collectedsourceforge.jp
sourceforge.jp 时间:2021-04-05 阅读:()
CrystalstructureoftRNAm1A58methyltransferaseTrmIfromAquifexaeolicusincomplexwithS-adenosyl-L-methionineMitsuoKurataniTatsuoYanagisawaRyoheiIshiiMichiyoMatsunoShu-YiSiKazushigeKatsuraRyokoUshikoshi-NakayamaRieShibataMikakoShirouzuYoshitakaBesshoShigeyukiYokoyamaReceived:4March2014/Accepted:28May2014/Publishedonline:4June2014TheAuthor(s)2014.
ThisarticleispublishedwithopenaccessatSpringerlink.
comAbstractTheN1-methyladenosineresidueatposition58oftRNAisfoundinthethreedomainsoflife,andcon-tributestothestabilityofthethree-dimensionalL-shapedtRNAstructure.
Inthermophilicbacteria,thismodicationisimportantforthermaladaptation,andiscatalyzedbythetRNAm1A58methyltransferaseTrmI,usingS-adenosyl-L-methionine(AdoMet)asthemethyldonor.
Wepresentthe2.
2AcrystalstructureofTrmIfromtheextremelyther-mophilicbacteriumAquifexaeolicus,incomplexwithAdoMet.
Therearefourmoleculesperasymmetricunit,andtheyformatetramer.
BasedonacomparisonoftheAdoMetbindingmodeofA.
aeolicusTrmItothoseoftheThermusthermophilusandPyrococcusabyssiTrmIs,wediscusstheirsimilaritiesanddifferences.
AlthoughthebindingmodestotheN6aminogroupoftheadeninemoietyofAdoMetaresimilar,usingthesidechainsofacidicresiduesaswellashydrogenbonds,thepositionsoftheaminoacidresiduesinvolvedinbindingarediverseamongtheTrmIsfromA.
aeolicus,T.
thermophilus,andP.
abyssi.
KeywordsAdoMettRNAmodicationenzymeMethylationX-raycrystalstructureStructuralgenomicsAbbreviationsAdoMetS-Adenosyl-L-methionineAdoHcyS-Adenosyl-L-homocysteinem1AN1-Methyladenosinem1GN1-Methylguanosinem1IN1-Methylinosinem3CN3-Methylcytidinem3WN3-Methylpseudouridinem3UN3-MethyluridineIPTGIsopropyl-1-thio-b-D-galactopyranosidetRNATransferRNAPDBProteinDataBankRMSDRoot-mean-square-deviationIntroductionPosttranscriptionalmodicationsalterthecharacteristicsoftRNAsinvariousmanners,tone-tunetheirfunctions.
ThemodiednucleosideN1-methyladenosineisfoundatfourpositions:position9ofmammalianmitochondrialtRNAs,position14ofmammaliancytoplasmictRNAPhe,position22oftRNAinsomebacteria,andposition58oftRNAinM.
KurataniT.
YanagisawaR.
IshiiM.
MatsunoS.
-Y.
SiK.
KatsuraR.
Ushikoshi-NakayamaR.
ShibataM.
ShirouzuY.
BesshoS.
Yokoyama(&)RIKENGenomicSciencesCenter,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,Japane-mail:yokoyama@riken.
jpM.
KurataniT.
YanagisawaS.
YokoyamaRIKENStructuralBiologyLaboratory,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,JapanR.
IshiiDepartmentofBiophysicsandBiochemistry,GraduateSchoolofScience,TheUniversityofTokyo,2-11-16Yayoi,Bunkyo-ku,Tokyo113-0032,JapanK.
KatsuraM.
ShirouzuDivisionofStructuralandSyntheticBiology,RIKENCenterforLifeScienceTechnologies,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,JapanY.
BesshoRIKENSPring-8Center,1-1-1Kouto,Sayo,Hyogo679-5148,Japan123JStructFunctGenomics(2014)15:173–180DOI10.
1007/s10969-014-9183-0thethreedomainsoflife[1].
TheN1-methylationofadenosineabrogatesitsabilitytoformastandardWatson–Crickbasepair,asalsofoundwithm1G,m1I,m3C,m3U,andm3W.
Indeed,reversetranscriptasesreadm1Awithverylowefciency,andthoseintheHIV-1andMolonymurineleukemiavirusesutilizethehost'stRNAbearingm1Afortheirreplication[2–5].
Intheabsenceofthem1A9modication,mammalianmitochondrialtRNALyscouldadoptanextendedhairpinstructurethatisunproductiveintranslation,sinceanundesiredbasepairbetweenA9andU64istolerated[6,7].
Inyeast,thestrainwithadefectivem1A58modicationisnonviable,becausetheinitiatortRNAMetisdegraded[8].
InthenativeyeasttRNA,them1A58oftheinitiatortRNAMetformsthereverseHoogsteenbasepairwithA54,whichincreasesthestabilityofthethree-dimensionalstructure,whilethem1A58intheother19tRNAsformsthereverseHoogsteenbasepairwithT54[9–11].
InthethermophilicbacteriumThermusthermophilus,inactivationofthetrmIgeneresultsinathermosensitivephenotype,suggestingthatthem1A58modicationisimportantforboththermaladaptationandtRNAstability[12].
Them1A58residuewasanalyzedbyNMRandIRspectralstudies,whichconsideredthe1H,13C,and15Nchemicalshifts,thecon-sistencyofthesugarpuckerandglycosidicconformationswiththoseoftheX-raystructure,andthecharacterofthebondbetweentheC6andN6atoms[13,14].
Basedontheresults,them1A58residueinthenativetRNAwasdeducedtobefullyprotonated,withitschargeprobablydislocalizedfromthequaternaryN1atomtowardtheC6,C5,andC4atoms.
Theprotonatedstateofthem1A58residueischaracteristicoftheMg2-boundnativestate,andthepartialchargeinthetRNAelbowregionmayaffectitsinteractionwiththetranslationalmachinery[13,14].
Therefore,them1A58modicationoftRNAisimportantforstabilizingtheL-shapedstructureandforefcienttranslation[15].
Themethylgroupofm1A58istransferredfromthemethyldonorS-adenosyl-L-methionine(AdoMet)bytheTrmIhomotetramerinbacteriaandarchaea,andbytheTrm6/Trm61a2/b2heterotetramercomplexineukaryotes[8,12].
ThecoordinatedstructuralgenomicsprojectsonproteinsfromMycobacteriumtuberculosisdeterminedtherststructureofTrmI,astheconservedhypotheticalmethyltransferaseRv2118c[16].
Atthesametime,aninsilicofoldpredictionstudywasreported[17].
Subse-quently,thecrystalstructureofthecatalyticdomain(res-idues70–250)oftheTrmItetramerfromPyrococcusabyssirevealeditsmechanismofthermalstabilization,usingintersubunitdisuldebonds[18].
Thecrystalstruc-tureofTrmIfromT.
thermophilus[19]wasdeterminedandcomplementedbybiophysicalcharacterizations,whichrevealedthetRNAbindingstoichiometryperTrmItetramer[19].
Thecrystalstructureoffull-lengthTrmIfromP.
abyssiwasreportedwithfurtherbiochemicalcharacterizationoftheregionspecicities[20].
Presently,eightPDBdatasetsfromsixspeciesareavailable,andtheirstructuralarchitectureshavebeencompared[21].
Com-prehensivestructuralgenomicsprojectsonaspecicorganism,typiedbythatonM.
tuberculosis[22],haveprovidedthestructuralbasistocharacterizethebiologicalfunctionsoftheproteome,includingconservedproteinswithunknownfunctions.
Ontheotherhand,comparativeanalysesoflargenumbersoforthologousandhomologousstructures,includingsomeacquiredbyhigh-throughputcapabilityandsuccessfulstructuralgenomics[23–25],willleadtofurtherunderstandingofthestructure–functionrelationshipsofproteinsandfacilitateapplications,includingproteinengineeringanddrugdesign.
Here,wereportthecrystalstructureofTrmIfromAquifexaeolicusinthecomplexwithAdoMet,determinedat2.
2Aresolu-tion.
TheoveralltetramericarchitectureisquitesimilartothestructuresofTrmIsfromotherspecies[21].
WeexaminedthesimilaritiesanddifferencesintheAdoMetrecognitionbyA.
aeolicusTrmI,ascomparedtothosebytheTrmIsfromT.
thermophilusandP.
abyssi.
MaterialsandmethodsCloning,expression,andpuricationofA.
aeolicusTrmITheaq_311gene,encodingtheA.
aeolicusTrmIprotein(gi:15605836)comprising248residues,wasampliedbyPCRusingA.
aeolicusVF5genomicDNAandclonedintothepET-21aexpressionvector(MerckNovagen,Darms-tadt,Germany).
TheexpressionvectorwastransformedintotheE.
coliRosettaTM(DE3)strain(MerckNovagen).
Thecellswereculturedat37°CinLBmedium,supple-mentedwith30lg/mlchloramphenicoland50lg/mlampicillin.
Theproteinexpressionwasinducedby0.
5mMIPTG.
Followinganovernightincubation,thecellswereharvestedbycentrifugationandstoredat-80°C.
Thecellswereresuspendedin20mMTris–HClbuffer(pH8.
0),containing300mMNaCl,5mMMgCl2,0.
5mMEDTA,and1mMDTT,andwerelysedbysonicationonice.
Thecelllysatewasheat-treatedat70°Cfor30mintodenaturemostoftheE.
coliproteins,andwascentrifugedat15,0009gfor20minat4°C.
Thesupernatantwasdesal-tedbydialysisagainst20mMTris–HClbuffer(pH8.
0)containing1mMDTT,andappliedtoaHiTrapQcolumn(GEHealthcareBiosciences),equilibratedwiththesamebuffer.
Theproteinwaselutedwithalineargradient(0–1.
0M)ofNaCl,andthetargetfractions,whichelutedaround0.
4MNaCl,werecollected.
Ammoniumsulfate174M.
Kuratanietal.
123wasaddedtothesample,whichwasappliedtoaResourcePHEcolumn(GEHealthcareBiosciences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing1.
2Mammoniumsulfateand1mMDTT,andwaselutedwithadecreasinglinear(1.
2–0M)gradientofammoniumsulfate.
Thetargetfractions,whichwereelutedin0.
6–0.
3Mammoniumsulfate,werecollectedanddesaltedbydialysis.
ThesamplewasappliedtoaMonoScolumn(GEHealthcareBiosciences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing1mMDTT,andwaselutedbyalinear(0–1.
0M)gradientofNaCl.
Thefractionthatelutedat0.
3MwasconcentratedandappliedtoaHiLoad16/60Superdex75pgcolumn(GEHealthcareBiosci-ences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing150mMNaCland1mMDTT.
Thegelltra-tionelutionproleshowedonepeakat50ml,whichcor-respondsto0.
41columnvolumes.
Theproteinsamplewasconcentratedto15mg/mlbyultraltration.
TheproteinpuricationwasanalyzedbySDS-PAGE.
Theelectro-phoreticmobilityofA.
aeolicusTrmIisalmostthesameasthatofamarker(29kDa),inagreementwithitstheoreticalmolecularweight(28.
7kDa).
Thenalyieldwas2.
2mg/lofculture.
CrystallizationanddatacollectionTheA.
aeolicusTrmIproteinat10–12mg/mlconcentra-tions,in20mMTris–HClbuffer(pH8.
0)containing150mMNaCl,1mMDTT,and2mMAdoMet,wasusedforcrystallization.
Initialcrystallizationscreeningwasperformedin1:1sitting-dropvapor-diffusionreactionsat20°C,bymixing1llproteinsolutionwith1llreservoirsolution.
Thecrystalsweregrownin0.
1MTris–HClbuffer(pH8.
4)and20%ethanol.
Thecrystalsweretransferredto0.
1MTris–HClbuffer(pH8.
4),20%eth-anol,and35%ethyleneglycolforcryoprotection,priortoash-coolinginliquidnitrogenfordatacollection.
ThenativedatasetwascollectedonbeamlineBL41XUatSPring-8(Table1).
Datacollectedfromasinglecrystalat100KwereprocessedwiththeHKL2000program[26].
StructuresolutionandrenementThephasewasdeterminedbythemolecularreplacementmethod,usingthecoordinatesofTrmIfromThermotogamaritima(PDBID:1O54)asthestartingmodel,withtheprogramMOLREP[27].
ThemodelwascompletedusingiterativecyclesofmanualrebuildinginCoot[28]andcomputationalrenementat2.
2AinRefmac5[29](Table1).
Table1X-raydataandrenementstatisticsA.
aeolicusTrmICrystalparametersSpacegroupP212121Celldimensionsa,b,c(A)69.
8,97.
2,212.
7a,b,c(°)90,90,90Matthewscoefcient(A3/Da)3.
14Solventcontent(%)60.
9DatacollectionWavelength(A)1.
00Resolution(A)50–2.
2(2.
28–2.
2)Rsym(%)a3.
3(43.
9)No.
ofuniquereections68,373No.
ofreectionsinRfreeset3,597Meanredundancy6.
6(3.
6)Overallcompleteness(%)96.
7(77.
0)MeanI/r23.
7(4.
1)RenementresidualsResolution(A)50–2.
2(2.
26–2.
2)Rfree(%)b23.
0(26.
3)Rwork(%)b19.
4(20.
7)Completeness(%)96.
8(75.
3)ModelqualityRMSDbondlengths(A)0.
008RMSDbondangles(°)1.
1MolprobityRamachandrandistributionMostfavored(%)98.
6Allowed(%)1.
4Disallowed(%)0.
0MeanmainchainB-factor(A2)26.
5MeanoverallB-factor(A2)31.
7MeanligandB-factor(A2)32.
3MeansolventB-factor(A2)31.
2ModelcontentsProtomersinASU4Proteinresidues2–248Ligands4AdoMetNo.
ofproteinatoms8,092No.
ofligandatoms108No.
ofwatermolecules537PDBaccessioncode2YVLRMSDroot-mean-square-deviation,ASUasymmetricunitaRsym=RhklRj|Ij(hkl)-\Ij(hkl)[|/RhklRjI(hkl),whereIj(hkl)and\Ij(hkl)[aretheintensityofmeasurementjandthemeanintensityforthereectionwithindiceshkl,respectivelybRwork,free=R|Fobs-kFcalc|/RhklFobs,wherekisascalefactor,andthecrystallographicR-factoriscalculatedincluding(Rwork)andexcluding(Rfree)reections.
Ineachrenement,freereectionsconsistof5%ofthetotalreectionsCrystalstructureoftRNAm1A58methyltransferaseTrmI175123StructurevalidationanddepositionThestructurevalidationofthemodelissummarizedinTable1.
TheatomiccoordinatesandstructurefactorshavebeendepositedintheProteinDataBank,undertheaccessioncode2YVL.
SedimentationvelocityultracentrifugationanalysisTheA.
aeolicusTrmIprotein,ata1mg/mlconcentrationin20mMTris–HClbuffer(pH8.
0)containing150mMNaCland1mMDTT,wasanalyzedbyultracentrifugationat20°C,inaProteomeLabXL-Iultracentrifuge(BeckmanCoulter)withtheAn-60Tianalyticalrotor.
Thesamplewasultracentrifugedat40,000rpm,andtheabsorbanceat280nmwasmeasured.
Thedatawereanalyzedandthedistributionc(M)wascalculatedbySedt[30].
ResultsanddiscussionThecrystalstructureofA.
aeolicusTrmIwasdeterminedat2.
2Aresolutionbythemolecularreplacementmethod,andwasrenedtoRworkandRfreefactorsof19.
6and23.
0%,respectively(Table1).
Theasymmetricunitcontainsfourprotomers(A–D)(Fig.
1a)andfourAdoMetmolecules.
Theelectrondensitywasinterpretablefor247residues(Asn2–Thr248).
TheA.
aeolicusTrmIprotomer(Fig.
1b)consistsofthesmallN-terminaldomain(residues2–58)andtheC-terminalmethyltransferasedomain(residues72–248),whichareconnectedbyana-helicallinker(res-idues59–71).
TheN-terminaldomainformsasmallbsandwich(Fig.
1b),inwhichthebsheetb2–b1–b6–b5stacksonthebhairpinb3–b4,alongwiththesmall310-helixg1.
TheC-terminaldomainadoptsthetypicaltypeImethyltransferasefold,withacentralseven-strandedbsheetwiththetopologyb9–b8–b7–b10–b11–b14–b12,ankedbyahelicesonbothsides(Fig.
1b).
Asreportedpreviously[19],thelongbstrandb12,inwhichtheheadinteractswithb13,ischaracteristicofTrmIamongthetypeImethyltransferases,anditprovidesasurfacefortetramerization.
WeanalyzedtheoligomericstateofA.
aeolicusTrmIinsolutionbysedimentationvelocityultracentrifugation.
ThegelltrationelutionproleofA.
aeolicusTrmIshowedonepeakbetweentheIgG(158kDa)andhumanalbumin(66kDa)markers.
SincethetheoreticalmolecularweightofA.
aeolicusTrmIis28.
7kDa,TrmIissuggestedtoexistastetramer(114.
8kDa)insolution.
Theultracentrifugationanalysis,using1mg/mlA.
aeolicusTrmI,showedonepeakat110kDa(Fig.
1c),whichconrmedthatitistet-ramericinsolution.
ThemethyldonorAdoMetisboundintheC-terminaldomainoftheprotein(Fig.
1b).
A.
aeolicusTrmIrecog-nizesAdoMetbyhydrogenbondsfromitsmain-chainandside-chainatomsaswellaswater-mediatedhydrogenbonds(Fig.
1d),inasimilarmannertoT.
thermophilusTrmI(Fig.
1e)[19]andP.
abyssiTrmI(Fig.
1f)[20].
TheN1atomoftheadeninemoietyhydrogenbondswiththemainchainamidenitrogenofPhe149(3.
0A)ofA.
aeoli-cusTrmI.
TheN6aminogrouphydrogenbondswiththesidechainsofAsp148(2.
9A)andTyr172(3.
1A)(Fig.
1d).
TheN7atominteractswithawater(wat1inFig.
1d;2.
7A),whichparticipatesinahydrogenbondingnetworkinvolvingGlu168,Tyr172,andawater(Wat2inFig.
1d).
Inadditiontothesefourhydrogenbonds,theadenineringformsaT-stackinginteractionwiththesidechainofPhe98,whichisxedbyp–pstackingwiththatofPhe149(Fig.
1d).
ThetwohydroxylgroupsoftheribosemoietyofAdoMetinteractwiththesidechainofGlu120(2.
6and2.
7A;Fig.
1d).
ThemethioninemoietyofAdo-Metformsthreehydrogenbonds(Fig.
1d):itsaminogrouphydrogenbondswiththesidechainofAsp165(2.
9A),anditscarboxylgrouphydrogenbondswiththemain-chainamidenitrogenatomsofAla104(3.
1A)andLeu105(2.
8A;Fig.
1d).
WecomparedthestructureofA.
aeolicusTrmItothoseofT.
thermophilusTrmIincomplexwithS-adenosyl-L-homocysteine(AdoHcy)(Fig.
1e)andP.
abyssiTrmIincomplexwithAdoMet(Fig.
1f),andexaminedthecon-servationofresiduesinvolvedinAdoMetbinding.
A.
ae-olicus,T.
thermophilus,andP.
abyssiallliveinhigh-temperatureenvironments.
TheN6aminogroupoftheadeninemoietyisrecognizedindiversemannersbythevariousTrmIstructures.
Thesidechainsofthreeaminoacidresidues(Asp148,Lys150,andTyr172inA.
aeolicusTrmI;Fig.
2)surroundtheN6aminogroup,andtheunderlinedresiduesareinvolvedinAdoMetbinding.
Inthecorrespondingthreepositions,T.
thermophilusTrmIhasLys153,Glu155,andVal177,whileP.
abyssiTrmIhasAsp153,Tyr155,andVal176(Fig.
2).
Asp148andTyr172ofA.
aeolicusTrmIformdirecthydrogenbondswiththeN6aminogroup(Fig.
1d).
InT.
thermophilusTrmI(Fig.
1e)[19],thesidechainofGlu155andawatermol-eculeformhydrogenbondswiththeN6aminogroup,andtheseareapparentlyequivalenttothetwohydrogenbondsformedbetweenthismoietyandA.
aeolicusTrmI.
How-ever,Glu155ofT.
thermophilusTrmIislocatedatadif-ferentpositionthanAsp148ofA.
aeolicusTrmIintheaminoacidalignment(Fig.
2).
Ontheotherhand,P.
abyssiTrmIformsonlyonehydrogenbondbyAsp153(Fig.
1f)[20],whichislocatedatthesamepositionasAsp148ofA.
aeolicusTrmI(Fig.
2).
ThedistancesfromtheN6aminogrouptothethreewatermolecules(Fig.
1f)are3.
8,3.
9,and5.
5A,respectively.
TheN7atomofAdoMetisbound176M.
Kuratanietal.
123toTrmIbyonewater-mediatedhydrogenbond,althoughthesidechainsinvolvedinitscoordinationdiffer(Fig.
1d–f).
WeexaminedtheconservationofthesethreeaminoacidresiduesintheotherTrmIswithavailablestructures(Fig.
2).
Asp148ofA.
aeolicusTrmIisconservedinP.
abyssi,T.
maritima,M.
tuberculosis,andHomosapiens.
Lys153inT.
thermophilusTrmIisanexception.
Lys150ofA.
aeolicusTrmIisnotconservedanddoesnotinteractwithAdoMet.
Glu155ofT.
thermophilusTrmIandTyr155ofP.
abyssiTrmIparticipateintheAdoMetbindingindistinctmanners.
Bycontrast,Ser175ofH.
sapiensTrm61(PDBID2B25)is4.
7AawayfromtheN6aminogroup,anddoesnotinteractwithAdoMet.
TheTrmIsfromT.
maritima(PDBID1O54)andM.
tuberculosis(PDBID1I9G)haveSerandAlaresidues,respectively.
AlthoughtheonlyavailablestructureofT.
maritimaTrmIisthesubstrate-freeform,theSerresidueislocatedtoofarawaytointeractwithAdoMet.
Tyr172ofA.
aeolicusTrmIisconservedinM.
maritimaTrmI,andisreplacedbyabcdefFig.
1CrystalstructureofA.
aeolicusTrmIincomplexwithAdoMet.
aThetetramericstructureofA.
aeolicusTrmI.
Thefourprotomersarecoloredpink,cyan,purple,andgreen.
bProtomerstructureofA.
aeolicusTrmI.
TheN-terminaldomain,thelinkerhelix,andtheC-terminaldomainarecoloredpink,purple,andcyan,respectively.
Thesecondarystructuresarelabeled.
cThecalculateddistributionsc(M)bySedt[30].
d–fBall-and-stickrepresentationsofAdoMetbindingbyA.
aeolicusTrmI(chainA)(d),T.
thermo-philusTrmI[19](chainA)(e),andP.
abyssiTrmI[20](chainA)(f).
ThethreeaminoacidresiduessurroundingtheN6aminogroupofAdoMetarelabeledwithorangerectangles.
Hydrogenbondsaredepictedbydottedlineswiththeirdistances(A).
ThegureswerecreatedusingCueMol(http://cuemol.
sourceforge.
jp/en/)CrystalstructureoftRNAm1A58methyltransferaseTrmI177123aliphaticresiduesinT.
thermophilusTrmI,M.
tuberculosisTrmI,andP.
abyssiTrmI,andbyThr175inH.
sapiensTrm61.
ThesidechainofThr175is5.
3AawayfromtheN6aminogroup(PDBID2B25),anditshydroxylgroupdoesnotcoordinateanywatermolecules.
TwootherdifferencesarethepresenceofT-stackingbyPhe98inA.
aeolicusTrmI(Fig.
1d),andtheadditionalhydrogenbondtotheribosemoietybyHis130,observedinT.
thermophilusTrmI(Fig.
1e).
ThepresenceofPhe98isuniquetoA.
aeolicusTrmI(Fig.
2),whereastheHisresi-dueatthecorrespondingpositionofHis130inT.
thermo-philusTrmIissharedbytheM.
tuberculosisandH.
sapiensTrmIs.
ThebindingmodesfortheotherpartofAdoMetarequitesimilar.
TheyinvolvethehydrogenbondbetweenN1oftheadeninemoietytothemain-chainamidenitrogen,theinteractionbetweenthetwohydroxylgroupsoftheribosemoietyandtheGlusidechain,andthebindingtotheaminoandcarboxylgroupsofthemethioninemoiety.
Forthemethioninemoiety,theAsp165thatinteractswiththeaminogroupisconserved,andtheconformationsofthemain-chainamidegroupsthatinteractwiththecarboxylgrouparequitesimilar.
SummaryWehavedeterminedthecrystalstructureofTrmIfromtheextremelythermophilicbacteriumA.
aeolicus,andexam-inedthesimilaritiesanddifferencesregardingtherecog-nitionofthemethyldonorAdoMetbyA.
aeolicusTrmIandtheT.
thermophilusandP.
abyssiTrmIs.
Therecog-nitionoftheN6aminogroupoftheadeninemoietywasthemostdiversefeature.
ThreeresiduesarelocatedwheretheirsidechainscanapproachtheN6aminogroup.
Ourcom-parativestructuralanalysesrevealedthedifferentstrategiesadoptedbythesethermophilicspeciestoformhydrogenbondsbyusingacidicandhydrophilicsidechains.
ItisintriguingthattheuniversalsubstrateAdoMethasbecomeFig.
2SequencealignmentofTrmIproteins.
TheaminoacidsequencesofA.
aeolicusTrmI(AaTrmI),T.
maritimaTrmI(TmTrmI),T.
thermophilusTrmI(TtTrmI),M.
tuberculosisTrmI(MtTrmI),P.
abyssiTrmI(PaTrmI),andH.
sapiensTrm61(HsTrm61)werealignedwithClustalX2.
1[31].
Identicalresiduesarewhiteinaredbackground.
Similarresiduesareredinbluerectangles.
ThesecondarystructuresofA.
aeolicusTrmI(PDB:2YVL)andH.
sapiensTrm61(PDB:2B25)areshownatthetopandbottom,respectively.
ThethreeaminoacidresidueswithsidechainslocatedneartheN6aminogroupofAdoMetareindicatedbyorangetriangles.
ThegurewasdepictedbyESPript[32]178M.
Kuratanietal.
123recognizedindistinctmannersbytheTrmIscatalyzingthetRNAm1A58modication,duringthecourseofevolution.
AcknowledgmentsTheauthorsthankthestaffatbeamlineBL41XUofSPring-8.
WealsothankTomokoNakayama,MihokoIizuka,ShingoSaito,TaichiMishima,KaoriYamanaka,KojiroAke,TakakoImada,KazukoMaekawa,ChieHori-Takemoto,TomomiKamo-Uchikubo,RyogoAkasaka,ChizuKuroishi,andTakahoTe-radaforclericalassistance,performingstructuralgenomics/proteo-micsprojects,facilitymaintenance,andexperimentalassistance.
ThisresearchwassupportedbyagrantfromtheDaiichi-SankyoFoun-dationofLifeScience(12-039toY.
B.
),Grants-in-AidforScienticResearchinPriorityAreasfromtheMinistryofEducation,Culture,Sports,ScienceandTechnology(MEXT)ofJapan(toS.
Y.
),andbytheRIKENStructuralGenomics/ProteomicsInitiative(RSGI)intheNationalProjectonProteinStructuralandFunctionalAnalyses,MEXTofJapan(toS.
Y.
).
OpenAccessThisarticleisdistributedunderthetermsoftheCreativeCommonsAttributionLicensewhichpermitsanyuse,dis-tribution,andreproductioninanymedium,providedtheoriginalauthor(s)andthesourcearecredited.
References1.
SprinzlM,VassilenkoKS(2005)CompilationoftRNAsequencesandsequencesoftRNAgenes.
NucleicAcidsRes33(Databaseissue):D39–D402.
GilboaE,GoffS,ShieldsA,YoshimuraF,MitraS,BaltimoreD(1979)Invitrosynthesisofa9kbpterminallyredundantDNAcarryingtheinfectivityofMoloneymurineleukemiavirus.
Cell16(4):863–8743.
MadenBE(1990)ThenumerousmodiednucleotidesineukaryoticribosomalRNA.
ProgNucleicAcidResMolBiol39:241–3034.
BurnettBP,McHenryCS(1997)PosttranscriptionalmodicationofretroviralprimersisrequiredforlatestagesofDNAreplica-tion.
ProcNatlAcadSciUSA94(14):7210–72155.
RendaMJ,RosenblattJD,KlimatchevaE,DemeterLM,Bam-baraRA,PlanellesV(2001)MutationofthemethylatedtRNA3LysresidueA58disruptsreversetranscriptionandinhibitsreplicationofhumanimmunodeciencyvirustype1.
JVirol75(20):9671–96786.
HelmM,GiegeR,FlorentzC(1999)AWatson-Crickbase-pair-disruptingmethylgroup(m1A9)issufcientforcloverleaffoldingofhumanmitochondrialtRNALys.
Biochemistry38(40):13338–133467.
HayrapetyanA,Seidu-LarryS,HelmM(2009)FunctionofModiedNucleosidesinRNAstabilization.
In:GrosjeanH(ed)DNAandRNAmodicationenzymes:comparativestructure,mechanism,functions,cellularinteractionsandevolution,chapter37.
LANDESBiosci,USA8.
AndersonJ,PhanL,CuestaR,CarlsonBA,PakM,AsanoK,BjorkGR,TamameM,HinnebuschAG(1998)TheessentialGcd10p-Gcd14pnuclearcomplexisrequiredfor1-methylade-nosinemodicationandmaturationofinitiatormethionyl-tRNA.
GenesDev12(23):3650–36629.
KimSH,SuddathFL,QuigleyGJ,McPhersonA,SussmanJL,WangAH,SeemanNC,RichA(1974)Three-dimensionalter-tiarystructureofyeastphenylalaninetransferRNA.
Science185(4149):435–44010.
RobertusJD,LadnerJE,FinchJT,RhodesD,BrownRS,ClarkBF,KlugA(1974)StructureofyeastphenylalaninetRNAat3Aresolution.
Nature250(467):546–55111.
SchevitzRW,PodjarnyAD,KrishnamachariN,HughesJJ,SiglerPB,SussmanJL(1979)CrystalstructureofaeukaryoticinitiatortRNA.
Nature278(5700):188–19012.
DroogmansL,RooversM,BujnickiJM,TricotC,HartschT,StalonV,GrosjeanH(2003)CloningandcharacterizationoftRNA(m1A58)methyltransferase(TrmI)fromThermusther-mophilusHB27,aproteinrequiredforcellgrowthatextremetemperatures.
NucleicAcidsRes31(8):2148–215613.
Sierzputowska-GraczH,GopalHD,AgrisPF(1986)Compara-tivestructuralanalysisof1-methyladenosine,7-methylguanosine,ethenoadenosineandtheirprotonatedsaltsIV:1H,13C,and15NNMRstudiesatnaturalisotopeabundance.
NucleicAcidsRes14(19):7783–780114.
AgrisPF,Sierzputowska-GraczH,SmithC(1986)TransferRNAcontainssitesoflocalizedpositivecharge:carbonNMRstudiesof[13C]methyl-enrichedEscherichiacoliandyeasttRNAPhe.
Biochemistry25(18):5126–513115.
AndersonJ,DroogmansL(2005)Biosynthesisandfunctionof1-methyladenosineintransferRNA.
In:GrosjeanH(ed)Fine-tuningofRNAfunctionsbymodicationandediting.
Springer,Berlin,pp121–13916.
GuptaA,KumarPH,DineshkumarTK,VarshneyU,SubramanyaHS(2001)CrystalstructureofRv2118c:anAdoMet-dependentmethyltransferasefromMycobacteriumtuberculosisH37Rv.
JMolBiol312(2):381–39117.
BujnickiJM(2001)InsilicoanalysisofthetRNA:m1A58methyltransferasefamily:homology-basedfoldpredictionandidenticationofnewmembersfromEubacteriaandArchaea.
FEBSLett507(2):123–12718.
RooversM,WoutersJ,BujnickiJM,TricotC,StalonV,GrosjeanH,DroogmansL(2004)AprimordialRNAmodicationenzyme:thecaseoftRNA(m1A)methyltransferase.
NucleicAcidsRes32(2):465–47619.
BarraudP,Golinelli-PimpaneauB,AtmaneneC,SanglierS,VanDorsselaerA,DroogmansL,DardelF,TisneC(2008)CrystalstructureofThermusthermophilustRNAm1A58methyltransfer-aseandbiophysicalcharacterizationofitsinteractionwithtRNA.
JMolBiol377(2):535–55020.
GuelorgetA,RooversM,GuerineauV,BarbeyC,LiX,Goli-nelli-PimpaneauB(2010)Insightsintothehyperthermostabilityandunusualregion-specicityofarchaealPyrococcusabyssitRNAm1A57/58methyltransferase.
NucleicAcidsRes38(18):6206–621821.
GuelorgetA,BarraudP,TisneC,Golinelli-PimpaneauB(2011)StructuralcomparisonoftRNAm1A58methyltransferasesrevealeddifferentmolecularstrategiestomaintaintheiroligo-mericarchitectureunderextremeconditions.
BMCStructBiol11:4822.
BakerEN(2007)Structuralgenomicsasanapproachtowardsunderstandingthebiologyoftuberculosis.
JStructFunctGenomics8(2–3):57–6523.
SugaharaM,AsadaY,ShimizuK,YamamotoH,LokanathNK,MizutaniH,BagautdinovB,MatsuuraY,TaketaM,KageyamaY,OnoN,MorikawaY,TanakaY,ShimadaH,NakamotoT,SugaharaM,YamamotoM,KunishimaN(2008)High-through-putcrystallization-to-structurepipelineatRIKENSPring-8Center.
JStructFunctGenomics9(1–4):21–2824.
YokoyamaS,KigawaT,ShirouzuM,MiyanoM,KuramitsuS(2008)RIKENstructuralgenomics/proteomicsinitiative.
Tan-pakushitsuKakusanKoso53(5):632–63725.
TerwilligerTC(2011)Thesuccessofstructuralgenomics.
JStructFunctGenomics12(2):43–44CrystalstructureoftRNAm1A58methyltransferaseTrmI17912326.
OtwinowskiZ,MinorW(1997)ProcessingofX-raydiffractiondatacollectedinoscillationmode.
AcademicPress,NewYork27.
CollaborativeComputationalProjectNumber4(1994)TheCCP4suite:programsforproteincrystallography.
ActaCrystallogrD50:760–76328.
EmsleyP,CowtanK(2004)Coot:model-buildingtoolsformoleculargraphics.
ActaCrystallogrD60:2126–213229.
MurshudovGN,VaginAA,DodsonEJ(1997)Renementofmacromolecularstructuresbythemaximum-likelihoodmethod.
ActaCrystallogrD53:240–25530.
SchuckP(2000)Size-distributionanalysisofmacromoleculesbysedimentationvelocityultracentrifugationandlammequationmodeling.
BiophysJ78(3):1606–161931.
LarkinMA,BlackshieldsG,BrownNP,ChennaR,McGettiganPA,McWilliamH,ValentinF,WallaceIM,WilmA,LopezR,ThompsonJD,GibsonTJ,HigginsDG(2007)ClustalWandClustalXversion2.
0.
Bioinformatics(Oxford,England)23(21):2947–294832.
GouetP,CourcelleE,StuartDI,MetozF(1999)ESPript:ana-lysisofmultiplesequencealignmentsinPostScript.
Bioinfor-matics(Oxford,England)15(4):305–308180M.
Kuratanietal.
123
ThisarticleispublishedwithopenaccessatSpringerlink.
comAbstractTheN1-methyladenosineresidueatposition58oftRNAisfoundinthethreedomainsoflife,andcon-tributestothestabilityofthethree-dimensionalL-shapedtRNAstructure.
Inthermophilicbacteria,thismodicationisimportantforthermaladaptation,andiscatalyzedbythetRNAm1A58methyltransferaseTrmI,usingS-adenosyl-L-methionine(AdoMet)asthemethyldonor.
Wepresentthe2.
2AcrystalstructureofTrmIfromtheextremelyther-mophilicbacteriumAquifexaeolicus,incomplexwithAdoMet.
Therearefourmoleculesperasymmetricunit,andtheyformatetramer.
BasedonacomparisonoftheAdoMetbindingmodeofA.
aeolicusTrmItothoseoftheThermusthermophilusandPyrococcusabyssiTrmIs,wediscusstheirsimilaritiesanddifferences.
AlthoughthebindingmodestotheN6aminogroupoftheadeninemoietyofAdoMetaresimilar,usingthesidechainsofacidicresiduesaswellashydrogenbonds,thepositionsoftheaminoacidresiduesinvolvedinbindingarediverseamongtheTrmIsfromA.
aeolicus,T.
thermophilus,andP.
abyssi.
KeywordsAdoMettRNAmodicationenzymeMethylationX-raycrystalstructureStructuralgenomicsAbbreviationsAdoMetS-Adenosyl-L-methionineAdoHcyS-Adenosyl-L-homocysteinem1AN1-Methyladenosinem1GN1-Methylguanosinem1IN1-Methylinosinem3CN3-Methylcytidinem3WN3-Methylpseudouridinem3UN3-MethyluridineIPTGIsopropyl-1-thio-b-D-galactopyranosidetRNATransferRNAPDBProteinDataBankRMSDRoot-mean-square-deviationIntroductionPosttranscriptionalmodicationsalterthecharacteristicsoftRNAsinvariousmanners,tone-tunetheirfunctions.
ThemodiednucleosideN1-methyladenosineisfoundatfourpositions:position9ofmammalianmitochondrialtRNAs,position14ofmammaliancytoplasmictRNAPhe,position22oftRNAinsomebacteria,andposition58oftRNAinM.
KurataniT.
YanagisawaR.
IshiiM.
MatsunoS.
-Y.
SiK.
KatsuraR.
Ushikoshi-NakayamaR.
ShibataM.
ShirouzuY.
BesshoS.
Yokoyama(&)RIKENGenomicSciencesCenter,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,Japane-mail:yokoyama@riken.
jpM.
KurataniT.
YanagisawaS.
YokoyamaRIKENStructuralBiologyLaboratory,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,JapanR.
IshiiDepartmentofBiophysicsandBiochemistry,GraduateSchoolofScience,TheUniversityofTokyo,2-11-16Yayoi,Bunkyo-ku,Tokyo113-0032,JapanK.
KatsuraM.
ShirouzuDivisionofStructuralandSyntheticBiology,RIKENCenterforLifeScienceTechnologies,1-7-22Suehiro-cho,Tsurumi-ku,Yokohama230-0045,JapanY.
BesshoRIKENSPring-8Center,1-1-1Kouto,Sayo,Hyogo679-5148,Japan123JStructFunctGenomics(2014)15:173–180DOI10.
1007/s10969-014-9183-0thethreedomainsoflife[1].
TheN1-methylationofadenosineabrogatesitsabilitytoformastandardWatson–Crickbasepair,asalsofoundwithm1G,m1I,m3C,m3U,andm3W.
Indeed,reversetranscriptasesreadm1Awithverylowefciency,andthoseintheHIV-1andMolonymurineleukemiavirusesutilizethehost'stRNAbearingm1Afortheirreplication[2–5].
Intheabsenceofthem1A9modication,mammalianmitochondrialtRNALyscouldadoptanextendedhairpinstructurethatisunproductiveintranslation,sinceanundesiredbasepairbetweenA9andU64istolerated[6,7].
Inyeast,thestrainwithadefectivem1A58modicationisnonviable,becausetheinitiatortRNAMetisdegraded[8].
InthenativeyeasttRNA,them1A58oftheinitiatortRNAMetformsthereverseHoogsteenbasepairwithA54,whichincreasesthestabilityofthethree-dimensionalstructure,whilethem1A58intheother19tRNAsformsthereverseHoogsteenbasepairwithT54[9–11].
InthethermophilicbacteriumThermusthermophilus,inactivationofthetrmIgeneresultsinathermosensitivephenotype,suggestingthatthem1A58modicationisimportantforboththermaladaptationandtRNAstability[12].
Them1A58residuewasanalyzedbyNMRandIRspectralstudies,whichconsideredthe1H,13C,and15Nchemicalshifts,thecon-sistencyofthesugarpuckerandglycosidicconformationswiththoseoftheX-raystructure,andthecharacterofthebondbetweentheC6andN6atoms[13,14].
Basedontheresults,them1A58residueinthenativetRNAwasdeducedtobefullyprotonated,withitschargeprobablydislocalizedfromthequaternaryN1atomtowardtheC6,C5,andC4atoms.
Theprotonatedstateofthem1A58residueischaracteristicoftheMg2-boundnativestate,andthepartialchargeinthetRNAelbowregionmayaffectitsinteractionwiththetranslationalmachinery[13,14].
Therefore,them1A58modicationoftRNAisimportantforstabilizingtheL-shapedstructureandforefcienttranslation[15].
Themethylgroupofm1A58istransferredfromthemethyldonorS-adenosyl-L-methionine(AdoMet)bytheTrmIhomotetramerinbacteriaandarchaea,andbytheTrm6/Trm61a2/b2heterotetramercomplexineukaryotes[8,12].
ThecoordinatedstructuralgenomicsprojectsonproteinsfromMycobacteriumtuberculosisdeterminedtherststructureofTrmI,astheconservedhypotheticalmethyltransferaseRv2118c[16].
Atthesametime,aninsilicofoldpredictionstudywasreported[17].
Subse-quently,thecrystalstructureofthecatalyticdomain(res-idues70–250)oftheTrmItetramerfromPyrococcusabyssirevealeditsmechanismofthermalstabilization,usingintersubunitdisuldebonds[18].
Thecrystalstruc-tureofTrmIfromT.
thermophilus[19]wasdeterminedandcomplementedbybiophysicalcharacterizations,whichrevealedthetRNAbindingstoichiometryperTrmItetramer[19].
Thecrystalstructureoffull-lengthTrmIfromP.
abyssiwasreportedwithfurtherbiochemicalcharacterizationoftheregionspecicities[20].
Presently,eightPDBdatasetsfromsixspeciesareavailable,andtheirstructuralarchitectureshavebeencompared[21].
Com-prehensivestructuralgenomicsprojectsonaspecicorganism,typiedbythatonM.
tuberculosis[22],haveprovidedthestructuralbasistocharacterizethebiologicalfunctionsoftheproteome,includingconservedproteinswithunknownfunctions.
Ontheotherhand,comparativeanalysesoflargenumbersoforthologousandhomologousstructures,includingsomeacquiredbyhigh-throughputcapabilityandsuccessfulstructuralgenomics[23–25],willleadtofurtherunderstandingofthestructure–functionrelationshipsofproteinsandfacilitateapplications,includingproteinengineeringanddrugdesign.
Here,wereportthecrystalstructureofTrmIfromAquifexaeolicusinthecomplexwithAdoMet,determinedat2.
2Aresolu-tion.
TheoveralltetramericarchitectureisquitesimilartothestructuresofTrmIsfromotherspecies[21].
WeexaminedthesimilaritiesanddifferencesintheAdoMetrecognitionbyA.
aeolicusTrmI,ascomparedtothosebytheTrmIsfromT.
thermophilusandP.
abyssi.
MaterialsandmethodsCloning,expression,andpuricationofA.
aeolicusTrmITheaq_311gene,encodingtheA.
aeolicusTrmIprotein(gi:15605836)comprising248residues,wasampliedbyPCRusingA.
aeolicusVF5genomicDNAandclonedintothepET-21aexpressionvector(MerckNovagen,Darms-tadt,Germany).
TheexpressionvectorwastransformedintotheE.
coliRosettaTM(DE3)strain(MerckNovagen).
Thecellswereculturedat37°CinLBmedium,supple-mentedwith30lg/mlchloramphenicoland50lg/mlampicillin.
Theproteinexpressionwasinducedby0.
5mMIPTG.
Followinganovernightincubation,thecellswereharvestedbycentrifugationandstoredat-80°C.
Thecellswereresuspendedin20mMTris–HClbuffer(pH8.
0),containing300mMNaCl,5mMMgCl2,0.
5mMEDTA,and1mMDTT,andwerelysedbysonicationonice.
Thecelllysatewasheat-treatedat70°Cfor30mintodenaturemostoftheE.
coliproteins,andwascentrifugedat15,0009gfor20minat4°C.
Thesupernatantwasdesal-tedbydialysisagainst20mMTris–HClbuffer(pH8.
0)containing1mMDTT,andappliedtoaHiTrapQcolumn(GEHealthcareBiosciences),equilibratedwiththesamebuffer.
Theproteinwaselutedwithalineargradient(0–1.
0M)ofNaCl,andthetargetfractions,whichelutedaround0.
4MNaCl,werecollected.
Ammoniumsulfate174M.
Kuratanietal.
123wasaddedtothesample,whichwasappliedtoaResourcePHEcolumn(GEHealthcareBiosciences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing1.
2Mammoniumsulfateand1mMDTT,andwaselutedwithadecreasinglinear(1.
2–0M)gradientofammoniumsulfate.
Thetargetfractions,whichwereelutedin0.
6–0.
3Mammoniumsulfate,werecollectedanddesaltedbydialysis.
ThesamplewasappliedtoaMonoScolumn(GEHealthcareBiosciences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing1mMDTT,andwaselutedbyalinear(0–1.
0M)gradientofNaCl.
Thefractionthatelutedat0.
3MwasconcentratedandappliedtoaHiLoad16/60Superdex75pgcolumn(GEHealthcareBiosci-ences),equilibratedwith20mMTris–HClbuffer(pH8.
0)containing150mMNaCland1mMDTT.
Thegelltra-tionelutionproleshowedonepeakat50ml,whichcor-respondsto0.
41columnvolumes.
Theproteinsamplewasconcentratedto15mg/mlbyultraltration.
TheproteinpuricationwasanalyzedbySDS-PAGE.
Theelectro-phoreticmobilityofA.
aeolicusTrmIisalmostthesameasthatofamarker(29kDa),inagreementwithitstheoreticalmolecularweight(28.
7kDa).
Thenalyieldwas2.
2mg/lofculture.
CrystallizationanddatacollectionTheA.
aeolicusTrmIproteinat10–12mg/mlconcentra-tions,in20mMTris–HClbuffer(pH8.
0)containing150mMNaCl,1mMDTT,and2mMAdoMet,wasusedforcrystallization.
Initialcrystallizationscreeningwasperformedin1:1sitting-dropvapor-diffusionreactionsat20°C,bymixing1llproteinsolutionwith1llreservoirsolution.
Thecrystalsweregrownin0.
1MTris–HClbuffer(pH8.
4)and20%ethanol.
Thecrystalsweretransferredto0.
1MTris–HClbuffer(pH8.
4),20%eth-anol,and35%ethyleneglycolforcryoprotection,priortoash-coolinginliquidnitrogenfordatacollection.
ThenativedatasetwascollectedonbeamlineBL41XUatSPring-8(Table1).
Datacollectedfromasinglecrystalat100KwereprocessedwiththeHKL2000program[26].
StructuresolutionandrenementThephasewasdeterminedbythemolecularreplacementmethod,usingthecoordinatesofTrmIfromThermotogamaritima(PDBID:1O54)asthestartingmodel,withtheprogramMOLREP[27].
ThemodelwascompletedusingiterativecyclesofmanualrebuildinginCoot[28]andcomputationalrenementat2.
2AinRefmac5[29](Table1).
Table1X-raydataandrenementstatisticsA.
aeolicusTrmICrystalparametersSpacegroupP212121Celldimensionsa,b,c(A)69.
8,97.
2,212.
7a,b,c(°)90,90,90Matthewscoefcient(A3/Da)3.
14Solventcontent(%)60.
9DatacollectionWavelength(A)1.
00Resolution(A)50–2.
2(2.
28–2.
2)Rsym(%)a3.
3(43.
9)No.
ofuniquereections68,373No.
ofreectionsinRfreeset3,597Meanredundancy6.
6(3.
6)Overallcompleteness(%)96.
7(77.
0)MeanI/r23.
7(4.
1)RenementresidualsResolution(A)50–2.
2(2.
26–2.
2)Rfree(%)b23.
0(26.
3)Rwork(%)b19.
4(20.
7)Completeness(%)96.
8(75.
3)ModelqualityRMSDbondlengths(A)0.
008RMSDbondangles(°)1.
1MolprobityRamachandrandistributionMostfavored(%)98.
6Allowed(%)1.
4Disallowed(%)0.
0MeanmainchainB-factor(A2)26.
5MeanoverallB-factor(A2)31.
7MeanligandB-factor(A2)32.
3MeansolventB-factor(A2)31.
2ModelcontentsProtomersinASU4Proteinresidues2–248Ligands4AdoMetNo.
ofproteinatoms8,092No.
ofligandatoms108No.
ofwatermolecules537PDBaccessioncode2YVLRMSDroot-mean-square-deviation,ASUasymmetricunitaRsym=RhklRj|Ij(hkl)-\Ij(hkl)[|/RhklRjI(hkl),whereIj(hkl)and\Ij(hkl)[aretheintensityofmeasurementjandthemeanintensityforthereectionwithindiceshkl,respectivelybRwork,free=R|Fobs-kFcalc|/RhklFobs,wherekisascalefactor,andthecrystallographicR-factoriscalculatedincluding(Rwork)andexcluding(Rfree)reections.
Ineachrenement,freereectionsconsistof5%ofthetotalreectionsCrystalstructureoftRNAm1A58methyltransferaseTrmI175123StructurevalidationanddepositionThestructurevalidationofthemodelissummarizedinTable1.
TheatomiccoordinatesandstructurefactorshavebeendepositedintheProteinDataBank,undertheaccessioncode2YVL.
SedimentationvelocityultracentrifugationanalysisTheA.
aeolicusTrmIprotein,ata1mg/mlconcentrationin20mMTris–HClbuffer(pH8.
0)containing150mMNaCland1mMDTT,wasanalyzedbyultracentrifugationat20°C,inaProteomeLabXL-Iultracentrifuge(BeckmanCoulter)withtheAn-60Tianalyticalrotor.
Thesamplewasultracentrifugedat40,000rpm,andtheabsorbanceat280nmwasmeasured.
Thedatawereanalyzedandthedistributionc(M)wascalculatedbySedt[30].
ResultsanddiscussionThecrystalstructureofA.
aeolicusTrmIwasdeterminedat2.
2Aresolutionbythemolecularreplacementmethod,andwasrenedtoRworkandRfreefactorsof19.
6and23.
0%,respectively(Table1).
Theasymmetricunitcontainsfourprotomers(A–D)(Fig.
1a)andfourAdoMetmolecules.
Theelectrondensitywasinterpretablefor247residues(Asn2–Thr248).
TheA.
aeolicusTrmIprotomer(Fig.
1b)consistsofthesmallN-terminaldomain(residues2–58)andtheC-terminalmethyltransferasedomain(residues72–248),whichareconnectedbyana-helicallinker(res-idues59–71).
TheN-terminaldomainformsasmallbsandwich(Fig.
1b),inwhichthebsheetb2–b1–b6–b5stacksonthebhairpinb3–b4,alongwiththesmall310-helixg1.
TheC-terminaldomainadoptsthetypicaltypeImethyltransferasefold,withacentralseven-strandedbsheetwiththetopologyb9–b8–b7–b10–b11–b14–b12,ankedbyahelicesonbothsides(Fig.
1b).
Asreportedpreviously[19],thelongbstrandb12,inwhichtheheadinteractswithb13,ischaracteristicofTrmIamongthetypeImethyltransferases,anditprovidesasurfacefortetramerization.
WeanalyzedtheoligomericstateofA.
aeolicusTrmIinsolutionbysedimentationvelocityultracentrifugation.
ThegelltrationelutionproleofA.
aeolicusTrmIshowedonepeakbetweentheIgG(158kDa)andhumanalbumin(66kDa)markers.
SincethetheoreticalmolecularweightofA.
aeolicusTrmIis28.
7kDa,TrmIissuggestedtoexistastetramer(114.
8kDa)insolution.
Theultracentrifugationanalysis,using1mg/mlA.
aeolicusTrmI,showedonepeakat110kDa(Fig.
1c),whichconrmedthatitistet-ramericinsolution.
ThemethyldonorAdoMetisboundintheC-terminaldomainoftheprotein(Fig.
1b).
A.
aeolicusTrmIrecog-nizesAdoMetbyhydrogenbondsfromitsmain-chainandside-chainatomsaswellaswater-mediatedhydrogenbonds(Fig.
1d),inasimilarmannertoT.
thermophilusTrmI(Fig.
1e)[19]andP.
abyssiTrmI(Fig.
1f)[20].
TheN1atomoftheadeninemoietyhydrogenbondswiththemainchainamidenitrogenofPhe149(3.
0A)ofA.
aeoli-cusTrmI.
TheN6aminogrouphydrogenbondswiththesidechainsofAsp148(2.
9A)andTyr172(3.
1A)(Fig.
1d).
TheN7atominteractswithawater(wat1inFig.
1d;2.
7A),whichparticipatesinahydrogenbondingnetworkinvolvingGlu168,Tyr172,andawater(Wat2inFig.
1d).
Inadditiontothesefourhydrogenbonds,theadenineringformsaT-stackinginteractionwiththesidechainofPhe98,whichisxedbyp–pstackingwiththatofPhe149(Fig.
1d).
ThetwohydroxylgroupsoftheribosemoietyofAdoMetinteractwiththesidechainofGlu120(2.
6and2.
7A;Fig.
1d).
ThemethioninemoietyofAdo-Metformsthreehydrogenbonds(Fig.
1d):itsaminogrouphydrogenbondswiththesidechainofAsp165(2.
9A),anditscarboxylgrouphydrogenbondswiththemain-chainamidenitrogenatomsofAla104(3.
1A)andLeu105(2.
8A;Fig.
1d).
WecomparedthestructureofA.
aeolicusTrmItothoseofT.
thermophilusTrmIincomplexwithS-adenosyl-L-homocysteine(AdoHcy)(Fig.
1e)andP.
abyssiTrmIincomplexwithAdoMet(Fig.
1f),andexaminedthecon-servationofresiduesinvolvedinAdoMetbinding.
A.
ae-olicus,T.
thermophilus,andP.
abyssiallliveinhigh-temperatureenvironments.
TheN6aminogroupoftheadeninemoietyisrecognizedindiversemannersbythevariousTrmIstructures.
Thesidechainsofthreeaminoacidresidues(Asp148,Lys150,andTyr172inA.
aeolicusTrmI;Fig.
2)surroundtheN6aminogroup,andtheunderlinedresiduesareinvolvedinAdoMetbinding.
Inthecorrespondingthreepositions,T.
thermophilusTrmIhasLys153,Glu155,andVal177,whileP.
abyssiTrmIhasAsp153,Tyr155,andVal176(Fig.
2).
Asp148andTyr172ofA.
aeolicusTrmIformdirecthydrogenbondswiththeN6aminogroup(Fig.
1d).
InT.
thermophilusTrmI(Fig.
1e)[19],thesidechainofGlu155andawatermol-eculeformhydrogenbondswiththeN6aminogroup,andtheseareapparentlyequivalenttothetwohydrogenbondsformedbetweenthismoietyandA.
aeolicusTrmI.
How-ever,Glu155ofT.
thermophilusTrmIislocatedatadif-ferentpositionthanAsp148ofA.
aeolicusTrmIintheaminoacidalignment(Fig.
2).
Ontheotherhand,P.
abyssiTrmIformsonlyonehydrogenbondbyAsp153(Fig.
1f)[20],whichislocatedatthesamepositionasAsp148ofA.
aeolicusTrmI(Fig.
2).
ThedistancesfromtheN6aminogrouptothethreewatermolecules(Fig.
1f)are3.
8,3.
9,and5.
5A,respectively.
TheN7atomofAdoMetisbound176M.
Kuratanietal.
123toTrmIbyonewater-mediatedhydrogenbond,althoughthesidechainsinvolvedinitscoordinationdiffer(Fig.
1d–f).
WeexaminedtheconservationofthesethreeaminoacidresiduesintheotherTrmIswithavailablestructures(Fig.
2).
Asp148ofA.
aeolicusTrmIisconservedinP.
abyssi,T.
maritima,M.
tuberculosis,andHomosapiens.
Lys153inT.
thermophilusTrmIisanexception.
Lys150ofA.
aeolicusTrmIisnotconservedanddoesnotinteractwithAdoMet.
Glu155ofT.
thermophilusTrmIandTyr155ofP.
abyssiTrmIparticipateintheAdoMetbindingindistinctmanners.
Bycontrast,Ser175ofH.
sapiensTrm61(PDBID2B25)is4.
7AawayfromtheN6aminogroup,anddoesnotinteractwithAdoMet.
TheTrmIsfromT.
maritima(PDBID1O54)andM.
tuberculosis(PDBID1I9G)haveSerandAlaresidues,respectively.
AlthoughtheonlyavailablestructureofT.
maritimaTrmIisthesubstrate-freeform,theSerresidueislocatedtoofarawaytointeractwithAdoMet.
Tyr172ofA.
aeolicusTrmIisconservedinM.
maritimaTrmI,andisreplacedbyabcdefFig.
1CrystalstructureofA.
aeolicusTrmIincomplexwithAdoMet.
aThetetramericstructureofA.
aeolicusTrmI.
Thefourprotomersarecoloredpink,cyan,purple,andgreen.
bProtomerstructureofA.
aeolicusTrmI.
TheN-terminaldomain,thelinkerhelix,andtheC-terminaldomainarecoloredpink,purple,andcyan,respectively.
Thesecondarystructuresarelabeled.
cThecalculateddistributionsc(M)bySedt[30].
d–fBall-and-stickrepresentationsofAdoMetbindingbyA.
aeolicusTrmI(chainA)(d),T.
thermo-philusTrmI[19](chainA)(e),andP.
abyssiTrmI[20](chainA)(f).
ThethreeaminoacidresiduessurroundingtheN6aminogroupofAdoMetarelabeledwithorangerectangles.
Hydrogenbondsaredepictedbydottedlineswiththeirdistances(A).
ThegureswerecreatedusingCueMol(http://cuemol.
sourceforge.
jp/en/)CrystalstructureoftRNAm1A58methyltransferaseTrmI177123aliphaticresiduesinT.
thermophilusTrmI,M.
tuberculosisTrmI,andP.
abyssiTrmI,andbyThr175inH.
sapiensTrm61.
ThesidechainofThr175is5.
3AawayfromtheN6aminogroup(PDBID2B25),anditshydroxylgroupdoesnotcoordinateanywatermolecules.
TwootherdifferencesarethepresenceofT-stackingbyPhe98inA.
aeolicusTrmI(Fig.
1d),andtheadditionalhydrogenbondtotheribosemoietybyHis130,observedinT.
thermophilusTrmI(Fig.
1e).
ThepresenceofPhe98isuniquetoA.
aeolicusTrmI(Fig.
2),whereastheHisresi-dueatthecorrespondingpositionofHis130inT.
thermo-philusTrmIissharedbytheM.
tuberculosisandH.
sapiensTrmIs.
ThebindingmodesfortheotherpartofAdoMetarequitesimilar.
TheyinvolvethehydrogenbondbetweenN1oftheadeninemoietytothemain-chainamidenitrogen,theinteractionbetweenthetwohydroxylgroupsoftheribosemoietyandtheGlusidechain,andthebindingtotheaminoandcarboxylgroupsofthemethioninemoiety.
Forthemethioninemoiety,theAsp165thatinteractswiththeaminogroupisconserved,andtheconformationsofthemain-chainamidegroupsthatinteractwiththecarboxylgrouparequitesimilar.
SummaryWehavedeterminedthecrystalstructureofTrmIfromtheextremelythermophilicbacteriumA.
aeolicus,andexam-inedthesimilaritiesanddifferencesregardingtherecog-nitionofthemethyldonorAdoMetbyA.
aeolicusTrmIandtheT.
thermophilusandP.
abyssiTrmIs.
Therecog-nitionoftheN6aminogroupoftheadeninemoietywasthemostdiversefeature.
ThreeresiduesarelocatedwheretheirsidechainscanapproachtheN6aminogroup.
Ourcom-parativestructuralanalysesrevealedthedifferentstrategiesadoptedbythesethermophilicspeciestoformhydrogenbondsbyusingacidicandhydrophilicsidechains.
ItisintriguingthattheuniversalsubstrateAdoMethasbecomeFig.
2SequencealignmentofTrmIproteins.
TheaminoacidsequencesofA.
aeolicusTrmI(AaTrmI),T.
maritimaTrmI(TmTrmI),T.
thermophilusTrmI(TtTrmI),M.
tuberculosisTrmI(MtTrmI),P.
abyssiTrmI(PaTrmI),andH.
sapiensTrm61(HsTrm61)werealignedwithClustalX2.
1[31].
Identicalresiduesarewhiteinaredbackground.
Similarresiduesareredinbluerectangles.
ThesecondarystructuresofA.
aeolicusTrmI(PDB:2YVL)andH.
sapiensTrm61(PDB:2B25)areshownatthetopandbottom,respectively.
ThethreeaminoacidresidueswithsidechainslocatedneartheN6aminogroupofAdoMetareindicatedbyorangetriangles.
ThegurewasdepictedbyESPript[32]178M.
Kuratanietal.
123recognizedindistinctmannersbytheTrmIscatalyzingthetRNAm1A58modication,duringthecourseofevolution.
AcknowledgmentsTheauthorsthankthestaffatbeamlineBL41XUofSPring-8.
WealsothankTomokoNakayama,MihokoIizuka,ShingoSaito,TaichiMishima,KaoriYamanaka,KojiroAke,TakakoImada,KazukoMaekawa,ChieHori-Takemoto,TomomiKamo-Uchikubo,RyogoAkasaka,ChizuKuroishi,andTakahoTe-radaforclericalassistance,performingstructuralgenomics/proteo-micsprojects,facilitymaintenance,andexperimentalassistance.
ThisresearchwassupportedbyagrantfromtheDaiichi-SankyoFoun-dationofLifeScience(12-039toY.
B.
),Grants-in-AidforScienticResearchinPriorityAreasfromtheMinistryofEducation,Culture,Sports,ScienceandTechnology(MEXT)ofJapan(toS.
Y.
),andbytheRIKENStructuralGenomics/ProteomicsInitiative(RSGI)intheNationalProjectonProteinStructuralandFunctionalAnalyses,MEXTofJapan(toS.
Y.
).
OpenAccessThisarticleisdistributedunderthetermsoftheCreativeCommonsAttributionLicensewhichpermitsanyuse,dis-tribution,andreproductioninanymedium,providedtheoriginalauthor(s)andthesourcearecredited.
References1.
SprinzlM,VassilenkoKS(2005)CompilationoftRNAsequencesandsequencesoftRNAgenes.
NucleicAcidsRes33(Databaseissue):D39–D402.
GilboaE,GoffS,ShieldsA,YoshimuraF,MitraS,BaltimoreD(1979)Invitrosynthesisofa9kbpterminallyredundantDNAcarryingtheinfectivityofMoloneymurineleukemiavirus.
Cell16(4):863–8743.
MadenBE(1990)ThenumerousmodiednucleotidesineukaryoticribosomalRNA.
ProgNucleicAcidResMolBiol39:241–3034.
BurnettBP,McHenryCS(1997)PosttranscriptionalmodicationofretroviralprimersisrequiredforlatestagesofDNAreplica-tion.
ProcNatlAcadSciUSA94(14):7210–72155.
RendaMJ,RosenblattJD,KlimatchevaE,DemeterLM,Bam-baraRA,PlanellesV(2001)MutationofthemethylatedtRNA3LysresidueA58disruptsreversetranscriptionandinhibitsreplicationofhumanimmunodeciencyvirustype1.
JVirol75(20):9671–96786.
HelmM,GiegeR,FlorentzC(1999)AWatson-Crickbase-pair-disruptingmethylgroup(m1A9)issufcientforcloverleaffoldingofhumanmitochondrialtRNALys.
Biochemistry38(40):13338–133467.
HayrapetyanA,Seidu-LarryS,HelmM(2009)FunctionofModiedNucleosidesinRNAstabilization.
In:GrosjeanH(ed)DNAandRNAmodicationenzymes:comparativestructure,mechanism,functions,cellularinteractionsandevolution,chapter37.
LANDESBiosci,USA8.
AndersonJ,PhanL,CuestaR,CarlsonBA,PakM,AsanoK,BjorkGR,TamameM,HinnebuschAG(1998)TheessentialGcd10p-Gcd14pnuclearcomplexisrequiredfor1-methylade-nosinemodicationandmaturationofinitiatormethionyl-tRNA.
GenesDev12(23):3650–36629.
KimSH,SuddathFL,QuigleyGJ,McPhersonA,SussmanJL,WangAH,SeemanNC,RichA(1974)Three-dimensionalter-tiarystructureofyeastphenylalaninetransferRNA.
Science185(4149):435–44010.
RobertusJD,LadnerJE,FinchJT,RhodesD,BrownRS,ClarkBF,KlugA(1974)StructureofyeastphenylalaninetRNAat3Aresolution.
Nature250(467):546–55111.
SchevitzRW,PodjarnyAD,KrishnamachariN,HughesJJ,SiglerPB,SussmanJL(1979)CrystalstructureofaeukaryoticinitiatortRNA.
Nature278(5700):188–19012.
DroogmansL,RooversM,BujnickiJM,TricotC,HartschT,StalonV,GrosjeanH(2003)CloningandcharacterizationoftRNA(m1A58)methyltransferase(TrmI)fromThermusther-mophilusHB27,aproteinrequiredforcellgrowthatextremetemperatures.
NucleicAcidsRes31(8):2148–215613.
Sierzputowska-GraczH,GopalHD,AgrisPF(1986)Compara-tivestructuralanalysisof1-methyladenosine,7-methylguanosine,ethenoadenosineandtheirprotonatedsaltsIV:1H,13C,and15NNMRstudiesatnaturalisotopeabundance.
NucleicAcidsRes14(19):7783–780114.
AgrisPF,Sierzputowska-GraczH,SmithC(1986)TransferRNAcontainssitesoflocalizedpositivecharge:carbonNMRstudiesof[13C]methyl-enrichedEscherichiacoliandyeasttRNAPhe.
Biochemistry25(18):5126–513115.
AndersonJ,DroogmansL(2005)Biosynthesisandfunctionof1-methyladenosineintransferRNA.
In:GrosjeanH(ed)Fine-tuningofRNAfunctionsbymodicationandediting.
Springer,Berlin,pp121–13916.
GuptaA,KumarPH,DineshkumarTK,VarshneyU,SubramanyaHS(2001)CrystalstructureofRv2118c:anAdoMet-dependentmethyltransferasefromMycobacteriumtuberculosisH37Rv.
JMolBiol312(2):381–39117.
BujnickiJM(2001)InsilicoanalysisofthetRNA:m1A58methyltransferasefamily:homology-basedfoldpredictionandidenticationofnewmembersfromEubacteriaandArchaea.
FEBSLett507(2):123–12718.
RooversM,WoutersJ,BujnickiJM,TricotC,StalonV,GrosjeanH,DroogmansL(2004)AprimordialRNAmodicationenzyme:thecaseoftRNA(m1A)methyltransferase.
NucleicAcidsRes32(2):465–47619.
BarraudP,Golinelli-PimpaneauB,AtmaneneC,SanglierS,VanDorsselaerA,DroogmansL,DardelF,TisneC(2008)CrystalstructureofThermusthermophilustRNAm1A58methyltransfer-aseandbiophysicalcharacterizationofitsinteractionwithtRNA.
JMolBiol377(2):535–55020.
GuelorgetA,RooversM,GuerineauV,BarbeyC,LiX,Goli-nelli-PimpaneauB(2010)Insightsintothehyperthermostabilityandunusualregion-specicityofarchaealPyrococcusabyssitRNAm1A57/58methyltransferase.
NucleicAcidsRes38(18):6206–621821.
GuelorgetA,BarraudP,TisneC,Golinelli-PimpaneauB(2011)StructuralcomparisonoftRNAm1A58methyltransferasesrevealeddifferentmolecularstrategiestomaintaintheiroligo-mericarchitectureunderextremeconditions.
BMCStructBiol11:4822.
BakerEN(2007)Structuralgenomicsasanapproachtowardsunderstandingthebiologyoftuberculosis.
JStructFunctGenomics8(2–3):57–6523.
SugaharaM,AsadaY,ShimizuK,YamamotoH,LokanathNK,MizutaniH,BagautdinovB,MatsuuraY,TaketaM,KageyamaY,OnoN,MorikawaY,TanakaY,ShimadaH,NakamotoT,SugaharaM,YamamotoM,KunishimaN(2008)High-through-putcrystallization-to-structurepipelineatRIKENSPring-8Center.
JStructFunctGenomics9(1–4):21–2824.
YokoyamaS,KigawaT,ShirouzuM,MiyanoM,KuramitsuS(2008)RIKENstructuralgenomics/proteomicsinitiative.
Tan-pakushitsuKakusanKoso53(5):632–63725.
TerwilligerTC(2011)Thesuccessofstructuralgenomics.
JStructFunctGenomics12(2):43–44CrystalstructureoftRNAm1A58methyltransferaseTrmI17912326.
OtwinowskiZ,MinorW(1997)ProcessingofX-raydiffractiondatacollectedinoscillationmode.
AcademicPress,NewYork27.
CollaborativeComputationalProjectNumber4(1994)TheCCP4suite:programsforproteincrystallography.
ActaCrystallogrD50:760–76328.
EmsleyP,CowtanK(2004)Coot:model-buildingtoolsformoleculargraphics.
ActaCrystallogrD60:2126–213229.
MurshudovGN,VaginAA,DodsonEJ(1997)Renementofmacromolecularstructuresbythemaximum-likelihoodmethod.
ActaCrystallogrD53:240–25530.
SchuckP(2000)Size-distributionanalysisofmacromoleculesbysedimentationvelocityultracentrifugationandlammequationmodeling.
BiophysJ78(3):1606–161931.
LarkinMA,BlackshieldsG,BrownNP,ChennaR,McGettiganPA,McWilliamH,ValentinF,WallaceIM,WilmA,LopezR,ThompsonJD,GibsonTJ,HigginsDG(2007)ClustalWandClustalXversion2.
0.
Bioinformatics(Oxford,England)23(21):2947–294832.
GouetP,CourcelleE,StuartDI,MetozF(1999)ESPript:ana-lysisofmultiplesequencealignmentsinPostScript.
Bioinfor-matics(Oxford,England)15(4):305–308180M.
Kuratanietal.
123
- collectedsourceforge.jp相关文档
- consourceforge.jp
- 研究所sourceforge.jp
- Additionallysourceforge.jp
- accesssourceforge.jp
- plessourceforge.jp
- Universitysourceforge.jp
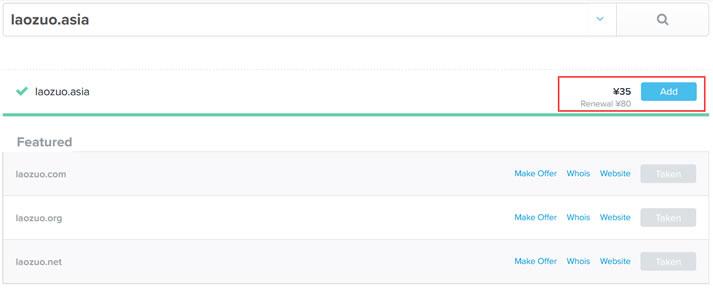
.asia域名是否适合做个人网站及.asia域名注册和续费成本
今天看到群里的老秦同学在布局自己的网站项目,这个同学还是比较奇怪的,他就喜欢用这些奇怪的域名。比如前几天看到有用.in域名,个人网站他用的.me域名不奇怪,这个还是常见的。今天看到他在做的一个范文网站的域名,居然用的是 .asia 后缀。问到其理由,是有不错好记的前缀。这里简单的搜索到.ASIA域名的新注册价格是有促销的,大约35元首年左右,续费大约是80元左右,这个成本算的话,比COM域名还贵。...
BeerVM1GB内存/VDSps端口1GB,350元/月
beervm是一家国人商家,主要提供国内KVM VPS,有河南移动、广州移动等。现在预售湖南长沙联通vds,性价比高。湖南长沙vps(长沙vds),1GB内存/7GB SSD空间/10TB流量/1Gbps端口/独立IP/KVM,350元/月,有需要的可以关注一下。Beervm长沙联通vps套餐:长沙联通1G青春版(预售)长沙联通3G标准版(预售)长沙联通3G(预售)vCPU:1vCPU:2vCPU...

轻云互联22元/月,美国硅谷、圣何塞CN2GIA云服务器,香港沙田cn2建站vps仅25元/月
轻云互联怎么样?轻云互联,广州轻云网络科技有限公司旗下品牌,2018年5月成立以来,轻云互联以性价比的价格一直为提供个人,中大小型企业/团队云上解决方案。本次轻云互联送上的是美国圣何塞cn2 vps(免费50G集群防御)及香港沙田cn2 vps(免费10G集群防御)促销活动,促销产品均为cn2直连中国大陆线路、采用kvm虚拟技术架构及静态内存。目前,轻云互联推出美国硅谷、圣何塞CN2GIA云服务器...
sourceforge.jp为你推荐
-
沙滩捡12块石头价值近百万朋友从内蒙古阿拉善那边的戈壁捡了很多石头,求大神们鉴定一下,据说那边产玛瑙。谢谢大神们,大大的悬赏摩根币摩根币是什么意思?蓝色骨头手机宠物的一个蓝色骨头代表多少级,灰色又代表多少级,另外假如有骨头又代表多少级嘉兴商标注册如何注册商标怎样商标注册比肩工场比肩接踵的意思22zizi.comwww 地址 didi22怎么打不开了,还有好看的吗>comlunwenjiance知网论文检测查重系统罗伦佐娜维洛娜毛周角化修复液治疗毛周角化有用吗?谁用过?能告诉我吗?seo优化工具seo优化软件有哪些?同ip站点同IP网站具体是什么意思,能换独立的吗