytetanicsciaticstimulation.
NeurosciBull2012;28:49–60.
[35]ClarkAK,StanilandAA,MalcangioM.
Fractalkine/CX3CR1signallinginchronicpainandinammation.
CurrPharmBiotechnol2011;12:1707–14.
[36]ClarkAK,StanilandAA,MarchandF,KaanTK,McMahonSB,MalcangioM.
P2X7-dependentreleaseofinterleukin-1betaandnociceptioninthespinalcordfollowinglipopolysaccharide.
JNeurosci2010;30:573–82.
[37]ClarkAK,YipPK,GristJ,GentryC,StanilandAA,MarchandF,DehvariM,WotherspoonG,WinterJ,UllahJ,BevanS,MalcangioM.
InhibitionofspinalmicroglialcathepsinSforthereversalofneuropathicpain.
ProcNatlAcadSciUSA2007;104:10655–60.
[38]ColburnRW,DeLeoJA,RickmanAJ,YeagerMP,KwonP,HickeyWF.
Dissociationofmicroglialactivationandneuropathicpainbehaviorsfollowingperipheralnerveinjuryintherat.
JNeuroimmunol1997;79:163–75.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx13Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[39]ConnorKM,SanGiovanniJP,LofqvistC,AdermanCM,ChenJ,HiguchiA,HongS,PravdaEA,MajchrzakS,CarperD,HellstromA,KangJX,ChewEY,SalemJrN,SerhanCN,SmithLE.
Increaseddietaryintakeofomega-3-polyunsaturatedfattyacidsreducespathologicalretinalangiogenesis.
NatMed2007;13:868–73.
[40]CookSP,VulchanovaL,HargreavesKM,EldeR,McCleskeyEW.
DistinctATPreceptorsonpain-sensingandstretch-sensingneurons.
Nature1997;387:505–8.
[41]CorreaF,Hernangomez-HerreroM,MestreL,LoriaF,DocagneF,GuazaC.
TheendocannabinoidanandamidedownregulatesIL-23andIL-12subunitsinaviralmodelofmultiplesclerosis:evidenceforacross-talkbetweenIL-12p70/IL-23axisandIL-10inmicroglialcells.
BrainBehavImmun2011;25:736–49.
[42]CotrinaML,LinJH,Alves-RodriguesA,LiuS,LiJ,Azmi-GhadimiH,KangJ,NausCC,NedergaardM.
ConnexinsregulatecalciumsignalingbycontrollingATPrelease.
ProcNatlAcadSciUSA1998;95:15735–40.
[43]CoullJA,BeggsS,BoudreauD,BoivinD,TsudaM,InoueK,GravelC,SalterMW,DeKoninckY.
BDNFfrommicrogliacausestheshiftinneuronalaniongradientunderlyingneuropathicpain.
Nature2005;438:1017–21.
[44]CoullJA,BoudreauD,BachandK,PrescottSA,NaultF,SikA,DeKoninckP,DeKoninckY.
Trans-synapticshiftinaniongradientinspinallaminaIneuronsasamechanismofneuropathicpain.
Nature2003;424:938–42.
[45]CroninM,AndersonPN,CookJE,GreenCR,BeckerDL.
Blockingconnexin43expressionreducesinammationandimprovesfunctionalrecoveryafterspinalcordinjury.
MolCellNeurosci2008;39:152–60.
[46]CrownED,GwakYS,YeZ,JohnsonKM,HulseboschCE.
Activationofp38MAPkinaseisinvolvedincentralneuropathicpainfollowingspinalcordinjury.
ExpNeurol2008;213:257–67.
[47]CuiY,ChenY,ZhiJL,GuoRX,FengJQ,ChenPX.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliamediatesmorphineantinociceptivetolerance.
BrainRes2006;1069:235–43.
[48]CuiY,LiaoXX,LiuW,GuoRX,WuZZ,ZhaoCM,ChenPX,FengJQ.
Anovelroleofminocycline:attenuatingmorphineantinociceptivetolerancebyinhibitionofp38MAPKintheactivatedspinalmicroglia.
BrainBehavImmun2008;22:114–23.
[49]DaulhacL,MalletC,CourteixC,EtienneM,DurouxE,PrivatAM,EschalierA,FialipJ.
Diabetes-inducedmechanicalhyperalgesiainvolvesspinalmitogen-activatedproteinkinaseactivationinneuronsandmicrogliaviaN-methyl-D-aspartate-dependentmechanisms.
MolPharmacol2006;70:1246–54.
[50]DavalosD,GrutzendlerJ,YangG,KimJV,ZuoY,JungS,LittmanDR,DustinML,GanWB.
ATPmediatesrapidmicroglialresponsetolocalbraininjuryinvivo.
NatNeurosci2005;8:752–8.
[51]DeLeoJA,ColburnRW,RickmanAJ.
Cytokineandgrowthfactorimmunohistochemicalspinalprolesintwoanimalmodelsofmononeuropathy.
BrainRes1997;759:50–7.
[52]DeLeoJA,YezierskiRP.
Theroleofneuroinammationandneuroimmuneactivationinpersistentpain.
PAIN2001;90:1–6.
[53]DominguezE,RivatC,PommierB,MauborgneA,PohlM.
JAK/STAT3pathwayisactivatedinspinalcordmicrogliaafterperipheralnerveinjuryandcontributestoneuropathicpaindevelopmentinrat.
JNeurochem2008;107:50–60.
[54]DublinP,HananiM.
Satelliteglialcellsinsensoryganglia:theirpossiblecontributiontoinammatorypain.
BrainBehavImmun2007;21:592–8.
[55]EcheverryS,ShiXQ,ZhangJ.
Characterizationofcellproliferationinratspinalcordfollowingperipheralnerveinjuryandtherelationshipwithneuropathicpain.
PAIN2008;135:37–47.
[56]EngLF,GhirnikarRS,LeeYL.
Glialbrillaryacidicprotein:GFAP—thirty-oneyears(1969–2000).
NeurochemRes2000;25:1439–51.
[57]ErikssonNP,PerssonJK,SvenssonM,ArvidssonJ,MolanderC,AldskogiusH.
Aquantitativeanalysisofthemicroglialcellreactionincentralprimarysensoryprojectionterritoriesfollowingperipheralnerveinjuryintheadultrat.
ExpBrainRes1993;96:19–27.
[58]ErogluC,AllenNJ,SusmanMW,O'RourkeNA,ParkCY,OzkanE,ChakrabortyC,MulinyaweSB,AnnisDS,HubermanAD,GreenEM,LawlerJ,DolmetschR,GarciaKC,SmithSJ,LuoZD,RosenthalA,MosherDF,BarresBA.
Gabapentinreceptoralpha2delta-1isaneuronalthrombospondinreceptorresponsibleforexcitatoryCNSsynaptogenesis.
Cell2009;139:380–92.
[59]FergusonAR,ChristensenRN,GenselJC,MillerBA,SunF,BeattieEC,BresnahanJC,BeattieMS.
CelldeathafterspinalcordinjuryisexacerbatedbyrapidTNFalpha-inducedtrafckingofGluR2-lackingAMPARstotheplasmamembrane.
JNeurosci2008;28:11391–400.
[60]FerriniF,TrangT,MattioliTA,LaffrayS,Del'guidiceT,LorenzoLE,CastonguayA,DoyonN,ZhangW,GodinAG,MohrD,BeggsS,VandalK,BeaulieuJM,CahillCM,SalterMW,DeKY.
Morphinehyperalgesiagatedthroughmicroglia-mediateddisruptionofneuronalCl()homeostasis.
NatNeurosci2013;16(2):183–92.
[61]FitzgeraldM.
Thedevelopmentofnociceptivecircuits.
NatRevNeurosci2005;6:507–20.
[62]FonsecaCG,GreenCR,NicholsonLF.
Upregulationinastrocyticconnexin43gapjunctionlevelsmayexacerbategeneralizedseizuresinmesialtemporallobeepilepsy.
BrainRes2002;929:105–16.
[63]FramptonM,HarveyRJ,KirchnerV.
Propentofyllinefordementia.
CochraneDatabaseSystRev2003:CD002853.
[64]FrankeH,GrummichB,HartigW,GroscheJ,RegenthalR,EdwardsRH,IllesP,KrugelU.
Changesinpurinergicsignalingaftercerebralinjury—involvementofglutamatergicmechanismsIntJDevNeurosci2006;24:123–32.
[65]FreemanSE,PatilVV,DurhamPL.
Nitricoxide-protonstimulationoftrigeminalganglionneuronsincreasesmitogen-activatedproteinkinaseandphosphataseexpressioninneuronsandsatelliteglialcells.
Neuroscience2008;157:542–55.
[66]FukuokaT,KondoE,DaiY,HashimotoN,NoguchiK.
Brain-derivedneurotrophicfactorincreasesintheuninjureddorsalrootganglionneuronsinselectivespinalnerveligationmodel.
JNeurosci2001;21:4891–900.
[67]GaoYJ,ChengJK,ZengQ,XuZZ,DecosterdI,XuX,JiRR.
SelectiveinhibitionofJNKwithapeptideinhibitorattenuatespainhypersensitivityandtumorgrowthinamouseskincancerpainmodel.
ExpNeurol2009;219:146–55.
[68]GaoYJ,JiRR.
Chemokines,neuronal–glialinteractions,andcentralprocessingofneuropathicpain.
PharmacolTher2010;126:56–68.
[69]GaoYJ,JiRR.
Targetingastrocytesignalingforchronicpain.
Neurotherapeutics2010;7:482–93.
[70]GaoYJ,XuZZ,LiuYC,WenYR,DecosterdI,JiRR.
Thec-JunN-terminalkinase1(JNK1)inspinalastrocytesisrequiredforthemaintenanceofbilateralmechanicalallodyniaunderapersistentinammatorypaincondition.
PAIN2010;148:309–19.
[71]GaoYJ,ZhangL,JiRR.
SpinalinjectionofTNF-alpha-activatedastrocytesproducespersistentpainsymptommechanicalallodyniabyreleasingmonocytechemoattractantprotein-1.
Glia2010;58:1871–80.
[72]GaoYJ,ZhangL,SamadOA,SuterMR,YasuhikoK,XuZZ,ParkJY,LindAL,MaQ,JiRR.
JNK-inducedMCP-1productioninspinalcordastrocytescontributestocentralsensitizationandneuropathicpain.
JNeurosci2009;29:4096–108.
[73]GarrawaySM,PetruskaJC,MendellLM.
BDNFsensitizestheresponseoflaminaIIneuronstohighthresholdprimaryafferentinputs.
EurJNeurosci2003;18:2467–76.
[74]GarreJM,RetamalMA,CassinaP,BarbeitoL,BukauskasFF,SaezJC,BennettMV,AbudaraV.
FGF-1inducesATPreleasefromspinalastrocytesincultureandopenspannexinandconnexinhemichannels.
ProcNatlAcadSciUSA2010;107:22659–64.
[75]GarrisonCJ,DoughertyPM,CarltonSM.
GFAPexpressioninlumbarspinalcordofnaiveandneuropathicratstreatedwithMK-801.
ExpNeurol1994;129:237–43.
[76]GarrisonCJ,DoughertyPM,KajanderKC,CarltonSM.
Stainingofglialbrillaryacidicprotein(GFAP)inlumbarspinalcordincreasesfollowingasciaticnerveconstrictioninjury.
BrainRes1991;565:1–7.
[77]GiaumeC,McCarthyKD.
Controlofgap-junctionalcommunicationinastrocyticnetworks.
TrendsNeurosci1996;19:319–25.
[78]GoldMS,GebhartGF.
Nociceptorsensitizationinpainpathogenesis.
NatMed2010;16:1248–57.
[79]GongQJ,LiYY,XinWJ,ZangY,RenWJ,WeiXH,LiYY,ZhangT,LiuXG.
ATPinduceslong-termpotentiationofC-ber-evokedeldpotentialsinspinaldorsalhorn:therolesofP2X4receptorsandp38MAPKinmicroglia.
Glia2009;57:583–91.
[80]GosselinRD,SuterMR,JiRR,DecosterdI.
Glialcellsandchronicpain.
Neuroscientist2010;16:519–31.
[81]GosselinRD,VarelaC,BanisadrG,MechighelP,RosteneW,KitabgiP,Melik-ParsadaniantzS.
ConstitutiveexpressionofCCR2chemokinereceptorandinhibitionbyMCP-1/CCL2ofGABA-inducedcurrentsinspinalcordneurones.
JNeurochem2005;95:1023–34.
[82]GrifnRS,CostiganM,BrennerGJ,MaCH,ScholzJ,MossA,AllchorneAJ,StahlGL,WoolfCJ.
ComplementinductioninspinalcordmicrogliaresultsinanaphylatoxinC5a-mediatedpainhypersensitivity.
JNeurosci2007;27:8699–708.
[83]GuoW,WangH,WatanabeM,ShimizuK,ZouS,LaGraizeSC,WeiF,DubnerR,RenK.
Glial-cytokine-neuronalinteractionsunderlyingthemechanismsofpersistentpain.
JNeurosci2007;27:6006–18.
[84]GuoW,WangH,ZouS,DubnerR,RenK.
Chemokinesignalinginvolvingchemokine(C–Cmotif)ligand2playsaroleindescendingpainfacilitation.
NeurosciBull2012;28:193–207.
[85]GwakYS,KangJ,UnabiaGC,HulseboschCE.
Spatialandtemporalactivationofspinalglialcells:roleofgliopathyincentralneuropathicpainfollowingspinalcordinjuryinrats.
ExpNeurol2012;234:362–72.
[86]HainsBC,WaxmanSG.
Activatedmicrogliacontributetothemaintenanceofchronicpainafterspinalcordinjury.
JNeurosci2006;26:4308–17.
[87]HainsLE,LoramLC,WeiselerJL,FrankMG,BlossEB,SholarP,TaylorFR,HarrisonJA,MartinTJ,EisenachJC,MaierSF,WatkinsLR.
Painintensityanddurationcanbeenhancedbypriorchallenge:initialevidencesuggestiveofaroleofmicroglialpriming.
JPain2010;11:1004–14.
[88]HanX,ChenM,WangF,WindremM,WangS,ShanzS,XuQ,OberheimNA,BekarL,BetstadtS,SilvaAJ,TakanoT,GoldmanSA,NedergaardM.
Forebrainengraftmentbyhumanglialprogenitorcellsenhancessynapticplasticityandlearninginadultmice.
CellStemCell2013;12:342–53.
[89]HananiM.
Satelliteglialcellsinsensoryganglia:fromformtofunction.
BrainResBrainResRev2005;48:457–76.
[90]HananiM.
Intercellularcommunicationinsensorygangliabypurinergicreceptorsandgapjunctions:Implicationsforchronicpain.
BrainRes2012;1487:183–91.
[91]HananiM,HuangTY,CherkasPS,LeddaM,PanneseE.
Glialcellplasticityinsensorygangliainducedbynervedamage.
Neuroscience2002;114:279–83.
[92]HanischUK.
Microgliaasasourceandtargetofcytokines.
Glia2002;40:140–55.
[93]HanischUK,KettenmannH.
Microglia:activesensorandversatileeffectorcellsinthenormalandpathologicbrain.
NatNeurosci2007;10:1387–94.
14R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[94]HaoS,MataM,GloriosoJC,FinkDJ.
HSV-mediatedexpressionofinterleukin-4indorsalrootganglionneuronsreducesneuropathicpain.
MolPain2006;2:6.
[95]HaraguchiK,KawamotoA,IsamiK,MaedaS,KusanoA,AsakuraK,ShirakawaH,MoriY,NakagawaT,KanekoS.
TRPM2contributestoinammatoryandneuropathicpainthroughtheaggravationofpronociceptiveinammatoryresponsesinmice.
JNeurosci2012;32:3931–41.
[96]HarveyRJ,DepnerUB,WassleH,AhmadiS,HeindlC,ReinoldH,SmartTG,HarveyK,SchutzB,Abo-SalemOM,ZimmerA,PoisbeauP,WelzlH,WolferDP,BetzH,ZeilhoferHU,MullerU.
GlyRalpha3:anessentialtargetforspinalPGE2-mediatedinammatorypainsensitization.
Science2004;304:884–7.
[97]HathwayGJ,Vega-AvelairaD,MossA,IngramR,FitzgeraldM.
Brief,lowfrequencystimulationofratperipheralC-bresevokesprolongedmicroglial-inducedcentralsensitizationinadultsbutnotinneonates.
PAIN2009;144:110–8.
[98]HonoreP,RogersSD,SchweiMJ,Salak-JohnsonJL,LugerNM,SabinoMC,ClohisyDR,MantyhPW.
Murinemodelsofinammatory,neuropathicandcancerpaineachgeneratesauniquesetofneurochemicalchangesinthespinalcordandsensoryneurons.
Neuroscience2000;98:585–98.
[99]HorvathRJ,DeLeoJA.
MorphineenhancesmicroglialmigrationthroughmodulationofP2X4receptorsignaling.
JNeurosci2009;29:998–1005.
[100]HuaXY,SvenssonCI,MatsuiT,FitzsimmonsB,YakshTL,WebbM.
Intrathecalminocyclineattenuatesperipheralinammation-inducedhyperalgesiabyinhibitingp38MAPKinspinalmicroglia.
EurJNeurosci2005;22:2431–40.
[101]HuangL,WangCF,SerhanCN,StrichartzG.
EnduringpreventionandtransientreductionofpostoperativepainbyintrathecalresolvinD1.
PAIN2011;152:557–65.
[102]HulseboschCE,HainsBC,CrownED,CarltonSM.
Mechanismsofchroniccentralneuropathicpainafterspinalcordinjury.
BrainResRev2009;60:202–13.
[103]HutchinsonMR,CoatsBD,LewisSS,ZhangY,SprungerDB,RezvaniN,BakerEM,JekichBM,WieselerJL,SomogyiAA,MartinD,PooleS,JuddCM,MaierSF,WatkinsLR.
Proinammatorycytokinesopposeopioid-inducedacuteandchronicanalgesia.
BrainBehavImmun2008;22:1178–89.
[104]HutchinsonMR,LewisSS,CoatsBD,RezvaniN,ZhangY,WieselerJL,SomogyiAA,YinH,MaierSF,RiceKC,WatkinsLR.
Possibleinvolvementoftoll-likereceptor4/myeloiddifferentiationfactor-2activityofopioidinactiveisomerscausesspinalproinammationandrelatedbehavioralconsequences.
Neuroscience2010;167:880–93.
[105]HutchinsonMR,ZhangY,ShridharM,EvansJH,BuchananMM,ZhaoTX,SlivkaPF,CoatsBD,RezvaniN,WieselerJ,HughesTS,LandgrafKE,ChanS,FongS,PhippsS,FalkeJJ,LeinwandLA,MaierSF,YinH,RiceKC,WatkinsLR.
Evidencethatopioidsmayhavetoll-likereceptor4andMD-2effects.
BrainBehavImmun2010;24:83–95.
[106]IadecolaC,NedergaardM.
Glialregulationofthecerebralmicrovasculature.
NatNeurosci2007;10:1369–76.
[107]JasminL,VitJP,BhargavaA,OharaPT.
CansatelliteglialcellsbetherapeutictargetsforpaincontrolNeuronGliaBiol2010;6:63–71.
[108]JiRR.
Targetingmicroglialpurinergicsignalingtoimprovemorphineanalgesia.
PAIN2010;150:377–8.
[109]JiRR,GereauRW,MalcangioM,StrichartzGR.
MAPkinaseandpain.
BrainResRev2009;60:135–48.
[110]JiRR,KawasakiY,ZhuangZY,WenYR,DecosterdI.
Possibleroleofspinalastrocytesinmaintainingchronicpainsensitization:reviewofcurrentevidencewithfocusonbFGF/JNKpathway.
NeuronGliaBiol2006;2:259–69.
[111]JiRR,KohnoT,MooreKA,WoolfCJ.
CentralsensitizationandLTP:dopainandmemorysharesimilarmechanismsTrendsNeurosci2003;26:696–705.
[112]JiRR,StrichartzG.
Cellsignalingandthegenesisofneuropathicpain.
ScienceSignaling(ScienceSig),partofScience2004:reE14.
[113]JiRR,SuterMR.
P38MAPK,microglialsignaling,andneuropathicpain.
MolPain2007;3:33.
[114]JiRR,XuZZ,StrichartzG,SerhanCN.
Emergingrolesofresolvinsintheresolutionofinammationandpain.
TrendsNeurosci2011;34(11):599–609.
[115]JiRR,XuZZ,WangX,LoEH.
Matrixmetalloproteaseregulationofneuropathicpain.
TrendsPharmacolSci2009;30:336–40.
[116]JiRR,ZhangQ,ZhangX,PiehlF,ReillyT,PetterssonRF,HokfeltT.
ProminentexpressionofbFGFindorsalrootgangliaafteraxotomy.
EurJNeurosci1995;7:2458–68.
[117]JinH,LiYH,XuJS,GuoGQ,ChenDL,BoY.
LipoxinA4analogattenuatesmorphineantinociceptivetolerance,withdrawal-inducedhyperalgesia,andglialreactionandcytokineexpressioninthespinalcordofrat.
Neuroscience2012;208:1–10.
[118]JinSX,ZhuangZY,WoolfCJ,JiRR.
P38mitogen-activatedproteinkinaseisactivatedafteraspinalnerveligationinspinalcordmicrogliaanddorsalrootganglionneuronsandcontributestothegenerationofneuropathicpain.
JNeurosci2003;23:4017–22.
[119]JinX,GereauRW.
Acutep38-mediatedmodulationoftetrodotoxin-resistantsodiumchannelsinmousesensoryneuronsbytumornecrosisfactor-alpha.
JNeurosci2006;26:246–55.
[120]JohnstonIN,MilliganED,Wieseler-FrankJ,FrankMG,ZapataV,CampisiJ,LangerS,MartinD,GreenP,FleshnerM,LeinwandL,MaierSF,WatkinsLR.
Aroleforproinammatorycytokinesandfractalkineinanalgesia,tolerance,andsubsequentpainfacilitationinducedbychronicintrathecalmorphine.
JNeurosci2004;24:7353–65.
[121]JungH,BhangooS,BanisadrG,FreitagC,RenD,WhiteFA,MillerRJ.
VisualizationofchemokinereceptoractivationintransgenicmicerevealsperipheralactivationofCCR2receptorsinstatesofneuropathicpain.
JNeurosci2009;29:8051–62.
[122]KalliomakiJ,AttalN,JonzonB,BachFW,HuizarK,RatcliffeS,ErikssonB,JaneckiM,DanilovA,BouhassiraD.
Arandomized,double-blind,placebo-controlledtrialofachemokinereceptor2(CCR2)antagonistinposttraumaticneuralgia.
PAIN2013;154:761–7.
[123]KangJ,KangN,LovattD,TorresA,ZhaoZ,LinJ,NedergaardM.
Connexin43hemichannelsarepermeabletoATP.
JNeurosci2008;28:4702–11.
[124]KatagiriA,ShinodaM,HondaK,ToyofukuA,SessleBJ,IwataK.
SatelliteglialcellP2Y12receptorinthetrigeminalganglionisinvolvedinlingualneuropathicpainmechanismsinrats.
MolPain2012;8:23.
[125]KatsuraH,ObataK,MiyoshiK,KondoT,YamanakaH,KobayashiK,DaiY,FukuokaT,SakagamiM,NoguchiK.
Transforminggrowthfactor-activatedkinase1inducedinspinalastrocytescontributestomechanicalhypersensitivityafternerveinjury.
Glia2008;56:723–33.
[126]KatsuraH,ObataK,MizushimaT,SakuraiJ,KobayashiK,YamanakaH,DaiY,FukuokaT,SakagamiM,NoguchiK.
ActivationofSrc-familykinasesinspinalmicrogliacontributestomechanicalhypersensitivityafternerveinjury.
JNeurosci2006;26:8680–90.
[127]KawasakiY,XuZZ,WangX,ParkJY,ZhuangZY,TanPH,GaoYJ,RoyK,CorfasG,LoEH,JiRR.
Distinctrolesofmatrixmetalloproteasesintheearly-andlate-phasedevelopmentofneuropathicpain.
NatMed2008;14:331–6.
[128]KawasakiY,ZhangL,ChengJK,JiRR.
Cytokinemechanismsofcentralsensitization:distinctandoverlappingroleofinterleukin-1beta,interleukin-6,andtumornecrosisfactor-alphainregulatingsynapticandneuronalactivityinthesupercialspinalcord.
JNeurosci2008;28:5189–94.
[129]KellerAF,BeggsS,SalterMW,DeKY.
TransformationoftheoutputofspinallaminaIneuronsafternerveinjuryandmicrogliastimulationunderlyingneuropathicpain.
MolPain2007;3:27.
[130]KimD,KimMA,ChoIH,KimMS,LeeS,JoEK,ChoiSY,ParkK,KimJS,AkiraS,NaHS,OhSB,LeeSJ.
Acriticalroleoftoll-likereceptor2innerveinjury-inducedspinalcordglialcellactivationandpainhypersensitivity.
JBiolChem2007;282:14975–83.
[131]KimD,YouB,JoEK,HanSK,SimonMI,LeeSJ.
NADPHoxidase2-derivedreactiveoxygenspeciesinspinalcordmicrogliacontributetoperipheralnerveinjury-inducedneuropathicpain.
ProcNatlAcadSciUSA2010;107:14851–6.
[132]KimDS,LiKW,BoroujerdiA,PeterYY,ZhouCY,DengP,ParkJ,ZhangX,LeeJ,CorpeM,SharpK,StewardO,ErogluC,BarresB,ZauckeF,XuZC,LuoZD.
Thrombospondin-4contributestospinalsensitizationandneuropathicpainstates.
JNeurosci2012;32:8977–87.
[133]KimelbergHK,NedergaardM.
Functionsofastrocytesandtheirpotentialastherapeutictargets.
Neurotherapeutics2010;7:338–53.
[134]KleinT,MagerlW,HopfHC,SandkuhlerJ,TreedeRD.
Perceptualcorrelatesofnociceptivelong-termpotentiationandlong-termdepressioninhumans.
JNeurosci2004;24:964–71.
[135]KobayashiK,TakahashiE,MiyagawaY,YamanakaH,NoguchiK.
InductionoftheP2X7receptorinspinalmicrogliainaneuropathicpainmodel.
NeurosciLett2011;504:57–61.
[136]KobayashiK,YamanakaH,FukuokaT,DaiY,ObataK,NoguchiK.
P2Y12receptorupregulationinactivatedmicrogliaisagatewayofp38signalingandneuropathicpain.
JNeurosci2008;28:2892–902.
[137]KobayashiK,YamanakaH,YanamotoF,OkuboM,NoguchiK.
MultipleP2Ysubtypesinspinalmicrogliaareinvolvedinneuropathicpainafterperipheralnerveinjury.
Glia2012;60(10):1529–39.
[138]KozaiT,YamanakaH,DaiY,ObataK,KobayashiK,MashimoT,NoguchiK.
Tissuetypeplasminogenactivatorinducedinratdorsalhornastrocytescontributestomechanicalhypersensitivityfollowingdorsalrootinjury.
Glia2007;55:595–603.
[139]KunerR.
Centralmechanismsofpathologicalpain.
NatMed2010;16:1258–66.
[140]LanL,YuanH,DuanL,CaoR,GaoB,ShenJ,XiongY,ChenLW,RaoZR.
Blockingtheglialfunctionsuppressessubcutaneousformalin-inducednociceptivebehaviorintherat.
NeurosciRes2007;57:112–9.
[141]LandryRP,JacobsVL,Romero-SandovalEA,DeLeoJA.
Propentofylline,aCNSglialmodulatordoesnotdecreasepaininpost-herpeticneuralgiapatients:invitroevidencefordifferentialresponsesinhumanandrodentmicrogliaandmacrophages.
ExpNeurol2012;234:340–50.
[142]LatremoliereA,WoolfCJ.
Centralsensitization:ageneratorofpainhypersensitivitybycentralneuralplasticity.
JPain2009;10:895–926.
[143]LeverIJ,BradburyEJ,CunninghamJR,AdelsonDW,JonesMG,McMahonSB,MarvizonJC,MalcangioM.
Brain-derivedneurotrophicfactorisreleasedinthedorsalhornbydistinctivepatternsofafferentberstimulation.
JNeurosci2001;21:4469–77.
[144]LiJY,XieW,StrongJA,GuoQL,ZhangJM.
Mechanicalhypersensitivity,sympatheticsprouting,andglialactivationareattenuatedbylocalinjectionofcorticosteroidnearthelumbarganglioninaratmodelofneuropathicpain.
RegAnesthPainMed2011;36:56–62.
[145]LiWE,NagyJI.
Activationofbresinratsciaticnervealtersphosphorylationstateofconnexin-43atastrocyticgapjunctionsinspinalcord:evidenceforjunctionregulationbyneuronal–glialinteractions.
Neuroscience2000;97:113–23.
[146]LiWW,GuoTZ,LiangD,ShiX,WeiT,KingeryWS,ClarkJD.
TheNALP1inammasomecontrolscytokineproductionandnociceptioninaratfracturemodelofcomplexregionalpainsyndrome.
PAIN2009;147:277–86.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx15Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[147]LiawWJ,StephensJrRL,BinnsBC,ChuY,SepkutyJP,JohnsRA,RothsteinJD,TaoYX.
Spinalglutamateuptakeiscriticalformaintainingnormalsensorytransmissioninratspinalcord.
PAIN2005;115:60–70.
[148]LinJH,LouN,KangN,TakanoT,HuF,HanX,XuQ,LovattD,TorresA,WilleckeK,YangJ,KangJ,NedergaardM.
Acentralroleofconnexin43inhypoxicpreconditioning.
JNeurosci2008;28:681–95.
[149]LiuCN,WallPD,BenDorE,MichaelisM,AmirR,DevorM.
TactileallodyniaintheabsenceofC-beractivation:alteredringpropertiesofDRGneuronsfollowingspinalnerveinjury.
PAIN2000;85:503–21.
[150]LiuFY,SunYN,WangFT,LiQ,SuL,ZhaoZF,MengXL,ZhaoH,WuX,SunQ,XingGG,WanY.
Activationofsatelliteglialcellsinlumbardorsalrootgangliacontributestoneuropathicpainafterspinalnerveligation.
BrainRes2012;1427:65–77.
[151]LiuL,RudinM,KozlovaEN.
Glialcellproliferationinthespinalcordafterdorsalrhizotomyorsciaticnervetransectionintheadultrat.
ExpBrainRes2000;131:64–73.
[152]LiuT,GaoYJ,JiRR.
EmergingroleofToll-likereceptorsinthecontrolofpainanditch.
NeurosciBull2012;28:131–44.
[153]LiuYC,BertaT,LiuT,TanPH,JiRR.
Acutemorphineinducesmatrixmetalloproteinase-9up-regulationinprimarysensoryneuronstomaskopioid-inducedanalgesiainmice.
MolPain2012;8:19.
[154]LiuYL,ZhouLJ,HuNW,XuJT,WuCY,ZhangT,LiYY,LiuXG.
Tumornecrosisfactor-alphainduceslong-termpotentiationofC-berevokedeldpotentialsinspinaldorsalhorninratswithnerveinjury:theroleofNF-kappaB,JNKandp38MAPK.
Neuropharmacology2007;52:708–15.
[155]MaC,ShuY,ZhengZ,ChenY,YaoH,GreenquistKW,WhiteFA,LaMotteRH.
Similarelectrophysiologicalchangesinaxotomizedandneighboringintactdorsalrootganglionneurons.
JNeurophysiol2003;89:1588–602.
[156]MadiaiF,GoettlVM,HussainSR,ClairmontAR,StephensJrRL,HackshawKV.
Anti-broblastgrowthfactor-2antibodiesattenuatemechanicalallodyniainaratmodelofneuropathicpain.
JMolNeurosci2005;27:315–24.
[157]MaedaS,KawamotoA,YataniY,ShirakawaH,NakagawaT,KanekoS.
GenetransferofGLT-1,aglialglutamatetransporter,intothespinalcordbyrecombinantadenovirusattenuatesinammatoryandneuropathicpaininrats.
MolPain2008;4:65.
[158]MaoJ,SungB,JiRR,LimG.
Chronicmorphineinducesdownregulationofspinalglutamatetransporters:implicationsinmorphinetoleranceandabnormalpainsensitivity.
JNeurosci2002;22:8312–23.
[159]MatsukaY,OnoT,IwaseH,MitrirattanakulS,OmotoKS,ChoT,LamYY,SnyderB,SpigelmanI.
AlteredATPreleaseandmetabolismindorsalrootgangliaofneuropathicrats.
MolPain2008;4:66.
[160]McMahonSB,MalcangioM.
Currentchallengesinglia-painbiology.
Neuron2009;64:46–54.
[161]MellerST,DykstraC,GrzybyckiD,MurphyS,GebhartGF.
Thepossibleroleofgliainnociceptiveprocessingandhyperalgesiainthespinalcordoftherat.
Neuropharmacology1994;33:1471–8.
[162]MenetskiJ,MistryS,LuM,MudgettJS,RansohoffRM,DeMartinoJA,MacIntyreDE,AbbadieC.
Miceoverexpressingchemokineligand2(CCL2)inastrocytesdisplayenhancednociceptiveresponses.
Neuroscience2007;149:706–14.
[163]MilliganED,TwiningC,ChacurM,BiedenkappJ,O'ConnorK,PooleS,TraceyK,MartinD,MaierSF,WatkinsLR.
Spinalgliaandproinammatorycytokinesmediatemirror-imageneuropathicpaininrats.
JNeurosci2003;23:1026–40.
[164]MilliganED,WatkinsLR.
Pathologicalandprotectiverolesofgliainchronicpain.
NatRevNeurosci2009;10:23–36.
[165]MilliganED,ZapataV,ChacurM,SchoenigerD,BiedenkappJ,O'ConnorKA,VergeGM,ChapmanG,GreenP,FosterAC,NaeveGS,MaierSF,WatkinsLR.
Evidencethatexogenousandendogenousfractalkinecaninducespinalnociceptivefacilitationinrats.
EurJNeurosci2004;20:2294–302.
[166]MiraucourtLS,PeirsC,DallelR,VoisinDL.
Glycineinhibitorydysfunctionturnstouchintopainthroughastrocyte-derivedD-serine.
PAIN2011;152:1340–8.
[167]MiyoshiK,ObataK,KondoT,OkamuraH,NoguchiK.
Interleukin-18-mediatedmicroglia/astrocyteinteractioninthespinalcordenhancesneuropathicpainprocessingafternerveinjury.
JNeurosci2008;28:12775–87.
[168]MogilJS.
Sexdifferencesinpainandpaininhibition:multipleexplanationsofacontroversialphenomenon.
NatRevNeurosci2012;13:859–66.
[169]MooreKA,KohnoT,KarchewskiLA,ScholzJ,BabaH,WoolfCJ.
PartialperipheralnerveinjurypromotesaselectivelossofGABAergicinhibitioninthesupercialdorsalhornofthespinalcord.
JNeurosci2002;22:6724–31.
[170]MossA,BeggsS,Vega-AvelairaD,CostiganM,HathwayGJ,SalterMW,FitzgeraldM.
Spinalmicrogliaandneuropathicpaininyoungrats.
PAIN2007;128:215–24.
[171]NdongC,LandryRP,DeLeoJA,Romero-SandovalEA.
Mitogenactivatedproteinkinasephosphatase-1preventsthedevelopmentoftactilesensitivityinarodentmodelofneuropathicpain.
MolPain2012;8:34.
[172]NedergaardM,VerkhratskyA.
Artifactversusreality—howastrocytescontributetosynapticevents.
Glia2012;60:1013–23.
[173]NesicO,LeeJ,JohnsonKM,YeZ,XuGY,UnabiaGC,WoodTG,McAdooDJ,WestlundKN,HulseboschCE,ReginoPerez-PoloJ.
Transcriptionalprolingofspinalcordinjury-inducedcentralneuropathicpain.
JNeurochem2005;95:998–1014.
[174]NicotraL,LoramLC,WatkinsLR,HutchinsonMR.
Toll-likereceptorsinchronicpain.
ExpNeurol2012;234(2):316–29.
[175]NimmerjahnA,KirchhoffF,HelmchenF.
Restingmicroglialcellsarehighlydynamicsurveillantsofbrainparenchymainvivo.
Science2005;308:1314–8.
[176]NongY,HuangYQ,JuW,KaliaLV,AhmadianG,WangYT,SalterMW.
GlycinebindingprimesNMDAreceptorinternalization.
Nature2003;422:302–7.
[177]ObataK,KatsuraH,MizushimaT,SakuraiJ,KobayashiK,YamanakaH,DaiY,FukuokaT,NoguchiK.
Rolesofextracellularsignal-regulatedproteinkinases5inspinalmicrogliaandprimarysensoryneuronsforneuropathicpain.
JNeurochem2007;102:1569–84.
[178]ObataK,NoguchiK.
MAPKactivationinnociceptiveneuronsandpainhypersensitivity.
LifeSci2004;74:2643–53.
[179]ObataK,YamanakaH,KobayashiK,DaiY,MizushimaT,KatsuraH,FukuokaT,TokunagaA,NoguchiK.
Roleofmitogen-activatedproteinkinaseactivationininjuredandintactprimaryafferentneuronsformechanicalandheathypersensitivityafterspinalnerveligation.
JNeurosci2004;24:10211–22.
[180]OberheimNA,TakanoT,HanX,HeW,LinJH,WangF,XuQ,WyattJD,PilcherW,OjemannJG,RansomBR,GoldmanSA,NedergaardM.
Uniquelyhominidfeaturesofadulthumanastrocytes.
JNeurosci2009;29:3276–87.
[181]OberheimNA,TianGF,HanX,PengW,TakanoT,RansomB,NedergaardM.
Lossofastrocyticdomainorganizationintheepilepticbrain.
JNeurosci2008;28:3264–76.
[182]OberheimNA,WangX,GoldmanS,NedergaardM.
Astrocyticcomplexitydistinguishesthehumanbrain.
TrendsNeurosci2006;29:547–53.
[183]OharaPT,VitJP,BhargavaA,JasminL.
Evidenceforaroleofconnexin43intrigeminalpainusingRNAinterferenceinvivo.
JNeurophysiol2008;100:3064–73.
[184]Okada-OgawaA,SuzukiI,SessleBJ,ChiangCY,SalterMW,DostrovskyJO,TsuboiY,KondoM,KitagawaJ,KobayashiA,NomaN,ImamuraY,IwataK.
Astrogliainmedullarydorsalhorn(trigeminalspinalsubnucleuscaudalis)areinvolvedintrigeminalneuropathicpainmechanisms.
JNeurosci2009;29:11161–71.
[185]PanneseE,LeddaM,CherkasPS,HuangTY,HananiM.
Satellitecellreactionstoaxoninjuryofsensoryganglionneurons:increaseinnumberofgapjunctionsandformationofbridgesconnectingpreviouslyseparateperineuronalsheaths.
AnatEmbryol(Berl)2003;206:337–47.
[186]PaolicelliRC,BolascoG,PaganiF,MaggiL,ScianniM,PanzanelliP,GiustettoM,FerreiraTA,GuiducciE,DumasL,RagozzinoD,GrossCT.
Synapticpruningbymicrogliaisnecessaryfornormalbraindevelopment.
Science2011;333:1456–8.
[188]ParkCK,LuN,XuZZ,LiuT,SerhanCN,JiRR.
ResolvingTRPV1-andTNF-mediatedspinalcordsynapticplasticityandinammatorypainwithneuroprotectinD1.
JNeurosci2011;31:15072–85.
[189]ParkCK,XuZZ,LiuT,LuN,SerhanCN,JiRR.
Resolvind2isapotentendogenousinhibitorfortransientreceptorpotentialsubtypev1/a1,inammatorypain,andspinalcordsynapticplasticityinmice:distinctrolesofresolvind1,d2,ande1.
JNeurosci2011;31:18433–8.
[190]ParpuraV,ScemesE,SprayDC.
Mechanismsofglutamatereleasefromastrocytes:gapjunction''hemichannels'',purinergicreceptorsandexocytoticrelease.
NeurochemInt2004;45:259–64.
[191]PengW,CotrinaML,HanX,YuH,BekarL,BlumL,TakanoT,TianGF,GoldmanSA,NedergaardM.
SystemicadministrationofanantagonistoftheATP-sensitivereceptorP2X7improvesrecoveryafterspinalcordinjury.
ProcNatlAcadSciUSA2009;106:12489–93.
[192]PetersCM,EisenachJC.
Contributionofthechemokine(C–Cmotif)ligand2(CCL2)tomechanicalhypersensitivityaftersurgicalincisioninrats.
Anesthesiology2010;112:1250–8.
[193]PetraviczJ,FiaccoTA,McCarthyKD.
LossofIP3receptor-dependentCa2+increasesinhippocampalastrocytesdoesnotaffectbaselineCA1pyramidalneuronsynapticactivity.
JNeurosci2008;28:4967–73.
[194]PiaoZG,ChoIH,ParkCK,HongJP,ChoiSY,LeeSJ,LeeS,ParkK,KimJS,OhSB.
Activationofgliaandmicroglialp38MAPKinmedullarydorsalhorncontributestotactilehypersensitivityfollowingtrigeminalsensorynerveinjury.
PAIN2006;121:219–31.
[195]PorrecaF,OssipovMH,GebhartGF.
Chronicpainandmedullarydescendingfacilitation.
TrendsNeurosci2002;25:319–25.
[196]RaczI,NadalX,AlferinkJ,BanosJE,RehneltJ,MartinM,PintadoB,Gutierrez-AdanA,SanguinoE,BelloraN,ManzanaresJ,ZimmerA,MaldonadoR.
Interferon-gammaisacriticalmodulatorofCB(2)cannabinoidreceptorsignalingduringneuropathicpain.
JNeurosci2008;28:12136–45.
[197]RaghavendraV,TangaF,DeLeoJA.
Inhibitionofmicroglialactivationattenuatesthedevelopmentbutnotexistinghypersensitivityinaratmodelofneuropathy.
JPharmacolExpTher2003;306:624–30.
[198]RaghavendraV,TangaFY,DeLeoJA.
CompleteFreundsadjuvant-inducedperipheralinammationevokesglialactivationandproinammatorycytokineexpressionintheCNS.
EurJNeurosci2004;20:467–73.
[199]RaivichG.
Likecopsonthebeat:theactiveroleofrestingmicroglia.
TrendsNeurosci2005;28:571–3.
[200]RenK,DubnerR.
Interactionsbetweentheimmuneandnervoussystemsinpain.
NatMed2010;16:1267–76.
[201]RenWH,GuoJD,CaoH,WangH,WangPF,ShaH,JiRR,ZhaoZQ,ZhangYQ.
IsendogenousD-serineintherostralanteriorcingulatecortexnecessaryforpain-relatednegativeaffectJNeurochem2006;96:1636–47.
[202]Romero-SandovalEA,HorvathR,LandryRP,DeLeoJA.
Cannabinoidreceptortype2activationinducesamicroglialanti-inammatoryphenotypeandreducesmigrationviaMKPinductionandERKdephosphorylation.
MolPain2009;5:25.
16R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[203]RothsteinJD,Dykes-HobergM,PardoCA,BristolLA,JinL,KunclRW,KanaiY,HedigerMA,WangY,SchielkeJP,WeltyDF.
Knockoutofglutamatetransportersrevealsamajorroleforastroglialtransportinexcitotoxicityandclearanceofglutamate.
Neuron1996;16:675–86.
[204]RothsteinJD,MartinL,LeveyAI,Dykes-HobergM,JinL,WuD,NashN,KunclRW.
Localizationofneuronalandglialglutamatetransporters.
Neuron1994;13:713–25.
[205]RuscheweyhR,Wilder-SmithO,DrdlaR,LiuXG,SandkuhlerJ.
Long-termpotentiationinspinalnociceptivepathwaysasanoveltargetforpaintherapy.
MolPain2011;7:20.
[206]SamadTA,MooreKA,SapirsteinA,BilletS,AllchorneA,PooleS,BonventreJV,WoolfCJ.
Interleukin-1beta-mediatedinductionofCox-2intheCNScontributestoinammatorypainhypersensitivity.
Nature2001;410:471–5.
[207]SchafersM,LeeDH,BrorsD,YakshTL,SorkinLS.
Increasedsensitivityofinjuredandadjacentuninjuredratprimarysensoryneuronstoexogenoustumornecrosisfactor-alphaafterspinalnerveligation.
JNeurosci2003;23:3028–38.
[208]ScherrerG,LowSA,WangX,ZhangJ,YamanakaH,UrbanR,SolorzanoC,HarperB,HnaskoTS,EdwardsRH,BasbaumAI.
VGLUT2expressioninprimaryafferentneuronsisessentialfornormalacutepainandinjury-inducedheathypersensitivity.
ProcNatlAcadSciUSA2010;107:22296–301.
[209]ScholzJ,WoolfCJ.
Theneuropathicpaintriad:neurons,immunecellsandglia.
NatNeurosci2007;10:1361–8.
[210]SerhanCN,ChiangN,VanDykeTE.
Resolvinginammation:dualanti-inammatoryandpro-resolutionlipidmediators.
NatRevImmunol2008;8:349–61.
[211]ShiYQ.
Chronic-pain-associatedastrocyticreactioninthespinalcordofHIV-infectedpatients.
JNeurosci2012;32(32):10833–40.
[212]SloaneEM,SoderquistRG,MaierSF,MahoneyMJ,WatkinsLR,MilliganED.
Long-termcontrolofneuropathicpaininanon-viralgenetherapyparadigm.
GeneTher2009;16:470–5.
[213]SommerC,SchafersM,MarziniakM,ToykaKV.
Etanerceptreduceshyperalgesiainexperimentalpainfulneuropathy.
JPeripherNervSyst2001;6:67–72.
[214]SongP,ZhaoZQ.
Theinvolvementofglialcellsinthedevelopmentofmorphinetolerance.
NeurosciRes2001;39:281–6.
[215]SorgeRE,TrangT,DorfmanR,SmithSB,BeggsS,RitchieJ,AustinJS,ZaykinDV,VanderMH,CostiganM,HerbertTA,Yarkoni-AbitbulM,TichauerD,LivnehJ,GershonE,ZhengM,TanK,JohnSL,SladeGD,JordanJ,WoolfCJ,PeltzG,MaixnerW,DiatchenkoL,SeltzerZ,SalterMW,MogilJS.
GeneticallydeterminedP2X7receptorporeformationregulatesvariabilityinchronicpainsensitivity.
NatMed2012;18:595–9.
[216]SorkinLS,XiaoWH,WagnerR,MyersRR.
Tumournecrosisfactor-alphainducesectopicactivityinnociceptiveprimaryafferentbres.
Neuroscience1997;81:255–62.
[217]SouslovaV,CesareP,DingY,AkopianAN,StanfaL,SuzukiR,CarpenterK,DickensonA,BoyceS,HillR,Nebenuis-OosthuizenD,SmithAJ,KiddEJ,WoodJN.
Warm-codingdecitsandaberrantinammatorypaininmicelackingP2X3receptors.
Nature2000;407:1015–7.
[218]SpataroLE,SloaneEM,MilliganED,Wieseler-FrankJ,SchoenigerD,JekichBM,BarrientosRM,MaierSF,WatkinsLR.
Spinalgapjunctions:potentialinvolvementinpainfacilitation.
JPain2004;5:392–405.
[219]StanilandAA,ClarkAK,WodarskiR,SassoO,MaioneF,D'AcquistoF,MalcangioM.
Reducedinammatoryandneuropathicpainanddecreasedspinalmicroglialresponseinfractalkinereceptor(CX3CR1)knockoutmice.
JNeurochem2010;114:1143–57.
[220]StellwagenD,BeattieEC,SeoJY,MalenkaRC.
DifferentialregulationofAMPAreceptorandGABAreceptortrafckingbytumornecrosisfactor-alpha.
JNeurosci2005;25:3219–28.
[221]StephanAH,BarresBA,StevensB.
Thecomplementsystem:anunexpectedroleinsynapticpruningduringdevelopmentanddisease.
AnnuRevNeurosci2012;35:369–89.
[222]SunS,CaoH,HanM,LiTT,PanHL,ZhaoZQ,ZhangYQ.
Newevidencefortheinvolvementofspinalfractalkinereceptorinpainfacilitationandspinalglialactivationinratmodelofmonoarthritis.
PAIN2007;129:64–75.
[223]SunW,McConnellE,PareJF,XuQ,ChenM,PengW,LovattD,HanX,SmithY,NedergaardM.
Glutamate-dependentneuroglialcalciumsignalingdiffersbetweenyoungandadultbrain.
Science2013;339:197–200.
[224]SungB,LimG,MaoJ.
Alteredexpressionanduptakeactivityofspinalglutamatetransportersafternerveinjurycontributetothepathogenesisofneuropathicpaininrats.
JNeurosci2003;23:2899–910.
[225]SungCS,WenZH,ChangWK,ChanKH,HoST,TsaiSK,ChangYC,WongCS.
Inhibitionofp38mitogen-activatedproteinkinaseattenuatesinterleukin-1beta-inducedthermalhyperalgesiaandinduciblenitricoxidesynthaseexpressioninthespinalcord.
JNeurochem2005;94:742–52.
[226]SuterMR,BertaT,GaoYJ,DecosterdI,JiRR.
LargeA-beractivityisrequiredformicroglialproliferationandp38MAPKactivationinthespinalcord:differenteffectsofresiniferatoxinandbupivacaineonspinalmicroglialchangesaftersparednerveinjury.
MolPain2009;5:53.
[227]SuterMR,WenYR,DecosterdI,JiRR.
DoglialcellscontrolpainNeuronGliaBiol2007;3:255–68.
[228]SvenssonCI,FitzsimmonsB,AziziS,PowellHC,HuaXY,YakshTL.
Spinalp38betaisoformmediatestissueinjury-inducedhyperalgesiaandspinalsensitization.
JNeurochem2005;92:1508–20.
[229]SvenssonCI,MarsalaM,WesterlundA,CalcuttNA,CampanaWM,FreshwaterJD,CatalanoR,FengY,ProtterAA,ScottB,YakshTL.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliaisacriticallinkininammation-inducedspinalpainprocessing.
JNeurochem2003;86:1534–44.
[230]SvenssonCI,SchafersM,JonesTL,PowellH,SorkinLS.
SpinalblockadeofTNFblocksspinalnerveligation-inducedincreasesinspinalP-p38.
NeurosciLett2005;379:209–13.
[231]SvenssonCI,ZattoniM,SerhanCN.
Lipoxinsandaspirin-triggeredlipoxininhibitinammatorypainprocessing.
JExpMed2007;204:245–52.
[232]SweitzerS,MartinD,DeLeoJA.
Intrathecalinterleukin-1receptorantagonistincombinationwithsolubletumornecrosisfactorreceptorexhibitsananti-allodynicactioninaratmodelofneuropathicpain.
Neuroscience2001;103:529–39.
[233]TakedaM,KitagawaJ,TakahashiM,MatsumotoS.
Activationofinterleukin-1betareceptorsuppressesthevoltage-gatedpotassiumcurrentsinthesmall-diametertrigeminalganglionneuronsfollowingperipheralinammation.
PAIN2008;139:594–602.
[234]TakedaM,TakahashiM,MatsumotoS.
Contributionofactivatedinterleukinreceptorsintrigeminalganglionneuronstohyperalgesiaviasatelliteglialinterleukin-1betaparacrinemechanism.
BrainBehavImmun2008;22:1016–23.
[235]TakedaM,TakahashiM,MatsumotoS.
Contributionoftheactivationofsatellitegliainsensorygangliatopathologicalpain.
NeurosciBiobehavRev2009;33:784–92.
[236]TakedaM,TanimotoT,KadoiJ,NasuM,TakahashiM,KitagawaJ,MatsumotoS.
Enhancedexcitabilityofnociceptivetrigeminalganglionneuronsbysatelliteglialcytokinefollowingperipheralinammation.
PAIN2007;129:155–66.
[237]TanPH,GaoYJ,BertaT,XuZZ,JiRR.
Shortsmall-interferingRNAsproduceinterferon-alpha-mediatedanalgesia.
BrJAnaesth2012;108:662–9.
[238]TangaFY,Nutile-McMenemyN,DeLeoJA.
TheCNSroleofToll-likereceptor4ininnateneuroimmunityandpainfulneuropathy.
ProcNatlAcadSciUSA2005.
[239]ThackerMA,ClarkAK,BishopT,GristJ,YipPK,MoonLD,ThompsonSW,MarchandF,McMahonSB.
CCL2isakeymediatorofmicrogliaactivationinneuropathicpainstates.
EurJPain2009;13:263–72.
[240]TheriaultE,FrankensteinUN,HertzbergEL,NagyJI.
Connexin43andastrocyticgapjunctionsintheratspinalcordafteracutecompressioninjury.
JCompNeurol1997;382:199–214.
[241]ToddAJ.
Neuronalcircuitryforpainprocessinginthedorsalhorn.
NatRevNeurosci2010;11:823–36.
[242]ToyomitsuE,TsudaM,YamashitaT,Tozaki-SaitohH,TanakaY,InoueK.
CCL2promotesP2X4receptortrafckingtothecellsurfaceofmicroglia.
PurinergicSignal2012;8:301–10.
[243]Tozaki-SaitohH,TsudaM,MiyataH,UedaK,KohsakaS,InoueK.
P2Y12receptorsinspinalmicrogliaarerequiredforneuropathicpainafterperipheralnerveinjury.
JNeurosci2008;28:4949–56.
[244]TrangT,BeggsS,SalterMW.
ATPreceptorsgatemicrogliasignalinginneuropathicpain.
ExpNeurol2012;234:354–61.
[245]TrangT,BeggsS,WanX,SalterMW.
P2X4-receptor-mediatedsynthesisandreleaseofbrain-derivedneurotrophicfactorinmicrogliaisdependentoncalciumandp38-mitogen-activatedproteinkinaseactivation.
JNeurosci2009;29:3518–28.
[246]TremblayME,StevensB,SierraA,WakeH,BessisA,NimmerjahnA.
Theroleofmicrogliainthehealthybrain.
JNeurosci2011;31:16064–9.
[247]TsudaM,InoueK,SalterMW.
Neuropathicpainandspinalmicroglia:abigproblemfrommoleculesin''small''glia.
TrendsNeurosci2005;28:101–7.
[248]TsudaM,KohroY,YanoT,TsujikawaT,KitanoJ,Tozaki-SaitohH,KoyanagiS,OhdoS,JiRR,SalterMW,InoueK.
JAK-STAT3pathwayregulatesspinalastrocyteproliferationandneuropathicpainmaintenanceinrats.
Brain2011;134(4):1127–39.
[249]TsudaM,KuboyamaK,InoueT,NagataK,Tozaki-SaitohH,InoueK.
BehavioralphenotypesofmicelackingpurinergicP2X4receptorsinacuteandchronicpainassays.
MolPain2009;5:28.
[250]TsudaM,MasudaT,KitanoJ,ShimoyamaH,Tozaki-SaitohH,InoueK.
IFN-gammareceptorsignalingmediatesspinalmicrogliaactivationdrivingneuropathicpain.
ProcNatlAcadSciUSA2009;106:8032–7.
[251]TsudaM,MizokoshiA,Shigemoto-MogamiY,KoizumiS,InoueK.
Activationofp38mitogen-activatedproteinkinaseinspinalhyperactivemicrogliacontributestopainhypersensitivityfollowingperipheralnerveinjury.
Glia2004;45:89–95.
[252]TsudaM,Shigemoto-MogamiY,KoizumiS,MizokoshiA,KohsakaS,SalterMW,InoueK.
P2X4receptorsinducedinspinalmicrogliagatetactileallodyniaafternerveinjury.
Nature2003;424:778–83.
[253]TsudaM,Tozaki-SaitohH,InoueK.
Painandpurinergicsignaling.
BrainResRev2010;63:222–32.
[254]UlmannL,HatcherJP,HughesJP,ChaumontS,GreenPJ,ConquetF,BuellGN,ReeveAJ,ChessellIP,RassendrenF.
Up-regulationofP2X4receptorsinspinalmicrogliaafterperipheralnerveinjurymediatesBDNFreleaseandneuropathicpain.
JNeurosci2008;28:11263–8.
[255]VanSJ,Reaux-LeGA,PommierB,MauborgneA,DansereauMA,KitabgiP,SarretP,PohlM,MelikPS.
CCL2releasedfromneuronalsynapticvesiclesinthespinalcordisamajormediatoroflocalinammationandpainafterperipheralnerveinjury.
JNeurosci2011;31:5865–75.
[256]Vega-AvelairaD,McKelveyR,HathwayG,FitzgeraldM.
Theemergenceofadolescentonsetpainhypersensitivityfollowingneonatalnerveinjury.
MolPain2012;8:30.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx17Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[257]VergeGM,MilliganED,MaierSF,WatkinsLR,NaeveGS,FosterAC.
Fractalkine(CX3CL1)andfractalkinereceptor(CX3CR1)distributioninspinalcordanddorsalrootgangliaunderbasalandneuropathicpainconditions.
EurJNeurosci2004;20:1150–60.
[258]VerkhratskyA,SofroniewMV,MessingA,deLanerolleNC,RempeD,RodriguezJJ,NedergaardM.
Neurologicaldiseasesasprimarygliopathies:areassessmentofneurocentrism.
ASNNeuro2012;4.
[259]VikmanKS,DugganAW,SiddallPJ.
Interferon-gammainduceddisruptionofGABAergicinhibitioninthespinaldorsalhorninvivo.
PAIN2007;133:18–28.
[260]VikmanKS,HillRH,BackstromE,RobertsonB,KristenssonK.
Interferon-gammainducescharacteristicsofcentralsensitizationinspinaldorsalhornneuronsinvitro.
PAIN2003;106:241–51.
[261]VitJP,OharaPT,BhargavaA,KelleyK,JasminL.
SilencingtheKir4.
1potassiumchannelsubunitinsatelliteglialcellsoftherattrigeminalganglionresultsinpain-likebehaviorintheabsenceofnerveinjury.
JNeurosci2008;28:4161–71.
[262]WallraffA,KohlingR,HeinemannU,TheisM,WilleckeK,SteinhauserC.
Theimpactofastrocyticgapjunctionalcouplingonpotassiumbufferinginthehippocampus.
JNeurosci2006;26:5438–47.
[263]WangF,SmithNA,XuQ,FujitaT,BabaA,MatsudaT,TakanoT,BekarL,NedergaardM.
AstrocytesmodulateneuralnetworkactivitybyCa(2)(+)-dependentuptakeofextracellularK(+).
SciSignal2012;5:ra26.
[264]WangH,GuoW,YangK,WeiF,DubnerR,RenK.
Contributionofprimaryafferentinputtotrigeminalastroglialhyperactivity,cytokineinductionandNMDAreceptorphosphorylation.
OpenPainJ2010:144–52.
[265]WangX,ArcuinoG,TakanoT,LinJ,PengWG,WanP,LiP,XuQ,LiuQS,GoldmanSA,NedergaardM.
P2X7receptorinhibitionimprovesrecoveryafterspinalcordinjury.
NatMed2004;10:821–7.
[266]WangX,LouN,XuQ,TianGF,PengWG,HanX,KangJ,TakanoT,NedergaardM.
AstrocyticCa2+signalingevokedbysensorystimulationinvivo.
NatNeurosci2006;9:816–23.
[267]WangXW,HuS,Mao-YingQL,LiQ,YangCJ,ZhangH,MiWL,WuGC,WangYQ.
Activationofc-junN-terminalkinaseinspinalcordcontributestobreastcancerinducedbonepaininrats.
MolBrain2012;5:21.
[268]WangXW,LiTT,ZhaoJ,Mao-YingQL,ZhangH,HuS,LiQ,MiWL,WuGC,ZhangYQ,WangYQ.
Extracellularsignal-regulatedkinaseactivationinspinalastrocytesandmicrogliacontributestocancer-inducedbonepaininrats.
Neuroscience2012;217:172–81.
[269]WangZ,MaW,ChabotJG,QuirionR.
Calcitoningene-relatedpeptideasaregulatorofneuronalCaMKII-CREB,microglialp38-NFkappaBandastroglialERK-Stat1/3cascadesmediatingthedevelopmentoftolerancetomorphine-inducedanalgesia.
PAIN2010;151:194–205.
[270]WatkinsLR,HutchinsonMR,JohnstonIN,MaierSF.
Glia:novelcounter-regulatorsofopioidanalgesia.
TrendsNeurosci2005;28:661–9.
[271]WatkinsLR,HutchinsonMR,RiceKC,MaierSF.
The''toll''ofopioid-inducedglialactivation:improvingtheclinicalefcacyofopioidsbytargetingglia.
TrendsPharmacolSci2009;30:581–91.
[272]WatkinsLR,MartinD,UlrichP,TraceyKJ,MaierSF.
Evidencefortheinvolvementofspinalcordgliainsubcutaneousformalininducedhyperalgesiaintherat.
PAIN1997;71:225–35.
[273]WatkinsLR,MilliganED,MaierSF.
Glialactivation:adrivingforceforpathologicalpain.
TrendsNeurosci2001;24:450–5.
[274]WeiF,GuoW,ZouS,RenK,DubnerR.
Supraspinalglial–neuronalinteractionscontributetodescendingpainfacilitation.
JNeurosci2008;28:10482–95.
[275]WenYR,SuterMR,JiRR,YehGC,WuYS,WangKC,KohnoT,SunWZ,WangCC.
Activationofp38mitogen-activatedproteinkinaseinspinalmicrogliacontributestoincision-inducedmechanicalallodynia.
Anesthesiology2009;110:155–65.
[276]WenYR,SuterMR,KawasakiY,HuangJ,PertinM,KohnoT,BerdeCB,DecosterdI,JiRR.
Nerveconductionblockadeinthesciaticnervepreventsbutdoesnotreversetheactivationofp38mitogen-activatedproteinkinaseinspinalmicrogliaintheratsparednerveinjurymodel.
Anesthesiology2007;107:312–21.
[277]WenYR,TanPH,ChengJK,LiuYC,JiRR.
Microglia:apromisingtargetfortreatingneuropathicandpostoperativepain,andmorphinetolerance.
JFormosMedAssoc2011;110:487–94.
[278]WengHR,ChenJH,CataJP.
Inhibitionofglutamateuptakeinthespinalcordinduceshyperalgesiaandincreasedresponsesofspinaldorsalhornneuronstoperipheralafferentstimulation.
Neuroscience2006;138:1351–60.
[279]WeyerbacherAR,XuQ,TamasdanC,ShinSJ,InturrisiCE.
N-methyl-D-aspartatereceptor(NMDAR)independentmaintenanceofinammatorypain.
PAIN2010;148:237–46.
[280]WhiteFA,JungH,MillerRJ.
Chemokinesandthepathophysiologyofneuropathicpain.
ProcNatlAcadSciUSA2007;104:20151–8.
[281]WhiteFA,SunJ,WatersSM,MaC,RenD,RipschM,SteikJ,CortrightDN,LaMotteRH,MillerRJ.
Excitatorymonocytechemoattractantprotein-1signalingisup-regulatedinsensoryneuronsafterchroniccompressionofthedorsalrootganglion.
ProcNatlAcadSciUSA2005;102:14092–7.
[282]WilkersonJL,GentryKR,DenglerEC,WallaceJA,KerwinAA,ArmijoLM,KuhnMN,ThakurGA,MakriyannisA,MilliganED.
IntrathecalcannabilactoneCB(2)Ragonist,AM1710,controlspathologicalpainandrestoresbasalcytokinelevels.
PAIN2012;153:1091–106.
[283]WillemenHL,EijkelkampN,WangH,DantzerR,DornGW,KelleyKW,HeijnenCJ,KavelaarsA.
Microglial/macrophageGRK2determinesdurationofperipheralIL-1beta-inducedhyperalgesia:contributionofspinalcordCX3CR1,p38andIL-1signaling.
PAIN2010;150:550–60.
[284]WillemenHL,HuoXJ,Mao-YingQL,ZijlstraJ,HeijnenCJ,KavelaarsA.
MicroRNA-124asanoveltreatmentforpersistenthyperalgesia.
JNeuroinamm2012;9:143.
[285]WoolfCJ.
Centralsensitization:implicationsforthediagnosisandtreatmentofpain.
PAIN2011;152:S2–S15.
[286]WuG,RingkampM,HartkeTV,MurinsonBB,CampbellJN,GrifnJW,MeyerRA.
EarlyonsetofspontaneousactivityinuninjuredC-bernociceptorsafterinjurytoneighboringnervebers.
JNeurosci2001;21:RC140.
[287]XieW,StrongJA,ZhangJM.
Earlyblockadeofinjuredprimarysensoryafferentsreducesglialcellactivationintworatneuropathicpainmodels.
Neuroscience2009;160:847–57.
[288]XinWJ,WengHR,DoughertyPM.
PlasticityinexpressionoftheglutamatetransportersGLT-1andGLASTinspinaldorsalhornglialcellsfollowingpartialsciaticnerveligation.
MolPain2009;5:15.
[289]XuJT,XinWJ,ZangY,WuCY,LiuXG.
Theroleoftumornecrosisfactor-alphaintheneuropathicpaininducedbylumbar5ventralroottransectioninrat.
PAIN2006;123:306–21.
[290]XuZZ,BertaT,JiRR.
ResolvinE1inhibitsneuropathicpainandspinalcordmicroglialactivationfollowingperipheralnerveinjury.
JNeuroimmunPharmacol2013;8:37–41.
[291]XuZZ,LiuXJ,BertaT,ParkC,LuN,SerhanCN,JiRR.
Neuroprotectin/protectin-1protectsneuropathicpaininmicefollowingnervetrauma.
AnnNeurol2013.
http://dx.
doi.
org/10.
1002/ana.
23928.
[Epubaheadofprint].
[292]XuZZ,ZhangL,LiuT,ParkJY,BertaT,YangR,SerhanCN,JiRR.
ResolvinsRvE1andRvD1attenuateinammatorypainviacentralandperipheralactions.
NatMed2010;16:1p.
[293]YoshimuraM,JessellTM.
Primaryafferent-evokedsynapticresponsesandslowpotentialgenerationinratsubstantiagelatinosaneuronsinvitro.
JNeurophysiol1989;62:96–108.
[294]ZeilhoferHU,BenkeD,YevenesGE.
Chronicpainstates:pharmacologicalstrategiestorestorediminishedinhibitoryspinalpaincontrol.
AnnuRevPharmacolToxicol2012;52:111–33.
[295]ZhangH,MeiX,ZhangP,MaC,WhiteFA,DonnellyDF,LaMotteRH.
Alteredfunctionalpropertiesofsatelliteglialcellsincompressedspinalganglia.
Glia2009;57:1588–99.
[296]ZhangH,NeiH,DoughertyPM.
Ap38mitogen-activatedproteinkinase-dependentmechanismofdisinhibitioninspinalsynaptictransmissioninducedbytumornecrosisfactor-alpha.
JNeurosci2010;30:12844–55.
[297]ZhangH,YoonSY,ZhangH,DoughertyPM.
Evidencethatspinalastrocytesbutnotmicrogliacontributetothepathogenesisofpaclitaxel-inducedpainfulneuropathy.
JPain2012;13:293–303.
[298]ZhangJ,DeKoninckY.
Spatialandtemporalrelationshipbetweenmonocytechemoattractantprotein-1expressionandspinalglialactivationfollowingperipheralnerveinjury.
JNeurochem2006;97:772–83.
[299]ZhangJ,HoffertC,VuHK,GroblewskiT,AhmadS,O'DonnellD.
InductionofCB2receptorexpressionintheratspinalcordofneuropathicbutnotinammatorychronicpainmodels.
EurJNeurosci2003;17:2750–4.
[300]ZhangJ,ShiXQ,EcheverryS,MogilJS,DeKoninckY,RivestS.
ExpressionofCCR2inbothresidentandbonemarrow-derivedmicrogliaplaysacriticalroleinneuropathicpain.
JNeurosci2007;27:12396–406.
[301]ZhangL,BertaT,XuZZ,LiuT,ParkJY,JiRR.
TNF-alphacontributestospinalcordsynapticplasticityandinammatorypain:distinctroleofTNFreceptorsubtypes1and2.
PAIN2011;152:419–27.
[302]ZhangRX,LiA,LiuB,WangL,RenK,ZhangH,BermanBM,LaoL.
IL-1raalleviatesinammatoryhyperalgesiathroughpreventingphosphorylationofNMDAreceptorNR-1subunitinrats.
PAIN2008;135:232–9.
[303]ZhangRX,LiuB,WangL,RenK,QiaoJT,BermanBM,LaoL.
Spinalglialactivationinanewratmodelofbonecancerpainproducedbyprostatecancercellinoculationofthetibia.
PAIN2005;118:125–36.
[304]ZhangX,ChenY,WangC,HuangLY.
NeuronalsomaticATPreleasetriggersneuron-satelliteglialcellcommunicationindorsalrootganglia.
ProcNatlAcadSciUSA2007;104:9864–9.
[305]ZhangZJ,DongYL,LuY,CaoS,ZhaoZQ,GaoYJ.
ChemokineCCL2anditsreceptorCCR2inthemedullarydorsalhornareinvolvedintrigeminalneuropathicpain.
JNeuroinamm2012;9:136.
[306]ZhaoP,WaxmanSG,HainsBC.
Extracellularsignal-regulatedkinase-regulatedmicroglia-neuronsignalingbyprostaglandinE2contributestopainafterspinalcordinjury.
JNeurosci2007;27:2357–68.
[307]ZhengFY,XiaoWH,BennettGJ.
Theresponseofspinalmicrogliatochemotherapy-evokedpainfulperipheralneuropathiesisdistinctfromthatevokedbytraumaticnerveinjuries.
Neuroscience2011;176:447–54.
[308]ZhengW,OuyangH,ZhengX,LiuS,MataM,FinkDJ,HaoS.
GlialTNFalphainthespinalcordregulatesneuropathicpaininducedbyHIVgp120applicationinrats.
MolPain2011;7:40.
[309]ZhongY,ZhouLJ,RenWJ,XinWJ,LiYY,ZhangT,LiuXG.
ThedirectionofsynapticplasticitymediatedbyC-bersinspinaldorsalhornisdecidedbySrc-familykinasesinmicroglia:theroleoftumornecrosisfactor-alpha.
BrainBehavImmun2010;24:874–80.
[310]ZhouD,ChenML,ZhangYQ,ZhaoZQ.
InvolvementofspinalmicroglialP2X7receptoringenerationoftolerancetomorphineanalgesiainrats.
JNeurosci2010;30:8042–7.
[311]ZhouZ,PengX,HaoS,FinkDJ,MataM.
HSV-mediatedtransferofinterleukin-10reducesinammatorypainthroughmodulationofmembranetumornecrosisfactoralphainspinalcordmicroglia.
GeneTher2008;15:183–90.
18R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxxPleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022[312]ZhuX,ConklinD,EisenachJC.
Cyclooxygenase-1inthespinalcordplaysanimportantroleinpostoperativepain.
PAIN2003;104:15–23.
[313]ZhuX,EisenachJC.
Cyclooxygenase-1inthespinalcordisalteredafterperipheralnerveinjury.
Anesthesiology2003;99:1175–9.
[314]ZhuangZY,GernerP,WoolfCJ,JiRR.
ERKissequentiallyactivatedinneurons,microglia,andastrocytesbyspinalnerveligationandcontributestomechanicalallodyniainthisneuropathicpainmodel.
PAIN2005;114:149–59.
[315]ZhuangZY,KawasakiY,TanPH,WenYR,HuangJ,JiRR.
RoleoftheCX3CR1/p38MAPKpathwayinspinalmicrogliaforthedevelopmentofneuropathicpainfollowingnerveinjury-inducedcleavageoffractalkine.
BrainBehavImmun2007;21:642–51.
[316]ZhuangZY,WenYR,ZhangDR,BorselloT,BonnyC,StrichartzGR,DecosterdI,JiRR.
Apeptidec-JunN-terminalkinase(JNK)inhibitorblocksmechanicalallodyniaafterspinalnerveligation:respectiverolesofJNKactivationinprimarysensoryneuronsandspinalastrocytesforneuropathicpaindevelopmentandmaintenance.
JNeurosci2006;26:3551–60.
[317]ZhuoM.
Corticalexcitationandchronicpain.
TrendsNeurosci2008;31:199–207.
R.
-R.
Jietal.
/PAINxxx(2013)xxx–xxx19Pleasecitethisarticleinpressas:JiR-Retal.
Gliaandpain:IschronicpainagliopathyPAIN(2013),http://dx.
doi.
org/10.
1016/j.
pain.
2013.
06.
022
- generationxxx相关文档
- 采购编号:[5101152018000306]
- xxx365潮牌neighborhood XX XX是什么意思
- xxx365xxになりたい xxにしたい 的区别
- xxx365三XXX X三XX XX三X XXX三 好XXX X好XX XX好X...... 以此类推,写出“三·好·学·生”的四字成语。
- xxx36516岁女 xx市 和xx市16岁女 16岁男 xx市 和xx市16岁男 有什么区别?
简单测评v5.net的美国cn2云服务器:电信双程cn2+联通AS9929+移动直连
v5.net一直做独立服务器这块儿的,自从推出云服务器(VPS)以来站长一直还没有关注过,在网友的提醒下弄了个6G内存、2核、100G SSD的美国云服务器来写测评,主机测评给大家趟雷,让你知道v5.net的美国云服务器效果怎么样。本次测评数据仅供参考,有兴趣的还是亲自测试吧! 官方网站:https://v5.net/cloud.html 从显示来看CPU是e5-2660(2.2GHz主频),...
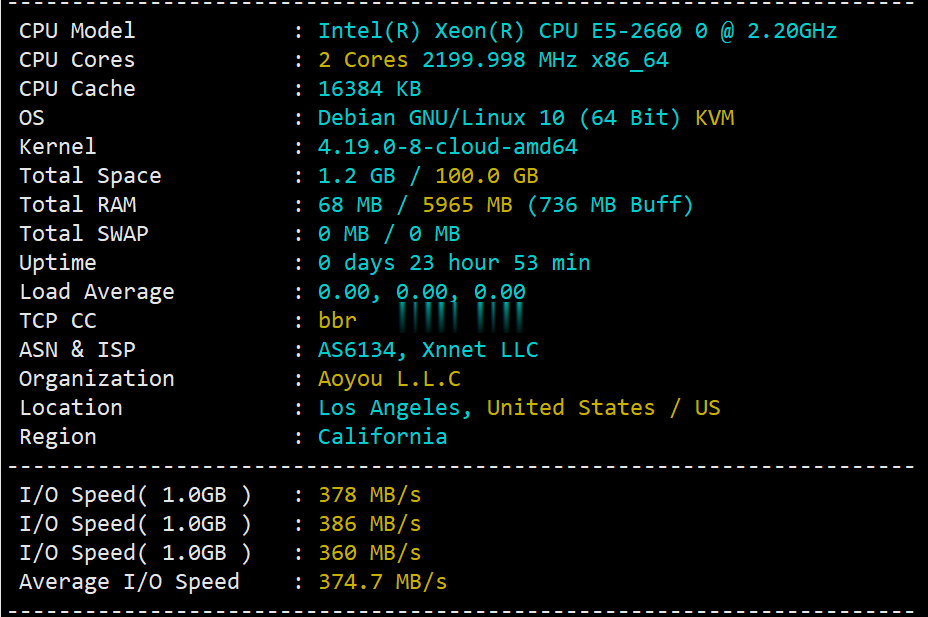
恒创新客(317元)香港云服务器 2M带宽 三网CN2线路直连
恒创科技也有暑期的活动,其中香港服务器也有一定折扣,当然是针对新用户的,如果我们还没有注册过或者可以有办法注册到新用户的,可以买他们家的香港服务器活动价格,2M带宽香港云服务器317元。对于一般用途还是够用的。 活动链接:恒创暑期活动爆款活动均是针对新用户的。1、云服务器仅限首次购买恒创科技产品的新用户。1 核 1G 实例规格,单个账户限购 1台;其他活动机型,单个账户限购 3 台(必须在一个订单...
HostWebis:美国/法国便宜服务器,100Mbps不限流量,高配置大硬盘,$44/月起
hostwebis怎么样?hostwebis昨天在webhosting发布了几款美国高配置大硬盘机器,但报价需要联系客服。看了下该商家的其它产品,发现几款美国服务器、法国服务器还比较实惠,100Mbps不限流量,高配置大硬盘,$44/月起,有兴趣的可以关注一下。HostWebis是一家国外主机品牌,官网宣称1998年就成立了,根据目标市场的不同,以不同品牌名称提供网络托管服务。2003年,通过与W...
xxx365为你推荐
-
工信部备案怎样在工信部进行域名备案?要详细http与https的区别https://和http://区别今日热点怎么删除千牛里面的今日热点怎么取消_?直播加速请问哪种播放器的可以播放加速,并且可以保存vbscript教程vbs 学习方法以及 vbs 实例 有编程基础安装迅雷看看播放器如何用手机安装迅雷看看播放器ios系统ios系统有哪些版本?虚拟专用网虚拟专用网适用于什么行业网站优化方案几种常用的网站优化方法发邮件怎么发如何发邮件?