sampleszpanel
zpanel 时间:2021-01-03 阅读:()
1SupplementaryMaterialfor:"ComparativeClinicalUtilityofTumorGenomicTestingandCell-FreeDNAinMetastaticBreastCancer"KaraN.
MaxwellMDPhD1,DanielleSoucier-Ernst2,EminTahirovicPhD3,AndreaB.
TroxelScD7,EricaCarpenterPhD1,2,ChristopherColameco2,CandaceClark2,MichaelFeldmanMDPhD4,BijalKakrecha1,MelissaLanger8,JoyLee2,DavidLewis2,DavidLiebermanMS4,JenniferJ.
D.
MorrissettePhD4,MattR.
PaulBS5,Tien-chiPanMS5,StephanieYee1,NatalieShih4,LewisA.
ChodoshMDPhD2,5,6,AngelaDeMicheleMDMSCE1,2,31DepartmentofMedicine,DivisionofHematology-Oncology;2AbramsonCancerCenter;3DepartmentofBiostatisticsandEpidemiology;4DepartmentofPathologyandLaboratoryMedicine;5DepartmentofCancerBiology;6DepartmentofMedicine,DivisionofEndocrinology,DiabetesandMetabolismatthePerelmanSchoolofMedicineattheUniversityofPennsylvania;7DepartmentofPopulationHealth,NYUSchoolofMedicine,8UniversityofMarylandSchoolofMedicineTableofContentsSupplementaryMethods…2SupplementaryFigure1…3SupplementaryFigure2…4SupplementaryFigure3…5SupplementaryFigure4…6SupplementaryTable1…7SupplementaryTable2…8SupplementaryTable3…9SupplementaryTable4…10SupplementaryTable5ExcelSupplementaryTable6…122SUPPLEMENTARYMETHODSDetailsonpatients,informedconsent,andstudydesignPatientsaged18andoverwitheithernewlysuspected,untreatedmetastaticbreastcancer,orprogressingdisease,wereeligibleiftheyhad:1)historyofhistologically-confirmedprimarybreastcancer;2)clinicalorimagingevidencesuggestiveofrecurrentmetastaticbreastcancerinalocal,regional,ordistantlocation;3)willingnesstoundergoand/orprovidetissuefromarecentbiopsyofrecurrenttumorforbothclinicalandresearchtesting;and4)willingnesstoundergobloodspecimencollection.
Patientswereexcludediftheywereonanticoagulationthatcouldnotbeinterruptedforthepurposeofstudyprocedures.
Genomictestingwaspaidforthroughinsurancecoverage,oraresearchfund.
Consentedpatientsunderwentclinicalbiopsyofametastaticsiteselectedbyreviewofallimagingbyaninterventionalradiologist(addinitials).
Selectionwasonthebasisofthemostsafelyaccessiblelesion.
Uptofourcorebiopsiesoftumorwereobtainedandanalyzedaccordingtoastandardalgorithmfor(1)histology,(2)estrogen,progesteroneandHer2receptorsbyASCO/CAPguidelines,and(3)CLIA-approvedtumorgenomictesting.
WholebloodsamplesweresentforprocessingandanalysisbyCLIA-approvedcfDNAgenomictesting.
Clinicaldatawereabstractedfromelectronicmedicalrecordsandprimaryreportsbytrainedresearchcoordinators.
SupplementaryFigure1:METAMORPHStudySchema.
DiagramoftheclinicalandresearchactivitiesintheMETAMORPHstudy.
PatientswithametastaticbreastcancerdiagnosisareinvitedtoenrollinMETAMORPH.
Theyundergoaclinicalbiopsy,aresearchblooddrawandanoptionalbonemarrowaspiration.
Theresearchblooddrawissentforfurtheranalysisofcirculatingtumorcells(CTC)anddisseminatedtumorcells(DTC)(datanotshown).
TheresearchblooddrawisalsosenttoGuardantHealthfortheGuardant360assay.
Thebiopsysampleissenttotheresearchtissuerepositoryforfurtheranalysisbywholeexomesequencing(WES)andRNAseq(datanotshown).
Thebiopsyisalsosentforsurgicalpathology(SurgPath)forreceptorstatusandgenomictumortestingattheCenterforPersonalizedDiagnosis(CPD).
3SupplementaryFigure2:METAMORPHConsortdiagram.
Seventypatientswereconsentedtothestudy.
Fourpatientsdidnotundergobiopsyduetoclinicianjudgementthatbiopsywouldnotbewarranted,providing66samplesfortumoranalysis.
Receptorswereobtainedfor62patientsandgenomictumorprofiling(CPDresults)wereobtainedfor53patients.
Receptorswerenotobtainedinsixpatientstherewasnotumorfoundonthehistologicalreviewforfivesubjectsandwasstillpendingforonesubjectatthetimeoftheanalysis.
CPDresultswerenotobtainedinninepatientsbecausefourhadnegativebiopsies,threefaileddecalcificationofbonematerial,andtwohadothersomatictestingperformed.
Threepatientsdidnotundergoblooddrawbecauseoflosstofollowuporrefusalofbiopsyproviding67samplesavailableforcfDNAanalysis.
Guardant360version1panelresultswereobtainedfor35patients.
Twenty-ninepatientsdidnothavecfDAsentforassayandthreehadtheversion2panelperformed.
Consentedtostudy(n=70)M0clinicalbiopsy(n=66)Reasonsnobx(n=4)M0Receptors(n=60)CPDResults(n=53)BloodsampleavailableforcfDNA(plasmaaliquots)(n=67)Guardantv1Results(n=35)Reasonsnobloodsample(n=3)ReasonsnoGuardantresults(n=32)ReasonsnoCPDresults(n=9)Reasonsnoreceptors(n=6)4140034150006140031150011140032140033140042150004150007140037140044140023140039140036140040150003150009140017140043150013150002HRStatus-PrimaryHRStatus-Metastasis#tumormutations/VUSsPatientIDPIK3CAHotspotPIK3CAOtherTP53ERBB2AKT1EGFRKITPDGFRAPTENHormoneReceptorStatusHR+Her2HRHer2+TNBCHR+Her2UnkA:AmplificationAAAAAAA341100000111111120212SupplementaryFigure3:Mutationalspectrainthetumorcohort.
Heatmapshowingthealterationsidentifiedbymetastatictumortestinginthetumorcohort.
Individualpatientidentifiers(ID)listedwithannotationsforreceptorstatusofprimary(ifapplicable)andofthebiopsiedmetastasis.
Totalnumberofalterationsidentifiedbyassay.
OnlyalterationsingenessharedbetweenCPD_FullandCPD_PPPareshown.
5a.
b.
SupplementaryFigure4:Analysisoftimetoprogressiononstandardtreatmentstratifiedbygenomicbiomarkers.
(a)PatientswerestratifiedintoaPIK3CAmutationpositiveorPIK3CAmutationnegativegroup.
TimefrominitiationoftreatmenttofirstprogressionwasanalyzedadjustingforreceptorstatusofthemetastaticbiopsyAND.
(b)PatientswerestratifiedintotwogroupsbasedonthepresenceorabsenceofmutationalheterogeneitydefinedashavingdifferentmutationsidentifiedinthecfDNAandmetDNAassays.
TimefrominitiationoftreatmenttofirstprogressionwasanalyzedadjustingforreceptorstatusofthemetastaticbiopsyAND.
67SupplementaryTable1:AssaysperformedpersampleinMETAMORPHstudyStudyIDSubgroupTumorAssaycfDNAAssay30113-13-0001tumor/cfDNACPD_FullGH_v130113-13-0003tumor/cfDNACPD_FullGH_v130113-13-0005tumor/cfDNACPD_FullGH_v130113-14-0001tumor/cfDNACPD_FullGH_v130113-14-0002tumor/cfDNACPD_FullGH_v130113-14-0004tumor/cfDNACPD_FullGH_v130113-14-0005tumor/cfDNACPD_FullGH_v130113-14-0006tumor/cfDNACPD_FullGH_v130113-14-0007tumor/cfDNACPD_FullGH_v130113-14-0008tumor/cfDNACPD_FullGH_v130113-14-0009tumor/cfDNACPD_FullGH_v130113-14-0010tumor/cfDNACPD_FullGH_v130113-14-0011tumor/cfDNACPD_FullGH_v130113-14-0013tumor/cfDNACPD_FullGH_v130113-14-0014tumor/cfDNACPD_FullGH_v130113-14-0015tumor/cfDNACPD_FullGH_v130113-14-0016tumor/cfDNACPD_FullGH_v130113-14-0019tumor/cfDNACPD_FullGH_v130113-14-0021tumor/cfDNACPD_FullGH_v130113-14-0024tumor/cfDNACPD_FullGH_v130113-14-0026tumor/cfDNACPD_FullGH_v130113-14-0027tumor/cfDNACPD_FullGH_v130113-14-0028tumor/cfDNACPD_FullGH_v130113-14-0029tumor/cfDNACPD_FullGH_v130113-14-0030tumor/cfDNACPD_FullGH_v130113-14-0018tumor/cfDNACPD_PPPGH_v130113-14-0020tumor/cfDNACPD_PPPGH_v130113-14-0022tumor/cfDNACPD_PPPGH_v130113-15-0010tumor/cfDNACPD_FullGH_v230113-15-0017tumor/cfDNACPD_FullGH_v230113-15-0012tumor/cfDNACPD_PPPGH_v230113-15-0014tumor/cfDNACPD_PPPGH_v230113-14-0017tumorCPD_Full30113-14-0031tumorCPD_Full30113-14-0032tumorCPD_Full30113-14-0033tumorCPD_Full30113-14-0034tumorCPD_Full30113-14-0036tumorCPD_Full30113-14-0039tumorCPD_Full30113-14-0040tumorCPD_Full30113-14-0042tumorCPD_Full30113-14-0043tumorCPD_Full30113-15-0002tumorCPD_Full30113-15-0003tumorCPD_Full30113-15-0004tumorCPD_Full30113-15-0006tumorCPD_Full30113-15-0007tumorCPD_Full30113-15-0009tumorCPD_Full30113-15-0013tumorCPD_Full30113-14-0023tumorCPD_PPP30113-14-0037tumorCPD_PPP30113-14-0044tumorCPD_PPP30113-15-0011tumorCPD_PPP30113-13-0006cfDNAGH_v230113-15-0015cfDNAGH_v230113-15-0018cfDNAGH_v28SupplementaryTable2:GenesfoundontheclinicalassaysusedinstudyGeneCPD_Full1CPD_PPPGH_v1GH_v22AKT1XXXXALKXXXXBRAFXXXXEGFRXXXXERBB2XXXXHRASXXXXIDH1XXXXKITXXXXKRASXXXXMETXXXXNOTCH1XXXXNRASXXXXPDGFRAXXXXPIK3CAXXXXPTENXXXXRETXXXXTP53XXXXAPCXXXATMXXXCDH1XXXCTNNB1XXXFBXW7XXXFGFR1XXXFGFR2XXXFGFR3XXXGNA11XXXGNAQXXXGNASXXXHNF1AXXXJAK2XXXJAK3XXXMLH1XXXMPLXXXNPM1XXXPTPN11XXXSMAD4XXXSMOXXXSRCXXXSTK11XXXVHLXXXARXXCDKN2AXXCSF1RXXXERBB4XXEZH2XXFLT3XXIDH2XXXKDRXXMAP2K1XXMYCXXPROCXRB1XXSMARCB1XXTERTXX1GenesarenotcoveredinfullontheCPDpanelsandmayincludeonlycertainexons.
2OthergenesontheGH_v2assayincludeARAF,ARID1A,BRCA1,BRCA2,CCND1,CCND2,CCNE1,CDK4,CDK6,CDKN2B,ESR1,GATA3,MAP2K2,NF1,NFE2L2,NTRK1,RAF1,RHEB,RHOA,RIT1,ROS19SupplementaryTable3:ReceptordiscordancebetweenprimaryandmetastaticbreasttumorbiopsiesCategoryn%Averagetimetorecurrence(months)HR+>HR-512.
5%89HR->HR+25.
0%228Her2+>Her2-12.
5%124Her2->Her2+00.
0%n/aConcordant3382.
5%7310SupplementaryTable4:ReanalysisoftumorsequencingdataforsinglenucleotidevariantsidentifiedcoveredandreportableonbothassaysbutdetectedonlybycfDNAassayPatientIDGeneVariantcfDNAAFTumorAADTumorTDTumorAAF30113-14-0001EGFRp.
K860R0.
40%851510.
16%30113-14-0001JAK3p.
G721R0.
50%05242nd30113-14-0002JAK2p.
V617F0.
20%1328690.
45%30113-14-0002RETp.
A641V0.
30%895240.
08%30113-14-0005NPM1p.
K292T0.
40%759870.
12%30113-14-0009TP53p.
R175G0.
60%740890.
17%30113-14-0018TP53p.
V272M0.
30%100742710.
13%30113-14-0020TP53p.
R175H0.
80%10309070.
03%30113-14-0024JAK2p.
V617F0.
30%120150.
05%30113-14-0026JAK2p.
V617F0.
30%1870250.
26%cfDNA:cellfreeDNA,AF:allelefrequency,AAD:alternatealleledepth,TD:totaldepth,AAF:alternateallelefrequency11SupplementaryTable5isincludedasanExcelspreadsheet12SupplementaryTable6:Multivariateanalysisofpanel-specificmutationalloadandTP53mutationstatuswithrespecttotimetoprogressiononstandardtherapyVariablecoefexp(coef)se(coef)zzPanel-specificmutationalloadmultivariateanalysisHighmutationalload1.
18283.
26350.
47722.
480.
013ReceptorHR+/Her2--0.
50950.
60080.
4822-1.
060.
291ReceptorHer2+-0.
61400.
54120.
6292-0.
980.
329Priortreatmentlines-0.
02510.
97520.
1027-0.
240.
807Chemotherapyorhormonaltherapy1.
05222.
86410.
57041.
840.
065PresenceofTP53mutationmultivariateanalysisPresenceofTP53mutation1.
04302.
83780.
40232.
590.
0095ReceptorHR+/Her2--0.
18840.
82820.
5051-0.
370.
7092ReceptorHer2+-0.
62500.
53530.
6226-1.
000.
3154Priortreatmentlines-0.
00050.
99950.
0963-0.
010.
9959Chemotherapyorhormonaltherapy0.
77032.
16050.
52601.
460.
1431
MaxwellMDPhD1,DanielleSoucier-Ernst2,EminTahirovicPhD3,AndreaB.
TroxelScD7,EricaCarpenterPhD1,2,ChristopherColameco2,CandaceClark2,MichaelFeldmanMDPhD4,BijalKakrecha1,MelissaLanger8,JoyLee2,DavidLewis2,DavidLiebermanMS4,JenniferJ.
D.
MorrissettePhD4,MattR.
PaulBS5,Tien-chiPanMS5,StephanieYee1,NatalieShih4,LewisA.
ChodoshMDPhD2,5,6,AngelaDeMicheleMDMSCE1,2,31DepartmentofMedicine,DivisionofHematology-Oncology;2AbramsonCancerCenter;3DepartmentofBiostatisticsandEpidemiology;4DepartmentofPathologyandLaboratoryMedicine;5DepartmentofCancerBiology;6DepartmentofMedicine,DivisionofEndocrinology,DiabetesandMetabolismatthePerelmanSchoolofMedicineattheUniversityofPennsylvania;7DepartmentofPopulationHealth,NYUSchoolofMedicine,8UniversityofMarylandSchoolofMedicineTableofContentsSupplementaryMethods…2SupplementaryFigure1…3SupplementaryFigure2…4SupplementaryFigure3…5SupplementaryFigure4…6SupplementaryTable1…7SupplementaryTable2…8SupplementaryTable3…9SupplementaryTable4…10SupplementaryTable5ExcelSupplementaryTable6…122SUPPLEMENTARYMETHODSDetailsonpatients,informedconsent,andstudydesignPatientsaged18andoverwitheithernewlysuspected,untreatedmetastaticbreastcancer,orprogressingdisease,wereeligibleiftheyhad:1)historyofhistologically-confirmedprimarybreastcancer;2)clinicalorimagingevidencesuggestiveofrecurrentmetastaticbreastcancerinalocal,regional,ordistantlocation;3)willingnesstoundergoand/orprovidetissuefromarecentbiopsyofrecurrenttumorforbothclinicalandresearchtesting;and4)willingnesstoundergobloodspecimencollection.
Patientswereexcludediftheywereonanticoagulationthatcouldnotbeinterruptedforthepurposeofstudyprocedures.
Genomictestingwaspaidforthroughinsurancecoverage,oraresearchfund.
Consentedpatientsunderwentclinicalbiopsyofametastaticsiteselectedbyreviewofallimagingbyaninterventionalradiologist(addinitials).
Selectionwasonthebasisofthemostsafelyaccessiblelesion.
Uptofourcorebiopsiesoftumorwereobtainedandanalyzedaccordingtoastandardalgorithmfor(1)histology,(2)estrogen,progesteroneandHer2receptorsbyASCO/CAPguidelines,and(3)CLIA-approvedtumorgenomictesting.
WholebloodsamplesweresentforprocessingandanalysisbyCLIA-approvedcfDNAgenomictesting.
Clinicaldatawereabstractedfromelectronicmedicalrecordsandprimaryreportsbytrainedresearchcoordinators.
SupplementaryFigure1:METAMORPHStudySchema.
DiagramoftheclinicalandresearchactivitiesintheMETAMORPHstudy.
PatientswithametastaticbreastcancerdiagnosisareinvitedtoenrollinMETAMORPH.
Theyundergoaclinicalbiopsy,aresearchblooddrawandanoptionalbonemarrowaspiration.
Theresearchblooddrawissentforfurtheranalysisofcirculatingtumorcells(CTC)anddisseminatedtumorcells(DTC)(datanotshown).
TheresearchblooddrawisalsosenttoGuardantHealthfortheGuardant360assay.
Thebiopsysampleissenttotheresearchtissuerepositoryforfurtheranalysisbywholeexomesequencing(WES)andRNAseq(datanotshown).
Thebiopsyisalsosentforsurgicalpathology(SurgPath)forreceptorstatusandgenomictumortestingattheCenterforPersonalizedDiagnosis(CPD).
3SupplementaryFigure2:METAMORPHConsortdiagram.
Seventypatientswereconsentedtothestudy.
Fourpatientsdidnotundergobiopsyduetoclinicianjudgementthatbiopsywouldnotbewarranted,providing66samplesfortumoranalysis.
Receptorswereobtainedfor62patientsandgenomictumorprofiling(CPDresults)wereobtainedfor53patients.
Receptorswerenotobtainedinsixpatientstherewasnotumorfoundonthehistologicalreviewforfivesubjectsandwasstillpendingforonesubjectatthetimeoftheanalysis.
CPDresultswerenotobtainedinninepatientsbecausefourhadnegativebiopsies,threefaileddecalcificationofbonematerial,andtwohadothersomatictestingperformed.
Threepatientsdidnotundergoblooddrawbecauseoflosstofollowuporrefusalofbiopsyproviding67samplesavailableforcfDNAanalysis.
Guardant360version1panelresultswereobtainedfor35patients.
Twenty-ninepatientsdidnothavecfDAsentforassayandthreehadtheversion2panelperformed.
Consentedtostudy(n=70)M0clinicalbiopsy(n=66)Reasonsnobx(n=4)M0Receptors(n=60)CPDResults(n=53)BloodsampleavailableforcfDNA(plasmaaliquots)(n=67)Guardantv1Results(n=35)Reasonsnobloodsample(n=3)ReasonsnoGuardantresults(n=32)ReasonsnoCPDresults(n=9)Reasonsnoreceptors(n=6)4140034150006140031150011140032140033140042150004150007140037140044140023140039140036140040150003150009140017140043150013150002HRStatus-PrimaryHRStatus-Metastasis#tumormutations/VUSsPatientIDPIK3CAHotspotPIK3CAOtherTP53ERBB2AKT1EGFRKITPDGFRAPTENHormoneReceptorStatusHR+Her2HRHer2+TNBCHR+Her2UnkA:AmplificationAAAAAAA341100000111111120212SupplementaryFigure3:Mutationalspectrainthetumorcohort.
Heatmapshowingthealterationsidentifiedbymetastatictumortestinginthetumorcohort.
Individualpatientidentifiers(ID)listedwithannotationsforreceptorstatusofprimary(ifapplicable)andofthebiopsiedmetastasis.
Totalnumberofalterationsidentifiedbyassay.
OnlyalterationsingenessharedbetweenCPD_FullandCPD_PPPareshown.
5a.
b.
SupplementaryFigure4:Analysisoftimetoprogressiononstandardtreatmentstratifiedbygenomicbiomarkers.
(a)PatientswerestratifiedintoaPIK3CAmutationpositiveorPIK3CAmutationnegativegroup.
TimefrominitiationoftreatmenttofirstprogressionwasanalyzedadjustingforreceptorstatusofthemetastaticbiopsyAND.
(b)PatientswerestratifiedintotwogroupsbasedonthepresenceorabsenceofmutationalheterogeneitydefinedashavingdifferentmutationsidentifiedinthecfDNAandmetDNAassays.
TimefrominitiationoftreatmenttofirstprogressionwasanalyzedadjustingforreceptorstatusofthemetastaticbiopsyAND.
67SupplementaryTable1:AssaysperformedpersampleinMETAMORPHstudyStudyIDSubgroupTumorAssaycfDNAAssay30113-13-0001tumor/cfDNACPD_FullGH_v130113-13-0003tumor/cfDNACPD_FullGH_v130113-13-0005tumor/cfDNACPD_FullGH_v130113-14-0001tumor/cfDNACPD_FullGH_v130113-14-0002tumor/cfDNACPD_FullGH_v130113-14-0004tumor/cfDNACPD_FullGH_v130113-14-0005tumor/cfDNACPD_FullGH_v130113-14-0006tumor/cfDNACPD_FullGH_v130113-14-0007tumor/cfDNACPD_FullGH_v130113-14-0008tumor/cfDNACPD_FullGH_v130113-14-0009tumor/cfDNACPD_FullGH_v130113-14-0010tumor/cfDNACPD_FullGH_v130113-14-0011tumor/cfDNACPD_FullGH_v130113-14-0013tumor/cfDNACPD_FullGH_v130113-14-0014tumor/cfDNACPD_FullGH_v130113-14-0015tumor/cfDNACPD_FullGH_v130113-14-0016tumor/cfDNACPD_FullGH_v130113-14-0019tumor/cfDNACPD_FullGH_v130113-14-0021tumor/cfDNACPD_FullGH_v130113-14-0024tumor/cfDNACPD_FullGH_v130113-14-0026tumor/cfDNACPD_FullGH_v130113-14-0027tumor/cfDNACPD_FullGH_v130113-14-0028tumor/cfDNACPD_FullGH_v130113-14-0029tumor/cfDNACPD_FullGH_v130113-14-0030tumor/cfDNACPD_FullGH_v130113-14-0018tumor/cfDNACPD_PPPGH_v130113-14-0020tumor/cfDNACPD_PPPGH_v130113-14-0022tumor/cfDNACPD_PPPGH_v130113-15-0010tumor/cfDNACPD_FullGH_v230113-15-0017tumor/cfDNACPD_FullGH_v230113-15-0012tumor/cfDNACPD_PPPGH_v230113-15-0014tumor/cfDNACPD_PPPGH_v230113-14-0017tumorCPD_Full30113-14-0031tumorCPD_Full30113-14-0032tumorCPD_Full30113-14-0033tumorCPD_Full30113-14-0034tumorCPD_Full30113-14-0036tumorCPD_Full30113-14-0039tumorCPD_Full30113-14-0040tumorCPD_Full30113-14-0042tumorCPD_Full30113-14-0043tumorCPD_Full30113-15-0002tumorCPD_Full30113-15-0003tumorCPD_Full30113-15-0004tumorCPD_Full30113-15-0006tumorCPD_Full30113-15-0007tumorCPD_Full30113-15-0009tumorCPD_Full30113-15-0013tumorCPD_Full30113-14-0023tumorCPD_PPP30113-14-0037tumorCPD_PPP30113-14-0044tumorCPD_PPP30113-15-0011tumorCPD_PPP30113-13-0006cfDNAGH_v230113-15-0015cfDNAGH_v230113-15-0018cfDNAGH_v28SupplementaryTable2:GenesfoundontheclinicalassaysusedinstudyGeneCPD_Full1CPD_PPPGH_v1GH_v22AKT1XXXXALKXXXXBRAFXXXXEGFRXXXXERBB2XXXXHRASXXXXIDH1XXXXKITXXXXKRASXXXXMETXXXXNOTCH1XXXXNRASXXXXPDGFRAXXXXPIK3CAXXXXPTENXXXXRETXXXXTP53XXXXAPCXXXATMXXXCDH1XXXCTNNB1XXXFBXW7XXXFGFR1XXXFGFR2XXXFGFR3XXXGNA11XXXGNAQXXXGNASXXXHNF1AXXXJAK2XXXJAK3XXXMLH1XXXMPLXXXNPM1XXXPTPN11XXXSMAD4XXXSMOXXXSRCXXXSTK11XXXVHLXXXARXXCDKN2AXXCSF1RXXXERBB4XXEZH2XXFLT3XXIDH2XXXKDRXXMAP2K1XXMYCXXPROCXRB1XXSMARCB1XXTERTXX1GenesarenotcoveredinfullontheCPDpanelsandmayincludeonlycertainexons.
2OthergenesontheGH_v2assayincludeARAF,ARID1A,BRCA1,BRCA2,CCND1,CCND2,CCNE1,CDK4,CDK6,CDKN2B,ESR1,GATA3,MAP2K2,NF1,NFE2L2,NTRK1,RAF1,RHEB,RHOA,RIT1,ROS19SupplementaryTable3:ReceptordiscordancebetweenprimaryandmetastaticbreasttumorbiopsiesCategoryn%Averagetimetorecurrence(months)HR+>HR-512.
5%89HR->HR+25.
0%228Her2+>Her2-12.
5%124Her2->Her2+00.
0%n/aConcordant3382.
5%7310SupplementaryTable4:ReanalysisoftumorsequencingdataforsinglenucleotidevariantsidentifiedcoveredandreportableonbothassaysbutdetectedonlybycfDNAassayPatientIDGeneVariantcfDNAAFTumorAADTumorTDTumorAAF30113-14-0001EGFRp.
K860R0.
40%851510.
16%30113-14-0001JAK3p.
G721R0.
50%05242nd30113-14-0002JAK2p.
V617F0.
20%1328690.
45%30113-14-0002RETp.
A641V0.
30%895240.
08%30113-14-0005NPM1p.
K292T0.
40%759870.
12%30113-14-0009TP53p.
R175G0.
60%740890.
17%30113-14-0018TP53p.
V272M0.
30%100742710.
13%30113-14-0020TP53p.
R175H0.
80%10309070.
03%30113-14-0024JAK2p.
V617F0.
30%120150.
05%30113-14-0026JAK2p.
V617F0.
30%1870250.
26%cfDNA:cellfreeDNA,AF:allelefrequency,AAD:alternatealleledepth,TD:totaldepth,AAF:alternateallelefrequency11SupplementaryTable5isincludedasanExcelspreadsheet12SupplementaryTable6:Multivariateanalysisofpanel-specificmutationalloadandTP53mutationstatuswithrespecttotimetoprogressiononstandardtherapyVariablecoefexp(coef)se(coef)zzPanel-specificmutationalloadmultivariateanalysisHighmutationalload1.
18283.
26350.
47722.
480.
013ReceptorHR+/Her2--0.
50950.
60080.
4822-1.
060.
291ReceptorHer2+-0.
61400.
54120.
6292-0.
980.
329Priortreatmentlines-0.
02510.
97520.
1027-0.
240.
807Chemotherapyorhormonaltherapy1.
05222.
86410.
57041.
840.
065PresenceofTP53mutationmultivariateanalysisPresenceofTP53mutation1.
04302.
83780.
40232.
590.
0095ReceptorHR+/Her2--0.
18840.
82820.
5051-0.
370.
7092ReceptorHer2+-0.
62500.
53530.
6226-1.
000.
3154Priortreatmentlines-0.
00050.
99950.
0963-0.
010.
9959Chemotherapyorhormonaltherapy0.
77032.
16050.
52601.
460.
1431
- sampleszpanel相关文档
- coverzpanel
- mixedzpanel
- NEUTRIKzpanel
- zpanel有什么免费的主机管理系统推荐
- zpanelVFP是什么意思
- zpanel支持多个PHP版本切换的虚拟主机管理系统有哪些?
hostkvm:7折优惠-香港VPS韩国VPS,8折优惠-日本软银、美国CN2 GIA、新加坡直连VPS
hostkvm本月对香港国际线路的VPS、韩国CN2+bgp线路的VPS正在做7折终身优惠,对日本软银线路、美国CN2 GIA线路、新加坡直连线路的VPS进行8折终身优惠促销。所有VPS从4G内存开始支持Windows系统,当然主流Linux发行版是绝对不会缺席的!官方网站:https://hostkvm.com香港国际线路、韩国,7折优惠码:2021summer日本、美国、新加坡,8折优惠码:2...

飞讯云E5-2678V3 64GB,湖北十堰100G高防物理机330元/月
飞讯云官网“飞讯云”是湖北飞讯网络有限公司旗下的云计算服务品牌,专注为个人开发者用户、中小型、大型企业用户提供一站式核心网络云端部署服务,促使用户云端部署化简为零,轻松快捷运用云计算。飞讯云是国内为数不多具有ISP/IDC双资质的专业云计算服务商,同时持有系统软件著作权证书、CNNIC地址分配联盟成员证书,通过了ISO27001信息安全管理体系国际认证、ISO9001质量保证体系国际认证。 《中华...
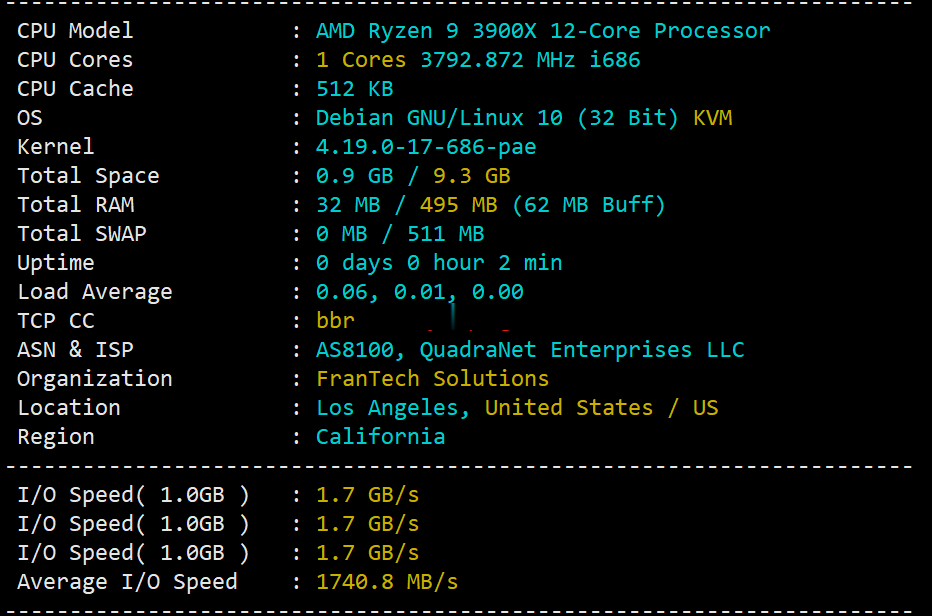
buyvm迈阿密机房VPS国内首发测评,高性能平台:AMD Ryzen 9 3900x+DDR4+NVMe+1Gbps带宽不限流量
buyvm的第四个数据中心上线了,位于美国东南沿海的迈阿密市。迈阿密的VPS依旧和buyvm其他机房的一样,KVM虚拟,Ryzen 9 3900x、DDR4、NVMe、1Gbps带宽、不限流量。目前还没有看见buyvm上架迈阿密的block storage,估计不久也会有的。 官方网站:https://my.frantech.ca/cart.php?gid=48 加密货币、信用卡、PayPal、...
zpanel为你推荐
-
域名价格域名费用大概是多少?美国虚拟空间国内虚拟空间与美国虚拟主机有什么不一样vps试用求永久免费vps服务器(要永久的)个人虚拟主机个人商城要选多大的虚拟主机?美国服务器托管美国服务器租用有那些机房,他们的优缺点是什么?海外域名外贸网站如何选择合适的海外域名?免费域名空间免费空间和免费域名重庆虚拟空间重庆虚拟主机租用那家好?虚拟主机是什么什么是虚拟主机?国内最好的虚拟主机国内安全性最好的虚拟主机空间商有哪些?