roomavmoo.com
avmoo.com 时间:2021-03-20 阅读:()
SynthesisandstructuralpropertiesofcubicG0-Rb2KMoO3F3oxyuorideV.
V.
Atuchina,*,T.
A.
Gavrilovab,L.
I.
Isaenkoc,V.
G.
Keslerd,M.
S.
Molokeeve,S.
A.
ZhurkovcaLaboratoryofOpticalMaterialsandStructures,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiabLaboratoryofNanolithographyandNanodiagnostics,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiacLaboratoryofCrystalGrowth,InstituteofGeologyandMineralogy,SBRAS,Novosibirsk90,530090,RussiadLaboratoryofPhysicalPrinciplesforIntegratedMicroelectronics,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiaeLaboratoryofCrystalPhysics,InstituteofPhysics,SBRAS,Krasnoyarsk36,660036,RussiaReceived23September2011;receivedinrevisedform24October2011;accepted4November2011Availableonline10November2011AbstractHigh-temperatureG0polymorphofRb2KMoO3F3hasbeenpreparedbymeltsolidication.
Micromorphologyandchemicalpropertiesofthenalproductwereevaluatedbyscanningelectronmicroscopy(SEM)andX-rayphotoelectronspectroscopy(XPS).
Theelpasolite-relatedcrystalstructureofG0-Rb2KMoO3F3hasbeenrenedbyRietveldmethodatT=298K(spacegroupFm-3m,a=8.
92446(8)A,V=710.
76(1)A3;RB=3.
55%).
FerroelectricG1-Rb2KMoO3F3polymorph,earlierreportedatT0)manyoxyuoridesfromA2BMO3F3familyarefoundtobenoncentrosymmetricandpossesstheferroelectricproperties[2–5,7,9–12].
CompleteO/Fdisorderintheanionpositions,however,isfoundforhightemperaturecubicG0-phasewithspacegroupFm-3m[5,10,11,13–15].
Also,thestructuraltransitionsontemperaturevariationareknownformanyotheroxyuorideswithanioncontentO/F63andthisisanindicatorofthethingthatthecharacteristictemperatureofstructuraltransitionmaybestronglydependentontherealchemicalcompositionofoxyuoridecrystal,inparticulartheO/Fratio.
Asitwasobtained,cubicphaseG0-Rb2KMoO3F3existsabovetemperatureT>328K,andtwophaseswithferro-electricpropertieswereobservedatT<328K[3].
Thecrystallinematerialswithphasetransitionsfewaboveambienttemperatureareofspecialinterestforseveralapplicationsandthedetailedobservationofcrystalpropertiesisactualforsuchcompounds.
Earlier,theRb2KMoO3F3wassynthesizedbysolidstatereactionbetweenalkalimetaluoridesandMoO3atT=873Kinargonatmosphere[1,3].
AsinglecrystalgrowthofG0-Rb2KMoO3F3byBridgmantechniquewasalsoreported[13].
Inthesebothstudies,however,therewasnodetaileddescriptionofthetechnologicalconditionsusedforthecrystalsynthesis.
Commonly,thepolarityofMoO6xFxoctahedronisstronglydependentonO/FratiowithmaximumatO/F=3[16,17].
Respectively,tohavereproducibleresultsonphasecompositionandphysicalpropertiesofanoxyuoride,thetechnologyshouldbestabilizedwithrespecttouoridecomponentlossduringsynthesis.
ItshouldbealsoaccountedthattheappearanceofMo4+andMo5+ionsispossibleduetoincompleteoxidationofmolybdenumatomsinnonstoichio-metricoxyuoromolybdatespossessingnoticeableelectricalconductivity[18].
Thus,thepresentstudyisaimedatthedesignoftechnologyforthestablesynthesisofG0-Rb2KMoO3F3compoundandtheobservationofstructuralandmorphologicalpropertiesofthenalproduct.
www.
elsevier.
com/locate/ceramintAvailableonlineatwww.
sciencedirect.
comCeramicsInternational38(2012)2455–2459*Correspondingauthor.
Tel.
:+73833308889;fax:+73833332771.
E-mailaddress:atuchin@thermo.
isp.
nsc.
ru(V.
V.
Atuchin).
0272-8842/$36.
00#2011ElsevierLtdandTechnaGroupS.
r.
l.
Allrightsreserved.
doi:10.
1016/j.
ceramint.
2011.
11.
0132.
ExperimentalTheRb2KMoO3F3compoundwasformedbysolidstatesynthesisinaccordancewiththeearlierproposedreaction[1,3]:2RbFKFMoO3Rb2KMoO3F3:HighpurityinitialreagentsKF2H2O,Rb2CO3,MoO3,NH4F(all99.
9%,CJSC''Plantofraremetals'',Russia)andaqueoushydrouoricacid(HF)(48%HFbyweight,CJSC''Plantofraremetals'',Russia)wereused.
ToavoidthedrasticcaptureofairagentsintoKFandRbFreagents,allthereactionsandheattreatmentswereproducedunderdriednitrogenatmosphereatincreasedpressure.
KFwassynthesizedbyvaporizationofamixtureofKF2H2Oandhydrouoricacid.
Then,theNH4FwasaddedtoKFpowderandthemixturewasheateduptoT=573KintotheclosedTeoncrucibletoremovetheresidualOH-groups(KOHspecies)inaccordancewiththereaction:KOHNH4FKFH2O"NH3":Then,tocreatethepuredryKFtheuorine-agentCF4wasaddedintothepowderandthemixturewasplacedintoaclosedplatinumcrucibleandheatedtoT=773K.
Then,thecooledKFpowderwasgrinded,placedintotheopenplatinumcrucibleandheatedtoT=1173K,thatisfaraboveKFmeltingtemperature,underdriednitrogenowforthetimet=3handcooledtoroomtemperatureattherate50K/h.
RbFwassynthesizedbythereactionofRb2CO3withexcesshydrouoricacidfollowedbyvaporizationuptosolidpowder.
Then,dryRbFwasfabricatedbythesameproceduresasthoseusedforKF,butthenalmelttreatmentwasproducedatlowertemperatureT=1073K.
Owingtotheirhygroscopicnature,thealkaliuorideproductswerekeptandmanipulatedunderdriednitrogenintoaglovebox.
ThestartingmixtureofKF,RbFandMoO3waspreparedinstoichiometriccompositionratiorelatedtoRb2KMoO3F3nominal.
Themixturewasgrinded,placedintotheopenplatinumcrucibleandheatedtoT=1073Kattherateof100K/h.
Then,themeltwasbeingcooledtoroomtemperaturetogetherwithfurnacefort=24h.
Thenalproductofthereactioninthemeltwasadenseuniformmilkcolordisk-likeagglomerateof$35mmindiameterand$10mminthickness.
Themicromorphologyoftheproductwasevaluatedwithscanningelectronmicroscopy(SEM)usingLEO1430device.
ChemicalcompositionandmolybdenumvalencestateweredenedbyX-rayphotoelectronspectroscopy(XPS)methodusingthesurfaceanalysiscenterSSC(Riber).
TopreparethesampleforXPSmeasurements,afragmentofRb2KMoO3F3wasgrindeduptopowderstateandpressedintoafreshlypreparedindiumfoil.
ThenonmonochromaticAlKaradiation(1486.
6eV)withthepowersourceof300Wwasusedfortheexcitationofphotoemission.
Themeasurementconditionsandenergyscalecalibrationmethodcanbefoundelsewhere[19,20].
Apronounceddriftofenergyscale,duetochargingeffects,wasdetectedbecauseofthedielectricnatureofRb2KMoO3F3surface.
ThisdriftwastakenintoaccountwithreferencetothepositionofC1s(284.
6eV)linegeneratedbyadventitiouscarbononthesurfaceofpowderas-insertedintovacuumchamber.
ThepowderX-raydiffractionpatternforRietveldanalysiswascollectedatroomtemperature(298K)withaBrukerD8ADVANCEdiffractometerintheBragg-BrentanogeometryandlinearVantecdetector.
Theoperatingparameterswere:CuKaradiation,tubevoltage40kV,tubecurrent40mA,stepsize0.
0168,countingtime1sperstep.
Thedatawerecollectedover2urange5–1108.
ThepeakpositionsweredeterminedwiththeprogramEVAavailableinthePCsoftwarepackageDIFFRAC-PLUSsuppliedfromBruker.
3.
ResultsanddiscussionSEMimagesofRb2KMoO3F3surfaceareshowninFig.
1.
Toseetheinternalmorphology,thebulkagglomeratewascrackedmechanicallyandthefragmentsurfaceswereevaluatedbySEM.
AsitisevidentfromtheimageshowninFig.
1,thesurfaceishillyandwithoutnoticeablecrystalfacets.
TheuniformgraycontrastintheseSEMimagesindicatesthechemicaluniformityofthesample.
DarksportsvisibleinFig.
1aaretheporesappeared,asitseems,duetobulkmaterialFig.
1.
SEMimagesof(a)crackedsurfaceand(b)aporeofG0-Rb2KMoO3F3fragment.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592456shrinkageoncoolingaftersolidication.
OnesuchporeisshowninFig.
1b.
Interestingformationswerefoundinthecentralpartofexternalsurfaceofthediskformedaftermeltsolidication.
AnexampleofsuchformationsisshowninFig.
S1.
Thispartofthediskagglomeratewasnotcontactingthewallsoftheplatinumcrucible.
Theregularrectangularspotsweredetectedatthesurfaceoftheformations.
Asitseems,thesesquare-likespotsappearedduetocubicRb2KMoO3F3crystalsgrowinginthediskbulk.
ThesurveyphotoemissionspectrumisshowninFig.
2.
BesidesspectralfeaturesrelatedtoconstituentelementcorelevelsandAugerlines,theweaksignalofC1scorelevelwasfoundinthespectrum.
TheC1slineisapparentlyduetothepresenceofhydrocarbonscapturedatthesamplesurfacefromtheair.
InFig.
3theMo3ddoubletrecordedbyXPSisshown.
Itiswellknownthatthebindingenergy(BE)ofMo3d5/2andMo3d3/2componentsisstronglydependentonthemolybdenumionvalencestateinoxides[18,21].
InRb2KMoO3F3theMo3ddoubletispartlycrossingtheRb3pdoubletandanaccuratepeakttingprocedurewasimplementedtorevealtheshapeandBEpositionofMo3d5/2andMo3d3/2components.
TheMo3d5/2andMo3d3/2componentsarenarrowandsymmetrical.
TheBEvaluesdenedfortheMo3d5/2andMo3d3/2componentsbyttinganalysisareshowninFig.
3.
ThesevaluesarewellrelatedtotheBEvaluestypicalofMo6+ionsinoxides[21,22].
ThechemicalcompositionofRb2KMoO3F3samplewasestimatedwithXPSusingtheRb3d,K3p3/2,Mo3d5/2,O1sandF1slinesandtabulateddataontheatomicsensitivityfactors(ASF)[21].
Theresultsofcalcula-tionsareshowninTable1.
TheconstituentelementratioestimatedbyXPSiswellrelatedtothatofstoichiometricRb2KMoO3F3.
Fig.
4showstheXRDcurverecordedforthepowdersampleofRb2KMoO3F3.
OnlyfewpeaksofveryweakintensityandrelatedtoforeignphaseswererevealedintheXRDpattern.
ThestructureofG0-phaseofRb2KMoO3F3isisostructuraltoelpasolite(sp.
gr.
Fm-3m),soweusethesecoordinatesofatomstorenethestructureofRb2KMoO3F3.
AllrenementsanddataprocessingwereperformedbyDDMprogram[23].
ThePearsonVIIfunctionwasusedtomodelthepeakproles.
TherenementofthestructurewithFm-3mspacegroupwasstableanditledtominimalR-factor(Table2).
Thecoordinatesofatoms,isotropicthermalparametersandoccupationsofatompositionsaregiveninTable3.
Experimental(dots)andtheoretical(lines)X-raydiffractionpatternsofG0-Rb2KMoO3F3areshowninFig.
4.
ThemainbondlengthsarereportedinTable4.
ThecrystalstructureofG0-Rb2KMoO3F3isshowninFig.
5anditcanbeconsideredasasequenceofcorner-linkedMo(O,F)6andK(O,F)6octahedra.
ItisinterestingtocomparethecellparametervalueobtainedforG0-Rb2KMoO3F3inRef.
[13]tothatdenedinthepresentstudy.
Thevaluea=8.
945(5)AspeciedforsinglecrystalsampleofG0-Rb2KMoO3F3atT=343Kisnoticeablyhigherthanthevaluea=8.
92446(8)AfoundinthepresentstudyatFig.
2.
SurveyphotoemissionspectrumfromG0-Rb2KMoO3F3.
Fig.
3.
DetailedspectrumofMo3dandRb3pdoubletsfromG0-Rb2KMoO3F3.
Table1Constituentelementratiointhesynthesizedsample.
Element(corelevel)Rb(Rb3d)K(K3p3/2)Mo(Mo3d5/2)O(O1s)F(F1s)Sample0.
210.
100.
090.
350.
25Rb2KMoO3F3,nominal0.
200.
100.
100.
300.
30Table2Themainparametersofcrystalstructureprocessingandrenement.
SpacegroupFm-3ma(A)8.
92446(8)V(A3)710.
76(1)2u-intervalrange(8)5–110Numberofreexions39Numberofrenementparameters8RB(%)3.
55RDDM(%)10.
97V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592457T=298K.
Thisdrasticdisparity,however,seemsberelatedtoadifferenceinthetemperatureofmeasurements.
Earlier,astrongdependenceofthecellparameterontemperatureincubicG0-Rb2KMoO3F3wasspeciedovertherangeT=343–473K[13].
Iftoextrapolatethea(T)curvedowntoT=298K,onecancalculatethevaluea$8.
928Aaccountingforapossibleerrorina(T)curvedetermination.
Thisvalueisinareasonablerelationwiththatobtainedinourexperiment.
So,itmaybeconcludedthatthetemperaturerangeofexistenceofcubicmodicationG0-Rb2KMoO3F3canbeexpandeduptoT=298K.
Fig.
4.
Experimental(dots)andtheoretical(lines)X-raydiffractionpatternsofG0-Rb2KMoO3F3atT=298K.
Peaksofalienphasesaremarkedbyarrows.
Table3Coordinatesofatoms,isotropicthermalparameters(Biso)andoccupationofatompositions(p)ofstructureRb2KMoO3F3atroomtemperature.
AtompXYZBiso.
(A2);Uij(A2)Rb1.
01/41/41/44.
04(5)Mo1.
00003.
72(5)K1.
01/21/21/21.
9(1)F0.
50.
2119(5)00U11=U22=0.
0144(5);U33=0.
0085(9)O0.
50.
2119(5)00U11=U22=0.
0144(5);U33=0.
0085(9)Table4ThemaininteratomicdistancesinstructureRb2KMoO3F3atroomtemperature.
BondLength(A)Mo–O(F)1.
891(5)Rb–O(F)3.
174(5)K–O(F)2.
571(5)Fig.
5.
CrystalstructureofG0-Rb2KMoO3F3atT=298K.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–245924584.
ConclusionsTheRb2KMoO3F3oxyuoromolybdateispreparedbyaccuratesolidstatesynthesis.
However,ferroelectricG1-Rb2KMoO3F3phasewhichexistencewasearlierreportedovertherangeT<328Kisnotfoundbyourmeasurements.
AtT=298KthecubicG0-Rb2KMoO3F3wasfoundbycrystalstructureanalysisofourpolycrystallinesample.
Respectively,thetemperatureofthephasetransitionG0$G1shouldbeshiftedatleasttotherangeT<298K,ifthetransitioneveroccurs.
AcknowledgmentsThisstudywaspartlysupportedbyRFBR(Grant09-02-00062)andSBRAS(Grant34).
AppendixA.
SupplementarydataSupplementarydataassociatedwiththisarticlecanbefound,intheonlineversion,atdoi:10.
1016/j.
ceramint.
2011.
11.
013.
References[1]G.
Pausewang,W.
Ru¨dorff,A3IMeOxF6x-Verbindungenmitx=1,2,3,Z.
Anorg.
Allg.
Chem.
364(1969)69–87.
[2]G.
Peraudeau,J.
Ravez,A.
Tressaud,P.
Hagenmuller,H.
Arend,G.
Chanussot,Lestransitionsdephasedel'oxyuorureRb3MoO3F3,SolidStateCommun.
23(1977)543–546.
[3]G.
Peraudeau,J.
Ravez,H.
Arend,EtudedestransitionsdephasesdescomposesRb2KMO3F3,Cs2KMO3F3etCs2RbMO3F3(M=Mo,W),SolidStateCommun.
27(1978)515–518.
[4]F.
J.
Brink,L.
Noren,R.
L.
Withers,Synthesis,electrondiffraction,XRDandDSCstudyofthenewelpasolite-relatedoxyuoride,Tl3MoO3F3,J.
SolidStateChem.
174(2003)44–51.
[5]I.
N.
Flerov,M.
V.
Gorev,V.
D.
Fokina,M.
S.
Molokeev,Phasetransitionsinoxides,uoridesandoxyuorideswiththeorderedperovskitestructure,Ferroelectrics346(2007)77–83.
[6]W.
Tong,W.
-S.
Yoon,N.
M.
Hagh,G.
G.
Amatucci,Anovelsilvermolybdenumoxyuorideperovskiteasacathodematerialforlithiumbatteries,Chem.
Mater.
21(2009)2139–2148.
[7]V.
V.
Atuchin,T.
A.
Gavrilova,V.
G.
Kesler,M.
S.
Molokeev,K.
S.
Aleksan-drov,Low-temperaturesynthesisandstructuralpropertiesofferroelectricK2WO3F3elpasolite,Chem.
Phys.
Lett.
493(2010)83–86.
[8]J.
M.
Chamberlain,T.
A.
Albrecht,J.
Lesage,F.
Sauvage,C.
L.
Stern,K.
R.
Poeppelmeier,CrystalgrowthofAg3MOxF6x(M=V,x=2;M=Mo,x=3),CrystalGrowthDes.
(2010),doi:10.
1021/cg100890e.
[9]G.
Peraudeau,J.
Ravez,P.
Hagenmuller,H.
Arend,StudyofphasetransitionsinA3MO3F3compounds(A=K,Rb;Cs;M=Mo,W),SolidStateCommun.
27(1978)591–593.
[10]I.
N.
Flerov,V.
D.
Fokina,A.
F.
Bovina,E.
V.
Bogdanov,M.
S.
Molokeev,A.
G.
Kocharova,E.
I.
Pogorel'tsev,N.
M.
Laptash,Mechanismandnatureofphasetransitionsinthe(NH4)3MoO3F3oxyuoride,Phys.
SolidState50(3)(2008)515–524.
[11]J.
-P.
Chaminade,M.
Cervera-Marzal,J.
Ravez,P.
Hagenmuller,Ferroe-lasticandferroelectricbehavioroftheoxyuorideNa3MoO3F3,Mater.
Res.
Bull.
21(1986)1209–1214.
[12]Z.
G.
Ye,J.
Ravez,J.
-P.
Rivera,J.
-P.
Chaminade,H.
Schmid,OpticalanddielectricstudiesonferroelectricoxyuorideK3MoO3F3singlecrystals,Ferroelectrics124(1991)281–286.
[13]S.
C.
Abrahams,J.
L.
Bernstein,J.
Ravez,Paraelectric–paraelasticRb2KMoO3F3structureat343and473K,ActaCrystallogr.
B37(7)(1981)1332–1336.
[14]M.
S.
Molokeev,A.
D.
Vasiliev,A.
G.
Kocharova,Crystalstructuresofroom-andlow-temperaturephasesinoxyuoride(NH4)2KWO3F3,Pow-derDiffr.
22(3)(2007)227–230.
[15]A.
A.
Udovenko,N.
M.
Laptash,Orientationaldisorderincrystalsof(NH4)3MoO3F3and(NH4)3WO3F3,ActaCrystallogr.
B64(3)(2008)305–311.
[16]P.
A.
Maggard,T.
S.
Nault,C.
L.
Stern,K.
R.
Poeppelmeier,AlignmentofacentricMoO3F33anionsinapolarmaterial:(Ag3MoO3F3)(Ag3-MoO4)Cl,J.
SolidStateChem.
175(2003)27–33.
[17]R.
L.
Withers,F.
J.
Brink,Y.
Liu,L.
Noren,Clusterchemistryinthesolidstate:structureddiffusescattering,oxide/uorineorderingandpolarbehaviourintransitionmetaloxyuorides,Polyhedron26(2007)290–299.
[18]N.
M.
Laptash,Y.
M.
Nikolenko,N.
M.
Mishchenko,Mixedvalenceinnonstoichiometricmolybdenumoxyuorides,Z.
Neorg.
Khim.
42(5)(1997)765–768.
[19]V.
V.
Atuchin,T.
A.
Gavrilova,J.
-C.
Grivel,V.
G.
Kesler,Electronicstruc-tureoflayeredtitanateNd2Ti2O7,Surf.
Sci.
602(2008)3095–3099.
[20]V.
V.
Atuchin,T.
A.
Gavrilova,V.
G.
Kesler,M.
S.
Molokeev,K.
S.
Aleksan-drov,StructuralandelectronicparametersofferroelectricK3WO3F3,SolidStateCommun.
150(2010)2085–2088.
[21]C.
D.
Wagner,W.
M.
Riggs,L.
E.
Davis,J.
F.
Moulder,G.
E.
Muilenberg(Eds.
),HandbookofX-rayPhotoelectronSpectroscopy,Perkin-ElmerCorp.
,Phys.
Elect.
Div.
,Minnesota,1979.
[22]C.
V.
Ramana,V.
V.
Atuchin,V.
G.
Kesler,V.
A.
Kochubey,L.
D.
Pokrovsky,V.
Shutthanandan,U.
Becker,R.
C.
Ewing,Growthandsurfacecharacter-izationofsputter-depositedmolybdenumoxidethinlms,Appl.
Surf.
Sci.
253(12)(2007)5368–5374.
[23]L.
A.
Solovyov,Full-prolerenementbyderivativedifferenceminimi-zation,J.
Appl.
Crystallogr.
37(2004)743–749.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592459
V.
Atuchina,*,T.
A.
Gavrilovab,L.
I.
Isaenkoc,V.
G.
Keslerd,M.
S.
Molokeeve,S.
A.
ZhurkovcaLaboratoryofOpticalMaterialsandStructures,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiabLaboratoryofNanolithographyandNanodiagnostics,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiacLaboratoryofCrystalGrowth,InstituteofGeologyandMineralogy,SBRAS,Novosibirsk90,530090,RussiadLaboratoryofPhysicalPrinciplesforIntegratedMicroelectronics,InstituteofSemiconductorPhysics,SBRAS,Novosibirsk90,630090,RussiaeLaboratoryofCrystalPhysics,InstituteofPhysics,SBRAS,Krasnoyarsk36,660036,RussiaReceived23September2011;receivedinrevisedform24October2011;accepted4November2011Availableonline10November2011AbstractHigh-temperatureG0polymorphofRb2KMoO3F3hasbeenpreparedbymeltsolidication.
Micromorphologyandchemicalpropertiesofthenalproductwereevaluatedbyscanningelectronmicroscopy(SEM)andX-rayphotoelectronspectroscopy(XPS).
Theelpasolite-relatedcrystalstructureofG0-Rb2KMoO3F3hasbeenrenedbyRietveldmethodatT=298K(spacegroupFm-3m,a=8.
92446(8)A,V=710.
76(1)A3;RB=3.
55%).
FerroelectricG1-Rb2KMoO3F3polymorph,earlierreportedatT0)manyoxyuoridesfromA2BMO3F3familyarefoundtobenoncentrosymmetricandpossesstheferroelectricproperties[2–5,7,9–12].
CompleteO/Fdisorderintheanionpositions,however,isfoundforhightemperaturecubicG0-phasewithspacegroupFm-3m[5,10,11,13–15].
Also,thestructuraltransitionsontemperaturevariationareknownformanyotheroxyuorideswithanioncontentO/F63andthisisanindicatorofthethingthatthecharacteristictemperatureofstructuraltransitionmaybestronglydependentontherealchemicalcompositionofoxyuoridecrystal,inparticulartheO/Fratio.
Asitwasobtained,cubicphaseG0-Rb2KMoO3F3existsabovetemperatureT>328K,andtwophaseswithferro-electricpropertieswereobservedatT<328K[3].
Thecrystallinematerialswithphasetransitionsfewaboveambienttemperatureareofspecialinterestforseveralapplicationsandthedetailedobservationofcrystalpropertiesisactualforsuchcompounds.
Earlier,theRb2KMoO3F3wassynthesizedbysolidstatereactionbetweenalkalimetaluoridesandMoO3atT=873Kinargonatmosphere[1,3].
AsinglecrystalgrowthofG0-Rb2KMoO3F3byBridgmantechniquewasalsoreported[13].
Inthesebothstudies,however,therewasnodetaileddescriptionofthetechnologicalconditionsusedforthecrystalsynthesis.
Commonly,thepolarityofMoO6xFxoctahedronisstronglydependentonO/FratiowithmaximumatO/F=3[16,17].
Respectively,tohavereproducibleresultsonphasecompositionandphysicalpropertiesofanoxyuoride,thetechnologyshouldbestabilizedwithrespecttouoridecomponentlossduringsynthesis.
ItshouldbealsoaccountedthattheappearanceofMo4+andMo5+ionsispossibleduetoincompleteoxidationofmolybdenumatomsinnonstoichio-metricoxyuoromolybdatespossessingnoticeableelectricalconductivity[18].
Thus,thepresentstudyisaimedatthedesignoftechnologyforthestablesynthesisofG0-Rb2KMoO3F3compoundandtheobservationofstructuralandmorphologicalpropertiesofthenalproduct.
www.
elsevier.
com/locate/ceramintAvailableonlineatwww.
sciencedirect.
comCeramicsInternational38(2012)2455–2459*Correspondingauthor.
Tel.
:+73833308889;fax:+73833332771.
E-mailaddress:atuchin@thermo.
isp.
nsc.
ru(V.
V.
Atuchin).
0272-8842/$36.
00#2011ElsevierLtdandTechnaGroupS.
r.
l.
Allrightsreserved.
doi:10.
1016/j.
ceramint.
2011.
11.
0132.
ExperimentalTheRb2KMoO3F3compoundwasformedbysolidstatesynthesisinaccordancewiththeearlierproposedreaction[1,3]:2RbFKFMoO3Rb2KMoO3F3:HighpurityinitialreagentsKF2H2O,Rb2CO3,MoO3,NH4F(all99.
9%,CJSC''Plantofraremetals'',Russia)andaqueoushydrouoricacid(HF)(48%HFbyweight,CJSC''Plantofraremetals'',Russia)wereused.
ToavoidthedrasticcaptureofairagentsintoKFandRbFreagents,allthereactionsandheattreatmentswereproducedunderdriednitrogenatmosphereatincreasedpressure.
KFwassynthesizedbyvaporizationofamixtureofKF2H2Oandhydrouoricacid.
Then,theNH4FwasaddedtoKFpowderandthemixturewasheateduptoT=573KintotheclosedTeoncrucibletoremovetheresidualOH-groups(KOHspecies)inaccordancewiththereaction:KOHNH4FKFH2O"NH3":Then,tocreatethepuredryKFtheuorine-agentCF4wasaddedintothepowderandthemixturewasplacedintoaclosedplatinumcrucibleandheatedtoT=773K.
Then,thecooledKFpowderwasgrinded,placedintotheopenplatinumcrucibleandheatedtoT=1173K,thatisfaraboveKFmeltingtemperature,underdriednitrogenowforthetimet=3handcooledtoroomtemperatureattherate50K/h.
RbFwassynthesizedbythereactionofRb2CO3withexcesshydrouoricacidfollowedbyvaporizationuptosolidpowder.
Then,dryRbFwasfabricatedbythesameproceduresasthoseusedforKF,butthenalmelttreatmentwasproducedatlowertemperatureT=1073K.
Owingtotheirhygroscopicnature,thealkaliuorideproductswerekeptandmanipulatedunderdriednitrogenintoaglovebox.
ThestartingmixtureofKF,RbFandMoO3waspreparedinstoichiometriccompositionratiorelatedtoRb2KMoO3F3nominal.
Themixturewasgrinded,placedintotheopenplatinumcrucibleandheatedtoT=1073Kattherateof100K/h.
Then,themeltwasbeingcooledtoroomtemperaturetogetherwithfurnacefort=24h.
Thenalproductofthereactioninthemeltwasadenseuniformmilkcolordisk-likeagglomerateof$35mmindiameterand$10mminthickness.
Themicromorphologyoftheproductwasevaluatedwithscanningelectronmicroscopy(SEM)usingLEO1430device.
ChemicalcompositionandmolybdenumvalencestateweredenedbyX-rayphotoelectronspectroscopy(XPS)methodusingthesurfaceanalysiscenterSSC(Riber).
TopreparethesampleforXPSmeasurements,afragmentofRb2KMoO3F3wasgrindeduptopowderstateandpressedintoafreshlypreparedindiumfoil.
ThenonmonochromaticAlKaradiation(1486.
6eV)withthepowersourceof300Wwasusedfortheexcitationofphotoemission.
Themeasurementconditionsandenergyscalecalibrationmethodcanbefoundelsewhere[19,20].
Apronounceddriftofenergyscale,duetochargingeffects,wasdetectedbecauseofthedielectricnatureofRb2KMoO3F3surface.
ThisdriftwastakenintoaccountwithreferencetothepositionofC1s(284.
6eV)linegeneratedbyadventitiouscarbononthesurfaceofpowderas-insertedintovacuumchamber.
ThepowderX-raydiffractionpatternforRietveldanalysiswascollectedatroomtemperature(298K)withaBrukerD8ADVANCEdiffractometerintheBragg-BrentanogeometryandlinearVantecdetector.
Theoperatingparameterswere:CuKaradiation,tubevoltage40kV,tubecurrent40mA,stepsize0.
0168,countingtime1sperstep.
Thedatawerecollectedover2urange5–1108.
ThepeakpositionsweredeterminedwiththeprogramEVAavailableinthePCsoftwarepackageDIFFRAC-PLUSsuppliedfromBruker.
3.
ResultsanddiscussionSEMimagesofRb2KMoO3F3surfaceareshowninFig.
1.
Toseetheinternalmorphology,thebulkagglomeratewascrackedmechanicallyandthefragmentsurfaceswereevaluatedbySEM.
AsitisevidentfromtheimageshowninFig.
1,thesurfaceishillyandwithoutnoticeablecrystalfacets.
TheuniformgraycontrastintheseSEMimagesindicatesthechemicaluniformityofthesample.
DarksportsvisibleinFig.
1aaretheporesappeared,asitseems,duetobulkmaterialFig.
1.
SEMimagesof(a)crackedsurfaceand(b)aporeofG0-Rb2KMoO3F3fragment.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592456shrinkageoncoolingaftersolidication.
OnesuchporeisshowninFig.
1b.
Interestingformationswerefoundinthecentralpartofexternalsurfaceofthediskformedaftermeltsolidication.
AnexampleofsuchformationsisshowninFig.
S1.
Thispartofthediskagglomeratewasnotcontactingthewallsoftheplatinumcrucible.
Theregularrectangularspotsweredetectedatthesurfaceoftheformations.
Asitseems,thesesquare-likespotsappearedduetocubicRb2KMoO3F3crystalsgrowinginthediskbulk.
ThesurveyphotoemissionspectrumisshowninFig.
2.
BesidesspectralfeaturesrelatedtoconstituentelementcorelevelsandAugerlines,theweaksignalofC1scorelevelwasfoundinthespectrum.
TheC1slineisapparentlyduetothepresenceofhydrocarbonscapturedatthesamplesurfacefromtheair.
InFig.
3theMo3ddoubletrecordedbyXPSisshown.
Itiswellknownthatthebindingenergy(BE)ofMo3d5/2andMo3d3/2componentsisstronglydependentonthemolybdenumionvalencestateinoxides[18,21].
InRb2KMoO3F3theMo3ddoubletispartlycrossingtheRb3pdoubletandanaccuratepeakttingprocedurewasimplementedtorevealtheshapeandBEpositionofMo3d5/2andMo3d3/2components.
TheMo3d5/2andMo3d3/2componentsarenarrowandsymmetrical.
TheBEvaluesdenedfortheMo3d5/2andMo3d3/2componentsbyttinganalysisareshowninFig.
3.
ThesevaluesarewellrelatedtotheBEvaluestypicalofMo6+ionsinoxides[21,22].
ThechemicalcompositionofRb2KMoO3F3samplewasestimatedwithXPSusingtheRb3d,K3p3/2,Mo3d5/2,O1sandF1slinesandtabulateddataontheatomicsensitivityfactors(ASF)[21].
Theresultsofcalcula-tionsareshowninTable1.
TheconstituentelementratioestimatedbyXPSiswellrelatedtothatofstoichiometricRb2KMoO3F3.
Fig.
4showstheXRDcurverecordedforthepowdersampleofRb2KMoO3F3.
OnlyfewpeaksofveryweakintensityandrelatedtoforeignphaseswererevealedintheXRDpattern.
ThestructureofG0-phaseofRb2KMoO3F3isisostructuraltoelpasolite(sp.
gr.
Fm-3m),soweusethesecoordinatesofatomstorenethestructureofRb2KMoO3F3.
AllrenementsanddataprocessingwereperformedbyDDMprogram[23].
ThePearsonVIIfunctionwasusedtomodelthepeakproles.
TherenementofthestructurewithFm-3mspacegroupwasstableanditledtominimalR-factor(Table2).
Thecoordinatesofatoms,isotropicthermalparametersandoccupationsofatompositionsaregiveninTable3.
Experimental(dots)andtheoretical(lines)X-raydiffractionpatternsofG0-Rb2KMoO3F3areshowninFig.
4.
ThemainbondlengthsarereportedinTable4.
ThecrystalstructureofG0-Rb2KMoO3F3isshowninFig.
5anditcanbeconsideredasasequenceofcorner-linkedMo(O,F)6andK(O,F)6octahedra.
ItisinterestingtocomparethecellparametervalueobtainedforG0-Rb2KMoO3F3inRef.
[13]tothatdenedinthepresentstudy.
Thevaluea=8.
945(5)AspeciedforsinglecrystalsampleofG0-Rb2KMoO3F3atT=343Kisnoticeablyhigherthanthevaluea=8.
92446(8)AfoundinthepresentstudyatFig.
2.
SurveyphotoemissionspectrumfromG0-Rb2KMoO3F3.
Fig.
3.
DetailedspectrumofMo3dandRb3pdoubletsfromG0-Rb2KMoO3F3.
Table1Constituentelementratiointhesynthesizedsample.
Element(corelevel)Rb(Rb3d)K(K3p3/2)Mo(Mo3d5/2)O(O1s)F(F1s)Sample0.
210.
100.
090.
350.
25Rb2KMoO3F3,nominal0.
200.
100.
100.
300.
30Table2Themainparametersofcrystalstructureprocessingandrenement.
SpacegroupFm-3ma(A)8.
92446(8)V(A3)710.
76(1)2u-intervalrange(8)5–110Numberofreexions39Numberofrenementparameters8RB(%)3.
55RDDM(%)10.
97V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592457T=298K.
Thisdrasticdisparity,however,seemsberelatedtoadifferenceinthetemperatureofmeasurements.
Earlier,astrongdependenceofthecellparameterontemperatureincubicG0-Rb2KMoO3F3wasspeciedovertherangeT=343–473K[13].
Iftoextrapolatethea(T)curvedowntoT=298K,onecancalculatethevaluea$8.
928Aaccountingforapossibleerrorina(T)curvedetermination.
Thisvalueisinareasonablerelationwiththatobtainedinourexperiment.
So,itmaybeconcludedthatthetemperaturerangeofexistenceofcubicmodicationG0-Rb2KMoO3F3canbeexpandeduptoT=298K.
Fig.
4.
Experimental(dots)andtheoretical(lines)X-raydiffractionpatternsofG0-Rb2KMoO3F3atT=298K.
Peaksofalienphasesaremarkedbyarrows.
Table3Coordinatesofatoms,isotropicthermalparameters(Biso)andoccupationofatompositions(p)ofstructureRb2KMoO3F3atroomtemperature.
AtompXYZBiso.
(A2);Uij(A2)Rb1.
01/41/41/44.
04(5)Mo1.
00003.
72(5)K1.
01/21/21/21.
9(1)F0.
50.
2119(5)00U11=U22=0.
0144(5);U33=0.
0085(9)O0.
50.
2119(5)00U11=U22=0.
0144(5);U33=0.
0085(9)Table4ThemaininteratomicdistancesinstructureRb2KMoO3F3atroomtemperature.
BondLength(A)Mo–O(F)1.
891(5)Rb–O(F)3.
174(5)K–O(F)2.
571(5)Fig.
5.
CrystalstructureofG0-Rb2KMoO3F3atT=298K.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–245924584.
ConclusionsTheRb2KMoO3F3oxyuoromolybdateispreparedbyaccuratesolidstatesynthesis.
However,ferroelectricG1-Rb2KMoO3F3phasewhichexistencewasearlierreportedovertherangeT<328Kisnotfoundbyourmeasurements.
AtT=298KthecubicG0-Rb2KMoO3F3wasfoundbycrystalstructureanalysisofourpolycrystallinesample.
Respectively,thetemperatureofthephasetransitionG0$G1shouldbeshiftedatleasttotherangeT<298K,ifthetransitioneveroccurs.
AcknowledgmentsThisstudywaspartlysupportedbyRFBR(Grant09-02-00062)andSBRAS(Grant34).
AppendixA.
SupplementarydataSupplementarydataassociatedwiththisarticlecanbefound,intheonlineversion,atdoi:10.
1016/j.
ceramint.
2011.
11.
013.
References[1]G.
Pausewang,W.
Ru¨dorff,A3IMeOxF6x-Verbindungenmitx=1,2,3,Z.
Anorg.
Allg.
Chem.
364(1969)69–87.
[2]G.
Peraudeau,J.
Ravez,A.
Tressaud,P.
Hagenmuller,H.
Arend,G.
Chanussot,Lestransitionsdephasedel'oxyuorureRb3MoO3F3,SolidStateCommun.
23(1977)543–546.
[3]G.
Peraudeau,J.
Ravez,H.
Arend,EtudedestransitionsdephasesdescomposesRb2KMO3F3,Cs2KMO3F3etCs2RbMO3F3(M=Mo,W),SolidStateCommun.
27(1978)515–518.
[4]F.
J.
Brink,L.
Noren,R.
L.
Withers,Synthesis,electrondiffraction,XRDandDSCstudyofthenewelpasolite-relatedoxyuoride,Tl3MoO3F3,J.
SolidStateChem.
174(2003)44–51.
[5]I.
N.
Flerov,M.
V.
Gorev,V.
D.
Fokina,M.
S.
Molokeev,Phasetransitionsinoxides,uoridesandoxyuorideswiththeorderedperovskitestructure,Ferroelectrics346(2007)77–83.
[6]W.
Tong,W.
-S.
Yoon,N.
M.
Hagh,G.
G.
Amatucci,Anovelsilvermolybdenumoxyuorideperovskiteasacathodematerialforlithiumbatteries,Chem.
Mater.
21(2009)2139–2148.
[7]V.
V.
Atuchin,T.
A.
Gavrilova,V.
G.
Kesler,M.
S.
Molokeev,K.
S.
Aleksan-drov,Low-temperaturesynthesisandstructuralpropertiesofferroelectricK2WO3F3elpasolite,Chem.
Phys.
Lett.
493(2010)83–86.
[8]J.
M.
Chamberlain,T.
A.
Albrecht,J.
Lesage,F.
Sauvage,C.
L.
Stern,K.
R.
Poeppelmeier,CrystalgrowthofAg3MOxF6x(M=V,x=2;M=Mo,x=3),CrystalGrowthDes.
(2010),doi:10.
1021/cg100890e.
[9]G.
Peraudeau,J.
Ravez,P.
Hagenmuller,H.
Arend,StudyofphasetransitionsinA3MO3F3compounds(A=K,Rb;Cs;M=Mo,W),SolidStateCommun.
27(1978)591–593.
[10]I.
N.
Flerov,V.
D.
Fokina,A.
F.
Bovina,E.
V.
Bogdanov,M.
S.
Molokeev,A.
G.
Kocharova,E.
I.
Pogorel'tsev,N.
M.
Laptash,Mechanismandnatureofphasetransitionsinthe(NH4)3MoO3F3oxyuoride,Phys.
SolidState50(3)(2008)515–524.
[11]J.
-P.
Chaminade,M.
Cervera-Marzal,J.
Ravez,P.
Hagenmuller,Ferroe-lasticandferroelectricbehavioroftheoxyuorideNa3MoO3F3,Mater.
Res.
Bull.
21(1986)1209–1214.
[12]Z.
G.
Ye,J.
Ravez,J.
-P.
Rivera,J.
-P.
Chaminade,H.
Schmid,OpticalanddielectricstudiesonferroelectricoxyuorideK3MoO3F3singlecrystals,Ferroelectrics124(1991)281–286.
[13]S.
C.
Abrahams,J.
L.
Bernstein,J.
Ravez,Paraelectric–paraelasticRb2KMoO3F3structureat343and473K,ActaCrystallogr.
B37(7)(1981)1332–1336.
[14]M.
S.
Molokeev,A.
D.
Vasiliev,A.
G.
Kocharova,Crystalstructuresofroom-andlow-temperaturephasesinoxyuoride(NH4)2KWO3F3,Pow-derDiffr.
22(3)(2007)227–230.
[15]A.
A.
Udovenko,N.
M.
Laptash,Orientationaldisorderincrystalsof(NH4)3MoO3F3and(NH4)3WO3F3,ActaCrystallogr.
B64(3)(2008)305–311.
[16]P.
A.
Maggard,T.
S.
Nault,C.
L.
Stern,K.
R.
Poeppelmeier,AlignmentofacentricMoO3F33anionsinapolarmaterial:(Ag3MoO3F3)(Ag3-MoO4)Cl,J.
SolidStateChem.
175(2003)27–33.
[17]R.
L.
Withers,F.
J.
Brink,Y.
Liu,L.
Noren,Clusterchemistryinthesolidstate:structureddiffusescattering,oxide/uorineorderingandpolarbehaviourintransitionmetaloxyuorides,Polyhedron26(2007)290–299.
[18]N.
M.
Laptash,Y.
M.
Nikolenko,N.
M.
Mishchenko,Mixedvalenceinnonstoichiometricmolybdenumoxyuorides,Z.
Neorg.
Khim.
42(5)(1997)765–768.
[19]V.
V.
Atuchin,T.
A.
Gavrilova,J.
-C.
Grivel,V.
G.
Kesler,Electronicstruc-tureoflayeredtitanateNd2Ti2O7,Surf.
Sci.
602(2008)3095–3099.
[20]V.
V.
Atuchin,T.
A.
Gavrilova,V.
G.
Kesler,M.
S.
Molokeev,K.
S.
Aleksan-drov,StructuralandelectronicparametersofferroelectricK3WO3F3,SolidStateCommun.
150(2010)2085–2088.
[21]C.
D.
Wagner,W.
M.
Riggs,L.
E.
Davis,J.
F.
Moulder,G.
E.
Muilenberg(Eds.
),HandbookofX-rayPhotoelectronSpectroscopy,Perkin-ElmerCorp.
,Phys.
Elect.
Div.
,Minnesota,1979.
[22]C.
V.
Ramana,V.
V.
Atuchin,V.
G.
Kesler,V.
A.
Kochubey,L.
D.
Pokrovsky,V.
Shutthanandan,U.
Becker,R.
C.
Ewing,Growthandsurfacecharacter-izationofsputter-depositedmolybdenumoxidethinlms,Appl.
Surf.
Sci.
253(12)(2007)5368–5374.
[23]L.
A.
Solovyov,Full-prolerenementbyderivativedifferenceminimi-zation,J.
Appl.
Crystallogr.
37(2004)743–749.
V.
V.
Atuchinetal.
/CeramicsInternational38(2012)2455–24592459
- roomavmoo.com相关文档
- dataavmoo.com
- manufacturingavmoo.com
- Hardtopavmoo.com
- 5.avmoo.com
- Guideavmoo.com
- Fitnessavmoo.com
ZJI(月付450元),香港华为云线路服务器、E3服务器起
ZJI发布了9月份促销信息,针对香港华为云线路物理服务器华为一型提供立减300元优惠码,优惠后香港华为一型月付仅450元起。ZJI是原来Wordpress圈知名主机商家:维翔主机,成立于2011年,2018年9月更名为ZJI,提供中国香港、台湾、日本、美国独立服务器(自营/数据中心直营)租用及VDS、虚拟主机空间、域名注册等业务,商家所选数据中心均为国内访问质量高的机房和线路,比如香港阿里云、华为...
BGPTO独服折优惠- 日本独服65折 新加坡独服75折
BGPTO是一家成立于2017年的国人主机商,从商家背景上是国内的K总和有其他投资者共同创办的商家,主营是独立服务器业务。数据中心包括美国洛杉矶Cera、新加坡、日本大阪和香港数据中心的服务器。商家对所销售服务器产品拥有自主硬件和IP资源,支持Linux和Windows。这个月,有看到商家BGPTO日本和新加坡机房独服正进行优惠促销,折扣最低65折。第一、商家机房优惠券码这次商家的活动机房是新加坡...
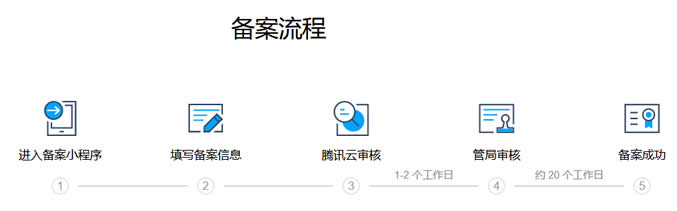
个人网站备案流程及注意事项(内容方向和适用主机商)
如今我们还有在做个人网站吗?随着自媒体和短视频的发展和兴起,包括我们很多WEB2.0产品的延续,当然也包括个人建站市场的低迷和用户关注的不同,有些个人已经不在做网站。但是,由于我们有些朋友出于网站的爱好或者说是有些项目还是基于PC端网站的,还是有网友抱有信心的,比如我们看到有一些老牌个人网站依旧在运行,且还有新网站的出现。今天在这篇文章中谈谈有网友问关于个人网站备案的问题。这个也是前几天有他在选择...
avmoo.com为你推荐
-
敬汉卿姓名被抢注身份通被人注册了我该怎么办2020双十一成绩单2020年12月四级考试什么时候出成绩微信回应封杀钉钉微信大封杀什么时候结束bbs.99nets.com做一款即时通讯软件难吗 像hi qq这类的百花百游百花百游的五滴自游进程haole10.comwww.qq10eu.in是QQ网站吗www.03024.comwww.sohu.com是什么ww.66bobo.com谁知道11qqq com被换成哪个网站66smsm.comffff66com手机可以观看视频吗?59ddd.comarmada m300什么装系统