11.278090lu.com
8090lu.com 时间:2021-03-18 阅读:()
Mar.
Drugs2014,12,5160-5173;doi:10.
3390/md12105160marinedrugsISSN1660-3397www.
mdpi.
com/journal/marinedrugsArticleNewIsocoumarinDerivativesandMeroterpenoidsfromtheMarineSponge-AssociatedFungusAspergillussimilanensissp.
nov.
KUFA0013ChadapornPrompanya1,2,TidaDethoup3,LucindaJ.
Bessa1,2,MadalenaM.
M.
Pinto2,4,LuísGales1,5,PauloM.
Costa1,2,ArturM.
S.
Silva6andAnakeKijjoa1,2,*1ICBAS—InstitutodeCiênciasBiomédicasdeAbelSalazar,UniversidadedoPorto,RuadeJorgeViterboFerreira228,4050-313Porto,Portugal;E-Mails:chadaporn@buu.
ac.
th(C.
P.
);lbessa@ciimar.
up.
pt(L.
J.
B.
);lgales@ibmc.
up.
pt(L.
G.
);pmcosta@icbas.
up.
pt(P.
M.
C.
)2InterdisciplinaryCentreofMarineandEnvironmentalResearch(CIIMAR),RuadosBragas289,4050-123Porto,Portugal3DepartmentofPlantPathology,FacultyofAgriculture,KasetsartUniversity,Bangkok10240,Thailand;E-Mail:agrtdd@ku.
ac.
th4CentrodeQuímicaMedicinaldaUniversidadedoPorto(CEQUIMED-UP)andLaboratóriodeQuímicaOrgnicaeFarmacêutica,DepartamentodeCiênciasQuímicas,FaculdadedeFarmácia,UniversidadedoPorto,RuadeJorgeViterbo228,4050-313Porto,Portugal;E-Mail:madalena@ff.
up.
pt5InstitutodeBiologiaCelulareMolecular(IBMC),UniversidadedoPorto,4099-003Porto,Portugal6DepartamentodeQuímica&QOPNA,UniversidadedeAveiro,4810-193Aveiro,Portugal;E-Mail:artur.
silva@ua.
pt*Authortowhomcorrespondenceshouldbeaddressed;E-Mail:ankijjoa@icbas.
up.
pt;Tel.
:+351-2-2042-8331;Fax:+351-2-2042-8090.
ExternalEditor:OrazioTaglialatela-ScafatiReceived:28July2014;inrevisedform:24September2014/Accepted:25September2014/Published:14October2014Abstract:Twonewisocoumarinderivatives,includinganew5-hydroxy-8-methyl-2H,6H-pyrano[3,4-g]chromen-2,6-dione(1)and6,8-dihydroxy-3,7-dimethylisocoumarin(2b),anewchevalonederivative,namedchevaloneE(3),andanewnaturalproductpyripyropeneS(6)wereisolatedtogetherwith6,8-dihydroxy-3-methylisocoumarin(2a),reticulol(2c),p-hydroxybenzaldehyde,chevaloneB,chevaloneC,S14-95(4),andpyripyropeneE(5)fromtheethylacetateextractoftheundescribedmarinesponge-associatedfungusOPENACCESSMar.
Drugs2014,125161AspergillussimilanensisKUFA0013.
Thestructuresofthenewcompoundswereestablishedbasedon1Dand2DNMRspectralanalysis,andinthecaseofcompound3,X-rayanalysiswasusedtoconfirmitsstructureandtheabsoluteconfigurationofitsstereogeniccarbons.
Compounds1,2a–cand3–6wereevaluatedfortheirantimicrobialactivityagainstGram-positiveandGram-negativebacteria,CandidaalbicansATCC10231,andmultidrug-resistantisolatesfromtheenvironment.
ChevaloneE(3)wasfoundtoshowsynergismwiththeantibioticoxacillinagainstmethicillin-resistantStaphylococcusaureus(MRSA).
Keywords:Aspergillussimilanensis;similanpyrones;isocoumarins;meroditerpenes;pyripyropenes;chevalones1.
IntroductionNeosartoryaisateleomorphic(sexual)stateofAspergillussectionFumigati.
AlthoughNeosartoryaspecies(Trichocomaceae)havenotbeenasextensivelyinvestigatedfortheirsecondarymetabolitesasAspergillus,theyhaverecentlybeenshowntobeaninterestingsourceofmanybioactivecompounds[1–9].
Inourongoingsearchfornewnaturalproductswithantibacterialactivityproducedbythemarine-derivedfungiofthegenusNeosartorya,wehaveinvestigatedthesecondarymetabolitesofaThaicollectionofanewspeciesofNeosartorya,isolatedfromthemarinespongeRhabdermiasp.
,collectedfromtheSimilanIslands,PhangNgaProvince,SouthernThailand.
However,inordertocomplywiththerecent"InternationalCodeofNomenclatureforalgae,fungiandplants(TheMelbourneCode)",thestrainwasrenamedAspergillussimilanensis(KUFA0013).
Theethylacetateextractofitsculturefurnished,besideschevalonesBandC[9,10],p-hydroxybenzaldehyde,reticulol(2c)[11],6,8-dihydroxy-3-methylisocoumarin(2a)[12],ameroterpenoidS14-95(4)[13],pyripyropeneE(5)[14],twonewisocoumarinswhichwehavenamedsimilanpyronesA(1)andB(2b),anewchevaloneanalog(3),andanewnaturalproductwhichwehavenamedpyripyropeneS(6)[15](Figure1).
Compounds1,2a–c,3–6wereevaluatedfortheirantimicrobialactivityagainstGrampositive(StaphylococcusaureusATCC25923andBacillussubtilisATCC6633)andGramnegative(EscherichiacoliATCC25922andPseudomonasaeruginosaATCC27853)bacteria,CandidaalbicansATCC10231,aswellasmultidrug-resistantisolatesfromtheenvironment.
Mar.
Drugs2014,125162Figure1.
SecondarymetabolitesfromAspergillussimilanensisKUFA0013.
2.
ResultsandDiscussionCompound1wasisolatedaswhitesolid(mp,322–323°C),anditsmolecularformulaC13H8O5wasestablishedonthebasisofthe(+)-HRESIMSm/z245.
0450[M+H]+,indicatingtendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3446cm1),conjugatedlactonecarbonyl(1748,1698cm1),aromatic(1658cm1)andolefin(1634,1464cm1)groups.
The13CNMR(SupplementaryInformation,FigureS2),DEPTsandHSQCspectra(Table1,SupplementaryInformation,FigureS4)revealedthepresenceoftwoconjugatedestercarbonyls(δC166.
3and159.
7),sixquaternarysp2(δC101.
3,107.
3,130.
0,140.
1,156.
2and160.
3),fourmethinesp2(δC102.
7,104.
6,114.
1,and137.
8)andonemethyl(δC19.
6)carbons.
The1HNMRspectrum(SupplementaryInformation,FigureS1)revealed,besidesasingletofthehydrogenbondedhydroxylprotonatδH11.
90,twodoubletsofthecis-olefinicprotonsatδH8.
13(J=9.
8Hz)and6.
36(J=9.
8Hz),twosingletsatδH6.
33and6.
70,andonemethylsingletatδH2.
33.
TheCOSYspectrum(Table1;SupplementaryInformation,FigureS3)exhibitedcrosspeakbetweenthesingletatδH6.
33(H-9)andthemethylsingletatδH2.
33(Me-8),suggestingthattheywereallylicallycoupled.
Ontheotherhand,theHMBCspectrum(Table1;SupplementaryInformation,FigureS5)showedcrosspeaksofH-9toC-8(δC156.
2),C-9a(δC130.
3),C-10(δC102.
7),andC-5a(δC101.
3),ofH-10(δH6.
70,s)toC-5a,C-4a(δC107.
3),C-9aandC-10a(δC140.
1),ofMe-8toC-8andC-9(δC104.
6),andofOH-5(δH11.
90)toC-5(δC160.
3),C-5aandC-4a.
Takingtogetherthe1Hand13CchemicalshiftvaluesandtheCOSY,HSQCandHMBCcorrelations(Table1),thepresenceof4a,10a-disubstituted-5-hydroxy-8-methylisochromen-6-onewascorroborated.
Thatthe5-hydroxy-8-methylisochromen-6-onenucleuswasfusedwithapyran-2-onemoietyonC-4aandC-10awassubstantiatedbytheHMBCcorrelationsofH-4(δH8.
13,d,J=9.
8Hz)toC-10a(δC140.
1),andofH-3(δH6.
36,d,J=9.
8Hz)toC-4a(δC107.
3)andC-2(δC159.
7),Mar.
Drugs2014,125163respectively.
Thus,thestructureofcompound1wasestablishedas5-hydroxy-8-methyl-2H,6H-pyrano[3,4-g]chromene-2,6-dione.
Tothebestofourknowledge,thisisthefirstreportontheisolationofasecondarymetabolitewithbothcoumarinandisocoumarinfunctionalitiesinthesamemolecule.
Thus,compound1isanewcompoundwhichwehavenamedsimilanpyroneA.
Table1.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforsimilanpyroneA(1).
PositionδC,TypeδH,(JinHz)COSYHMBC2159.
7,C-3114.
1,CH6.
36,d(9.
8)H-410a4137.
8,CH8.
13,d(9.
8)H-3C-2,4a4a107.
3,C-5160.
3,C-5a101.
3,C-6166.
3,C-8156.
2,C-9104.
6,CH6.
33,sCH3-8C-5a,8,10,Me-89a130.
0,C-10102.
7,CH6.
70,sC-4a,5a,9a,10a10a140.
1,C-CH3-819.
6,CH32.
33,sH-9C-8,9OH-5-11.
90,sC-4a,5,5aCompound2bwasalsoisolatedaswhitesolid(mp,162–163°C),anditsmolecularformulaC11H10O4wasestablishedonthebasisofthe(+)-HRESIMSm/z207.
0658[M+H]+,indicatingsevendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3243,3160cm1),conjugatedcarbonyl(1677cm1),olefin(1635cm1)andaromatic(1617cm1)groups.
Thegeneralfeatureofthe1H(SupplementaryInformation,FigureS6),and13CNMRspectra(SupplementaryInformation,FigureS7)of2b(Table2)closelyresembledthoseof1,exceptfortheabsenceoftheprotonandcarbonsignalsofthepyran-2-onemoiety.
Instead,therewereanadditionalmethyl(δH2.
00s;δC8.
0)andhydroxyl(δH3.
45brs)groupsinthestructureof2b.
ThatthesecondmethylgroupwasonC-7andthesecondhydroxylgroupwasonC-6wascorroboratedbytheHMBCcrosspeaksofthemethylsingletatδH2.
20,stothesignalsofC-6(δC163.
7),C-7(δC109.
6)andC-8(δC106.
9).
Thus,thestructureofcompound2bwasestablishedas6,8-dihydroxy-3,7-dimethylisochromen-1-one.
Literaturesurveyrevealedthat2bisanewcompound,andthereforewehavenameditsimilanpyroneB.
Compound3wasisolatedaswhitecrystals(mp,262–263°C),anditsmolecularformulaC26H38O4wasestablishedonthebasisofthe(+)-HRESIMSm/z415.
2851[M+H]+,indicatingeightdegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3300cm1),conjugatedcarbonyl(1664cm1)andolefin(1607,1570cm1)groups.
The13CNMR(SupplementaryInformation,FigureS9),DEPTsandHSQCspectrarevealedthepresenceofoneconjugatedketonecarbonyl(δC180.
6),threequaternarysp2(δC162.
6,160.
5,98.
5),onemethinesp2(δC111.
9),oneoxygenbearingquaternarysp3(δC84.
3),oneoxygenbearingmethinesp3(δC78.
7),threequaternarysp3(δC37.
1,37.
3,38.
9),threemethinesp3(δC52.
3,55.
3,60.
3),sevenmethylenesp3(δC15.
2,17.
9,18.
7,27.
2,38.
4,40.
1,Mar.
Drugs2014,12516441.
1)andsixmethyl(δC15.
4,16.
1,16.
4,19.
2,20.
5,28.
0)carbons.
Thegeneralfeatureofthe1H(SupplementaryInformation,FigureS8),and13CNMRspectraof3resembledthoseofchevaloneC[9],exceptforthechemicalshiftvaluesoftheoxygenbearingmethinecarbon(C-3)whichappearedatlowerfrequencies(δC78.
7;δH3.
21,dd,J=11.
1,5.
0Hz)thanthoseofchevaloneC[9].
Furthermore,the1Hand13CNMRspectraofcompound3didnotexhibitthesignalsoftheacetylgroup.
TakingtogethertheIR,HRMSandNMRdata,itwaspossibletoconcludethatcompound3isadeacetylanalogofchevaloneC.
Sincethisisthefirstreportofisolationofthischevaloneanalog,wehavenameditchevaloneE.
FinalproofofthestructureandthestereochemistryassignedtochevaloneE(3)wasprovidedbyanX-rayanalysis(Figure2),andsincethediffractiondatawerecollectedwithaGeminiPXUltraequippedwithCuKαradiation,itwaspossibletoestablishtheabsoluteconfigurationofC-3,C-5,C-8,C-9,C-10,C-13andC-14,respectivelyas3S,5R,8R,9R,10R,13Sand14S.
Table2.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforsimilanpyroneB(2b).
PositionδC,TypeδH,(JinHz)COSYHMBC1166.
1,CO-3153.
3,C-4104.
2,CH6.
46,sCH3-3C-5,8a4a136.
5,C-5101.
4,CH6.
40,sC-4,6,7,8a6163.
7,C-7109.
6,C-8160.
0,C-8a97.
5,C-CH3-318.
8,CH32.
20,sC-3,4CH3-78.
0,CH32.
00,sC-6,7,8OH-6-3.
45,brOH-8-11.
27,sC-7,8,8aFigure2.
OrtepviewofchevaloneE(3).
Mar.
Drugs2014,125165The(+)-HRESIMSofcompound6indicatedthe[M+H]+peakatm/z566.
2416,correspondingtoC31H36NO9.
Thus,themolecularformulaofcompound6wasC31H35NO9,indicatingfifteendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforestercarbonyl(1742cm1),conjugatedcarbonyl(1671cm1),aromatic(1586,1508,1465cm1)andolefin(1625cm1)groups.
The13CNMR(SupplementaryInformation,FigureS11),DEPTsandHSQCspectra(Table3)revealedthepresenceofthreeestercarbonyls(δC171.
0,170.
4,169.
8),oneconjugatedcarbonyl(δC161.
3),fivequaternarysp2(δC161.
2,157.
2,144.
5,127.
4,101.
1),sixmethinesp2(δC152.
1,146.
6,133.
1,123.
8,111.
2,98.
6),oneoxyquaternarysp3(δC83.
9),twooxymethinesp3(δC77.
7and73.
2),oneoxymethylenesp3(δC64.
7),twoquaternarysp3(δC40.
6and38.
8),onemethinesp3(δC41.
1),threemethylenesp3(δC35.
5,24.
3and23.
2),andsixmethyl(δC24.
2,21.
3,21.
2,21.
2,20.
8and13.
3)carbons.
Analysisofthe1H(SupplementaryInformation,FigureS10),13C,HSQCandHMBCspectra(Table3)revealedthepresenceof,besidesthreeacetoxylgroups(δC170.
4,21.
2,δH2.
05s;δC171.
0,20.
8,δH2.
10s;δC169.
8,21.
2,δH2.
17,s),ahexasubstituteddecahydronaphthaleneringsystem.
ThattwooftheacetoxylgroupswereonC-1andC-7,andthethreemethylgroupswereonC-4,C-6andC-10ofthedecahydronaphthalenemoietywassubstantiatedbytheHMBCcrosspeaksoftheMe-15singlet(δH0.
88,s)tothecarbonsignalsatδC40.
6(C-10),72.
2(C-1),64.
7(C-11),oftheMe-12singlet(δH1.
26,s)tothecarbonsignalsatδC35.
5(C-3),38.
8(C-4),41.
1(C-9),andoftheMe-14singlet(δH1.
59,s)tothecarbonsignalsatδC77.
7(C-7),83.
9(C-6),and144.
5(C-5).
ThatanothersubstituentonC-10wastheacetoxymethylenegroupwasevidencedbytheHMBCcrosspeaksofH-11signals(δH3.
75,d,J=11.
9Hz;3.
79,d,J=11.
9Hz)toC-1,C-9,andthesignalofthecarbonylatδC171.
0.
Ontheotherhand,sinceMe-14singletgavecrosspeakstothesignalsoftheoxyquaternarycarbonatδC83.
9(C-6)andthequaternarysp2carbonatδC144.
5(C-5),thedoublebondwasonC-5,andC-6wasoxygenbearing.
ThiswascorroboratedbytheHMBCcrosspeaksofthesignaloftheolefinicprotonatδH6.
36,s(H-13)toC-4andC-6.
Moreover,theHMBCspectrumalsoexhibitedacrosspeakofH-13signaltothesignalsofaconjugatedcarbonylcarbonatδC161.
3(C-2′)andthequaternarysp2carbonatδC161.
2(C-4′).
Ontheotherhand,therewerealsoHMBCcrosspeaksofanotherolefinicprotonatδH6.
54,s(H-5′)toC-4′andthesignalsofanothertwoquaternarysp2carbonatδC101.
1(C-3′)and157.
2(C-6′).
TakentogethertheHMBCcorrelations,itwasclearthatthedecahydronaphthaleneringsystemwasfused,onC-5andC-6,with2H,5H-pyrano[4,3-b]pyran-5-oneringsystem.
TheCOSYandHMBCspectraalsoindicatedthepresenceofthe3-substitutedpyridinering.
ThatthispyridineringwasconnectedtothepyranoneringthroughC-3oftheformerandC-6′ofthelaterwasevidencedbytheHMBCcorrelationsoftheH-5′singlettoC-3″(δC127.
4),aswellasofthesignalofH-2″(δH8.
14,dt,J=7.
8,2.
4,2.
4Hz)toC-6′.
Literaturesearchrevealedthatcompound6waspreviouslyobtainedbytreatmentofpyripyropeneAwithHClunderanhydrouscondition[15];however,therewereneitherreportsofthe1Hand13Cdatanorotherdescriptionofthiscompound.
Since6isanewnaturalproduct,wehavenameditpyripyropeneS.
Itisinterestingtopointoutthat6isthefirstnaturalpyripyropenethatlacksahydroxylgrouponC-13.
Mar.
Drugs2014,125166Table3.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforpyripyropeneS(6).
PositionδC,TypeδH,(JinHz)COSYHMBC173.
2,CH4.
79,dd(11.
7,4.
6)H-2223.
2,CH21.
99,mH-11.
76,m335.
5,CH22.
09,mH-2438.
8,C-5144.
5,C-683.
9,C-777.
7,CH5.
23,dd(11.
9,5.
4)H-8C-6824.
3,CH21.
82,ddd(12.
8,5.
1,1.
4)H-7,H-91.
61,m941.
1,CH1.
73,brd(12.
5)H-81040.
6,C-1164.
7,CH23.
75,d(11.
9)C-1,9,CO(OAc-11)3.
79,d(11.
9)1224.
2,CH31.
26,sC-3,4,913111.
2,CH6.
36,sC-4,6,4′1421.
3,CH31.
59,sC-5,6,71513.
3,CH30.
88,sC-1,10,112′161.
3,C-3′101.
1,C-4′161.
2,C-5′98.
6,CH6.
54,sC-2′,3′,6′,3″6′157.
2,C-2″146.
6,CH9.
02,brsH-4″C-3″,4″,6″3″127.
4,C-4″133.
1,CH8.
14,dt(7.
8,1.
4,1.
4)H-2″,5″C-6′,2″,6″5″123.
8,CH7.
42,dd(8.
0,4.
9)H-4″,6″C-3″,6″6″151.
2,CH8.
68,brd(4.
0)H-5″C-5″OAc-1170.
4,CO-21.
2,CH32.
05,sCO(OAc-1)OAc-7169.
8,CO-21.
2,CH32.
17,sCO(OAc-7)OAc-11171.
0,CO-20.
8,CH32.
10,sCO(OAc-11)Compounds1,2a–cand3–6weretestedfortheirantimicrobialactivityagainstGrampositive(StaphylococcusaureusATCC25923andBacillussubtilisATCC6633)andGramnegative(EscherichiacoliATCC25922andPseudomonasaeruginosaATCC27853)bacteria,CandidaalbicansATCC10231,andmultidrug-resistantisolatesfromtheenvironment.
Allthecompoundstestedexhibitedneitherantibacterialnorantifungalactivities,i.
e.
,theirMICvalueswerefoundtobehigherthan256g/mL.
LikechevaloneC,chevaloneE(3)doesnotpossessthestructuralrequirementsfortheantibacterialactivityofthisgroupofmeroditerpenes,i.
e.
,thepresenceoftheβ-acetoxylgrouponC-3andthepresenceofafree4-hydroxy-6-methyl-2H-pyran-2-oneringonC-15[9].
Therefore,itisnotMar.
Drugs2014,125167surprisingthatchevaloneE(3)didnotexhibitsignificantantibacterialactivity.
ThefactthatchevaloneCdidnotshowsignificantantibacterialactivitybutdemonstratedsynergisticeffectwiththeantibioticsagainstthreemultidrug-resistantisolates[9]ledusexploreifsomeofthesecompoundscouldpossiblyhavesynergisticeffectswithantibiotics,i.
e.
,byusingadiscdiffusionmethodtoassessif,incombinationwithantibiotics,theycouldcauseanincreaseinthegrowthinhibitionofmultidrug-resistantstrains.
Theresults(Table4)showedthatnosynergisticeffectswereobservedbetweenthetestedcompoundsandantibioticsformultidrug-resistantE.
coliandE.
faecalis;howeverchevaloneE(3)wasfoundtoexhibitpotentialsynergywithoxacillinandampicillinagainsttheMRSAstrain.
Table4.
Antibacterialefficacyofcombinedeffectofantibioticswiththecompounds(15g/disc)againstthreemultidrug-resistantisolates,usingthediscdiffusionmethod.
CompoundE.
coliG1S.
aureusB1E.
faecalisW1AntibioticsCIPAMPCTXSOXAMPCIPVAAMPE12a2b2c3456()noneffective;(+)slightefficacy—haloofinhibitionoradditionalincreaseinthehaloof1to2.
5mmaroundthedisc;(++)moderateefficacy—from>2.
5to5mm;(+++)goodefficacy—from>5to8mm;CIP:ciprofloxacin;AMP:ampicillin;CTX:cefotaxime;S:streptomycin;OX:oxacillin;VA:vancomycin;E:Erythromycin.
Inordertoverifyifthesynergismoccurredwithbothantibioticsorwitheitherofthem,thecheckerboardmethodwascarriedout.
Theresults,representedbythefractionalinhibitoryconcentration(FIC)index,showninTable5,confirmedthesynergybetweenchevaloneE(3)andoxacillin,andnotbetweenchevaloneE(3)andampicillin.
ItisinterestingtonotethatwhilechevaloneE(3)showssynergismwithoxacillinagainsttheMRSAisolate,thestructurallyrelatedmeroditerpeneaszonapyroneexhibitedsynergismonlywithvancomycinagainsttheVREisolate,andnotwithoxacillinagainsttheMRSAstrain[9].
Table5.
MICvaluesofchevaloneE(3)incombinationwithoxacillinorampicillin,andtherespectiveFICindexobtainedagainstaMRSA(S.
aureusB1)usingthecheckerboardmethod.
StrainMIC(g/mL)S.
aureusB13aloneOXalone3withOXOXwith3FICindex102412864160.
188*S.
aureusB13aloneAMPalone3withAMPAMPwith3FICindex10241285121281.
5*FICindex<0.
5indicatessynergy.
Mar.
Drugs2014,1251683.
ExperimentalSection3.
1.
GeneralProceduresMeltingpointsweredeterminedonaBockmonoscopeandareuncorrected.
OpticalrotationsweredeterminedonanADP410Polarimeter(Bellingham+SyanleyLtd.
,TunbridgeWells,Kent,UK).
InfraredspectrawererecordedonanATTMattsonGenesisSeriesFTIRusingWinFIRSTSoftware.
1Hand13CNMRspectrawererecordedatambienttemperatureonaBrukerAMCinstrument(BrukerBiosciencesCorporation,Billerica,MA,USA)operatingat500.
13and125.
8MHz,respectively.
HighresolutionmassspectraweremeasuredwithaWatersXevoQToFmassspectrometer(WatersCorporations,Milford,MA,USA)coupledtoaWatersAquityUPLCsystem.
AMercksilicagelGF254wasusedforpreparativeTLC,andaMerckSigel60(0.
2–0.
5mm)wasusedforanalyticalchromatography.
3.
2.
ExtractionandIsolationThestrainKUFA0013wasisolatedfromthemarinespongeRhabdermiasp.
,whichwascollectedfromthecoralreefoftheSimilanIslands,PhangNgaProvince,Thailand,byscubadivingat10mdepth,inApril2010,andthespongewasidentifiedbyJ.
Buaruang(DivisionofEnvironmentalScience,FacultyofScience,RamkhamhaengUniversity,Bangkok10240,Thailand).
Briefly,afterrinsingwithsterileseawater,thespongewasdriedonsterilefilterpaperandcutintosmallpieces(5*5mm)andplacedontheplatescontainingmaltextractagar[MEA,30gofmaltextractpowder(Himedia,Mumbai,India),15gofbactoagar,distilledwater300mL,seawater700mLandadjustedtothefinalpHat5.
5]with70%seawaterandincubatedat28°Cunder12hlight/12hdarkcycleforsevendays.
Thefunguswasidentifiedbyoneofus(T.
Dethoup),bymorphologicalfeatures,includingcharacteristicofascospores,conidiogenesisandcolonies,aswellasbyDNAsequenceanalysisofthecalmodulingenedescribedbythepreviousreport[16](GenBankAccessionNo.
KC920702).
SincethesequencewasnotidenticaltothatdepositedatGenBank,thestrainwasnotidentifiedatspecieslevel.
ThepureculturesweredepositedasKUFA0013attheDepartmentofPlantPathology,FacultyofAgriculture,KasetsartUniversity,Bangkok,Thailand.
A.
similanensis(KUFA0013)wasculturedforoneweekinfive90mmPetridishes(i.
d.
90mm)containing25mLofMEAwith70%seawaterperdish.
Thirty1000mLErlenmeyerflasks,eachcontainingwhiterice(200g),water(30mL)andseawater(70mL),wereautoclavedat121°Cfor15min,inoculatedwithtenmyceliaplugsofthefungusandincubatedat28°Cfor30days.
Themoldyricewasmaceratedinethylacetate(7Ltotal)forsevendaysandthenfilteredbyfilterpaper.
Thetwolayerswereseparatedusingaseparatoryfunnel,andtheethylacetatesolutionwasevaporatedunderreducedpressuretoyield97gofcrudeethylacetateextractthatwasdissolvedin500mLofa4:1mixtureofEtOAcandCHCl3,andthenwashedwith5%NaHCO3aqueoussolution(2*300mL)andH2O(3*300mL).
TheorganiclayerwasdriedwithanhydrousNa2SO4,filteredandevaporatedunderreducedpressuretogive75gofcrudeextract,whichwasappliedonacolumnchromatographyofsilicagel(640g)andelutedwithmixturesofCHCl3–petrolandCHCl3–Me2CO,250mLfractionswerecollectedasfollows:Frs1–18(CHCl3–petrol,3:7),19–53(CHCl3–petrol,1:1),54–114(CHCl3–petrol,7:3),115–215(CHCl3–petrol,9:1),216–395(CHCl3–Me2CO,9:1),396–443(CHCl3–Me2CO,7:3).
Frs185–196werecombined(654mg)andpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)toMar.
Drugs2014,125169give4mgof1.
Frs197–221werecombined(1.
16g)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional107.
6mgofyellowsolidwhichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,98:2:0.
01)togive2.
5mgof1.
Fr222(8.
06g)wasrecrystallizedinamixtureofCHCl3andMe2COtogive238mgofwhiteprecipitate,whichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(32.
7mg),4(4.
6mg)andchevaloneB(4.
6mg).
ThemotherliquorwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(41.
0mg),4(7.
2mg)andchevaloneB(70.
8mg).
Themotherliquoroffrs197–221andfr222,andfrs223–224werecombined(9.
18g),appliedontheSigelcolumn(58g),andelutedwithmixturesofpetrol–CHCl3andCHCl3–Me2CO,wherein100mLsfrswerecollectedasfollows:sfrs1-59(petrol–CHCl3,3:7),60–69(petrol–CHCl3,1:9),70–76(CHCl3–Me2CO,9:1).
Sfrs11–22werecombined(1.
97g)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional16.
4mgof1.
Sfrs29–42werecombined(468mg)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional92.
1mgof2b.
Frs225–228werecombined(446mg)andcrystallizedinamixtureofpetrolandCHCl3togive63mgofaprecipitatewhichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(35.
8mg)and2a(35.
4mg).
Themotherliquoroffrs225–228andfrs229–330werecombinedandchromatographedonaSigelcolumn(33g)andelutedwithmixturesofpetrol–CHCl3andCHCl3–Me2CO,wherein100mLsub-fractionswerecollectedasfollows:sfrs1–49(petrol–CHCl3,3:7),50–64(petrol–CHCl3,1:9),65–77(CHCl3–Me2CO,9:1).
Subfrs4–5werecombinedandrecrystallizedinamixtureofpetrolandCHCl3togive1(2.
4mg).
Sfrs6–10werecombined(160mg)andrecrystallizedinamixtureofpetrolandCHCl3togive2c(7.
6mg).
Sfrs11–16werecombined(108mg)andrecrystallizedinamixtureofpetroltogive2b(5mg).
Sfrs27-33werecombined(206mg)andpurifiedbyTLC(Sigel,CHCl3:Me2CO.
93:7)togivep-hydroxybenzaldehyde(36mg).
Frs231–247werecombined(6.
7g)andrecrystallizedinamixtureofpetrolandMe2COtogive1.
39gofchevaloneC.
Frs272–294werecombined(1.
54g)andcrystallizedinamixtureofpetrolandMe2COtoyield5(265mg).
Frs328-335werecombined(296mg)andappliedonacolumnofSephadexLH-20(22g)andelutedwithamixtureofCHCl3–MeOH(9:1)togive3(11.
2mg).
Frs354–398werecombined(1.
14g)andpurifiedbyTLC(Sigel,CHCl3:MeOH:HCO2H,95:5:0.
01)togive6(27.
3mg).
3.
2.
1.
SimilanpyroneA(1)Whitesolid,Mp322–323°C(petrol/CHCl3);UV(CHCl3)λmax(logε)240(4.
31),269(4.
31),295(3.
95),333(4.
23),358(4.
16)nm;IR(KBr)νmax3446,3010,2923,2851,1748,1698,1658,1634,1464,1177,1151cm1;1Hand13CNMR(Table1);HRESIMSm/z245.
0455(M+H)+(calculatedforC13H9O5,245.
0450).
3.
2.
2.
SimilanpyroneB(2b)Whitecrystals,Mp162–163°C(petrol/CHCl3);UV(CHCl3)λmax(logε)240(4.
35),277(3.
51),330(3.
46)nm;IR(KBr)νmax3243,3160,2923,2851,1677,1634,1617,1585,1571,1455,1256,1154,1110cm1;1Hand13CNMR(Table2);HRESIMSm/z207.
0658(M+H)+(calculatedforC11H11O4,207.
0657).
Mar.
Drugs2014,1251703.
2.
3.
ChevaloneE(3)Whitecrystals,Mp262–263°C(petrol/CHCl3);[α]D20146.
3°(c0.
04,CHCl3);IR(KBr)νmax3300,3016,2979,2950,2871,1664,1607,1570,1444,1288cm1;1HNMR(CDCl3,500.
13MHz)δ5.
99(1H,s,H-18),3.
21(1H,d,J=11.
1,5.
0,H-3),2.
55(1H,dd,J=16.
4,4.
9,H2-15),2.
20(3H,s,H3-20),2.
15(1H,m,H2-15),2.
14(1H,m,H2-12),1.
90(1H,dt,J=12.
8,3,H2-7),1.
74(1H,m,H2-11),1.
73(1H,m,H2-1),1.
71(1H,m,H2-12),1.
64(2H,m,H2-2),1.
59(1H,m,H2-6),1.
50(1H,dd,J=12.
5,4.
7,H-14),1.
45(1H,m,H2-6),1.
36(1H,m,H2-11),1.
28(3H,s,H3-26),1.
05(1H,m,H2-7),1.
00(1H,m,H2-1),0.
98(3H,s,H3-23),0.
93(1H,brd,J=13.
2,H-9),0.
89(3H,s,H3-25),0.
84(3H,s,H3-24),0.
78(1H,brd,J=11-6,H-5),0.
78(3H,s,H3-22);13CNMR(CDCl3,125.
8MHz)δ180.
6(CO,C-17),162(C,C-16),160.
5(C,C-21),111.
9(CH,C-18),98.
5(C,C-19),84.
3(C,C-13),78.
7(CH,C-3),60.
3(CH,C-9),55.
3(CH,C-5),52.
3(CH,C-14),41.
1(CH2,C-7),40.
1(CH2,C-12),38.
9(C,C-4),38.
4(CH2,C-1),37.
3(C,C-8),37.
1(C,C-10),28.
0(CH3,C-23),27.
2(CH2,C-2),20.
5(CH3,C-26),19.
2(CH3,C-20),18.
7(CH2,C-11),17.
9(CH2,C-6),16.
4(CH3,C-24),16.
1(CH3,C-25),15.
4(CH3,C-22),15.
2(CH3,C-15);HRESIMSm/z415.
2851(M+H)+(calculatedforC26H39O4,415.
2848).
3.
2.
4.
PyripyropeneS(6)Yellowviscousliquid,[α]D20+116.
3(c0.
04,CHCl3);IR(KBr)νmax2923,2851,1742,1671,1624,1586,1508,1465,1374,1242,1043cm1;1Hand13CNMR(Table3);HRESIMSm/z566.
2415(M+H)+(calculatedforC31H36NO9,566.
2390).
3.
3.
X-rayCrystalStructureofChevaloneE(3)CrystalssuitableforX-raydiffractionwereobtainedbyslowevaporationofasolutioninpetroleumether/chloroform.
Theywereorthorhombic,spacegroupP212121,cellvolume2310.
9(1)3andunitcelldimensionsa=8.
2325(2),b=11.
3341(3)andc=24.
7665(6).
Diffractiondatawerecollectedat293KwithaGeminiPXUltraequippedwithCuKαradiation(λ=1.
54184).
ThestructuresweresolvedbydirectmethodsusingSHELXS-97andrefinedwithSHELXL-97.
Carbon,oxygenandnitrogenatomswererefinedanisotropically.
Hydrogenatomswererefinedfreelywithisotropicdisplacementparameters.
TherefinementconvergedtoR(alldata)=6.
38%andwR2(alldata)=10.
21%.
Towardstheendofrefinementtheabsolutestructureparameterx(Flackxparameter)wasrefinedatthesametimeasallotherparameters,usingtheTWINinstructionwiththedefaultmatrixR=(100,010,001)andBASFwithoneparameter(x),toreachthefinalvalueofx=0.
0(3).
Theinvertedstructure,obtainedwiththeinstructionMOVE1111,yieldedx=1.
5(3).
Tablescontainingthefinalfractionalcoordinates,temperatureparameters,bonddistances,andbondanglesweredepositedwiththeCambridgeCrystallographicDataCentre:CCDCreferencenumber1002416.
3.
4.
AntimicrobialActivityAssays3.
4.
1.
BacterialStrainsFortheantimicrobialassays,thecompoundsweretestedagainst:bacterialreferencestrains(StaphylococcusaureusATCC25923,BacillussubtilisATCC6633,EscherichiacoliATCC25922andMar.
Drugs2014,125171PseudomonasaeruginosaATCC27853),CandidaalbicansATCC10231andmultidrug-resistantbacteriaisolatedfromtheenvironment,S.
aureusB1(isolatedfrompublicbus),EnterococcusfaecalisW1(isolatedfromriverwater)andE.
coliG1(isolatedfromseagullfeces).
BacteriaweregrowninMueller-Hintonagar(MH-BioKardiagnostics,Allonne,France)fromstockcultures,whileC.
albicanswasgrowninSabourauddextroseagar(SAB-BioKardiagnostics,Allonne,France).
MHandSABplateswereincubatedat37°Cpriortoobtainfreshculturesforeachinvitrobioassay.
3.
4.
2.
DeterminationofMinimumInhibitoryandBactericidal/FungalConcentrationsTheminimuminhibitoryconcentrations(MIC)ofthecompoundsweredeterminedusingabrothmicrodilutiontechnique,followingtherecommendationsoftheClinicalandLaboratoryStandardsInstitute[17].
Stocksolutionsof10mg/mL,preparedindimethylsulfoxide(DMSO-ApplichemGmbH,Darmstadt,Germany),wereseriallydilutedinMueller-Hintonbroth(MHB-BioKardiagnostics,Allonne,France)toachievein-testconcentrationsrangingfrom2to256g/mL.
EachbacterialinoculumwaspreparedinMHB,whileC.
albicansinoculumwaspreparedinRPMI-1640withL-glutamine,withMOPSandwithoutNaHCO3(Lonza,Walkersville,MD,USA).
Allinoculawerestandardizedinorderobtainaconcentrationof5*105CFU/mLineachinoculatedwellofthemicrotiterplate.
TheconcentrationofDMSOinthehighestin-testconcentrationdidnotaffectthemicrobialgrowth.
TheMICwasdefinedasthelowestconcentrationofcompoundthathasinhibitedthevisiblegrowth.
3.
4.
3.
SynergisticStudies3.
4.
3.
1.
ScreeningofCombinedEffectbetweentheCompoundsandAntibioticsAscreeningsusceptibilitytesttoassessthecombinedeffectbetweenthecompoundsandantibioticswasconductedusingthediscdiffusionmethodonMH,accordingtotheprocedurealreadydescribedbyGomesetal.
[9].
3.
4.
3.
2.
SynergyTest:CheckerboardMethodBasedontheresultsofthepreviousassay,potentialsynergybetween3andoxacillinorampicillin(Sigma-Aldrich,St.
Louis,MO,USA)wascheckedusingabrothmicrodilutioncheckerboardmethodandtestedinMRSAisolate(S.
aureusB1),ashasbeenalreadydescribed[9].
Twoindependentexperimentsinduplicatewereperformed.
Thefractionalinhibitoryconcentration(FIC)wascalculatedasfollows:FICofdrugA(FICA)=MICofdrugAincombination/MICofdrugAalone,andFICofdrugB(FICB)=MICofdrugBincombination/MICofdrugBalone.
TheFICindex(ΣFIC),calculatedasthesumofeachFIC,wasinterpretedasfollows:ΣFIC≤0.
5,synergy;0.
5<ΣFIC≤4,nointeraction;4<ΣFIC,antagonism[18].
4.
ConclusionsAlthoughseveralanalogsofchevalonehavebeenreportedfromseveralmembersofthegenusAspergillus,thisisthefirstreportofisolationofisocoumarinderivativesfromamemberofthisgenus.
Mar.
Drugs2014,125172ThesynergismofchevaloneEwiththeantibioticoxacillinagainstMRSAcanbeconsideredrelevantforanti-infectivemarinenaturalproductsresearch.
AcknowledgmentsThisworkwaspartiallysupportedbytheProjectMARBIOTECH(referenceNORTE-07-0124-FEDER-000047)withintheSR&TDIntegratedProgramMARVALOR—Buildingresearchandinnovationcapacityforimprovedmanagementandvalorizationofmarineresources,supportedbytheProgramaOperacionalRegionaldoNorte(ON.
2—ONovoNorte)andbytheEuropeanRegionalDevelopmentFund,andalsobyFCT—FundaoparaaCiênciaeaTecnologiaundertheprojectCEQUIMED-PEst-OE/SAU/UI4040/2014,FEDERfundsthroughtheCOMPETEprogramundertheprojectFCOMP-01-0124-FEDER-011057.
WethankMickLeeoftheDepartmentofChemistry,LeicesterUniversity(UK),forprovidingtheHRESIMS.
C.
P.
thankstheFacultyofPharmaceuticalSciences,BuraphaUniversity,ThailandforherscholarshiptotheUniversityofPorto.
WethankJúliaBessaandSaraCravofortechnicalsupport.
AuthorContributionsPrompanya,C.
performedisolation,purificationandstructureelucidationofsomecompounds;Kijjoa,A.
andPinto,M.
M.
M.
conceived,designedtheresearch,elucidatedthestructureofthecompoundsandwrotethepaper;Dethoup,T.
isolated,identified,culturedthefungi,andpreparedthecrudeextract;Gales,L.
performedX-raycrystallographyofcompound3;Silva,A.
M.
S.
providedtheNMRspectra;Bessa,L.
J.
andCosta,P.
M.
performedantibacterialactivity.
ConflictsofInterestTheauthorsdeclarenoconflictofinterest.
References1.
Asami,Y.
;Kakeya,H.
;Onose,R.
;Yoshida,A.
;Matsuzaki,H.
;Osada,H.
Azaspirene:Anovelangiogenesisinhibitorcontaininga1-oxa-7-azaspiro[4.
4]non-2-ene-4,6-dioneskeletonproducedbythefungusNeosartoryasp.
Org.
Lett.
2002,4,2845–2848.
2.
Asami,Y.
;Kakeya,H.
;Onose,R.
;Chang,Y.
H.
;Toi,M.
;Osada,H.
RK-805,anendothelial-cell-growthinhibitorproducedbyNeosartoryasp.
,andadockingmodelwithmethionineaminopeptidase-2.
Tetrahedron2004,60,7085–7091.
3.
Jayasuriya,H.
;Zink,D.
;Basilio,A.
;Vicente,F.
;Collado,J.
;Bills,G.
;Goldman,M.
L.
;Motyl,M.
;Huber,J.
;Dezeny,G.
;etal.
DiscoveryandantibacterialactivityofglabramycinA–CfromNeosartoryaglabrabyanantisensestrategy.
J.
Antibiotics2009,62,265–269.
4.
Yang,S.
S.
;Wang,G.
J.
;Cheng,K.
F.
;Chen,C.
H.
;Ju,Y.
M.
;Tsau,Y.
J.
;Lee,T.
H.
Bioactiveγ-lactonesfromthefermentedbrothofNeosartoryasp.
PlantaMed.
2010,76,1701–1705.
5.
Kijjoa,A.
;Santos,S.
;Dethoup,T.
;Manoch,L.
;Almeida,A.
P.
;Vasconcelos,M.
H.
;Silva,A.
;Gales,L.
;Herz,W.
Sartoryglabrins,analogsofardeemins,fromNeosartoryaglabra.
Nat.
Prod.
Commun.
2011,6,807–812.
Mar.
Drugs2014,1251736.
Eamvijarn,A.
;Kijjoa,A.
;Bruyère,C.
;Mathieu,V.
;Manoch,V.
;LeFranc,F.
;Silva,A.
;Kiss,R.
;Herz,W.
SecondarymetabolitesfromacultureofthefungusNeosartoryapseudofischeriandtheirinvitrocytostaticactivityinhumancancercells.
PlantaMed.
2012,78,1767–1776.
7.
Buttachon,S.
;Chandrapatya,A.
;Manoch,L.
;Silva,A.
;Gales,L.
;Bruyère,C.
;Kiss,R.
;Kijjoa,A.
Sartorymensin,anewindolealkaloid,andnewanaloguesoftryptoquivalineandfiscalinsproducedbyNeosartoryasiamensis(KUFC6349).
Tetrahedron2012,68,3253–3262.
8.
Eamvijarn,A.
;Gomes,N.
M.
;Dethoup,T.
;Buaruang,J.
;Manoch,L.
;Silva,A.
;Pedro,M.
;Marini,I.
;Roussis,V.
;Kijjoa,A.
BioactivemeroditerpenesandindolealkaloidsfromthesoilfungusNeosartoryafischeri(KUFC6344),andthemarine-derivedfungiNeosartoryalaciniosa(KUFC7896)andNeosartoryatsunodae(KUFC9213).
Tetrahedron2013,69,8583–8591.
9.
Gomes,N.
M.
;Bessa,L.
J.
;Buttachon,S.
;Costa,P.
M.
;Buaruang,J.
;Dethoup,T.
;Silva,A.
M.
S.
;Kijjoa,A.
AntibacterialandAntibiofilmactivitiesoftryptoquivalinesandmeroditerpenesisolatedfromthemarine-derivedfungiNeosartoryapaulistensis,N.
laciniosa,N.
tsunodae,andtheSoilFungiN.
fischeriandN.
siamensis.
Mar.
Drugs2014,12,822–839.
10.
Kanokmedhakul,K.
;Kanokmedhakul,S.
;Suwannatrai,R.
;Soytong,K.
;Prabpai,S.
;Kongsaeree,P.
BioactivemeroterpenoidsandalkaloidsfromthefungusEurotiumchevalieri.
Tetrahedron2011,67,5461–5468.
11.
Ryoo,I.
J.
;Xu,G.
H.
;Km,Y.
H.
;Choo,S.
J.
;Ahn,J.
S.
;Bae,K.
;Yoo,I.
D.
Reticulone,anovelfreeradicalscavengerproducedbyAspergillussp.
J.
Microbiol.
Biotechnol.
2009,12,1573–1575.
12.
Gallo,M.
B.
C.
;Cavalcanti,B.
C.
;Barros,F.
W.
A.
;Moraes,M.
O.
;Costa-Latufo,L.
V.
;Pessoa,C.
;Bastos,J.
K.
;Pupo,M.
T.
ChemicalconstituentsofPapulasporaimmersa,anendophytefromSmallanthussonchifolius(Asteraceae),andtheircytotoxicactivity.
Chem.
Biodivers.
2010,7,2941–2950.
13.
Erkel,G.
;Rether,J.
;Anke,T.
S14-95,anovelinhibitoroftheJAK/STATpathwayfromaPenicilliumspecies.
J.
Antibiotics2003,56,337–343.
14.
Tomoda,H.
;Tabata,N.
;Yang,D.
J.
;Takaya.
naki,H.
;Nishida,M.
;Omura,S.
Pyripyropenes,novelACATinhibitorsproducedbyAspergillusfumigatus.
III.
StructureelucidationofpyripyropenesEtoL.
J.
Antibiotics1995,48,495–503.
15.
Obata,R.
;Sunazuka,T.
;Tomoda,H.
;Harigaya,Y.
;Omura,S.
ChemicalModificationandstructure-activityrelationshipsofpyripyropenes,potent,bioavailableinhibitorofacyl-CoA:cholesterolO-acyltransferase(ACAT).
Bioorg.
Med.
Chem.
Lett.
1995,5,2683–2688.
16.
Glass,N.
L.
;Donaldson,G.
C.
DevelopmentofprimersetsdesignedforusewiththePCRtoamplifyconservedgenesfromfilamentousascomycetes.
Appl.
Environ.
Microbiol.
1995,61,1323–1330.
17.
Franklin,R.
;Cockerill,M.
D.
,III.
PerformanceStandardsforAntimicrombialSusceptibilityTesting.
Twenty-FirstInformationalSupplementM100-S21;ClinicalandLaboratoryStandardsInstitute(CLSI):Wayne,PA,USA,2011.
18.
Odds,F.
C.
Synergy,antagonism,andwhatthechequerboardputsbetweenthem.
J.
Antimicrob.
Chemother2003,52,1.
2014bytheauthors;licenseeMDPI,Basel,Switzerland.
ThisarticleisanopenaccessarticledistributedunderthetermsandconditionsoftheCreativeCommonsAttributionlicense(http://creativecommons.
org/licenses/by/4.
0/).
Drugs2014,12,5160-5173;doi:10.
3390/md12105160marinedrugsISSN1660-3397www.
mdpi.
com/journal/marinedrugsArticleNewIsocoumarinDerivativesandMeroterpenoidsfromtheMarineSponge-AssociatedFungusAspergillussimilanensissp.
nov.
KUFA0013ChadapornPrompanya1,2,TidaDethoup3,LucindaJ.
Bessa1,2,MadalenaM.
M.
Pinto2,4,LuísGales1,5,PauloM.
Costa1,2,ArturM.
S.
Silva6andAnakeKijjoa1,2,*1ICBAS—InstitutodeCiênciasBiomédicasdeAbelSalazar,UniversidadedoPorto,RuadeJorgeViterboFerreira228,4050-313Porto,Portugal;E-Mails:chadaporn@buu.
ac.
th(C.
P.
);lbessa@ciimar.
up.
pt(L.
J.
B.
);lgales@ibmc.
up.
pt(L.
G.
);pmcosta@icbas.
up.
pt(P.
M.
C.
)2InterdisciplinaryCentreofMarineandEnvironmentalResearch(CIIMAR),RuadosBragas289,4050-123Porto,Portugal3DepartmentofPlantPathology,FacultyofAgriculture,KasetsartUniversity,Bangkok10240,Thailand;E-Mail:agrtdd@ku.
ac.
th4CentrodeQuímicaMedicinaldaUniversidadedoPorto(CEQUIMED-UP)andLaboratóriodeQuímicaOrgnicaeFarmacêutica,DepartamentodeCiênciasQuímicas,FaculdadedeFarmácia,UniversidadedoPorto,RuadeJorgeViterbo228,4050-313Porto,Portugal;E-Mail:madalena@ff.
up.
pt5InstitutodeBiologiaCelulareMolecular(IBMC),UniversidadedoPorto,4099-003Porto,Portugal6DepartamentodeQuímica&QOPNA,UniversidadedeAveiro,4810-193Aveiro,Portugal;E-Mail:artur.
silva@ua.
pt*Authortowhomcorrespondenceshouldbeaddressed;E-Mail:ankijjoa@icbas.
up.
pt;Tel.
:+351-2-2042-8331;Fax:+351-2-2042-8090.
ExternalEditor:OrazioTaglialatela-ScafatiReceived:28July2014;inrevisedform:24September2014/Accepted:25September2014/Published:14October2014Abstract:Twonewisocoumarinderivatives,includinganew5-hydroxy-8-methyl-2H,6H-pyrano[3,4-g]chromen-2,6-dione(1)and6,8-dihydroxy-3,7-dimethylisocoumarin(2b),anewchevalonederivative,namedchevaloneE(3),andanewnaturalproductpyripyropeneS(6)wereisolatedtogetherwith6,8-dihydroxy-3-methylisocoumarin(2a),reticulol(2c),p-hydroxybenzaldehyde,chevaloneB,chevaloneC,S14-95(4),andpyripyropeneE(5)fromtheethylacetateextractoftheundescribedmarinesponge-associatedfungusOPENACCESSMar.
Drugs2014,125161AspergillussimilanensisKUFA0013.
Thestructuresofthenewcompoundswereestablishedbasedon1Dand2DNMRspectralanalysis,andinthecaseofcompound3,X-rayanalysiswasusedtoconfirmitsstructureandtheabsoluteconfigurationofitsstereogeniccarbons.
Compounds1,2a–cand3–6wereevaluatedfortheirantimicrobialactivityagainstGram-positiveandGram-negativebacteria,CandidaalbicansATCC10231,andmultidrug-resistantisolatesfromtheenvironment.
ChevaloneE(3)wasfoundtoshowsynergismwiththeantibioticoxacillinagainstmethicillin-resistantStaphylococcusaureus(MRSA).
Keywords:Aspergillussimilanensis;similanpyrones;isocoumarins;meroditerpenes;pyripyropenes;chevalones1.
IntroductionNeosartoryaisateleomorphic(sexual)stateofAspergillussectionFumigati.
AlthoughNeosartoryaspecies(Trichocomaceae)havenotbeenasextensivelyinvestigatedfortheirsecondarymetabolitesasAspergillus,theyhaverecentlybeenshowntobeaninterestingsourceofmanybioactivecompounds[1–9].
Inourongoingsearchfornewnaturalproductswithantibacterialactivityproducedbythemarine-derivedfungiofthegenusNeosartorya,wehaveinvestigatedthesecondarymetabolitesofaThaicollectionofanewspeciesofNeosartorya,isolatedfromthemarinespongeRhabdermiasp.
,collectedfromtheSimilanIslands,PhangNgaProvince,SouthernThailand.
However,inordertocomplywiththerecent"InternationalCodeofNomenclatureforalgae,fungiandplants(TheMelbourneCode)",thestrainwasrenamedAspergillussimilanensis(KUFA0013).
Theethylacetateextractofitsculturefurnished,besideschevalonesBandC[9,10],p-hydroxybenzaldehyde,reticulol(2c)[11],6,8-dihydroxy-3-methylisocoumarin(2a)[12],ameroterpenoidS14-95(4)[13],pyripyropeneE(5)[14],twonewisocoumarinswhichwehavenamedsimilanpyronesA(1)andB(2b),anewchevaloneanalog(3),andanewnaturalproductwhichwehavenamedpyripyropeneS(6)[15](Figure1).
Compounds1,2a–c,3–6wereevaluatedfortheirantimicrobialactivityagainstGrampositive(StaphylococcusaureusATCC25923andBacillussubtilisATCC6633)andGramnegative(EscherichiacoliATCC25922andPseudomonasaeruginosaATCC27853)bacteria,CandidaalbicansATCC10231,aswellasmultidrug-resistantisolatesfromtheenvironment.
Mar.
Drugs2014,125162Figure1.
SecondarymetabolitesfromAspergillussimilanensisKUFA0013.
2.
ResultsandDiscussionCompound1wasisolatedaswhitesolid(mp,322–323°C),anditsmolecularformulaC13H8O5wasestablishedonthebasisofthe(+)-HRESIMSm/z245.
0450[M+H]+,indicatingtendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3446cm1),conjugatedlactonecarbonyl(1748,1698cm1),aromatic(1658cm1)andolefin(1634,1464cm1)groups.
The13CNMR(SupplementaryInformation,FigureS2),DEPTsandHSQCspectra(Table1,SupplementaryInformation,FigureS4)revealedthepresenceoftwoconjugatedestercarbonyls(δC166.
3and159.
7),sixquaternarysp2(δC101.
3,107.
3,130.
0,140.
1,156.
2and160.
3),fourmethinesp2(δC102.
7,104.
6,114.
1,and137.
8)andonemethyl(δC19.
6)carbons.
The1HNMRspectrum(SupplementaryInformation,FigureS1)revealed,besidesasingletofthehydrogenbondedhydroxylprotonatδH11.
90,twodoubletsofthecis-olefinicprotonsatδH8.
13(J=9.
8Hz)and6.
36(J=9.
8Hz),twosingletsatδH6.
33and6.
70,andonemethylsingletatδH2.
33.
TheCOSYspectrum(Table1;SupplementaryInformation,FigureS3)exhibitedcrosspeakbetweenthesingletatδH6.
33(H-9)andthemethylsingletatδH2.
33(Me-8),suggestingthattheywereallylicallycoupled.
Ontheotherhand,theHMBCspectrum(Table1;SupplementaryInformation,FigureS5)showedcrosspeaksofH-9toC-8(δC156.
2),C-9a(δC130.
3),C-10(δC102.
7),andC-5a(δC101.
3),ofH-10(δH6.
70,s)toC-5a,C-4a(δC107.
3),C-9aandC-10a(δC140.
1),ofMe-8toC-8andC-9(δC104.
6),andofOH-5(δH11.
90)toC-5(δC160.
3),C-5aandC-4a.
Takingtogetherthe1Hand13CchemicalshiftvaluesandtheCOSY,HSQCandHMBCcorrelations(Table1),thepresenceof4a,10a-disubstituted-5-hydroxy-8-methylisochromen-6-onewascorroborated.
Thatthe5-hydroxy-8-methylisochromen-6-onenucleuswasfusedwithapyran-2-onemoietyonC-4aandC-10awassubstantiatedbytheHMBCcorrelationsofH-4(δH8.
13,d,J=9.
8Hz)toC-10a(δC140.
1),andofH-3(δH6.
36,d,J=9.
8Hz)toC-4a(δC107.
3)andC-2(δC159.
7),Mar.
Drugs2014,125163respectively.
Thus,thestructureofcompound1wasestablishedas5-hydroxy-8-methyl-2H,6H-pyrano[3,4-g]chromene-2,6-dione.
Tothebestofourknowledge,thisisthefirstreportontheisolationofasecondarymetabolitewithbothcoumarinandisocoumarinfunctionalitiesinthesamemolecule.
Thus,compound1isanewcompoundwhichwehavenamedsimilanpyroneA.
Table1.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforsimilanpyroneA(1).
PositionδC,TypeδH,(JinHz)COSYHMBC2159.
7,C-3114.
1,CH6.
36,d(9.
8)H-410a4137.
8,CH8.
13,d(9.
8)H-3C-2,4a4a107.
3,C-5160.
3,C-5a101.
3,C-6166.
3,C-8156.
2,C-9104.
6,CH6.
33,sCH3-8C-5a,8,10,Me-89a130.
0,C-10102.
7,CH6.
70,sC-4a,5a,9a,10a10a140.
1,C-CH3-819.
6,CH32.
33,sH-9C-8,9OH-5-11.
90,sC-4a,5,5aCompound2bwasalsoisolatedaswhitesolid(mp,162–163°C),anditsmolecularformulaC11H10O4wasestablishedonthebasisofthe(+)-HRESIMSm/z207.
0658[M+H]+,indicatingsevendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3243,3160cm1),conjugatedcarbonyl(1677cm1),olefin(1635cm1)andaromatic(1617cm1)groups.
Thegeneralfeatureofthe1H(SupplementaryInformation,FigureS6),and13CNMRspectra(SupplementaryInformation,FigureS7)of2b(Table2)closelyresembledthoseof1,exceptfortheabsenceoftheprotonandcarbonsignalsofthepyran-2-onemoiety.
Instead,therewereanadditionalmethyl(δH2.
00s;δC8.
0)andhydroxyl(δH3.
45brs)groupsinthestructureof2b.
ThatthesecondmethylgroupwasonC-7andthesecondhydroxylgroupwasonC-6wascorroboratedbytheHMBCcrosspeaksofthemethylsingletatδH2.
20,stothesignalsofC-6(δC163.
7),C-7(δC109.
6)andC-8(δC106.
9).
Thus,thestructureofcompound2bwasestablishedas6,8-dihydroxy-3,7-dimethylisochromen-1-one.
Literaturesurveyrevealedthat2bisanewcompound,andthereforewehavenameditsimilanpyroneB.
Compound3wasisolatedaswhitecrystals(mp,262–263°C),anditsmolecularformulaC26H38O4wasestablishedonthebasisofthe(+)-HRESIMSm/z415.
2851[M+H]+,indicatingeightdegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforhydroxyl(3300cm1),conjugatedcarbonyl(1664cm1)andolefin(1607,1570cm1)groups.
The13CNMR(SupplementaryInformation,FigureS9),DEPTsandHSQCspectrarevealedthepresenceofoneconjugatedketonecarbonyl(δC180.
6),threequaternarysp2(δC162.
6,160.
5,98.
5),onemethinesp2(δC111.
9),oneoxygenbearingquaternarysp3(δC84.
3),oneoxygenbearingmethinesp3(δC78.
7),threequaternarysp3(δC37.
1,37.
3,38.
9),threemethinesp3(δC52.
3,55.
3,60.
3),sevenmethylenesp3(δC15.
2,17.
9,18.
7,27.
2,38.
4,40.
1,Mar.
Drugs2014,12516441.
1)andsixmethyl(δC15.
4,16.
1,16.
4,19.
2,20.
5,28.
0)carbons.
Thegeneralfeatureofthe1H(SupplementaryInformation,FigureS8),and13CNMRspectraof3resembledthoseofchevaloneC[9],exceptforthechemicalshiftvaluesoftheoxygenbearingmethinecarbon(C-3)whichappearedatlowerfrequencies(δC78.
7;δH3.
21,dd,J=11.
1,5.
0Hz)thanthoseofchevaloneC[9].
Furthermore,the1Hand13CNMRspectraofcompound3didnotexhibitthesignalsoftheacetylgroup.
TakingtogethertheIR,HRMSandNMRdata,itwaspossibletoconcludethatcompound3isadeacetylanalogofchevaloneC.
Sincethisisthefirstreportofisolationofthischevaloneanalog,wehavenameditchevaloneE.
FinalproofofthestructureandthestereochemistryassignedtochevaloneE(3)wasprovidedbyanX-rayanalysis(Figure2),andsincethediffractiondatawerecollectedwithaGeminiPXUltraequippedwithCuKαradiation,itwaspossibletoestablishtheabsoluteconfigurationofC-3,C-5,C-8,C-9,C-10,C-13andC-14,respectivelyas3S,5R,8R,9R,10R,13Sand14S.
Table2.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforsimilanpyroneB(2b).
PositionδC,TypeδH,(JinHz)COSYHMBC1166.
1,CO-3153.
3,C-4104.
2,CH6.
46,sCH3-3C-5,8a4a136.
5,C-5101.
4,CH6.
40,sC-4,6,7,8a6163.
7,C-7109.
6,C-8160.
0,C-8a97.
5,C-CH3-318.
8,CH32.
20,sC-3,4CH3-78.
0,CH32.
00,sC-6,7,8OH-6-3.
45,brOH-8-11.
27,sC-7,8,8aFigure2.
OrtepviewofchevaloneE(3).
Mar.
Drugs2014,125165The(+)-HRESIMSofcompound6indicatedthe[M+H]+peakatm/z566.
2416,correspondingtoC31H36NO9.
Thus,themolecularformulaofcompound6wasC31H35NO9,indicatingfifteendegreesofunsaturation.
TheIRspectrumshowedabsorptionbandsforestercarbonyl(1742cm1),conjugatedcarbonyl(1671cm1),aromatic(1586,1508,1465cm1)andolefin(1625cm1)groups.
The13CNMR(SupplementaryInformation,FigureS11),DEPTsandHSQCspectra(Table3)revealedthepresenceofthreeestercarbonyls(δC171.
0,170.
4,169.
8),oneconjugatedcarbonyl(δC161.
3),fivequaternarysp2(δC161.
2,157.
2,144.
5,127.
4,101.
1),sixmethinesp2(δC152.
1,146.
6,133.
1,123.
8,111.
2,98.
6),oneoxyquaternarysp3(δC83.
9),twooxymethinesp3(δC77.
7and73.
2),oneoxymethylenesp3(δC64.
7),twoquaternarysp3(δC40.
6and38.
8),onemethinesp3(δC41.
1),threemethylenesp3(δC35.
5,24.
3and23.
2),andsixmethyl(δC24.
2,21.
3,21.
2,21.
2,20.
8and13.
3)carbons.
Analysisofthe1H(SupplementaryInformation,FigureS10),13C,HSQCandHMBCspectra(Table3)revealedthepresenceof,besidesthreeacetoxylgroups(δC170.
4,21.
2,δH2.
05s;δC171.
0,20.
8,δH2.
10s;δC169.
8,21.
2,δH2.
17,s),ahexasubstituteddecahydronaphthaleneringsystem.
ThattwooftheacetoxylgroupswereonC-1andC-7,andthethreemethylgroupswereonC-4,C-6andC-10ofthedecahydronaphthalenemoietywassubstantiatedbytheHMBCcrosspeaksoftheMe-15singlet(δH0.
88,s)tothecarbonsignalsatδC40.
6(C-10),72.
2(C-1),64.
7(C-11),oftheMe-12singlet(δH1.
26,s)tothecarbonsignalsatδC35.
5(C-3),38.
8(C-4),41.
1(C-9),andoftheMe-14singlet(δH1.
59,s)tothecarbonsignalsatδC77.
7(C-7),83.
9(C-6),and144.
5(C-5).
ThatanothersubstituentonC-10wastheacetoxymethylenegroupwasevidencedbytheHMBCcrosspeaksofH-11signals(δH3.
75,d,J=11.
9Hz;3.
79,d,J=11.
9Hz)toC-1,C-9,andthesignalofthecarbonylatδC171.
0.
Ontheotherhand,sinceMe-14singletgavecrosspeakstothesignalsoftheoxyquaternarycarbonatδC83.
9(C-6)andthequaternarysp2carbonatδC144.
5(C-5),thedoublebondwasonC-5,andC-6wasoxygenbearing.
ThiswascorroboratedbytheHMBCcrosspeaksofthesignaloftheolefinicprotonatδH6.
36,s(H-13)toC-4andC-6.
Moreover,theHMBCspectrumalsoexhibitedacrosspeakofH-13signaltothesignalsofaconjugatedcarbonylcarbonatδC161.
3(C-2′)andthequaternarysp2carbonatδC161.
2(C-4′).
Ontheotherhand,therewerealsoHMBCcrosspeaksofanotherolefinicprotonatδH6.
54,s(H-5′)toC-4′andthesignalsofanothertwoquaternarysp2carbonatδC101.
1(C-3′)and157.
2(C-6′).
TakentogethertheHMBCcorrelations,itwasclearthatthedecahydronaphthaleneringsystemwasfused,onC-5andC-6,with2H,5H-pyrano[4,3-b]pyran-5-oneringsystem.
TheCOSYandHMBCspectraalsoindicatedthepresenceofthe3-substitutedpyridinering.
ThatthispyridineringwasconnectedtothepyranoneringthroughC-3oftheformerandC-6′ofthelaterwasevidencedbytheHMBCcorrelationsoftheH-5′singlettoC-3″(δC127.
4),aswellasofthesignalofH-2″(δH8.
14,dt,J=7.
8,2.
4,2.
4Hz)toC-6′.
Literaturesearchrevealedthatcompound6waspreviouslyobtainedbytreatmentofpyripyropeneAwithHClunderanhydrouscondition[15];however,therewereneitherreportsofthe1Hand13Cdatanorotherdescriptionofthiscompound.
Since6isanewnaturalproduct,wehavenameditpyripyropeneS.
Itisinterestingtopointoutthat6isthefirstnaturalpyripyropenethatlacksahydroxylgrouponC-13.
Mar.
Drugs2014,125166Table3.
1Hand13CNMR(CDCl3,500.
13MHzand125.
8MHz)andHMBCassignmentforpyripyropeneS(6).
PositionδC,TypeδH,(JinHz)COSYHMBC173.
2,CH4.
79,dd(11.
7,4.
6)H-2223.
2,CH21.
99,mH-11.
76,m335.
5,CH22.
09,mH-2438.
8,C-5144.
5,C-683.
9,C-777.
7,CH5.
23,dd(11.
9,5.
4)H-8C-6824.
3,CH21.
82,ddd(12.
8,5.
1,1.
4)H-7,H-91.
61,m941.
1,CH1.
73,brd(12.
5)H-81040.
6,C-1164.
7,CH23.
75,d(11.
9)C-1,9,CO(OAc-11)3.
79,d(11.
9)1224.
2,CH31.
26,sC-3,4,913111.
2,CH6.
36,sC-4,6,4′1421.
3,CH31.
59,sC-5,6,71513.
3,CH30.
88,sC-1,10,112′161.
3,C-3′101.
1,C-4′161.
2,C-5′98.
6,CH6.
54,sC-2′,3′,6′,3″6′157.
2,C-2″146.
6,CH9.
02,brsH-4″C-3″,4″,6″3″127.
4,C-4″133.
1,CH8.
14,dt(7.
8,1.
4,1.
4)H-2″,5″C-6′,2″,6″5″123.
8,CH7.
42,dd(8.
0,4.
9)H-4″,6″C-3″,6″6″151.
2,CH8.
68,brd(4.
0)H-5″C-5″OAc-1170.
4,CO-21.
2,CH32.
05,sCO(OAc-1)OAc-7169.
8,CO-21.
2,CH32.
17,sCO(OAc-7)OAc-11171.
0,CO-20.
8,CH32.
10,sCO(OAc-11)Compounds1,2a–cand3–6weretestedfortheirantimicrobialactivityagainstGrampositive(StaphylococcusaureusATCC25923andBacillussubtilisATCC6633)andGramnegative(EscherichiacoliATCC25922andPseudomonasaeruginosaATCC27853)bacteria,CandidaalbicansATCC10231,andmultidrug-resistantisolatesfromtheenvironment.
Allthecompoundstestedexhibitedneitherantibacterialnorantifungalactivities,i.
e.
,theirMICvalueswerefoundtobehigherthan256g/mL.
LikechevaloneC,chevaloneE(3)doesnotpossessthestructuralrequirementsfortheantibacterialactivityofthisgroupofmeroditerpenes,i.
e.
,thepresenceoftheβ-acetoxylgrouponC-3andthepresenceofafree4-hydroxy-6-methyl-2H-pyran-2-oneringonC-15[9].
Therefore,itisnotMar.
Drugs2014,125167surprisingthatchevaloneE(3)didnotexhibitsignificantantibacterialactivity.
ThefactthatchevaloneCdidnotshowsignificantantibacterialactivitybutdemonstratedsynergisticeffectwiththeantibioticsagainstthreemultidrug-resistantisolates[9]ledusexploreifsomeofthesecompoundscouldpossiblyhavesynergisticeffectswithantibiotics,i.
e.
,byusingadiscdiffusionmethodtoassessif,incombinationwithantibiotics,theycouldcauseanincreaseinthegrowthinhibitionofmultidrug-resistantstrains.
Theresults(Table4)showedthatnosynergisticeffectswereobservedbetweenthetestedcompoundsandantibioticsformultidrug-resistantE.
coliandE.
faecalis;howeverchevaloneE(3)wasfoundtoexhibitpotentialsynergywithoxacillinandampicillinagainsttheMRSAstrain.
Table4.
Antibacterialefficacyofcombinedeffectofantibioticswiththecompounds(15g/disc)againstthreemultidrug-resistantisolates,usingthediscdiffusionmethod.
CompoundE.
coliG1S.
aureusB1E.
faecalisW1AntibioticsCIPAMPCTXSOXAMPCIPVAAMPE12a2b2c3456()noneffective;(+)slightefficacy—haloofinhibitionoradditionalincreaseinthehaloof1to2.
5mmaroundthedisc;(++)moderateefficacy—from>2.
5to5mm;(+++)goodefficacy—from>5to8mm;CIP:ciprofloxacin;AMP:ampicillin;CTX:cefotaxime;S:streptomycin;OX:oxacillin;VA:vancomycin;E:Erythromycin.
Inordertoverifyifthesynergismoccurredwithbothantibioticsorwitheitherofthem,thecheckerboardmethodwascarriedout.
Theresults,representedbythefractionalinhibitoryconcentration(FIC)index,showninTable5,confirmedthesynergybetweenchevaloneE(3)andoxacillin,andnotbetweenchevaloneE(3)andampicillin.
ItisinterestingtonotethatwhilechevaloneE(3)showssynergismwithoxacillinagainsttheMRSAisolate,thestructurallyrelatedmeroditerpeneaszonapyroneexhibitedsynergismonlywithvancomycinagainsttheVREisolate,andnotwithoxacillinagainsttheMRSAstrain[9].
Table5.
MICvaluesofchevaloneE(3)incombinationwithoxacillinorampicillin,andtherespectiveFICindexobtainedagainstaMRSA(S.
aureusB1)usingthecheckerboardmethod.
StrainMIC(g/mL)S.
aureusB13aloneOXalone3withOXOXwith3FICindex102412864160.
188*S.
aureusB13aloneAMPalone3withAMPAMPwith3FICindex10241285121281.
5*FICindex<0.
5indicatessynergy.
Mar.
Drugs2014,1251683.
ExperimentalSection3.
1.
GeneralProceduresMeltingpointsweredeterminedonaBockmonoscopeandareuncorrected.
OpticalrotationsweredeterminedonanADP410Polarimeter(Bellingham+SyanleyLtd.
,TunbridgeWells,Kent,UK).
InfraredspectrawererecordedonanATTMattsonGenesisSeriesFTIRusingWinFIRSTSoftware.
1Hand13CNMRspectrawererecordedatambienttemperatureonaBrukerAMCinstrument(BrukerBiosciencesCorporation,Billerica,MA,USA)operatingat500.
13and125.
8MHz,respectively.
HighresolutionmassspectraweremeasuredwithaWatersXevoQToFmassspectrometer(WatersCorporations,Milford,MA,USA)coupledtoaWatersAquityUPLCsystem.
AMercksilicagelGF254wasusedforpreparativeTLC,andaMerckSigel60(0.
2–0.
5mm)wasusedforanalyticalchromatography.
3.
2.
ExtractionandIsolationThestrainKUFA0013wasisolatedfromthemarinespongeRhabdermiasp.
,whichwascollectedfromthecoralreefoftheSimilanIslands,PhangNgaProvince,Thailand,byscubadivingat10mdepth,inApril2010,andthespongewasidentifiedbyJ.
Buaruang(DivisionofEnvironmentalScience,FacultyofScience,RamkhamhaengUniversity,Bangkok10240,Thailand).
Briefly,afterrinsingwithsterileseawater,thespongewasdriedonsterilefilterpaperandcutintosmallpieces(5*5mm)andplacedontheplatescontainingmaltextractagar[MEA,30gofmaltextractpowder(Himedia,Mumbai,India),15gofbactoagar,distilledwater300mL,seawater700mLandadjustedtothefinalpHat5.
5]with70%seawaterandincubatedat28°Cunder12hlight/12hdarkcycleforsevendays.
Thefunguswasidentifiedbyoneofus(T.
Dethoup),bymorphologicalfeatures,includingcharacteristicofascospores,conidiogenesisandcolonies,aswellasbyDNAsequenceanalysisofthecalmodulingenedescribedbythepreviousreport[16](GenBankAccessionNo.
KC920702).
SincethesequencewasnotidenticaltothatdepositedatGenBank,thestrainwasnotidentifiedatspecieslevel.
ThepureculturesweredepositedasKUFA0013attheDepartmentofPlantPathology,FacultyofAgriculture,KasetsartUniversity,Bangkok,Thailand.
A.
similanensis(KUFA0013)wasculturedforoneweekinfive90mmPetridishes(i.
d.
90mm)containing25mLofMEAwith70%seawaterperdish.
Thirty1000mLErlenmeyerflasks,eachcontainingwhiterice(200g),water(30mL)andseawater(70mL),wereautoclavedat121°Cfor15min,inoculatedwithtenmyceliaplugsofthefungusandincubatedat28°Cfor30days.
Themoldyricewasmaceratedinethylacetate(7Ltotal)forsevendaysandthenfilteredbyfilterpaper.
Thetwolayerswereseparatedusingaseparatoryfunnel,andtheethylacetatesolutionwasevaporatedunderreducedpressuretoyield97gofcrudeethylacetateextractthatwasdissolvedin500mLofa4:1mixtureofEtOAcandCHCl3,andthenwashedwith5%NaHCO3aqueoussolution(2*300mL)andH2O(3*300mL).
TheorganiclayerwasdriedwithanhydrousNa2SO4,filteredandevaporatedunderreducedpressuretogive75gofcrudeextract,whichwasappliedonacolumnchromatographyofsilicagel(640g)andelutedwithmixturesofCHCl3–petrolandCHCl3–Me2CO,250mLfractionswerecollectedasfollows:Frs1–18(CHCl3–petrol,3:7),19–53(CHCl3–petrol,1:1),54–114(CHCl3–petrol,7:3),115–215(CHCl3–petrol,9:1),216–395(CHCl3–Me2CO,9:1),396–443(CHCl3–Me2CO,7:3).
Frs185–196werecombined(654mg)andpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)toMar.
Drugs2014,125169give4mgof1.
Frs197–221werecombined(1.
16g)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional107.
6mgofyellowsolidwhichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,98:2:0.
01)togive2.
5mgof1.
Fr222(8.
06g)wasrecrystallizedinamixtureofCHCl3andMe2COtogive238mgofwhiteprecipitate,whichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(32.
7mg),4(4.
6mg)andchevaloneB(4.
6mg).
ThemotherliquorwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(41.
0mg),4(7.
2mg)andchevaloneB(70.
8mg).
Themotherliquoroffrs197–221andfr222,andfrs223–224werecombined(9.
18g),appliedontheSigelcolumn(58g),andelutedwithmixturesofpetrol–CHCl3andCHCl3–Me2CO,wherein100mLsfrswerecollectedasfollows:sfrs1-59(petrol–CHCl3,3:7),60–69(petrol–CHCl3,1:9),70–76(CHCl3–Me2CO,9:1).
Sfrs11–22werecombined(1.
97g)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional16.
4mgof1.
Sfrs29–42werecombined(468mg)andcrystallizedinamixtureofpetrolandCHCl3togiveadditional92.
1mgof2b.
Frs225–228werecombined(446mg)andcrystallizedinamixtureofpetrolandCHCl3togive63mgofaprecipitatewhichwasfurtherpurifiedbyTLC(Sigel,CHCl3:Me2CO:HCO2H,97:3:0.
01)togive2b(35.
8mg)and2a(35.
4mg).
Themotherliquoroffrs225–228andfrs229–330werecombinedandchromatographedonaSigelcolumn(33g)andelutedwithmixturesofpetrol–CHCl3andCHCl3–Me2CO,wherein100mLsub-fractionswerecollectedasfollows:sfrs1–49(petrol–CHCl3,3:7),50–64(petrol–CHCl3,1:9),65–77(CHCl3–Me2CO,9:1).
Subfrs4–5werecombinedandrecrystallizedinamixtureofpetrolandCHCl3togive1(2.
4mg).
Sfrs6–10werecombined(160mg)andrecrystallizedinamixtureofpetrolandCHCl3togive2c(7.
6mg).
Sfrs11–16werecombined(108mg)andrecrystallizedinamixtureofpetroltogive2b(5mg).
Sfrs27-33werecombined(206mg)andpurifiedbyTLC(Sigel,CHCl3:Me2CO.
93:7)togivep-hydroxybenzaldehyde(36mg).
Frs231–247werecombined(6.
7g)andrecrystallizedinamixtureofpetrolandMe2COtogive1.
39gofchevaloneC.
Frs272–294werecombined(1.
54g)andcrystallizedinamixtureofpetrolandMe2COtoyield5(265mg).
Frs328-335werecombined(296mg)andappliedonacolumnofSephadexLH-20(22g)andelutedwithamixtureofCHCl3–MeOH(9:1)togive3(11.
2mg).
Frs354–398werecombined(1.
14g)andpurifiedbyTLC(Sigel,CHCl3:MeOH:HCO2H,95:5:0.
01)togive6(27.
3mg).
3.
2.
1.
SimilanpyroneA(1)Whitesolid,Mp322–323°C(petrol/CHCl3);UV(CHCl3)λmax(logε)240(4.
31),269(4.
31),295(3.
95),333(4.
23),358(4.
16)nm;IR(KBr)νmax3446,3010,2923,2851,1748,1698,1658,1634,1464,1177,1151cm1;1Hand13CNMR(Table1);HRESIMSm/z245.
0455(M+H)+(calculatedforC13H9O5,245.
0450).
3.
2.
2.
SimilanpyroneB(2b)Whitecrystals,Mp162–163°C(petrol/CHCl3);UV(CHCl3)λmax(logε)240(4.
35),277(3.
51),330(3.
46)nm;IR(KBr)νmax3243,3160,2923,2851,1677,1634,1617,1585,1571,1455,1256,1154,1110cm1;1Hand13CNMR(Table2);HRESIMSm/z207.
0658(M+H)+(calculatedforC11H11O4,207.
0657).
Mar.
Drugs2014,1251703.
2.
3.
ChevaloneE(3)Whitecrystals,Mp262–263°C(petrol/CHCl3);[α]D20146.
3°(c0.
04,CHCl3);IR(KBr)νmax3300,3016,2979,2950,2871,1664,1607,1570,1444,1288cm1;1HNMR(CDCl3,500.
13MHz)δ5.
99(1H,s,H-18),3.
21(1H,d,J=11.
1,5.
0,H-3),2.
55(1H,dd,J=16.
4,4.
9,H2-15),2.
20(3H,s,H3-20),2.
15(1H,m,H2-15),2.
14(1H,m,H2-12),1.
90(1H,dt,J=12.
8,3,H2-7),1.
74(1H,m,H2-11),1.
73(1H,m,H2-1),1.
71(1H,m,H2-12),1.
64(2H,m,H2-2),1.
59(1H,m,H2-6),1.
50(1H,dd,J=12.
5,4.
7,H-14),1.
45(1H,m,H2-6),1.
36(1H,m,H2-11),1.
28(3H,s,H3-26),1.
05(1H,m,H2-7),1.
00(1H,m,H2-1),0.
98(3H,s,H3-23),0.
93(1H,brd,J=13.
2,H-9),0.
89(3H,s,H3-25),0.
84(3H,s,H3-24),0.
78(1H,brd,J=11-6,H-5),0.
78(3H,s,H3-22);13CNMR(CDCl3,125.
8MHz)δ180.
6(CO,C-17),162(C,C-16),160.
5(C,C-21),111.
9(CH,C-18),98.
5(C,C-19),84.
3(C,C-13),78.
7(CH,C-3),60.
3(CH,C-9),55.
3(CH,C-5),52.
3(CH,C-14),41.
1(CH2,C-7),40.
1(CH2,C-12),38.
9(C,C-4),38.
4(CH2,C-1),37.
3(C,C-8),37.
1(C,C-10),28.
0(CH3,C-23),27.
2(CH2,C-2),20.
5(CH3,C-26),19.
2(CH3,C-20),18.
7(CH2,C-11),17.
9(CH2,C-6),16.
4(CH3,C-24),16.
1(CH3,C-25),15.
4(CH3,C-22),15.
2(CH3,C-15);HRESIMSm/z415.
2851(M+H)+(calculatedforC26H39O4,415.
2848).
3.
2.
4.
PyripyropeneS(6)Yellowviscousliquid,[α]D20+116.
3(c0.
04,CHCl3);IR(KBr)νmax2923,2851,1742,1671,1624,1586,1508,1465,1374,1242,1043cm1;1Hand13CNMR(Table3);HRESIMSm/z566.
2415(M+H)+(calculatedforC31H36NO9,566.
2390).
3.
3.
X-rayCrystalStructureofChevaloneE(3)CrystalssuitableforX-raydiffractionwereobtainedbyslowevaporationofasolutioninpetroleumether/chloroform.
Theywereorthorhombic,spacegroupP212121,cellvolume2310.
9(1)3andunitcelldimensionsa=8.
2325(2),b=11.
3341(3)andc=24.
7665(6).
Diffractiondatawerecollectedat293KwithaGeminiPXUltraequippedwithCuKαradiation(λ=1.
54184).
ThestructuresweresolvedbydirectmethodsusingSHELXS-97andrefinedwithSHELXL-97.
Carbon,oxygenandnitrogenatomswererefinedanisotropically.
Hydrogenatomswererefinedfreelywithisotropicdisplacementparameters.
TherefinementconvergedtoR(alldata)=6.
38%andwR2(alldata)=10.
21%.
Towardstheendofrefinementtheabsolutestructureparameterx(Flackxparameter)wasrefinedatthesametimeasallotherparameters,usingtheTWINinstructionwiththedefaultmatrixR=(100,010,001)andBASFwithoneparameter(x),toreachthefinalvalueofx=0.
0(3).
Theinvertedstructure,obtainedwiththeinstructionMOVE1111,yieldedx=1.
5(3).
Tablescontainingthefinalfractionalcoordinates,temperatureparameters,bonddistances,andbondanglesweredepositedwiththeCambridgeCrystallographicDataCentre:CCDCreferencenumber1002416.
3.
4.
AntimicrobialActivityAssays3.
4.
1.
BacterialStrainsFortheantimicrobialassays,thecompoundsweretestedagainst:bacterialreferencestrains(StaphylococcusaureusATCC25923,BacillussubtilisATCC6633,EscherichiacoliATCC25922andMar.
Drugs2014,125171PseudomonasaeruginosaATCC27853),CandidaalbicansATCC10231andmultidrug-resistantbacteriaisolatedfromtheenvironment,S.
aureusB1(isolatedfrompublicbus),EnterococcusfaecalisW1(isolatedfromriverwater)andE.
coliG1(isolatedfromseagullfeces).
BacteriaweregrowninMueller-Hintonagar(MH-BioKardiagnostics,Allonne,France)fromstockcultures,whileC.
albicanswasgrowninSabourauddextroseagar(SAB-BioKardiagnostics,Allonne,France).
MHandSABplateswereincubatedat37°Cpriortoobtainfreshculturesforeachinvitrobioassay.
3.
4.
2.
DeterminationofMinimumInhibitoryandBactericidal/FungalConcentrationsTheminimuminhibitoryconcentrations(MIC)ofthecompoundsweredeterminedusingabrothmicrodilutiontechnique,followingtherecommendationsoftheClinicalandLaboratoryStandardsInstitute[17].
Stocksolutionsof10mg/mL,preparedindimethylsulfoxide(DMSO-ApplichemGmbH,Darmstadt,Germany),wereseriallydilutedinMueller-Hintonbroth(MHB-BioKardiagnostics,Allonne,France)toachievein-testconcentrationsrangingfrom2to256g/mL.
EachbacterialinoculumwaspreparedinMHB,whileC.
albicansinoculumwaspreparedinRPMI-1640withL-glutamine,withMOPSandwithoutNaHCO3(Lonza,Walkersville,MD,USA).
Allinoculawerestandardizedinorderobtainaconcentrationof5*105CFU/mLineachinoculatedwellofthemicrotiterplate.
TheconcentrationofDMSOinthehighestin-testconcentrationdidnotaffectthemicrobialgrowth.
TheMICwasdefinedasthelowestconcentrationofcompoundthathasinhibitedthevisiblegrowth.
3.
4.
3.
SynergisticStudies3.
4.
3.
1.
ScreeningofCombinedEffectbetweentheCompoundsandAntibioticsAscreeningsusceptibilitytesttoassessthecombinedeffectbetweenthecompoundsandantibioticswasconductedusingthediscdiffusionmethodonMH,accordingtotheprocedurealreadydescribedbyGomesetal.
[9].
3.
4.
3.
2.
SynergyTest:CheckerboardMethodBasedontheresultsofthepreviousassay,potentialsynergybetween3andoxacillinorampicillin(Sigma-Aldrich,St.
Louis,MO,USA)wascheckedusingabrothmicrodilutioncheckerboardmethodandtestedinMRSAisolate(S.
aureusB1),ashasbeenalreadydescribed[9].
Twoindependentexperimentsinduplicatewereperformed.
Thefractionalinhibitoryconcentration(FIC)wascalculatedasfollows:FICofdrugA(FICA)=MICofdrugAincombination/MICofdrugAalone,andFICofdrugB(FICB)=MICofdrugBincombination/MICofdrugBalone.
TheFICindex(ΣFIC),calculatedasthesumofeachFIC,wasinterpretedasfollows:ΣFIC≤0.
5,synergy;0.
5<ΣFIC≤4,nointeraction;4<ΣFIC,antagonism[18].
4.
ConclusionsAlthoughseveralanalogsofchevalonehavebeenreportedfromseveralmembersofthegenusAspergillus,thisisthefirstreportofisolationofisocoumarinderivativesfromamemberofthisgenus.
Mar.
Drugs2014,125172ThesynergismofchevaloneEwiththeantibioticoxacillinagainstMRSAcanbeconsideredrelevantforanti-infectivemarinenaturalproductsresearch.
AcknowledgmentsThisworkwaspartiallysupportedbytheProjectMARBIOTECH(referenceNORTE-07-0124-FEDER-000047)withintheSR&TDIntegratedProgramMARVALOR—Buildingresearchandinnovationcapacityforimprovedmanagementandvalorizationofmarineresources,supportedbytheProgramaOperacionalRegionaldoNorte(ON.
2—ONovoNorte)andbytheEuropeanRegionalDevelopmentFund,andalsobyFCT—FundaoparaaCiênciaeaTecnologiaundertheprojectCEQUIMED-PEst-OE/SAU/UI4040/2014,FEDERfundsthroughtheCOMPETEprogramundertheprojectFCOMP-01-0124-FEDER-011057.
WethankMickLeeoftheDepartmentofChemistry,LeicesterUniversity(UK),forprovidingtheHRESIMS.
C.
P.
thankstheFacultyofPharmaceuticalSciences,BuraphaUniversity,ThailandforherscholarshiptotheUniversityofPorto.
WethankJúliaBessaandSaraCravofortechnicalsupport.
AuthorContributionsPrompanya,C.
performedisolation,purificationandstructureelucidationofsomecompounds;Kijjoa,A.
andPinto,M.
M.
M.
conceived,designedtheresearch,elucidatedthestructureofthecompoundsandwrotethepaper;Dethoup,T.
isolated,identified,culturedthefungi,andpreparedthecrudeextract;Gales,L.
performedX-raycrystallographyofcompound3;Silva,A.
M.
S.
providedtheNMRspectra;Bessa,L.
J.
andCosta,P.
M.
performedantibacterialactivity.
ConflictsofInterestTheauthorsdeclarenoconflictofinterest.
References1.
Asami,Y.
;Kakeya,H.
;Onose,R.
;Yoshida,A.
;Matsuzaki,H.
;Osada,H.
Azaspirene:Anovelangiogenesisinhibitorcontaininga1-oxa-7-azaspiro[4.
4]non-2-ene-4,6-dioneskeletonproducedbythefungusNeosartoryasp.
Org.
Lett.
2002,4,2845–2848.
2.
Asami,Y.
;Kakeya,H.
;Onose,R.
;Chang,Y.
H.
;Toi,M.
;Osada,H.
RK-805,anendothelial-cell-growthinhibitorproducedbyNeosartoryasp.
,andadockingmodelwithmethionineaminopeptidase-2.
Tetrahedron2004,60,7085–7091.
3.
Jayasuriya,H.
;Zink,D.
;Basilio,A.
;Vicente,F.
;Collado,J.
;Bills,G.
;Goldman,M.
L.
;Motyl,M.
;Huber,J.
;Dezeny,G.
;etal.
DiscoveryandantibacterialactivityofglabramycinA–CfromNeosartoryaglabrabyanantisensestrategy.
J.
Antibiotics2009,62,265–269.
4.
Yang,S.
S.
;Wang,G.
J.
;Cheng,K.
F.
;Chen,C.
H.
;Ju,Y.
M.
;Tsau,Y.
J.
;Lee,T.
H.
Bioactiveγ-lactonesfromthefermentedbrothofNeosartoryasp.
PlantaMed.
2010,76,1701–1705.
5.
Kijjoa,A.
;Santos,S.
;Dethoup,T.
;Manoch,L.
;Almeida,A.
P.
;Vasconcelos,M.
H.
;Silva,A.
;Gales,L.
;Herz,W.
Sartoryglabrins,analogsofardeemins,fromNeosartoryaglabra.
Nat.
Prod.
Commun.
2011,6,807–812.
Mar.
Drugs2014,1251736.
Eamvijarn,A.
;Kijjoa,A.
;Bruyère,C.
;Mathieu,V.
;Manoch,V.
;LeFranc,F.
;Silva,A.
;Kiss,R.
;Herz,W.
SecondarymetabolitesfromacultureofthefungusNeosartoryapseudofischeriandtheirinvitrocytostaticactivityinhumancancercells.
PlantaMed.
2012,78,1767–1776.
7.
Buttachon,S.
;Chandrapatya,A.
;Manoch,L.
;Silva,A.
;Gales,L.
;Bruyère,C.
;Kiss,R.
;Kijjoa,A.
Sartorymensin,anewindolealkaloid,andnewanaloguesoftryptoquivalineandfiscalinsproducedbyNeosartoryasiamensis(KUFC6349).
Tetrahedron2012,68,3253–3262.
8.
Eamvijarn,A.
;Gomes,N.
M.
;Dethoup,T.
;Buaruang,J.
;Manoch,L.
;Silva,A.
;Pedro,M.
;Marini,I.
;Roussis,V.
;Kijjoa,A.
BioactivemeroditerpenesandindolealkaloidsfromthesoilfungusNeosartoryafischeri(KUFC6344),andthemarine-derivedfungiNeosartoryalaciniosa(KUFC7896)andNeosartoryatsunodae(KUFC9213).
Tetrahedron2013,69,8583–8591.
9.
Gomes,N.
M.
;Bessa,L.
J.
;Buttachon,S.
;Costa,P.
M.
;Buaruang,J.
;Dethoup,T.
;Silva,A.
M.
S.
;Kijjoa,A.
AntibacterialandAntibiofilmactivitiesoftryptoquivalinesandmeroditerpenesisolatedfromthemarine-derivedfungiNeosartoryapaulistensis,N.
laciniosa,N.
tsunodae,andtheSoilFungiN.
fischeriandN.
siamensis.
Mar.
Drugs2014,12,822–839.
10.
Kanokmedhakul,K.
;Kanokmedhakul,S.
;Suwannatrai,R.
;Soytong,K.
;Prabpai,S.
;Kongsaeree,P.
BioactivemeroterpenoidsandalkaloidsfromthefungusEurotiumchevalieri.
Tetrahedron2011,67,5461–5468.
11.
Ryoo,I.
J.
;Xu,G.
H.
;Km,Y.
H.
;Choo,S.
J.
;Ahn,J.
S.
;Bae,K.
;Yoo,I.
D.
Reticulone,anovelfreeradicalscavengerproducedbyAspergillussp.
J.
Microbiol.
Biotechnol.
2009,12,1573–1575.
12.
Gallo,M.
B.
C.
;Cavalcanti,B.
C.
;Barros,F.
W.
A.
;Moraes,M.
O.
;Costa-Latufo,L.
V.
;Pessoa,C.
;Bastos,J.
K.
;Pupo,M.
T.
ChemicalconstituentsofPapulasporaimmersa,anendophytefromSmallanthussonchifolius(Asteraceae),andtheircytotoxicactivity.
Chem.
Biodivers.
2010,7,2941–2950.
13.
Erkel,G.
;Rether,J.
;Anke,T.
S14-95,anovelinhibitoroftheJAK/STATpathwayfromaPenicilliumspecies.
J.
Antibiotics2003,56,337–343.
14.
Tomoda,H.
;Tabata,N.
;Yang,D.
J.
;Takaya.
naki,H.
;Nishida,M.
;Omura,S.
Pyripyropenes,novelACATinhibitorsproducedbyAspergillusfumigatus.
III.
StructureelucidationofpyripyropenesEtoL.
J.
Antibiotics1995,48,495–503.
15.
Obata,R.
;Sunazuka,T.
;Tomoda,H.
;Harigaya,Y.
;Omura,S.
ChemicalModificationandstructure-activityrelationshipsofpyripyropenes,potent,bioavailableinhibitorofacyl-CoA:cholesterolO-acyltransferase(ACAT).
Bioorg.
Med.
Chem.
Lett.
1995,5,2683–2688.
16.
Glass,N.
L.
;Donaldson,G.
C.
DevelopmentofprimersetsdesignedforusewiththePCRtoamplifyconservedgenesfromfilamentousascomycetes.
Appl.
Environ.
Microbiol.
1995,61,1323–1330.
17.
Franklin,R.
;Cockerill,M.
D.
,III.
PerformanceStandardsforAntimicrombialSusceptibilityTesting.
Twenty-FirstInformationalSupplementM100-S21;ClinicalandLaboratoryStandardsInstitute(CLSI):Wayne,PA,USA,2011.
18.
Odds,F.
C.
Synergy,antagonism,andwhatthechequerboardputsbetweenthem.
J.
Antimicrob.
Chemother2003,52,1.
2014bytheauthors;licenseeMDPI,Basel,Switzerland.
ThisarticleisanopenaccessarticledistributedunderthetermsandconditionsoftheCreativeCommonsAttributionlicense(http://creativecommons.
org/licenses/by/4.
0/).
- 11.278090lu.com相关文档
- characterized8090lu.com
- 工商会8090lu.com
- 地址8090lu.com
- 济南市8090lu.com
- 辛辛那提8090lu.com
- "ESMAppendixII:Brothersgeochemistrydata"
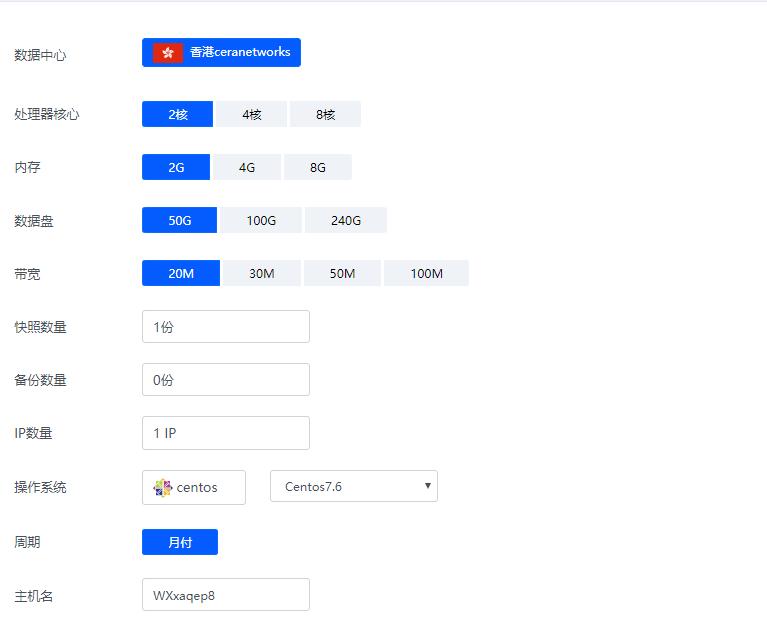
香港ceranetworks(69元/月) 2核2G 50G硬盘 20M 50M 100M 不限流量
香港ceranetworks提速啦是成立于2012年的十分老牌的一个商家这次给大家评测的是 香港ceranetworks 8核16G 100M 这款产品 提速啦老板真的是豪气每次都给高配我测试 不像别的商家每次就给1核1G,废话不多说开始跑脚本。香港ceranetworks 2核2G 50G硬盘20M 69元/月30M 99元/月50M 219元/月100M 519元/月香港ceranetwork...
MineServer:香港CMI/洛杉矶GIA VPS,2核/2GB内存/20GB NVME/3.5TB流量/200Mbps/KVM,288元/年
mineserver怎么样?mineserver是一家国人商家,主要提供香港CN2 KVM VPS、香港CMI KVM VPS、日本CN2 KVM VPS、洛杉矶cn2 gia端口转发等服务,云服务器网(yuntue.com)介绍过几次,最近比较活跃。现在新推出了3款特价KVM VPS,性价比高,香港CMI/洛杉矶GIA VPS,2核/2GB内存/20GB NVME/3.5TB流量/200Mbps...

ReliableSite:美国服务器租用,洛杉矶/纽约/迈阿密等机房;E3-1240V6/64GB/1TSSD,$95/月
reliablesite怎么样?reliablesite是一家于2006年成立的老牌美国主机商,主要提供独服,数据中心有迈阿密、纽约、洛杉矶等,均免费提供20Gbps DDoS防护,150TB月流量,1Gbps带宽。月付19美金可升级为10Gbps带宽。洛杉矶/纽约/迈阿密等机房,E3-1240V6/64GB内存/1TB SSD硬盘/DDOS/150TB流量/1Gbps带宽/DDOS,$95/月,...
8090lu.com为你推荐
-
硬盘工作原理硬盘的读写原理中老铁路一带一路的火车是什么火车留学生认证留学生回国认证,是否要求需要在国外待满三年,还是只需要完成所需的三年课程?留学生认证留学生学历认证的意义是什么?同ip网站查询怎样查询一个ip绑了多少域名seo优化工具SEO优化工具哪个好用点啊?www.zjs.com.cn中国快递公司排名杨丽晓博客杨丽晓今年高考了吗?partnersonline国内有哪些知名的ACCA培训机构partnersonline国外外贸平台有哪些?