populationo3o4o5
o3o4o5 com 时间:2021-03-02 阅读:()
AssociationforBiologyLaboratoryEducation(ABLE)~http://www.
zoo.
utoronto.
ca/able69Chapter5PCR-BasedDetectionofGeneticallyModifiedFoodsDianaL.
BrandnerMadisonAreaTechnicalCollege3550AndersonSt.
Madison,WI53704dbrandner@madison.
tec.
wi.
usDianaL.
BrandnerhasbeenanInstructionalAssistantintheBiotechnologyLaboratoryTechnicianProgramatMadisonAreaTechnicalCollege,since1990.
ShereceivedherB.
S.
inBiology,ChemistryandSecondaryScienceEducationfromtheUniversityofWisconsin-StevensPoint.
2002MadisonAreaTechnicalCollegeReprintedFrom:Brandner,D.
L.
2002.
PCR-Baseddetectionofgeneticallymodifiedfoods.
Pages69-84,inTestedstudiesforlaboratoryteaching,Volume23(M.
A.
O'Donnell,Editor).
Proceedingsofthe23rdWorkshop/ConferenceoftheAssociationforBiologyLaboratoryEducation(ABLE),392pages.
-Copyrightpolicy:http://www.
zoo.
utoronto.
ca/able/volumes/copyright.
htmAlthoughthelaboratoryexercisesinABLEproceedingsvolumeshavebeentestedanddueconsiderationhasbeengiventosafety,individualsperformingtheseexercisesmustassumeallresponsibilityforrisk.
TheAssociationforBiologyLaboratoryEducation(ABLE)disclaimsanyliabilitywithregardstosafetyinconnectionwiththeuseoftheexercisesinitsproceedingsvolumes.
70GeneticallyModifiedFoodsContentsIntroduction.
70Synopsis.
70Objectives70Level71LengthoftheLab.
71PreparationTimeRequired.
71ConceptInformation.
71Materials75NotesforInstructor.
77DirectionsforSettingUptheLab.
77SafetyProcedures.
78TeachingTips.
78FurtherReading80StudentTeamLogistics.
80PedagogicalInformation.
80StudentOutline80Protocol.
80DataAnalysisandInterpretation82ExpectedResults.
82Acknowledgements.
83LiteratureCited.
83AppendixA:Reagent&EquipmentVendors84IntroductionSynopsisStudentsperformDNAisolationonfoodproducts(cornorsoy/organicandnonorganic)andDNAamplificationbypolymerasechainreaction(PCR)onfoodDNAtodetectthepresenceofgeneticmodification.
Thestudentswillusegeneticallymodifiedreferencestandardsascontrolsandsampleswillbeanalyzedusingagarosegelelectrophoresis.
ObjectivesAttheendofthislab,studentswillbeableto:Discussamethodfordetectinggeneticmodificationinfood.
DescribeamethodforisolatingDNA.
DescribeamethodofamplifyingDNA.
DescribeamethodforseparatingDNAbysizeusingagarosegelelectrophoresis.
LevelHighSchool:AdvancedTwo-yearcollege:Intermediate,AdvancedFour-yearcollege:Intermediate,AdvancedGeneticalyModifiedFoods71LengthofLabAsuggestedtimeallotmentfollows:Day1Introductionandselectionoffoodstobetested.
Day2IsolateDNAfromfood.
(Ifsteps1-5arepreparedaheadoftime)Day3Setupandrunpolymerasechainreaction.
Day4Loadandrunagarosegels,stainandphotographDay5InterpretresultsPreparationTimeRequired10-15hoursdependingifallreagentsareonhand.
ConceptInformationIntroductionGeneticallymodifiedfoodsareofteninthenews.
Whilegeneticmodificationshavemadeimprovementsinmanycropsandhelpedtoincreaseyields,manygroupshaveraisedloudprotestagainst"tinkering"withcropplants.
IntheU.
S.
,however,geneticallymodifiedfoodshavebeenintroducedtothemarketwithlittlefanfare.
ForsomecropsintheUSoverhalfoftheacresplantedaregeneticallymodifiedvarieties(USDA/NASS2000).
Muchoftheworld,incontrast,hasexperiencedstrongandincreasingresistancetotheintroductionofanygeneticallymodifiedfoodstothemarketplace.
TheEuropeanUnionandothercountriesrequirecertificationthatfoodsenteringtheircountriesbeGeneticallyModifiedOrganism(GMO)freeorcontainminimallimits.
Inthefallof2000,geneticallymodifiedfoodscaughttheattentionoftheUSpresswhenitwasrevealedbyawatchdoggroupthatTacoBellbrandtacoshellscontainedatypeofgeneticallymodifiedcornthatwasnotapprovedforhumanconsumptionbytheUSDA.
Anationwiderecallofcornproductswasorderedafterindependentverificationoftheearlierresults.
Theparticularcornmodificationinthesefoodswasnotapproved,butthepresslargelyignoredthefactthattheUSDA,FDA,andEPAalreadyapprovemanygeneticallymodifiedfoods.
Withtheavailabilityofsimpletestslikethisonetodetectgeneticmodificationinfoodproducts,publicawarenesswillcontinuetoincrease.
BenefitsofGeneticallyModifiedFoodsPeoplehavebeenmodifyingcropplantssincethedawnofagriculture.
Yearafteryearancientpeopleselectedandsavedseedsfromplantsdisplayingspecifictraits.
Later,withcrossbreedingandthedevelopmentofhybridplants,traditionalplantbreedingemerged.
Todaymoderntechniquesinbiotechnologyallowplantbreederstointroduceveryspecifictraitsviaparticulargenesintoplants.
Insertedgenesmaycomefromthesamespeciesofplant,fromotherplantspecies,orevenfromanimalsorbacteria.
Geneticmodificationofcropscanproducefourgeneralbenefits:1)agricultural--increasedyield,2)environmental--reduceduseofpesticides,herbicides,andfuel,3)nutritional--improvedqualityoffoods,and4)diseaseprevention--foodsthatworklikeediblevaccines.
Agriculturalbenefitsincludenewmethodstoimproveproductivityandprofitabilitywhileatthesametimereducingrelianceonpesticidesandherbicides(Table1).
Monsantohasseveralproductsthatcontainagenemakingplantstolerantoftheherbicideglyphosate(tradenameRoundup).
Glyphosatekillsplantsbyblockingthepathwayforsynthesizingessentialaminoacids(Clark2000).
72GeneticallyModifiedFoodsTable1.
SelectedGeneticallyModifiedCropsCurrentlyAllowedintheUSFoodSupply,March2000.
(Source:USFood&DrugAdministration)ProductCompanyEngineeredTraitName&YearCornNovartisBttoxintocontrolinsectpestsBt111996CornNovartisBttoxintocontrolinsectpestsKnockOut1995CornDow/MycogenBttoxintocontrolinsectpestsNaureGard1995CornMonsanto/DeKalbBttoxintocontrolinsectpestsBt-Xtra1997CanolaMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1999CottonMonsantoBttoxintocontrolinsectpestsBollgard1995CottonMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1996PapayaCornellUniv.
/Univ.
HawaiiResistpapayaringspotvirusSunup,Rainbow1997PotatoMonsantoBttoxintocontrolinsectpestsNewLeaf1995SoybeanMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1995SugarbeetAventisResistglyphosateherbicidetocontrolweedsNameunknown2000TomatoMonsanto/CalgeneAlteredripeningtoenhancefreshmarketvalueFlavrSavr1994BiotechcompanieshavemodifiedcropsbyintroducingagenethatproducesBttoxintocontrolinsectpestsinlieuofpesticideapplication.
ThesourceofthegeneisthebacteriumBacillusthuringiensis(Bt),whichhaslongbeenusedforinsectcontrol.
Organicfarmersusethebacteriumbecauseitkillsplant-eatinginsectsbutnotbeneficialinsectslikebees.
InsectsingesttheBtprotein,whichbindstotheepithelialcellsofthemidgut,killingtheinsect.
BecausetheBtproteinisbrokendowninanacidicgut,vertebratesarenotaffected.
Btdoesnotcontaminategroundwaterandisconsiderednontoxictohumansandlivestock(Ramanujan2000).
Scientistsareworkingtoengineerresistancetocertainplantpathogens.
ResearchersattheUniversityofHawaiiandCornellUniversityhavedevelopedtwonewvarietiesofpapayawhichareresistanttothepapayaringspotvirus(PRSV).
PapayaisHawaii'ssecondlargestfruitcropandanimportantcropinthetropicseverywhere.
Worldwidethediseaseisaseriousthreatbecauseitisrapidlytransmittedandcanquicklydestroyentireplantations.
Themodificationusedinpapayaiscalled'pathogenderivedresistance',whereagenefromthepathogenisinsertedtofightthepathogenitself(Tennant1994).
Geneticmodificationcanimprovefoodquality.
Consumersbenefitbyhavingfoodsavailabletothemwithincreasedvitamin,mineral,andnutritionalcontent.
Goldenriceisanimportantexample.
Theyellowcoloredgrainsareproducedbyricegeneticallyalteredtomakebeta-carotene,apigmentthebodyconvertstoVitaminA.
VitaminAdeficiencyleadstoblindnessandimmunesystemimpairment,especiallyinchildren.
ThisvitamindeficiencycontributestothedeathofmorethanamillionchildreneachyearinAsia,Africa,andLatinAmerica(Rusting2000).
Foodsthatcanbeengineeredtocombathumandiseaseofferenormousadvancesinpublichealth.
Oneday,childrenmaygetimmunizedbyeatingfoodssuchasbananas,potatoesandtomatoes.
Themodifiedplantscouldbegrownlocallyatlowcosteliminatingproblemsofvaccinetransportandrefrigeration.
Ediblevaccineswouldnotrequiresyringesorotherequipment,whichoftencontributetoinfectionanddiseasespreaduponreuse(Landridge2000).
ResearchersatLomaLindaUniversitySchoolofMedicinehavealreadysucceededininsertingagenefromthecholeraGeneticalyModifiedFoods73bacteriumintopotatoes.
Themodifiedpotatoesproduceanontoxiccomponentofthecholeratoxinthattriggerstheproductionofantibodiesagainstcholerawheneaten(Arakawa1998).
Finally,manymodificationscontributetoincreasingtheamountoffoodproducedworldwide.
Astheworldpopulationcontinuestoincrease,itisundeniablethattheproblemoffeedingeveryonewillrequiregreaterfoodproduction.
Increasedcropyieldsthroughgeneticmodificationcanworktowardthatend.
RisksofGeneticallyModifiedFoodsSomepeoplearguethatalongwiththebenefitsofgeneticallymodifyingfoodcomerisks.
Suchrisksmayinclude:exposuretopossibleallergensandtoxins,harmtotheenvironment,antibioticresistance,andthespreadofintroducedgenestonon-targetplantsbyoutcrossingandpollendrift(Obrycki2001).
InNovember2000theFoodandDrugAdministrationrecalled300supermarketandrestaurantproductsmadewithStarLinkcorn.
StarLink,producedbyAventis,ResearchTrianglePark,N.
C.
,containsthegeneCry9C,whichprotectstheplantsagainstinsectpests.
TheEPAhadapprovedStarLinkcornin1998withthestipulationthatitwasnotforhumanconsumption.
StudieshadshownthattheCry9Cproteinproducedinthemodifiedcornwasheatstableandresistanttostomachacidsandenzymes,allcharacteristicsofhumanallergens,hencetherestrictiononhumanuse.
AventisfailedtokeepStarLinkcornseparatefromapprovedandnongeneticallymodifiedcornsotheunapprovedcornenteredthemarketinitiatingthemassiverecall(USDA2000).
AdifferentproblemarosefortheTerraPrimaorganiccornchipcompanyinHudson,Wisconsin,in1995.
Despitestrictpracticesbyitsorganiccorngrowers,itwasdiscoveredthatsomeofTerraPrima'sApacheTortillachipsshowedtracesofBtcorn.
GenetictestingrevealedthatpollenfromacropofNovartisBtcornplantedmorethanaquarter-mileawayhadcontaminatedanorganiccornfieldofoneofTerraPrima'ssuppliers.
Becauseofthecontaminationbypollendrift,TerraPrimarecalledanddestroyed90,000bagsofchips,asignificantmonetarylosstothesmallcompany(Ramanujan2000).
MethodsforGeneticallyModifyingFoodsInordertogeneticallymodifyfoodcrops,oneneedsareliablemeansofintroducingnewgeneticmaterialintothehost.
TherearethreemainmethodsforintroducingforeignDNA:biologicalvectors(Ti-plasmidfromAgrobacterium),physicalmethods(particlegunandelectroporation),andchemicalmethods(polyethyleneglycolandcalciumchloride)(Hemmer1997).
Ofthethreemethods,thebiologicalvectorsystemisusedmostoften.
Thisisabinaryvectorsystem.
OnevectorcontainstheDNAtobetransferred(thetransgene)andisintroducedalongwiththesecondvector,theTiplasmidofAgrobacteriumtumefaciens,whichcontainsgenesencodingthenecessarymechanismforthegenetictransfertotakeplace(McBride1990).
74GeneticallyModifiedFoodsInorderforthetransgenetoworkeffectivelyinitsnewhostitneedstobecontrolledbyapromotersequenceandaterminatorsequence.
Thisgroupingiscalledagenecassette(Figure1).
Manypotentialpromoterelementshavebeenidentified,butthemostcommonlyusedistheCaMV35Spromoterderivedfromthephytopathogeniccauliflowermosaicvirus(Spoth2000).
TheNOSterminatorfromtheTiplasmidinAgrobacteriumtumefaciensisthemostcommonterminator.
PromoterTransgeneTerminatorGeneCassetteFigure1.
Agenecassette.
RegulationofGeneticallyModifiedFoodsTheU.
S.
governmentworkstoensurenewagriculturalbiotechnologyproductsaresafeforanimalandhumanhealthandsafefortheenvironment(USDA2000).
Threeagenciesareinvolved:theFoodandDrugAdministration(FDA),EnvironmentalProtectionAgency(EPA),andUnitedStatesDepartmentofAgriculture(USDA)/AnimalandPlantHealthInspectionService(APHIS).
UnderFDAregulations,companiesarelegallyobligedtoensurethatanyfoodproductmeetsthesafetystandardsofthelaw.
Thisappliesequallytobothconventionalandgeneticallymodifiedfood.
TheFDAwillpullproductsoffthemarketiftheydonotmeetsafetystandards(USDA2000).
TheEPAapprovesnewherbicidesandpesticides(USDA2000).
Companieswishingtofieldtestormovebiotechnology-derivedplantsmustobtainAPHISapprovalunderUSDAregulations.
AnAPHIS"determinationofnon-regulatedstatus"mustbeobtainedbycompaniesbeforeacropcanbeproducedonawidescaleandsoldcommercially(USDA2000).
Over5000fieldtrialshavebeenconductedsince1987withAPHISapproval.
About40GMOproductshavemetallfederalregulatoryrequirementsandaresoldcommercially(USDA2000).
InothercountriesregulationsforGMOfoodsvary.
Somecountrieshaveadoptedlabelingregulations.
Suchregulationsnecessitateappropriatetechniquestoidentifythepresenceorabsenceofgeneticmodificationsofoodcanbelabeledproperly(Zimmermann1998).
Manycountriesandcommunitiesaredevelopingandstandardizingmethodsforthedetectionofgeneticallymodifiedfoods.
IntheU.
S.
theUSDAGrainInspection,PackersandStockyardsAdministration(GIPSA)isestablishingabiotechreferencelaboratoryinKansasCity,MO.
Thereferencelabwillevaluateandverifyanalyticaltechniquesforthedetectionofgeneticallyenhancedgrainsandgrainproducts.
Thelabwillalsoaccreditindependentanalyticallaboratoriesthattestforthepresenceofgeneticmodification.
Thisnewreferencelabwillalleviatetestingproblemsthatoccurbecausecurrentlythereisnostandardizationofreferencematerials,samplingmethods,orextractionproceduresfordetectiontests(USDA/ERS2000).
DetectionofGeneticallyModifiedFoodsSeveralcompaniesintheUnitedStatesandEuropetestfoodsforgeneticmodification.
Methodsvaryforthedifferentcompaniesandcountries,buttherearetwobasicmeansfordetectinggeneticmodification.
Onemethodtestsfoodfortheproductofthetransgene,usuallyaprotein.
TheothermethodtestsforthepresenceofDNAfromthetransgeneoranotherportionofthegenecassette.
ProteinsareassayedusinganELISA(EnzymeLinkedImmunoSorbentAssay).
Inthetestanenzymeislinkedtoanantibodyboundtotheprotein,whichthenreactswithacoloredsubstrateGeneticalyModifiedFoods75enablingdetectionofaspecificprotein.
ELISAsusuallycostlessthanDNAtests,offerquickerresults,andcansometimesbedoneonsite.
ThebigdrawbackisthatELISAsdonotworkwellonprocessedfoodsbecauseheatduringprocessingcandestroytheprotein.
Incontrast,DNAtestsaremoreexpensive,cannotbedoneonsite,andtakeseveralhourstocomplete.
Importantly,however,DNAtestsareveryaccurate,workonprocessedfoods,andcanbequantified.
AboutThisLabThislabhasthreeparts:1)isolationofDNAfromfood,2)PCR,and3)visualizationoftheresultsusingagarosegelelectrophoresis.
Thelabusesapolymerasechainreaction(PCR)testtodetectforeignDNAingeneticallymodifiedfood,anditusesprimersthataredesignedtoamplifyDNAfromtheCaMV35Spromotorcomponentofthegenecassette.
AmplificationofthispromoteryieldsDNAfragments195basepairs(bp)inlength.
SincealmostalltransgeniccropscontaintheCaMV35SpromoteritoffersagoodtargetforDNAtesting.
Apositivetestforthispromoterisnotalwaysconclusive,however.
PlantsinthecabbagefamilyshouldbetreatedcarefullybecausetheseplantsmaybenaturallyinfectedwiththeCaulimovirus,thesourceoftheCaMV35Spromoter.
InsuchcasesfurtherPCRtestsshouldberunwithprimersdesignedtoamplifythespecifictransgeneDNA.
ForplantsfromotherfamiliestheriskofinfectionbytheCaulimovirusisverysmall(DGJRC1998).
Thefollowinglabactivityhasbeenmodifiedforuseinthepre-collegeorcollegeclassroom.
ThemethodofDNAisolationandPCRarebasedoncurrentlyvalidatedtestsusedintheEuropeanUnion(DGJRC1998).
ThislabisdesignedtobeusedwithcornproductsandcertifiedreferencestandardmaizepowderfromtheInstituteforReferenceMaterialsandMeasurements.
Effortshavebeenmadetokeepcoststoaminimum,however,PCRlabactivitiesaregenerallynotconsideredtobeshoestring.
ThisactivityassumestheinstructorhaspreviousexperienceandknowledgeofPCRandthenecessarypreparationinvolved.
Instructorsshouldallowseverallabperiodstocompletethislabactivity.
MaterialsEquipmentMicropipettors(100-1000L,10-100L,and0.
5-10L)AnalyticalbalanceAutoclavepHmeterSpatulasThermometer55-60Cincubator70CwaterbathordribathTimersVortexerMicrocentrifuge14,000xgMinifugeMicrocentrifugetuberacksVac-ManLaboratoryVacuumManifold(PromegaA7231)VacuumlineorvacuumpumpLabmarkersThermalcyclerHorizontalgelelectrophoresisboxesGelelectrophoresispowersuppliesUVtransilluminatorStainingtraysCameraAssortedbeakers,graduatedcylindersandstoragebottlesStirbarsMagneticstirplateHotplateormicrowaveovenHeat-proofglovesRefrigeratorFreezerGogglesorsafetyglassesAssociationforBiologyLaboratoryEducation(ABLE)~http://www.
zoo.
utoronto.
ca/able76MaterialsandConsumableLabSuppliesInstituteforReferenceMaterialsandMeasurements(IRMM)-certifiedreferencestandardsFlukaBiochemika63194MaizePowderMZ-SetavailablefromSigmaDriedfoodproductscontainingcornmealorsoyflowerexamplesinclude:cornmeal,cornflour,cornchips,soyflour,organiccornmeal,ororganicsoyflour.
Microcentrifugetubes1.
5-mL(sterile)Sterilesticks(Fisher01-340)Nucleasefreewater(PromegaP1193)WeighboatsAerosolresistantpipettips(100-1000L,10-100L,and0.
5-10L)Tris-HCl(SigmaT5941)NaCl(SigmaS9888)EDTA(disodiumsalt)(SigmaE1644)SDS(Sigmal4509)Guanidine-HCl(SigmaG9284)ProteinaseK(PromegaV3021)Labelingtape3-mlsyringes(sterile)Wizardminicolumns(PromegaA7211)Wizardresin(PromegaA7181)Isopropanol(SigmaI9516)PCRprimersCaMV35SPromoterAmershamPharmaciaReady-To-GoPCRbeads(27-9555-01)Sterilemineraloil(SigmaM5904)4%precastagarosegels(BioWhittakerMolecularApplications54926)TBEelectrophoresisbuffer(SigmaT4415)PCRMarker,50-2000bp(Novagen69278-3)Blue/OrangeLoadingDye,6X(PromegaG1881)DeionizedordistilledwaterFilmEthidiumbromide10mg/mL(SigmaE1510)GlovesLabcoatsorapronsEquipmentandMaterialsforEachTeamofFourStudentsEquipmentMicropipettors(100-1000L,10-100L,and0.
5-10L)TimerMicrocentrifugetuberackLabmarkerHorizontalgelelectrophoresisboxStainingtrayGogglesorsafetyglassesMaterialsandConsumableLabSuppliesCertifiedreferencesforthepositiveand0%controlFoodproducts(4)Microcentrifugetubes,1.
5-mL(sterile)severalSterilesticks1/foodsample4mLnucleasefreewaterWeighboatsAerosolresistantpipettips(100-1000L,10-100L,and0.
5-10L)6mLextractionbuffer700LGuanidine-HCl300LProteinaseKLabelingtape3-mLsyringes(6)Wizardminicolumns(6)6mLWizardresin12mL80%Isopropanol7LofeachPCRprimerAmershamPharmaciaReady-To-GoPCRbeads(7)TBEelectrophoresisbufferGeneticalyModifiedFoods77Agarose6LPCRMarker,50-2000bp40LBlue/OrangeLoadingDye,6XGloves,severalpairperstudentLabcoatorapron,1perstudentNotesforInstructorDirectionsforSettingUptheLabSolutionPreparationExtractionbuffer10mMTris-HClatpH7.
5150mMNaCl2mMEDTA1%SDSStoreatroomtemperatureforamaximumofthreemonths.
5MGuanidine-HClAdd80mLofdistilledwaterto47.
8gofguanidinehydrochlorideinaflask,andstiruntilcompletelydissolved.
BTVto100mLwithdistilledwaterandautoclave.
Storeatroomtemperatureforamaximumofthreemonths.
20mg/mLProteinaseKAdd5mLnucleasewaterto100mgProteinaseKinasteriletubeorflask.
Aliquotandstoreat-20Cforamaximumofsixmonths.
80%Isopropanol80mLof100%isopropanol20mLsteriledistilledwaterMakes100mLOligonucleotidePrimerSequencesPrimersareavailablefromseveralcompaniesandpricesvary.
Diluteprimerstoafinalconcentrationof25pmol/Linnucleasefreewater.
Storeinfreezerforoneyear.
35SPromotorSense:5'GCTCCTACAAATGCCATCA3'Antisense:5'GATAGTGGGATTGTGCGTCA3'TBEelectrophoresisbufferFollowmanufacturerdirectionsfor1Xsolution.
PCRMarkerUse6Lpergellane.
Storeinrefrigerator.
EthidiumBromidestain1g/mL78GeneticallyModifiedFoodsAdd50Lof10mg/mLethidiumbromideto500mLofdeionizedordistilledwater.
Storeinunbreakableopaquebottlesatroomtemperature.
LabelbottleCaution:EthidiumBromideSafetyProceduresAlllaboratoryproceduresshouldbeconductedwithgloves,goggles,andaprons.
Washhandsbeforeandattheconclusionofthelab.
Ifthepowerison,electricalshockmayresultfromtouchingthebufferorelectrophoresisequipment.
Neverleavetheelectrophoresispowerunitonwithoutsupervision.
Thereisariskoffireifthebufferleaksoutorifthebuffershouldevaporatecompletelyduringelectrophoresis.
Neverleavestirplatesorhotplatesonwithoutsupervision.
Besurethatstudentsarefamiliarwiththeoperatinginstructionsandsafetyprecautionsbeforetheyuseanycentrifuge.
Usecautionwithhotliquidsandglassware.
Wearheat-proofglovestomovehotglasswareandmetals.
Ethidiumbromideisamutagenandcancer-suspectagent.
Weargloveswhenhandlingthestainandstainedgels.
Designateanethidiumstainworkarea.
Disposeofstainproperly(seeMicklosandFreyer1990,pp.
256-257).
CheckMSDS(MaterialSafetyDataSheets)forallchemicalsandreagentsinthelabbeforepreparingandrunningthelab.
NeverlookatanunshieldedUVlightsource.
WearafullfaceUVshieldandcoverallexposedskinifyourUVlightsourceisunshielded.
TeachingTipsForhelpdetermininghowtoreconstituteyouroligoneucleotideprimersequences.
Seethefollowingwebsites:www.
lifetech.
comTechOnLine,FAQs–CalculationsforCustomOligosorwww.
genosys.
comCustomOligos,FAQ–YouAndYourOligosIfyoudon'tusetheAmershamPharmaciaReady-To-GoPCRbeadspreparethefollowingPCRmastermix.
Notethattheprimerconcentrationsaredifferent.
SinglePCR(L)10Reactions(L)DNAtemplate5--Primer1(50pmol/L)110Primer2(50pmol/L)11010XReactionBuffer10100MgCl2(25mMSolution)660PCRNucleotideMix(10mM)220TaqDNAPolymerase(5u/L)0.
55NucleaseFreeWater74.
5745TotalVolume100950GeneticalyModifiedFoods79ThermalcyclerProfileforPCRUsingCaMV35SDenaturation3min/94C,amplification20sec/94C,40sec/54C,60sec/72C,for40cyclesandafinalextensionof3min/72C.
Fortycyclestakesapproximately2.
5hrtocompletewiththePerkinElmerThermalcycler480.
Crushfoodproductslikecornchipsbeforethelabsotheyareeasiertoweigh.
Ifyoudon'thaveaccesstoavacuumsource.
ThereisanalternativetotheVac-ManLaboratoryVacuumManifold.
Use3-mLsyringesandplungersinstead.
Ifyoucan'tuseethidiumbromidestain,alternativesincludeCarolinaBLUDNAStain,Ward'sDNAStainandEdvotekDNAInstaStain.
Whenusingastainwithlesssensitivitythanethidiumbromide,loadmoreoftheDNAsampleintothegelwells.
ForinformationonthedisposalofethidiumbromidestainconsultMicklos(1990)orHorn(1993).
Ondaytwotheinstructormayneedtopreparethefoodsamplesaheadoftimeforsteps1-5oftheDNAIsolationportion,ifclasstimeislimited.
Useaerosolresistantpipettipsforallpipettingtopreventcrosscontaminationbetweensamples.
HighlyprocessedfoodscontainlessandpoorerqualityDNAthanlessprocessedfoods.
TheInstituteforReferenceMaterialsandMeasurements(IRMM)-certifiedreferencestandardsMaizePowderMZ-Setcontainsatleast1gofeachfourcontrolstandards:0%,0.
1%,0.
5%and2%GMOdrypowder.
Blue/OrangeLoadingDye,6XisaconvenientmarkerdyecontainingorangeG,bromophenolblueandxylenecyanol.
Thedyeisprovidedinapremixedreadytouseform.
Storeat–20C.
Afewminutesafterthepowersupplyisturnedonandcurrentisappliedtothegel,theloadingdyeshouldbeseenmovingtowardthepositiveelectrodeendofthegelbox.
TheBlue/Orangeloadingdyewilleventuallyseparateintothreebandcolors.
TheyellowishorangebandisorangeG.
Thepurplishbandisbromophenolblueandtheaquacoloredbandisxylenecyanol.
BromophenolbluedyemigratesatapproximatelythesamerateasaDNAfragmentof300bpandxylenecyanolmigratesatapproximatelythesamerateasaDNAfragmentof9000bp.
Whenaliquotingsmallamountsofliquidforstudents,eachaliquotshouldcontainslightlymorethanwillberequiredforthelab.
Togetthecontentsofthetubeinthebottomofthetube,tapthetubeonthecountertoporcentrifugeforacoupleseconds.
Theinstructormaywanttoconsiderdispensingtheprimersintoeachstudent'ssampletubeduetotheverysmallquantity.
PreparenegativecontrolsamplesforthePCRportionofthelab.
Anegativecontrolisacheckonthereagentsusedinthislabactivity.
PreparethesampleexactlythesameastheothersbutdonotaddDNA.
Makeupthevolumedifferencewithwater.
IfaPCRproductispresentaftertheamplification,DNAhascontaminatedthereagents.
ThePCRMarkerconsistsofeightfragmentsthatrangeinsizefrom50–2000bp.
Havestudentsmakeachartofallthesamplesbeingtestedandcontrolstorecordtheresultsoftheclass.
80GeneticallyModifiedFoodsFurtherReadingBiotechnologyInstitute.
2000.
GeneticallyModifiedFoodCrops.
YourWorld:Biotechnology&You,10(1).
Mullis,K.
B.
1990.
Theunusualoriginofthepolymerasechainreaction.
ScientificAmerican,262(4):56-65.
[journalarticle]Nottingham,Stephen.
1998EatYourGenes:Howgeneticallymodifiedfoodisenteringourdiet.
NewYork:ZedBooksLtd.
[book]PBS.
2001.
HarvestOfFear:ShouldWeGrowGMCropsANova/FrontlineSpecialReport.
Online:http://www.
pbs.
org/wgbh/harvest/Videocopiesareavailable.
Seidman,L.
&C.
Moore.
1999.
BasicLaboratoryMethodsforBiotechnologyUpperSaddleRiver,NJ.
PrenticeHall,751pages.
[ISBN0-13-795535-9][book]StudentTeamLogisticsOrganizetheclassintoteamsof3-4students.
Haveeachteambringinfourfoodsamplesfortesting.
Haveeachteamprepareone2%positivecontrolsampleandone0%controlsample.
PedagogicalInformationThefollowingisachartofconceptscoveredinthislabandstudentmisconceptionsoftheconcepts:CorrectConceptMisconceptionNobandonthegelmayindicatethat:-nogeneticmodificationhastakenplace.
-thetechniqueusedinthegeneticmodificationwasnotdetectedbythisdetectionmethod.
-theamountofthefoodsamplewasn'tbigenoughtodetectgeneticmodification.
-theprocedurewasn'tworkingproperly.
Nobandonthegelindicatesthatthefoodproducthasnotbeengeneticallymodified.
Thedetectionmethodusedinthislabcanindicatewhethergeneticmodificationutilizedthe35Spromoter.
Thedetectionmethodusedinthislabcanindicatewhethergeneticmodificationofanykindhastakenplaceandwhatgenehasbeenused.
StudentOutlineProtocolDNAIsolation1.
Wearingglovesselectyourfoodsampleorcontrolsampleandweighout0.
1goffoodandplaceitintoadisposablemicrocentrifugetube.
2Add200Lofnucleasefreewatertothetube.
3.
Useasterilesticktohomogenizethefoodsampletoasmoothslurry.
4.
Add860Lofextractionbuffer,100Lof5MGuanidine-HCland40Lof20mg/mLProteinaseKtothetubecontainingthehomogenate.
Vortextube.
5.
Incubateat55-60Cfor3hourswithintermittentmixing.
6.
Allowsamplestocoolatroomtemperaturefor10minutes.
7.
Centrifuge10minutesat14,000xginamicrocentrifuge.
GeneticalyModifiedFoods818.
Foreachsample,attachonelabeled3-mLsyringebarreltotheLuer-LokextensionofaWizardminicolumnandattachthisminicolumn/syringebarrelassemblytotheVac-ManLaboratoryVacuumManifold.
9.
Checktoensureallstopcocksareclosedbeforeproceeding.
10.
Add1mLofWizardresintoeachminicolumn/syringeassembly.
11.
Carefullyremove300Loftheclearedsupernatantfromeachsampleandtransferittothebarreloftheminicolumn/syringeassemblycontainingtheWizardresin.
12.
Openthestopcocksandapplyavacuumtopulltheresin/supernatantmixintotheminicolumn.
Whentheentiresamplehaspassedthroughthecolumn,closethestopcockandturnoffthevacuum.
InthissteptheDNAwillsticktothecolumn.
13.
Add2mLof80%isopropanoltoeachminicolumnandreapplythevacuumtodrawthesolutionthroughtheminicolumn.
Thisstepwashesthecolumn.
14.
Removethesyringebarrelandtransfertheminicolumntoa1.
5mLmicrocentrifugetube.
Centrifugetheminicolumnat10,000xginamicrocentrifugefor2minutestoremoveanyresidualalcohol.
15.
Transfertheminicolumntoanewmicrocentrifugetube,add50Lof70Cnucleasefreewatertothecolumnandallowittointeractwiththeresinfor1minute.
ThisstepelutestheDNAfromthecolumn.
16.
ElutetheDNAbycentrifugationat10,000xgfor1minuteinamicrocentrifuge.
17.
YoumaystophereandstoretheDNAintherefrigeratorforaboutaweek.
Forlongerperiods,storeitinthefreezer.
PCRDNAAmplification1.
WearingglovesobtainoneReady-To-GoPCRbeadina0.
5mLtubeforeachsampletobetested.
Checkthatthebeadineachtubeisvisibleatthebottomofthetube.
Labelthetubeandplaceitinarack.
SeeSettingUpReactions(Table2).
Table2.
SettingUpReactionsForeachgroup:Tube#Tubew/beadDNAPrimermixH2OMineralOilGelLane#Sample#195L2L18L1dropSample#295L2L18L1dropSample#395L2L18L1dropSample#495L2L18L1drop0%Standard95L2L18L1drop2%Standard95L2L18L1dropNeg.
Control=NODNA90L2L23L1drop2.
Add5LoftemplateDNAtothetube.
Changepipettipsbetweensamples.
3.
Add2Lofprimermixtothetube.
4.
Add18Lofnucleasefreewater.
Capthetubeandgentlyvortex,thencentrifugebrieflytocollectthecontentsatthebottomofthetube.
Thetotalvolumeinthetubeshouldbe25L.
5.
Overlaythereactionwith1dropofmineraloil,ifitisrequiredforyourthermalcycler.
6.
Placeyourtubeinthethermalcyclerandstartthereaction.
Whileyouarewaitingfortheamplificationreactionyoumaybegintoprepareyouragarosegelforanalysis.
Whenthe82GeneticallyModifiedFoodsreactioniscomplete.
Youmaystopandfreezethereactiontubesorcontinuewithgelelectrophoresis.
AgaroseGelElectrophoresisAnalysis1.
Wearingglovesobtainone4%agaroseTBEgelwitheightwells,agelboxcontainingTBE1Xbuffer,andapowersupply.
2.
Add5LloadingdyetothetubeswithyourPCRsamples,standardsandnegativecontrol.
3.
Load15Lofeachsample,standardandnegativecontrolintoseparatewellsofyourgel,avoidmixingmineraloilwiththesample.
SavealaneforthePCRmarkeroneachgel.
Recordwhereeachsampleislocatedonthegel.
4.
Add1LloadingdyetoyourPCRmarker.
Loadthemarkerintoonewellofthegel.
5.
Attachthegelboxtothepowersupply,turnthepoweron,andsetto100-150volts.
Electrophoresefor40-60minutesoruntilthebromophenolbluebandhastraveledone-thirdthelengthofthegel.
Voltsettingsandtimewillvarywithdifferentequipment.
6.
Wearinggloves,carefullyremovethegelandputitintoastainingtray.
Coverthegelwithethidiumbromidestainandstainfor5-10minutes.
7.
Afterstaining,decanttheethidiumbromidestainfromthestainingtraybackintothestoragebottle.
8.
Rinsethegelwithtapwater,inthetray,forseveralminutestoremovebackgroundethidiumbromidestainfromthegel.
9.
Viewonultraviolettransilluminatorandphotograph.
Recordyourresultsandsharewithallothergroups.
DataAnalysisandInterpretationShareacopyofyourresultswithalltheothergroups.
Areanybandsinthe180-195bprangeHowmanylanesshowPCRbandsDoesthe0%controlstandardshowaPCRbandShoulditWhyDoesyournegativecontrolshowaPCRbandShoulditWhyDoesyourpositivecontrolstandardshowaPCRbandShoulditWhyCanyouseethePCRMarkerladderHowmanybandsshouldtherebeAreallthebandsvisibleDoyourorganicfoodsamplesshowaPCRbandIftheydowhatdoesthismeanExpectedResultsNoDNAfragmentsshouldappearinthenegativecontrollaneexceptbandsatorbelow50bpinlengthareartifactsoftheprimers.
Bandsof195bpindicatepresenceoftheCaMV35Spromoter.
NoDNAfragmentsof195bpshouldappearinthe0%controlstandardlane.
A195bpfragmentwillappearinthe2%controlstandardlane.
Studentsamplesmayormaynotshowabandat195bp.
Theoretically,organicfoodproductsshouldbefreeofgeneticallymodifiedfoods.
However,organicdefinitionsvary.
Ifaproductindicatesthatitis100%organic,thereshouldbenoband.
Non-organiccornproductswillprobablycontaindetectablelevelsofgeneticallymodifiedcorn(Figure2).
GeneticalyModifiedFoods83Figure2.
Expectedelectrophoresisresults.
AcknowledgementsSupportforthedevelopmentofthislabcamefromtheBiotechnologyLaboratoryTechnicianProgramandstaffatMATC.
SpecialthankstoNoreenWarrenandThomasBrandner.
LiteratureCitedArakawa,T.
,D.
K.
X.
Chong,andW.
H.
R.
Landridge.
1998.
Efficacyofafoodplant-basedoralcholeratoxinBsubunitvaccine.
NatureBiotechnology16(3):292.
Clark,D.
andL.
Russell.
2000.
MolecularBiologyMadeSimpleandFun.
Vienna,IL:CacheRiverPress,pages218-220.
DGJRC.
1998.
DirectorateGeneralsEuropeanCommissionsJointResearchCentre,EnvironmentInstitute,ConsumerProtection&FoodUnit.
Screeningmethodfortheidentificationofgeneticallymodifiedorganisms(GMO)infood.
FDA.
2000.
U.
S.
FoodandDrugAdministrationCenterforFoodSafety&AppliedNutrition.
FoodsDerivedfromNewPlantVarietiesDerivedthroughRecombinantDNATechnology.
Online:http://vm.
cfsan.
fda.
gov/~lrd/biocon.
htmlHemmer,W.
1997.
FoodsDerivedfromGeneticallyModifiedOrganismsandDetectionMethods.
BATS.
Online:www.
bats.
ch/abstr/297/intro.
htm84GeneticallyModifiedFoodsHorn,T.
M.
1993.
WorkingwithDNA&BacteriainPrecollegeScienceClassrooms.
Reston,VA:NationalAssociationofBiologyTeachers,pages14-17.
Landridge,W.
H.
R.
2000.
EdibleVaccines.
ScientificAmerican283(3):66-71.
McBride,K.
,andK.
Summerfelt.
1990.
ImprovedbinaryvectorsforAgrobacterium-mediatedplanttransformation.
PlantMolecularBiology14:269-276.
Micklos,D.
,andG.
Freyer.
1990.
DNAScience:AfirstcourseinrecombinantDNAtechnology.
ColdSpringHarborLaboratoryPress/CarolinaBiologicalSupplyCompany,pages256-257.
Obrycki,J.
J.
,J.
E.
Losey,O.
R.
Taylor,andL.
C.
H.
Jesse.
2001.
TransgenicInsecticidalCorn:BeyondInsecticidalToxicitytoEcologicalComplexity.
BioScience51(5):353-361.
Ramanujan,K.
andD.
Blum.
2000.
GeneticallyModifiedFood101.
WisconsinAcademyReview46(4):49-50.
Rusting,R.
2000.
MovingAgainstMalnutrition.
ScientificAmerican283(3):70.
Spoth,B.
,andE.
Strauss.
2000.
ScreeningforGeneticallyModifiedOrganismsinFoodUsingPromega'sWizardResins,QualityMonitoringofFoods,ApplicationNotes071.
PromegaCorporation,Madison,Wisconsin.
Tennant,P.
F.
,C.
Gonsalves,K.
-S.
Ling,M.
Fitch,R.
Manshardt,J.
L.
Slightom,andD.
Gonsalves.
1994.
DifferentialProtectionAgainstPapayaRingspotVirusIsolatesinCoatProteinGeneTransgenicPapayaandClassicallyCross-ProtectedPapaya.
TheAmericanPhytopathologicalSociety84(11):1359-1365.
USDA.
2000.
UnitedStatesDepartmentofAgriculture,AgriculturalBiotechnology.
FrequentlyAskedQuestions.
Online:www.
usda.
gov/agencies/biotech/faq.
htmlUSDA/ERS.
2000.
UnitedStatesDepartmentofAgriculture.
EconomicResearchService,BiotechCornandSoybeans:changingMarketsandtheGovernment'sRole.
Online:http://www.
ers.
gov/whatsnew/issues/biotechmarkets/USDA/NASS.
2000.
UnitedStatesDepartmentofAgriculture,NationalAgriculturalStatisticsService,FarmerReportedGeneticallyModifiedVarieties.
AcreageJune2000,AgriculturalStatisticsBoard,Online:www.
usda.
mannlib.
cornell.
edu/reports/nassr/field/pcp-bba/acrg0600.
pdfZimmermann,A.
,J.
Luthy,andU.
Pauli.
1998.
Quantitativeandqualitativeevaluationofninedifferentextractionmethodsfornucleicacidsonsoyabeanfoodsamples.
ZLebensmUntersForsch,A207:81-90.
AppendixAReagentandEquipmentVendorswww.
sigma-aldrich.
comwww.
apbiotech.
comwww.
promega.
comwww.
fishersci.
comwww.
vwrsp.
comwww.
nabt.
orgwww.
lifetech.
comwww.
genosys.
comwww.
novagen.
comwww.
carolina.
comwww.
edvotek.
comwww.
wardsci.
com
zoo.
utoronto.
ca/able69Chapter5PCR-BasedDetectionofGeneticallyModifiedFoodsDianaL.
BrandnerMadisonAreaTechnicalCollege3550AndersonSt.
Madison,WI53704dbrandner@madison.
tec.
wi.
usDianaL.
BrandnerhasbeenanInstructionalAssistantintheBiotechnologyLaboratoryTechnicianProgramatMadisonAreaTechnicalCollege,since1990.
ShereceivedherB.
S.
inBiology,ChemistryandSecondaryScienceEducationfromtheUniversityofWisconsin-StevensPoint.
2002MadisonAreaTechnicalCollegeReprintedFrom:Brandner,D.
L.
2002.
PCR-Baseddetectionofgeneticallymodifiedfoods.
Pages69-84,inTestedstudiesforlaboratoryteaching,Volume23(M.
A.
O'Donnell,Editor).
Proceedingsofthe23rdWorkshop/ConferenceoftheAssociationforBiologyLaboratoryEducation(ABLE),392pages.
-Copyrightpolicy:http://www.
zoo.
utoronto.
ca/able/volumes/copyright.
htmAlthoughthelaboratoryexercisesinABLEproceedingsvolumeshavebeentestedanddueconsiderationhasbeengiventosafety,individualsperformingtheseexercisesmustassumeallresponsibilityforrisk.
TheAssociationforBiologyLaboratoryEducation(ABLE)disclaimsanyliabilitywithregardstosafetyinconnectionwiththeuseoftheexercisesinitsproceedingsvolumes.
70GeneticallyModifiedFoodsContentsIntroduction.
70Synopsis.
70Objectives70Level71LengthoftheLab.
71PreparationTimeRequired.
71ConceptInformation.
71Materials75NotesforInstructor.
77DirectionsforSettingUptheLab.
77SafetyProcedures.
78TeachingTips.
78FurtherReading80StudentTeamLogistics.
80PedagogicalInformation.
80StudentOutline80Protocol.
80DataAnalysisandInterpretation82ExpectedResults.
82Acknowledgements.
83LiteratureCited.
83AppendixA:Reagent&EquipmentVendors84IntroductionSynopsisStudentsperformDNAisolationonfoodproducts(cornorsoy/organicandnonorganic)andDNAamplificationbypolymerasechainreaction(PCR)onfoodDNAtodetectthepresenceofgeneticmodification.
Thestudentswillusegeneticallymodifiedreferencestandardsascontrolsandsampleswillbeanalyzedusingagarosegelelectrophoresis.
ObjectivesAttheendofthislab,studentswillbeableto:Discussamethodfordetectinggeneticmodificationinfood.
DescribeamethodforisolatingDNA.
DescribeamethodofamplifyingDNA.
DescribeamethodforseparatingDNAbysizeusingagarosegelelectrophoresis.
LevelHighSchool:AdvancedTwo-yearcollege:Intermediate,AdvancedFour-yearcollege:Intermediate,AdvancedGeneticalyModifiedFoods71LengthofLabAsuggestedtimeallotmentfollows:Day1Introductionandselectionoffoodstobetested.
Day2IsolateDNAfromfood.
(Ifsteps1-5arepreparedaheadoftime)Day3Setupandrunpolymerasechainreaction.
Day4Loadandrunagarosegels,stainandphotographDay5InterpretresultsPreparationTimeRequired10-15hoursdependingifallreagentsareonhand.
ConceptInformationIntroductionGeneticallymodifiedfoodsareofteninthenews.
Whilegeneticmodificationshavemadeimprovementsinmanycropsandhelpedtoincreaseyields,manygroupshaveraisedloudprotestagainst"tinkering"withcropplants.
IntheU.
S.
,however,geneticallymodifiedfoodshavebeenintroducedtothemarketwithlittlefanfare.
ForsomecropsintheUSoverhalfoftheacresplantedaregeneticallymodifiedvarieties(USDA/NASS2000).
Muchoftheworld,incontrast,hasexperiencedstrongandincreasingresistancetotheintroductionofanygeneticallymodifiedfoodstothemarketplace.
TheEuropeanUnionandothercountriesrequirecertificationthatfoodsenteringtheircountriesbeGeneticallyModifiedOrganism(GMO)freeorcontainminimallimits.
Inthefallof2000,geneticallymodifiedfoodscaughttheattentionoftheUSpresswhenitwasrevealedbyawatchdoggroupthatTacoBellbrandtacoshellscontainedatypeofgeneticallymodifiedcornthatwasnotapprovedforhumanconsumptionbytheUSDA.
Anationwiderecallofcornproductswasorderedafterindependentverificationoftheearlierresults.
Theparticularcornmodificationinthesefoodswasnotapproved,butthepresslargelyignoredthefactthattheUSDA,FDA,andEPAalreadyapprovemanygeneticallymodifiedfoods.
Withtheavailabilityofsimpletestslikethisonetodetectgeneticmodificationinfoodproducts,publicawarenesswillcontinuetoincrease.
BenefitsofGeneticallyModifiedFoodsPeoplehavebeenmodifyingcropplantssincethedawnofagriculture.
Yearafteryearancientpeopleselectedandsavedseedsfromplantsdisplayingspecifictraits.
Later,withcrossbreedingandthedevelopmentofhybridplants,traditionalplantbreedingemerged.
Todaymoderntechniquesinbiotechnologyallowplantbreederstointroduceveryspecifictraitsviaparticulargenesintoplants.
Insertedgenesmaycomefromthesamespeciesofplant,fromotherplantspecies,orevenfromanimalsorbacteria.
Geneticmodificationofcropscanproducefourgeneralbenefits:1)agricultural--increasedyield,2)environmental--reduceduseofpesticides,herbicides,andfuel,3)nutritional--improvedqualityoffoods,and4)diseaseprevention--foodsthatworklikeediblevaccines.
Agriculturalbenefitsincludenewmethodstoimproveproductivityandprofitabilitywhileatthesametimereducingrelianceonpesticidesandherbicides(Table1).
Monsantohasseveralproductsthatcontainagenemakingplantstolerantoftheherbicideglyphosate(tradenameRoundup).
Glyphosatekillsplantsbyblockingthepathwayforsynthesizingessentialaminoacids(Clark2000).
72GeneticallyModifiedFoodsTable1.
SelectedGeneticallyModifiedCropsCurrentlyAllowedintheUSFoodSupply,March2000.
(Source:USFood&DrugAdministration)ProductCompanyEngineeredTraitName&YearCornNovartisBttoxintocontrolinsectpestsBt111996CornNovartisBttoxintocontrolinsectpestsKnockOut1995CornDow/MycogenBttoxintocontrolinsectpestsNaureGard1995CornMonsanto/DeKalbBttoxintocontrolinsectpestsBt-Xtra1997CanolaMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1999CottonMonsantoBttoxintocontrolinsectpestsBollgard1995CottonMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1996PapayaCornellUniv.
/Univ.
HawaiiResistpapayaringspotvirusSunup,Rainbow1997PotatoMonsantoBttoxintocontrolinsectpestsNewLeaf1995SoybeanMonsantoResistglyphosateherbicidetocontrolweedsRoundupReady1995SugarbeetAventisResistglyphosateherbicidetocontrolweedsNameunknown2000TomatoMonsanto/CalgeneAlteredripeningtoenhancefreshmarketvalueFlavrSavr1994BiotechcompanieshavemodifiedcropsbyintroducingagenethatproducesBttoxintocontrolinsectpestsinlieuofpesticideapplication.
ThesourceofthegeneisthebacteriumBacillusthuringiensis(Bt),whichhaslongbeenusedforinsectcontrol.
Organicfarmersusethebacteriumbecauseitkillsplant-eatinginsectsbutnotbeneficialinsectslikebees.
InsectsingesttheBtprotein,whichbindstotheepithelialcellsofthemidgut,killingtheinsect.
BecausetheBtproteinisbrokendowninanacidicgut,vertebratesarenotaffected.
Btdoesnotcontaminategroundwaterandisconsiderednontoxictohumansandlivestock(Ramanujan2000).
Scientistsareworkingtoengineerresistancetocertainplantpathogens.
ResearchersattheUniversityofHawaiiandCornellUniversityhavedevelopedtwonewvarietiesofpapayawhichareresistanttothepapayaringspotvirus(PRSV).
PapayaisHawaii'ssecondlargestfruitcropandanimportantcropinthetropicseverywhere.
Worldwidethediseaseisaseriousthreatbecauseitisrapidlytransmittedandcanquicklydestroyentireplantations.
Themodificationusedinpapayaiscalled'pathogenderivedresistance',whereagenefromthepathogenisinsertedtofightthepathogenitself(Tennant1994).
Geneticmodificationcanimprovefoodquality.
Consumersbenefitbyhavingfoodsavailabletothemwithincreasedvitamin,mineral,andnutritionalcontent.
Goldenriceisanimportantexample.
Theyellowcoloredgrainsareproducedbyricegeneticallyalteredtomakebeta-carotene,apigmentthebodyconvertstoVitaminA.
VitaminAdeficiencyleadstoblindnessandimmunesystemimpairment,especiallyinchildren.
ThisvitamindeficiencycontributestothedeathofmorethanamillionchildreneachyearinAsia,Africa,andLatinAmerica(Rusting2000).
Foodsthatcanbeengineeredtocombathumandiseaseofferenormousadvancesinpublichealth.
Oneday,childrenmaygetimmunizedbyeatingfoodssuchasbananas,potatoesandtomatoes.
Themodifiedplantscouldbegrownlocallyatlowcosteliminatingproblemsofvaccinetransportandrefrigeration.
Ediblevaccineswouldnotrequiresyringesorotherequipment,whichoftencontributetoinfectionanddiseasespreaduponreuse(Landridge2000).
ResearchersatLomaLindaUniversitySchoolofMedicinehavealreadysucceededininsertingagenefromthecholeraGeneticalyModifiedFoods73bacteriumintopotatoes.
Themodifiedpotatoesproduceanontoxiccomponentofthecholeratoxinthattriggerstheproductionofantibodiesagainstcholerawheneaten(Arakawa1998).
Finally,manymodificationscontributetoincreasingtheamountoffoodproducedworldwide.
Astheworldpopulationcontinuestoincrease,itisundeniablethattheproblemoffeedingeveryonewillrequiregreaterfoodproduction.
Increasedcropyieldsthroughgeneticmodificationcanworktowardthatend.
RisksofGeneticallyModifiedFoodsSomepeoplearguethatalongwiththebenefitsofgeneticallymodifyingfoodcomerisks.
Suchrisksmayinclude:exposuretopossibleallergensandtoxins,harmtotheenvironment,antibioticresistance,andthespreadofintroducedgenestonon-targetplantsbyoutcrossingandpollendrift(Obrycki2001).
InNovember2000theFoodandDrugAdministrationrecalled300supermarketandrestaurantproductsmadewithStarLinkcorn.
StarLink,producedbyAventis,ResearchTrianglePark,N.
C.
,containsthegeneCry9C,whichprotectstheplantsagainstinsectpests.
TheEPAhadapprovedStarLinkcornin1998withthestipulationthatitwasnotforhumanconsumption.
StudieshadshownthattheCry9Cproteinproducedinthemodifiedcornwasheatstableandresistanttostomachacidsandenzymes,allcharacteristicsofhumanallergens,hencetherestrictiononhumanuse.
AventisfailedtokeepStarLinkcornseparatefromapprovedandnongeneticallymodifiedcornsotheunapprovedcornenteredthemarketinitiatingthemassiverecall(USDA2000).
AdifferentproblemarosefortheTerraPrimaorganiccornchipcompanyinHudson,Wisconsin,in1995.
Despitestrictpracticesbyitsorganiccorngrowers,itwasdiscoveredthatsomeofTerraPrima'sApacheTortillachipsshowedtracesofBtcorn.
GenetictestingrevealedthatpollenfromacropofNovartisBtcornplantedmorethanaquarter-mileawayhadcontaminatedanorganiccornfieldofoneofTerraPrima'ssuppliers.
Becauseofthecontaminationbypollendrift,TerraPrimarecalledanddestroyed90,000bagsofchips,asignificantmonetarylosstothesmallcompany(Ramanujan2000).
MethodsforGeneticallyModifyingFoodsInordertogeneticallymodifyfoodcrops,oneneedsareliablemeansofintroducingnewgeneticmaterialintothehost.
TherearethreemainmethodsforintroducingforeignDNA:biologicalvectors(Ti-plasmidfromAgrobacterium),physicalmethods(particlegunandelectroporation),andchemicalmethods(polyethyleneglycolandcalciumchloride)(Hemmer1997).
Ofthethreemethods,thebiologicalvectorsystemisusedmostoften.
Thisisabinaryvectorsystem.
OnevectorcontainstheDNAtobetransferred(thetransgene)andisintroducedalongwiththesecondvector,theTiplasmidofAgrobacteriumtumefaciens,whichcontainsgenesencodingthenecessarymechanismforthegenetictransfertotakeplace(McBride1990).
74GeneticallyModifiedFoodsInorderforthetransgenetoworkeffectivelyinitsnewhostitneedstobecontrolledbyapromotersequenceandaterminatorsequence.
Thisgroupingiscalledagenecassette(Figure1).
Manypotentialpromoterelementshavebeenidentified,butthemostcommonlyusedistheCaMV35Spromoterderivedfromthephytopathogeniccauliflowermosaicvirus(Spoth2000).
TheNOSterminatorfromtheTiplasmidinAgrobacteriumtumefaciensisthemostcommonterminator.
PromoterTransgeneTerminatorGeneCassetteFigure1.
Agenecassette.
RegulationofGeneticallyModifiedFoodsTheU.
S.
governmentworkstoensurenewagriculturalbiotechnologyproductsaresafeforanimalandhumanhealthandsafefortheenvironment(USDA2000).
Threeagenciesareinvolved:theFoodandDrugAdministration(FDA),EnvironmentalProtectionAgency(EPA),andUnitedStatesDepartmentofAgriculture(USDA)/AnimalandPlantHealthInspectionService(APHIS).
UnderFDAregulations,companiesarelegallyobligedtoensurethatanyfoodproductmeetsthesafetystandardsofthelaw.
Thisappliesequallytobothconventionalandgeneticallymodifiedfood.
TheFDAwillpullproductsoffthemarketiftheydonotmeetsafetystandards(USDA2000).
TheEPAapprovesnewherbicidesandpesticides(USDA2000).
Companieswishingtofieldtestormovebiotechnology-derivedplantsmustobtainAPHISapprovalunderUSDAregulations.
AnAPHIS"determinationofnon-regulatedstatus"mustbeobtainedbycompaniesbeforeacropcanbeproducedonawidescaleandsoldcommercially(USDA2000).
Over5000fieldtrialshavebeenconductedsince1987withAPHISapproval.
About40GMOproductshavemetallfederalregulatoryrequirementsandaresoldcommercially(USDA2000).
InothercountriesregulationsforGMOfoodsvary.
Somecountrieshaveadoptedlabelingregulations.
Suchregulationsnecessitateappropriatetechniquestoidentifythepresenceorabsenceofgeneticmodificationsofoodcanbelabeledproperly(Zimmermann1998).
Manycountriesandcommunitiesaredevelopingandstandardizingmethodsforthedetectionofgeneticallymodifiedfoods.
IntheU.
S.
theUSDAGrainInspection,PackersandStockyardsAdministration(GIPSA)isestablishingabiotechreferencelaboratoryinKansasCity,MO.
Thereferencelabwillevaluateandverifyanalyticaltechniquesforthedetectionofgeneticallyenhancedgrainsandgrainproducts.
Thelabwillalsoaccreditindependentanalyticallaboratoriesthattestforthepresenceofgeneticmodification.
Thisnewreferencelabwillalleviatetestingproblemsthatoccurbecausecurrentlythereisnostandardizationofreferencematerials,samplingmethods,orextractionproceduresfordetectiontests(USDA/ERS2000).
DetectionofGeneticallyModifiedFoodsSeveralcompaniesintheUnitedStatesandEuropetestfoodsforgeneticmodification.
Methodsvaryforthedifferentcompaniesandcountries,buttherearetwobasicmeansfordetectinggeneticmodification.
Onemethodtestsfoodfortheproductofthetransgene,usuallyaprotein.
TheothermethodtestsforthepresenceofDNAfromthetransgeneoranotherportionofthegenecassette.
ProteinsareassayedusinganELISA(EnzymeLinkedImmunoSorbentAssay).
Inthetestanenzymeislinkedtoanantibodyboundtotheprotein,whichthenreactswithacoloredsubstrateGeneticalyModifiedFoods75enablingdetectionofaspecificprotein.
ELISAsusuallycostlessthanDNAtests,offerquickerresults,andcansometimesbedoneonsite.
ThebigdrawbackisthatELISAsdonotworkwellonprocessedfoodsbecauseheatduringprocessingcandestroytheprotein.
Incontrast,DNAtestsaremoreexpensive,cannotbedoneonsite,andtakeseveralhourstocomplete.
Importantly,however,DNAtestsareveryaccurate,workonprocessedfoods,andcanbequantified.
AboutThisLabThislabhasthreeparts:1)isolationofDNAfromfood,2)PCR,and3)visualizationoftheresultsusingagarosegelelectrophoresis.
Thelabusesapolymerasechainreaction(PCR)testtodetectforeignDNAingeneticallymodifiedfood,anditusesprimersthataredesignedtoamplifyDNAfromtheCaMV35Spromotorcomponentofthegenecassette.
AmplificationofthispromoteryieldsDNAfragments195basepairs(bp)inlength.
SincealmostalltransgeniccropscontaintheCaMV35SpromoteritoffersagoodtargetforDNAtesting.
Apositivetestforthispromoterisnotalwaysconclusive,however.
PlantsinthecabbagefamilyshouldbetreatedcarefullybecausetheseplantsmaybenaturallyinfectedwiththeCaulimovirus,thesourceoftheCaMV35Spromoter.
InsuchcasesfurtherPCRtestsshouldberunwithprimersdesignedtoamplifythespecifictransgeneDNA.
ForplantsfromotherfamiliestheriskofinfectionbytheCaulimovirusisverysmall(DGJRC1998).
Thefollowinglabactivityhasbeenmodifiedforuseinthepre-collegeorcollegeclassroom.
ThemethodofDNAisolationandPCRarebasedoncurrentlyvalidatedtestsusedintheEuropeanUnion(DGJRC1998).
ThislabisdesignedtobeusedwithcornproductsandcertifiedreferencestandardmaizepowderfromtheInstituteforReferenceMaterialsandMeasurements.
Effortshavebeenmadetokeepcoststoaminimum,however,PCRlabactivitiesaregenerallynotconsideredtobeshoestring.
ThisactivityassumestheinstructorhaspreviousexperienceandknowledgeofPCRandthenecessarypreparationinvolved.
Instructorsshouldallowseverallabperiodstocompletethislabactivity.
MaterialsEquipmentMicropipettors(100-1000L,10-100L,and0.
5-10L)AnalyticalbalanceAutoclavepHmeterSpatulasThermometer55-60Cincubator70CwaterbathordribathTimersVortexerMicrocentrifuge14,000xgMinifugeMicrocentrifugetuberacksVac-ManLaboratoryVacuumManifold(PromegaA7231)VacuumlineorvacuumpumpLabmarkersThermalcyclerHorizontalgelelectrophoresisboxesGelelectrophoresispowersuppliesUVtransilluminatorStainingtraysCameraAssortedbeakers,graduatedcylindersandstoragebottlesStirbarsMagneticstirplateHotplateormicrowaveovenHeat-proofglovesRefrigeratorFreezerGogglesorsafetyglassesAssociationforBiologyLaboratoryEducation(ABLE)~http://www.
zoo.
utoronto.
ca/able76MaterialsandConsumableLabSuppliesInstituteforReferenceMaterialsandMeasurements(IRMM)-certifiedreferencestandardsFlukaBiochemika63194MaizePowderMZ-SetavailablefromSigmaDriedfoodproductscontainingcornmealorsoyflowerexamplesinclude:cornmeal,cornflour,cornchips,soyflour,organiccornmeal,ororganicsoyflour.
Microcentrifugetubes1.
5-mL(sterile)Sterilesticks(Fisher01-340)Nucleasefreewater(PromegaP1193)WeighboatsAerosolresistantpipettips(100-1000L,10-100L,and0.
5-10L)Tris-HCl(SigmaT5941)NaCl(SigmaS9888)EDTA(disodiumsalt)(SigmaE1644)SDS(Sigmal4509)Guanidine-HCl(SigmaG9284)ProteinaseK(PromegaV3021)Labelingtape3-mlsyringes(sterile)Wizardminicolumns(PromegaA7211)Wizardresin(PromegaA7181)Isopropanol(SigmaI9516)PCRprimersCaMV35SPromoterAmershamPharmaciaReady-To-GoPCRbeads(27-9555-01)Sterilemineraloil(SigmaM5904)4%precastagarosegels(BioWhittakerMolecularApplications54926)TBEelectrophoresisbuffer(SigmaT4415)PCRMarker,50-2000bp(Novagen69278-3)Blue/OrangeLoadingDye,6X(PromegaG1881)DeionizedordistilledwaterFilmEthidiumbromide10mg/mL(SigmaE1510)GlovesLabcoatsorapronsEquipmentandMaterialsforEachTeamofFourStudentsEquipmentMicropipettors(100-1000L,10-100L,and0.
5-10L)TimerMicrocentrifugetuberackLabmarkerHorizontalgelelectrophoresisboxStainingtrayGogglesorsafetyglassesMaterialsandConsumableLabSuppliesCertifiedreferencesforthepositiveand0%controlFoodproducts(4)Microcentrifugetubes,1.
5-mL(sterile)severalSterilesticks1/foodsample4mLnucleasefreewaterWeighboatsAerosolresistantpipettips(100-1000L,10-100L,and0.
5-10L)6mLextractionbuffer700LGuanidine-HCl300LProteinaseKLabelingtape3-mLsyringes(6)Wizardminicolumns(6)6mLWizardresin12mL80%Isopropanol7LofeachPCRprimerAmershamPharmaciaReady-To-GoPCRbeads(7)TBEelectrophoresisbufferGeneticalyModifiedFoods77Agarose6LPCRMarker,50-2000bp40LBlue/OrangeLoadingDye,6XGloves,severalpairperstudentLabcoatorapron,1perstudentNotesforInstructorDirectionsforSettingUptheLabSolutionPreparationExtractionbuffer10mMTris-HClatpH7.
5150mMNaCl2mMEDTA1%SDSStoreatroomtemperatureforamaximumofthreemonths.
5MGuanidine-HClAdd80mLofdistilledwaterto47.
8gofguanidinehydrochlorideinaflask,andstiruntilcompletelydissolved.
BTVto100mLwithdistilledwaterandautoclave.
Storeatroomtemperatureforamaximumofthreemonths.
20mg/mLProteinaseKAdd5mLnucleasewaterto100mgProteinaseKinasteriletubeorflask.
Aliquotandstoreat-20Cforamaximumofsixmonths.
80%Isopropanol80mLof100%isopropanol20mLsteriledistilledwaterMakes100mLOligonucleotidePrimerSequencesPrimersareavailablefromseveralcompaniesandpricesvary.
Diluteprimerstoafinalconcentrationof25pmol/Linnucleasefreewater.
Storeinfreezerforoneyear.
35SPromotorSense:5'GCTCCTACAAATGCCATCA3'Antisense:5'GATAGTGGGATTGTGCGTCA3'TBEelectrophoresisbufferFollowmanufacturerdirectionsfor1Xsolution.
PCRMarkerUse6Lpergellane.
Storeinrefrigerator.
EthidiumBromidestain1g/mL78GeneticallyModifiedFoodsAdd50Lof10mg/mLethidiumbromideto500mLofdeionizedordistilledwater.
Storeinunbreakableopaquebottlesatroomtemperature.
LabelbottleCaution:EthidiumBromideSafetyProceduresAlllaboratoryproceduresshouldbeconductedwithgloves,goggles,andaprons.
Washhandsbeforeandattheconclusionofthelab.
Ifthepowerison,electricalshockmayresultfromtouchingthebufferorelectrophoresisequipment.
Neverleavetheelectrophoresispowerunitonwithoutsupervision.
Thereisariskoffireifthebufferleaksoutorifthebuffershouldevaporatecompletelyduringelectrophoresis.
Neverleavestirplatesorhotplatesonwithoutsupervision.
Besurethatstudentsarefamiliarwiththeoperatinginstructionsandsafetyprecautionsbeforetheyuseanycentrifuge.
Usecautionwithhotliquidsandglassware.
Wearheat-proofglovestomovehotglasswareandmetals.
Ethidiumbromideisamutagenandcancer-suspectagent.
Weargloveswhenhandlingthestainandstainedgels.
Designateanethidiumstainworkarea.
Disposeofstainproperly(seeMicklosandFreyer1990,pp.
256-257).
CheckMSDS(MaterialSafetyDataSheets)forallchemicalsandreagentsinthelabbeforepreparingandrunningthelab.
NeverlookatanunshieldedUVlightsource.
WearafullfaceUVshieldandcoverallexposedskinifyourUVlightsourceisunshielded.
TeachingTipsForhelpdetermininghowtoreconstituteyouroligoneucleotideprimersequences.
Seethefollowingwebsites:www.
lifetech.
comTechOnLine,FAQs–CalculationsforCustomOligosorwww.
genosys.
comCustomOligos,FAQ–YouAndYourOligosIfyoudon'tusetheAmershamPharmaciaReady-To-GoPCRbeadspreparethefollowingPCRmastermix.
Notethattheprimerconcentrationsaredifferent.
SinglePCR(L)10Reactions(L)DNAtemplate5--Primer1(50pmol/L)110Primer2(50pmol/L)11010XReactionBuffer10100MgCl2(25mMSolution)660PCRNucleotideMix(10mM)220TaqDNAPolymerase(5u/L)0.
55NucleaseFreeWater74.
5745TotalVolume100950GeneticalyModifiedFoods79ThermalcyclerProfileforPCRUsingCaMV35SDenaturation3min/94C,amplification20sec/94C,40sec/54C,60sec/72C,for40cyclesandafinalextensionof3min/72C.
Fortycyclestakesapproximately2.
5hrtocompletewiththePerkinElmerThermalcycler480.
Crushfoodproductslikecornchipsbeforethelabsotheyareeasiertoweigh.
Ifyoudon'thaveaccesstoavacuumsource.
ThereisanalternativetotheVac-ManLaboratoryVacuumManifold.
Use3-mLsyringesandplungersinstead.
Ifyoucan'tuseethidiumbromidestain,alternativesincludeCarolinaBLUDNAStain,Ward'sDNAStainandEdvotekDNAInstaStain.
Whenusingastainwithlesssensitivitythanethidiumbromide,loadmoreoftheDNAsampleintothegelwells.
ForinformationonthedisposalofethidiumbromidestainconsultMicklos(1990)orHorn(1993).
Ondaytwotheinstructormayneedtopreparethefoodsamplesaheadoftimeforsteps1-5oftheDNAIsolationportion,ifclasstimeislimited.
Useaerosolresistantpipettipsforallpipettingtopreventcrosscontaminationbetweensamples.
HighlyprocessedfoodscontainlessandpoorerqualityDNAthanlessprocessedfoods.
TheInstituteforReferenceMaterialsandMeasurements(IRMM)-certifiedreferencestandardsMaizePowderMZ-Setcontainsatleast1gofeachfourcontrolstandards:0%,0.
1%,0.
5%and2%GMOdrypowder.
Blue/OrangeLoadingDye,6XisaconvenientmarkerdyecontainingorangeG,bromophenolblueandxylenecyanol.
Thedyeisprovidedinapremixedreadytouseform.
Storeat–20C.
Afewminutesafterthepowersupplyisturnedonandcurrentisappliedtothegel,theloadingdyeshouldbeseenmovingtowardthepositiveelectrodeendofthegelbox.
TheBlue/Orangeloadingdyewilleventuallyseparateintothreebandcolors.
TheyellowishorangebandisorangeG.
Thepurplishbandisbromophenolblueandtheaquacoloredbandisxylenecyanol.
BromophenolbluedyemigratesatapproximatelythesamerateasaDNAfragmentof300bpandxylenecyanolmigratesatapproximatelythesamerateasaDNAfragmentof9000bp.
Whenaliquotingsmallamountsofliquidforstudents,eachaliquotshouldcontainslightlymorethanwillberequiredforthelab.
Togetthecontentsofthetubeinthebottomofthetube,tapthetubeonthecountertoporcentrifugeforacoupleseconds.
Theinstructormaywanttoconsiderdispensingtheprimersintoeachstudent'ssampletubeduetotheverysmallquantity.
PreparenegativecontrolsamplesforthePCRportionofthelab.
Anegativecontrolisacheckonthereagentsusedinthislabactivity.
PreparethesampleexactlythesameastheothersbutdonotaddDNA.
Makeupthevolumedifferencewithwater.
IfaPCRproductispresentaftertheamplification,DNAhascontaminatedthereagents.
ThePCRMarkerconsistsofeightfragmentsthatrangeinsizefrom50–2000bp.
Havestudentsmakeachartofallthesamplesbeingtestedandcontrolstorecordtheresultsoftheclass.
80GeneticallyModifiedFoodsFurtherReadingBiotechnologyInstitute.
2000.
GeneticallyModifiedFoodCrops.
YourWorld:Biotechnology&You,10(1).
Mullis,K.
B.
1990.
Theunusualoriginofthepolymerasechainreaction.
ScientificAmerican,262(4):56-65.
[journalarticle]Nottingham,Stephen.
1998EatYourGenes:Howgeneticallymodifiedfoodisenteringourdiet.
NewYork:ZedBooksLtd.
[book]PBS.
2001.
HarvestOfFear:ShouldWeGrowGMCropsANova/FrontlineSpecialReport.
Online:http://www.
pbs.
org/wgbh/harvest/Videocopiesareavailable.
Seidman,L.
&C.
Moore.
1999.
BasicLaboratoryMethodsforBiotechnologyUpperSaddleRiver,NJ.
PrenticeHall,751pages.
[ISBN0-13-795535-9][book]StudentTeamLogisticsOrganizetheclassintoteamsof3-4students.
Haveeachteambringinfourfoodsamplesfortesting.
Haveeachteamprepareone2%positivecontrolsampleandone0%controlsample.
PedagogicalInformationThefollowingisachartofconceptscoveredinthislabandstudentmisconceptionsoftheconcepts:CorrectConceptMisconceptionNobandonthegelmayindicatethat:-nogeneticmodificationhastakenplace.
-thetechniqueusedinthegeneticmodificationwasnotdetectedbythisdetectionmethod.
-theamountofthefoodsamplewasn'tbigenoughtodetectgeneticmodification.
-theprocedurewasn'tworkingproperly.
Nobandonthegelindicatesthatthefoodproducthasnotbeengeneticallymodified.
Thedetectionmethodusedinthislabcanindicatewhethergeneticmodificationutilizedthe35Spromoter.
Thedetectionmethodusedinthislabcanindicatewhethergeneticmodificationofanykindhastakenplaceandwhatgenehasbeenused.
StudentOutlineProtocolDNAIsolation1.
Wearingglovesselectyourfoodsampleorcontrolsampleandweighout0.
1goffoodandplaceitintoadisposablemicrocentrifugetube.
2Add200Lofnucleasefreewatertothetube.
3.
Useasterilesticktohomogenizethefoodsampletoasmoothslurry.
4.
Add860Lofextractionbuffer,100Lof5MGuanidine-HCland40Lof20mg/mLProteinaseKtothetubecontainingthehomogenate.
Vortextube.
5.
Incubateat55-60Cfor3hourswithintermittentmixing.
6.
Allowsamplestocoolatroomtemperaturefor10minutes.
7.
Centrifuge10minutesat14,000xginamicrocentrifuge.
GeneticalyModifiedFoods818.
Foreachsample,attachonelabeled3-mLsyringebarreltotheLuer-LokextensionofaWizardminicolumnandattachthisminicolumn/syringebarrelassemblytotheVac-ManLaboratoryVacuumManifold.
9.
Checktoensureallstopcocksareclosedbeforeproceeding.
10.
Add1mLofWizardresintoeachminicolumn/syringeassembly.
11.
Carefullyremove300Loftheclearedsupernatantfromeachsampleandtransferittothebarreloftheminicolumn/syringeassemblycontainingtheWizardresin.
12.
Openthestopcocksandapplyavacuumtopulltheresin/supernatantmixintotheminicolumn.
Whentheentiresamplehaspassedthroughthecolumn,closethestopcockandturnoffthevacuum.
InthissteptheDNAwillsticktothecolumn.
13.
Add2mLof80%isopropanoltoeachminicolumnandreapplythevacuumtodrawthesolutionthroughtheminicolumn.
Thisstepwashesthecolumn.
14.
Removethesyringebarrelandtransfertheminicolumntoa1.
5mLmicrocentrifugetube.
Centrifugetheminicolumnat10,000xginamicrocentrifugefor2minutestoremoveanyresidualalcohol.
15.
Transfertheminicolumntoanewmicrocentrifugetube,add50Lof70Cnucleasefreewatertothecolumnandallowittointeractwiththeresinfor1minute.
ThisstepelutestheDNAfromthecolumn.
16.
ElutetheDNAbycentrifugationat10,000xgfor1minuteinamicrocentrifuge.
17.
YoumaystophereandstoretheDNAintherefrigeratorforaboutaweek.
Forlongerperiods,storeitinthefreezer.
PCRDNAAmplification1.
WearingglovesobtainoneReady-To-GoPCRbeadina0.
5mLtubeforeachsampletobetested.
Checkthatthebeadineachtubeisvisibleatthebottomofthetube.
Labelthetubeandplaceitinarack.
SeeSettingUpReactions(Table2).
Table2.
SettingUpReactionsForeachgroup:Tube#Tubew/beadDNAPrimermixH2OMineralOilGelLane#Sample#195L2L18L1dropSample#295L2L18L1dropSample#395L2L18L1dropSample#495L2L18L1drop0%Standard95L2L18L1drop2%Standard95L2L18L1dropNeg.
Control=NODNA90L2L23L1drop2.
Add5LoftemplateDNAtothetube.
Changepipettipsbetweensamples.
3.
Add2Lofprimermixtothetube.
4.
Add18Lofnucleasefreewater.
Capthetubeandgentlyvortex,thencentrifugebrieflytocollectthecontentsatthebottomofthetube.
Thetotalvolumeinthetubeshouldbe25L.
5.
Overlaythereactionwith1dropofmineraloil,ifitisrequiredforyourthermalcycler.
6.
Placeyourtubeinthethermalcyclerandstartthereaction.
Whileyouarewaitingfortheamplificationreactionyoumaybegintoprepareyouragarosegelforanalysis.
Whenthe82GeneticallyModifiedFoodsreactioniscomplete.
Youmaystopandfreezethereactiontubesorcontinuewithgelelectrophoresis.
AgaroseGelElectrophoresisAnalysis1.
Wearingglovesobtainone4%agaroseTBEgelwitheightwells,agelboxcontainingTBE1Xbuffer,andapowersupply.
2.
Add5LloadingdyetothetubeswithyourPCRsamples,standardsandnegativecontrol.
3.
Load15Lofeachsample,standardandnegativecontrolintoseparatewellsofyourgel,avoidmixingmineraloilwiththesample.
SavealaneforthePCRmarkeroneachgel.
Recordwhereeachsampleislocatedonthegel.
4.
Add1LloadingdyetoyourPCRmarker.
Loadthemarkerintoonewellofthegel.
5.
Attachthegelboxtothepowersupply,turnthepoweron,andsetto100-150volts.
Electrophoresefor40-60minutesoruntilthebromophenolbluebandhastraveledone-thirdthelengthofthegel.
Voltsettingsandtimewillvarywithdifferentequipment.
6.
Wearinggloves,carefullyremovethegelandputitintoastainingtray.
Coverthegelwithethidiumbromidestainandstainfor5-10minutes.
7.
Afterstaining,decanttheethidiumbromidestainfromthestainingtraybackintothestoragebottle.
8.
Rinsethegelwithtapwater,inthetray,forseveralminutestoremovebackgroundethidiumbromidestainfromthegel.
9.
Viewonultraviolettransilluminatorandphotograph.
Recordyourresultsandsharewithallothergroups.
DataAnalysisandInterpretationShareacopyofyourresultswithalltheothergroups.
Areanybandsinthe180-195bprangeHowmanylanesshowPCRbandsDoesthe0%controlstandardshowaPCRbandShoulditWhyDoesyournegativecontrolshowaPCRbandShoulditWhyDoesyourpositivecontrolstandardshowaPCRbandShoulditWhyCanyouseethePCRMarkerladderHowmanybandsshouldtherebeAreallthebandsvisibleDoyourorganicfoodsamplesshowaPCRbandIftheydowhatdoesthismeanExpectedResultsNoDNAfragmentsshouldappearinthenegativecontrollaneexceptbandsatorbelow50bpinlengthareartifactsoftheprimers.
Bandsof195bpindicatepresenceoftheCaMV35Spromoter.
NoDNAfragmentsof195bpshouldappearinthe0%controlstandardlane.
A195bpfragmentwillappearinthe2%controlstandardlane.
Studentsamplesmayormaynotshowabandat195bp.
Theoretically,organicfoodproductsshouldbefreeofgeneticallymodifiedfoods.
However,organicdefinitionsvary.
Ifaproductindicatesthatitis100%organic,thereshouldbenoband.
Non-organiccornproductswillprobablycontaindetectablelevelsofgeneticallymodifiedcorn(Figure2).
GeneticalyModifiedFoods83Figure2.
Expectedelectrophoresisresults.
AcknowledgementsSupportforthedevelopmentofthislabcamefromtheBiotechnologyLaboratoryTechnicianProgramandstaffatMATC.
SpecialthankstoNoreenWarrenandThomasBrandner.
LiteratureCitedArakawa,T.
,D.
K.
X.
Chong,andW.
H.
R.
Landridge.
1998.
Efficacyofafoodplant-basedoralcholeratoxinBsubunitvaccine.
NatureBiotechnology16(3):292.
Clark,D.
andL.
Russell.
2000.
MolecularBiologyMadeSimpleandFun.
Vienna,IL:CacheRiverPress,pages218-220.
DGJRC.
1998.
DirectorateGeneralsEuropeanCommissionsJointResearchCentre,EnvironmentInstitute,ConsumerProtection&FoodUnit.
Screeningmethodfortheidentificationofgeneticallymodifiedorganisms(GMO)infood.
FDA.
2000.
U.
S.
FoodandDrugAdministrationCenterforFoodSafety&AppliedNutrition.
FoodsDerivedfromNewPlantVarietiesDerivedthroughRecombinantDNATechnology.
Online:http://vm.
cfsan.
fda.
gov/~lrd/biocon.
htmlHemmer,W.
1997.
FoodsDerivedfromGeneticallyModifiedOrganismsandDetectionMethods.
BATS.
Online:www.
bats.
ch/abstr/297/intro.
htm84GeneticallyModifiedFoodsHorn,T.
M.
1993.
WorkingwithDNA&BacteriainPrecollegeScienceClassrooms.
Reston,VA:NationalAssociationofBiologyTeachers,pages14-17.
Landridge,W.
H.
R.
2000.
EdibleVaccines.
ScientificAmerican283(3):66-71.
McBride,K.
,andK.
Summerfelt.
1990.
ImprovedbinaryvectorsforAgrobacterium-mediatedplanttransformation.
PlantMolecularBiology14:269-276.
Micklos,D.
,andG.
Freyer.
1990.
DNAScience:AfirstcourseinrecombinantDNAtechnology.
ColdSpringHarborLaboratoryPress/CarolinaBiologicalSupplyCompany,pages256-257.
Obrycki,J.
J.
,J.
E.
Losey,O.
R.
Taylor,andL.
C.
H.
Jesse.
2001.
TransgenicInsecticidalCorn:BeyondInsecticidalToxicitytoEcologicalComplexity.
BioScience51(5):353-361.
Ramanujan,K.
andD.
Blum.
2000.
GeneticallyModifiedFood101.
WisconsinAcademyReview46(4):49-50.
Rusting,R.
2000.
MovingAgainstMalnutrition.
ScientificAmerican283(3):70.
Spoth,B.
,andE.
Strauss.
2000.
ScreeningforGeneticallyModifiedOrganismsinFoodUsingPromega'sWizardResins,QualityMonitoringofFoods,ApplicationNotes071.
PromegaCorporation,Madison,Wisconsin.
Tennant,P.
F.
,C.
Gonsalves,K.
-S.
Ling,M.
Fitch,R.
Manshardt,J.
L.
Slightom,andD.
Gonsalves.
1994.
DifferentialProtectionAgainstPapayaRingspotVirusIsolatesinCoatProteinGeneTransgenicPapayaandClassicallyCross-ProtectedPapaya.
TheAmericanPhytopathologicalSociety84(11):1359-1365.
USDA.
2000.
UnitedStatesDepartmentofAgriculture,AgriculturalBiotechnology.
FrequentlyAskedQuestions.
Online:www.
usda.
gov/agencies/biotech/faq.
htmlUSDA/ERS.
2000.
UnitedStatesDepartmentofAgriculture.
EconomicResearchService,BiotechCornandSoybeans:changingMarketsandtheGovernment'sRole.
Online:http://www.
ers.
gov/whatsnew/issues/biotechmarkets/USDA/NASS.
2000.
UnitedStatesDepartmentofAgriculture,NationalAgriculturalStatisticsService,FarmerReportedGeneticallyModifiedVarieties.
AcreageJune2000,AgriculturalStatisticsBoard,Online:www.
usda.
mannlib.
cornell.
edu/reports/nassr/field/pcp-bba/acrg0600.
pdfZimmermann,A.
,J.
Luthy,andU.
Pauli.
1998.
Quantitativeandqualitativeevaluationofninedifferentextractionmethodsfornucleicacidsonsoyabeanfoodsamples.
ZLebensmUntersForsch,A207:81-90.
AppendixAReagentandEquipmentVendorswww.
sigma-aldrich.
comwww.
apbiotech.
comwww.
promega.
comwww.
fishersci.
comwww.
vwrsp.
comwww.
nabt.
orgwww.
lifetech.
comwww.
genosys.
comwww.
novagen.
comwww.
carolina.
comwww.
edvotek.
comwww.
wardsci.
com
- populationo3o4o5相关文档
- Slowo3o4o5
- Insertiono3o4o5
- packageo3o4o5
- Temperatureo3o4o5
- specificationo3o4o5
- capacitanceo3o4o5
TmhHost香港三网CN2 GIA月付45元起,美国CN2 GIA高防VPS季付99元起
TmhHost是一家国内正规公司,具备ISP\ICP等资质,主营国内外云服务器及独立服务器租用业务,目前,商家新上香港三网CN2 GIA线路VPS及国内镇江BGP高防云主机,其中香港三网CN2 GIA线路最低每月45元起;同时对美国洛杉矶CN2 GIA线路高防及普通VPS进行优惠促销,优惠后美国洛杉矶Cera机房CN2 GIA线路高防VPS季付99元起。香港CN2 GIA安畅机房,三网回程CN2 ...

hosthatch:14个数据中心15美元/年
hosthatch在做美国独立日促销,可能你会说这操作是不是晚了一个月?对,为了准备资源等,他们拖延到现在才有空,这次是针对自己全球14个数据中心的VPS。提前示警:各个数据中心的网络没有一个是针对中国直连的,都会绕道而且ping值比较高,想买的考虑清楚再说!官方网站:https://hosthatch.com所有VPS都基于KVM虚拟,支持PayPal在内的多种付款方式!芝加哥(大硬盘)VPS5...
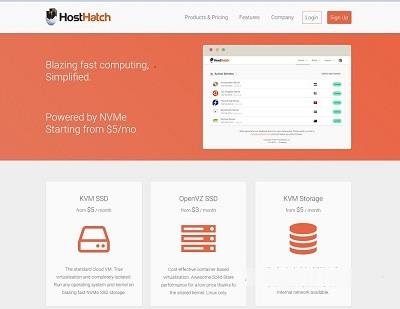
MineServer:洛杉矶CN2 GIA VPS/512MB内存/20GB NVME/800GB流量/200Mbps/KVM,58元/季
mineserver怎么样?mineserver是一家国人商家,主要提供香港CN2 KVM VPS、香港CMI KVM VPS、日本CN2 KVM VPS、洛杉矶cn2 gia端口转发等服务,之前介绍过几次,最近比较活跃。这家新推出了洛杉矶CN2 GIA VPS,512MB内存/20GB NVME/800GB流量/200Mbps/KVM,58元/季,并且进行了带宽升级,同时IP更改为美国IP。点击...

o3o4o5 com为你推荐
-
ptrPTR指的是什么?可以发外链的论坛有直接能带链接的论坛?天府热线劲舞团(四川天府热线)为什么越来越卡了??1433端口怎么去看1433端口网站联盟网络联盟是什么意思godaddy美国GODADDY 域名支持域名别名解析吗?硬盘人移动硬盘的优缺点神雕侠侣礼包大全神雕侠侣手游每天送的元宝买什么合适神雕侠侣礼包大全神雕侠侣手游版四重大礼包怎么得到啊?mate8价格华为mate8手机参数配置如何,多少元