cessfulhttp
http代理服务器软件 时间:2021-04-09 阅读:()
METHODOLOGYARTICLEOpenAccessAone-stepcloningmethodfortheconstructionofsomaticcellgenetargetingvectors:applicationtoproductionofhumanknockoutcelllinesYiLiu,ShangzeLi,HuihuiZhang,ZurongWan,XiaodongZhangandRunleiDu*AbstractBackground:Genetargetingisapowerfulmethodthatcanbeusedforexaminingthefunctionsofgenes.
Traditionally,theconstructionofknockout(KO)vectorsrequiresanamplificationsteptoobtaintwohomologous,largefragmentsofgenomicDNA.
Restrictionenzymesthatcutatuniquerecognitionssitesandnumerouscloningstepsarethencarriedout;thisisoftenatime-consumingandfrustratingprocess.
Results:Wehavedevelopedaone-stepcloningmethodfortheinsertionoftwoarmsintoaKOvectorusingexonucleaseIII.
Wemodifiedanadeno-associatedvirusKOshuttlevector(pTK-LoxP-NEO-AAV)toyieldpAAV-LIC,whichcontainedtwocassettesatthetwomultiple-cloningsites.
ThevectorwasdigestedwithEcoRVtogivetwofragments.
Thetwohomologousarms,whichhadanoverlapof16baseswiththeendsofthevectorfragments,wereamplifiedbypolymerasechainreaction.
Afterpurification,thefourfragmentsweremixedandtreatedwithexonucleaseIII,thentransformedintoEscherichiacolitoobtainthedesiredclones.
Usingthismethod,weconstructedSirT1andHDAC2KOvectors,whichwereusedtoestablishSirT1KOcellsfromthecolorectalcancercellline(HCT116)andHDAC2KOcellsfromthecolorectalcancercellline(DLD1).
Conclusions:Ourmethodisafast,simple,andefficienttechniqueforcloning,andhasgreatpotentialforhigh-throughputconstructionofKOvectors.
BackgroundGenetargetingisapowerfulmethodfortheproductionofgeneticallymodifiedcelllinesoranimals,allowingforthevariousfunctionsofgenestobestudied.
Inmice,severalthousandgeneshavebeendisruptedusinghom-ologousrecombination.
However,whenthesemethodshavebeenappliedtohumansomaticcellstheyhavegen-erallybeenineffectivebecauseofverylowtargetingeffi-ciencies[1].
AlthoughRNAinterferencecanreducetheexpressionofagene,interpretationofsuchexperimentscanbeunreliablebecauseofnon-specifictargetingorin-completeinactivationofgenes.
Therefore,webelieveitisessentialtoimprovetheefficiencyofknockout(KO)approachesinhumansomaticcells.
Recombinantadeno-associatedviruses(rAAVs),suchashumanparvovirus,possessasingle-strandedDNAgenomeofaround4.
7kb.
Hirata[2]andPorteus[3]foundthatrAAVscanbeusedtotargetgenesinhumancelllines.
UseoftheserAAVsresultedinhighertarget-ingfrequenciesthanthoseobtainedwithconventionalplasmidvectors[4,5].
Thewild-typehumanparvovirusgenomecontainstwoopenreadingframes(ORFs),designatedrepandcap,flankedbytwoinvertedterminalrepeats.
TherepORFencodesproteinsinvolvedinviralreplication,andthecapORFencodesproteinsnecessaryforviralpackaging.
InrAAV-mediatedKOvectorstheseORFsaredeletedandreplacedwithaneomycinresistantgeneflankedbytwohomologyarms.
ThestagesinvolvedincarryingoutrAAV-mediatedgeneKOinclude:(i)de-signandconstructionoftheAAVKOvector;(ii)collect-inganinfectiousrAAVstock;(iii)infectingtheappropriatecellline;(iv)screeningforhomologousrecombinants;and(v)iterativelytargetingthemultiplealleles.
Ittakesatleastthreemonthsjusttotargetthefirstallele[6].
Apartfromthelowefficiencyofhomologousrecom-bination,arate-limitingstepingenetargetingofhuman*Correspondence:runleidu@whu.
edu.
cnEqualcontributorsCollegeofLifeSciences,WuhanUniversity,Wuhan,Hubei,PRChina2012Liuetal.
;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
Liuetal.
BMCBiotechnology2012,12:71http://www.
biomedcentral.
com/1472-6750/12/71somaticcelllinesisassemblyofthegenetargetingcon-struct.
Traditionally,thisrequiresanamplificationsteptoobtaintwolargehomologousfragmentsofgenomicDNA,followedbyrestrictionendonucleasedigestion,andthennumerouscloningsteps.
Itisanextremelytime-consumingprocessandlimitedbytheavailableuniquerestrictionenzymesitesinthevectorandinthetwoamplifiedhomologousfragments.
Phage-basedEscherichiacolihomologousrecombinationsystems[7-9]havebeendevelopedthatnowmakeitpossibletosubcloneormodifyDNAclonedintoplasmids,bacterialartificialchromosomes(BACs),orP1-derivedartificialchromosomes(PACs)withouttheneedforrestrictionenzymesorDNAligases.
However,theserecombinationsystemsrequirelonghomologyarmsandusuallyonecanonlyinsertonefragmentatthetime.
TraditionalDNAcloningsuffersfromseverallimita-tions,includingpoorligationefficiency,alongwithade-pendenceontheavailabilityofuniquerestrictionsitesinboththeinsertandvector.
Forthisreason,manymeth-odsregardingdirectionalsubcloninghavebeendevel-oped[10,11].
ThesemethodsincludetheuseofuracilDNAN-glycosylase[12],T4DNApolymerase[13],en-zymaticassembly[14-16]orexonucleaseIII(ExoIII)[17]togeneratelongcompatiblecohesiveendsbetweentheDNAinsertandcloningvector.
Ithasalsobeenfoundthatbygeneratinglongercohesiveends,theannealedDNAcomplexbecomesmorestableandtheligationre-actioncanbeomitted.
Thistechniquehascometobeknownasligation-independentcloning(LIC),andhasbeendemonstratedtohaveahighefficiency.
DevelopingmoreLICmethodswillgivetheresearchersmorechioceaccordingtothedifferentsituations.
Toincreasecloningefficiencyandligationoffourfrag-mentssimultaneously,wedevelopedaone-stepLICmethodforconstructionofKOvectors.
Usingthismethod,itbecomeseasytoconstructKOvectorsintwodays.
Inthispaper,wehaveoutlinedourstrategyformodifyingpTK-LoxP-NEO-AAV,anddemonstratedsuc-cessfulconstructionofaSirT1andHDAC2KOvector.
Oncewehadmadethevectors,wewereabletoobtainSirT1KOcellsfromthecolorectalcancercelllineHCT116andHDAC2KOcellsfromthecolorectalcan-cercelllineDLD1.
ResultsanddiscussionConstructionandcharacterizationofapAAV-LICvectorAssemblyofgene-targetingconstructsisarate-limitingstepwhentargetinggenesinhumansomaticcelllines.
Otherlimitationsincludemulti-stepligationsandtheavailabilityofuniquerestrictionsitesintheinsertandvector.
Toovercomemanyoftheselimitationswemodi-fiedanAAVshuttlevectorintoaone-stepLICvector.
Thiswasachievedbyintroducingtwofixedcassettesintothemultiple-cloningsite(MCS)oftheshuttlevec-tor(Figure1).
Thetwocassettesinvolvedthreeele-ments:stickyendscomplementarytothecloningsiteinpTK-LoxP-NEO-AAV,allowingforinsertionofthecas-settes;EcoRVsitesinthemiddleofthecassette,whichcouldbeusedtocuttheLICvectorintotwofragmentscontainingbluntends;and14-to18-mersequencesthatcouldproducelongstickyendswhenExoIIIwasused.
First,cassetteAwasinsertedintopTK-LoxP-NEO-AAVusingEcoRVandSalIdigestion.
BecausethecassetteenddidnotincludetheEcoRVpalindromicsequence,thisprocessrestoredtheSalIsiteanddestroyedtheori-ginalEcoRVsite.
Followingconfirmationbysequencing,cassetteBwasinsertedintothevectornowcontainingcassetteA.
ThiswasfacilitatedbydigestionwithNheIandSpeIdigestion.
Themodificationstothevectorwereagainconfirmedbysequencing,andwedesignatedthedesignedvectoraspAAV-LIC.
ConstructionofaSirT1andHDAC2KOvectorsWeusedpAAV-LICtoconstructSirT1andHDAC2KOvectorbyone-stepLIC(Figure2).
ThepAAV-LICvectorwasdigestedwithEcoRV,resultingintwofragments(ap-proximately1kband3.
1kb).
Thesefragmentswererecoveredfromagarosegelsfollowingelectrophoresisandpurifiedusinggelextractionkits.
Thesmallerfrag-mentwasfoundtocontainthethymidinekinase(TK)promoter,theLoxPsequenceandtheneomycinORF.
ThelargerfragmentwasfoundtocontaintheAAVbackbone,necessaryforplasmidreplicationinE.
coli,andforviralpackaging.
ThetwohomologousarmsforSirT1andHDAC2KOvectorconstructionwereobtainedbypolymerasechainreaction(PCR)andpurifiedusinggelextractionkits.
ThePCRprimersusedweredesignedtoaddadditional14-to18-mersequencesoverlappingwiththeendsofdigestedvectorfragments.
Polymerases,includingTaqpolymerase,possesstheabilitytoadddATPatthe30endsofPCRproducts,therebyinhibitingthe30exo-nucleaseactivityofExoIII.
ThereforewerecommendusingpfuorPrimeStarpolymeraseswhenamplifyinghomologousarms,astheyresultinampliconswithbluntends.
The30exonucleaseactivityofExoIIIisveryhighat37°C.
Togenerate16-meroverhangsatastableandlowrate,itisnecessarytooptimizereactiontemperatureandtime.
Accordingtoourexperience,toensurestablecloning,1hat0°Cor30minat4°Carebothsufficient.
Usingotherconditionsproveddifficult,withboththenumberofcoloniesandtherateofpositivetransfor-mantsacquiredhighlyvariable.
Toproducecomplemen-tarystickyends,thepurifiedhomologousarmsandtwovectorfragmentsweremixedandchilledat0°Cfor10min,thentreatedwith20UofExoIIIat0°Cfor1h.
TheLiuetal.
BMCBiotechnology2012,12:71Page2of7http://www.
biomedcentral.
com/1472-6750/12/71reactionwasterminatedusingEDTAorbyheatingto65°C.
AftertransformationintoDH5αE.
coli,enzymaticbacterialDNArepairmachineryhelpedtofacilitateligationendogenously.
ColonyPCRandsequencingwasusedtoverifythepositivetransformants.
ScreeningbyPCRshowedthat9ofthe12coloniesanalyzedwerepositivetransformants(Figure3a).
SequencingresultsfurtherconfirmedthePCRresults(Figure3bandc).
TheresultingKOvectorwasdesignatedastheAAV-SirT1KOvector.
AlsowegottheAAV-HDAC2KOvector.
UseofgenetargetingtogenerateSirT1-nullHCT116cellsandHDAC2-nullDLD1cellsToconfirmitsabilitytobeusedinthedevelopmentofKOcelllines,thetargetingAAVswerepackagedin293TcellsbytransfectingequalamountsoftheSirT1KOtargetingvectortargetingvector,pHelperandpRC.
ThenHCT116cellswereinfectedwiththeSirT1target-ingvirusesandselectedwithgeneticin.
Aftertwoweeks,theneomycin-resistantcloneswerescreenedforhom-ologousrecombinationbygenomicPCR.
Wescreened100clonesandobtained4positives.
Toremovetheneomycin-resistantgene,twoofthecorrectlytargetedcloneswereinfectedwithadenovirusesexpressingCre-recombinase.
UsinggenomicPCR,wescreened24clones,with10ofthemfoundtobepositivescontaininganadditional250bpfragmentinwhichtheLoxPsitewasinserted.
UpuntilthispointwehadsucceededinobtainingheterozygousKOclones.
Todeletebothalleles,heterozygousKOcloneswereinfectedwiththesametargetingvirusandtheneomycinresistancegenewasexcised.
FinallyweobtainedhomozygousKOclonesandtheKOcelllineswereconfirmedbywesternblot(Figure4a).
Usethesameprotocol,HDAC2targetingvirusesweremadeandDLD1cellswereinfectedwiththeHDAC2targetingviruses.
AlsoweobtainedHDAC2-nullDLD1cellsandconfirmedbywesternblot(Figure4b).
ConclusionsAsoutlinedabove,wesuccessfullyconstructedtheAAV-SirT1andAAV-HDAC2KOvectorsandobtainedSirT1andHDAC2KOcelllines.
Comparedwiththetrad-itional'digestion-ligation'vectorconstructionmethod,ourapproachisnotlimitedbytheavailabilityofrestric-tionsites;therefore,somaticcellgeneKOvectorscanbeeasilyconstructedwithin2–3days.
Additionally,ourmethoddoesnotrequiretheuseofanysinglespecificrestrictionenzyme,andhasgreatpotentialforthehigh-throughputconstructionofgeneKOvectors.
How-ever,italsohassomelimitations,suchasthepotentialFigure1SchematicdepictingthemodificationofpTK-LoxP-NEO-AAVintopAAV-LICbyinsertionoftwodesignedLICadaptors.
ThetwoLICadaptorscontainEcoRVsitesandanadditional11–15bpsequencenecessaryforLICcloning.
ThetwoEcoRVrestrictionsequencesareunderlinedandanarrowindicatesthedigestionsites.
LeftbluntendandrightoverhangsequencesforEcoRVandSalIincassetteAallowforannealingoftheLICadaptorintotherightarmMCSofthepTK-LoxP-NEO-AAVplasmid.
OverhangsequencesforNheIandSpeIincassetteBallowforannealingoftheLICadaptorintotheleftarmMCS.
Liuetal.
BMCBiotechnology2012,12:71Page3of7http://www.
biomedcentral.
com/1472-6750/12/71errorscanbeproducedduringamplificationbyPCRanditneedhighefficiencycompetentcellsforthetransformation.
MethodsMaterialsandbacterialstrainsRestrictionenzymes,Pfupolymerase,ExoIIIandDNAligationkitswerepurchasedfromTakara(Dalian,China).
Kitsforplasmidminipreps,DNApurificationandgenomeextractionwerepurchasedfromTiangen(Beijing,China).
PrimerswerepurchasedfromGenscript(Nanjing,China).
TheE.
coliDH5αstrainwasusedforplasmidpreparationandallrestriction-basedcloning.
CellcultureHEK-293TcellswereculturedinDulbecco'smodifiedEagle'smedium(DMEM)containing10%fetalbovineserum(FBS)and100units/mlpenicillin-streptomycin.
Thecolorectalcancercellline,HCT116,wasculturedinMcCoy's5Acontaining10%FBSand100units/mlFigure2ConstructionofasomaticcellKOvectorviaaone-stepcloningmethod.
CassetteAisindicatedbytworectanglesandcassetteBbytwoechelons.
ThehomologousarmswereamplifiedusingPCRwithprimerscontainingthe15–18ntsequenceatthe50endandsequencesspecificfortheExoIII-generatedoverhangsinpAAV-LIC.
E2–6:exons2–6;NEO:neomycinresistancegene;TK:thymidinekinasepromoter.
Liuetal.
BMCBiotechnology2012,12:71Page4of7http://www.
biomedcentral.
com/1472-6750/12/71penicillin-streptomycin.
McCoy's5Amediumwaspur-chasedfromAppliChem(SeajetScientificCo.
,Ltd.
,Beijing,CHINA).
AllothercellculturereagentswereobtainedfromLifeTechnologiesCorporation(Shanghai,CHINA).
ConstructionofthemodifiedAAVshuttlevectorTheAAVshuttlevector,pTK-LoxP-NEO-AAV,wasmodifiedtoproducethepAAV-LICvector.
Twopairsofuniqueprimersweredesignedforcreating'LICzones'.
TheseprimerpairswerecassetteA(sense,50-CATGGGTGGTGGGATATCTTATACTTCCAAG-30;andantisense,50-TCGACTTGGAAGTATAAGATATCCCACCACCCATG-30)andcassetteB(sense,50-CTAGCATGGTGAGAATTTATGATATCCCCACCCTCCCAGA-30;andantisense,50-CTAGTCTGGGAGGGTGGGGATATCATAAATTCTCACCATG-30).
TheunderlinedsequenceindicatestherecognitionsiteforEcoRV.
Primersweredilutedto5mMwithdouble-distilledwater.
Senseandantisenseprimers(3μlofeach)forcas-setteAorcassetteBweremixedwith3μlofbuffer3Figure3ColonyPCRandsequencingresultsforthepAAV-SirT1geneKOplasmid.
(a)ColonyscreeningbyPCR.
Leftarm(>1kb)andrightarm(http://www.
biomedcentral.
com/1472-6750/12/71(NEB)and21μlofsterilewater.
Thereactionwasincu-batedat94°Cfor10min,thencooledslowlytoroomtemperature.
CassetteAorBfusionproductswereinsertedintothedigestedpTK-LoxP-NEO-AAVvectorusingDNAligationkits.
Recombinantswereverifiedbyrestrictionanalysisandsequencing.
GeneralprimersforAAVgene-targetingvectorsThehomologousarmisaround1kb,andwedesignedprimersusingprimer3(http://frodo.
wi.
mit.
edu/primer3/).
ToinsertarmsintothepAAV-LICbackbone,thepri-mersneededtocontaincomplementarysequencestotheExoIII-generatedoverhangsinpAAV-LIC.
ThedesignedandsynthesizedprimerswereLeftArmSense(50-ATGGTGAGAATTTATGAT-30),LeftArmAnti-sense(50-TGGGAGGGTGGGGAT-30),RightArmSense(50-ATGGGTGGTGGGAT-30),andRightArmAntisense(50-TTGGAAGTATAAGAT-30).
ConstructionoftheSirT1AAVKOvectorPlasmidpAAV-LIC(2μg)wassubjectedtoovernightdi-gestionwithEcoRVina50μlreactionat37°C.
Thetwofragmentsproducedwerepurifiedusingagelextractionkitanddilutedto50ng/μlinTEbuffer(pH8.
0).
TwohomologousarmswereamplifiedfromgenomicDNAextractedfromHCT116cells.
Twopairsofuniquepri-mersweredesigned:(a)SirT1,leftarm,forward(50-ATGGTGAGAATTTATGATCGCCTGTTTGTATCCT-TCCTGAC-30)andreverse(50-TGGGAGGGTGGG-GATCAGAGATGGCTGGAATTGTCC–30);and(b)SirT1,rightarm,forward(50–ATGGGTGGTGGGAT-CAGCCTTGTCAGATAAGGAAG–30)andreverse(50–TTGGAAGTATAAGATCACCTTAGCTCGGCAGTTCTT–30);(c)HDAC2,leftarm,forward(50-ATGGTGAGAATTTATGATAGCCAGCAGCATTGCTGCAG30)andreverse(50–TGGGAGGGTGGGGATAGCCGCGGAACCCAGCGCC30);and(d)HDAC2,rightarm,forward(50–ATGGGTGGTGGGATAAGTCTGCTACTACTACGAC30)andreverse(50–TTGGAAGTATAAGATCCCCAAAGCAGGGTCTAT30).
TheunderlinedsequencesindicatethedesignedprimersfromtheSirT1genomeDNAorHDAC2genomeDNA.
ThePCRswereperformedusingpfupolymerase,withampliconselectrophoresedandthenrecoveredbygelextractionkits.
Recoveredfragmentsweredilutedto100ng/μlinTEbuffer.
Thevectorfragmentsandampliconsweremixedinonereactiontube.
Thelargefragment(1μl),smallfrag-ment(2μl),leftarm(2μl)andrightarm(2μl)weremixedwith1μlof10*ExoIIIbufferand1μlofsterilewater.
Thetubewaspre-chilledonicefor2min,andthen1μlofExoIII(20units)wasaddedtothetubeandgentlymixed.
Aftera1hincubationat0°C,1μlof0.
5MEDTAwasaddedtostopthereaction.
Theenzymewasinactivatedat65°Cfor5min.
Aftercooling,themixturewastransformedintoE.
coliDH5αandculturedat37°Covernight.
PositivetransformantswereidentifiedbycolonyPCRandsequencing.
SomaticcellgenetargetingSomaticcellgenetargetingwasconductedasdescribedpreviously[5].
Briefly,thetargetingAAVswerepackagedin293TcellsbytransfectingequalamountsofSirT1orHDAC2KOtargetingvector,pHelperandpRCplasmids(1mgeach).
After72h,scrapedthetransfectedcellsandsuspendedthecellsintosterilephosphate-bufferedsaline,thenfreezedandthawedthepelletthreecycles,finallyspinedthelysatetoremovecelldebrisanddividedthesupernatantcontainingrAAVintoseveralaliquotsandfrozenat-80°C.
HCT116orDLD1cellswereinfectedwiththeSirT1orHDAC2targetingvirusesandselectedwithgeneticinfortwoweeks.
Thegeneticin-resistantcloneswerethenscreenedforhom-ologousrecombinationbygenomicPCRwithprimersderivedfromtheneomycinresistancegene(50-GTTGTGCCCAGTCATAGCCG-30)andtheupstreamregionofthelefthomologousarm(SirT1:50-GAAAGTTCCCCACATCTGCT-30;HDAC2:50-TGCTCCAATCTTCCAGTGTCT-30).
Positivecloneswereconfirmedbygen-omicPCR,withprimersderivedfromtheneomycinre-sistancegene(50-TCTGGATTCATCGACTGTGG-30)andthedownstreamregionoftherighthomolo-gousarm(SirT1:50-TCACTCCTCCAGGGCTAAAA-30;HDAC250-CACCTAGGAACAGCCTTTGC-30).
Cor-rectlytargetedcloneswereinfectedwithadenovirusesexpressingCre-recombinasetodeletetheselectabledrugmarker.
Toselectcloneswithsuccessfuldeletionoftheselectabledrugmarker,genomicPCRwasemployedtoamplifyanapproximate250bpfragmentinwhichtheLoxPsitewasinserted,usingspecificprimers(SirT1:50-TAGGTGTGTGTCGCATCCAT-30and50-CCTGTTCCAGCGTGTCTATGT-30;HDAC2:50-CATGGCGTACAGTCAAGGAG-30and50-CAAATGTCGGTCCCTCCTC-30).
TheheterozygousKOcloneswereinfectedwiththesametargetingvirustotargetthesecondalleleandtheneomycinresistancegenewasexcisedasdescribedearl-ier.
FinalconfirmationinthegenerationoftheKOcelllineswasdoneusingwesternblotting.
CompetinginterestsTheauthorsdeclaretheyhavenocompetinginterests.
Authors'contributionsRDconceivedtheprojectanddesignedtheexperimentsalongwithYL.
YL,HZandSLperformedthelaboratoryworkandanalyzedtheresults.
XZandRDwrotethemanuscript.
Allauthorsreadandapprovedthefinalmanuscript.
AcknowledgementsThisworkwassupportedbygrantsfromtheNationalBasicResearchProgramofChina(2011CB944404),theNationalNaturalScienceFoundationofChina(30971499),theTrans-CenturyTrainingProgrammeFoundationforLiuetal.
BMCBiotechnology2012,12:71Page6of7http://www.
biomedcentral.
com/1472-6750/12/71theTalentsbytheStateEducationCommission(NCET-10-0655),andtheFundamentalResearchFundsfortheCentralUniversities(204275771).
Received:21June2012Accepted:2October2012Published:9October2012References1.
SedivyJM,DutriauxA:Genetargetingandsomaticcellgenetics–arebirthoracomingofageTrendsGenet1999,15(3):88–90.
2.
HirataR,ChamberlainJ,DongR,RussellDW:Targetedtransgeneinsertionintohumanchromosomesbyadeno-associatedvirusvectors.
NatBiotechnol2002,20(7):735–738.
3.
PorteusMH,CathomenT,WeitzmanMD,BaltimoreD:Efficientgenetargetingmediatedbyadeno-associatedvirusandDNAdouble-strandbreaks.
MolCellBiol2003,23(10):3558–3565.
4.
ZhangX,GuoC,ChenY,ShulhaHP,SchnetzMP,LaFramboiseT,BartelsCF,MarkowitzS,WengZ,ScacheriPC,etal:Epitopetaggingofendogenousproteinsforgenome-wideChIP-chipstudies.
NatMethods2008,5(2):163–165.
5.
ZhangP,ZhaoY,ZhuX,SedwickD,ZhangX,WangZ:Cross-talkbetweenphospho-STAT3andPLCgamma1playsacriticalroleincolorectaltumorigenesis.
MolCancerRes2011,9(10):1418–1428.
6.
RagoC,VogelsteinB,BunzF:Geneticknockoutsandknockinsinhumansomaticcells.
NatProtocols2007,2(11):2734–2746.
7.
LiuP,JenkinsNA,CopelandNG:Ahighlyefficientrecombineering-basedmethodforgeneratingconditionalknockoutmutations.
GenomeRes2003,13(3):476–484.
8.
AngrandPO,DaigleN,vanderHoevenF,ScholerHR,StewartAF:SimplifiedgenerationoftargetingconstructsusingETrecombination.
NucleicAcidsRes1999,27(17):e16.
9.
ZhangY,BuchholzF,MuyrersJP,StewartAF:AnewlogicforDNAengineeringusingrecombinationinEscherichiacoli.
NatGenet1998,20(2):123–128.
10.
LiC,WenA,ShenB,LuJ,HuangY,ChangY:FastCloning:ahighlysimplified,purification-free,sequence-andligation-independentPCRcloningmethod.
BMCBiotechnol2011,11:92.
11.
LiMZ,ElledgeSJ:SLIC:amethodforsequence-andligation-independentcloning.
MethodsMolBiol2012,852:51–59.
12.
Geu-FloresF,Nour-EldinHH,NielsenMT,HalkierBA:USERfusion:arapidandefficientmethodforsimultaneousfusionandcloningofmultiplePCRproducts.
NucleicAcidsRes2007,35(7):e55.
13.
DuR,LiS,ZhangX:AmodifiedplasmidvectorpCMV-3Tag-LICforrapid,reliable,ligation-independentcloningofpolymerasechainreactionproducts.
AnalBiochem2011,408(2):357–359.
14.
GibsonDG,YoungL,ChuangRY,VenterJC,HutchisonCA3rd:SmithHO:EnzymaticassemblyofDNAmoleculesuptoseveralhundredkilobases.
NatMethods2009,6(5):343–345.
15.
GibsonDG,SmithHO,HutchisonCA3rd,VenterJC,MerrymanC:Chemicalsynthesisofthemousemitochondrialgenome.
NatMethods2010,7(11):901–903.
16.
GibsonDG:SynthesisofDNAfragmentsinyeastbyone-stepassemblyofoverlappingoligonucleotides.
NucleicAcidsRes2009,37(20):6984–6990.
17.
HsiaoK:ExonucleaseIIIinducedligase-freedirectionalsubcloningofPCRproducts.
NucleicAcidsRes1993,21(23):5528–5529.
doi:10.
1186/1472-6750-12-71Citethisarticleas:Liuetal.
:Aone-stepcloningmethodfortheconstructionofsomaticcellgenetargetingvectors:applicationtoproductionofhumanknockoutcelllines.
BMCBiotechnology201212:71.
SubmityournextmanuscripttoBioMedCentralandtakefulladvantageof:ConvenientonlinesubmissionThoroughpeerreviewNospaceconstraintsorcolorgurechargesImmediatepublicationonacceptanceInclusioninPubMed,CAS,ScopusandGoogleScholarResearchwhichisfreelyavailableforredistributionSubmityourmanuscriptatwww.
biomedcentral.
com/submitLiuetal.
BMCBiotechnology2012,12:71Page7of7http://www.
biomedcentral.
com/1472-6750/12/71
Traditionally,theconstructionofknockout(KO)vectorsrequiresanamplificationsteptoobtaintwohomologous,largefragmentsofgenomicDNA.
Restrictionenzymesthatcutatuniquerecognitionssitesandnumerouscloningstepsarethencarriedout;thisisoftenatime-consumingandfrustratingprocess.
Results:Wehavedevelopedaone-stepcloningmethodfortheinsertionoftwoarmsintoaKOvectorusingexonucleaseIII.
Wemodifiedanadeno-associatedvirusKOshuttlevector(pTK-LoxP-NEO-AAV)toyieldpAAV-LIC,whichcontainedtwocassettesatthetwomultiple-cloningsites.
ThevectorwasdigestedwithEcoRVtogivetwofragments.
Thetwohomologousarms,whichhadanoverlapof16baseswiththeendsofthevectorfragments,wereamplifiedbypolymerasechainreaction.
Afterpurification,thefourfragmentsweremixedandtreatedwithexonucleaseIII,thentransformedintoEscherichiacolitoobtainthedesiredclones.
Usingthismethod,weconstructedSirT1andHDAC2KOvectors,whichwereusedtoestablishSirT1KOcellsfromthecolorectalcancercellline(HCT116)andHDAC2KOcellsfromthecolorectalcancercellline(DLD1).
Conclusions:Ourmethodisafast,simple,andefficienttechniqueforcloning,andhasgreatpotentialforhigh-throughputconstructionofKOvectors.
BackgroundGenetargetingisapowerfulmethodfortheproductionofgeneticallymodifiedcelllinesoranimals,allowingforthevariousfunctionsofgenestobestudied.
Inmice,severalthousandgeneshavebeendisruptedusinghom-ologousrecombination.
However,whenthesemethodshavebeenappliedtohumansomaticcellstheyhavegen-erallybeenineffectivebecauseofverylowtargetingeffi-ciencies[1].
AlthoughRNAinterferencecanreducetheexpressionofagene,interpretationofsuchexperimentscanbeunreliablebecauseofnon-specifictargetingorin-completeinactivationofgenes.
Therefore,webelieveitisessentialtoimprovetheefficiencyofknockout(KO)approachesinhumansomaticcells.
Recombinantadeno-associatedviruses(rAAVs),suchashumanparvovirus,possessasingle-strandedDNAgenomeofaround4.
7kb.
Hirata[2]andPorteus[3]foundthatrAAVscanbeusedtotargetgenesinhumancelllines.
UseoftheserAAVsresultedinhighertarget-ingfrequenciesthanthoseobtainedwithconventionalplasmidvectors[4,5].
Thewild-typehumanparvovirusgenomecontainstwoopenreadingframes(ORFs),designatedrepandcap,flankedbytwoinvertedterminalrepeats.
TherepORFencodesproteinsinvolvedinviralreplication,andthecapORFencodesproteinsnecessaryforviralpackaging.
InrAAV-mediatedKOvectorstheseORFsaredeletedandreplacedwithaneomycinresistantgeneflankedbytwohomologyarms.
ThestagesinvolvedincarryingoutrAAV-mediatedgeneKOinclude:(i)de-signandconstructionoftheAAVKOvector;(ii)collect-inganinfectiousrAAVstock;(iii)infectingtheappropriatecellline;(iv)screeningforhomologousrecombinants;and(v)iterativelytargetingthemultiplealleles.
Ittakesatleastthreemonthsjusttotargetthefirstallele[6].
Apartfromthelowefficiencyofhomologousrecom-bination,arate-limitingstepingenetargetingofhuman*Correspondence:runleidu@whu.
edu.
cnEqualcontributorsCollegeofLifeSciences,WuhanUniversity,Wuhan,Hubei,PRChina2012Liuetal.
;licenseeBioMedCentralLtd.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/2.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycited.
Liuetal.
BMCBiotechnology2012,12:71http://www.
biomedcentral.
com/1472-6750/12/71somaticcelllinesisassemblyofthegenetargetingcon-struct.
Traditionally,thisrequiresanamplificationsteptoobtaintwolargehomologousfragmentsofgenomicDNA,followedbyrestrictionendonucleasedigestion,andthennumerouscloningsteps.
Itisanextremelytime-consumingprocessandlimitedbytheavailableuniquerestrictionenzymesitesinthevectorandinthetwoamplifiedhomologousfragments.
Phage-basedEscherichiacolihomologousrecombinationsystems[7-9]havebeendevelopedthatnowmakeitpossibletosubcloneormodifyDNAclonedintoplasmids,bacterialartificialchromosomes(BACs),orP1-derivedartificialchromosomes(PACs)withouttheneedforrestrictionenzymesorDNAligases.
However,theserecombinationsystemsrequirelonghomologyarmsandusuallyonecanonlyinsertonefragmentatthetime.
TraditionalDNAcloningsuffersfromseverallimita-tions,includingpoorligationefficiency,alongwithade-pendenceontheavailabilityofuniquerestrictionsitesinboththeinsertandvector.
Forthisreason,manymeth-odsregardingdirectionalsubcloninghavebeendevel-oped[10,11].
ThesemethodsincludetheuseofuracilDNAN-glycosylase[12],T4DNApolymerase[13],en-zymaticassembly[14-16]orexonucleaseIII(ExoIII)[17]togeneratelongcompatiblecohesiveendsbetweentheDNAinsertandcloningvector.
Ithasalsobeenfoundthatbygeneratinglongercohesiveends,theannealedDNAcomplexbecomesmorestableandtheligationre-actioncanbeomitted.
Thistechniquehascometobeknownasligation-independentcloning(LIC),andhasbeendemonstratedtohaveahighefficiency.
DevelopingmoreLICmethodswillgivetheresearchersmorechioceaccordingtothedifferentsituations.
Toincreasecloningefficiencyandligationoffourfrag-mentssimultaneously,wedevelopedaone-stepLICmethodforconstructionofKOvectors.
Usingthismethod,itbecomeseasytoconstructKOvectorsintwodays.
Inthispaper,wehaveoutlinedourstrategyformodifyingpTK-LoxP-NEO-AAV,anddemonstratedsuc-cessfulconstructionofaSirT1andHDAC2KOvector.
Oncewehadmadethevectors,wewereabletoobtainSirT1KOcellsfromthecolorectalcancercelllineHCT116andHDAC2KOcellsfromthecolorectalcan-cercelllineDLD1.
ResultsanddiscussionConstructionandcharacterizationofapAAV-LICvectorAssemblyofgene-targetingconstructsisarate-limitingstepwhentargetinggenesinhumansomaticcelllines.
Otherlimitationsincludemulti-stepligationsandtheavailabilityofuniquerestrictionsitesintheinsertandvector.
Toovercomemanyoftheselimitationswemodi-fiedanAAVshuttlevectorintoaone-stepLICvector.
Thiswasachievedbyintroducingtwofixedcassettesintothemultiple-cloningsite(MCS)oftheshuttlevec-tor(Figure1).
Thetwocassettesinvolvedthreeele-ments:stickyendscomplementarytothecloningsiteinpTK-LoxP-NEO-AAV,allowingforinsertionofthecas-settes;EcoRVsitesinthemiddleofthecassette,whichcouldbeusedtocuttheLICvectorintotwofragmentscontainingbluntends;and14-to18-mersequencesthatcouldproducelongstickyendswhenExoIIIwasused.
First,cassetteAwasinsertedintopTK-LoxP-NEO-AAVusingEcoRVandSalIdigestion.
BecausethecassetteenddidnotincludetheEcoRVpalindromicsequence,thisprocessrestoredtheSalIsiteanddestroyedtheori-ginalEcoRVsite.
Followingconfirmationbysequencing,cassetteBwasinsertedintothevectornowcontainingcassetteA.
ThiswasfacilitatedbydigestionwithNheIandSpeIdigestion.
Themodificationstothevectorwereagainconfirmedbysequencing,andwedesignatedthedesignedvectoraspAAV-LIC.
ConstructionofaSirT1andHDAC2KOvectorsWeusedpAAV-LICtoconstructSirT1andHDAC2KOvectorbyone-stepLIC(Figure2).
ThepAAV-LICvectorwasdigestedwithEcoRV,resultingintwofragments(ap-proximately1kband3.
1kb).
Thesefragmentswererecoveredfromagarosegelsfollowingelectrophoresisandpurifiedusinggelextractionkits.
Thesmallerfrag-mentwasfoundtocontainthethymidinekinase(TK)promoter,theLoxPsequenceandtheneomycinORF.
ThelargerfragmentwasfoundtocontaintheAAVbackbone,necessaryforplasmidreplicationinE.
coli,andforviralpackaging.
ThetwohomologousarmsforSirT1andHDAC2KOvectorconstructionwereobtainedbypolymerasechainreaction(PCR)andpurifiedusinggelextractionkits.
ThePCRprimersusedweredesignedtoaddadditional14-to18-mersequencesoverlappingwiththeendsofdigestedvectorfragments.
Polymerases,includingTaqpolymerase,possesstheabilitytoadddATPatthe30endsofPCRproducts,therebyinhibitingthe30exo-nucleaseactivityofExoIII.
ThereforewerecommendusingpfuorPrimeStarpolymeraseswhenamplifyinghomologousarms,astheyresultinampliconswithbluntends.
The30exonucleaseactivityofExoIIIisveryhighat37°C.
Togenerate16-meroverhangsatastableandlowrate,itisnecessarytooptimizereactiontemperatureandtime.
Accordingtoourexperience,toensurestablecloning,1hat0°Cor30minat4°Carebothsufficient.
Usingotherconditionsproveddifficult,withboththenumberofcoloniesandtherateofpositivetransfor-mantsacquiredhighlyvariable.
Toproducecomplemen-tarystickyends,thepurifiedhomologousarmsandtwovectorfragmentsweremixedandchilledat0°Cfor10min,thentreatedwith20UofExoIIIat0°Cfor1h.
TheLiuetal.
BMCBiotechnology2012,12:71Page2of7http://www.
biomedcentral.
com/1472-6750/12/71reactionwasterminatedusingEDTAorbyheatingto65°C.
AftertransformationintoDH5αE.
coli,enzymaticbacterialDNArepairmachineryhelpedtofacilitateligationendogenously.
ColonyPCRandsequencingwasusedtoverifythepositivetransformants.
ScreeningbyPCRshowedthat9ofthe12coloniesanalyzedwerepositivetransformants(Figure3a).
SequencingresultsfurtherconfirmedthePCRresults(Figure3bandc).
TheresultingKOvectorwasdesignatedastheAAV-SirT1KOvector.
AlsowegottheAAV-HDAC2KOvector.
UseofgenetargetingtogenerateSirT1-nullHCT116cellsandHDAC2-nullDLD1cellsToconfirmitsabilitytobeusedinthedevelopmentofKOcelllines,thetargetingAAVswerepackagedin293TcellsbytransfectingequalamountsoftheSirT1KOtargetingvectortargetingvector,pHelperandpRC.
ThenHCT116cellswereinfectedwiththeSirT1target-ingvirusesandselectedwithgeneticin.
Aftertwoweeks,theneomycin-resistantcloneswerescreenedforhom-ologousrecombinationbygenomicPCR.
Wescreened100clonesandobtained4positives.
Toremovetheneomycin-resistantgene,twoofthecorrectlytargetedcloneswereinfectedwithadenovirusesexpressingCre-recombinase.
UsinggenomicPCR,wescreened24clones,with10ofthemfoundtobepositivescontaininganadditional250bpfragmentinwhichtheLoxPsitewasinserted.
UpuntilthispointwehadsucceededinobtainingheterozygousKOclones.
Todeletebothalleles,heterozygousKOcloneswereinfectedwiththesametargetingvirusandtheneomycinresistancegenewasexcised.
FinallyweobtainedhomozygousKOclonesandtheKOcelllineswereconfirmedbywesternblot(Figure4a).
Usethesameprotocol,HDAC2targetingvirusesweremadeandDLD1cellswereinfectedwiththeHDAC2targetingviruses.
AlsoweobtainedHDAC2-nullDLD1cellsandconfirmedbywesternblot(Figure4b).
ConclusionsAsoutlinedabove,wesuccessfullyconstructedtheAAV-SirT1andAAV-HDAC2KOvectorsandobtainedSirT1andHDAC2KOcelllines.
Comparedwiththetrad-itional'digestion-ligation'vectorconstructionmethod,ourapproachisnotlimitedbytheavailabilityofrestric-tionsites;therefore,somaticcellgeneKOvectorscanbeeasilyconstructedwithin2–3days.
Additionally,ourmethoddoesnotrequiretheuseofanysinglespecificrestrictionenzyme,andhasgreatpotentialforthehigh-throughputconstructionofgeneKOvectors.
How-ever,italsohassomelimitations,suchasthepotentialFigure1SchematicdepictingthemodificationofpTK-LoxP-NEO-AAVintopAAV-LICbyinsertionoftwodesignedLICadaptors.
ThetwoLICadaptorscontainEcoRVsitesandanadditional11–15bpsequencenecessaryforLICcloning.
ThetwoEcoRVrestrictionsequencesareunderlinedandanarrowindicatesthedigestionsites.
LeftbluntendandrightoverhangsequencesforEcoRVandSalIincassetteAallowforannealingoftheLICadaptorintotherightarmMCSofthepTK-LoxP-NEO-AAVplasmid.
OverhangsequencesforNheIandSpeIincassetteBallowforannealingoftheLICadaptorintotheleftarmMCS.
Liuetal.
BMCBiotechnology2012,12:71Page3of7http://www.
biomedcentral.
com/1472-6750/12/71errorscanbeproducedduringamplificationbyPCRanditneedhighefficiencycompetentcellsforthetransformation.
MethodsMaterialsandbacterialstrainsRestrictionenzymes,Pfupolymerase,ExoIIIandDNAligationkitswerepurchasedfromTakara(Dalian,China).
Kitsforplasmidminipreps,DNApurificationandgenomeextractionwerepurchasedfromTiangen(Beijing,China).
PrimerswerepurchasedfromGenscript(Nanjing,China).
TheE.
coliDH5αstrainwasusedforplasmidpreparationandallrestriction-basedcloning.
CellcultureHEK-293TcellswereculturedinDulbecco'smodifiedEagle'smedium(DMEM)containing10%fetalbovineserum(FBS)and100units/mlpenicillin-streptomycin.
Thecolorectalcancercellline,HCT116,wasculturedinMcCoy's5Acontaining10%FBSand100units/mlFigure2ConstructionofasomaticcellKOvectorviaaone-stepcloningmethod.
CassetteAisindicatedbytworectanglesandcassetteBbytwoechelons.
ThehomologousarmswereamplifiedusingPCRwithprimerscontainingthe15–18ntsequenceatthe50endandsequencesspecificfortheExoIII-generatedoverhangsinpAAV-LIC.
E2–6:exons2–6;NEO:neomycinresistancegene;TK:thymidinekinasepromoter.
Liuetal.
BMCBiotechnology2012,12:71Page4of7http://www.
biomedcentral.
com/1472-6750/12/71penicillin-streptomycin.
McCoy's5Amediumwaspur-chasedfromAppliChem(SeajetScientificCo.
,Ltd.
,Beijing,CHINA).
AllothercellculturereagentswereobtainedfromLifeTechnologiesCorporation(Shanghai,CHINA).
ConstructionofthemodifiedAAVshuttlevectorTheAAVshuttlevector,pTK-LoxP-NEO-AAV,wasmodifiedtoproducethepAAV-LICvector.
Twopairsofuniqueprimersweredesignedforcreating'LICzones'.
TheseprimerpairswerecassetteA(sense,50-CATGGGTGGTGGGATATCTTATACTTCCAAG-30;andantisense,50-TCGACTTGGAAGTATAAGATATCCCACCACCCATG-30)andcassetteB(sense,50-CTAGCATGGTGAGAATTTATGATATCCCCACCCTCCCAGA-30;andantisense,50-CTAGTCTGGGAGGGTGGGGATATCATAAATTCTCACCATG-30).
TheunderlinedsequenceindicatestherecognitionsiteforEcoRV.
Primersweredilutedto5mMwithdouble-distilledwater.
Senseandantisenseprimers(3μlofeach)forcas-setteAorcassetteBweremixedwith3μlofbuffer3Figure3ColonyPCRandsequencingresultsforthepAAV-SirT1geneKOplasmid.
(a)ColonyscreeningbyPCR.
Leftarm(>1kb)andrightarm(http://www.
biomedcentral.
com/1472-6750/12/71(NEB)and21μlofsterilewater.
Thereactionwasincu-batedat94°Cfor10min,thencooledslowlytoroomtemperature.
CassetteAorBfusionproductswereinsertedintothedigestedpTK-LoxP-NEO-AAVvectorusingDNAligationkits.
Recombinantswereverifiedbyrestrictionanalysisandsequencing.
GeneralprimersforAAVgene-targetingvectorsThehomologousarmisaround1kb,andwedesignedprimersusingprimer3(http://frodo.
wi.
mit.
edu/primer3/).
ToinsertarmsintothepAAV-LICbackbone,thepri-mersneededtocontaincomplementarysequencestotheExoIII-generatedoverhangsinpAAV-LIC.
ThedesignedandsynthesizedprimerswereLeftArmSense(50-ATGGTGAGAATTTATGAT-30),LeftArmAnti-sense(50-TGGGAGGGTGGGGAT-30),RightArmSense(50-ATGGGTGGTGGGAT-30),andRightArmAntisense(50-TTGGAAGTATAAGAT-30).
ConstructionoftheSirT1AAVKOvectorPlasmidpAAV-LIC(2μg)wassubjectedtoovernightdi-gestionwithEcoRVina50μlreactionat37°C.
Thetwofragmentsproducedwerepurifiedusingagelextractionkitanddilutedto50ng/μlinTEbuffer(pH8.
0).
TwohomologousarmswereamplifiedfromgenomicDNAextractedfromHCT116cells.
Twopairsofuniquepri-mersweredesigned:(a)SirT1,leftarm,forward(50-ATGGTGAGAATTTATGATCGCCTGTTTGTATCCT-TCCTGAC-30)andreverse(50-TGGGAGGGTGGG-GATCAGAGATGGCTGGAATTGTCC–30);and(b)SirT1,rightarm,forward(50–ATGGGTGGTGGGAT-CAGCCTTGTCAGATAAGGAAG–30)andreverse(50–TTGGAAGTATAAGATCACCTTAGCTCGGCAGTTCTT–30);(c)HDAC2,leftarm,forward(50-ATGGTGAGAATTTATGATAGCCAGCAGCATTGCTGCAG30)andreverse(50–TGGGAGGGTGGGGATAGCCGCGGAACCCAGCGCC30);and(d)HDAC2,rightarm,forward(50–ATGGGTGGTGGGATAAGTCTGCTACTACTACGAC30)andreverse(50–TTGGAAGTATAAGATCCCCAAAGCAGGGTCTAT30).
TheunderlinedsequencesindicatethedesignedprimersfromtheSirT1genomeDNAorHDAC2genomeDNA.
ThePCRswereperformedusingpfupolymerase,withampliconselectrophoresedandthenrecoveredbygelextractionkits.
Recoveredfragmentsweredilutedto100ng/μlinTEbuffer.
Thevectorfragmentsandampliconsweremixedinonereactiontube.
Thelargefragment(1μl),smallfrag-ment(2μl),leftarm(2μl)andrightarm(2μl)weremixedwith1μlof10*ExoIIIbufferand1μlofsterilewater.
Thetubewaspre-chilledonicefor2min,andthen1μlofExoIII(20units)wasaddedtothetubeandgentlymixed.
Aftera1hincubationat0°C,1μlof0.
5MEDTAwasaddedtostopthereaction.
Theenzymewasinactivatedat65°Cfor5min.
Aftercooling,themixturewastransformedintoE.
coliDH5αandculturedat37°Covernight.
PositivetransformantswereidentifiedbycolonyPCRandsequencing.
SomaticcellgenetargetingSomaticcellgenetargetingwasconductedasdescribedpreviously[5].
Briefly,thetargetingAAVswerepackagedin293TcellsbytransfectingequalamountsofSirT1orHDAC2KOtargetingvector,pHelperandpRCplasmids(1mgeach).
After72h,scrapedthetransfectedcellsandsuspendedthecellsintosterilephosphate-bufferedsaline,thenfreezedandthawedthepelletthreecycles,finallyspinedthelysatetoremovecelldebrisanddividedthesupernatantcontainingrAAVintoseveralaliquotsandfrozenat-80°C.
HCT116orDLD1cellswereinfectedwiththeSirT1orHDAC2targetingvirusesandselectedwithgeneticinfortwoweeks.
Thegeneticin-resistantcloneswerethenscreenedforhom-ologousrecombinationbygenomicPCRwithprimersderivedfromtheneomycinresistancegene(50-GTTGTGCCCAGTCATAGCCG-30)andtheupstreamregionofthelefthomologousarm(SirT1:50-GAAAGTTCCCCACATCTGCT-30;HDAC2:50-TGCTCCAATCTTCCAGTGTCT-30).
Positivecloneswereconfirmedbygen-omicPCR,withprimersderivedfromtheneomycinre-sistancegene(50-TCTGGATTCATCGACTGTGG-30)andthedownstreamregionoftherighthomolo-gousarm(SirT1:50-TCACTCCTCCAGGGCTAAAA-30;HDAC250-CACCTAGGAACAGCCTTTGC-30).
Cor-rectlytargetedcloneswereinfectedwithadenovirusesexpressingCre-recombinasetodeletetheselectabledrugmarker.
Toselectcloneswithsuccessfuldeletionoftheselectabledrugmarker,genomicPCRwasemployedtoamplifyanapproximate250bpfragmentinwhichtheLoxPsitewasinserted,usingspecificprimers(SirT1:50-TAGGTGTGTGTCGCATCCAT-30and50-CCTGTTCCAGCGTGTCTATGT-30;HDAC2:50-CATGGCGTACAGTCAAGGAG-30and50-CAAATGTCGGTCCCTCCTC-30).
TheheterozygousKOcloneswereinfectedwiththesametargetingvirustotargetthesecondalleleandtheneomycinresistancegenewasexcisedasdescribedearl-ier.
FinalconfirmationinthegenerationoftheKOcelllineswasdoneusingwesternblotting.
CompetinginterestsTheauthorsdeclaretheyhavenocompetinginterests.
Authors'contributionsRDconceivedtheprojectanddesignedtheexperimentsalongwithYL.
YL,HZandSLperformedthelaboratoryworkandanalyzedtheresults.
XZandRDwrotethemanuscript.
Allauthorsreadandapprovedthefinalmanuscript.
AcknowledgementsThisworkwassupportedbygrantsfromtheNationalBasicResearchProgramofChina(2011CB944404),theNationalNaturalScienceFoundationofChina(30971499),theTrans-CenturyTrainingProgrammeFoundationforLiuetal.
BMCBiotechnology2012,12:71Page6of7http://www.
biomedcentral.
com/1472-6750/12/71theTalentsbytheStateEducationCommission(NCET-10-0655),andtheFundamentalResearchFundsfortheCentralUniversities(204275771).
Received:21June2012Accepted:2October2012Published:9October2012References1.
SedivyJM,DutriauxA:Genetargetingandsomaticcellgenetics–arebirthoracomingofageTrendsGenet1999,15(3):88–90.
2.
HirataR,ChamberlainJ,DongR,RussellDW:Targetedtransgeneinsertionintohumanchromosomesbyadeno-associatedvirusvectors.
NatBiotechnol2002,20(7):735–738.
3.
PorteusMH,CathomenT,WeitzmanMD,BaltimoreD:Efficientgenetargetingmediatedbyadeno-associatedvirusandDNAdouble-strandbreaks.
MolCellBiol2003,23(10):3558–3565.
4.
ZhangX,GuoC,ChenY,ShulhaHP,SchnetzMP,LaFramboiseT,BartelsCF,MarkowitzS,WengZ,ScacheriPC,etal:Epitopetaggingofendogenousproteinsforgenome-wideChIP-chipstudies.
NatMethods2008,5(2):163–165.
5.
ZhangP,ZhaoY,ZhuX,SedwickD,ZhangX,WangZ:Cross-talkbetweenphospho-STAT3andPLCgamma1playsacriticalroleincolorectaltumorigenesis.
MolCancerRes2011,9(10):1418–1428.
6.
RagoC,VogelsteinB,BunzF:Geneticknockoutsandknockinsinhumansomaticcells.
NatProtocols2007,2(11):2734–2746.
7.
LiuP,JenkinsNA,CopelandNG:Ahighlyefficientrecombineering-basedmethodforgeneratingconditionalknockoutmutations.
GenomeRes2003,13(3):476–484.
8.
AngrandPO,DaigleN,vanderHoevenF,ScholerHR,StewartAF:SimplifiedgenerationoftargetingconstructsusingETrecombination.
NucleicAcidsRes1999,27(17):e16.
9.
ZhangY,BuchholzF,MuyrersJP,StewartAF:AnewlogicforDNAengineeringusingrecombinationinEscherichiacoli.
NatGenet1998,20(2):123–128.
10.
LiC,WenA,ShenB,LuJ,HuangY,ChangY:FastCloning:ahighlysimplified,purification-free,sequence-andligation-independentPCRcloningmethod.
BMCBiotechnol2011,11:92.
11.
LiMZ,ElledgeSJ:SLIC:amethodforsequence-andligation-independentcloning.
MethodsMolBiol2012,852:51–59.
12.
Geu-FloresF,Nour-EldinHH,NielsenMT,HalkierBA:USERfusion:arapidandefficientmethodforsimultaneousfusionandcloningofmultiplePCRproducts.
NucleicAcidsRes2007,35(7):e55.
13.
DuR,LiS,ZhangX:AmodifiedplasmidvectorpCMV-3Tag-LICforrapid,reliable,ligation-independentcloningofpolymerasechainreactionproducts.
AnalBiochem2011,408(2):357–359.
14.
GibsonDG,YoungL,ChuangRY,VenterJC,HutchisonCA3rd:SmithHO:EnzymaticassemblyofDNAmoleculesuptoseveralhundredkilobases.
NatMethods2009,6(5):343–345.
15.
GibsonDG,SmithHO,HutchisonCA3rd,VenterJC,MerrymanC:Chemicalsynthesisofthemousemitochondrialgenome.
NatMethods2010,7(11):901–903.
16.
GibsonDG:SynthesisofDNAfragmentsinyeastbyone-stepassemblyofoverlappingoligonucleotides.
NucleicAcidsRes2009,37(20):6984–6990.
17.
HsiaoK:ExonucleaseIIIinducedligase-freedirectionalsubcloningofPCRproducts.
NucleicAcidsRes1993,21(23):5528–5529.
doi:10.
1186/1472-6750-12-71Citethisarticleas:Liuetal.
:Aone-stepcloningmethodfortheconstructionofsomaticcellgenetargetingvectors:applicationtoproductionofhumanknockoutcelllines.
BMCBiotechnology201212:71.
SubmityournextmanuscripttoBioMedCentralandtakefulladvantageof:ConvenientonlinesubmissionThoroughpeerreviewNospaceconstraintsorcolorgurechargesImmediatepublicationonacceptanceInclusioninPubMed,CAS,ScopusandGoogleScholarResearchwhichisfreelyavailableforredistributionSubmityourmanuscriptatwww.
biomedcentral.
com/submitLiuetal.
BMCBiotechnology2012,12:71Page7of7http://www.
biomedcentral.
com/1472-6750/12/71
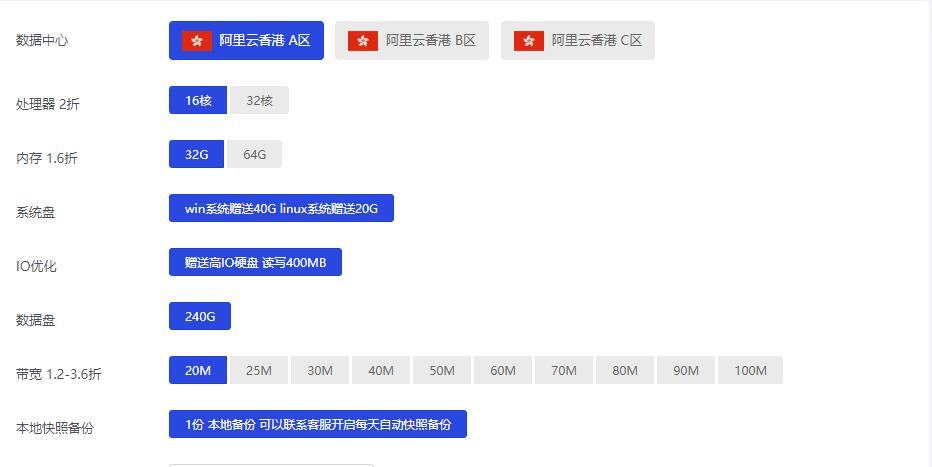
阿里云香港 16核32G 20M 999元/月
阿里云香港配置图提速啦是成立于2012年的十分老牌的一个商家这次给大家评测的是 阿里云香港 16核32G 20M 这款产品,单单说价格上就是十分的离谱原价8631元/月的现价只要 999元 而且还有个8折循环优惠。废话不多说直接进入正题。优惠时间 2021年8月20日-2021年9月20日 优惠码 wn789 8折优惠阿里云香港BGP专线 16核32G 10M带宽 优惠购买 399元购买链接阿里云...
Sharktech:鲨鱼机房1Gbps无限流量美国服务器;丹佛$49/月起,洛杉矶$59/月起
sharktech怎么样?sharktech鲨鱼机房(Sharktech)我们也叫它SK机房,是一家成立于2003年的老牌国外主机商,提供的产品包括独立服务器租用、VPS主机等,自营机房在美国洛杉矶、丹佛、芝加哥和荷兰阿姆斯特丹等,主打高防产品,独立服务器免费提供60Gbps/48Mpps攻击防御。机房提供1-10Gbps带宽不限流量服务器,最低丹佛/荷兰机房每月49美元起,洛杉矶机房最低59美元...
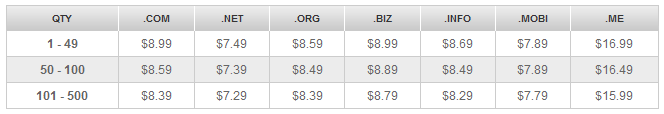
NameSilo域名优惠码活动
NameSilo是通过之前的感恩节优惠活动中认识到这家注册商的,于是今天早上花了点时间专门了解了NameSilo优惠码和商家的详细信息。该商家只销售域名,他们家的域名销售价格还是中规中矩的,没有像godaddy域名标价和使用优惠之后的价格悬殊很大,而且其特色就是该域名平台提供免费的域名停放、免费隐私保护等功能。namesilo新注册域名价格列表,NameSilo官方网站:www.namesilo....
http代理服务器软件为你推荐
-
操作httpthinksns在thinksns 中集成UCenter过程中,按照教程做的,但是出现 通信失败,请问如何处理,谢谢iproute网关怎么设置?苹果appstore宕机apple id登陆不了app store怎么办搜狗360因为我做百度,搜狗,360,神马竞价推广已经有一年多了,所以请问下,网上有哪些平台可以接竞价的单呢?重庆网站制作我想做个网站,我是重庆的人。想在本地找个做网站的公司,请教一下在重庆那个公司比较好一点,,,,谢谢银花珠树晓来看关于下雪景的诗句泉州商标注册请问泉州商标注册要怎么办理?在哪办理?discuz伪静态discuz怎么才能把专题目录也实现伪静态的方法详解团购程序什么是团购 团购的目的与流程